Abstract
Chemical stimulation of the kidney increases sympathetic activity and blood pressure in rats. The hypothalamic paraventricular nucleus (PVN) is important in mediating the excitatory renal reflex (ERR). In this study, we examined the role of molecular signaling in the PVN in mediating the capsaicin-induced ERR and sympathetic activation. Bilateral PVN microinjections were performed in rats under anesthesia. The ERR was elicited by infusion of capsaicin into the cortico-medullary border of the right kidney. The reflex was evaluated as the capsaicin-induced changes in left renal sympathetic nerve activity and mean arterial pressure. Blockade of angiotensin type 1 receptors with losartan or inhibition of angiotensin-converting enzyme with captopril in the PVN abolished the capsaicin-induced ERR. Renal infusion of capsaicin significantly increased NAD(P)H oxidase activity and superoxide anion production in the PVN, which were prevented by ipsilateral renal denervation or microinjection of losartan into the PVN. Furthermore, either scavenging of superoxide anions or inhibition of NAD(P)H oxidase in the PVN abolished the capsaicin-induced ERR. We conclude that the ERR induced by renal infusion of capsaicin is mediated by angiotensin type 1 receptor-related NAD(P)H oxidase activation and superoxide anion production within the PVN.
Keywords: Renal reflex, Sympathetic activity, Blood pressure, Paraventricular nucleus, Angiotensin, Reactive oxygen species
Introduction
Sympathetic over-activity contributes to the development of chronic heart failure, hypertension, and chronic kidney disease [1–3], and the kidneys play important roles in the sympathetic activation in these diseases [4, 5]. It is well known that the renal nerve contains both afferents and efferents. Chemoreceptors in the kidney can be activated by capsaicin, bradykinin, adenosine, and hypertonic NaCl in the renal tissue and pelvis, while mechanoreceptors mainly respond to changes in pelvic pressure [6]. The renal afferent sensory signals contribute to the sympathetic activation in hypertension and chronic heart failure [7, 8]. Renal denervation has been used as an interventional approach to chronic kidney disease and some cardiovascular diseases [9, 10]. Recently, we showed that renal infusion of capsaicin, angiotensin II (Ang II), bradykinin, or adenosine activates renal afferents, and then causes increases in sympathetic activity and blood pressure, indicating that chemical stimulation of the kidney results in an excitatory renal reflex (ERR) in rats, resulting in sympathetic activation and pressor responses [11].
Afferent activity from the kidneys is closely associated with several brain sites involved in the modulation of cardiovascular and sympathetic activity, including the nucleus of the solitary tract, rostral ventrolateral medulla, paraventricular nucleus of the hypothalamus (PVN), preoptic area, and subfornical organ [4]. The PVN receives inputs from various visceral receptors including arterial baroreceptors and pulmonary/cardiac vagal afferents [12], cardiac sympathetic afferents [13], adipose afferents [14], and renal afferents [11]. The PVN is critical in the integration and regulation of sympathetic and cardiovascular activity by sending its descending projections to the rostral ventrolateral medulla and spinal intermediolateral column [15, 16]. Renal afferent inputs increase sympathetic activity [7], and renal nerve stimulation excites some neurons in the PVN [17]. An imbalance of excitatory and inhibitory synaptic inputs in the PVN is the basis of the enhanced sympathetic activity in hypertension [7, 18]. We recently showed that chemical stimulation of the kidneys promotes c-Fos expression in the PVN, and lesioning PVN neurons with kainic acid abolishes the sympathetic activation and pressor responses to renal infusion of capsaicin, suggesting that the PVN is a crucial central nucleus in the ERR pathway [11]. Angiotensin type 1 receptors (AT1Rs) are densely distributed in the PVN, and are involved in the control of sympathetic activity [19, 20]. We previously showed that AT1Rs in the PVN mediate the cardiac sympathetic afferent reflex (CSAR), a sympatho-excitatory reflex induced by chemical stimulation of sympathetic afferent endings innervating the heart [13, 21]. Both N-methyl-D-aspartate receptors (NMDARs) and non-NMDARs in the PVN mediate the adipose afferent reflex (AAR), by which afferent activity from white adipose tissue increases sympathetic outflow and blood pressure [22]. However, the molecular signaling in the PVN that mediates the ERR was not known. The aim of this study was to determine the molecular signaling mechanism in the PVN that mediates the ERR.
Methods
Animals and General Procedures
Experiments were carried out on 196 male Sprague-Dawley rats weighing 280 g–320 g. The experimental procedures were approved by the Experimental Animal Care and Use Committee, and complied with the Guide for the Care and Use of Laboratory Animals. The animals were kept under a 12-h dark-light cycle with free access to tap water and chow. The experiments were performed on rats anaesthetized by intraperitoneal injection of α-chloralose (40 mg/kg) combined with urethane (800 mg/kg). Corneal and paw withdrawal reflexes were used to assess the depth of anesthesia [23]. The right carotid artery and trachea were exposed via a midline neck incision, the trachea was cannulated, and positive-pressure ventilation was applied with a small animal ventilator (model 51600, Stoelting, Chicago, IL). The right carotid artery was cannulated to record pressure. The blood pressure, heart rate, and renal sympathetic nerve activity (RSNA) were simultaneously recorded with a data acquisition system (8SP, ADInstruments, Bella Vista, NSW, Australia). Following the surgery, the animal was allowed to stabilize for at least 30 min. At the end of each experiment, animals were euthanized by overdose with pentobarbital sodium (100 mg/kg, i.v.).
PVN Microinjection
Each rat was fixed prone in a stereotaxic frame (Stoelting, Chicago, IL). The coordinates for microinjections into the PVN were 1.8 mm caudal to bregma, 0.4 mm lateral to the midline, and 7.9 mm below the dorsal surface [21]. Glass micropipettes with tip size 50 μm were used for bilateral PVN microinjections of 50 nL on each side completed in 1 min. After experiments, the same volume of Evans Blue was microinjected into the PVN for histological identification of the injection sites [24]. The visible spread of dye was < 300 μm in diameter. Data were excluded for microinjection sites outside the PVN.
RSNA Recording
Recordings were made for the left RSNA as we reported previously [25]. Briefly, the left flank was incised to expose the renal artery and nerve. The nerve was carefully isolated and cut at its distal end to abolish afferent activity. The central end of the nerve was laid on silver electrodes immersed in mineral oil at 37°C and its activity was amplified with a band-pass filter at 100-3000 Hz using a differential amplifier (model DP-304, Warner Instruments, Hamden, CT). The signals were integrated at a 100-ms time constant using LabChart 8 (ADInstruments). After each experiment, the background noise was recorded after cutting the central end of the nerve. The RSNA value was calculated as the measured RSNA value minus the background noise value. The effects of chemicals on RSNA were calculated as a percentage change of the control value.
Renal Infusion of Capsaicin to Induce the ERR
As we previously reported, the ERR was elicited by renal infusion of capsaicin [11]. Briefly, the right flank was incised to expose the right kidney. A stainless-steel tube (0.31 mm OD) was horizontally inserted into the kidney from right to left for renal infusion. The insertion was stopped when the tube encountered a slight resistance, indicating that its tip had reached the cortico-medullary border, about 2 mm below the renal surface. The outer end of the tube was connected to a programmable pressure injector (Model PM2000B, MDI Inc., Lakewood, NJ) through a PE50 polyethylene catheter. The ERR was elicited by infusion of capsaicin (1 nmol/μL) into the kidney at 1.0 μL/min for 20 min. An equal amount of vehicle was infused as a control. The ERR was evaluated by the capsaicin-induced RSNA and mean arterial pressure (MAP) responses.
Measurement of Superoxide Anions and NAD(P)H Oxidase Activity
Coronal sections 450 µm thick were cut at the PVN level on a cryostat microtome (Model CM1900, Leica, Wetzlar, Germany), and the bilateral PVN areas were punched out using a 15-gauge needle. The punched tissue was homogenized and centrifuged in lysis buffer. Total protein concentration was determined with the Bradford assay (BCA; Pierce, Santa Cruz, CA).
Superoxide anion content was assessed by the lucigenin-derived chemiluminescence method [26–28]; the photon emission was started by adding dark-adapted lucigenin (5 μmol/L). NAD(P)H oxidase activity was determined by the same method [29–31]; the photon emission was started by adding both dark-adapted lucigenin and NAD(P)H (100 μmol/L). Light emission was measured ten times in 10 min using a luminometer (Model 20/20n, Turner, CA). The values were expressed as relative mean light units/min per mg protein. Background chemiluminescence in the buffer containing lucigenin (5 μmol/L) was also measured.
Detection of Superoxide Anions in the PVN
Superoxide anions in the PVN were detected with the specific fluorogenic probe dihydroethidium (DHE), as we reported previously [32, 33]. Experimental samples and corresponding control samples were processed in parallel. The settings of the detector and laser were kept constant during experiments. The DHE fluorescence was detected under a fluorescence microscope (DP70, Olympus Optical, Tokyo, Japan).
Chemicals
Capsaicin was from MedChem Express (Monmouth Junction, NJ). Ang II, losartan, captopril, tempol, apocynin, N-acetylcysteine (NAC), diethyldithiocarbamic acid (DETC), capsazepine, diphenyleneiodonium (DPI), and allopurinol were from Sigma (St. Louis, MO). AP5 and CNQX were from Apexbio (Houston, TX). Capsaicin, captopril, apocynin, or DPI was dissolved in PBS with 1% DMSO. The vehicle (Veh) was used as control. All other chemicals were dissolved in PBS, and PBS served as control. The concentrations of losartan, captopril, and other compounds were selected according to their dose-effect relationships or our previous data [21, 24].
Statistics
RSNA and MAP changes were assessed by averaging the values for 1 min at the maximal chemically-induced responses. The statistical significance of differences between groups was evaluated by one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test. Data are expressed as the mean ± SEM. A P-value < 0.05 was considered statistically significant.
Results
Effects of Ang II, Losartan, and Captopril on the ERR
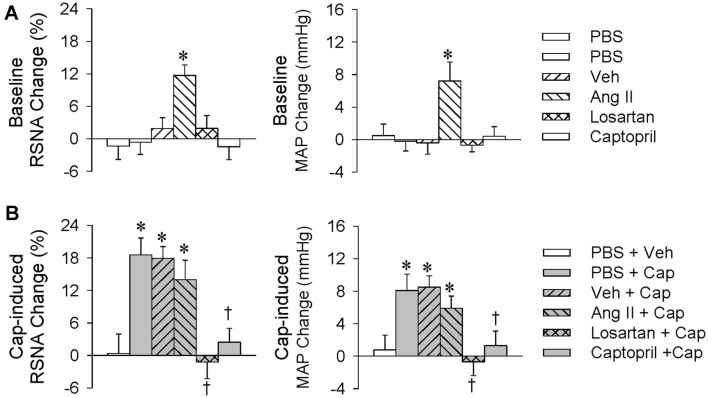
Microinjection of PBS, Ang II (0.3 nmol), losartan (10 nmol), or captopril (10 nmol) into the PVN was carried out 10 min before renal infusion of vehicle or capsaicin (1 nmol/min, 20 min). The renal infusion site, rate, and duration as well as the dose of capsaicin were determined based on our previous study [11]. Representative recordings showed that renal infusion of capsaicin induced the ERR, as evidenced by increased RSNA and MAP (Fig. 1A), which were prevented by pretreatment of the PVN with losartan, an antagonist of AT1Rs (Fig. 1B). Microinjection of Ang II into the PVN increased the baseline RSNA and MAP, but losartan or the angiotensin-converting enzyme inhibitor captopril had no significant effects on these baseline values (Fig. 2A). To compare the renal infusion-induced RSNA and MAP changes, they were calculated as the difference between the values before and after the renal infusion. Microinjection of Ang II into the PVN failed to have a greater effect on the capsaicin-induced RSNA and MAP changes than those caused by microinjection of PBS (RSNA: + 14.1% ± 3.5% versus + 18.2% ± 2.3%, P > 0.05; MAP: + 5.9 ± 1.5 mmHg vs + 8.1 ± 2.0 mmHg, P > 0.05; Fig. 2B). To compare the RSNA and MAP changes caused by PVN microinjection plus renal infusion, the total RSNA and MAP changes were calculated as the difference between the values before the PVN microinjection and those after the renal infusion. The total RSNA and MAP changes due to PVN microinjection of Ang II plus renal infusion of capsaicin were also not significantly greater than those caused by PVN microinjection of PBS plus renal infusion of capsaicin (RSNA: + 23.5% ± 3.6% vs +18.9% ± 3.6%, P > 0.05; MAP: + 11.7 ± 1.7 mmHg vs +8.0 ± 1.8 mmHg, P > 0.05). Importantly, the capsaicin-induced ERR was almost abolished by PVN pretreatment with losartan or captopril (Fig. 2B). These findings suggest that the capsaicin-induced ERR is mediated by Ang II and its AT1Rs in the PVN. Microinjection of losartan into anterior hypothalamic areas close to the PVN failed to abolish the ERR, suggesting that the effect of losartan on the ERR is not due to diffusion.
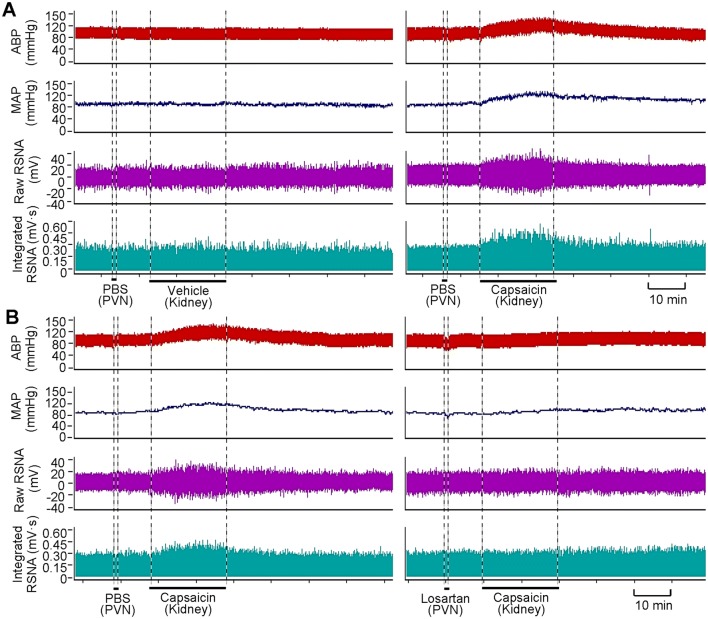
Fig. 1.
Representative recordings showing the capsaicin-induced excitatory renal reflex. A Renal infusion of capsaicin at 1 nmol/min for 20 min causes reflex changes of arterial blood pressure (ABP), mean arterial pressure (MAP) and contralateral renal sympathetic nerve activity (RSNA). B Effect of pretreatment with PVN microinjection of losartan (10 nmol) on the reflex. The pretreatment was carried out 10 min before renal infusion of vehicle (PBS) or capsaicin.
Fig. 2.
Roles of Ang II in the PVN in the capsaicin-induced excitatory renal reflex. The reflex was induced by renal infusion of capsaicin (Cap) at 1 nmol/min for 20 min. The PVN microinjection was carried out 10 min before renal infusion. A Baseline RSNA and MAP changes caused by the PVN microinjection of Ang II (0.3 nmol), losartan (10 nmol), or captopril (10 nmol) (*P < 0.05 vs PBS or Veh). B Effects of PVN microinjection of Ang II, losartan, or captopril on the capsaicin-induced reflex (*P < 0.05 vs PBS + Veh; †P < 0.05 vs PBS + Cap or Veh + Cap). Values are the mean ± SEM; n = 6 per group.
Effects of Tempol, NAC, and DETC on the ERR
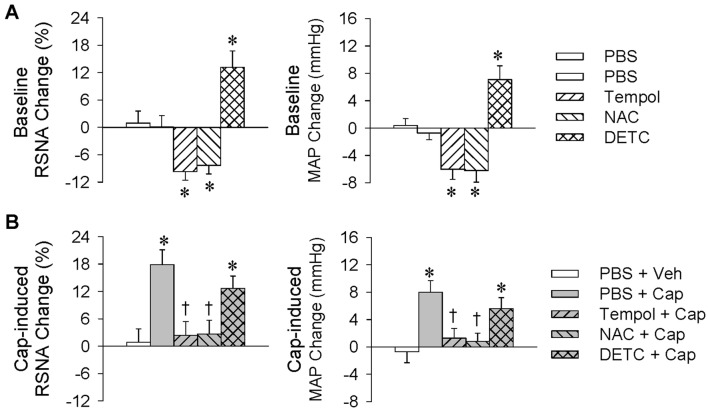
Our previous study has shown that reactive oxygen species (ROS) in the PVN mediate the effect of Ang II on the CSAR in hypertensive rats [24]. In this study, microinjection of PBS, tempol (20 nmol), NAC (40 nmol), or DETC (10 nmol) into the PVN was carried out 10 min before renal infusion of vehicle or capsaicin (1 nmol/min, 20 min). Microinjection of the ROS scavenger tempol or NAC into the PVN reduced the baseline RSNA and MAP values, while DETC, a superoxide dismutase inhibitor, increased these values (Fig. 3A). The capsaicin-induced ERR was almost abolished by pretreatment with PVN microinjection of tempol or NAC. Microinjection of DETC into the PVN failed to have greater effects on the capsaicin-induced RSNA and MAP changes than those caused by microinjection of PBS (RSNA: +12.7% ± 2.7% vs +17.9% ± 3.3%, P > 0.05; MAP: +5.4 ± 1.7 mmHg vs +8.0 ± 1.7 mmHg, P > 0.05; Fig. 3B). The total RSNA and MAP changes due to PVN microinjection of DETC plus renal infusion of capsaicin were significantly greater than those caused by PVN microinjection of PBS plus renal infusion of capsaicin (RSNA: +29.6% ± 3.5% vs +19.4% ± 2.8%, P < 0.05; MAP: +11.9 ± 1.4 mmHg vs +7.1 ± 1.4 mmHg, P < 0.05). These results suggest that superoxide anions in the PVN mediate the capsaicin-induced ERR.
Fig. 3.
Roles of reactive oxygen species in the PVN in the capsaicin-induced excitatory renal reflex. The reflex was induced by renal infusion of capsaicin (Cap) at 1 nmol/min for 20 min. The PVN microinjection was carried out 10 min before renal infusion. A Baseline RSNA and MAP changes caused by the PVN microinjection of tempol (20 nmol), NAC (40 nmol), or DETC (10 nmol) (*P < 0.05 vs PBS). B Effects of PVN microinjection of tempol, NAC, or DETC on the reflex (*P < 0.05 vs PBS + Veh; †P < 0.05 vs PBS + Cap). Values are the mean ± SEM; n = 6 per group.
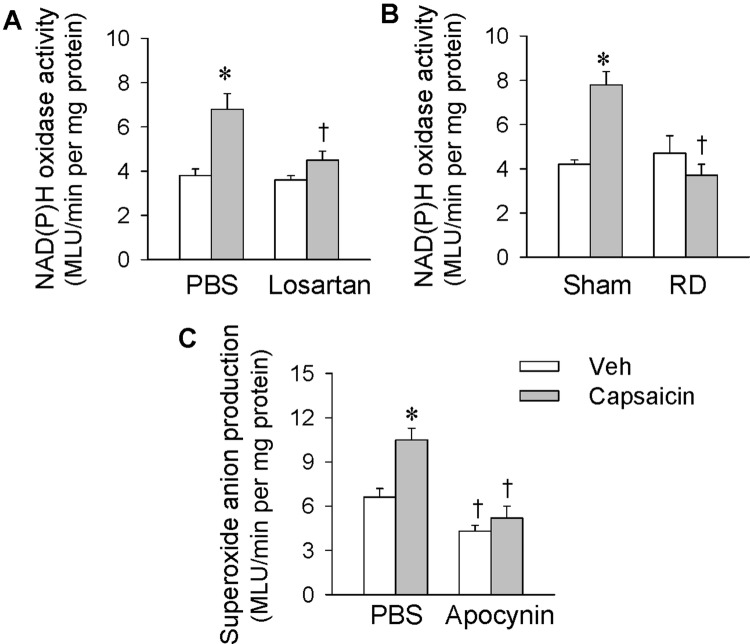
Effects of Losartan and Renal Denervation on Superoxide Anion Production
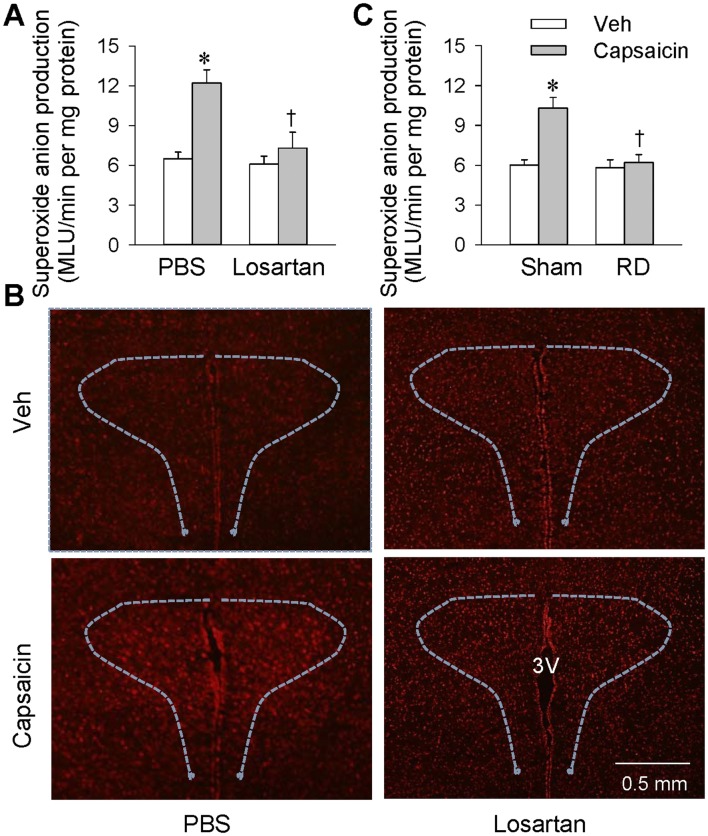
Superoxide anion production in the PVN was determined in order to provide further evidence about their importance in mediating the capsaicin-induced ERR. The PVN microinjection of PBS or losartan (10 nmol) was carried out 10 min before renal infusion of capsaicin (1 nmol/min for 20 min), and tissue samples for measurements were prepared immediately after the infusion. Renal infusion of capsaicin promoted superoxide anion production in the bilateral PVN, and this was prevented by bilateral losartan pretreatment (Fig. 4A and B). Furthermore, right renal denervation (RD) was carried out 60 min before ipsilateral renal infusion of capsaicin, and tissue samples were prepared immediately afterwards. Ipsilateral RD abolished the increase in superoxide anions in the bilateral PVN elicited by renal infusion of capsaicin, indicating that this superoxide anion production mediates the effects of renal infusion of capsaicin via afferent signals from the kidney rather than changes in circulating hormones or other secondary effects (Fig. 4C).
Fig. 4.
Effects of losartan and renal denervation (RD) on superoxide anion production in the PVN. A Effects of PVN microinjection of losartan (10 nmol) or PBS 10 min before renal infusion of capsaicin at 1 nmol/min for 20 min (*P < 0.05 vs Veh; †P < 0.05 vs PBS). B Representative images of dihydroethidium staining showing the changes in ROS levels in the PVN after renal infusion of capsaicin. C Effects of right RD 60 min before ipsilateral renal infusion of capsaicin (*P < 0.05 vs Veh; †P < 0.05 vs Sham). Values are the mean ± SEM; n = 6 per group.
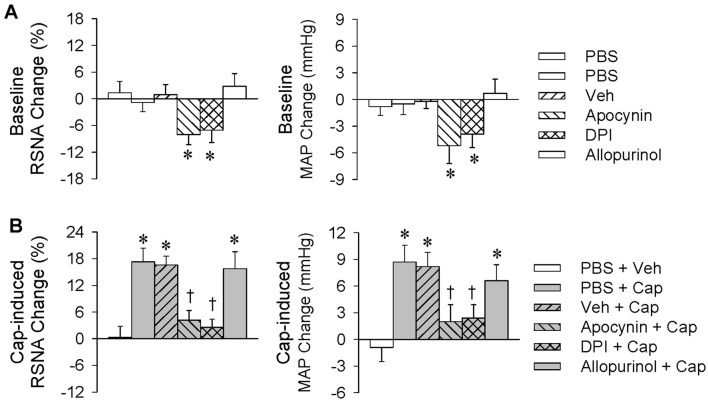
Effects of Apocynin, DPI, and Allopurinol
Inhibitors of NAD(P)H oxidase and xanthine oxidase were each used to determine the origin of superoxide anions in the PVN. Microinjection of PBS, vehicle, apocynin (1 nmol), DPI (1.5 nmol), or allopurinol (10 nmol) into the PVN was carried out 10 min before renal infusion of vehicle or capsaicin (1 nmol/min, 20 min). Microinjection of the NAD(P)H oxidase inhibitor apocynin or DPI reduced the baseline RSNA and MAP, but the xanthine oxidase inhibitor allopurinol had no significant effects on these values (Fig. 5A). Bilateral microinjection of apocynin or DPI attenuated the capsaicin-induced ERR, while allopurinol had no significant effect (Fig. 5B). These results suggest that the capsaicin-induced ERR involves NAD(P)H oxidase rather than xanthine oxidase in the PVN.
Fig. 5.
Roles of NAD(P)H oxidase in the PVN in the capsaicin-induced excitatory renal reflex. The reflex was induced by renal infusion of capsaicin (Cap) at 1 nmol/min for 20 min. The PVN microinjection was carried out 10 min before renal infusion. A Baseline RSNA and MAP changes caused by PVN microinjection of apocynin (1 nmol), DPI (1.5 nmol), or allopurinol (10 nmol) (*P < 0.05 vs PBS or Veh). B Effects of PVN microinjection of apocynin, DPI, or allopurinol on the reflex (*P < 0.05 vs PBS + Veh; †P < 0.05 vs PBS + Cap). Values are the mean ± SEM; n = 6 per group.
Effects of Losartan and Renal Denervation on NAD(P)H Oxidase Activity
Microinjection of PBS, losartan (10 nmol), or apocynin (1 nmol) into the PVN was carried out 10 min before renal infusion of vehicle or capsaicin (1 nmol/min, 20 min). A right RD was carried out 60 min before the ipsilateral renal infusion and tissue samples were prepared immediately afterwards. Renal infusion of capsaicin increased NAD(P)H oxidase activity in the bilateral PVN, and this was prevented by bilateral losartan pretreatment (Fig. 6A). Ipsilateral RD abolished the NAD(P)H oxidase activation in the bilateral PVN induced by renal infusion of capsaicin (Fig. 6B). Moreover, pretreatment with apocynin abolished the capsaicin-induced superoxide anion production in the PVN (Fig. 6C). These results indicate that the NAD(P)H oxidase is responsible for the capsaicin-induced production of superoxide anions in the PVN.
Fig. 6.
Effects of losartan and renal denervation (RD) on NAD(P)H oxidase activity in the PVN and effects of apocynin on superoxide anion production in the PVN. Renal infusion of capsaicin was carried out at 1 nmol/min for 20 min. A Effects of PVN microinjection of losartan on NAD(P)H oxidase activity in the PVN. The microinjection of PBS or losartan (10 nmol) was carried out 10 min before renal infusion of capsaicin. B Effects of right RD on NAD(P)H oxidase activity in the PVN. The RD was carried out 60 min before ipsilateral renal infusion of capsaicin. C Effects of apocynin on the capsaicin-induced superoxide anion production in the PVN (*P < 0.05 vs Veh; †P < 0.05 vs PBS or Sham; MLU, mean light units). Values are the mean ± SEM; n = 6 per group.
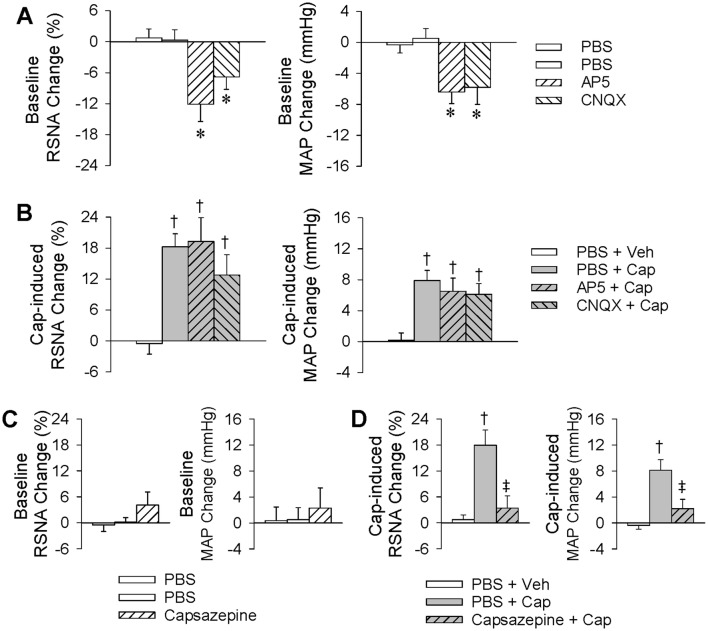
Effects of PVN Microinjection of AP5 or CNQX, or Renal Infusion of Capsazepine, on the ERR
Ionotropic glutamate receptors are divided into NMDARs and non-NMDARs. Our previous studies have shown that both types in the PVN mediate the AAR [22], so it was of interest to determine whether they are also involved in the ERR. Renal infusion of capsaicin (1 nmol/min, 20 min) was carried out to induce the ERR 10 min after treatment with the NMDAR antagonist AP5 (9 nmol) or the non-NMDAR antagonist CNQX (9 nmol). We found that either AP5 or CNQX inhibited the baseline RSNA and reduced the baseline blood pressure (Fig. 7A), but had no significant effect on the ERR (Fig. 7B). These results indicate that ionotropic glutamate receptors are not involved in the capsaicin-induced ERR. On the other hand, transient receptor potential vanilloid subtype 1 receptors (TRPV1Rs) act as sensory mediators, and are activated by capsaicin, heat, and endogenous ligands [34–36]. We therefore used capsazepine, a competitive antagonist of TRPV1Rs to determine whether the capsaicin-induced ERR is mediated by these receptors. Capsaicin (1 nmol/min for 20 min) was infused into the kidney to induce the ERR immediately after the renal infusion of capsazepine (20 nmol/min for 10 min). Capsazepine had no significant effect on the baseline RSNA and MAP (Fig. 7C), but almost completely abolished the ERR (Fig. 7D), suggesting that the capsaicin-induced ERR is mediated by renal TRPV1Rs.
Fig. 7.
Effects of PVN microinjection of AP5 or CNQX, or renal infusion of capsazepine, on the capsaicin-induced excitatory renal reflex. The reflex was induced by renal infusion of capsaicin (Cap) at 1 nmol/min for 20 min. A Baseline RSNA and MAP changes caused by PVN microinjection of AP5 (9 nmol) or CNQX (9 nmol). B Effects of PVN microinjection of AP5 or CNQX on the capsaicin-induced reflex. The PVN microinjection was carried out 10 min before renal infusion. C Baseline RSNA and MAP changes caused by the renal infusion of capsazepine. D Effects of renal infusion of capsazepine on the reflex. Renal infusion of Veh or capsaicin for 20 min was carried out immediately after renal infusion of PBS or capsazepine for 10 min (*P < 0.05 vs PBS; †P < 0.05 vs PBS + Veh; ‡P < 0.05 vs PBS + Cap). Values are the mean ± SEM; n = 6 per group.
Discussion
We recently showed that chemical stimulation of the kidney with capsaicin elicits the ERR, leading to increases in sympathetic activity and blood pressure. Stimulation of renal afferents with capsaicin promotes c-Fos expression in the bilateral PVN, while lesioning of the bilateral PVN with kainic acid abolishes this capsaicin-induced reflex. Thus, the PVN is an important component of the ERR neural circuitry [11]. The primary novel findings in this study are that the ERR induced by renal infusion of capsaicin is mediated by AT1Rs in the hypothalamic PVN. AT1R-dependent NAD(P)H oxidase activation and superoxide anion production in the PVN contribute to the ERR.
It is known that Ang II in the PVN causes sympathetic activation and a pressor response via the activation of AT1Rs [37]. Abundant AT1Rs have been found in the bilateral PVN [38], and they are upregulated in chronic heart failure [39] and hypertension [21]. We found that blockade of AT1Rs or inhibition of Ang II production in the PVN abolished the ERR, indicating that the reflex is mediated by endogenous Ang II and AT1Rs in the PVN. Although PVN microinjection of Ang II increased the baseline RSNA and MAP, it failed to further augment the sympathetic activation and pressor response induced by renal infusion of capsaicin. A possible explanation is that, given the administration of exogenous Ang II, release of endogenous Ang II due to renal infusion of capsaicin may be unable to induce a further large increase in the Ang II content of the PVN. This might be why the total changes caused by the PVN microinjection of Ang II plus renal infusion of capsaicin were not significantly greater than those caused by Ang II or capsaicin alone. These results support the idea that Ang II in the PVN is involved in the ERR. It has been shown that Ang II and AT1Rs in the PVN mediate the CSAR [40], but this does not mean that most sympatho-excitatory reflexes due to peripheral afferent activity are mediated by Ang II and its AT1Rs in the PVN. An exception is that the AAR induced by infusion of capsaicin into white adipose tissue causes sympathetic activation that is mediated by ionotropic glutamate receptors in the PVN rather than AT1Rs [22].
Tempol mimics the role of superoxide dismutase in scavenging superoxide anions [41], and NAC is another antioxidant with superoxide scavenging properties [42]. Ang II is an important stimulus for superoxide anion production in the brain [43] and vasculature [44], and superoxide anion production in the PVN plays a role in the sympathetic activation in hypertension [45]. Furthermore, our previous study has shown that superoxide anions in the PVN contribute to the enhanced CSAR in chronic heart failure [25], so we further investigated whether this mechanism is involved in the ERR. PVN microinjection of tempol or NAC reduced the baseline RSNA and MAP, and prevented the capsaicin-induced ERR. Inhibition of superoxide dismutase with DETC increased the baseline RSNA and MAP rather than the capsaicin-induced ERR. However, the total RSNA and MAP responses to DETC plus capsaicin were significantly greater than those caused by Ang II or capsaicin alone. More importantly, renal infusion of capsaicin increased superoxide anion production in the PVN, which was prevented by pretreatment with bilateral microinjection of losartan into the PVN or ipsilateral renal denervation. These findings provide evidence that the capsaicin-induced ERR is mediated by the AT1R activation-dependent production of superoxide anion in the PVN.
NAD(P)H oxidase is expressed in neurons throughout the brain [46], and ROS derived from it play a major role in Ang II signaling [47]. Apocynin prevents the assembly of NAD(P)H oxidase, thereby inhibiting its oxidase activity and superoxide anion formation [48]. Inhibition of NAD(P)H oxidase with apocynin in the PVN reduced the baseline RSNA and MAP, and almost completely abolished the ERR. Similar results were obtained after PVN microinjection of another NAD(P)H oxidase, DPI. However, inhibition of xanthine oxidase with allopurinol in the PVN had no significant effects on the ERR. Renal infusion of capsaicin increased NAD(P)H oxidase activity in the PVN, which was prevented by the bilateral microinjection of losartan or ipsilateral RD. Furthermore, apocynin abolished the superoxide anion production induced by renal infusion of capsaicin. These findings indicate that NAD(P)H oxidase is a key source of the AT1R-mediated superoxide anion production in the PVN. It is notable that the ERR is mediated by Ang II–AT1R–NAD(P)H oxidase–ROS rather than NMDARs and non-NMDARs in the PVN, similar to the signaling pathway of the CSAR [21]. However, the signaling pathway of the ERR is different from that of the AAR, which is mediated by both NMDARs and non-NMDARs. In this study, we only focused on the signaling pathway in the PVN, but other central nuclei such as the rostral ventrolateral medulla and the nucleus of the solitary tract may also be involved in the ERR, and this deserves further investigation.
Sympathetic over-activity contributes greatly to the pathogenesis and development of several diseases including chronic kidney disease, chronic heart failure, and hypertension [6]. It has been shown that enhanced renal afferent activity in 2-kidney 1-clip hypertensive mice increases blood pressure and sympathetic nerve activity [49], while renal afferent denervation in the rat prevents the progression of chronic renal failure [50]. Chronic intermittent hypobaric hypoxia attenuates renal vascular hypertension [51], while renal afferent signaling plays a role in chronic intermittent hypoxia-induced hypertension, which is involved in human obstructive sleep apnea syndrome [52]. Increased afferent activity from the kidney might play important roles in the sympathetic over-activity in these diseases, and this needs further investigation. Ang II, AT1Rs, NAD(P)H oxidase and superoxide anion production in the PVN may be critical targets in interventions for ERR-related sympathetic activation, and might attenuate some cardiovascular diseases with excessive sympathetic activation.
In conclusion, the capsaicin-induced ERR is mediated by Ang II and AT1Rs, and their downstream production of NAD(P)H oxidase-derived superoxide anion in the PVN.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31871148, 91639105, 31571167, and 31571168).
Conflict of interest
The authors declare no competing or financial interests.
References
- 1.Grassi G, Ram VS. Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens. 2016;10:457–466. doi: 10.1016/j.jash.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Zhang DY, Anderson AS. The sympathetic nervous system and heart failure. Cardiol Clin. 2014;32:33–45. doi: 10.1016/j.ccl.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaur J, Young BE, Fadel PJ. Sympathetic overactivity in chronic kidney disease: consequences and mechanisms. Int J Mol Sci. 2017;18:E1682. doi: 10.3390/ijms18081682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol. 2015;308:R79–R95. doi: 10.1152/ajpregu.00351.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bie P, Evans RG. Normotension, hypertension and body fluid regulation: brain and kidney. Acta Physiol (Oxf) 2017;219:288–304. doi: 10.1111/apha.12718. [DOI] [PubMed] [Google Scholar]
- 6.Frame AA, Carmichael CY, Wainford RD. Renal afferents. Curr Hypertens Rep. 2016;18:69. doi: 10.1007/s11906-016-0676-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng H, Patel KP. Integration of renal sensory afferents at the level of the paraventricular nucleus dictating sympathetic outflow. Auton Neurosci. 2017;204:57–64. doi: 10.1016/j.autneu.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdulla MH, Johns EJ. The innervation of the kidney in renal injury and inflammation: a cause and consequence of deranged cardiovascular control. Acta Physiol (Oxf) 2017;220:404–416. doi: 10.1111/apha.12856. [DOI] [PubMed] [Google Scholar]
- 9.Veelken R, Schmieder RE. Renal denervation–implications for chronic kidney disease. Nat Rev Nephrol. 2014;10:305–313. doi: 10.1038/nrneph.2014.59. [DOI] [PubMed] [Google Scholar]
- 10.Carlstrom M. Therapeutic value of renal denervation in cardiovascular disease? Acta Physiol (Oxf) 2017;220:11–13. doi: 10.1111/apha.12816. [DOI] [PubMed] [Google Scholar]
- 11.Ye C, Qiu Y, Zhang F, Chen AD, Zhou H, Wang JJ, et al. Chemical stimulation of renal tissue induces sympathetic activation and pressor response via hypothalamic paraventricular nucleus. Neurosci Bull. 2019 doi: 10.1007/s12264-019-00417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pyner S. The paraventricular nucleus and heart failure. Exp Physiol. 2014;99:332–339. doi: 10.1113/expphysiol.2013.072678. [DOI] [PubMed] [Google Scholar]
- 13.Chen WW, Xiong XQ, Chen Q, Li YH, Kang YM, Zhu GQ. Cardiac sympathetic afferent reflex and its implications for sympathetic activation in chronic heart failure and hypertension. Acta Physiol (Oxf) 2015;213:778–794. doi: 10.1111/apha.12447. [DOI] [PubMed] [Google Scholar]
- 14.Xiong XQ, Chen WW, Zhu GQ. Adipose afferent reflex: sympathetic activation and obesity hypertension. Acta Physiol (Oxf) 2014;210:468–478. doi: 10.1111/apha.12182. [DOI] [PubMed] [Google Scholar]
- 15.Badoer E. The role of the hypothalamic PVN in the regulation of renal sympathetic nerve activity and blood flow during hyperthermia and in heart failure. Am J Physiol Renal Physiol. 2010;298:F839–F846. doi: 10.1152/ajprenal.00734.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J, Ren XS, Kang Y, Dai HB, Ding L, Tong N, et al. Intermedin in paraventricular nucleus attenuates sympathoexcitation and decreases TLR4-mediated sympathetic activation via adrenomedullin receptors in rats with obesity-related hypertension. Neurosci Bull. 2019;35:34–46. doi: 10.1007/s12264-018-0292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu B, Zheng H, Liu X, Patel KP. Activation of afferent renal nerves modulates RVLM projecting PVN neurons. Am J Physiol Heart Circ Physiol. 2015;308:1103–1111. doi: 10.1152/ajpheart.00862.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou JJ, Ma HJ, Shao JY, Pan HL, Li DP. Impaired hypothalamic regulation of sympathetic outflow in primary hypertension. Neurosci Bull. 2019;35:124–132. doi: 10.1007/s12264-018-0316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cato MJ, Toney GM. Angiotensin II excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: an in vitro patch-clamp study in brain slices. J Neurophysiol. 2005;93:403–413. doi: 10.1152/jn.01055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu XJ, Zhao YN, Hou YK, Li HB, Xia WJ, Gao HL, et al. Chronic intracerebroventricular infusion of metformin inhibits salt-sensitive hypertension via attenuation of oxidative stress and neurohormonal excitation in rat paraventricular nucleus. Neurosci Bull. 2019;35:57–66. doi: 10.1007/s12264-018-0308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen AD, Zhang SJ, Yuan N, Xu Y, De W, Gao XY, et al. AT1 receptors in paraventricular nucleus contribute to sympathetic activation and enhanced cardiac sympathetic afferent reflex in renovascular hypertensive rats. Exp Physiol. 2011;96:94–103. doi: 10.1113/expphysiol.2010.054353. [DOI] [PubMed] [Google Scholar]
- 22.Cui BP, Li P, Sun HJ, Ding L, Zhou YB, Wang JJ, et al. Ionotropic glutamate receptors in paraventricular nucleus mediate adipose afferent reflex and regulate sympathetic outflow in rats. Acta Physiol (Oxf) 2013;209:45–54. doi: 10.1111/apha.12125. [DOI] [PubMed] [Google Scholar]
- 23.Zhang LL, Ding L, Zhang F, Gao R, Chen Q, Li YH, et al. Salusin-beta in rostral ventrolateral medulla increases sympathetic outflow and blood pressure via superoxide anions in hypertensive rats. J Hypertens. 2014;32:1059–1067. doi: 10.1097/HJH.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 24.Han Y, Fan ZD, Yuan N, Xie GQ, Gao J, De W, et al. Superoxide anions in paraventricular nucleus mediate the enhanced cardiac sympathetic afferent reflex and sympathetic activity in renovascular hypertensive rats. J Appl Physiol. 2011;110:646–652. doi: 10.1152/japplphysiol.00908.2010. [DOI] [PubMed] [Google Scholar]
- 25.Han Y, Shi Z, Zhang F, Yu Y, Zhong MK, Gao XY, et al. Reactive oxygen species in the paraventricular nucleus mediate the cardiac sympathetic afferent reflex in chronic heart failure rats. Eur J Heart Fail. 2007;9:967–973. doi: 10.1016/j.ejheart.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Pereira SC, Parente JM, Belo VA, Mendes AS, Gonzaga NA, do Vale GT, et al. Quercetin decreases the activity of matrix metalloproteinase-2 and ameliorates vascular remodeling in renovascular hypertension. Atherosclerosis 2018, 270: 146–153. [DOI] [PubMed]
- 27.Li Y, Zhu H, Kuppusamy P, Zweier JL, Trush MA. Mitochondrial electron transport chain-derived superoxide exits macrophages: implications for mononuclear cell-mediated pathophysiological processes. React Oxyg Species (Apex) 2016;1:81–98. doi: 10.20455/ros.2016.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harms JE, Kuczmarski JM, Kim JS, Thomas GD, Kaufman MP. The role played by oxidative stress in evoking the exercise pressor reflex in health and simulated peripheral artery disease. J Physiol. 2017;595:4365–4378. doi: 10.1113/JP273816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheu ML, Shen CC, Chen YS, Chiang CK. Ochratoxin A induces ER stress and apoptosis in mesangial cells via a NADPH oxidase-derived reactive oxygen species-mediated calpain activation pathway. Oncotarget. 2017;8:19376–19388. doi: 10.18632/oncotarget.14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciarcia R, Damiano S, Florio A, Spagnuolo M, Zacchia E, Squillacioti C, et al. The protective effect of apocynin on cyclosporine A-induced hypertension and nephrotoxicity in rats. J Cell Biochem. 2015;116:1848–1856. doi: 10.1002/jcb.25140. [DOI] [PubMed] [Google Scholar]
- 31.Xu S, Zhao Y, Jin C, Yu L, Ding F, Fu G, et al. PKC/NADPH oxidase are involved in the protective effect of pioglitazone in high homocysteine-induced paracrine dyfunction in endothelial progenitor cells. Am J Transl Res. 2017;9:1037–1048. [PMC free article] [PubMed] [Google Scholar]
- 32.Sun HJ, Zhao MX, Ren XS, Liu TY, Chen Q, Li YH, et al. Salusin-beta promotes vascular smooth muscle cell migration and intimal hyperplasia after vascular injury via ROS/NFkB/MMP-9 pathway. Antioxid Redox Signal. 2016;24:1045–1057. doi: 10.1089/ars.2015.6475. [DOI] [PubMed] [Google Scholar]
- 33.Zhao MX, Zhou B, Ling L, Xiong XQ, Zhang F, Chen Q, et al. Salusin-beta contributes to oxidative stress and inflammation in diabetic cardiomyopathy. Cell Death Dis. 2017;8:e2690. doi: 10.1038/cddis.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong B, Wang DH. N-oleoyldopamine, a novel endogenous capsaicin-like lipid, protects the heart against ischemia-reperfusion injury via activation of TRPV1. Am J Physiol Heart Circ Physiol. 2008;295:H728–H735. doi: 10.1152/ajpheart.00022.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore C, Gupta R, Jordt SE, Chen Y, Liedtke WB. Regulation of pain and itch by TRP channels. Neurosci Bull. 2018;34:120–142. doi: 10.1007/s12264-017-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiu IM. Infection, pain, and itch. Neurosci Bull. 2018;34:109–119. doi: 10.1007/s12264-017-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li YF, Wang W, Mayhan WG, Patel KP. Angiotensin-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1035–R1043. doi: 10.1152/ajpregu.00338.2004. [DOI] [PubMed] [Google Scholar]
- 38.Ma H, Chen SR, Chen H, Pan HL. Endogenous AT1 receptor-protein kinase C activity in the hypothalamus augments glutamatergic input and sympathetic outflow in hypertension. J Physiol. 2019;597:4325–4340. doi: 10.1113/JP278427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang HJ, Zhang F, Zhang Y, Gao XY, Wang W, Zhu GQ. AT1 receptor in paraventricular nucleus mediates the enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure. Auton Neurosci. 2005;121:56–63. doi: 10.1016/j.autneu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Zhu GQ, Gao L, Patel KP, Zucker IH, Wang W. ANG II in the paraventricular nucleus potentiates the cardiac sympathetic afferent reflex in rats with heart failure. J Appl Physiol. 2004;97:1746–1754. doi: 10.1152/japplphysiol.00573.2004. [DOI] [PubMed] [Google Scholar]
- 41.Yamada J, Yoshimura S, Yamakawa H, Sawada M, Nakagawa M, Hara S, et al. Cell permeable ROS scavengers, Tiron and Tempol, rescue PC12 cell death caused by pyrogallol or hypoxia/reoxygenation. Neurosci Res. 2003;45:1–8. doi: 10.1016/s0168-0102(02)00196-7. [DOI] [PubMed] [Google Scholar]
- 42.Elbini Dhouib I, Jallouli M, Annabi A, Gharbi N, Elfazaa S, Lasram MM. A minireview on N-acetylcysteine: An old drug with new approaches. Life Sci 2016, 151: 359–363. [DOI] [PubMed]
- 43.Capettini LS, Montecucco F, Mach F, Stergiopulos N, Santos RA, da Silva RF. Role of Renin-Angiotensin system in inflammation, immunity and aging. Curr Pharm Des. 2012;18:963–970. doi: 10.2174/138161212799436593. [DOI] [PubMed] [Google Scholar]
- 44.Masi S, Uliana M, Virdis A. Angiotensin II and vascular damage in hypertension: Role of oxidative stress and sympathetic activation. Vascul Pharmacol. 2019;115:13–17. doi: 10.1016/j.vph.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Yu XJ, Miao YW, Li HB, Su Q, Liu KL, Fu LY, et al. Blockade of endogenous angiotensin-(1-7) in hypothalamic paraventricular nucleus attenuates high salt-induced sympathoexcitation and hypertension. Neurosci Bull. 2019;35:47–56. doi: 10.1007/s12264-018-0297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–198. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- 47.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 48.Wang K, Zhu ZF, Chi RF, Li Q, Yang ZJ, Jie X, et al. The NADPH oxidase inhibitor apocynin improves cardiac sympathetic nerve terminal innervation and function in heart failure. Exp Physiol. 2019;104:1638–1649. doi: 10.1113/EP087552. [DOI] [PubMed] [Google Scholar]
- 49.Ong J, Kinsman BJ, Sved AF, Rush BM, Tan RJ, Carattino MD, et al. Renal sensory nerves increase sympathetic nerve activity and blood pressure in 2-kidney 1-clip hypertensive mice. J Neurophysiol. 2019;122:358–367. doi: 10.1152/jn.00173.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campese VM, Kogosov E, Koss M. Renal afferent denervation prevents the progression of renal disease in the renal ablation model of chronic renal failure in the rat. Am J Kidney Dis. 1995;26:861–865. doi: 10.1016/0272-6386(95)90456-5. [DOI] [PubMed] [Google Scholar]
- 51.Li N, Guan Y, Tian YM, Ma HJ, Zhang X, Zhang Y, et al. Chronic intermittent hypobaric hypoxia ameliorates renal vascular hypertension through up-regulating NOS in nucleus tractus solitarii. Neurosci Bull. 2019;35:79–90. doi: 10.1007/s12264-018-00330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.AlMarabeh S, Abdulla MH, O’Halloran KD. Is aberrant reno-renal reflex control of blood pressure a contributor to chronic intermittent hypoxia-induced hypertension? Front Physiol. 2019;10:465. doi: 10.3389/fphys.2019.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]