Abstract
We report an extremely premature infant with necrotizing cellulitis. After minor trauma to the left arm when removing an adhesive sensor, patient developed rapidly progressive cellulitis, which evolved into a necrotic ulcer. Microbiological studies (mass spectroscopy and molecular assay) identified Rhizopus arrhizus as the responsible fungus.
Keywords: Mucormycosis, Necrotizing cellulitis, Premature infant, Diagnosis
Introduction
Mucormycosis (formerly zygomycosis) is an invasive infection caused by fungi that belong to the Mucorales order [1]. Unlike other opportunistic agents, Mucorales can infect a broad range of hosts, including immunocompetent patients without apparent underlying conditions [2]. In neonates, mucormycosis is a rare but frequently life-threatening infection. The most common infection sites include the gastrointestinal tract, followed by the skin, lungs, and rhinocerebral spaces [[3], [4], [5]]. Cutaneous lesions due to mucormycosis can be caused by an infection originating in the skin or secondary to dissemination from another site [4,5].
Case presentation
A newborn female was born at 24 weeks + 3 days via emergent cesarean delivery secondary to breech presentation and cord prolapse with a birth weight of 645 g. The infant was born to a 36-year-old, HIV negative mother with an Apgar score of 4 and 8 at 1 and 5 min, respectively. Management protocol for the extremely preterm infant was initiated, including mechanical ventilation, central umbilical catheters, total parenteral nutrition, empiric antibiotics, and skin sensors for monitoring. During stay in the neonatal intensive care unit, she developed late-onset sepsis secondary to Escherichia coli bacteremia (on day of life 9), requiring antimicrobial therapy with cefepime for 10 days. On day of life 20 and after removal of an adhesive patch located in the proximal third of the left arm, she presented a small skin abrasion. The lesion evolved with erythema, induration, and a plaque with a necrotic center. Over the next 48 h, the lesion progressed to an ulcer with extension to subcutaneous cell tissue and progression of the necrotic area, despite intensive treatment by the wound care team with healings, hydrating dermal wound dressings with sodium alginate and carboxymethylcellulose, as hydrocolloids to cover the lesion (Fig. 1). Additionally, the infant also presented clinical deterioration consisting of thermic instability, metabolic acidosis, hyperglycemia, and hypotension. Based on the clinical features and time of evolution of the lesion cutaneous mucormicosis was suspected. Patient underwent skin biopsy for microbiological and pathological studies, and empiric antifungal treatment was initiated with liposomal amphotericin B (L-AmB); fungal biomarkers such as serum galactomannan and 1,3 beta-D-glucan were not performed. Her general condition deteriorated progressively despite therapy with L-AmB and multisystemic support. She evolved into refractory shock, metabolic acidosis, and renal failure. The critical clinical condition did not allow extensive surgical debridement of the affected tissue and patient died 36 h later.
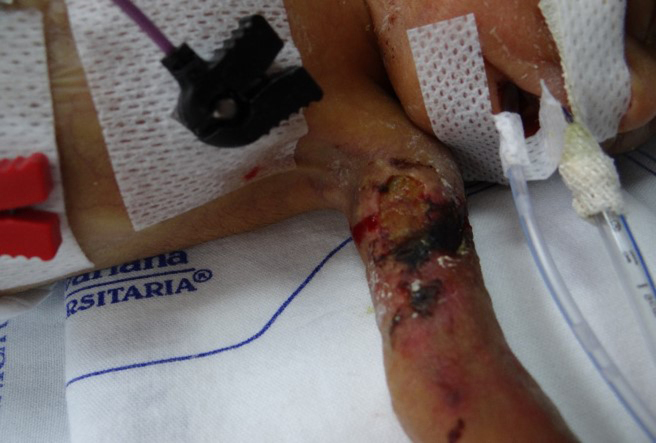
Fig. 1.
Lesion on the left arm characterized by cellulitis and ulcer with tissue necrosis consistent with primary cutaneous mucormycosis.
Fungal cultures obtained during skin biopsy, revealed the growth of a mold that was classified by phenotypic characteristics as Rhizopus spp. (Fig. 2). The histopathology report described the presence of broad aseptate hyphae with right angle branching, suggestive of Mucorales (Fig. 3). Mass spectroscopy (matrix-assisted laser desorption/ ionization-time mass spectrometry of flight mass spectroscopy MALDI-TOF MS, BD™ Buker MALDI Biotyper™System) and polymerase chain reaction (PCR) plus sequencing from culture (panfungal PCR, sequencing region D1/ D2 -24S RNA long subunit-, internal transcribed spacer region) [6] identified Rhizopus arrhizus (formerly R. oryzae) as the fungus responsible for the infection. Fungal blood cultures collected before starting L-AmB were negative.
Fig. 2.
Direct evaluation of tissue culture with lactophenol blue 400×, showing structures compatible with Rhizopus spp.
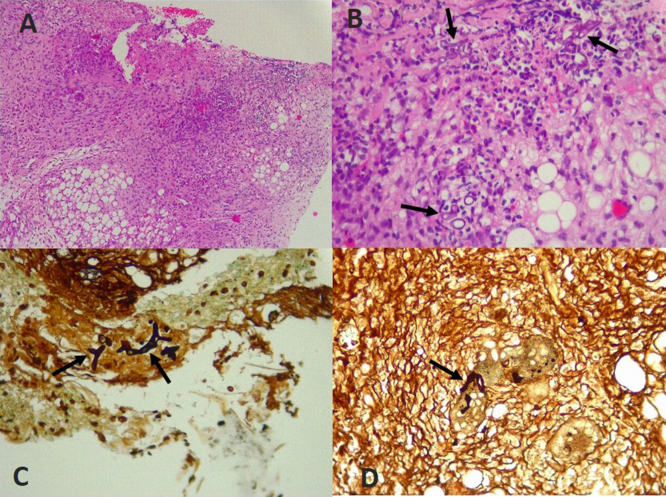
Fig. 3.
Histopathology of skin biopsy: (A) Necrotic ulcer extending up to the subcutaneous cellular tissue (Hematoxylin & Eosin –HE- staining 10×). (B) Base of the ulcer with few broad and aseptate hyphae (HE, 40×). (C, D) Broad aseptate hyphae with right angle branching seen on skin ulcer (Grocott's methenamine silver staining 40×).
Discussion
The term mucormycosis (formerly zygomycosis) applies to infections caused by the fungal order Mucorales, subphylum Mucormycotina, being Mucoraceae the most important family, which comprises the most common isolated species including Rhizopus, Mucor, and Lichtheimia, amongst others [1]. Although these fungi are ubiquitous in the environment and have little intrinsic pathogenicity for the normal host, invasive and fatal infections can occur under certain clinical conditions such as immunocompromised hosts and patients with diabetes [7]. Preterm neonates are at greater risk for invasive infections due to immaturity in innate and adaptative immune systems [4,8].
Predisposing factors for mucormycosis in neonates include prematurity, low birth weight, parenteral nutrition use, antibiotic use, orogastric tubes, among others [2,4,5]. Moreover, primary cutaneous mucormycosis has been associated with abrasion of the skin or mucous membranes associated with the use of tongue depressors, adhesive tape, venous catheters, and skin sensors. Although antibiotics are reported as a possible risk factor for mucormycosis there is no clear explanation between the use of antibiotics and primary cutaneous mucormycosis [4,[7], [8], [9], [10], [11], [12], [13]].
In neonates, the most common site of infection is the gastrointestinal tract, followed by cutaneous, pulmonary, and rhinocerebral involvement [[3], [4], [5]]. Cutaneous mucormycosis may present as a primary cutaneous infection or secondary to disseminated forms and/or rhinocerebral mucormycosis [1,13].
In primary cutaneous mucormycosis the infection begins after minor local skin trauma. After inoculation, the fungus penetrates the skin with vascular invasion associated with thrombosis of the vessels and the appearance of infarctions and necrotic surrounding tissue [2,5,8]. In our case, due to the temporal relationship between skin abrasion of the left arm, the rapid clinical evolution, together with the microbiological and histopathological findings, a primary skin infection was suggested. However, secondary skin involvement cannot be ruled out as part of disseminated infection, since an autopsy was not performed.
Primary cutaneous mucormycosis has two different clinical presentations. Superficial infection occurs in healthy hosts and presents as a vesicle or pustule that evolves to an erythematous plaque that can eventually ulcerate. In the gangrenous form, a painful papule appears and rapidly progresses to necrotizing cellulitis or necrotic plaque. In the latter case, the infection can spread to nearby or distant tissues [5].
In both settings, the characteristic finding of the skin infection is a necrotic eschar at the site of the lesion that may or may not be present, especially in the superficial form. Formation of skin abscesses has also been described as a variant in neonates [10,13].
According to Roilides E et al. and Oh D et al., the most common genera identified in case reports of mucormycosis in neonates and children are Rhizopus spp., Mucor spp., Lichtheimia spp. (Absidia spp.), and Rhizomucor spp. At the species level, R. microsporus, R. arrhizus (R. oryzae), and Lichtheimia corymbifera (Absidia corymbifera) were the most frequently reported [2,4,5].
The diagnosis of mucormycosis should always be considered when acute necrotizing cellulitis occurs in an immunosuppressed individual [13]. The differential diagnosis includes other fungal infections such as cutaneous aspergillosis, hyalohyphomycosis caused by Fusarium, and other filamentous fungi, necrotizing fasciitis, clostridial gas gangrene, sepsis-associated purpura fulminans, bacterial cellulitis, and pyoderma gangrenosum [1,11,13].
Culture and histopathology remain the basis for the diagnosis of mucormycosis in most cases. Appropriate specimen handling during tissue biopsy and laboratory processing is essential to achieve the best yield. Mucorales usually grow well in routine culture media. Typical histopathological features such as the presence of broad, hyaline and aseptate hyphae, associated with vascular invasion and necrotic tissue usually confirm the diagnosis of mucormycosis [1,[12], [13], [14]]. However, PCR assays and mass spectrometry tests (MALDI-TOF MS) allow a faster and more accurate diagnosis, achieving the identification of the fungus up to the species level, not always possible by traditional methods [1,14,15]. In our patient, despite the unfavorable outcome, both culture and pathology were consistent with the presumed diagnosis. The pathogen identification by traditional microbiology (Rhizopus spp.), correlated with the identification by PCR and mass spectrometry (R. arrhizus).
The management of necrotizing cellulitis due to Mucorales depends on the underlying disease, the control of predisposing factors, and the disease severity. In general, the mainstay of treatment consist of surgical debridement and anti-fungal treatment with L-AmB associated or not with another agent [1,5,7,13,14]. Ischemic necrosis of infected tissue prevents the arrival of leukocytes and anti-fungal agents to the site of infection, which limits their efficacy. According to Francis JR et al., pooled data of pediatric patients with mucormycosis have shown that children who receive antifungal therapy and surgical debridement had a lower risk of death than patients who received antifungal therapy alone (18.5 % vs. 60 %) [14].
In summary, this is the first case of necrotizing cellulitis due to Mucorales in an extremely premature infant diagnosed in our facility. High suspicion in patients with predisposing factors, as well as prompt recognition and an aggressive approach, are required to achieve infection control. Traditional diagnostic methods (microbiology and histopathology) can be complemented with modern tests such as mass spectrometry and molecular studies to obtain a rapid and accurate etiological diagnosis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent
This case report was approved by the Institutional Ethics Committee (Clínica Universitaria Bolivariana and Universidad Pontificia Bolivariana).
CRediT authorship contribution statement
Álvaro Hoyos: Conceptualization, Writing - review & editing, Supervision. María Adelaida Mejía: Conceptualization, Writing - review & editing. Verónica Herrera: Conceptualization, Formal analysis, Investigation, Validation, Writing - review & editing, Visualization. Andrés Soto: Visualization, Investigation. Clara Rico: Writing - review & editing.
Declaration of Competing Interest
The authors have no conflicting interests to declare in this article.
Acknowledgements
To Alejandro Díaz-Díaz for his critical review.
Contributor Information
Álvaro Hoyos, Email: alvaromicro@hotmail.com.
María Adelaida Mejía, Email: mariaad.mejia@upb.edu.co.
Verónica Herrera, Email: bronikherrera2@gmail.com.
Andrés Soto, Email: piperock-80@hotmail.com.
References
- 1.Bonifaz A., Tirado-Sánchez A., Calderon L., Ponce R.M. Cutaeous mucormycosis: mycological, clinical, and therapeutic aspects. Curr Fungal Infect Rep. 2015;9:229–237. doi: 10.1111/myc.12233. [DOI] [Google Scholar]
- 2.Roilides E., Zaoutis T.E., Walsh T.J. Invasive zygomycosis in neonates and chlidren. Clin Microbiol Infect. 2009;15:50–54. doi: 10.1111/j.1469-0691.2009.02981.x. [DOI] [PubMed] [Google Scholar]
- 3.Inoue S., Odaka A., Hashimoto D., Hoshi R., Kurishima C., Kunikata T. Rare case of disseminated neonatal zygomycosis mimicking necrotizing enterocolitis whit necrotizing fasciitis. J Pediatr Surg. 2011;46:E29–E32. doi: 10.1016/j.jpedsurg.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Roilides E., Zaoutis T.E., Katragkou A., Benjamin D.K., Walsh T.J. Zygomycosis in neonates: an uncommon but life-threatening infection. Am J Perinatol. 2009;26:565–573. doi: 10.1055/s-0029-1220775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh D., Notrica D. Primary cutaneus mucormycosis in infant and neonates: case report and review of the literatura. J Pediatr Surg. 2002;37:1607–1611. doi: 10.1053/jpsu.2002.36193. [DOI] [PubMed] [Google Scholar]
- 6.CLSI . CLSI guideline MM18. 1st ed. Clinical and laboratory Standards Institute; Wayne,PA: 2008. Interpretative criteria for identification of bacteria and fungi by targeted DNA sequencing. [Google Scholar]
- 7.Arisoy A.E., Arisoy E.S., Correa-Calderon A., Kaplan S.L. Rhizopus necrotizing cellulitis in a preterm infant: a case report and review of the literatura. Pediatr Infect Dis J. 1993;12:1029–1031. [PubMed] [Google Scholar]
- 8.Brooks D., Abdessalam S., Davies H.D., Aldrich A.M., Bedrnicek J., Gollehon N. Invasive cutaneus mucormycosis in an extremely preterm infant. J Pediatr Surg Case Rep. 2018;35:52–56. doi: 10.1016/j.epsc.2018.05.018. [DOI] [Google Scholar]
- 9.Mitchell S.J., Gray J., Morgan M.E.I., Hocking M.D., Durbin G.M. Nosocomial infection with Rhizopus microsporus in preterm infantes: association with wooden tongue depressores. Lancet. 1996;348:441–443. doi: 10.1016/s0140-6736(96)05059-3. [DOI] [PubMed] [Google Scholar]
- 10.Ng P.C., Dear P.R.F. Phycomycotic abscesses in a preterm infant. Arch Dis Child. 1989;64:862–863. doi: 10.1136/adc.64.6.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennis J.E., Rhodes K.H., Cooney D.R., Roberts G.D. Nosocomial Rhizopus infection (zygomycosis) in children. J Pediatr. 1980;96:824–828. doi: 10.1016/s0022-3476(80)80550-6. [DOI] [PubMed] [Google Scholar]
- 12.Craig N.M., Lueder F.L., Pensler J.M., Bean B.S., Petrick M.L., Thompson R.B. Disseminated Rhizopus infection in a premature infant. Pediatr Dermatol. 1994;11:346–350. doi: 10.1111/j.1525-1470.1994.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 13.du Plessis P.J., Wentzel L.F., Delport S.D. Zygomatic necrotizing cellulitis in a premature infant. Dermatology. 1997;195:179–181. doi: 10.1159/000245728. [DOI] [PubMed] [Google Scholar]
- 14.Francis J.R., Villanueva P., Bryant P., Blyth C.C. Mucormycosis in children: review and recommendations for Management. J Pediatric Infect Dis Soc. 2018;7:159–164. doi: 10.1093/jpids/pix107. [DOI] [PubMed] [Google Scholar]
- 15.Walsh T.J., McCarthy M.W. The expanding use of matrix-assisted laser desorption/ionization-time of flight mass espectroscopy in the diagnosis of patients with mycotic diseases. Expert Rev Mol Diagn. 2019;19:241–248. doi: 10.1080/14737159.2019.1574572. [DOI] [PubMed] [Google Scholar]