Abstract
Astrocytes are the most abundant cell type in the central nervous system (CNS). They provide trophic support for neurons, modulate synaptic transmission and plasticity, and contribute to neuronal dysfunction. Many transgenic mouse lines have been generated to obtain astrocyte-specific expression of inducible Cre recombinase for functional studies; however, the expression patterns of inducible Cre recombinase in these lines have not been systematically characterized. We generated a new astrocyte-specific Aldh1l1-CreERT2 knock-in mouse line and compared the expression pattern of Cre recombinase between this and five widely-used transgenic lines (hGfap-CreERT2 from The Jackson Laboratory and The Mutant Mouse Resource and Research Center, Glast-CreERT2, Cx30-CreERT2, and Fgfr3-iCreERT2) by crossing with Ai14 mice, which express tdTomato fluorescence following Cre-mediated recombination. In adult Aldh1l1-CreERT2:Ai14 transgenic mice, tdTomato was detected throughout the CNS, and five novel morphologically-defined types of astrocyte were described. Among the six evaluated lines, the specificity of Cre-mediated recombination was highest when driven by Aldh1l1 and lowest when driven by hGfap; in the latter mice, co-staining between tdTomato and NeuN was observed in the hippocampus and cortex. Notably, evident leakage was noted in Fgfr3-iCreERT2 mice, and the expression level of tdTomato was low in the thalamus when Cre recombinase expression was driven by Glast and in the capsular part of the central amygdaloid nucleus when driven by Cx30. Furthermore, tdTomato was clearly expressed in peripheral organs in four of the lines. Our results emphasize that the astrocyte-specific CreERT2 transgenic lines used in functional studies should be carefully selected.
Electronic supplementary material
The online version of this article (10.1007/s12264-019-00451-z) contains supplementary material, which is available to authorized users.
Keywords: Astrocytes, Cre recombinase, Expression pattern, Aldh1l1, Morphology
Introduction
Astrocytes are the largest group of glial cells, accounting for 20%–40% of the total number of glial cells in the human brain [1–3]. For a long time, they have been assumed to be a homogenous population [1], but a growing body of evidence suggests that astrocytes exhibit high heterogeneity across and within brain regions [4–8]. This heterogeneity is reflected in their morphologies, gene expression profiles, and functions [9]. Particularly, our understanding of astrocytic functions is increasing dramatically, and many more novel functions have recently been discovered and assigned [7, 10–13].
To elucidate the role of astrocytes in vivo, many transgenic mouse lines have been generated to achieve the astrocyte-specific expression of Cre recombinase in order to manipulate the gene functions and/or the activity of astrocytes by using the Cre-loxp system [14]. However, most existing astrocyte Cre lines, such as glial fibrillary acidic protein (GFAP)-Cre, do not meet the requirement of astrocyte-specific expression of Cre recombinase, in part due to the fact that most of the astrocyte promoters used to drive Cre recombinase expression are also expressed by non-astrocytes during early development [15–17]. Thus, there is a persistent need to develop new tools to target astrocytes efficiently with high spatial and temporal specificity [18]. To this end, many astrocyte-specific CreERT2 transgenic mouse lines, such as human Gfap (hGfap)-CreERT2, glutamate aspartate transporter (Glast)-CreERT2, connexin 30 (Cx30)-CreERT2, fibroblast growth factor receptor 3 (Fgfr3)-iCreERT2, and aldehyde dehydrogenase 1 family member L1 (Aldh1l1)-CreERT2, have been generated and characterized [19–23]. However, so far, no studies have been carried out to systematically compare the spatial and temporal expression of Cre recombinase in these astrocyte-specific CreERT2 transgenic mouse lines, making it difficult to choose an ideal mouse line for functional studies of astrocytes in the central nervous system (CNS).
Aldh1l1 is responsible for formate oxidation in vivo and catalyzes the conversion of 10-formyltetrahydrofolate, NADP, and water to tetrahydrofolate, NADPH, and CO2 [24, 25]. It is expressed primarily by glia, especially astrocytes and ventricular ependymal cells [26] and is considered to be a highly specific marker for astrocytes with a substantially broader expression pattern than the traditional astrocyte marker GFAP. Moreover, Aldh1l1 has not been detected in oligodendrocytes, oligodendrocyte precursor cells, or neurons based on transcriptome data [27, 28]. These studies suggest that Aldh1l1 is a highly useful genetic locus to express tamoxifen-inducible CreERT2 for spatiotemporally-controlled astrocyte-specific genetic manipulation.
To achieve astrocyte-specific expression of inducible Cre recombinase, we used CRISPR-Cas9 technology to generate knock-in mice driving CreERT2 expression by the endogenous Aldh1l1 promotor. Using this mouse line, we systematically characterized the morphology of astrocytes and compared the specificity and efficiency of Cre-mediated recombination between this and five widely-used astrocyte-specific CreERT2 lines.
Materials and Methods
Animals
Fgfr3-iCreERT2 transgenic mice were a gift from Professor William D. Richardson (University College London, UK). Two hGfap-CreERT2 transgenic mice were purchased from The Jackson Laboratory (The Jackson Laboratory, Bar Harbor, ME, No: 012849) [29] and the Mutant Mouse Resource and Research Center (Mutant Mouse Resource and Research Center, USA, No: 016992-MU) [19]. Glast-CreERT2 and Cx30-CreERT2 transgenic mice were generously provided by Professor Frank W. Pfrieger (University of Strasbourg, France) [18]. To evaluate the expression patterns of inducible Cre recombinase, we crossed the astrocyte-specific CreERT2 mice with a fluorescent reporter line (Ai14, The Jackson Laboratory, Stock No: 007914). All mice were maintained under specific pathogen-free conditions. Three to four mice were housed in a cage with an individual ventilated caging system at (24 ± 1) °C. The mice were maintained under standard laboratory conditions (12-h light–dark cycle, lights on from 07:00 to 19:00) with free access to food and water unless otherwise indicated. All experiments were conducted in accordance with the regulations for the administration of affairs concerning experimental animals (China) and approved by the Southern Medical University Animal Ethics Committee.
Construction, Generation, and Screening of the Aldh1l1-CreERT2 Knock-in Mouse Line
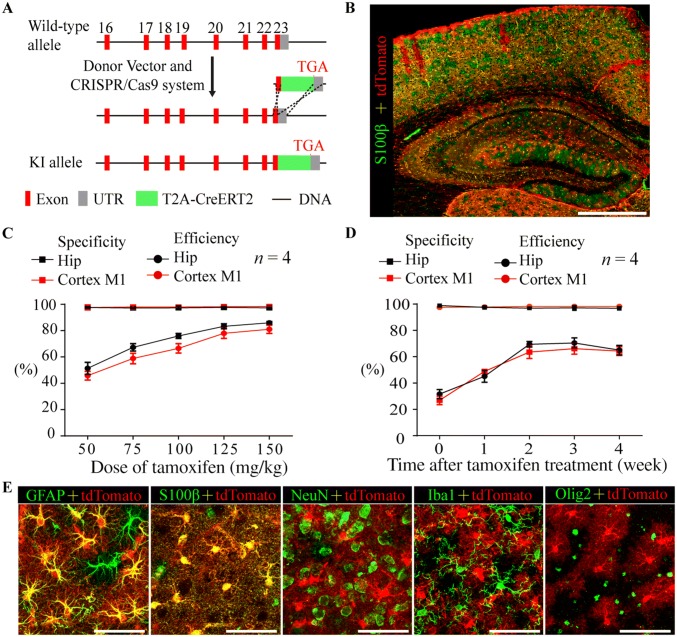
The Aldh1l1-CreERT2 knock-in mouse line was generated by the Model Animal Research Center of Nanjing University (Nanjing, China). The targeting strategy is shown in Fig. 1A. Briefly, Aldh1l1-CreERT2 knock-in mice were generated via a CRISPR/Cas9 system [30, 31] using Cas9 mRNA, sgRNA (CCAGGTCTTGTCCCCAATACTGG), and a donor, which were co-injected into C57BL/6 zygotes using microinjection methods. Then, these zygotes were transplanted into pseudopregnant mice [32]. sgRNA-directed Cas9 endonuclease cleavage occurred near the termination codon and created a double-strand break [33]. These breaks were subsequently repaired and resulted in a T2A-CreERT2 insertion before the stop codon of the Aldh1l1 gene. The mice were screened through PCR analysis using specific primers (Fig. S1A–C).
Fig. 1.
Creation and characterization of Aldh1l1-CreERT2 knock-in mice. A Schematic showing the generation of Aldh1l1-CreERT2 knock-in mice via the CRISPR/Cas9 system. B Representative montage of the cortex and hippocampus in an Aldh1l1-CreERT2:Ai14 double-transgenic mouse injected with tamoxifen and stained for S100β (green) (scale bar, 200 µm). C Graph showing the specificity (percentage of tdTomato-positive cells expressing S100β) and efficiency (percentage of S100β-positive cells expressing tdTomato) of Cre-mediated recombination in the hippocampus and M1 cortex of Aldh1l1-CreERT2:Ai14 transgenic mice as a function of the dose of tamoxifen (mean ± SEM, n = 4). D As in (C) but shown as a function of time after treatment with 100 mg/kg tamoxifen (mean ± SEM, n = 4). E Representative high-magnification images of cortical astrocytes in Aldh1l1-CreERT2:Ai14 transgenic mice showing co-staining with GFAP and S100β but not NeuN, Iba1, or Olig2 (scale bars, 50 µm).
Tamoxifen Treatment
Tamoxifen (Sigma-Aldrich, St. Louis, MO, #T5648) was used to induce CreERT2-mediated recombination. Tamoxifen was dissolved in 10% ethanol (Sigma-Aldrich, #32205) and 90% sunflower oil (Sigma-Aldrich, #S5007) to reach a final concentration of 20 mg/mL. Adult mice (postnatal days P56–60) were given an intraperitoneal (i.p.) injection of 100 mg/kg tamoxifen (unless otherwise stated) once a day for 7 consecutive days. Expression of Cre recombinase was assessed 2 weeks after the last dose of tamoxifen (unless otherwise stated). In mice aged P7, P14, and P21, two i.p. doses of 100 mg/kg tamoxifen were given 24 h apart, and the expression of Cre recombinase was assessed 5 days later.
Virus Injection
The recombinant adeno-associated virus (AAV) vectors (AAV2/9 CAG-DIO-GFP, titers 1 × 1013 particles per mL) were packaged by SunBio (Shanghai, China). Mice were deeply anesthetized with 1% sodium pentobarbital (i.p.) (Sigma-Aldrich, #P3761) and placed in a stereotaxic instrument (RWD Life Science Inc., Shenzhen, China). The scalp was removed, and a 1-mm diameter hole was drilled through the skull with a dental drill at the following coordinates: CA1 (anterior, − 2.0 mm; lateral, ± 1.5 mm; ventral, − 1.5 mm), CA3 (anterior, − 2.0 mm; lateral, ± 2.5 mm; ventral, − 2.0 mm), and dentate gyrus (DG) (anterior, − 2.0 mm; lateral, ± 1.15 mm; ventral, − 2.0 mm). The AAV solution was injected using a Hamilton syringe with a microinjector pump (KDS, Stoelting, Wood Dale, IL); in all experiments, 0.3 µL of virus solution was injected at 0.05 mL/min. After the injection was completed, the needle was raised 0.1 mm and maintained in position for an additional 10 min to allow the virus to diffuse at the injection site; the needle was then slowly withdrawn [34]. One week later, tamoxifen was administered as described above.
Tissue Sections
For tissue fixation, animals were deeply anesthetized with 1% sodium pentobarbital (Sigma, #P3761) and then intracardially perfused with 4 °C saline (0.9% NaCl) followed by 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). After dissection, the tissues were postfixed overnight in 4% PFA in PBS at 4 °C and then in 30% sucrose in PBS at 4 °C. The tissues were subsequently embedded in OCT compound (Tissue-Tek, UK, #4583) and cryo-sectioned (Leica, Germany, #CM1850-1-1) at 20 µm for immunohistochemical staining or 100 µm for morphological analysis. For each brain area, one in every seven slides was collected into a 5-mL centrifuge tube. Tissue slides were stored at − 80 °C or washed three times in PBS prior to staining.
Immunohistochemistry
The sections were washed in PBS and then incubated while shaking for 2 h in PBS containing 5% BSA and 1% Triton X-100 at room temperature. They were then washed three times for 5 min each in PBS, incubated with primary antibodies overnight at 4 °C, washed three times for 5 min each with PBS, incubated with secondary antibodies for 2 h at room temperature, and finally washed three times for 5 min each with PBS. The following primary antibodies were used: anti-NeuN (1:500, Cell Signaling Technology, Beverly, MA, #24307), anti-GFAP (1:500, Cell Signaling Technology, #3670S), anti-calcium-binding protein B (S100β, 1:100, Abcam, UK, #ab52642), anti-Iba1 (1:500, Wako, Japan, #019-19741), anti-Olig2 (1:500, Abcam, #Ab42453), anti-SOX2 (1:1000, Abcam, #ab97959), anti-doublecortin (DCX, 1:500, Cell Signaling Technology, #4604), anti-excitatory amino acid transporter 1 (Glast, 1:50, Proteintech, USA, #20785-1-AP), anti-parvalbumin (PV, 1:5000, Swant, Switzerland, #PV27) and anti-Aldh1l1 (1:50, Proteintech, #17390-1-AP); and the following secondary antibodies were used: goat anti-mouse IgG with Alexa Fluor 594 or 488 (1:2000, Life Technologies, Carlsbad, CA, #A11005 or #A11001) and goat anti-rabbit IgG (H + L) with Alexa Fluor 594 or 488 (1:2000, Life Technologies, #A11012 or #A11008). Sections were placed on coverslips, embedded in Vectashield mounting medium (Vectorlabs, Burlingame, CA, #H-1200) and inspected by confocal microscopy (Nikon C2, Japan).
Cell Counting
Three to five sections containing each brain area were obtained from each mouse, and three to four mice were used in each experiment. Image allocations were blinded for analysis. Cells labeled by specific markers were counted using the “Cell Counter” plugin in ImageJ 1.50i software. Identification of cells was ensured by nuclear DAPI labeling. The specificity of Cre recombinase activity in each mouse line was defined as the number of marker- and tdTomato-positive cells divided by the total number of tdTomato+ cells. The efficiency was defined as the number of tdTomato+ cells divided by the total number of marker-positive cells.
Morphological Analysis
To sparsely label and evaluate the morphological architecture of astrocytes, laser confocal 3D imaging was applied in Aldh1l1-CreERT2:Ai14 mice treated with a low dose of tamoxifen (50 mg/kg). After perfusion-based fixation, tissues were cut into 100 µm-thick coronal/sagittal sections on a cryostat microtome (Leica, CM1850-1-1). To increase the light transmittance of the tissue, the slides were washed in PBS and then incubated while shaking for 2 days in PBS containing 5% BSA and 2% Triton X-100 at 4 °C. The solution was changed once a day. Single cells were imaged by confocal microscopy (Nikon C2) using a 40 × or 60 × objective. Image stacks were obtained at 100-nm steps to obtain optimal z-axis resolution. Morphometric parameters were analyzed using the image analysis software Imaris 8.1 (Bitplane, Belfast, UK).
Statistical Analysis
Student’s t test and one-way ANOVA were used to compare the means of two and multiple independent samples, respectively, using SPSS 22.0 software (Chicago, IL). The number of experimental animals is indicated by ‘n’. Data shown in the text and figures are expressed as the mean ± standard error of the mean (SEM) (unless otherwise stated). P < 0.05 was considered statistically significant, and GraphPad Prism 6.0 (La Jolla, CA) was used to generate graphs.
Results
Generation and Characterization of Aldh1l1-CreERT2 Knock-in Mouse Line
To obtain a knock-in mouse line expressing inducible Cre recombinase in astrocytes with high selectivity and efficiency, T2A-CreERT2, a fragment obtained from a donor, was inserted before the stop codon of the endogenous Aldh1l1 gene using the CRISPR/Cas9 system (Fig. 1A). The Aldh1l1-CreERT2 knock-in mice were identified through PCR analysis (Fig. S1A–C). The sizes of the PCR products obtained from the 5’ homology arm, the 3′ homology arm, and WT tissue were 2126 bp, 1457 bp, and 452 bp, respectively (Fig. S1B, C). Homozygotes and heterozygotes displayed no alterations in growth, fertility, or behavior.
The expression pattern of inducible Cre recombinase in the CNS was visualized by crossing Aldh1l1-CreERT2 knock-in mice with an Ai14 reporter line following tamoxifen treatment [35] (Table 1, Fig. S2A). In adult Aldh1l1-CreERT2:Ai14 transgenic mice, the Cre reporter tdTomato was not detected in the absence of tamoxifen treatment (Fig. S3B), indicating the absence of leaky reporter expression. Tamoxifen-induced Cre recombination occurred throughout the CNS with high specificity in astrocytes as shown by co-localization of tdTomato with the astrocyte markers GFAP and S100β (Fig. 1C–E; Table 1). To examine the expression patterns of endogenous Aldh1l1, we stained for Aldh1l1 in the M1 cortex and hippocampus of adult Aldh1l1-CreERT2:Ai14 mice. Almost all tdTomato+ cells were Aldh1l1+ (~ 98%); however, Aldh1l1+/tdTomato− cells were also occasionally observed, similar to the results with S100β (Fig. S1D, E). This suggested that the expression pattern of Cre recombinase faithfully recapitulated that of the endogenous Aldh1l1 promoter. The efficiency of Cre recombination was dependent on the dose of tamoxifen (Fig. 1C) and approached a plateau at two weeks after treatment with 100 mg/kg tamoxifen (Fig. 1B, D), while the specificity was stable regardless of the dose or time after treatment (Fig. 1C, D). Increasing the dose of tamoxifen to > 150 mg/kg resulted in significant weight loss in mice (data not shown).
Table 1.
Astroglia-specific induction of Cre recombination by tamoxifen (%).
| Brain region | GFAP | S100β | NeuN | Iba1 | Olig2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Efficiency | Specificity | Efficiency | Specificity | Efficiency | Specificity | Efficiency | Specificity | Efficiency | Specificity | |
| Olfactory bulb | – | – | 57.84 ± 7.62 | 77.84 ± 0.36 | 0 | 0 | 1.38 ± 0.36 | 0.81 ± 0.13 | 6.1 ± 4.01 | 7.42 ± 4.51 |
| Cortex | – | – | 54.05 ± 1.41 | 96.12 ± 1.37 | 0.09 ± 0.01 | 0.59 ± 0.07 | 1.57 ± 0.43 | 1.23 ± 1.51 | 2.58 ± 1.46 | 4.23 ± 1.29 |
| Hippocampus | 66.86 ± 1.48 | 95.84 ± 1.02 | 64.75 ± 1.32 | 97.51 ± 1.3 | 0 | 0 | 2.44 ± 1.79 | 1.51 ± 0.85 | 1.64 ± 0.7 | 2.38 ± 1.31 |
| BLA | – | – | 62.96 ± 2.72 | 96.94 ± 1.51 | 0 | 0 | 1.30 ± 1.54 | 1.15 ± 1.40 | 3.17 ± 1.94 | 4.55 ± 2.01 |
| PFC | – | – | 59.75 ± 2.41 | 96.71 ± 0.24 | 0 | 0 | 1.67 ± 1.13 | 1.52 ± 1.44 | 2.98 ± 1.68 | 3.15 ± 1.88 |
| VTA | 53.57 ± 3.58 | 95.6 ± 1.09 | 0 | 0 | 2.71 ± 1.01 | 2.14 ± 1.94 | 2.95 ± 1.62 | 3.44 ± 1.28 | ||
| Thalamus | – | – | 95.28 ± 2.64 | 97.16 ± 1.18 | 0 | 0 | 3.57 ± 1.8 | 2.63 ± 1.51 | 3.21 ± 1.93 | 3.98 ± 1.75 |
| Hypothalamus | 69.01 ± 3.75 | 44.67 ± 6.02 | 61.03 ± 7.7 | 76.37 ± 1.6 | 0 | 0 | 3.92 ± 0.94 | 2.82 ± 1.45 | 3.08 ± 1.55 | 3.81 ± 1.42 |
| Midbrain | – | – | 64.82 ± 3.77 | 95.39 ± 2.48 | 0 | 0 | 1.97 ± 1.54 | 1.55 ± 1.81 | 2.96 ± 1.08 | 3.17 ± 1.26 |
| Cerebellum (Bergmann glia) | – | – | 92.17 ± 0.41 | 97.29 ± 1.49 | 0 | 0 | 2.53 ± 0.53 | 1.91 ± 0.47 | 2.24 ± 1.06 | 2.51 ± 1.67 |
| Medulla | – | – | 50.23 ± 0.63 | 90.54 ± 2.59 | 0 | 0 | 0.88 ± 0.15 | 0.92 ± 0.36 | 2.85 ± 1.92 | 3.11 ± 1.83 |
| Spinal cord | 79.18 ± 4.76 | 56.17 ± 2.57 | 46.94 ± 3.38 | 78.51 ± 7.15 | 0 | 0 | 2.40 ± 0.61 | 1.69 ± 0.63 | 1.84 ± 1.05 | 2.47 ± 1.68 |
Aldh1l1-CreERT2:Ai14 transgenic mice were induced by tamoxifen, and immunofluorescence with GFAP, S100β, NeuN and Iba1, then imaged by confocal laser scanning microscopy. The specificity of Cre recombinase activity was defined as the number of marker- and tdTomato-positive cells divided by the total number of tdTomato+ cells. The efficiency was defined as the number of tdTomato+ cells divided by the total number of marker-positive cells. Data are shown as mean ± SEM, n = 3.
To assess whether inducible Cre recombinase was expressed in cells other than astrocytes, we also co-stained for markers of neurons (NeuN), microglia (Iba1), and oligodendrocytes (Olig2) in adult Aldh1l1-CreERT2:Ai14 mice (Fig. 1E; Table 1). We only very occasionally observed co-localization of tdTomato and NeuN in the M1 region of cortex, but no co-labeling was found in other cortical areas (Fig. 3A; Table 1). A small number of tdTomato+ cells were also Iba1+ (Table 1). In contrast, the percentage of tdTomato+ cells that co-expressed Olig2 was relatively high, especially in the olfactory bulb (Table 1), possibly because astrocytes and oligodendrocytes share a common origin, and because Olig2 is expressed in glial-restricted precursors giving rise to oligodendrocyte or astrocyte precursors [36, 37].
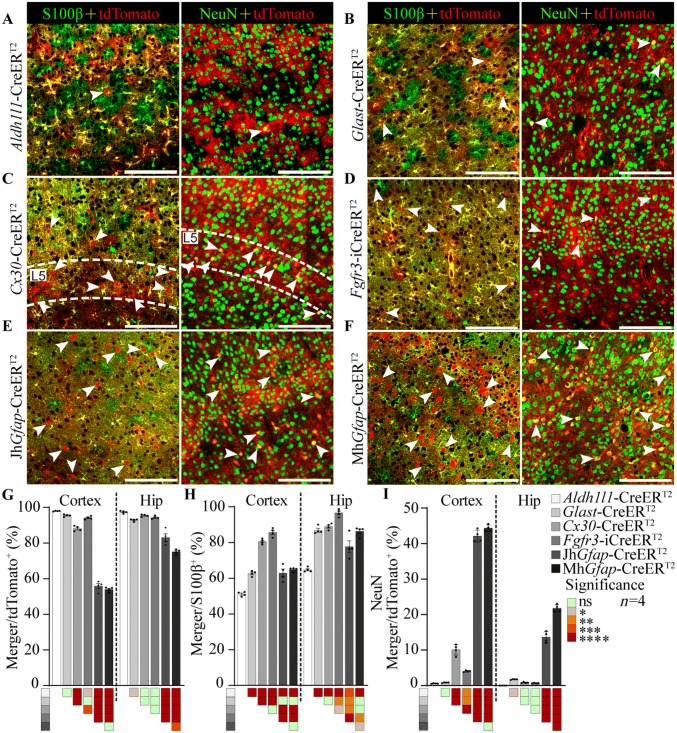
Fig. 3.
Comparison of the specificity and efficiency of CreERT2-mediated recombination in the cortex and hippocampus among astrocyte-targeting transgenic mice. A–F Representative staining for S100β or NeuN (green) in the M1 cortex of Aldh1l1- (A), Glast- (B), Cx30- (C), Fgfr3-i (D), JhGfap- (E), and MhGfap- CreERT2:Ai14 (F) double-transgenic mice (arrowheads indicate single cells positive for tdTomato (left) and co-staining with NeuN (right); scale bars, 50 µm). G, H Specificity (G) and efficiency (H) of CreERT2-mediated recombination in the M1 cortex and hippocampus in astrocyte-specific CreERT2:Ai14 transgenic mice. I Percentage of tdTomato-positive cells expressing NeuN in the M1 cortex and hippocampus in astrocyte-specific CreERT2:Ai14 transgenic mice. In G–I, data are shown as the mean ± SEM, n = 4; colored squares, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (one-way ANOVA followed by Tukey’s test).
Previous studies have reported that the expression level of Aldh1l1 is highest during early development, but decreases after one month of age [38]. To further examine the astrocyte targeting specificity and efficiency of Aldh1l1-CreERT2 knock-in mice during development, Aldh1l1-CreERT2:Ai14 mice aged P7, P14, and P21 were used. The results showed that, while the specificity of Cre recombinase expression was high (> 90%) during early development (Fig. S2A, B), the efficiency was lower in young mice than in adults (Fig. S2A, C), possibly due to the low dose of tamoxifen administered to young mice.
As an alternative approach to reporter lines, we also studied the specificity of Cre targeting using AAV-mediated transduction of a Cre-dependent GFP construct (AAV2/9-CAG-DIO-GFP). Cre-induced expression of GFP was observed in the hippocampus in Aldh1l1-CreERT2 knock-in mice after intracranial injections of AAV followed by tamoxifen treatment. GFP was consistently co-expressed with S100β in CA1 (95.08% ± 0.88% of GFP+ cells, n = 3) and CA3 (98.69% ± 0.31% of GFP+ cells, n = 3) (Fig. S1G) but not with NeuN. However, 9.36% ± 1.38% of GFP+ cells co-expressed NeuN in the DG region (Fig. S1F), possibly because the virus transduced neuronal precursor cells in neurogenic areas.
Morphological Diversity of Astrocytes in Aldh1l1-CreERT2:Ai14 Mice
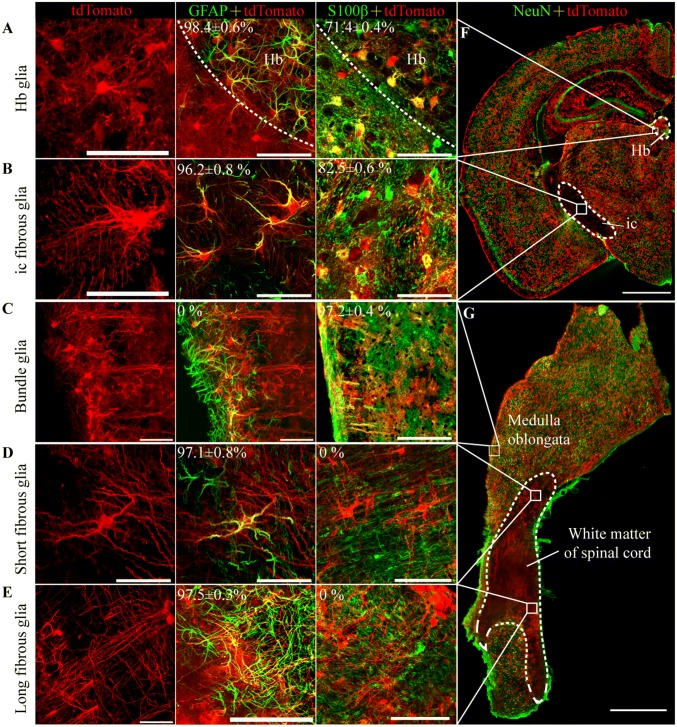
Astrocytes have highly variable and complex morphologies. To examine this aspect, we sparsely labeled astrocytes with tdTomato by treating Aldh1l1-CreERT2:Ai14 mice with a low dose of tamoxifen (50 mg/kg). tdTomato labeled not only protoplasmic astrocytes in grey matter and fibrous astrocytes in white matter, but also perivascular glia, which were closely opposed to blood vessels through their end-feet (Fig. S3C). We identified all the morphologically-defined types of astrocyte that were reported previously, including pia mater glia, tanycytes, marginal astrocytes, and Bergmann glia (data not shown) [3, 5, 39]. In addition, we identified five novel morphologically-distinct types that have never been reported; these were habenular nucleus (Hb) glia, inner capsule (ic) fibrous glia, bundle glia, and spinal cord short/long fibrous glia (Fig. 2A–E).
Fig. 2.
Characterization of five novel astrocyte morphologies in Aldh1l1- CreERT2:Ai14 transgenic mice. A Habenular nucleus (Hb) glia co-stained with GFAP (98.4% ± 0.6%) or S100β (71.4% ± 0.4%) but not NeuN (scale bar, 50 µm). B–E As in (A), but in inner capsule (ic) fibrous glia (B), bundle glia (C), short fibrous glia (D), and long fibrous glia (E) (scale bars, 50 µm). (F) Distribution of Hb astrocytes and ic fibrous glia (scale bar, 1 mm). G Distribution of bundle glia, short fibrous glia, and long fibrous glia in the sagittal plane (scale bar, 1 mm).
Nearly all Hb glia expressed GFAP (98.4% ± 0.6%, n = 3), but only 71.4% ± 0.4% were co-stained with S100β (Fig. 2A; Table 2). These cells filled the Hb, in which they had dense and distinct boundaries, but relatively small diameters (26.5 µm ± 3.1 µm) and they covered an area (715.0 µm2 ± 209.8 µm2) smaller than that of the other fibrous glia types (Fig. 2A; Table 2). Their processes formed clusters that were interspersed among each other and that surrounded the cell bodies of neurons.
Table 2.
Quantification of characteristics of the novel morphologically-defined types of astrocytes in the Aldh1l1-CreERT2:Ai14 transgenic mice.
| Cell type | Distribution | Efficiency (%) | Cell number | Max diameter (µm) | Body area (µm2) | Territory area (µm2) | Branch number | Branch series | ||
|---|---|---|---|---|---|---|---|---|---|---|
| GFAP | S100B | NeuN | ||||||||
| Hb glia | Hb | 98.4 ± 0.6 | 71.4 ± 0.4 | 0 | 12 | 26.45 ± 3.05 | 57.78 ± 11.22 | 714.95 ± 209.79 | 5.08 ± 1.08 | 2.58 ± 0.51 |
| ic fibrous glia | ic | 96.2 ± 0.8 | 82.5 ± 0.6 | 0 | 16 | 45.81 ± 11.55 | 71.28 ± 18.42 | 2618.11 ± 408.64 | 4.96 ± 0.71 | 3.1 ± 0.86 |
| Bundle glia* | Medulla oblongata | 0 | 97.2 ± 0.4 | 0 | 12 | 122.42 ± 10.5 | 76.72 ± 14.89 | 3724.11 ± 591.47 | 8.81 ± 2.44 | 1 |
| Spinal cord short fibrous glia | Spinal cord | 97.1 ± 0.8 | 0 | 0 | 16 | 90.84 ± 32.16 | 109.73 ± 23.37 | 11,039.79 ± 2731.6 | 3.5 ± 1.22 | 2.83 ± 0.41 |
| Spinal cord long fibrous glia | Spinal cord | 97.5 ± 0.3 | 0 | 0 | 14 | 171 ± 42.75 | 95.63 ± 13.57 | 11,080.35 ± 6708.94 | 2.25 ± 0.5 | 2.75 ± 0.5 |
The efficiency was defined as the number of tdTomato+ cells divided by the total number of marker-positive cells. Hb Habenular nucleus, ic inner capsule. Data are shown as mean ± SD.
Most of the ic fibrous glia co-expressed GFAP (96.2% ± 0.8%, n = 3), and 82.5% ± 0.6% of them also co-expressed S100β (Fig. 2B; Table 2). These cells were located in the internal capsule, which contains axons that project to and from the cerebral cortex (Fig. 2G). Most of the ic fibrous glia had ~ 5 branches (Table 2) that did not cover ‘‘bushy’’ territories. The processes of adjacent cells formed a network that was flattened and oriented perpendicular to the sagittal plane (Fig. 2B) indicating that these cells support axonal functions.
Bundle glia co-localized with S100β but not with GFAP (Fig. 2C). These cells were located at the surface of the dorsal medulla oblongata and ran as bundles into the medulla. Bundle glia had the most abundant branches (8.81 ± 2.44 branches/cell) but had no secondary branches (Table 2).
Spinal cord short/long fibrous glia co-expressed GFAP but not S100β (Fig. 2D, E). These cells were located in the white matter of the spinal cord, with the shorter more superficial than the longer (Fig. 2G). The short fibrous glia had thicker branches than other fibrous glia, and the long fibrous glia had the longest branches (171 µm ± 42.75 µm) among all morphologically-defined types of astrocyte (Table 2).
Differences in Inducible Cre Recombination Among Astrocyte-specific CreERT2 Lines
Astrocyte-specific gene recombination has been widely used to study the functions of genes in astrocytes [14, 40], but very little is known about the differences in the expression patterns of inducible Cre recombinase driven by different promoters. To address this issue, we crossed five widely-used astrocyte-specific CreERT2 lines [hGfap-CreERT2 (from the MMRRC, MhGfap-CreERT2), hGfap-CreERT2 (from The Jackson Laboratory, JhGfap-CreERT2), Glast-CreERT2, Cx30-CreERT2 and Fgfr3-iCreERT2] with Ai14 reporter mice to create MhGfap-CreERT2:Ai14, JhGfap-CreERT2:Ai14, Glast-CreERT2:Ai14, Cx30-CreERT2:Ai14, and Fgfr3-iCreERT2:Ai14 mice.
All the transgenic lines were treated with 100 mg/kg tamoxifen for 7 days at P56–P60, and the specificity and efficiency of CreERT2-mediated recombination was then assessed in the M1 cortex and hippocampus 2 weeks after the last dose of tamoxifen (Figs. 3A–F and S4–6). The Aldh1l1- and Glast-CreERT2 lines had the highest specificity in the M1 cortex as shown by co-staining for S100β and NeuN (one-way ANOVA, P < 0.05) (Fig. 3G, I). Similarly high specificity was detected in the Aldh1l1-, Cx30-, and Fgfr3-iCreERT2 lines in the hippocampus (Fig. 3G, I). Unexpectedly, the JhGfap- and MhGfap-CreERT2 lines had the lowest specificity, as many tdTomato+ cells were also NeuN+ in the M1 cortex and hippocampus (one-way ANOVA, P < 0.0001) (Fig. 3I). These mouse lines were not used in subsequent experiments. In the Glast-CreERT2 line, the specificity was high in the cortex, but there was co-localization between tdTomato and NeuN in the DG (Fig. S6B). In the Cx30-CreERT2 line, some neurons in the M1 cortex also expressed tdTomato, and these cells were distributed mainly in layer V (Fig. 3C, G–I). The Aldh1l1 line showed the lowest expression efficiency and the Fgfr3-iCreERT2 line the highest in both the cortex and hippocampus (Fig. 3A, G–I).
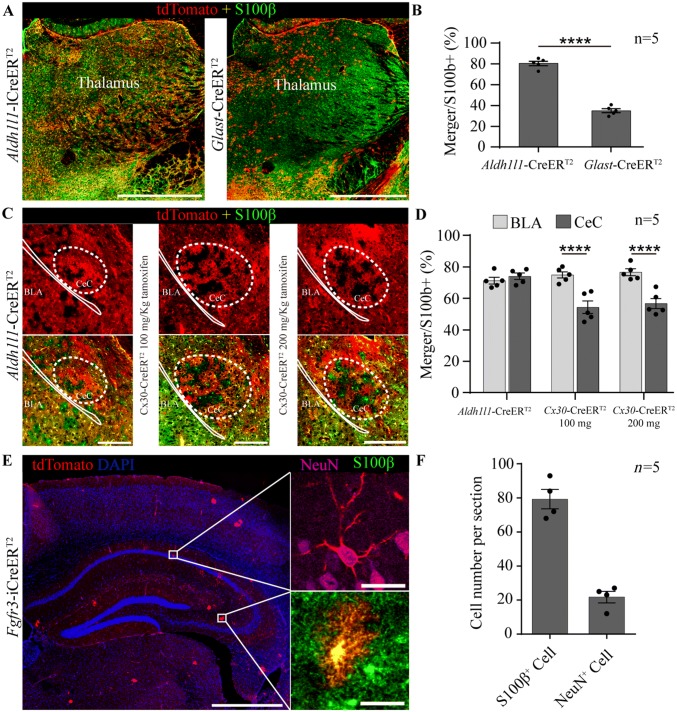
Some unique properties of the astrocyte-specific CreERT2 lines were identified. In the Glast-CreERT2 line, the expression efficiency of Cre-mediated recombination was lowest in the thalamus (Figs. 4A, B and S7A) and recombination occurred mainly in areas of the thalamus that lacked PV-positive cells (Fig. S7B). In the Cx30-CreERT2 line, “cavitation areas” in the capsular part of the central amygdaloid nucleus (CeC) were observed (54.31% ± 3.95%, n = 5), even at higher doses (56.70% ± 3.21%, n = 5; Fig. 4C, D). Leaky Cre recombination in neurons and astrocytes not treated with tamoxifen was detected only in the Fgfr3-iCreERT2 line (Fig. 4E, F). In addition, many tdTomato+ cells were observed in the choroid plexus of the Aldh1l1-CreERT2 line, while tdTomato+ ependymal cells were observed in the Cx30-CreERT2 and Fgfr3-iCreERT2 lines, and to a lesser extent, in the Glast-CreERT2 line (Fig. S7C).
Fig. 4.
Unique properties of astrocyte-specific CreERT2:Ai14 transgenic mice. A Representative staining for S100β in the thalamus of Aldh1l1- and Glast- CreERT2:Ai14 mice (scale bars, 1 mm). B Bar graphs showing that the efficiency of CreERT2-mediated recombination in Glast-CreERT2:Ai14 mice was lower than in Aldh1l1-CreERT2:Ai14 mice in the thalamus. C Representative staining for S100β in the basal lateral amygdala (BLA) and capsular part of the central amygdaloid nucleus (CeC) in Aldh1l1- and Cx30-CreERT2:Ai14 mice treated with 100 mg/kg or 200 mg/kg tamoxifen once per day for 5 consecutive days (scale bars, 200 µm). D Bar graphs showing the efficiency of CreERT2-mediated recombination in Cx30-CreERT2:Ai14 mice was significantly reduced in the CeC. E Representative staining for NeuN and S100β in Fgfr3-iCreERT2:Ai14 mice not treated with tamoxifen [scale bars, 1 mm (right) and 10 µm (left)]. F Bar graphs showing the numbers of astrocytes (S100β+, 79.25 ± 5.71 cells) and neurons (NeuN+, 21.75 ± 3.35 cells) per hippocampal section in Fgfr3-iCreERT2:Ai14 mice not treated with tamoxifen. In B, D, and F, the data are shown as the mean ± SEM; ****P < 0.0001, Student’s t test.
Cre-mediated Recombination in Neural Stem Cells in Adult Astrocyte-specific CreERT2:Ai14 Mice
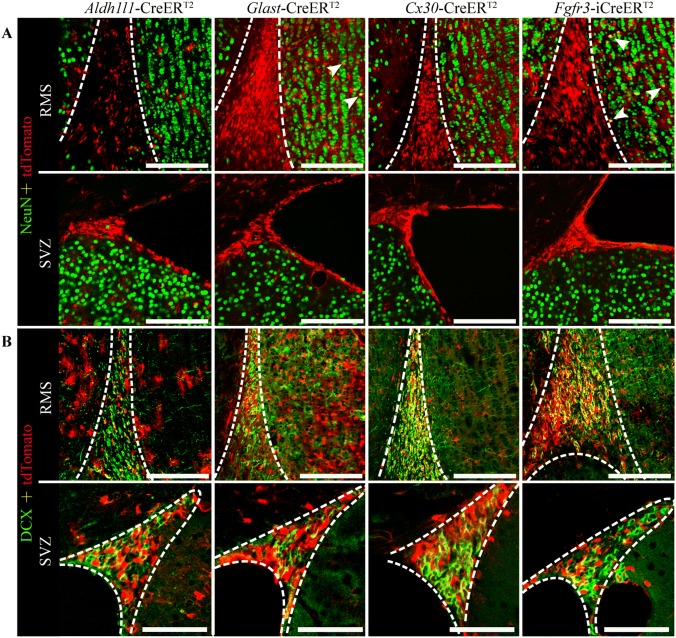
Neural stem cells persist in the rostral migratory stream (RMS), subventricular zone (SVZ), and subgranular zone (SGZ) of the adult rodent brain. Stem cells in the SVZ and RMS migrate to the cortex and the olfactory bulb, where they become mature neurons or astrocytes in adult mice [41, 42]. In addition, neuroblasts from the SGZ migrate and differentiate into DG granule cells [43]. It has been reported that Aldh1L1, Glast, and Fgfr3 are expressed in neural stem cells in adult animals [18, 20, 44]. To examine Cre-mediated recombination in neural stem cells in adult astrocyte-specific CreERT2 lines, we co-stained tissue from the RMS, SVZ (Fig. 5A, B), and SGZ (Figs. S5A–D and S1H) with NeuN (marker for mature neurons) or DCX (marker for neuronal precursor cells). As described above and reported previously [18], in the Glast-CreERT2 line, many cells in the DG were co-stained with NeuN (Figs. 3I, S6B), but this was not observed in the DG of the Aldh1l1-CreERT2 line (Fig. S6A–D), and almost no cells in the Aldh1l1-CreERT2 line were co-stained for DCX (1.66% ± 0.13% of DCX+ cells, n = 4; Fig. S1H). In addition, some cells in the granular cell layer were co-stained with NeuN in both the Glast-CreERT2 and Fgfr3-iCreERT2 lines (Fig. 5A). As expected, Aldh1l1-CreERT2 mediated recombination in neural stem cells of the RMS and SVZ, as demonstrated by the co-localization of tdTomato with DCX. Similar results were obtained in the Glast-, Cx30-, and Fgfr3-iCreERT2 lines (Fig. 5B).
Fig. 5.
Cre-mediated recombination in neural stem cells in astrocyte-specific CreERT2:Ai14 transgenic mice. A Representative staining for NeuN (green) in the rostral migratory stream (RMS) and subventricular zone (SVZ) in Aldh1l1-, Glast-, Cx30-, and Fgfr3-i CreERT2:Ai14 mice (arrows indicate cells co-stained with NeuN; scale bars, 50 µm). B Representative staining for DCX (green) in the RMS and SVZ in Aldh1l1-, Glast-, Cx30-, and Fgfr3-i CreERT2:Ai14 mice (scale bars, 50 µm).
Cre-mediated Recombination in Peripheral Organs of Astrocyte-specific CreERT2 Mice
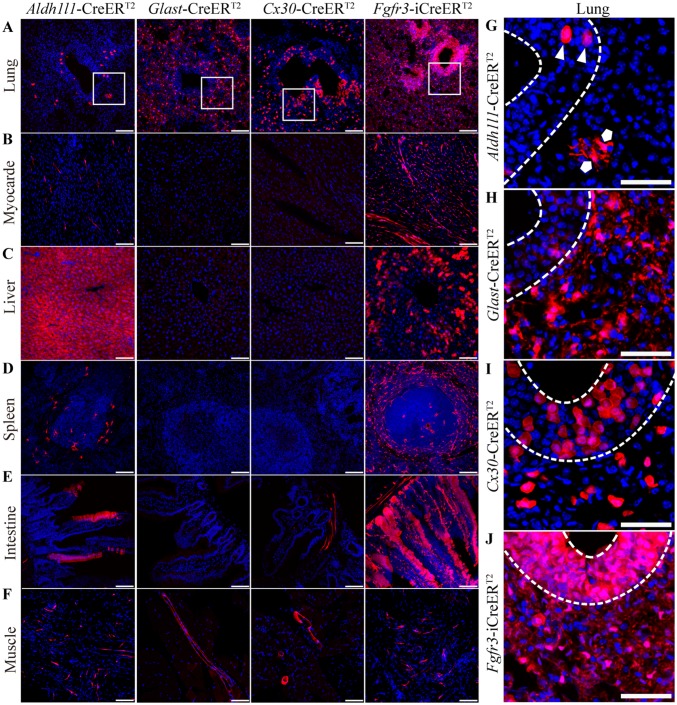
Previous studies have suggested that Aldh1l1 is expressed in organs such as the spleen and liver, as well as interstitial cells [45, 46]. Consistent with these reports, we found a high level of Cre reporter expression in the liver of mice in the Aldh1l1-CreERT2 line (Fig. 6C), but only moderate levels of tdTomato expression in the lung (Fig. 6A), heart (Fig. 6B), spleen (Fig. 6D), intestine (Fig. 6E), and muscle (Fig. 6F). In the Fgfr3-iCreERT2 line, many tdTomato+ cells were found in the liver, spleen, lung, small intestine, and heart (Fig. 6A–F). In the Glast- and Cx30-CreERT2 lines, there were many tdTomato+ cells in the lungs (Fig. 6A, G–J) and a few in blood vessel walls in muscles (Fig. 6F). In addition, sporadic tdTomato+ cells were present on the blood vessel walls of the small intestine in the Cx30-CreERT2 line (Fig. 6E).
Fig. 6.
Cre-mediated recombination in the organs of astrocyte-specific CreERT2:Ai14 transgenic mice. A–F Recombination in the lung (A), myocardium (B), liver (C), spleen (D), intestine (E), and muscle (F) in Aldh1l1-, Glast-, Cx30-, and Fgfr3-i CreERT2:Ai14 mice (scale bars, 100 µm). G–J Representative high-magnification images of lung in the Aldh1l1-, Glast-, Cx30-, and Fgfr3-i CreERT2:Ai14 mice. Two morphologically distinct cell types, round (triangles) and branching cells (pentagons), were sparsely distributed in the Aldh1l1-CreERT2:Ai14 mice (G) but densely distributed in the Fgfr3-i CreERT2:Ai14 mice (J); however, only branching cells were observed in the Glast-CreERT2:Ai14 mice (H), and only round cells in the Cx30-CreERT2:Ai14 mice (I) (scale bars, 50 µm).
Surprisingly, morphologically distinct cells were found to express tdTomato in the lungs of CreERT2 lines (Fig. 6G–J). In the Aldh1l1-CreERT2 line, two different cell types were sparsely labeled with tdTomato; these were called branching cells and round cells and were located in the lung lining and on the surface of the trachea, respectively. Interestingly, we found that branching cells were specifically labeled in the Glast-CreERT2 line (Fig. 6H), while round cells were specifically labeled in the Cx30-CreERT2 line (Fig. 6I).
Discussion
In this study, we generated a new astrocyte-specific Aldh1l1-CreERT2 knock-in mouse line using CRISPR-Cas9 technology and assessed the efficiency and specificity of Cre recombinase in the whole CNS. Five novel morphologically-defined types of astrocyte were then characterized using this line. By comparing the Aldh1l1-CreERT2 line with five widely-used astrocyte-specific CreERT2 transgenic lines, we discovered that Aldh1l1-CreERT2 knock-in mice had the highest astrocyte specificity, highlighting its utility for future functional studies; however, the relatively low efficiency may limit its practical application.
In Aldh1l1-CreERT2 knock-in mice, Cre recombination covered almost all brain areas, showed no leakage, and had a high degree of astrocyte specificity. While the expected insertion site was detected by sequencing (data not shown), we cannot exclude the possibility that CRISPR-mediated insertion of CreER may result in non-specific modification of the endogenous genome. However, Cre-positive cells were almost all co-labeled with Aldh1l1, suggesting that Cre recombination expression is strictly controlled by the activity of the endogenous Aldh1l1 promoter. Recently, two other lines of Aldh1l1-CreERT2 mice were independently generated by two labs using bacterial artificial chromosome (BAC)-based transgene technology [22, 23]. One group inserted the CreERT2 cDNA into the start codon of the ALDH1L1 gene, but the specificity and efficiency of the inducible Cre recombinase in the whole CNS were not evaluated through crossing with a fluorescent reporter line [23]. The other group substituted the open reading frame of exon 2 with the CreERT2 cDNA, resulting in low expression specificity in cortex (12% of Cre+ cells were S100β−) and cerebellum, in which 3.3% of PV+ cells in the molecular layer showed Cre recombination. In addition, Cre recombination was also observed in some neurons in the DG and olfactory bulb [22]. These differences may result from the fact that CreERT2 was inserted into different locations. Moreover, BAC-based transgenes are randomly inserted, which may affect the expression of other genes, and multiple copies of the gene are commonly inserted [47, 48]. In contrast, CRISPR/Cas9-based gene knock-in is a fixed-point insertion and usually a single copy of the gene is inserted, having less effect on other endogenous genes [49, 50].
Our study provides new evidence for the diversity of astrocyte morphology. Reichenbach and Wolburg described 12 subtypes of astrocyte based on morphology [3, 39, 51]. Emsley and Macklis imaged astrocytes in hGfap-GFP mice to provide a morphological characterization of this cell type in the whole CNS and showed region-specific differences [5]. However, they did not describe these morphological features quantitatively or in detail. By evaluating astrocytes sparsely-labeled with tdTomato due to treatment with a low dose of tamoxifen (50 mg/kg) in adult Aldh1l1-CreERT2:Ai14 mice, we identified all of the morphologically-defined types of astrocyte reported previously [3–5, 39] and additionally described five new morphologically-distinct types: Hb glia with crossing processes, ic fibrous glial cells perpendicular to coronal sections, bundle glial cells in the medulla, and spinal cord short and long fibrous glia (Fig. 2A–F; Table 2). Whether these cell types have different physiological characteristics and functions needs further study.
Our data showed that different astrocyte-specific promoters give rise to different Cre recombination patterns. The highest specificity was obtained when Cre recombinase was driven by Aldh1l1, suggesting that this line is ideal for functional research; however, its lower efficiency may miss some astrocyte subtypes. When inducible Cre recombinase was driven by Glast or Cx30, it was expressed at low levels in the thalamus or CeC, respectively. The data obtained in the Glast-CreERT2 line were consistent with previous reports [18], and the expression of Cre recombinase was concentrated in areas negative for PV in the thalamus (Fig. S7B). It has been suggested that there are two astrocyte subtypes in the thalamus, similar to the Glast+ and Glast− astrocytes in the cortex [8], and coordinated with the different neurons around them. In addition, Cx30− astrocytes lack gap junctions and do not express Cx30 but instead express other connexins in the CeC that are relevant to specific functions [52, 53]. When driven by Fgfr3, Cre recombinase showed leakage throughout the brain, and this may trigger developmental effects. However, this leakage does not occur in Fgfr3-iCreERT2:R26R-EYFP mice [20]. This discrepancy may be due to the different genetic backgrounds of the transgenic mice [54]. The R26R-EYFP mice were a mixture of 129X1/SvJ and C57BL/6 J strains [55], but Ai14 mice were congenic on the C57BL/6 J genetic background and showed stronger fluorescence than R26R-EYFP [35]. It should be noted that the lowest specificity among all these lines was achieved by hGfap (Fig. 3G, I). A possible explanation is that Gfap promoter sequences exert direct activity in neurons [56]. These findings put into question conclusions that are obtained with hGfap-CreERT2 lines, especially in behavioral and electrophysiological experiments.
In tissues outside the brain, inducible Cre recombinase was expressed at high levels in the liver (Fig. 6C) when driven by Aldh1l1, potentially because the Aldh1l1 CpG island is methylation-free in DNA extracted from hepatocytes [45]. Therefore, future studies on Aldh1l1-CreERT2 line-driven knockouts should consider potential effects on the liver. Interestingly, inducible Cre recombinase occurred in two morphologically distinct lung cells, one each in the Glast- and Cx30-CreERT2 lines. These data suggest that astrocyte-specific Cre recombination may also affect peripheral tissues.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by Grants from the National Natural Science Foundation of China (31430032, 31830033, 81971080, and 81671356), the Program for Changjiang Scholars and Innovative Research Teams in University (IRT_16R37), the Science and Technology Program of Guangdong (2018B030334001), and the Guangzhou Science and Technology Project (201707020027, 201704020116). Thanks to Professor William D. Richardson (University College London, UK) for the Fgfr3-iCreERT2 line.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Contributor Information
Jian-Ming Yang, Email: jimmyyoung@smu.edu.cn.
Tian-Ming Gao, Email: tgao@smu.edu.cn.
References
- 1.Herculano-Houzel S. The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 2014;62:1377–1391. doi: 10.1002/glia.22683. [DOI] [PubMed] [Google Scholar]
- 2.Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature. 2010;468:214–222. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]
- 3.Verkhratsky AN, Butt A. Glial Physiology and Pathophysiology. Chichester, West Sussex, UK; Hoboken, NJ, USA: Wiley-Blackwell, 2013.
- 4.Bailey MS, Shipley MT. Astrocyte subtypes in the rat olfactory bulb: morphological heterogeneity and differential laminar distribution. J Comp Neurol. 1993;328:501–526. doi: 10.1002/cne.903280405. [DOI] [PubMed] [Google Scholar]
- 5.Emsley JG, Macklis JD. Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol. 2006;2:175–186. doi: 10.1017/S1740925X06000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buosi AS, Matias I, Araujo APB, Batista C, Gomes FCA. Heterogeneity in synaptogenic profile of astrocytes from different brain regions. Mol Neurobiol. 2018;55:751–762. doi: 10.1007/s12035-016-0343-z. [DOI] [PubMed] [Google Scholar]
- 7.Ben Haim L, Rowitch DH. Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci. 2017;18:31–41. doi: 10.1038/nrn.2016.159. [DOI] [PubMed] [Google Scholar]
- 8.Morel L, Men Y, Chiang MSR, Tian Y, Jin S, Yelick J, et al. Intracortical astrocyte subpopulations defined by astrocyte reporter mice in the adult brain. Glia. 2019;67:171–181. doi: 10.1002/glia.23545. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q, Jie W, Liu JH, Yang JM, Gao TM. An astroglial basis of major depressive disorder? An overview. Glia. 2017;65:1227–1250. doi: 10.1002/glia.23143. [DOI] [PubMed] [Google Scholar]
- 11.Cui Y, Yang Y, Ni Z, Dong Y, Cai G, Foncelle A, et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature. 2018;554:323–327. doi: 10.1038/nature25752. [DOI] [PubMed] [Google Scholar]
- 12.Tso CF, Simon T, Greenlaw AC, Puri T, Mieda M, Herzog ED. Astrocytes regulate daily rhythms in the suprachiasmatic nucleus and behavior. Curr Biol. 2017;27:1055–1061. doi: 10.1016/j.cub.2017.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niu B, Zhang T, Hu HQ, Cao BZ. Transcriptome sequencing reveals astrocytes as a therapeutic target in heat-stroke. Neurosci Bull. 2017;33:627–640. doi: 10.1007/s12264-017-0156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahn HM, Scheller A, Kirchhoff F. Genetic control of astrocyte function in neural circuits. Front Cell Neurosci. 2015;9:310. doi: 10.3389/fncel.2015.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo ZB, Su YY, Lou HF. GFAP-positive progenitor cell production is concentrated in specific encephalic regions in young adult mice. Neurosci Bull. 2018;34:769–778. doi: 10.1007/s12264-018-0228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casper KB, McCarthy KD. GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol Cell Neurosci. 2006;31:676–684. doi: 10.1016/j.mcn.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Hirrlinger PG, Scheller A, Braun C, Hirrlinger J, Kirchhoff F. Temporal control of gene recombination in astrocytes by transgenic expression of the tamoxifen-inducible DNA recombinase variant CreERT2. Glia. 2006;54:11–20. doi: 10.1002/glia.20342. [DOI] [PubMed] [Google Scholar]
- 18.Slezak M, Goritz C, Niemiec A, Frisen J, Chambon P, Metzger D, et al. Transgenic mice for conditional gene manipulation in astroglial cells. Glia. 2007;55:1565–1576. doi: 10.1002/glia.20570. [DOI] [PubMed] [Google Scholar]
- 19.Casper KB, Jones K, McCarthy KD. Characterization of astrocyte-specific conditional knockouts. Genesis. 2007;45:292–299. doi: 10.1002/dvg.20287. [DOI] [PubMed] [Google Scholar]
- 20.Young KM, Mitsumori T, Pringle N, Grist M, Kessaris N, Richardson WD. An Fgfr3-iCreER(T2) transgenic mouse line for studies of neural stem cells and astrocytes. Glia. 2010;58:943–953. doi: 10.1002/glia.20976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori T, Tanaka K, Buffo A, Wurst W, Kuhn R, Gotz M. Inducible gene deletion in astroglia and radial glia–a valuable tool for functional and lineage analysis. Glia. 2006;54:21–34. doi: 10.1002/glia.20350. [DOI] [PubMed] [Google Scholar]
- 22.Winchenbach J, Duking T, Berghoff SA, Stumpf SK, Hulsmann S, Nave KA, et al. Inducible targeting of CNS astrocytes in Aldh1l1-CreERT2 BAC transgenic mice. F1000Research. 2016;5:2934. doi: 10.12688/f1000research.10509.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivasan R, Lu TY, Chai H, Xu J, Huang BS, Golshani P, et al. New transgenic mouse lines for selectively targeting astrocytes and studying calcium signals in astrocyte processes in situ and in vivo. Neuron. 2016;92:1181–1195. doi: 10.1016/j.neuron.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KM, Lan Q, Kricker A, Purdue MP, Grulich AE, Vajdic CM, et al. One-carbon metabolism gene polymorphisms and risk of non-Hodgkin lymphoma in Australia. Hum Genet. 2007;122:525–533. doi: 10.1007/s00439-007-0431-2. [DOI] [PubMed] [Google Scholar]
- 25.Lim U, Wang SS, Hartge P, Cozen W, Kelemen LE, Chanock S, et al. Gene-nutrient interactions among determinants of folate and one-carbon metabolism on the risk of non-Hodgkin lymphoma: NCI-SEER case-control study. Blood. 2007;109:3050–3059. doi: 10.1182/blood-2006-07-034330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neymeyer V, Tephly TR, Miller MW. Folate and 10-formyltetrahydrofolate dehydrogenase (FDH) expression in the central nervous system of the mature rat. Brain Res. 1997;766:195–204. doi: 10.1016/s0006-8993(97)00528-3. [DOI] [PubMed] [Google Scholar]
- 27.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, et al. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26:8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen B, Zhang J, Wu H, Wang J, Ma K, Li Z, et al. Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res. 2013;23:720–723. doi: 10.1038/cr.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Y, Zhang X, Shen B, Lu Y, Chen W, Ma J, et al. Generating rats with conditional alleles using CRISPR/Cas9. Cell Res. 2014;24:122–125. doi: 10.1038/cr.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui L, Zhang Z, Sun F, Duan X, Wang M, Di K, et al. Transcervical embryo transfer in mice. J Am Assoc Lab Anim Sci. 2014;53:228–231. [PMC free article] [PubMed] [Google Scholar]
- 33.Wei C, Liu J, Yu Z, Zhang B, Gao G, Jiao R. TALEN or Cas9 - rapid, efficient and specific choices for genome modifications. J Genet Genomics. 2013;40:281–289. doi: 10.1016/j.jgg.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Chen YH, Lan YJ, Zhang SR, Li WP, Luo ZY, Lin S, et al. ErbB4 signaling in the prelimbic cortex regulates fear expression. Transl Psychiatry. 2017;7:e1168. doi: 10.1038/tp.2017.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Wu Y, Lee JC, Xue H, Pevny LH, Kaprielian Z, et al. Oligodendrocyte and astrocyte development in rodents: an in situ and immunohistological analysis during embryonic development. Glia. 2002;40:25–43. doi: 10.1002/glia.10111. [DOI] [PubMed] [Google Scholar]
- 37.Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, et al. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y, Vidensky S, Jin L, Jie C, Lorenzini I, Frankl M, et al. Molecular comparison of GLT1 + and ALDH1L1 + astrocytes in vivo in astroglial reporter mice. Glia. 2011;59:200–207. doi: 10.1002/glia.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matyash V, Kettenmann H. Heterogeneity in astrocyte morphology and physiology. Brain Res Rev. 2010;63:2–10. doi: 10.1016/j.brainresrev.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Tran CHT, Peringod G, Gordon GR. Astrocytes integrate behavioral state and vascular signals during functional hyperemia. Neuron. 2018;100(1133–1148):e1133. doi: 10.1016/j.neuron.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 41.Chaboub LS, Deneen B. Developmental origins of astrocyte heterogeneity: the final frontier of CNS development. Dev Neurosci. 2012;34:379–388. doi: 10.1159/000343723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH. Astrocyte development and heterogeneity. Cold Spring Harb Perspect Biol. 2014;7:a020362. doi: 10.1101/cshperspect.a020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obernier K, Alvarez-Buylla A. Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain. Development. 2019;146:dev156059. doi: 10.1242/dev.156059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foo LC, Dougherty JD. Aldh1L1 is expressed by postnatal neural stem cells in vivo. Glia. 2013;61:1533–1541. doi: 10.1002/glia.22539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oleinik NV, Krupenko NI, Krupenko SA. Epigenetic silencing of ALDH1L1, a metabolic regulator of cellular proliferation, in cancers. Genes Cancer. 2011;2:130–139. doi: 10.1177/1947601911405841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boesmans W, Rocha NP, Reis HJ, Holt M, Vanden Berghe P. The astrocyte marker Aldh1L1 does not reliably label enteric glial cells. Neurosci Lett. 2014;566:102–105. doi: 10.1016/j.neulet.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 47.Beil J, Buch T. Generation of bacterial artificial chromosome (BAC) transgenic mice. Methods Mol Biol. 2014;1194:157–169. doi: 10.1007/978-1-4939-1215-5_8. [DOI] [PubMed] [Google Scholar]
- 48.Yang XW, Gong S. An overview on the generation of BAC transgenic mice for neuroscience research. Curr Protoc Neurosci 2005, Chapter 5: 5–20. [DOI] [PubMed]
- 49.Kesavan G, Chekuru A, Machate A, Brand M. CRISPR/Cas9-mediated zebrafish knock-in as a novel strategy to study midbrain-hindbrain boundary development. Front Neuroanat. 2017;11:52. doi: 10.3389/fnana.2017.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech L, Kempton HR, et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell. 2014;159:440–455. doi: 10.1016/j.cell.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reichenbach A, Wolburg H. Neuroglia, 2nd ed. Oxford, UK: Oxford University Press, 2004.
- 52.Pannasch U, Dossi E, Ezan P, Rouach N. Astroglial Cx30 sustains neuronal population bursts independently of gap-junction mediated biochemical coupling. Glia. 2019;67:1104–1112. doi: 10.1002/glia.23591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clasadonte J, Haydon PG. Connexin 30 controls the extension of astrocytic processes into the synaptic cleft through an unconventional non-channel function. Neurosci Bull. 2014;30:1045–1048. doi: 10.1007/s12264-014-1476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshiki A, Moriwaki K. Mouse phenome research: implications of genetic background. ILAR J. 2006;47:94–102. doi: 10.1093/ilar.47.2.94. [DOI] [PubMed] [Google Scholar]
- 55.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su M, Hu H, Lee Y, d’Azzo A, Messing A, Brenner M. Expression specificity of GFAP transgenes. Neurochem Res. 2004;29:2075–2093. doi: 10.1007/s11064-004-6881-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.