Abstract
In the current study, we sought to investigate whether T-type Ca2+ channels (TCCs) in the brain are involved in generating post-anesthetic hyperexcitatory behaviors (PAHBs). We found that younger rat pups (postnatal days 9–11) had a higher incidence of PAHBs and higher PAHB scores than older pups (postnatal days 16–18) during emergence from sevoflurane anesthesia. The power spectrum of the theta oscillations (4 Hz–8 Hz) in the prefrontal cortex was significantly enhanced in younger pups when PAHBs occurred, while there were no significant changes in older pups. Both the power of theta oscillations and the level of PAHBs were significantly reduced by the administration of TCC inhibitors. Moreover, the sensitivity of TCCs in the medial dorsal thalamic nucleus to sevoflurane was found to increase with age by investigating the kinetic properties of TCCs in vitro. TCCs were activated by potentiated GABAergic depolarization with a sub-anesthetic dose of sevoflurane (1%). These data suggest that (1) TCCs in the brain contribute to the generation of PAHBs and the concomitant electroencephalographic changes; (2) the stronger inhibitory effect of sevoflurane contributes to the lack of PAHBs in older rats; and (3) the contribution of TCCs to PAHBs is not mediated by a direct effect of sevoflurane on TCCs.
Keywords: Emergence agitation, Neonatal rat, General anesthesia, Sevoflurane, T-type calcium channel, Theta wave
Introduction
With the development of new anesthetic formulations and techniques, general anesthesia has become widely available in pediatric surgery. Sevoflurane is a popular volatile anesthetic used in pediatric surgery because of its many advantages including hemodynamic stability, sweet smell, lack of respiratory irritation, and rapid onset of action [1]. However, a high risk of emergence agitation (EA) or delirium induced by sevoflurane has been extensively reported [2].
EA is a well-documented phenomenon in the immediate postoperative period in humans, particularly in preschool-age children [3]. This condition manifests as an unconscious state accompanied by a series of hyperexcitatory behaviors that often spontaneously resolve [4]. However, EA can be urgent when children become uncontrollable during emergence from general anesthesia, and may hurt themselves by accident. EA unavoidably contributes to extension in hospital stay, and may place additional burdens on both patients and medical staff [5]. Furthermore, EA may be associated with postoperative psychological problems in children, such as eating or sleeping disorders, anxiety, apathy, and aggression [6]. Although many adjunct agents (e.g., dexmedetomidine, remifentanil, ketamine, and nalbuphine) have demonstrated some effectiveness against EA [7–9], the underlying mechanism is not yet clear.
In a previous study, we reported EA-like behaviors, which we named post-anesthetic hyperexcitatory behaviors (PAHBs), during emergence from sevoflurane anesthesia in neonatal rats [10]. We developed a scale for evaluating such behaviors and demonstrated that PAHBs present features similar to EA in humans. We found that sevoflurane-induced PAHBs are closely associated with GABAergic (GABA, γ-aminobutyric acid) depolarization/excitation in the neocortical neurons of neonatal rats. However, sevoflurane-potentiated depolarizing potentials cannot activate action potentials alone because of the limited reversal potential of GABA. Nonetheless, these potentials are able to elicit action potentials, presumably by evoking other subthreshold events. Low-threshold Ca2+ spikes, which are mediated by the T-type Ca2+ channel (TCC), result in a neuron reaching the threshold for burst firing in the central nervous system [11]. Therefore, we sought to investigate whether TCCs are involved in generating PAHBs in neonatal rats.
Materials and Methods
Animal Care
Pregnant Sprague-Dawley rats from the Key Laboratory of Brain Functional Genomics affiliated with East China Normal University (Shanghai, China) were housed in a temperature-controlled (22°C–24°C) vivarium on a 12 h/12 h light-dark cycle. Male pups born of these rats were used. The day of birth was defined as postnatal day 0 (P0). The experimental procedures described below were in accordance with the ARRIVE (Animals in Research: Reporting In Vivo Experiments) guidelines and were approved by the Animal Care and Use Committee of Fudan University in Shanghai (201802130S). All possible efforts were made to minimize the number of animals and their suffering.
PAHB Model and Assessment
Neonatal Sprague-Dawley rats were used in the behavioral test and were divided into younger (P9–P11) and older pups (P16–P18). PAHB modeling and scoring were the same as we described in the previous study [10]. In brief, anesthesia was induced in rat pups with 6% (v/v) sevoflurane gas over 1 min and was then maintained with 3% sevoflurane gas for 9 min. These gases were generated with a calibrated sevoflurane and oxygen-fed commercial vaporizer (Penlon, Abingdon, OX14, UK) and were supplied to the anesthesia chamber at 3 L/min. PAHB scores were based on behavioral type [0, none; 1, trembling; 2, head bobbing or stereotypy; 3, unilateral forelimb or hindlimb clonus; 4, bilateral forelimb or hindlimb clonus; 5, side-to-side rolling; 6, wandering (i.e., scrambling along the monitoring chamber walls)], and a bonus score of 0, 0.5, or 1 was given on the basis of its subgrade (0, mild; 0.5, moderate; 1, severe). The sum of the main and bonus scores constituted the behavioral score. We also introduced an open field system to evaluate PAHBs. The observation apparatus consisted of four plastic boxes (26 cm × 26 cm) with a field bordered by 37-cm high sidewalls. Movement distance and movement duration were monitored in each rat for 6 min and analyzed using the Truscan system (Coulbourn, Holliston, MA).
Electroencephalogram (EEG) Recording
To determine the changes in cortical activity in the frontal lobe during the peri-anesthesia period, rat pups were fitted with instrumentation for EEG recording. A head rack and recording electrodes were mounted on each pup under sevoflurane anesthesia 6 h prior to EEG recording. The entire surgical process took no more than 15 min. Each pup’s head was fixed on a turntable with a respiratory mask connected to the calibrated sevoflurane and oxygen-fed commercial vaporizer (Penlon Sigma Elite). EEG recording was generally composed of four stages that lasted 10 min each, acclimation, awakening (baseline recording), anesthesia, and emergence. The EEG signals were amplified by a Model 3000 amplifier (A.M, Carlsborg, WA) and digitized and sampled at 200-µs intervals (Digidata 1440, pClamp 10.2; Molecular Devices, San Jose, CA). The power values were calculated from power spectra generated using Fourier analysis in MatLab software (MathWorks, Natick, MA).
Test of Drug Effects on PAHBs and EEGs
To investigate the potential roles of TCCs in generating PAHBs, we examined the effects of the TCC inhibitors NNC 55-0396 and ethosuximide on PAHBs and EEGs. Intracerebroventricular microinjection (ICVM) of saline or NNC 55-0396 (0.1 μg/kg body weight) was performed 5 min prior to sevoflurane anesthesia. For ICVM, a 26-gauge cannula made of polyethylene tubing (PE-10: inner diameter 0.28 mm and outer diameter 0.61 mm) was inserted into the lateral cerebral ventricle (stereotaxic coordinates: 0.4 mm lateral to the midline, 0.4 mm posterior to bregma, and 2.5 mm ventral to the skull surface [12]) and secured to the skull with surgical glue 24 h before the behavioral test or EEG recording. Ethosuximide (0.2 g/kg body weight) was administered intraperitoneally 5 min before sevoflurane anesthesia.
Thalamic Slice Preparation
Animals were anesthetized with sodium pentobarbitone (100 mg/kg body weight, i.p.), and the brain was quickly removed and submerged in ice-cold artificial cerebrospinal fluid (ACSF, in mmol/L: 124 NaCl, 1.3 MgSO4, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2.4 CaCl2, and 10 glucose). After being chilled for 1–2 min, the brain was trimmed to a block containing the thalamus. Then, coronal slices (400 μm) were cut in ice-cold ACSF using a vibro-slicer (Leica VT1000, Buffalo Grove, IL). Slices containing the medial dorsal thalamic nucleus (MDTN) were transferred to a gas interface recording chamber perfused with aerated ACSF (95% O2/5% CO2; 22°C–24°C) containing (in μmol/L) 30 bicuculline methiodide, 2 nimodipine, 3 ω-conotoxin MVIIC, and 0.5 tetrodotoxin to screen out TCC currents [13]. To record GABAA receptor-mediated responses in isolation, we included AP-5 [DL-2-amino-5-phosphonopentanoic acid, an NMDA (N–methyl-D-aspartate) receptor antagonist, 100 μmol/L], DNQX (6,7-dinitroquinoxaline-2,3-dione, a non-NMDA receptor antagonist, 20 μmol/L), and CGP 54626 hydrochloride (a GABAB receptor antagonist, 1 μmol/L) in the perfusion medium. ACSF for recording was perfused by a peristaltic pump-driven or gravity-fed bath-perfusion system at 0.5–1 mL/min. Humidified 95% O2/5% CO2 was continuously blown over the slices to further ensure the adequate oxygenation of cells in the tissue.
Intracellular Recording
Voltage-clamp recordings were obtained from neurons in MDTN slices equilibrated for 1 h–6 h in the recording chamber. Whole-cell recordings (series resistance, 6 MΩ–12 MΩ) of TCC currents were obtained with micropipettes (tip diameter, 1.5 µm–2.0 µm; resistance, 4 MΩ–6 MΩ) filled with an internal solution (pH 7.3) composed of (in mmol/L) 140 K-gluconate, 10 HEPES, 2 MgCl2, 1 CaCl2, 11 EGTA, and 2 K2ATP, while the internal solution for gramicidin (50 μg/mL)-perforated patch clamp recording (series resistance, 30 MΩ–90 MΩ) was composed of (in mmol/L) 143 K-gluconate, 2 KCl, 10 HEPES, and 0.5 EGTA, pH 7.2–7.3. GABAA receptor-mediated postsynaptic potentials were elicited by electrically stimulating a site near the recorded cell with a custom-made, bipolar tungsten electrode; constant current (0.1 mA–0.5 mA) or voltage (5 V–20 V) pulses (biphasic square wave, 0.5-ms duration) were used for stimulation. Voltage errors resulting from series resistance were compensated offline for voltage-clamp recordings and online for current-clamp recordings by using a bridge circuit. The signals from neurons were amplified by an Axoclamp-700B amplifier (Molecular Devices) (bandwidth filter set to 10 kHz for current-clamp recordings and 1 kHz for voltage-clamp recordings) and were digitized and sampled at 50-µs intervals (Digidata 1440, pClamp 10.2; Molecular Devices). Activation/inactivation kinetic curves were fitted with the Boltzmann equation, where Imax is the maximum current, V1/2 is the half-activation/inactivation potential, and k is the steepness constant.
Drugs
All drugs used in this study were from Sigma-Aldrich (St. Louis, MO), except for sevoflurane (Abbott, Queenborough, Kent, UK). For the direct application of sevoflurane to recorded neurons, we prepared sevoflurane-containing solutions by bubbling gas mixtures of sevoflurane and 95% O2/5% CO2 generated by the calibrated commercial vaporizer in ACSF for >30 min. The concentration of sevoflurane in the ACSF bubbled with gas mixtures containing 1% sevoflurane, as determined by gas chromatography, was 0.184 mmol/L, which was 92%–94% of the theoretical concentration. The sevoflurane-containing solutions with or without NNC 55-0396 (20 μmol/L) were focally applied to recorded neurons by the “Y-tube” method. To minimize the loss of sevoflurane, we used high-quality polytetrafluorethylene tubing and valves.
Statistical Analysis
Numerical data are expressed as the mean ± SEM. Student’s t-test was used to compare two independent datasets with normal distributions, while paired t-tests were used to compare two dependent datasets with normal distributions. Two-way analysis of variance (ANOVA) and pairwise comparison with the Bonferroni method were performed to compare multiple independent datasets. The categorical data were compared using Fisher’s exact test. P ≤ 0.05 was considered significant.
Results
Enhanced Spontaneous Movements in Younger Pups During Emergence from Sevoflurane Anesthesia
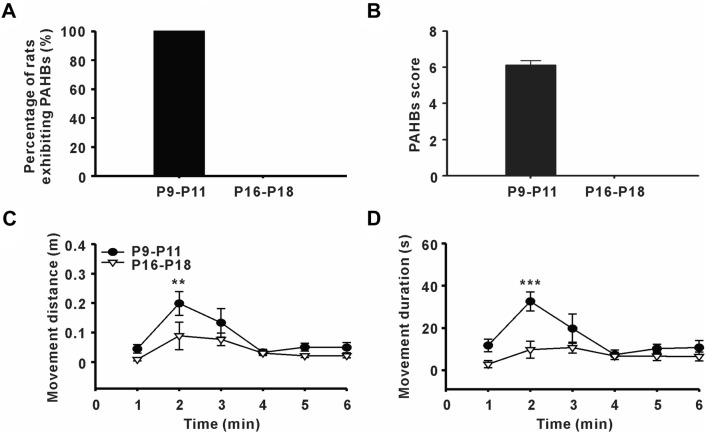
Coincident with our previous findings [10], younger (P9–P11) pups had a high incidence of PAHBs (8 of 8 animals) and high PAHB scores (6.1 ± 0.25), while older pups (P16–P18) were placid during emergence from sevoflurane anesthesia (Fig. 1A, B).
Fig. 1.
Enhanced spontaneous movements in younger pups during emergence from sevoflurane anesthesia. A Incidence of post-anesthetic hyperexcitatory behaviors (PAHBs) in younger rat pups (P9–P11) and older rat pups (P16–P18) (n = 8 per group). B PAHB scores in younger and older pups. C, D Movement distance and movement duration in younger and older pups after sevoflurane withdrawal in the open field test (n = 8 per group; **P ≤ 0.01, ***P ≤ 0.001 vs P16–P18, two-way ANOVA followed by Bonferroni’s t-test).
To obtain a more objective evaluation, we examined PAHBs in the open-field test. According to the analysis of the results for movement distance and movement duration in the open field, we found that younger pups showed an eruption of spontaneous movement in the second minute after sevoflurane withdrawal. However, this phenomenon did not occur in older pups (movement distance in the second minute: P9–P11 vs P16–P18 = 0.2 ± 0.04 m vs 0.09 ± 0.05 m, P = 0.003; movement duration in the second minute: P9–P11 vs P16–P18 = 32.6 ± 4.53 s vs 9.8 ± 4.06 s, P ≤ 0.001, n = 8, two-way ANOVA followed by Bonferroni’s t-test, Fig. 1C, D).
Involvement of TCCs in Generating PAHBs
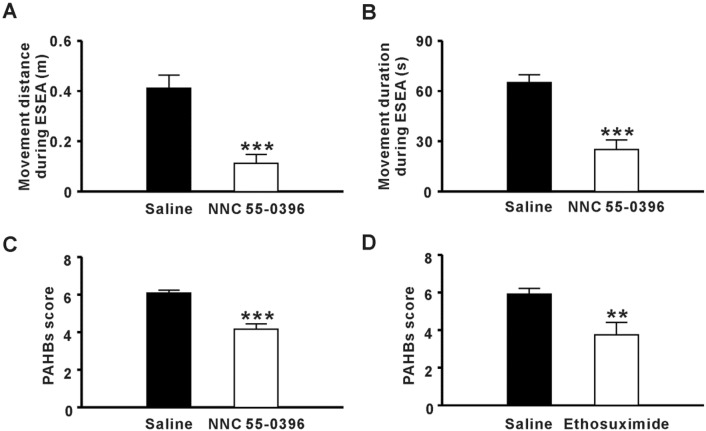
To investigate the contribution of TCCs to generating PAHBs, we delivered NNC 55-0396, a selective TCC inhibitor by ICVM [14]. We found that spontaneous movements 1 min–3 min after sevoflurane withdrawal were significantly inhibited (movement distance: Saline vs NNC 55-0396 = 0.4 ± 0.05 m vs 0.1 ± 0.04 m, P ≤0.001; movement duration: Saline vs NNC 55-0396 = 65.1 ± 4.50 s vs 25.0 ± 5.69 s, n = 6–7, P ≤ 0.001, Student’s t-test, Fig. 2A, B). Moreover, the PAHB score of the NNC 55-0396 group was much lower than that of the saline group (Saline vs NNC 55-0396 = 6.1 ± 0.25 vs 4.2 ± 0.28, n = 6 per group, P ≤ 0.001, Student’s t-test, Fig. 2C). We also found that PAHBs were controlled by the intraperitoneal administration of ethosuximide, an anti-absence seizure drug that functions by blocking TCC currents [15] (PAHB score: saline vs ethosuximide = 5.9 ± 0.30 vs 3.8 ± 0.66, n = 6 per group, P = 0.009, Student’s t-test, Fig. 2D). These data indicated that TCCs in the brain are involved in generating PAHBs.
Fig. 2.
Involvement of T-type Ca2+ channels in generating post-anesthetic hyperexcitatory behaviors (PAHBs). A, B Effect of NNC 55-0396 on movement distance and movement duration during early stage of emergence from anesthesia (ESEA) (n = 6-7; ***P < 0.001 vs saline, Student’s t-test). C Effect of NNC 55-0396 on PAHB score (n = 6 per group; ***P ≤ 0.001 vs saline Student’s t-test). D Effect of ethosuximide on PAHB score (n = 6 per group; **P ≤ 0.01 vs saline, Student’s t-test).
Potentiated Theta Oscillations in EEG When Generating PAHBs
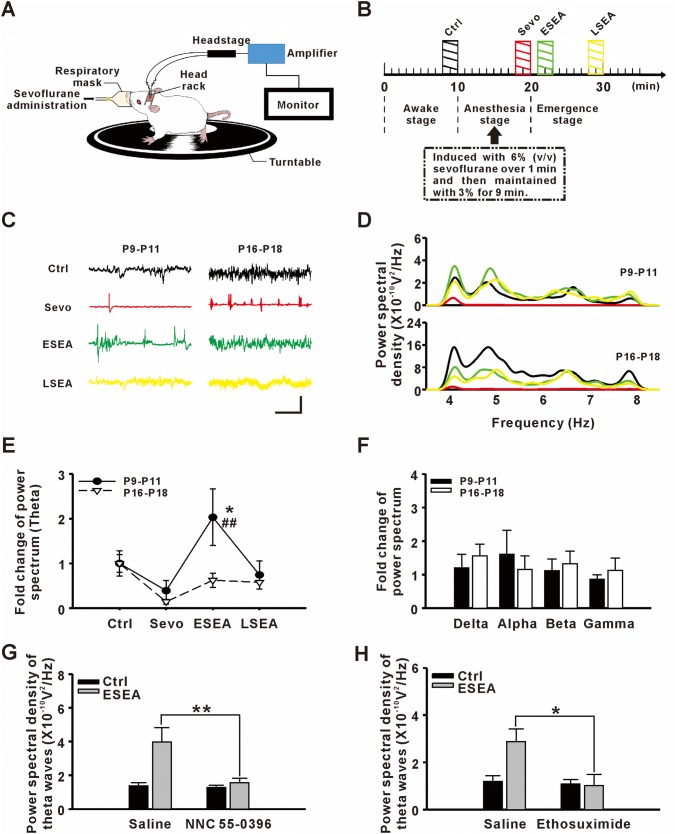
It has been demonstrated that TCCs allow for continuous rhythmic bursts in the thalamus [16]. Synchronized bursting from masses of neurons in the thalamus produces abnormal theta oscillations that propagate to the neocortex through thalamo-cortical efferents [17]. So, we further recorded EEG during the peri-anesthesia period to determine whether abnormal activity occurs during PAHBs (Fig. 3A, B).
Fig. 3.
Potentiated theta oscillations in the frontal lobe when generating post-anesthetic hyperexcitatory behaviors (PAHBs). A Sketch of EEG recording during the peri-anesthesia period. B Schematic of the EEG recording process. Shaded bars indicate periods of EEG analysis: Ctrl (black, baseline recording), Sevo (red, recording under sevoflurane anesthesia), ESEA (green, recording at the early stage of emergence from anesthesia), and LSEA (yellow, recording at a later stage of emergence from anesthesia). C Samples of EEGs from different groups and stages (scale bars; 0.2 mV and 5 s; color coding as in B). D Power spectra of theta oscillations in different groups and stages. E Fold-changes of power spectra of theta oscillations (n = 8–10; *P ≤ 0.05 vs Ctrl; ##P ≤ 0.01 vs P16-P18, two-way ANOVA followed by Bonferroni’s t-test). F Fold changes of power spectra of delta, alpha, beta, and gamma oscillations. G Effect of NNC 55-0396 on the power spectrum of theta oscillations at ESEA (n = 7–8; **P ≤ 0.01 vs saline, Student’s t-test). H Effect of ethosuximide on the power spectrum of theta oscillations at ESEA (n = 6 per group; *P ≤ 0.05 vs saline, Student’s t-test).
One to three minutes after sevoflurane withdrawal (early stage of emergence from anesthesia, ESEA), the period in which PAHBs clustered, younger pups showed potentiated long-term bursts in the frontal lobe, a specific EEG pattern that rarely occurred under other conditions or in the older pups (Fig. 3C). Spectral analysis showed that the power of the theta oscillations was significantly higher in younger pups during ESEA than during the awakening stage, while there were no significant changes in the older pups (Fig. 3D, E; Table 1). Moreover, there were no significant changes in the other frequency bands in either group (Fig. 3F; Table 1).
Table 1.
Relative power in different frequency bands in the waking state (Ctrl) and the early stage of emergence from anesthesia (ESEA).
| Relative power spectrum | Delta (0–4 Hz) | Thetaa (4–8 Hz) | Alpha (8–13 Hz) | Beta (13–30 Hz) | Gamma (30–80 Hz) | |
|---|---|---|---|---|---|---|
| P9–P11 (n = 10) | Ctrl | 1.0 ± 0.30 | 1.0 ± 0.28 | 1.0 ± 0.29 | 1.0 ± 0.26 | 1.0 ± 0.23 |
| ESEA | 1.2 ± 0.40 | 2.0 ± 0.63 | 1.6 ± 0.72 | 1.1 ± 0.35 | 0.9 ± 0.14 | |
| P16–P18 (n = 8) | Ctrl | 1.0 ± 0.42 | 1.0 ± 0.20 | 1.0 ± 0.24 | 1.0 ± 0.28 | 1.0 ± 0.38 |
| ESEA | 1.6 ± 0.35 | 0.6 ± 0.16 | 1.2 ± 0.40 | 1.3 ± 0.38 | 1.1 ± 0.37 | |
aP9–11: Ctrl vs ESEA, P = 0.022; P16–18: Ctrl vs ESEA, P = 0.446; ESEA: P9–11 vs P16–18, P = 0.004, two-way ANOVA followed by Bonferroni’s t-test
Next, we investigated whether blocking TCCs could affect the abnormal EEG activity during ESEA. As expected, the potentiated power of theta oscillations was attenuated by both ICVM with NNC 55-0396 and the intraperitoneal administration of ethosuximide [Saline vs NNC 55-0396 = (4.0 ± 0.84) × 10−10 V2/Hz vs (1.6 ± 0.28) × 10−10 V2/Hz, n = 7–8, P = 0.009; saline vs ethosuximide = (2.9 ± 0.54) × 10−10 V2/Hz vs (1.0 ± 0.47) × 10−10 V2/Hz, n = 6 per group, P = 0.027, Student’s t-test, Fig. 3G, H]. These results suggested that potentiated theta oscillations in the frontal lobe are relevant to PAHBs.
Effect of Sub-anesthetic Dose of Sevoflurane on TCCs
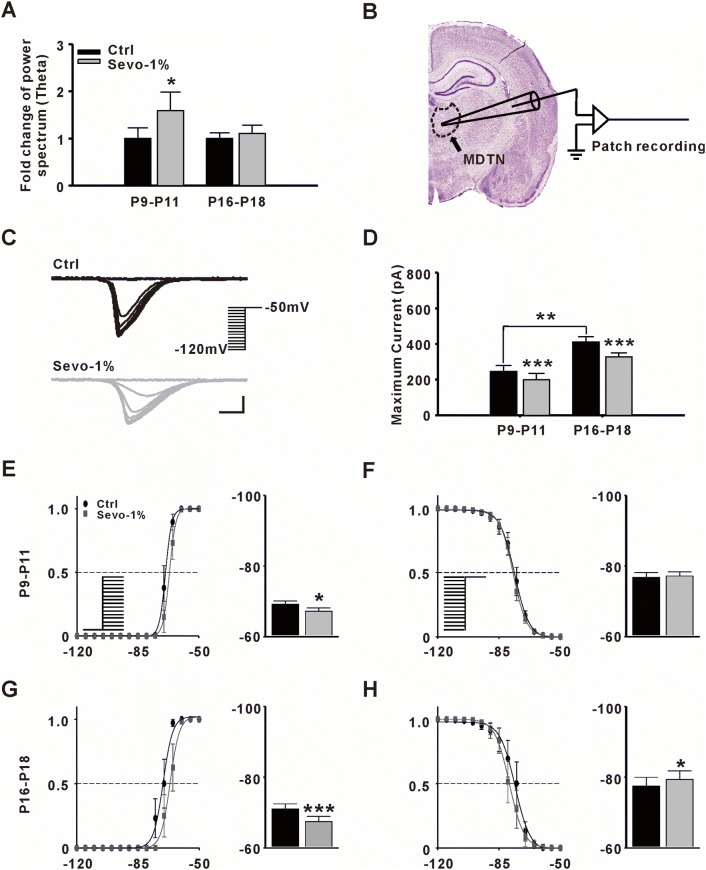
According to our previous study, PAHBs can be continually induced by 1% sevoflurane administration [10]. Here, we found that theta oscillations in young pups were also potentiated during 1% sevoflurane administration (Ctrl vs Sevo-1% = 1.0 ± 0.22 vs 1.6 ± 0.40, n = 6, P = 0.032, paired t-test, Fig. 4A). Therefore, we investigated whether a sub-anesthetic dose (1%) of sevoflurane activated TCCs in younger rats. Because TCCs are expressed at high levels in thalamic relay cells [18], we performed in vitro patch clamp recording in the MDTN (Fig. 4B). Our results showed that the relative amplitude of the maximum TCC current in MDTN neurons was attenuated by 1% sevoflurane in both groups (P9–P11: Ctrl vs Sevo-1% = 1.0 ± 0.14 vs 0.8 ± 0.14, n = 8, P ≤ 0.001; P16–P18: Ctrl vs Sevo-1% = 1.0 ± 0.07 vs 0.8 ± 0.05, n = 8, P ≤ 0.001, paired t-test, Fig. 4C, D).
Fig. 4.
Effect of sevoflurane on T-type Ca2+ channel (TCC) currents. A Fold-changes in power spectrum of theta oscillations with 1% sevoflurane (Sevo-1%). A two-minute period of EEG was analyzed before and after a 10-min Sevo-1% application (n = 6, *P ≤ 0.05 vs Ctrl, paired t-test). B Sketch of in vitro patch clamp recording in the medial dorsal thalamic nucleus (MDTN). C Samples of evoked TCC currents from MDTN neurons before (black) and after (gray) Sevo-1% application. TCC currents were gradually inactivated by transient depolarization from various holding potentials to −50 mV (scale bars, 100 pA and 100 ms). D Fold-changes in maximum TCC currents after Sevo-1% application in different groups (n = 8 per group, **P ≤0.01 vs P9–P11, Student’s t-test; ***P ≤ 0.001 vs Ctrl, paired t-test). E, F Steady-state activation/inactivation of TCC currents recorded from the MDTN in younger rat pups (P9–P11) (left panels, activation/inactivation curves; right panels, half-activation/inactivation potentials; n = 8 per group; *P ≤ 0.05 vs Ctrl, paired t-test). G, H Steady-state activation/inactivation of TCC currents recorded from the MDTN in older rat pups (P16–P18) (n = 8 per group; *P ≤ 0.05, ***P ≤ 0.001 vs Ctrl, paired t-test).
To further investigate the kinetic properties of TCCs, we fitted the activation/inactivation curves of the ion channels and found that the activation curves were only slightly right-shifted by 1% sevoflurane application in P9–P11 rats (half activation potential: Ctrl vs Sevo-1% = 69.1 ± 1.01 mV vs 67.2 ± 0.99 mV, P = 0.039; half inactivation potential: Ctrl vs Sevo-1% = 76.8 ± 1.38 mV vs 77.2 ± 1.16 mV, P = 0.76, paired t-test, Fig. 4E, F). Both activation (right-shifted) and inactivation curves (left-shifted) were clearly changed in P16–P18 rats, showing that the kinetic properties of TCCs were significantly inhibited by 1% sevoflurane (half activation potential: Ctrl vs Sevo-1% = 71.0 ± 1.48 mV vs 67.5 ± 1.46 mV, P ≤ 0.001; half inactivation potential: Ctrl vs Sevo-1% = 77.5 ± 2.46 mV vs 79.4 ± 2.39 mV, P = 0.016, paired t-test, Fig. 4G, H). In brief, the sensitivity of TCCs to sevoflurane increased with age. In other words, the stronger inhibitory effect of sevoflurane might contribute to the lack of PAHBs in older rats.
Effect of Sub-anesthetic Dose of Sevoflurane on Neuronal Activity in the MDTN
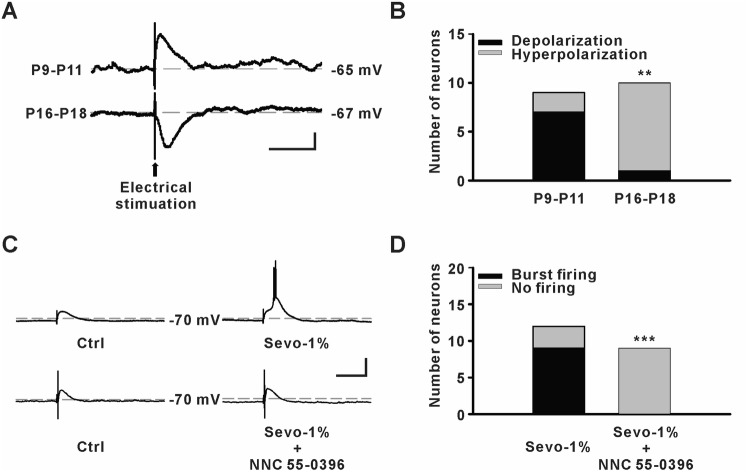
In the introduction, we hypothesized that GABAergic depolarization might be able to elicit action potentials by evoking TCC current. In order to test this possibility, we examined the fast GABAergic postsynaptic potentials and the effects of 1% sevoflurane on neuronal activity in the MDTN using gramicidin-perforated patch clamp recording. We found that 7 of 9 cells in younger pups were depolarized in GABAergic potential, while this occurred in only 1 of 10 cells in older pups (P = 0.005, Fisher’s exact test, Fig. 5A, B). Moreover, burst firing was elicited in 9 of 12 MDTN neurons by 1% sevoflurane administration in younger pups, while this firing was completely suppressed (0 of 9 neurons) by NNC 55-0396 (P = 0.001, Fisher’s exact test, Fig. 5C, D). These data indicate that the contribution of TCCs to PAHB is not mediated by the direct effect of sevoflurane on TCCs. Nonetheless, TCCs contribute to neuronal excitability in the presence of a sub-anesthetic concentration of sevoflurane.
Fig. 5.
Effect of sevoflurane on neuronal activity in the medial dorsal thalamic nucleus (MDTN). A Samples of evoked GABAergic potentials from MDTN neurons (scale bars, 5 mV and 200 ms). B Numbers of GABAergic depolarization in MDTN neurons (P9–11: n = 7 of 9 neurons; P16–18: n = 1 of 10 neurons, **P ≤ 0.01, Fisher’s exact test). C Samples of success or failure to evoke burst firing by 1% sevoflurane (Sevo-1%) without or with NNC 55-0396 (20 μmol/L), respectively (scale bars, 20 mV and 200 ms). D Numbers of MDTN neurons showing sevoflurane-evoked burst firing (P9–11: n = 9 of 12 neurons; P16–18: n = 0 of 9 neurons, ***P ≤ 0.001, Fisher’s exact test).
Discussion
PAHBs were found in an animal model in our previous work, and when we attempted to compare them to EA in humans, we found that their incidence depends on the age of the animal, type of anesthetic, and duration of anesthesia [10]. These characteristics conform to the features of EA in humans. To evaluate PAHBs, we initially developed a scale that was a modified version of a scale used for quantifying seizure behaviors [19]. Scale methods can sometimes be influenced by subjectivity and can be scored differently by different investigators. Therefore, we introduced an open field system to evaluate the PAHBs beyond the use of this score. We found that younger rats with a high incidence of PAHBs showed an eruption of spontaneous movement in the second minute after sevoflurane withdrawal. Concurrently, we recorded potentiated EEG bursting patterns and enhanced theta oscillation power in the same time window, indicating that sevoflurane-induced abnormalities in EEG are related to PAHBs.
A clinical study reported increased frontal lobe cortical functional connectivity in children with EA during emergence from sevoflurane anesthesia [20]. The authors suggested that internally and externally imposed stimuli may result in increased network connectivity. Meanwhile, a variety of EEG patterns occurred during the emergence stage. Although they did not summarize specific EEG patterns for EA in children, their results revealed that theta activity was evident in 3 of the 4 cases.
EEG activity encompasses the synchronous signals of thousands or millions of neurons from the same cortical area. It has been well documented that sevoflurane causes epileptiform EEG activity in both humans and rodents [21, 22]. Thalamo-cortical dysrhythmia is a theoretical framework that can be used to explain many neurological disorders, including epilepsy [23]. Thalamo-cortical dysrhythmia is caused by an imbalance of synaptic inputs, including GABAergic transmission, to thalamic nuclei. Such conditions may create membrane hyperpolarization to de-inactivate TCCs. Therefore, low-threshold Ca2+ spikes could be elicited via TCCs to drive thalamic neurons into a low-frequency bursting mode [24].
Bursting is a common type of neuronal firing in the MDTN [25]. Due to the low threshold, burst firing may amplify sensory-signal transmission from the MDTN to the neocortex to avoid signal attenuation [26]. The MDTN makes reciprocal connections with cells in the prefrontal, limbic, and motor cortices [27], such that a functional imbalance within this neural circuit induced by general anesthetics may lead to abnormal behaviors. Therefore, according to our findings that blocking TCCs attenuated PAHBs and the concomitant EEG changes, we speculated that TCCs may contribute to the generation of PAHBs by potentiating theta activity in the brain. Since TCC inhibitors completely prevented the increased theta waves but only partially prevented the PAHB behaviors after sevoflurane anesthesia, we considered that theta activity might only partially contribute to PAHBs, and other mechanisms independent of the theta activity might contribute.
However, some questions remain unanswered. Why do such hyperexcitatory behaviors (EA or PAHBs) occur more frequently in children or younger pups? Why do they occur during emergence from general anesthesia?
It has been demonstrated that GABAergic action shifts from depolarization/excitation to hyperpolarization/inhibition during the maturation of the central nervous system [28]. In previous work, we showed that sevoflurane-induced PAHBs are strongly associated with GABAergic depolarization/excitation in the neocortical neurons of neonatal rats [10]. However, the depolarizing GABAergic postsynaptic potentials potentiated by sevoflurane in most of these cortical neurons, as well as in neurons in the MDTN (in 7 of 9 cells, EGABA = –60.3 ± 3.04 mV), were subthreshold. In other words, action potentials cannot be activated by GABAergic depolarization alone. Nonetheless, these GABAergic potentials are able to elicit action potentials, presumably by evoking low-threshold Ca2+ spikes that are mediated by TCCs, as it has been demonstrated that TCCs are the molecular determinant of the excitatory effects of GABA in the peripheral somatosensory system [29]. We found that TCCs were less sensitive to sevoflurane in younger than in older pups. Together with GABAergic depolarization, the neurophysiological properties of TCCs may determine the high incidence of PAHBs in younger pups. In addition, the stronger inhibitory effect of sevoflurane might contribute to the lack of PAHBs in older rats.
In addition to the hyperpolarizing inhibition, there is another form of inhibition called shunting, which may reduce excitatory synaptic responses and inactivate voltage-gated ion channels via a local increase in conductance across the plasma membrane [30]. Therefore, a high concentration of GABA would increase the tonic conductance of the plasma membrane, resulting in shunting-mediated inhibition [31]. Sevoflurane acts as a GABAA receptor agonist that potentiates the GABAA channel current [32]. Isoflurane, a volatile anesthetic that has actions similar to those of sevoflurane, is able to shunt voltage-gated Na+ and Ca2+ channels, including TCCs [33]. Therefore, sevoflurane may shunt TCCs via GABAA receptors during deep anesthesia. However, we found that ~80% of T-type currents remained active under a sub-anesthetic dose (1%) of sevoflurane.
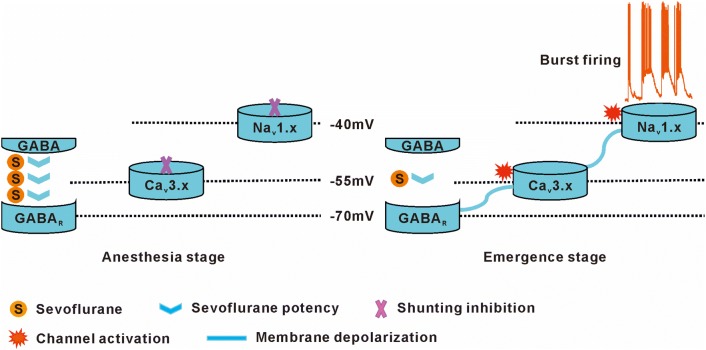
According to the results noted above, we hypothesize the following. In a young pup, sevoflurane inhibits neuronal activity via shunting inhibition during deep anesthesia. When sevoflurane is withdrawn, the excitatory voltage channels recover from inhibition, and potentiated GABAergic depolarization by a sub-anesthetic dose of sevoflurane triggers TCCs, such that burst firing is elicited by the TCC current (Fig. 6).
Fig. 6.
The hypothesis of sevoflurane-induced post-anesthetic hyperexcitatory behaviors.
In the present study, we revealed a possible role of TCCs in the brain in generating hyperexcitatory behaviors during emergence from sevoflurane anesthesia in neonatal rats. However, ICVM or intraperitoneal injection of TCC inhibitors are limited in not providing the contributions of the exact subtypes and their locations. Thus, a gene-editing technique should be used for further investigation. Nonetheless, our conclusion provides a new target for EA management in the clinical setting. For instance, ethosuximide may be considered a candidate for further clinical study.
Acknowledgements
This work was supported by the National Natural Science Foundation, Beijing, People’s Republic of China (81671058 and 81730031 to YW and 81401089 to MD); the National Research Foundation of Korea grants funded by the Republic of Korea (2019R1I1A1A01057744 to YK); and the Foundation of Shanghai Municipal Science and Technology Commission (19ZR1407500 to FS).
Conflict of interest
The authors claim that there are no conflicts of interest.
Footnotes
Feng-Yan Shen, Byung-Gun Lim and Wen Wen have contributed equally to this work.
Contributor Information
Young-Beom Kim, Email: floweransi@korea.ac.kr.
Ying-Wei Wang, Email: wangyingwei@yahoo.com.
References
- 1.Sakai EM, Connolly LA, Klauck JA. Inhalation anesthesiology and volatile liquid anesthetics: focus on isoflurane, desflurane, and sevoflurane. Pharmacotherapy. 2005;25:1773–1788. doi: 10.1592/phco.2005.25.12.1773. [DOI] [PubMed] [Google Scholar]
- 2.Costi D, Cyna AM, Ahmed S, Stephens K, Strickland P, Ellwood J, et al. Effects of sevoflurane versus other general anaesthesia on emergence agitation in children. Cochrane Database Syst Rev. 2014;12(9):CD007084. doi: 10.1002/14651858.CD007084.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore AD, Anghelescu DL. Emergence delirium in pediatric anesthesia. Paediatr Drugs. 2017;19:11–20. doi: 10.1007/s40272-016-0201-5. [DOI] [PubMed] [Google Scholar]
- 4.Veyckemans F. Excitation phenomena during sevoflurane anaesthesia in children. Curr Opin Anaesthesiol. 2001;14:339–343. doi: 10.1097/00001503-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Vlajkovic GP, Sindjelic RP. Emergence delirium in children: many questions, few answers. Anesth Analg. 2007;104:84–91. doi: 10.1213/01.ane.0000250914.91881.a8. [DOI] [PubMed] [Google Scholar]
- 6.Kain ZN, Caldwell-Andrews AA, Weinberg ME, Mayes LC, Wang SM, Gaal D, et al. Sevoflurane versus halothane: postoperative maladaptive behavioral changes: a randomized, controlled trial. Anesthesiology. 2005;102:720–726. doi: 10.1097/00000542-200504000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Hauber JA, Davis PJ, Bendel LP, Martyn SV, McCarthy DL, Evans MC, et al. Dexmedetomidine as a rapid bolus for treatment and prophylactic prevention of emergence agitation in anesthetized children. Anesth Analg. 2015;121:1308–1315. doi: 10.1213/ANE.0000000000000931. [DOI] [PubMed] [Google Scholar]
- 8.Aouad MT, Al-Alami AA, Nasr VG, Souki FG, Zbeidy RA, Siddik-Sayyid SM. The effect of low-dose remifentanil on responses to the endotracheal tube during emergence from general anesthesia. Anesth Analg. 2009;108:1157–1160. doi: 10.1213/ane.0b013e31819b03d8. [DOI] [PubMed] [Google Scholar]
- 9.Dalens BJ, Pinard AM, Letourneau DR, Albert NT, Truchon RJ. Prevention of emergence agitation after sevoflurane anesthesia for pediatric cerebral magnetic resonance imaging by small doses of ketamine or nalbuphine administered just before discontinuing anesthesia. Anesth Analg. 2006;102:1056–1061. doi: 10.1213/01.ane.0000200282.38041.1f. [DOI] [PubMed] [Google Scholar]
- 10.Lim BG, Shen FY, Kim YB, Kim WB, Kim YS, Han HC, et al. Possible role of GABAergic depolarization in neocortical neurons in generating hyperexcitatory behaviors during emergence from sevoflurane anesthesia in the rat. ASN Neuro. 2014;6:127–136. doi: 10.1042/AN20140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chemin J, Cazade M, Lory P. Modulation of T-type calcium channels by bioactive lipids. Pflug Arch. 2014;466:689–700. doi: 10.1007/s00424-014-1467-5. [DOI] [PubMed] [Google Scholar]
- 12.Varma A, He J, Weissfeld L, Devaskar SU. Postnatal intracerebroventricular exposure to neuropeptide Y causes weight loss in female adult rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1560–R1566. doi: 10.1152/ajpregu.00557.2001. [DOI] [PubMed] [Google Scholar]
- 13.Shen FY, Chen ZY, Zhong W, Ma LQ, Chen C, Yang ZJ, et al. Alleviation of neuropathic pain by regulating T-type calcium channels in rat anterior cingulate cortex. Mol Pain. 2015;11:8. doi: 10.1186/s12990-015-0008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang L, Keyser BM, Tagmose TM, Hansen JB, Taylor JT, Zhuang H, et al. NNC 55-0396 [(1S,2S)-2-(2-(N-[(3-benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluoro-1,2, 3,4-tetrahydro-1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochloride]: a new selective inhibitor of T-type calcium channels. J Pharmacol Exp Ther. 2004;309:193–199. doi: 10.1124/jpet.103.060814. [DOI] [PubMed] [Google Scholar]
- 15.Huguenard JR. Block of T-type Ca(2+) channels is an important action of succinimide antiabsence drugs. Epilepsy Curr. 2002;2:49–52. doi: 10.1046/j.1535-7597.2002.00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contreras D. The role of T-channels in the generation of thalamocortical rhythms. CNS Neurol Disord Drug Targets. 2006;5:571–585. doi: 10.2174/187152706779025526. [DOI] [PubMed] [Google Scholar]
- 17.van Wijngaarden JB, Zucca R, Finnigan S, Verschure PF. The impact of cortical lesions on thalamo-cortical network dynamics after acute ischaemic stroke: a combined experimental and theoretical study. PLoS Comput Biol. 2016;12:e1005048. doi: 10.1371/journal.pcbi.1005048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- 19.Bough KJ, Gudi K, Han FT, Rathod AH, Eagles DA. An anticonvulsant profile of the ketogenic diet in the rat. Epilepsy Res. 2002;50:313–325. doi: 10.1016/S0920-1211(02)00086-4. [DOI] [PubMed] [Google Scholar]
- 20.Martin JC, Liley DT, Harvey AS, Kuhlmann L, Sleigh JW, Davidson AJ. Alterations in the functional connectivity of frontal lobe networks preceding emergence delirium in children. Anesthesiology. 2014;121:740–752. doi: 10.1097/ALN.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 21.Constant I, Seeman R, Murat I. Sevoflurane and epileptiform EEG changes. Paediatr Anaesth. 2005;15:266–274. doi: 10.1111/j.1460-9592.2004.01538.x. [DOI] [PubMed] [Google Scholar]
- 22.Mohanram A, Kumar V, Iqbal Z, Markan S, Pagel PS. Repetitive generalized seizure-like activity during emergence from sevoflurane anesthesia. Can J Anaesth. 2007;54:657–661. doi: 10.1007/BF03022961. [DOI] [PubMed] [Google Scholar]
- 23.Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 25.Wei H, Bonjean M, Petry HM, Sejnowski TJ, Bickford ME. Thalamic burst firing propensity: a comparison of the dorsal lateral geniculate and pulvinar nuclei in the tree shrew. J Neurosci. 2011;31:17287–17299. doi: 10.1523/JNEUROSCI.6431-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hull C, Isaacson JS, Scanziani M. Postsynaptic mechanisms govern the differential excitation of cortical neurons by thalamic inputs. J Neurosci. 2009;29:9127–9136. doi: 10.1523/JNEUROSCI.5971-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saalmann YB. Intralaminar and medial thalamic influence on cortical synchrony, information transmission and cognition. Front Syst Neurosci. 2014;8:83. doi: 10.3389/fnsys.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci. 2014;15:637–654. doi: 10.1038/nrn3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aptel H, Hilaire C, Pieraut S, Boukhaddaoui H, Mallie S, Valmier J, et al. The Cav3.2/alpha1H T-type Ca2+ current is a molecular determinant of excitatory effects of GABA in adult sensory neurons. Mol Cell Neurosci. 2007;36:293–303. doi: 10.1016/j.mcn.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- 31.Song I, Savtchenko L, Semyanov A. Tonic excitation or inhibition is set by GABA(A) conductance in hippocampal interneurons. Nat Commun. 2011;2:376. doi: 10.1038/ncomms1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lecker I, Yin Y, Wang DS, Orser BA. Potentiation of GABAA receptor activity by volatile anaesthetics is reduced by alpha5GABAA receptor-preferring inverse agonists. Br J Anaesth. 2013;110(Suppl 1):i73–i81. doi: 10.1093/bja/aet038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ries CR, Puil E. Mechanism of anesthesia revealed by shunting actions of isoflurane on thalamocortical neurons. J Neurophysiol. 1999;81:1795–1801. doi: 10.1152/jn.1999.81.4.1795. [DOI] [PubMed] [Google Scholar]