Highlights
-
•
Immunotherapy is a new option for head and neck squamous cell carcinoma.
-
•
Very limited data are available for concurrent radio-immunotherapy in recurrent disease.
-
•
Our case report adds to this very limited series, focusing on oligopression under immunotherapy.
Keywords: Head and neck cancer, Immunotherapy, Re-irradiation, Oligoprogression, Intensity modulated radiotherapy, Nivolumab
Abstract
Immune checkpoint inhibitors (ICIs) represent a recently introduced class of agents active in head and neck squamous cell carcinoma (HNSCC). For a subgroup of patients with recurrent or metastatic disease, long-term benefit can be achieved: maintaining a sustained response to immunotherapy is therefore a critical factor for its efficacy at an individual level. In analogy to targeted agents, a limited pattern of progression, or “oligoprogression”, can occur. For locally recurrent HNSCC, the potential biologic interplay between the efficacy of ICIs and the design of radiation fields chosen for primary treatment is currently unknown. Here, we report on a patient who presented two subsequent oligoprogressions successfully treated with re-irradiation without interrupting Nivolumab. Both oligoprogressive lesions developed in previously unirradiated areas. We hypothesize the existence of a synergistic effect with optimal spatial cooperation between ICIs and re-irradiation for oligoprogressive disease under immunotherapy.
1. Introduction
Over the last 5 years, the therapeutic armamentarium of head and neck squamous cell carcinoma (HNSCC) has been enriched by the advent of immunotherapy. Currently, two drugs are FDA-approved in the recurrent/metastatic (R/M) setting: immune checkpoint inhibitors (ICIs) Nivolumab and Pembrolizumab are both indicated for use in platinum-refractory HNSCC, whereas Pembrolizumab can also be administered in first-line treatment as single agent for patients with Combined Positive Score (CPS) > 20 or in combination with platinum-5 FU chemotherapy for patients with CPS > 1, based on the results of Checkmate 141 [1], Keynote 040 [2] and 048 [3] trials, respectively. Spurred by biologic rationale [4] and pre-clinical evidence [5], several clinical trials were launched to investigate the potential synergistic effect of immunotherapy and radiotherapy (RT) in locally advanced and R/M HNSCC, however in clinical practice their combination is at present investigational. Amongst many yet unresolved questions regarding suitable RT total dose and fractionation, optimal timing of delivery and sequence of treatment in respect to immunotherapy, two main aspects are worth considering in locally recurrent HNSCC. First, is there a potential interplay between the efficacy of ICI’s, or the lack thereof, and the extent of radiation fields designed for primary RT? Second, can re-irradiation be safely performed once the patient has already been exposed to immunotherapy or concurrently with it? Here, we report on two subsequent marginal oligo-progressions developed under Nivolumab which were re-irradiated without interrupting the ICI.
2. Case
A 54-year old woman with previous tobacco exposure (10 pk/years) presented in February 2014 with a painless lump in the neck of 3 cm in right level IB. After proper workup, she was diagnosed with a squamous cell carcinoma of unknown primary of the head and neck and underwent a type 3 modified radical neck dissection with ipsilateral tonsillectomy. The pathologic report showed a 2.5 cm, p16 negative, single metastatic lymph node with extranodal extension (ENE) (pTxN1 according to TNM 7th edition). Adjuvant concurrent chemo-radiotherapy was completed in June 2014. Intensity modulated radiotherapy (IMRT) was delivered with Tomotherapy through a sequential plan, encompassing the following structures: the right submandibular lodge and ipsilateral level IB (high risk volume), oral cavity, bilateral palatine tonsils, level IA, right levels II, III and left level IB (intemediate risk), nasopharynx, base of tongue, aryepiglottic folds, pyriform sinuses, right level IV, V and left levels II and III (low risk) receiving 66 Gy, 60 Gy and 50 Gy at conventional fractionation, respectively (Fig. 1). A total of 280 mg/m2 of Cisplatin was also administered in 7 weekly introductions. The patient was free of disease until May 2017, when mucosal emergence of the primary tumor occurred: a 1 cm ulcerated lesion developed in the right upper retromolar trigone. One month after its transoral resection with clear margins, a lump developed in left level IIA. In July 2017 the patient underwent a left type 3 modified radical neck dissection which yielded 3 positive lymph nodes out of 20 located at levels IB with ENE, IIA and V, respectively (pTxN3b according to TNM 8th edition). A feasibility study for adjuvant re-irradiation was proposed but ultimately not performed for patient’s refusal. After three months from salvage surgery, a multifocal loco-regional relapse was diagnosed: necrotic bilateral retropharyngeal nodes (maximum diameter of 17 mm), a 1.2 × 1.7 cm pre-laryngeal lesion and a rounded lymph node of 12 mm at left level 3 were reported. First line chemotherapy was started in accordance with the Extreme regimen [6]. A partial remission was obtained after 3 cycles, with good tolerance. Overall, after 6 cycles and 10 maintenance bi-weekly doses of Cetuximab, in September 2018 progressive disease was observed: the target lesions were now a 2 cm right infra-parotid adenopathy and the known pre-laryngeal lesion which was more than 30% larger than in previous CT. Thus, second-line Nivolumab was started, at a fixed dose of 240 mg every 2 weeks. After 7 cycles, the patient noticed a fast growing lymphadenopathy in the left supraclavicular fossa. Over just 2 weeks, a fixed mass of 6 cm had developed (Fig. 2). A CT scan (11/1/19) confirmed the focal oligoprogression (Fig. 3), showing a marked regression of the other two pathologic findings and no distant lesions. The site of progressive disease was marginal to the primary IMRT field, since left level 4 had not been encompassed in the low-risk volume (Supplementary Fig. 1). A full re-irradiation course of 66 Gy at conventional fractionation was delivered with Volumetric Modulated Arc Therapy (VMAT) (Supplementary Fig. 2): the treatment was completed on 11/3/19 without interrupting Nivolumab (cycles 9 through 12). The toxicity profile was optimal, with a G1 radiation dermatitis but no unexpected side effects. Over the following weeks, a steady regression of the mass was observed. After less than two months from the end of re-irradiation, a similar pattern of rapid swelling occurred in the right parotid gland, an intentionally spared area of the first RT course (Supplementary Fig. 3). Taking into account the dosimetric summation of previous plans, we decided to re-irradiate this second oligoprogressive lesion with VMAT delivering 30 Gy at conventional fractionation, keeping the patient on Nivolumab (Supplementary Fig. 4). Right before the start of treatment on 17/5/19, the overlying skin was infiltrated, with a 4 × 3 cm lesion (Supplementary Fig. 5). During treatment, a 2 cm ulcerative loss developed, requiring the application of daily wound dressings. The treatment was completed on 10/6/19, with mild toxicity. A complete healing of the skin developed over the following weeks. Apart from the known left supraclavicular adenopathy, now down to 1 cm, no other evidence of disease was found at last contrast-enhanced CT scan of head, neck, thorax and upper abdomen on 22/2/20 (Fig. 4 and Supplementary Fig. 6). So far, 36 cycles of Nivolumab have been administered (last on 7/04/20), with G1 hypothyroidism and G2 diarrhea as worst immune-related adverse events.
Fig. 1.
Shaded surface display of first IMRT plan. Volumes encompassed by PTVs 50, 60 and 66 Gy drawn as red, blue and aqua green halos, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
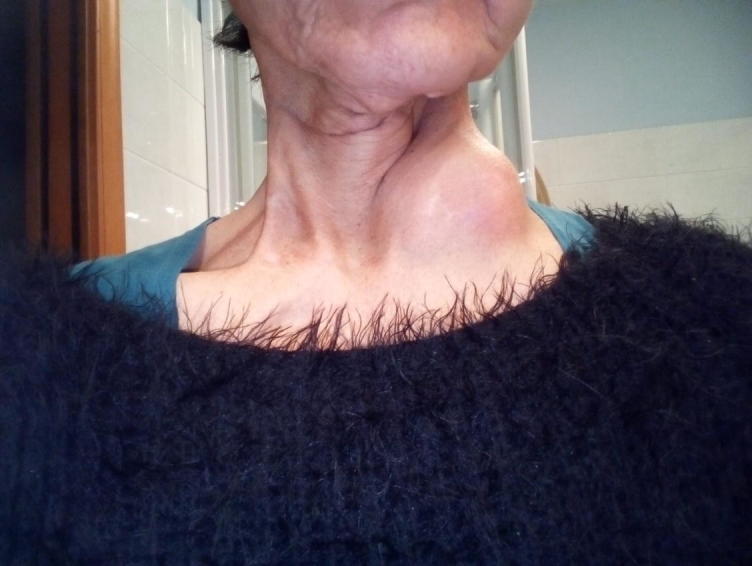
Left supraclavicular mass.
Fig. 3.
CT scan showing the oligoprogressive site in the left level 4 and supraclavicular fossa (coronal view).
Fig. 4.
Complete response in the left supraclavicular mass.
3. Discussion
To the best of our knowledge, this is the first report on two subsequent rounds of re-irradiation for loco-regional oligoprogressive recurrences under immunotherapy in HNSCC. It has been hypothesized [7] that the classical “extended – field “ approach of head and neck radiotherapy may be detrimental to the efficacy of ICI’s by inducing a chronic loco-regional immunosuppression. Along this line of thinking, some authors suggested [7], [8] that a re-thinking in the design of elective clinical target volumes may be hypothesized, with the aim of not depleting all lymph node levels in the head and neck region. In contrast to this theory, some preclinical evidence [9] points more towards a potential “in-situ vaccination” of RT. In R/M HNSCC treated with ICI’s, hyperprogression is a rare phenomenon. However, in one of the largest series published so far [10], there was a significant correlation between the development of an accelerated tumor growth with a pattern of regional recurrence, potentially implying a causative relationship with the previous extent of irradiated fields, based on unknown mechanisms. In oncogene-addicted non-small cell lung cancer, the use of local ablative therapy for oligoprogressive disease is an established approach [11], allowing for prolonged disease control and continuation of targeted therapy. In light of the introduction of ICI’s into a new continuum of care for R/M HNSCC [12], this concept may be extrapolated to selected cases experiencing oligoprogression under immune checkpoint blockade.
Recently, the safety [13] and potential synergistic effect of combining palliative RT with immunotherapy have been highlighted [14], [15]. Very limited data focus on R/M HNSCC. In these few reports [16], [17], [18], [19], [20], excellent clinical responses were observed with no major side effects (Supplementary Table 1). Our case report adds to this very limited series: the addition of state of the art re-irradiation to Nivolumab allowed to tackle the only macroscopic site of disease progression, defer the need of a further line of treatment and provide sustained clinical benefit. Oligoprogressive disease is still a relatively unrecognized entity in R/M HNSCC: our approach recalled the use of local ablative therapy for focal disease progression known for NSCLC since a few years [21]. Interestingly, the two oligoprogressive lesions were marginal recurrences in respect to the extended field design chosen as adjuvant treatment for a p16 negative squamous cell carcinoma of unknown primary. As already pointed out, the left supraclavicular fossa and right parotid gland had received minimal and moderate dose from the first irradiation (5 Gy and 22.1 Gy mean dose, respectively). Whereas 66 Gy could have been effective for a 6-cm mass by itself, one could speculate whether 30 Gy in 15 fractions would lead to a long-lasting local control. Interestingly, this dose regimen was experimentally tested in an aggressive dose de-escalation study on adjuvant chemo-radiotherapy for HPV positive oropharyngeal cancer [22], [23]. Nevertheless, in both oligorecurrent lesions, the marked clinical response we observed may potentially reflect a “radiosensitizing” effect of Nivolumab, particularly when given concurrently to radiation. In this perspective, an optimal spatial cooperation may have occurred, both at a local and systemic level. Apart from the known PD-L1 positive status of our patient (Supplementary Fig. 7), no predictive biomarkers are available to support the exceptional response we reported. In order to exploit this potential synergistic effect on a large scale, several gaps of knowledge on biologic interplay must be filled for further development. Nevertheless, we believe that very preliminary reports on selected cases like ours warrant prospective investigations on the combination of RT and immunotherapy in HNSCC.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2020.04.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
Coronal view of dose distribution of first IMRT plan: thick pink line corresponding to 47.5 Gy (95% isodose of PTV 50); thick violet line corresponding to 57 Gy (95% isodose of PTV 60); thick aqua green line corresponding to 62.7 Gy (95% isodose of PTV 66).
Supplementary figure 2.
Coronal view of dose distribution of first re-irradiation: PTV 66 Gy drawn in blue halo. Thick blue line corresponding to 66 Gy; yellow line corresponding to 85 Gy; dark green line corresponding to 90 Gy; red reline corresponding to 100 Gy; dark brown line corresponding to 110 Gy. Cumulative dose-volume histogram showed point maximum doses of 103 Gy, 90 Gy and 85 Gy to 0.5 cc of left common carotid artery, larynx and inferior constrictor muscle, respectively.
Supplementary figure 3.

CT scan showing the oligoprogressive site in the right parotid gland.
Supplementary figure 4.
Coronal view of dose distribution of second re-irradiation: PTV 30 Gy drawn in light green halo. Light green line corresponding to 30 Gy; pale pink line corresponding to 50 Gy; blue line corresponding to 66 Gy ;yellow line corresponding to 85 Gy; dark green line corresponding to 90 Gy; red line corresponding to 95 Gy; dark brown line corresponding to 110 Gy. Cumulative dose-volume histogram showed point maximum doses of 98 Gy, 93 Gy and 87 Gy to 0.5 cc of mandible, right external carotid artery and right internal carotid artery, respectively.
Supplementary figure 5.

Infiltrating lesion in the right parotid gland.
Supplementary figure 6.

Complete response in the right parotid gland.
Supplementary figure 7.
Immunohistochemistry staining of PD-L1(bottom panels) with matched hematoxylin and eosin samples (top panels). Membranous and cytoplasmic positivity for PD-L1 staining in 70% of tumor cells.
Case reports published on the combination of local treatments (RT and photodynamic therapy) with immunotherapy. Abbreviations: IT, immunotherapy; SCCUP, squamous cell carcinoma of unknown primary; na, not available.
References
- 1.Ferris R.L., Blumenschein G., Jr, Fayette J., Guigay J., Colevas A.D., Licitra L. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen E.E.W., Soulières D., Le Tourneau C., Dinis J., Licitra L., Ahn M.J. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156–167. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 3.Burtness B., Harrington K.J., Greil R., Soulières D., Tahara M., de Castro G., Jr Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 4.Loi M., Desideri I., Greto D., Mangoni M., Sottili M., Meattini I. Radiotherapy in the age of cancer immunology: Current concepts and future developments. Crit Rev Oncol Hematol. 2017;112:1–10. doi: 10.1016/j.critrevonc.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Dovedi S.J., Adlard A.L., Lipowska-Bhalla G., McKenna C., Jones S., Cheadle E.J. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–5468. doi: 10.1158/0008-5472. [DOI] [PubMed] [Google Scholar]
- 6.Vermorken J.B., Mesia R., Rivera F., Remenar E., Kawecki A., Rottey S. Platinum-based Chemotherapy plus Cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 7.Sharabi A.B., Lim M., DeWeese T.L., Drake C.G. Radiation and checkpoint blockade immunotherapy: Radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498–509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 8.Bonomo P., Desideri I., Loi M., Mangoni M., Sottili M., Marrazzo L. Anti PD-L1 DUrvalumab combined with Cetuximab and RadiOtherapy in locally advanced squamous cell carcinoma of the head and neck: a phase I/II study (DUCRO) Clin Transl Radiat Oncol. 2018;9:42–47. doi: 10.1016/j.ctro.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Saada-Bouzid E., Defaucheux C., Karabajakian A., Coloma V.P., Servois V., Paoletti X. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28(7):1605–1611. doi: 10.1093/annonc/mdx178. [DOI] [PubMed] [Google Scholar]
- 11.Gomez D.R., Tang C., Zhang J., Blumenschein G.R., Hernandez M., Lee J.J. Therapy or observation for patients with oligometastatic non-small cel lung cancer: long-term results of a multi-institutional phase II, randomized study. J Clin Oncol. 2019;37(18):1558–1565. doi: 10.1200/JCO.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Argiris A., Harrington K.J., Tahara M., Schulten J., Chomette P., Ferreira Castro A. Evidence-based treatment options in recurrent and/or metastatic squamous cell carcinoma of the head and neck. Front Oncol. 2017;7:72. doi: 10.3389/fonc.2017.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang W.L., Pike L.R.G., Royce T.J., Mahal B.A., Loeffler J.S. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat Rev Clin Oncol. 2018;15(8):477–494. doi: 10.1038/s41571-018-0046-7. [DOI] [PubMed] [Google Scholar]
- 14.Levy A., Massard C., Soria J.C., Deutsch E. Concurrent irradiation with the anti-programmed cell death ligand-1 immune checkpoint blocker durvalumab: Single centre subset analysis from a phase 1/2 trial. Eur J Cancer. 2016;68:156–162. doi: 10.1016/j.ejca.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Amin N.P., Zainib M., Parker S.M., Agarwal M., Mattes M.D. Multi-institutional report on toxicities of concurrent nivolumab and radiation therapy. Adv Radiat Oncol. 2018;3(3):399–404. doi: 10.1016/j.adro.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagasaka M., Zaki M., Kim H., Raza S.N., Yoo G., Lin H.S. PD1/PD-L1 inhibition as a potential radiosensitizer in head and neck squamous cell carcinoma: a case report. J Immunother Cancer. 2016;4:83. doi: 10.1186/s40425-016-0187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos L.L., Oliveira J., Monteiro E., Santos J., Sarmento C. Treatment of head and neck cancer with photodynamic therapy with Redaporfin: a clinical case report. Case Rep Oncol. 2018;11(3):769–776. doi: 10.1159/000493423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atiq S.O., Atiq O.O., Atiq M.O., Phillips K.C., Jacks B.B., Moreno M. The role of immunotherapy and radiation therapy in tumor chemosensitivity in advanced head and neck cancer. Am J Case Rep. 2018;19:1241–1244. doi: 10.12659/AJCR.910224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finazzi T., Rordorf T., Ikenberg K., Huber G.F., Guckenberger M., Garcia Schueler H.I. Radiotherapy-induced anti-tumor immune response and immune related adverse events in a case of recurrent nasopharyngeal carcinoma undergoing anti-PD-1 immunotherapy. BMC Cancer. 2018;18(1):395. doi: 10.1186/s12885-018-4295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denaro N., Merlano M.C. Unexpected response with palliative conventional therapy in head and neck squamous cell carcinoma after anti-programmed death-1 progression. Head Neck. 2019;41(3):E42–E47. doi: 10.1002/hed.25418. [DOI] [PubMed] [Google Scholar]
- 21.Weickhardt A.J., Scheier B., Burke J.M., Gan G., Lu X., Bunn P.A., Jr Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7(12):1807–1814. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma D.J., Price K.A., Moore E.J., Patel S.H., Hinni M.L., Garcia J.J. P Phase II evaluation of aggressive dose deescalation for adjuvant chemoradiotherapy in human papillomavirus associated oropharynx squamous cell carcinoma. J Clin Oncol. 2019;37:1909–1918. doi: 10.1200/JCO.19.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garden A.S. Not all 30-Gy regimens are equal. J Clin Oncol. 2019;37(36):3558–3559. doi: 10.1200/JCO.19.01666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Case reports published on the combination of local treatments (RT and photodynamic therapy) with immunotherapy. Abbreviations: IT, immunotherapy; SCCUP, squamous cell carcinoma of unknown primary; na, not available.









