Recently, a paper published in Science reported discovery of sensory peripheral glial cells [1]. This paper demonstrated a rather unexpected property of non-myelinating Schwann cells (also known as Remak cells) dwelling in the subepidermal border of the skin [2]. These glial cells and their interactions with nociceptive nerve terminals were characterized using a variety of genetic labelling tools, transmission electron microscopy, immunocytochemistry, and electrophysiology. The morphological and functional data presented by Abdo and colleagues show that these subcutaneous glial cells form a network in the subepidermal border; moreover, these Schwann cells are intimately associated with nociceptive nerve endings to form the glio-neuronal sensory organ. These glial cells contribute to pain sensation; their optogenetic stimulation triggers electrical activity in nociceptive nerves and pain-related behaviors. Thus the new type of peripheral glia, the nociceptive Schwann cells was identified.
The discovery of a peripheral sensory glio-neuronal organ highlights an unexpected evolutionary link with primordial glia, which are associated with peripheral sensations in several invertebrates. The evolutionary history of neuroglia, supportive homeostatic and defensive neural cells, began with the emergence of central (the “brain”) and peripheral nervous systems, which accompanied the transition from Ctenophora and Cnidarians (which possess a diffuse nervous system) to more advanced life forms [3, 4]. The primordial supportive glia found in earthworms and flatworms are represented by several subtypes, which are mostly associated with peripheral nerves (neurilemmal, subneurilemmal, and periaxonal sheath-forming glia), and cells esheathing neurons (supporting-nutrifying-glia) [3, 4]. In the roundworm Caenorhabditis elegans, the majority of glial cells are however associated with sensory neurons. The nervous system of C. elegans contains 50 glial cells of ectodermal (i.e. neural) origin and 6 mesodermal supportive cells, the latter providing a link between some neurons and muscle cells [5–7]. Most of the glial cells (46 out of 50) in C. elegans act as an integral part of the sensory system. These 46 glial cells comprise 26 socket cells and 20 sheath cells that are associated with neuronal terminals and form specific sensory organs known as sensilla [7]. The remaining four glial cells, defined as cephalic sheath cells, serve a dual function: their anterior processes form sensilla localized in the lips of the worm, whereas their posterior processes ensheath the nerve ring (the C. elegans “brain”) where they contribute to the formation of the neuropil [8]. These cephalic sheath cells can be considered as the primeval ancestors of astrocytes [9].
The peripheral glia of C. elegans have numerous functions; they establish the location of sensilla, regulate the size and morphological appearance of sensory neuronal terminals, provide for homeostatic control of the sensory organs, and (arguably) may even regulate neuronal activity. The developmental program of sensilla formation is impaired in the absence of glia (which can be selectively ablated in C. elegans); glia-secreted factors control sensory dendrite attachment during the migration of neurons in development, while the proper development of sensory structures requires the expression of gene sets both in neurons and in glia [8, 10]. Most unusual though was the finding that the C. elegans sheath and socket glia express several types of mechanosensitive degenerin/epithelial Na+ channel (in particular, acid-sensitive degenerin (ACD)-1 and degenerin linked to mechanosensation (DELM)–1,2 channels) [11, 12]. These channels are required for sensory function (in particular, they mediate touch sensitivity) and for several types of foraging behavior in worms [12]. Thus, from very early evolutionary stages the ancestral neuroglia were associated with the function of the sensory nervous system, and formed peripheral sensory organs.
The sensilla operate as sensory organs throughout invertebrates. In Drosophila melanogaster for example, taste and olfactory sensilla combine neuronal elements (2 neurons per olfactory sensillum and 2–4 per taste sensillum), and several supportive (presumably peripheral glial) cells [13, 14]. At the same time, mechanosensitive peripheral structures in Drosophila (trichoid sensilla, also known as bristles and cuticular campaniform sensilla) seem to be devoid of glia [15].
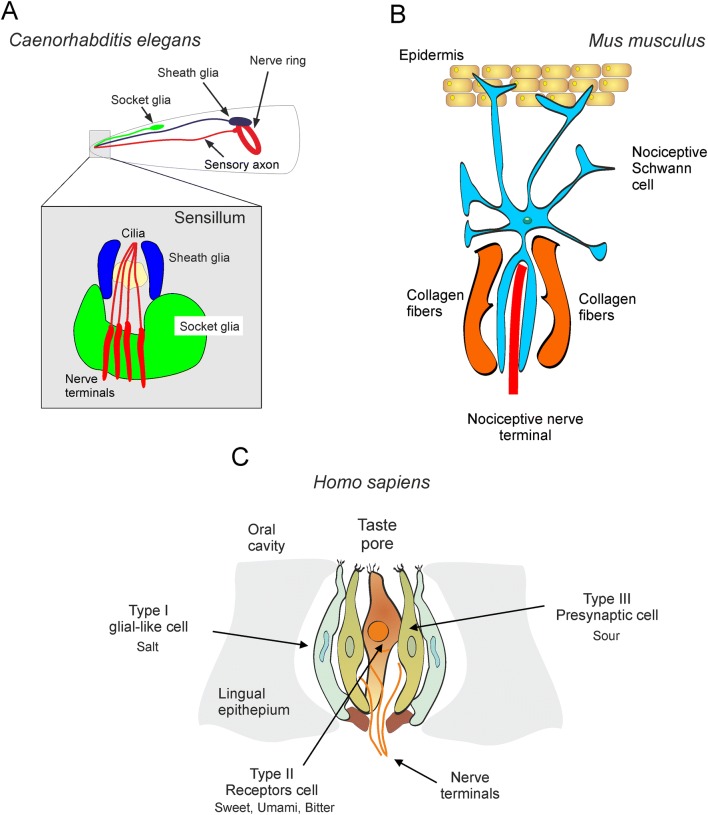
In mammals, peripheral glia are generally considered to be responsible for the insulation and myelination of axons (myelinating and non-myelinating Schwann cells), for covering neuro-muscular junctions (perisynaptic Schwann cells), and for maintaining homoeostasis in peripheral ganglia (satellite glia). The discovery of nociceptive Schwann cells [1] prompts a fundamental extension of the repertoire of peripheral glial functions. The subcutaneous glial cells are mechanoreceptors which, upon activation, cause membrane depolarization; this depolarization is apparently conducted to nerve endings (by an as-yet unknown transduction cascade) and instigate pain-associated behaviors. Thus, nociceptive Schwann cells together with nerve endings form a peripheral sensory organ, which bears a surprising semblance to sensilla in C. elegans (Fig. 1A, B). This evolutionary conservation of the fundamental principle behind the architecture of peripheral sensory machinery—that is, the formation of a glial-neuronal complex—is most exciting indeed.
Fig. 1.
Glial-neuronal sensory organs. A Sensilla in C. elegans. B Glial-neuronal sensory organ in the skin of mouse. C Glial-neuronal organisation of the taste bud in humans (redrawn from [20]).
Hitherto, the glial contribution to sensory organs in mammals has been documented only for special senses. In the retina, Muller glial cells not only provide for mechanical integrity and local homeostatic support, but also act as light-guides [16]. In the olfactory epithelium, glia-like sustentacular supporting cells insulate and support neurons, regulate ionic homeostasis, phagocytose dead cells, and secrete numerous trophic and signaling molecules [17]. The glial cells are an important part of taste-bud structure and function [18]; they are thought to sense the salty taste (Fig. 1C). From now on we shall consider the glial cell as a key element of skin sensory organs involved in nociception.
Many questions and future research directions arise from the discovery of nociceptive glia. It is of course of immense interest to reveal the mechanism through which the depolarization of nociceptive Schwann cells excites neuronal endings. It is also of importance to discern the sensory modalities (apart from mechanosensitivity) which are mediated through the glia. Another obvious fundamental question is whether the glial-neural sensory organs are operational in higher primates and humans, and the contributions of these organs to pain and other sensory perceptions. Could nociceptive glial cells connect not only to non-myelinated fibers but also to fast A-fibers mediating ultra-fast pain in humans? [19]. Ultimately, we need to determine whether nociceptive peripheral glia can be a substrate for pain-related pathologies and a target for therapeutic interventions. Be all this as it may, the surprising evolutionary connection between the roundworm and mammals opens new avenues for understanding the basic principles of neuronal-glial interactions and further extends the roles played by glia in the function of the nervous system.
Acknowledgements
The authors’ research was supported by National Key R&D Program of China(2019YFC1709101), the Project First-Class Disciplines Development of Chengdu University of Traditional Chinese Medicine (CZYHW1901), the National Natural Science Foundation of China (81774437 and 81973969), and Science and Technology Program of Sichuan Province, China (2019YFH0108 and 2018SZ0257).
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Yong Tang, Email: tangyong@cdutcm.edu.cn.
Alexei Verkhratsky, Email: Alexej.Verkhratsky@manchester.ac.uk.
References
- 1.Abdo H, Calvo-Enrique L, Lopez JM, Song J, Zhang MD, Usoskin D, et al. Specialized cutaneous Schwann cells initiate pain sensation. Science. 2019;365:695–699. doi: 10.1126/science.aax6452. [DOI] [PubMed] [Google Scholar]
- 2.Cauna N. The free penicillate nerve endings of the human hairy skin. J Anat. 1973;115:277–288. [PMC free article] [PubMed] [Google Scholar]
- 3.Hartline DK. The evolutionary origins of glia. Glia. 2011;59:1215–1236. doi: 10.1002/glia.21149. [DOI] [PubMed] [Google Scholar]
- 4.Verkhratsky A, Ho MS, Parpura V. Evolution of neuroglia. Adv Exp Med Biol. 2019;1175:15–44. doi: 10.1007/978-981-13-9913-8_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans.? 2UU. J Comp Neurol. 1975;160:313–337. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- 6.Stout RF, Jr, Verkhratsky A, Parpura V. Caenorhabditis elegans glia modulate neuronal activity and behavior. Front Cell Neurosci. 2014;8:67. doi: 10.3389/fncel.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oikonomou G, Shaham S. The glia of Caenorhabditis elegans. Glia. 2011;59:1253–1263. doi: 10.1002/glia.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oikonomou G, Shaham S. On the morphogenesis of glial compartments in the sensory organs of Caenorhabditis elegans. Worm. 2012;1:51–55. doi: 10.4161/worm.19343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verkhratsky A, Nedergaard M. Physiology of Astroglia. Physiol Rev. 2018;98:239–389. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perens EA, Shaham S. C. elegans daf-6 encodes a patched-related protein required for lumen formation. Dev Cell. 2005;8:893–906. doi: 10.1016/j.devcel.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Apicella A, Jr, Lee SK, Ezcurra M, Slone RD, Goldmit M, et al. A glial DEG/ENaC channel functions with neuronal channel DEG-1 to mediate specific sensory functions in C. elegans. EMBO J. 2008;27:2388–2399. doi: 10.1038/emboj.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han L, Wang Y, Sangaletti R, D’Urso G, Lu Y, Shaham S, et al. Two novel DEG/ENaC channel subunits expressed in glia are needed for nose-touch sensitivity in Caenorhabditis elegans. J Neurosci. 2013;33:936–949. doi: 10.1523/JNEUROSCI.2749-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chai PC, Cruchet S, Wigger L, Benton R. Sensory neuron lineage mapping and manipulation in the Drosophila olfactory system. Nat Commun. 2019;10:643. doi: 10.1038/s41467-019-08345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amrein H. Chemosensory Transduction. Cambridge: Academic Press; 2016. Mechanism of Taste Perception in Drosophila; pp. 245–269. [Google Scholar]
- 15.Tuthill JC, Wilson RI. Mechanosensation and adaptive motor control in insects. Curr Biol. 2016;26:R1022–R1038. doi: 10.1016/j.cub.2016.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franze K, Grosche J, Skatchkov SN, Schinkinger S, Foja C, Schild D, et al. Muller cells are living optical fibers in the vertebrate retina. Proc Natl Acad Sci U S A. 2007;104:8287–8292. doi: 10.1073/pnas.0611180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegg CC, Irwin M, Lucero MT. Calcium store-mediated signaling in sustentacular cells of the mouse olfactory epithelium. Glia. 2009;57:634–644. doi: 10.1002/glia.20792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gravina SA, Yep GL, Khan M. Human biology of taste. Ann Saudi Med. 2013;33:217–222. doi: 10.5144/0256-4947.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagi SS, Marshall AG, Makdani A, Jarocka E, Liljencrantz J, Ridderstrom M, et al. An ultrafast system for signaling mechanical pain in human skin. Sci Adv. 2019;5:eaaw1297. doi: 10.1126/sciadv.aaw1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burnstock G, Verkhratsky A. Purinergic Signalling in the Nervous System. Heidelberg: Springer Verlag; 2012. p. 715. [Google Scholar]