Microglia, the brain’s “busy bees”, continuously survey the microenvironment by extending and retracting their ramified processes to maintain brain homeostasis [1, 2]. Upon disease or injury, microglia quickly transform their morphology and extend their processes towards the disease/injury sites to clear damage [2]. The mechanisms underlying the high motility of microglial processes and the rapid morphological transformation of microglia have been extensively investigated. However, studies on microglial dynamics in vivo have predominantly been carried out in anesthetized animals, and how microglia behave under awake conditions remained unknown. Using two-photon microscopy to track microglia dynamics in awake mice, two independent studies published recently [3, 4] have demonstrated that during wakefulness, microglia exhibit shorter arborization, reduced motility, and diminished responsiveness to injury compared to those under anesthesia. Moreover, both studies showed that norepinephrine (NE), a neurotransmitter released from the axon terminals of locus coeruleus (LC) neurons, directly regulates microglial motility through β2-adrenergic receptor (β2-AR) signaling in the awake condition [3, 4].
To determine whether microglial dynamics differ in the awake and anesthetized conditions, Stowell et al. [3] and Liu et al. [4] carefully analyzed microglial dynamics under both conditions, using the CX3CR1GFP/+ transgenic mouse line in which microglia are fluorescently labeled by GFP. Surprisingly, both groups found that microglia in the awake mice survey a smaller territory with shorter arbors, reduced motility, and attenuated responsiveness to acute injury than those in brains anesthetized with isoflurane or a fentanyl cocktail [3, 4]. To exclude the possibility that general anesthesia-related circulatory and respiratory effects influence microglial activity, Liu et al. [4] tracked microglial responses using different anesthetics such as ketamine/xylazine and urethane. In parallel, since the fentanyl cocktail produces a slow-wave-dominated state with both analgesic and sedative effects, Stowell et al. [3] recorded the microglial dynamics in mice anesthetized with the sedative-only dexmedetomidine (DEX). Consistently, both groups reported enhanced velocity and surveillance of microglial processes within 10 min–15 min of anesthesia induction, regardless of the type of anesthetic, suggesting that anesthesia may relieve an inhibitory signal affecting microglial dynamics during wakefulness.
Given that general anesthesia reduces neuronal activity, Liu et al. [4] investigated whether the enhanced microglial surveillance during anesthesia results from the suppressed neuronal activity. They imaged the dynamics of microglial processes in the barrel cortex of mice with unilaterally trimmed whiskers. Thirty minutes after trimming, the neuronal activity in the contralateral barrel cortex was substantially reduced based on Ca2+ imaging from neurons expressing GCamp6 (a fluorescent sensor reflecting the intracellular Ca2+ level). Meanwhile, microglia also robustly increased their process areas and surveyed greater territories in the contralateral but not in the ipsilateral barrel cortex. To directly address whether neuronal activity regulates microglial process dynamics, Liu et al. [4] suppressed the neuronal network activity with muscimol (a GABAa receptor agonist), tetrodotoxin (an inhibitor of voltage-gated Na+ channels), or optogenetic activation of GABAergic neurons. They found that reduced neuronal activity was accompanied by increased process dynamics and surveillance of microglia, reminiscent of the changes during anesthesia, suggesting that reduced neuronal activity triggers enhanced microglial dynamics and surveillance.
To reveal the mechanisms that underlie the differences between microglial dynamics in the awake and anesthetized states, both studies tested whether P2Y12 and fractalkine-CX3CR1 signaling, important pathways known to regulate microglial dynamics [2], are involved and found that neither contributed to the changes. Since NE is an important mediator of wakefulness and DEX is known to reduce NE release from the LC, Stowell et al. [3] tested whether NE regulates microglial responses. They ablated NE neurons in the LC using the neurotoxin N-(2-choloroethyl)-N-ethyl-2-bromobenzylamine (DSP4) and found that pre-ablation of LC projections eliminated the DEX-elicited increased arborization and surveillance of microglia. Conversely, optogenetic stimulation of NE release from LC axons reduced microglia arborization and surveillance in DEX-treated animals, suggesting that endogenous NE release from the LC inhibits microglial dynamics and surveillance during wakeful states. Liu et al. [4] reached the same conclusion by applying a wide range of neurotransmitters known to be reduced during anesthesia, including acetylcholine, dopamine, NE, and serotonin to the imaging area. They found that only NE prevented the anesthesia-enhanced microglial process surveillance. On the contrary, inhibition of NE release from NE neurons in the LC by chemogenetic manipulation in awake mice enhanced the microglial process dynamics, further supporting the hypothesis that NE is the inhibitory signal that limits microglial surveillance during wakefulness.
NE exerts diverse actions through a wide range of receptors. Interestingly, microglia express much higher levels of β2-ARs than other brain cell types [5] and previous studies in vitro have suggested that β2-AR signaling inhibits microglial chemotaxis towards ATP [6]. Stowell et al. [3] then treated anesthetized mice with a selective β2-AR agonist, clenbuterol, and found significantly reduced microglial motility and persistent process retraction, mimicking the changes in the awake condition [3]. By contrast, both studies showed that antagonizing β2-ARs with ICI-118, 551 in awake mice produced changes that resemble the anesthetized state with enhanced microglial motility and parenchymal surveillance [3, 4]. Moreover, the effects of clenbuterol were absent in β2-AR-ablated microglia [3], suggesting that endogenous NE acts directly on β2-ARs to inhibit microglial ramification and process motility during wakefulness.
Microglia shape neuronal circuits by modulating synapse formation, plasticity, and pruning through process arborization and surveillance [7]. Notably, Liu et al. [4] reported that enhanced microglial process surveillance increases microglia–dendrite interactions under anesthetized conditions, indicating that changes in microglial surveillance may result in modifications in synaptic plasticity. To determine whether NE–β2-AR signaling regulates microglia-mediated synaptic plasticity, Stowell et al. [3] examined ocular dominance plasticity (ODP) after 4 days of monocular deprivation when β2-AR was pharmacologically activated or inhibited. Interestingly, chronic activation of β2-ARs by clenbuterol impaired ODP; however, chronic blockade of β2-ARs by ICI-118, 551 had no effect on ODP, suggesting that sustained stimulation of β2-ARs interferes with microglia–dendrite contacts, but β2-ARs may not be required for ODP. These data provide functional significance for β2-ARs-regulated microglia dynamics in the modulation of synaptic plasticity (Fig. 1).
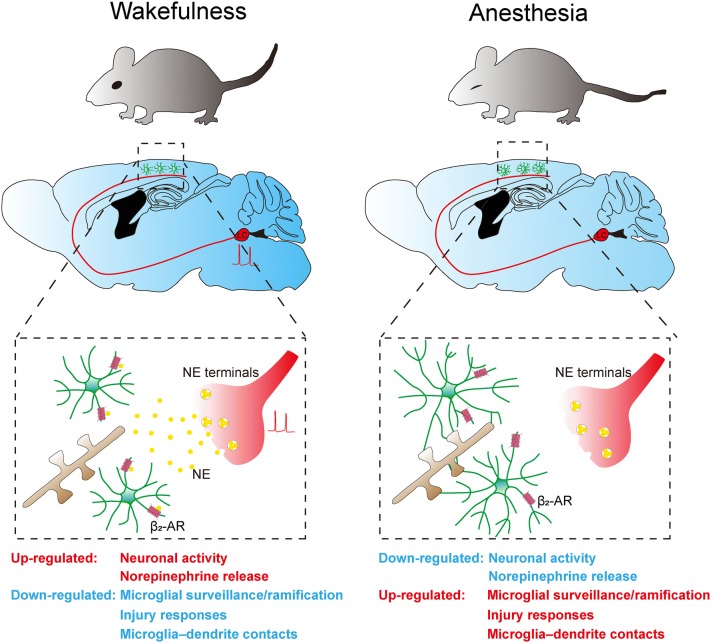
Fig. 1.
Regulation of microglial dynamics by norepinephrine (NE)-β2 adrenergic receptor (β2-AR) signaling. During wakefulness, tonic activation of NE neurons in the LC releases NE in the brain. Binding of NE to the β2-ARs on microglia restricts microglial surveillance and ramification, injury responses, and microglia–dendrite contacts. Under anesthesia, reduced NE neuronal activity decreases the levels of NE in the brain, releasing the inhibition of microglia by β2-AR signaling, thereby enhancing microglial surveillance and ramification, injury responses, and microglia-dendrite contacts.
Although microglial dynamics have been described for more than a decade, these two studies are the first to uncover the different behaviors of microglia under anesthetized and awake conditions. Despite the fact that microglia express a wide range of neurotransmitter receptors [8], these studies are the first to reveal how microglia tune their dynamics in vivo in response to the release of a neurotransmitter, NE. Together, these results highlight the importance of real-time imaging in awake animals and reveal the impact of neuronal activity on microglial dynamics in different states. These studies also raise important questions regarding whether the loss or gain of NE signaling in different contexts contributes to the aberrant microglial functions implicated in multiple diseases. For example, loss of NE neurons in the LC, an early sign in Alzheimer’s and Parkinson’s diseases [9], may enhance microglial dynamics and surveillance, whereas chronic stress or sleep deprivation may inhibit microglial surveillance by increased NE release [10]. Whether and how NE–β2-AR signaling contributes to pathology through aberrant regulation of microglial activity in these conditions needs further attention and investigation.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31671057) and the Fundamental Research Funds for the Central Universities (2019FZA7009).
References
- 1.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 2.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 3.Stowell RD, Sipe GO, Dawes RP, Batchelor HN, Lordy KA, Whitelaw BS, et al. Noradrenergic signaling in the wakeful state inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. Nat Neurosci. 2019;22:1782–1792. doi: 10.1038/s41593-019-0514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu YU, Ying Y, Li Y, Eyo UB, Chen T, Zheng J, et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat Neurosci. 2019;22:1771–1781. doi: 10.1038/s41593-019-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gyoneva S, Traynelis SF. Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J Biol Chem. 2013;288:15291–15302. doi: 10.1074/jbc.M113.458901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B. Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol. 2015;36:605–613. doi: 10.1016/j.it.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Heneka MT, Nadrigny F, Regen T, Martinez-Hernandez A, Dumitrescu-Ozimek L, Terwel D, et al. Locus coeruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc Natl Acad Sci U S A. 2010;107:6058–6063. doi: 10.1073/pnas.0909586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev. 2009;89:535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]