Highlights
-
•
Local recurrence of prostate cancer may be treated with salvage therapies.
-
•
Focal salvage high dose rate brachytherapy provides good biochemical control.
-
•
Severe genitourinary and gastrointestinal toxicities are low.
Keywords: Prostate cancer, Local recurrence, High dose rate brachytherapy, Focal salvage brachytherapy
Abstract
Introduction
Isolated local recurrence of prostate cancer following primary radiotherapy or brachytherapy may be treated with focal salvage high dose rate brachytherapy, although there remains an absence of high quality evidence to support this approach.
Methods
Men with prostate cancer treated consecutively between 2015 and 2018 using 19 Gy in a single fraction high dose rate brachytherapy (HDR) for locally recurrent prostate cancer were identified from an institutional database. Univariable analysis was performed to evaluate the relationship between patient, disease and treatment factors with biochemical progression free survival (bPFS).
Results
43 patients were eligible for evaluation. Median follow up duration was 26 months (range 1–60). Median bPFS was 35 months (95% confidence interval 25.6–44.4). Kaplan-Meier estimates for bPFS at 1, 2 and 3 years post salvage were 95.2%, 70.6% and 41.8% respectively. On univariable Cox regression analysis, only nadir PSA was significantly associated with bPFS although the majority of patients were also treated with androgen deprivation therapy. Only one late grade 3 genitourinary toxicity was observed.
Conclusion
Focal salvage HDR brachytherapy may provide good biochemical control with a low risk of severe toxicity. Further evaluation within clinical trials are needed to establish its role in the management of locally recurrent prostate cancer.
1. Introduction
Several modalities are available for the treatment of localised prostate cancer (PCa) including radical prostatectomy (RP), external beam radiotherapy (EBRT), brachytherapy and combination brachytherapy/EBRT. Despite technical advancements in the delivery of dose-escalated radiation to the prostate, locally persistent/recurrent disease may occur following primary treatment [1], [2], [3], [4].
Most patients with recurrent PCa are treated with palliative androgen deprivation therapy (ADT) [5]. However, for patients with confirmed isolated local disease, salvage therapies including low dose rate (LDR) and high dose rate (HDR) brachytherapy, high-intensity focussed ultrasound (HIFU), cryoablation and RP represent a radical alternative with the aim of local disease control and preventing development of metastases [6], [7].
Given the concerns regarding the toxicity of whole-gland salvage therapies, it has been suggested that focal salvage therapy using brachytherapy could be an effective but better tolerated alternative and some prospective early phase studies of focal salvage HDR brachytherapy have been reported [8], [9], [10]. Advancements in multiparametric magnetic resonance imaging (mp-MRI), positron emission tomography combined with computed tomography (PET-CT) and template-guided biopsies help to localise of the specific area of disease within the prostate to permit precise delivery of focal treatments [11], [12]. At this stage however, there remains limited clinical data and an absence of high quality evidence to support the use of focal salvage brachytherapy [8], [9], [10], [13], [14], [15].
We therefore present our efficacy and toxicity outcomes for patients treated at our institution with focal salvage HDR brachytherapy for locally recurrent PCa.
2. Methods
2.1. Patient population
Between 2015 and 2018, 43 patients consecutively treated for locally recurrent PCa using focal salvage HDR brachytherapy at a single centre were retrospectively identified from an institutional database. Primary disease was classified using National Comprehensive Cancer Network (NCCN) criteria version 4.2019. Intermediate risk disease was defined as the presence of one or more of the following factors: T2b-2c disease, prostate specific antigen (PSA) ≥ 10–20 ng/ml or Gleason score 7 (ISUP Grade 2 or 3). High risk disease was defined as the presence of one or more of the following factors: ≥T3a disease, PSA ≥ 20 ng/ml or Gleason score ≥ 8 (ISUP Grade 4 or 5). Radiological staging used the AJCC TNM 7 system. Relapse following primary treatment was identified by serial measurement of PSA. Radiological confirmation of locally recurrent disease was determined using a combination of mp-MRI pelvis (which includedT2-weighted (T2W), dynamic contrast-enhanced (DWE) and diffusion weighted (DWI) sequences) and whole body PET-CT (fludeoxyglucose 18 (18F) choline or 18F-fluorocyclobutane-1-carboxylic acid fluciclovine (FACBC, fluciclovine) PET-CT). Histological confirmation of locally recurrent PCa was identified using transperineal biopsy.
2.2. Treatment
2.2.1. Primary treatment
All patients received primary treatment using EBRT or LDR brachytherapy. Primary treatment with RP, prostate volume greater than 50 cc, IPSS score greater than 15 and medical unfitness for general anaesthesia were exclusion criteria.
2.2.2. HDR brachytherapy technique:
Focal salvage HDR brachytherapy was performed using transrectal ultrasound (TRUS) guidance under general anaesthesia. The gross tumour volume (GTV) was identified based on cognitive fusion of mp-MRI, PET-CT images, template-guided biopsy results and TRUS images. Metal catheters were inserted via transperineal approach to ensure adequate coverage of the GTV. The GTV, urethra and rectum were outlined on TRUS images by a consultant radiologist. The PTV was generated by an isotropic expansion of 3 mm in all directions but constrained by the rectum posteriorly. Treatment plans were generated using dose-volume histogram (DVH)-based inverse optimisation in Oncentra Prostate™ v4.2, with minor manual adjustments to dwell times applied if necessary to meet the plan objectives and constraints. The PTV dose objectives were 19 Gy in a single fraction to 100% isodose and D90 focal PTV > 17.1 Gy (90%). The organ at risk (OAR) constraints for the rectum were V100% =0, and D2cc < 12.35 Gy (65%) and for the urethra a hard constraint of D10% <20.9 Gy (110%).
2.2.3. Androgen deprivation therapy (ADT)
The majority of patients received ADT, either by 3 monthly LHRH agonist subcutaneous injection (Prostap 11.25 mg or Zoladex 10.8 mg) or bicalutamide 150 mg once daily tablet. Duration of ADT varied between 6 and 36 months in patients with hormone naïve PCa and continued to be administered in patients with castrate-resistant PCa. Where it was commenced for this relapse, ADT was started as neoadjuvant therapy 3 months prior to the BT implant and the remaining course was given as adjuvant therapy.
2.2.4. Follow up
As per our departmental protocol, patients were followed up using PSA at 6 monthly intervals for the first 3 years post treatment and annually thereafter. Relapse following focal salvage HDR brachytherapy was classified as either biochemical or radiological (whichever occurred earlier). Biochemical relapse was defined as a PSA of plus 2 ng/ml above the nadir value post salvage treatment. Radiological relapse was defined as radiological evidence of disease progression. Acute and late genitourinary (GU) and gastrointestinal (GI) toxicities were evaluated. Acute toxicity was defined as occurring less than 3 months following salvage treatment.
2.3. Study endpoints
The primary endpoint of this study was to evaluate biochemical progression free survival (bPFS) following focal salvage HDR brachytherapy. The secondary endpoint was to report the incidence of GU and GI toxicities, evaluated using Common Terminology Criteria for Adverse Events version 4.0 (CTCAE, National Cancer Institute).
2.4. Statistical analysis
Descriptive statistics were performed to evaluate patient, disease and treatment characteristics for both primary and recurrent disease. Statistical analysis was performed using IBM SPSS® version 21 (IBM, USA). Duration of follow up was calculated from the date of the HDR brachytherapy procedure. Actuarial bPFS survival curves were generated using the Kaplan-Meier method. Univariable Cox regression analyses were performed for the following variables to test for association with bPFS: primary disease risk category; primary tumour T stage; presenting PSA; primary tumour ISUP score (evaluated as individual scores and as groups 1–2, 3 and 4–5); primary prostate treatment; time to relapse after primary treatment; recurrent tumour ISUP score; ADT administered with salvage HDR brachytherapy or not; nadir PSA post salvage and D90 focal PTV value. A p value of <0.05 was taken to indicate a statistically significant association between the evaluated factor and bPFS.
3. Results
3.1. Primary treatment
A summary of baseline patient, disease and treatment characteristics is shown in Table 1. Median time between completion of primary treatment and first relapse (defined as either PSA plus 2 ng/ml above the nadir value post primary treatment or histological evidence of locally recurrent disease where PSA had not reached plus 2 ng/ml above the nadir value) was 70 months (95% confidence interval (CI) 60–80). First identifiable failure of primary treatment was biochemical in 39 patients (91%) and histological in 4 patients (9%).
Table 1.
Baseline patient, disease and treatment characteristics.
| Characteristic | Number (%) |
|---|---|
| Number of patients | 43 |
| Median age (range) | 70 (62–81) |
| Median presenting PSA (range) | 10.5 (3.4–178) |
| Primary tumour T stage | |
| T1 | 13 (31%) |
| T2 | 18 (42%) |
| T3a | 4 (9%) |
| T3b | 7 (16%) |
| T4 | 1 (2%) |
| Primary tumour ISUP grade | |
| 1 | 21 (49%) |
| 2 | 14 (33%) |
| 3 | 4 (9%) |
| 4 | 2 (5%) |
| 5 | 2 (5%) |
| Primary tumour risk category | |
| Low | 13 (30%) |
| Intermediate | 17 (40%) |
| High | 13 (30%) |
| Primary prostate cancer treatment | |
| EBRT | 15 (35%) |
| LDR brachytherapy | 28 (65%) |
| Hormone therapy with primary treatment | |
| None | 26 (60%) |
| 6 months | 5 (12%) |
| 2–3 years | 10 (23%) |
| >3 years | 1 (2%) |
| Unknown | 1 (2%) |
3.2. Focal salvage HDR brachytherapy
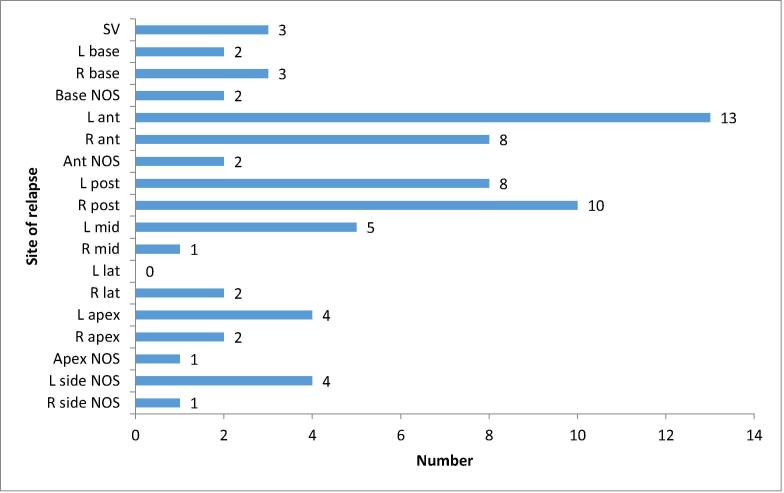
43 patients were evaluable. A summary of disease and treatment characteristics prior to focal salvage HDR brachytherapy is shown in Table 2. Staging investigations prior to salvage treatment included both MRI pelvis and PET-CT, MRI pelvis alone and PET-CT alone in 26, 9 and 8 patients respectively. All patients had histological confirmation of locally recurrent disease. In 37 cases (86%), this was done by trans-perineal biopsies from four quadrants (left and right superior and inferior) ± targeted biopsies. 3 patients (7%) underwent full template-guided biopsies. Fig. 1 illustrates the site of relapse for all 43 patients as evaluated by biopsy.
Table 2.
Characteristics of disease relapse prior to focal salvage HDR brachytherapy.
| Characteristic | Number (%) |
|---|---|
| Type of failure of primary treatment | |
| Biochemical | 39 (91%) |
| Histological | 4 (9%) |
| Median PSA at relapse (range) | 3.1 (1.1–7.5) |
| Type of biopsy | |
| Full template | 3 (7%) |
| Four quadrants | 24 (56%) |
| Four quadrants + targeted | 13 (30%) |
| Targeted only | 2 (5%) |
| TRUS | 1 (2%) |
| Re-biopsy ISUP grade | |
| 1 | 2 (5%) |
| 2 | 13 (30%) |
| 3 | 11 (26%) |
| 4 | 6 (14%) |
| 5 | 7 (16%) |
| Ungradable | 4 (9%) |
| ISUP upgraded at re-biopsy | |
| Yes | 27 (63%) |
| No | 12 (28%) |
| Ungradable | 4 (9%) |
| Imaging prior to salvage HDR brachytherapy | |
| MRI alone | 9 (21%) |
| PET-CT alone | 8 (19%) |
| Both MRI and PET-CT | 26 (60%) |
| Dose/fractionation of focal salvage HDR brachytherapy | 19 Gy in one fraction (100%) |
| ADT with focal salvage HDR brachytherapy | |
| None | 11 (26%) |
| 6 months | 19 (44%) |
| 2–3 years | 9 (21%) |
| Castrate resistant- continued hormone therapy | 4 (9%) |
Fig. 1.
Distribution of sites of relapse in 43 patients as evaluated by biopsy prior to focal salvage HDR brachytherapy. Note that the total number of relapses exceeds 43 since multiple sites were positive in some patients. L, left; R, right; ant, anterior gland; mid, mid gland; post, posterior gland; SV, seminal vesicle; NOS, not otherwise specified.
All patients were treated using 19 Gy in a single fraction. ADT was administered in 74% of cases- for 6 months in 44% and between 2 and 3 years in 21% of cases. 4 patients (9%) were castrate-resistant at time of salvage treatment and continued on ADT.
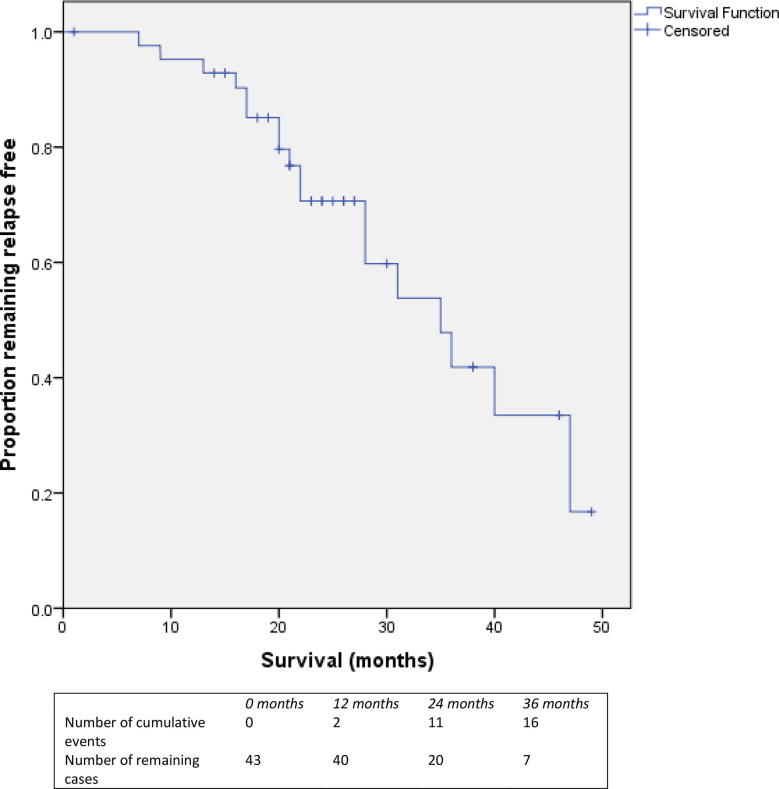
Median duration of follow up was 26 months (interquartile range 21.5–41.5, range 1–60). Median bPFS following focal salvage HDR brachytherapy was 35 months (95% CI 25.6–44.4) and is illustrated in Fig. 2. Kaplan-Meier estimates for bPFS at 1, 2 and 3 years post salvage were 95.2%, 70.6% and 41.8% respectively. Of the patient, disease and treatment-related factor evaluated using univariable Cox regression analysis, only nadir PSA was significantly associated with bPFS. Median nadir PSA post salvage was 0.24 ng/ml (range 0.01–2.8). But in patients with nadir PSA < 0.2 ng/ml, the median bPFS was 47 months compared with 28 months for those with nadir PSA > 0.2 ng/ml (HR 4.47, 95% CI 1.4–14.2, p = 0.06). No other factor evaluated by univariable analysis was significantly associated with bPFS. There appeared to be a non-significant trend to improved bPFS post salvage for patients with time to relapse after primary treatment longer than 30 months. Median bPFS was for those with a relapse free period > 30 months and < 30 months was 36 versus 21 months respectively (p = 0.288).
Fig. 2.
Kaplan-Meier survival plot showing biochemical progression free survival for the entire cohort (n = 43 patients).
17 patients experienced further disease progression following salvage HDR. Of these, the first treatment failure was biochemical progression in 14 patients and radiological evidence of progression in 3 patients. Median PSA at time of further relapse was 2.7 ng/ml (range 1.5–9.8). At the time of last follow up, the majority of patients who relapsed had either received no further treatment or were treated with ADT with palliative intent. Of patients who had undergone restaging imaging after disease progression was first detected, six patients had local recurrence alone, one had both local and regional nodal recurrence and one patient had developed distant metastatic disease. No patient had died. Table 3 summarises outcomes and further treatments following focal salvage HDR brachytherapy.
Table 3.
Outcomes following focal salvage HDR brachytherapy.
| Outcome | Number (%) |
|---|---|
| Median (range) nadir PSA | 0.27 (0.01–2.8) |
| Further disease relapse | |
| None | 25 (58%) |
| Biochemical progression | 9 (21%) |
| Local recurrence | 6 (14%) |
| Local and nodal recurrence | 1 (2%) |
| Distant metastatic disease | 1 (2%) |
| Median (range) PSA at time of relapse | 2.7 (1.83–16.5) |
| Further treatments received | |
| None | 12 (67% of 18 patients with relapse) |
| ADT/maximum androgen blockade | 4 (22%) |
| Enzalutamide/abiraterone | 2 (11%) |
| Chemotherapy | 1 (6%) |
| Radionuclide therapy | 1 (6%) |
3.3. HDR dosimetry
The planning dose objectives and achieved dosimetry are indicated in Table 4. The focal PTV D90 aim was adhered to by 95% of plans. Achievable coverage was dependent on the location of the Focal-PTV with respect to the organs at risk and in particular the urethra. All plans fulfilled the dose constraint for the rectum and the hard dose constraint for the urethra.
Table 4.
Planning objectives and achieved dosimetric parameters for focal salvage brachytherapy delivered in a single fraction of 19 Gy.
| Planning objective | Median value (range) | % of plans that adhered to objectives | |
|---|---|---|---|
| Focal PTV Volume | 19.4 cm3 (8.5–43.0 cm3) |
||
| Focal-PTV D90% (Gy) | >17.1 Gy | 18.6 Gy (15.1–20.4 Gy) |
95% |
| Focal PTV V19Gy (%) | >90% | 88.5% (64.0–95.9%) |
35%* |
| Urethra D10% (Gy) (Hard constraint**) | <20.9 Gy | 16.96 Gy (6.3–20.8 Gy) |
100% |
| Rectum D2cm3 (Gy) | <12.35 Gy | 8.3 Gy (3.1–12.1 Gy) |
100% |
* Note clinical protocol limited coverage to hard constraint of the urethra.
** Soft constraint for Urethra D10% was <14.25 Gy for patients treated before 23/02/2015 (50% of patients met soft constraint) and <17.1 Gy for patients treated after 23/02/2015 (61% of patients met soft constraint).
3.4. Toxicity
Incidences of acute and late GU and GI toxicities are shown in Table 5. ≤grade 2 acute GU and GI toxicities occurred in 39 (91%) and 6 (14%) patients respectively. ≤grade 2 late GU and GI toxicities occurred in 28 (65%) and 6 (14%) patients respectively. Only one (2%) grade 3 late GU toxicity was observed in a patient who underwent urethrotomy for a urethral stricture at 36 months.
Table 5.
Toxicity following focal salvage HDR brachytherapy.
| Toxicity | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| Acute genitourinary | 12 (28%) | 27 (63%) | 0 |
| Acute gastrointestinal | 6 (14%) | 0 | 0 |
| Late genitourinary | 10 (23%) | 18 (42%) | 1 (2%) |
| Late gastrointestinal | 6 (14%) | 0 | 0 |
4. Discussion
This single-centre, retrospective study represents one of the largest reported series of patients treated using focal salvage HDR brachytherapy for locally recurrent PCa. We evaluated bPFS and toxicity outcomes and these suggest that it is feasible to obtain good biochemical control with relatively low morbidity using this technique. Despite interest in whole gland and focal salvage HDR and LDR brachytherapy, there remains an absence of high quality evidence to guide their use in locally relapsed PCa. The European Association of Urology (EAU) PCa guidelines recommend salvage brachytherapy is not offered as a treatment for proven local recurrence because it is still experimental and requires further evaluation within clinical trials [6]. In a recent Delphi consensus study of salvage brachytherapy, agreement was reached for only 22% of questions concerning treatment technique, dosimetry and use of ADT and for approximately 50% of questions regarding patient selection and pre-treatment investigations [16].
Direct comparison of the findings of our study with other studies of salvage brachytherapy is challenging due to variations in the numbers of patients included, risk categories of primary disease, differences in pre-treatment diagnostic imaging and biopsy techniques, use of HDR versus LDR brachytherapy, different dose and fractionation schedules of HDR, use of whole gland versus focal gland treatments, use of different imaging-based planning techniques, use and duration of ADT and varying durations of follow up. Nevertheless, our median bPFS of 35 months is comparable to the prospective study of focal salvage HDR brachytherapy by Murgic et al. where median bPFS was 33 months [10]. In addition, our 2 and 3 year estimates for bPFS of 70.6% and 41.8% are comparable to the findings of the study by Chitmanee et al. where 2 and 3 year bPFS was 63% and 46% respectively [8]. Our 3 year estimate for bPFS of 41.8% is lower than the study by Murgic et al. (61%), although that study only evaluated 15 patients and our cohort included a greater proportion of patients with high risk characteristics which could potentially explain the lower result seen in our study [10]. Other studies of focal salvage HDR brachytherapy either included only small numbers or had limited follow up [9], [15]. Few studies of focal salvage LDR brachytherapy exist but this could be a promising technique with 3 year estimates for bPFS reported between 60 and 71% [13], [14].
In comparison, studies of whole gland salvage HDR or LDR brachytherapy have reported 3 and 5 year estimates of bPFS ranging from 46 to 88% and 20–87% respectively [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]. However, toxicity with whole gland salvage brachytherapy remains a concern. While our study is retrospective and did not include patient reported outcome measures (PROMs) and therefore is at risk of underrepresenting actual levels of toxicity, we only had one patient (2%) with a grade ≥ 3 GU toxicity. Our toxicity rates are similar to previous studies of focal salvage HDR and LDR brachytherapy [8], [9], [10], [13], [14], [15]. As a comparison, the rates of grade ≥ 3 GU and GI toxicities reported in previous studies of whole gland salvage brachytherapy are as high as 47% and 20% respectively [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38].
There is no clear consensus about the role of ADT with focal salvage HDR brachytherapy. In our study, where the use of and duration of ADT was at clinician discretion, 74% of patients received ADT and this was for 6 months in most cases. ADT was used in many previous studies of whole gland salvage brachytherapy but it was not used in several studies of focal salvage HDR and LDR brachytherapy [7], [10], [13], [14], [39], [40]. The rationale for this approach could be that salvage brachytherapy might avoid/delay the need to commence ADT and its associated toxicities and impact on quality of life [10].
There are few high quality studies directly comparing different imaging techniques in the recurrent PCa setting meaning that the optimal restaging imaging to select patients for salvage brachytherapy remains uncertain [11]. However, the majority of recent studies have focussed on the use of mp-MRI and PET-CT for local and distant staging. The poor sensitivity of computed tomography (CT) and isotope bone scintigraphy for the detection of metastatic PCa at PSA levels < 10 ng/ml has been previously demonstrated [41]. Since these imaging techniques were used to select patients in many of the previous studies of salvage brachytherapy, it has been suggested that this could be one reason for subsequent biochemical failure [10]. The majority of patients in our study were restaged after primary treatment failure using mp-MRI (81%) and PET-CT (79%). There is increasing evidence of high detection rates using mp-MRI for the evaluation of local recurrence, including after primary radiotherapy [11], [12]. Regarding metastatic disease, the best detection rates, especially at low PSA values and for small volume disease, may be seen with 68 Ga PSMA PET-CT [11], [42]. It might be that the optimum strategy for restaging prior to salvage brachytherapy is a combination of mp-MRI and 68 Ga PSMA PET-CT to evaluate local recurrence and exclude regional/distant metastases respectively [11].
In our study, the majority of patients underwent transperineal biopsies from four quadrants (± targeted biopsies) of the prostate with only 7% evaluated by transperineal prostate mapping (TPM). This meant that a precise description of the site of relapse provided by TPM was not possible in the majority of patients. The optimal method for histological confirmation of locally recurrent PCa remains unclear but there is increasing evidence to support the use of MRI-targeted biopsies and MRI/ultrasound fusion-targeted biopsies in the primary diagnostic setting [43], [44], [45], [46]. These techniques appear to detect more clinically significant cancers using fewer biopsy cores compared with standard biopsy techniques. In the recurrent PCa setting, a recent study compared MRI-targeted biopsies with TPM and reported similar detection rates for higher grade lesions but that TPM found a greater proportion of lower grade disease [12].
There remain uncertainties regarding which patients are most likely to benefit from salvage brachytherapy. In the treatment of PCa, local control appears to be associated with a reduction in subsequent development of distant metastatic disease and PCSM, which provides a rationale for treating isolated local recurrences with salvage therapies [47], [48]. However, there is a risk that patients with higher risk disease may be less likely to benefit from radical, and potentially toxic, salvage therapies since they are more likely to have occult micrometastatic disease that is simply not identified due to the limitations of current staging investigations [4], [49], [50]. Stratifying patients based on PSA kinetics may help predict the likely response to salvage brachytherapy. The PSA nadir post salvage was identified as a predictor of bPFS in previous studies [8], [22], [24], [32]. This is consistent with the results of our study, where nadir PSA < 0.2 ng/ml was associated with improved bPFS post salvage. However, a potential alternative explanation for this finding is that the majority of patients in our study received ADT in combination with focal salvage brachytherapy. Previous authors also reported that baseline PSA < 10 predicted for bPFS, although we were unable to replicate this finding in our study [20], [23], [25]. Time to relapse post primary treatment may also indicate the likely response to salvage brachytherapy since it was previously reported that a relapse free period > 30 months was associated with better bPFS [21], [23], [24]. We saw a trend regarding the same finding in our study, although the result was not statistically significant. In a recent meta-analysis of studies reporting outcomes after primary RT, patients with interval to biochemical failure < 18 months or Gleason score ≥ 8 (ISUP grade 4/5) had a higher risk of death from any cause and PCSM [51]. The authors recommended that these criteria could be used to classify patients with biochemical failure into low and high risk categories. This information could aid decision making regarding the potential benefits and toxicities from salvage therapy.
It is uncertain what the optimum dose/fractionation schedule is for focal salvage HDR brachytherapy. In the primary disease setting, there is concern that HDR monotherapy using 19 Gy in a single fraction provides insufficient disease control in comparison to fractionated regimens such as 27 Gy in 2 fractions [8], [52], [53], [54]. Despite its convenience, there has to be concern that single fraction treatments could also be inadequate in the setting of local recurrence. Therefore, future trials should evaluate fractionated rather than single fraction focal salvage HDR brachytherapy.
Strengths of this study include its relatively large numbers of patients in comparison to the majority of previous studies, especially studies of focal salvage brachytherapy. There was also consistency in terms of dose and fractionation for all patients in the series. Limitations of this study include its retrospective design and modest duration of follow up. Not all of our patients were restaged after primary treatment failure with both mp-MRI and PET-CT meaning that we could have underestimated the extent of local recurrence and distant disease. We have reported estimates of bPFS at 3 years whereas some other studies of whole gland salvage brachytherapy have described outcomes at 5 years or longer. This reflects the relatively recent development of focal salvage brachytherapy. We have evaluated association of various factors with bPFS using univariable analyses but given the relatively small numbers of patients in our study we did not perform a multivariable analysis. We also cannot be certain about our rates of local control and distant metastases for patients with biochemical failure post salvage and therefore are unable to report clinical progression free survival as an endpoint. This is because we performed serial measurements of PSA to evaluate response to salvage treatment rather than reassessment imaging/biopsies and at the time of biochemical failure restaging investigations were not consistently performed. Finally, although we describe clinician-assessment toxicities, we did not include PROMs and therefore could have underestimated actual levels of GU and GI toxicity.
5. Conclusions
In our retrospective study, focal salvage HDR brachytherapy appeared to achieve reasonable bPFS with low levels of severe GU and GI toxicity. Further evaluation within a clinical trial is required to establish its role in the treatment of locally recurrent PCa.
6. Sources of support
No specific funding was received for this study. This work was undertaken in Leeds Teaching Hospitals NHS Trust which receives funding from NHS England. The views expressed in this publication are those of the authors and not necessarily those of NHS England. F Slevin reports grants from Cancer Research UK Centres Network Accelerator Award Grant (A21993) to the Advanced Radiotherapy Technologies Network (ART-NET) consortium during the conduct of this study. AM Henry reports grants from Cancer Research UK (108036) during the conduct of this study; grants from NIHR (111218), grants from MRC (107154) and grants from Sir John Fisher Foundation (charity, no grant number) outside the submitted work.
Declaration of Competing Interest
None.
Contributor Information
Finbar Slevin, Email: finbarslevin@nhs.net.
Samantha Hodgson, Email: samantha.hodgson1@nhs.net.
Sree Lakshmi Rodda, Email: sreerodda@nhs.net.
Peter Bownes, Email: p.bownes@nhs.net.
David Bottomley, Email: davidm.bottomley@nhs.net.
Ese Adiotomre, Email: ese.adiotomre@nhs.net.
Bashar Al-Qaisieh, Email: bashar.al-qaisieh@nhs.net.
Emma Dugdale, Email: emma.dugdale@nhs.net.
Oliver Hulson, Email: oliverhulson@nhs.net.
Joshua Mason, Email: joshua.mason@nhs.net.
Jonathan Smith, Email: jonathan.smith18@nhs.net.
Ann M. Henry, Email: A.Henry@leeds.ac.uk.
References
- 1.Agarwal P.K., Sadetsky N., Konety B.R. Treatment failure after primary and salvage therapy for prostate cancer. Cancer. 2008;112(2):307–314. doi: 10.1002/cncr.23161. [DOI] [PubMed] [Google Scholar]
- 2.Lazarev S., Thompson M.R., Stone N.N. Low-dose-rate brachytherapy for prostate cancer: outcomes at >10 years of follow-up. BJU Int. 2018;121(5):781–790. doi: 10.1111/bju.14122. [DOI] [PubMed] [Google Scholar]
- 3.Zelefsky M.J., Kuban D.A., Levy L.B. Multi-institutional analysis of long-term outcome for stages T1–T2 prostate cancer treated with permanent seed implantation. Int J Rad Oncol Biol Phys. 2007;67(2):327–333. doi: 10.1016/j.ijrobp.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 4.Zumsteg Z.S., Spratt D.E., Romesser P.B. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur Urol. 2015;67(6):1009–1016. doi: 10.1016/j.eururo.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran H., Kwok J., Pickles T. Underutilization of local salvage therapy after radiation therapy for prostate cancer11Funding: UBC Summer Student Research Program. Urol Oncol. 2014;32(5):701–706. doi: 10.1016/j.urolonc.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 6.European Association of Urology. Prostate cancer. 2019. Available from: <https://uroweb.org/guideline/prostate-cancer/#note_484> [Accessed: 25/06/2019].
- 7.Steele E.M., Holmes J.A. A review of salvage treatment options for disease progression after radiation therapy for localized prostate cancer. Urol Oncol. 2019;37(9):582–598. doi: 10.1016/j.urolonc.2019.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Chitmanee PY, Tsang Hm Tharmalingam, et al. Single-dose focal salvage high dose rate brachytherapy for locally recurrent prostate cancer. Clin Oncol. [DOI] [PubMed]
- 9.Maenhout M., Peters M., van Vulpen M. Focal MRI-guided salvage high-dose-rate brachytherapy in patients with radiorecurrent. Prostate Cancer. 2017;16(6):1194–1201. doi: 10.1177/1533034617741797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murgic J., Morton G., Loblaw A. Focal salvage high dose-rate brachytherapy for locally recurrent prostate cancer after primary radiation therapy failure: results from a prospective clinical trial. Int J Rad Oncol Biol Phys. 2018;102(3):561–567. doi: 10.1016/j.ijrobp.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 11.De Visschere P.J.L., Standaert C., Fütterer J.J. A systematic review on the role of imaging in early recurrent prostate cancer. Eur Urol Oncol. 2019;2(1):47–76. doi: 10.1016/j.euo.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Kanthabalan A., Abd-Alazeez M., Arya M. Transperineal magnetic resonance imaging-targeted biopsy versus transperineal template prostate mapping biopsy in the detection of localised radio-recurrent prostate cancer. Clin Oncol (R Coll Radiol). 2016;28(9):568–576. doi: 10.1016/j.clon.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 13.Hsu C.C., Hsu H., Pickett B. Feasibility of MR imaging/MR spectroscopy-planned focal partial salvage permanent prostate implant (PPI) for localized recurrence after initial PPI for prostate cancer. Int J Rad Oncol Biol Phys. 2013;85(2):370–377. doi: 10.1016/j.ijrobp.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Peters M., Maenhout M., van der Voort J.R.N., van Zyp Focal salvage iodine-125 brachytherapy for prostate cancer recurrences after primary radiotherapy: a retrospective study regarding toxicity, biochemical outcome and quality of life. Radiother Oncol. 2014;112(1):77–82. doi: 10.1016/j.radonc.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Zamboglou C., Rischke H.-C., Meyer P.T. Single fraction multimodal image guided focal salvage high-dose-rate brachytherapy for recurrent prostate cancer. Cancer. 2016;8(3):241–248. doi: 10.5114/jcb.2016.61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaljouw E., Pieters B.R., Kovács G. A Delphi consensus study on salvage brachytherapy for prostate cancer relapse after radiotherapy, a Uro-GEC study. Radiother Oncol. 2016;118(1):122–130. doi: 10.1016/j.radonc.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Aaronson D.S., Yamasaki I., Gottschalk A. Salvage permanent perineal radioactive-seed implantation for treating recurrence of localized prostate adenocarcinoma after external beam radiotherapy. BJU Int. 2009;104(5):600–604. doi: 10.1111/j.1464-410X.2009.08445.x. [DOI] [PubMed] [Google Scholar]
- 18.Baumann B.C., Baumann J.C., Christodouleas J.P. Salvage of locally recurrent prostate cancer after external beam radiation using reduced-dose brachytherapy with neoadjuvant plus adjuvant androgen deprivation. Brachytherapy. 2017;16(2):291–298. doi: 10.1016/j.brachy.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Beyer D.C. Permanent brachytherapy as salvage treatment for recurrent prostate cancer. Urology. 1999;54(5):880–883. doi: 10.1016/s0090-4295(99)00241-1. [DOI] [PubMed] [Google Scholar]
- 20.Burri R.J., Stone N.N., Unger P. Long-term outcome and toxicity of salvage brachytherapy for local failure after initial radiotherapy for prostate cancer. Int J Rad Oncol Biol Phys. 2010;77(5):1338–1344. doi: 10.1016/j.ijrobp.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 21.Chen C.P., Weinberg V., Shinohara K. Salvage HDR brachytherapy for recurrent prostate cancer after previous definitive radiation therapy: 5-year outcomes. Int J Rad Oncol Biol Phys. 2013;86(2):324–329. doi: 10.1016/j.ijrobp.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Grado G.L., Collins J.M., Kriegshauser J.S. Salvage brachytherapy for localized prostate cancer after radiotherapy failure11Dr. Grado, Ms. Balch, and Ms. Grado are currently in private radiation oncology/prostate brachytherapy practice in Scottsdale, Arizona; Dr. Grado is also currently affiliated with Carle Clinic, Urbana, Illinois. Urology. 1999;53(1):2–10. doi: 10.1016/s0090-4295(98)00492-0. [DOI] [PubMed] [Google Scholar]
- 23.Henríquez I., Sancho G., Hervás A. Salvage brachytherapy in prostate local recurrence after radiation therapy: predicting factors for control and toxicity. Rad Oncol. 2014;9(1):102. doi: 10.1186/1748-717X-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henríquez López I., González-San Segundo C., Vegas J.O. Salvage brachytherapy for locally-recurrent prostate cancer after radiation therapy: a comparison of efficacy and toxicity outcomes with high-dose rate and low-dose rate brachytherapy. Radiother Oncol. 2019 doi: 10.1016/j.radonc.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Kollmeier M.A., McBride S., Taggar A. Salvage brachytherapy for recurrent prostate cancer after definitive radiation therapy: a comparison of low-dose-rate and high-dose-rate brachytherapy and the importance of prostate-specific antigen doubling time. Brachytherapy. 2017;16(6):1091–1098. doi: 10.1016/j.brachy.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Koutrouvelis P., Hendricks F., Lailas N. Salvage reimplantation in patient with local recurrent prostate carcinoma after brachytherapy with three dimensional computed tomography-guided permanent pararectal implant. Technol Cancer Res Treat. 2003;2(4):339–344. doi: 10.1177/153303460300200409. [DOI] [PubMed] [Google Scholar]
- 27.Loening S.A., Turner J.W. Use of percutaneous transperineal 198Au seeds to treat recurrent prostate adenocarcinoma after failure of definitive radiotherapy. Prostate. 1993;23(4):283–290. doi: 10.1002/pros.2990230403. [DOI] [PubMed] [Google Scholar]
- 28.Moman M.R., van der Poel H.G., Battermann J.J. Treatment outcome and toxicity after salvage 125-I implantation for prostate cancer recurrences after primary 125-I implantation and external beam radiotherapy. Brachytherapy. 2010;9(2):119–125. doi: 10.1016/j.brachy.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen P.L., Chen M.H., D'Amico A.V. Magnetic resonance image-guided salvage brachytherapy after radiation in select men who initially presented with favorable-risk prostate cancer: a prospective phase 2 study. Cancer. 2007;110(7):1485–1492. doi: 10.1002/cncr.22934. [DOI] [PubMed] [Google Scholar]
- 30.Peters M., van der Voort van Zyp J.R., Moerland M.A. Multivariable model development and internal validation for prostate cancer specific survival and overall survival after whole-gland salvage Iodine-125 prostate brachytherapy. Radiother Oncol. 2016;119(1):104–110. doi: 10.1016/j.radonc.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Peters M., van der Voort J.R.N., van Zyp M.A., Moerland Development and internal validation of a multivariable prediction model for biochemical failure after whole-gland salvage iodine-125 prostate brachytherapy for recurrent prostate cancer. Brachytherapy. 2016;15(3):296–305. doi: 10.1016/j.brachy.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Shimbo M., Inoue K., Koike Y. Salvage <sup>125</sup>I seed implantation for prostate cancer with postradiation local recurrence. Urol Int. 2013;90(3):294–300. doi: 10.1159/000346322. [DOI] [PubMed] [Google Scholar]
- 33.Vargas C., Swartz D., Vashi A. Salvage brachytherapy for recurrent prostate cancer. Brachytherapy. 2014;13(1):53–58. doi: 10.1016/j.brachy.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Wallner K.E., Nori D., Morse M.J. 125-iodine reimplantation for locally progressive prostatic carcinoma. J Urol. 1990;144:704–706. doi: 10.1016/s0022-5347(17)39560-5. [DOI] [PubMed] [Google Scholar]
- 35.Wojcieszek P., Szlag M., Głowacki G. Salvage high-dose-rate brachytherapy for locally recurrent prostate cancer after primary radiotherapy failure. Radiother Oncol. 2016;119(3):405–410. doi: 10.1016/j.radonc.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 36.Wong W.W., Buskirk S.J., Schild S.E. Combined prostate brachytherapy and short-term androgen deprivation therapy as salvage therapy for locally recurrent prostate cancer after external beam irradiation. J Urol. 2006;176(5):2020–2024. doi: 10.1016/j.juro.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Yamada Y., Kollmeier M.A., Pei X. A Phase II study of salvage high-dose-rate brachytherapy for the treatment of locally recurrent prostate cancer after definitive external beam radiotherapy. Brachytherapy. 2014;13(2):111–116. doi: 10.1016/j.brachy.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crook J.M., Zhang P., Pisansky T.M. A prospective phase 2 trial of transperineal ultrasound-guided brachytherapy for locally recurrent prostate cancer after external beam radiation therapy (NRG/RTOG0526): initial report of late toxicity outcome. Int J Rad Oncol Biol Phys. 2017;99(2 Suppl):S1. doi: 10.1016/j.ijrobp.2018.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ben-Josef E., Lawrence T.S. Using a bigger hammer: the role of stereotactic body radiotherapy in the management of oligometastases. J Clin Oncol. 2009;27(10):1537–1539. doi: 10.1200/JCO.2008.21.7299. [DOI] [PubMed] [Google Scholar]
- 40.Maenhout M., Peters M., van Vulpen M. Focal MRI-guided salvage high-dose-rate brachytherapy in patients with radiorecurrent prostate cancer. Technol Cancer Res Treat. 2017;16(6):1194–1201. doi: 10.1177/1533034617741797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hovels A.M., Heesakkers R.A., Adang E.M. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63(4):387–395. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 42.Perera M., Papa N., Roberts M. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 2019 doi: 10.1016/j.eururo.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 43.Kasivisvanathan V., Rannikko A.S., Borghi M. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378(19):1767–1777. doi: 10.1056/NEJMoa1801993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kasivisvanathan V., Stabile A., Neves J.B. Magnetic resonance imaging-targeted biopsy versus systematic biopsy in the detection of prostate cancer: a systematic review and meta-analysis. Eur Urol. 2019;76(3):284–303. doi: 10.1016/j.eururo.2019.04.043. [DOI] [PubMed] [Google Scholar]
- 45.Moore C.M., Robertson N.L., Arsanious N. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol. 2013;63(1):125–140. doi: 10.1016/j.eururo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Valerio M., Donaldson I., Emberton M. Detection of clinically significant prostate cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: a systematic review. Eur Urol. 2015;68(1):8–19. doi: 10.1016/j.eururo.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 47.Fuks Z., Leibel S.A., Wallner K.E. The effect of local control on metastatic dissemination in carcinoma of the prostate: long-term results in patients treated with 1251 implantation. Int J Rad Oncol Biol Phys. 1991;21(3):537–547. doi: 10.1016/0360-3016(91)90668-t. [DOI] [PubMed] [Google Scholar]
- 48.Zelefsky M.J., Reuter V.E., Fuks Z. Influence of local tumor control on distant metastases and cancer related mortality after external beam radiotherapy for prostate cancer. J Urol. 2008;179(4):1368–1373. doi: 10.1016/j.juro.2007.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morgan P.B., Hanlon A.L., Horwitz E.M. Timing of biochemical failure and distant metastatic disease for low-, intermediate-, and high-risk prostate cancer after radiotherapy. Cancer. 2007;110(1):68–80. doi: 10.1002/cncr.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou P., Chen M.-H., McLeod D. Predictors of prostate cancer-specific mortality after radical prostatectomy or radiation. Therapy. 2005;23(28):6992–6998. doi: 10.1200/JCO.2005.01.2906. [DOI] [PubMed] [Google Scholar]
- 51.Van den Broeck T., van den Bergh R.C.N., Arfi N. Prognostic value of biochemical recurrence following treatment with curative intent for prostate cancer: a systematic review. Eur Urol. 2019;75(6):967–987. doi: 10.1016/j.eururo.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Mendez L.C., Ravi A., Chung H. Pattern of relapse and dose received by the recurrent intraprostatic nodule in low- to intermediate-risk prostate cancer treated with single fraction 19 Gy high-dose-rate brachytherapy. Brachytherapy. 2018;17(2):291–297. doi: 10.1016/j.brachy.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Prada P.J., Cardenal J., Blanco A.G. High-dose-rate interstitial brachytherapy as monotherapy in one fraction for the treatment of favorable stage prostate cancer: toxicity and long-term biochemical results. Radiother Oncol. 2016;119(3):411–416. doi: 10.1016/j.radonc.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 54.Siddiqui Z.A., Gustafson G.S., Ye H. Five-year outcomes of a single-institution prospective trial of 19-Gy single-fraction high-dose-rate brachytherapy for low- and intermediate-risk prostate cancer. Int J Rad Oncol Biol Phys. 2019;104(5):1038–1044. doi: 10.1016/j.ijrobp.2019.02.010. [DOI] [PubMed] [Google Scholar]