Abstract
The applications of nanotechnology are wide ranging, and developing functional nanomaterials for agri-food applications from nature-derived polymers is widely conceived as a sustainable approach that is safer for human and animal consumption. In light of this, this review focuses on the advances in the development of nano-delivery systems using nature-derived polymers for agri-food applications. The review opens with a section detailing the different types of nature-derived polymers currently being used in various applications in the agri-food industry with a special mention on microbial extracellular polymeric materials. The major applications of nano-delivery systems in the food sector, such as food fortification and food preservation, as well as in the agricultural sector for controlled release of agrochemicals using nature-derived polymers are discussed. The review ends with a perspective on the safety and public perception of nano-enabled foods with a concluding remark on future directions of incorporating nano-delivery systems for agri-food purposes.
Subject Areas: Nanotechnology, Agricultural Science, Food Science, Polymers
Graphical Abstract
Nanotechnology; Agricultural Science; Food Science; Polymers
Introduction
Nanotechnology has now been an intricate part of humanity, and more so in the recent decade. Even before the phenomenal wonders of nanomaterials were uncovered by science, native nanomaterials have always existed around us, be it in the smoke emanating from a fire or as volcanic ashes from an eruption, or even casein micelles in milk that help to stabilize milk fats (Griffin et al., 2017). With the advancement of tools to visualize and manipulate these tiny structures, nanomaterials have inevitably penetrated every aspect of human life, starting with fabrics (e.g., antibacterial socks) (Rivero et al., 2015) to more complex applications such as in the aerospace, agri-food, automobile, biomedical, and wastewater industries. The usage and exploitation of nanomaterials provide superior properties not observed in larger size scales, and with the boom of nanotechnology, the global market for this industry is estimated to be $ 125.7 billion by 2024 (Global Industry Analysts Inc, 2019).
With an exploding global population that is estimated to hit eight billion in 2024 (Max et al., 2019), it is foreseeable that this will drive a surge in food demand that will put tremendous pressure on the agri-food industry. In fact, the current $5 trillion-worth agri-food industry will only continue to trend upward along with an expected 70% increase in global caloric demand and a corresponding increase in crop demand of at least 100% (Lutz et al., 2015, Adisa et al., 2019). There is now a strong belief that nanotechnology will bring significant impact to the agri-food sector, especially in addressing food safety and sustainability, i.e., improving nutritional and food security, and even enhancing agricultural productivity. In fact, nanotechnology was introduced into both the agricultural and food industries since 2003, where it has been used in food processing and preservation, to increase crop productivity, to improve animal feeds, and for environmental monitoring (He et al., 2019).
Some of the world's leading food companies, such as H.J. Heinz, Nestlé, Hershey, Unilever, and Kraft, are now investing on nano-enabled foods and food packaging, where the growth of the nanofood sector is expected to surge beyond $20.4 billion by 2020 (Momin and Joshi, 2015). An example of a nano-enabled food would be the low-fat ice creams from Nestlé that uses nanoemulsions to achieve a low-fat content (Wales Maurya, 2019). In terms of food preservation, Ag, SiO2, and TiO2 nanoparticles are added into food packaging materials to impart antimicrobial and hygroscopic properties (Bajpai et al., 2018, Xing et al., 2019). On the agricultural front, the main intent of using nanomaterials is to enhance agricultural yields via effective pest control and fertilization with the use of nanosensors, nanopesticides, and nanofertilizers (Prasad et al., 2017). Although nano-enabled products of herbicides, pesticides, and sensors are still very much in the research and development phase, a few commercial products comprising mainly of nanoparticles of micro and macro nutrients for plant growth, such as NanoPhos, NanoK, NanoZn, and Kocide 3000, are already in the market (He et al., 2019).
Although the US Food and Drug Administration (FDA)-approved engineered nanomaterials (ENMs) are found in a variety of consumer products, its usage in the agri-food sector has yet to be widely adopted. The main reason for this stems from toxicity concerns of persistent ENMs, as these may not degrade safely both in the physiological or natural environment (He et al., 2018). The release of non-degradable ENMs would continue to be cycled in the environment leading to what is being termed as a nanomaterials cycle (Hochella et al., 2019). As such, there is a pressing need for biodegradable alternatives, especially for agri-food applications, and naturally occurring biopolymer is one such alternative. Nature-derived biopolymers are polymeric macromolecular structures such as polysaccharides, proteins, and lipids that can be extracted from plants, animals, and microorganisms. Depending on the source, these biopolymers have varying degrees of branching structures that impart different properties and functions. Being renewable, eco-friendly, sustainable, and cheap, these biopolymers could also be engineered into nanomaterials with enhanced properties (Majeed et al., 2013, Mondal et al., 2019).
Given the surge in interest in exploring the use of biopolymeric nanomaterials in the agri-food sector, the aim of this article is to provide an overview of the development of nano-delivery systems based on nature-derived polymers for agricultural and food applications. More specifically, this review article will highlight the types of nature-derived polymers that have been explored and how they are applied. For agri-food applications, encapsulation technologies are often required, where such technologies are meant to protect active ingredients by minimizing interactions of the encapsulates with the environment, either through a coating or a matrix material (Sinha et al., 2019). These protective technologies can also be tuned to impart controlled release capabilities. Nature-derived biopolymeric nano- and sub-micron-sized particles are generally preferred for encapsulation as these particles can be safely incorporated into the food matrix without affecting their sensory properties (Valencia et al., 2019). In the agricultural sector, biopolymer-based nanoparticles help local delivery of fertilizers and pesticides without polluting soil and air. The immense impact of encapsulation technologies in the food industry is apparent with the global food encapsulation market projected to be $54.846 billion by 2024 (Inkwood Research, 2019). This article will also feature several notable applications in food, i.e., encapsulation of nutraceuticals and for food preservation, as well as agriculture, i.e., encapsulation of fertilizers and pesticides. A section on public receptivity of nanomaterials and the general perception of the toxicity of nanomaterials is also included toward the end of this review.
Nature-Derived Polymers and Their Current Uses
Polymer science was instrumental in advancing various developments in diverse fields. Both synthetic and natural polymers have now become an integral part of the food industry right from food packaging applications to the employment of edible polymers for food formulations. Although nature-derived polymers, e.g., polysaccharides and proteins, can be environmentally degraded into their monomers over time, the same may not be the case of synthetic polymers. The persistence of synthetic polymers poses huge problems to the environment. Some examples could be the rising concerns over the bioaccumulation and environmental contamination of polyfluoroalkyl substances and micro/nanoplastics. In this section, we shall focus on the use of bio-based polymers for agri-food applications, where such bio-based polymers can be either naturally derived from plants, animals, food waste or fabricated via biological process such as by living microorganisms.
Nature-Derived Polymers for Food Application
Food is naturally composed of polymers, i.e., polysaccharides, proteins, and also fats. The discussion here would focus on the use of these natural polymers in altering the structure and function of food. The main polymers in food formulation are mainly animal proteins (e.g., gelatin, egg protein, milk proteins-casein, lactoglobulin), plant proteins (e.g., zein), and a variety of polysaccharides (e.g., starch, chitosan, alginate, pectin, cellulose, gum arabic, carrageenan). These edible polymers have been used in modifying the properties of food, as a thickening or gelling agent, for emulsion stabilization, and even to enhance satiety (Rayner et al., 2016). Polysaccharides are the most commonly used thickeners owing to their highly branched structures that can interact well with water and have good water solubility. This provides a large hydrodynamic volume that is essential for thickening. Similarly, because of their good water-holding capacity, the polymeric chains of polysaccharides like alginate and carrageenan form continuous networks leading to the formation of gels, stabilized by ionic bridges such as calcium (Ca) and potassium (K) (Lovegrove et al., 2017). Proteins are used as emulsion stabilizers due to their amphiphilic nature, that can help stabilize oil-water interfaces. Different applications for each of these polymers in the food industry are tabulated in Table 1.
Table 1.
Commercially Used Nature-Derived Polymers in the Agri-Food Sector
| Food |
Agriculture |
||||||
|---|---|---|---|---|---|---|---|
| Stabilizer (Rayner et al., 2016) | Thickener (Lovegrove et al., 2017) | Gelling (Lovegrove et al., 2017) | Food Packaging (Grujić et al., 2017) | CRF (Akalin and Pulat, 2019, Jiao et al., 2018, Perez and Francois, 2016, Tang et al., 2017) | Pesticides (Neri-Badang and Chakraborty, 2019, Campos et al., 2015) | Water Retention (Thombare et al., 2018, Demitri et al., 2013) | |
| Alginate | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Carrageenan | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Cellulose | ✓ | ✓ | ✓ | ✓ | |||
| Chitosan | ✓ | ✓ | ✓ | ||||
| Curdlan | |||||||
| Cyclodextrin | ✓ | ||||||
| Gelatin | ✓ | ✓ | ✓ | ||||
| Guar gum | ✓ | ||||||
| Pectin | ✓ | ||||||
| Starch | ✓ | ✓ | ✓ | ||||
| Xanthan gum | ✓ | ✓ | |||||
Another area where polymers have been used extensively in the food industry is in food packaging. With an alarming surge in plastic waste globally, there is now a shift in the attitude of manufacturers to consider greener and sustainable sources—nature-derived polymers (Mangaraj et al., 2019). There is now greater interest in exploring natural polymers for food packaging, by identifying cost-effective alternatives that provide properties similar to that of synthetic polymers. In addition, there has been extensive research on the use of edible polymers as smart edible food packaging that promises to maintain food freshness for a longer period or to preserve the nutritional values of food. Starch and thermoplastic starch are some of the commonly used materials in food packaging aside from other nature-derived polymers such as cellulose, chitosan, whey protein, zein, and carrageenan. BioBag is one such example of a starch-based commercial bio-packaging available in the market. These polymers can work as effective coating materials to improve the barrier, mechanical, and thermal properties of the biopolymer films as well as enhance food preservation at lower costs (Grujić et al., 2017). Nonetheless, some challenges such as postharvest loss of nutrition and quality of fresh foods remain to be resolved for fresh food products when transportation or storage time is prolonged, which may be due to reactions between fresh products and these nature-derived polymers (He et al., 2019).
Nature-Derived Polymers for Agricultural Application
Modern agriculture is seeking alternatives for the use of agrochemicals via green nanotechnology or renewable nanomaterials. Synthetic polymers such as polycaprolactone, polyethylene, polyvinyl alcohol, and acrylate-based polymers are used to achieve slow release of fertilizers to improve soil conditions (as superabsorbent polymers [SAPs]) and also as mulch to cover the soil to prevent loss of moisture (Ekebafe et al., 2011, Steinmetz et al., 2016). These synthetic polymers may not be readily biodegradable, and environmental concerns relating to the degradation of these materials could arise. For instance, polyethylene and polypropylene used in agriculture can persist in the soil for prolonged periods, causing negative impacts to the environment and living systems. The outlook toward synthetic polymers is therefore shifting toward natural polymers, such as polysaccharides (Milani et al., 2017). The use of nature-derived polymers is perceived as a means to reduce environmental pollution because of their biodegradability, via various enzymatic actions by microorganisms and non-enzymatic mechanisms including chemical hydrolysis (Puoci et al., 2008). Some of the areas in which such nature-derived polymers are beginning to be used are in controlled release fertilizers (CRFs), for pesticide encapsulation, and as water-retaining agents. These applications with suitable examples have been discussed in the following paragraphs.
CRFs use a polymer coating on traditional fertilizers thereby providing a slower release of plant nutrients and in turn improving nutrient utilization efficiency (NUE). A range of CRFs are available in the market under the brand names such as Osmocote, Nutricote, and Plantacote (Parsons, 2017). CRF technology uses the principle of osmosis to control the release of essential nutrients such as nitrogen (N), phosphorus (P), and K, commonly referred to as NPK and other trace elements concealed within a biopolymeric or synthetic polymer coating. The main criteria for a CRF to be commercialized economically is that it should have a minimum of 85% encapsulation efficiency of the fertilizer that will enable the diffusion of nutrients when the pellets come into contact with water (Majeed et al., 2015). Some examples of biopolymers studied for use in CRF are starch (Naz and Sulaiman, 2014), chitosan (Perez and Francois, 2016), gelatin (Tang et al., 2017), lignin (Jiao et al., 2018), cellulose, and k-carrageenan (Akalin and Pulat, 2019) as outlined in Table 1. Some of these biopolymers such as chitosan and cellulose are poorly soluble in water, whereas starch can be chemically modified to possess various properties, which make them ideal for agricultural uses. The significant advantage of natural polymers is that they can be degraded by soil microorganisms resulting in environmentally non-toxic products, compared with their non-degradable synthetic counterparts (Siracusa, 2019).
Nature-derived polymers are also being studied for pesticide encapsulation. Seltima is an example of a commercial product using polymers for encapsulation of pesticides. Launched by BASF to protect rice plants, the product uses a humidity responsive controlled release technology to release pesticides selectively to the leaves of rice while avoiding release of the chemicals into the water in paddy fields thereby greatly reducing the contamination of water sources (Huang et al., 2018).
Another application where polymeric materials are currently used in agriculture is for water retention in drought-prone and semi-arid regions. Almost all of the current polymers used commercially for this purpose are acrylate-based SAPs. Although many studies are ongoing for the generation of smart and semi-synthetic SAPs, problems of non-biocompatibility, non-biodegradability, and non-renewal persist, leading to the search of natural SAPs. Hence, substantial research is underway to improve the properties of natural hydrogels like chitosan, alginate, pectin, and gellan, with longer lifespans, in order to replace synthetic polymers for water retention purposes (Mignon et al., 2019).
Extracellular Polymeric Materials
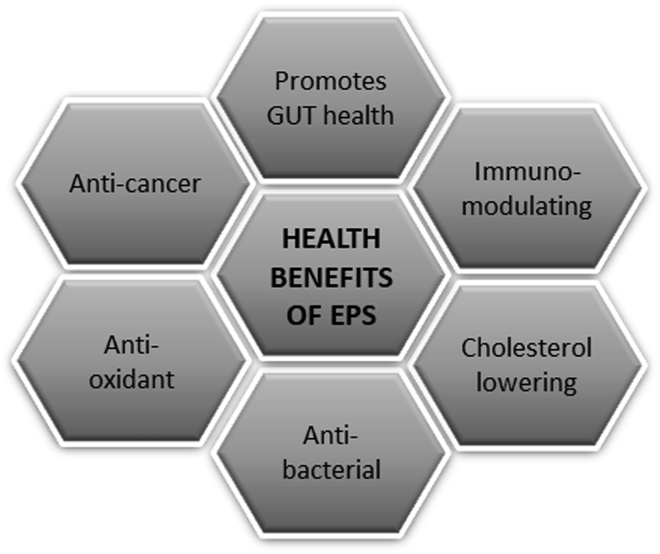
Although starch, cellulose, chitosan, and alginate derived from plant sources are some examples of commonly used biopolymers, this section aims to mention a special group of polymers secreted by microbes. Alginate, cellulose, chitosan, and dextran are some examples of polysaccharides that have been obtained from microbial origin. Although the knowledge of microbial secretion of polysaccharides has been present for long (Paul et al., 1986), their applications are only recently realized and have seen dramatic growth (Jindal and Khattar, 2018). A recent review outlines the many health-benefitting properties of not just the probiotic bacteria but also some of the exopolysaccharides (EPSs) secreted by these probiotic bacteria such as dextran, levan, and kefiran as shown in Figure 1 (Rahbar Saadat et al., 2019). Xanthan and Gellan are FDA-approved microbial polymers used as thickeners and stabilizers in the food industry. Dextran has been used as an anticoagulant and plasma volume expander in medical field. Some EPSs have been shown to have applications in soil remediation in removing heavy metal contaminants (Sardar et al., 2018). Although these materials have been used and are being further researched for their incorporation into the agri-food sector, how this nature-derived microbial EPSs can be put to use as a nano-encapsulating delivery system in food and agriculture would be an interesting scope for discussion (discussed in section Nutraceutical Encapsulation for Food Preservation). Being of polysaccharide origin, these materials would be possible candidates for fabricating nanoencapsulates. In fact, EPS secreted by microbial communities to protect themselves from the surrounding environment have been shown to possess antimicrobial properties. This promising property of EPS could be used advantageously in food packaging applications to protect against spoilage from pathogenic strains and prolong the shelf life of food (Moscovici, 2015).
Figure 1.
Graphic Showing the Various Possible Benefits of EPS
The main challenge in EPS production industrially is the proper maintenance of culture conditions such that the consistency of the product is maintained. Cost is another important consideration in terms of energy requirements during the fermentation process as well as solvents used in the extraction and purification stages. Using cheaper substrates such as biomass from food wastes is one way of reducing the cost, which can also make the production sustainable (Özcan and Toksoy Oner, 2015).
Nano-Delivery Systems Based on Nature-Derived Polymers
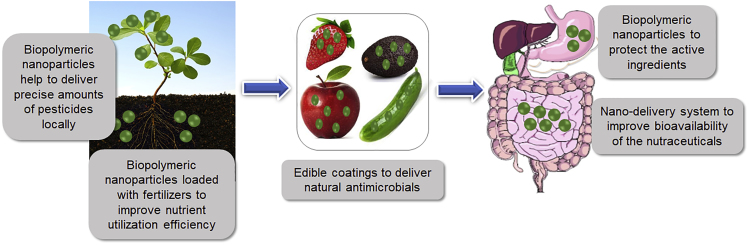
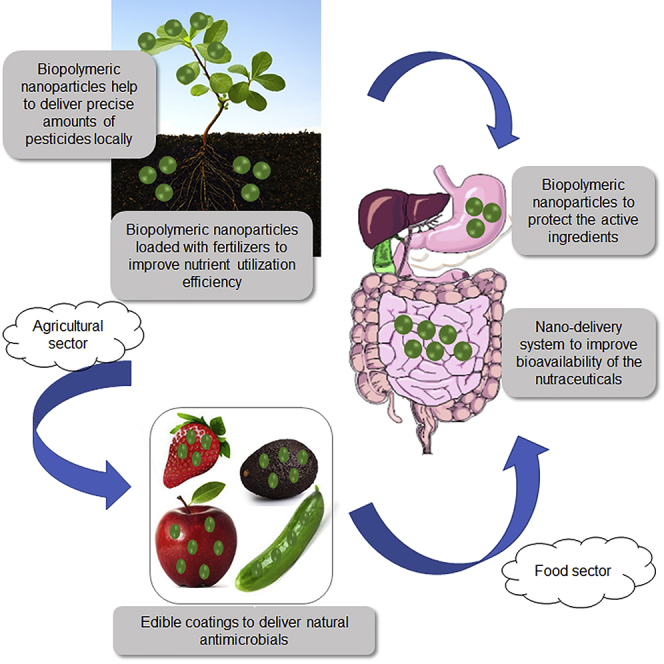
Nanomaterials based on nature-derived biopolymers are gaining significant attention for their potential applications in both food and agriculture sectors owing to their advantageous properties as well as sustainability by minimizing ecological impacts. The current limitation encountered by inorganic nanomaterials like TiO2, ZnO, and SiO2 and organic nanomaterials like carbon nanostructures is the lack of robust information on their toxicity as most of the toxicity studies conducted are in vitro rather than in vivo (He et al., 2019, Dorier et al., 2017). Some of the in vivo studies conducted are also short-term studies, investigating the effects of high-dose exposure. A comprehensive study on the chronic exposure of such nanomaterials would be more representative of realistic conditions (Bandala and Berli, 2019). This, combined with the lack of proper regulation and legislation, has now shifted the focus to nature-derived polymeric nanoparticles owing to their biocompatible and low or negligible toxic properties. Figure 2 summarizes the different applications of nature-derived nano-delivery systems in the agri-food sector discussed below.
Figure 2.
Applications of Biopolymeric Nano-Delivery Systems in the Agri-Food Sector
Nano-Delivery Systems in Food
Nano-delivery systems for food applications could be formulated as nanocapsules, nanospheres, and nanoemulsions using many well-reported techniques such as solvent evaporation, emulsification, precipitation, coacervation, electrospraying, and spray drying (Jafarizadeh et al., 2019). A variety of nature-derived polymers have been fabricated into nano-delivery systems using the above-mentioned techniques to encapsulate bioactive molecules such as nutraceuticals, antimicrobials, antioxidants, and flavors (Luo and Hu, 2017). These nanoparticles can then be introduced into the food matrix to function as nutrient supplements, preservatives, and flavor enhancers. The most important advantage of using nanoparticles for encapsulation in comparison with their micron-sized counterparts is that nano-sized particles do not generally affect the organoleptic properties of food as opposed to the micron-sized particles. Another distinct advantage is that, owing to their increased surface-volume ratio, nanoparticles offer increased bioavailability of the encapsulated nutrient.
Nutraceutical Encapsulation for Food Functionalization
Some of the commonly studied bioactive agents for nanoencapsulation could be categorized as vitamins (D, A, C, B); minerals (Ca, iron [Fe]); carotenoids (beta carotene, lycopene, astaxanthin, lutein); polyphenols (resveratrol, curcumin, catechins), and fatty acids (omega-3). The recently investigated biopolymeric delivery systems for encapsulation of nutraceuticals have been tabulated in Table 2. Chitosan is the most widely reported natural polymer for nanoencapsulation of nutraceuticals (Tan et al., 2016, Akbari-Alavijeh et al., 2020), given its mucoadhesive property and the ability of these particles below 200 nm to enhance penetration by opening the tight junctions in the intestinal epithelium. Ionic gelation is the most commonly reported method for fabricating chitosan nanoparticles (Calvo et al., 1997). Other methods of fabricating chitosan nanoparticles for food applications include electrospraying (Yilmaz et al., 2019) and complex coacervation (Azevedo et al., 2014, Ren et al., 2019).
Table 2.
Biopolymeric Delivery Systems for Encapsulation of Nutraceuticals
| Bioactive Compound Category | Bioactive Compound | Nature-Derived Polymer Used | Advantage of Nanoencapsulation | Reference |
|---|---|---|---|---|
| Polyphenols | Curcumin | Chitosan and gum arabica | Improved stability and antioxidant activity of curcumin | (Tan et al., 2016) |
| Curcumin | Soluble soya bean polysaccharides | Improved the anti-cancer property of curcumin | (Pan et al., 2018) | |
| Resveratrol | Chitosan/γ-poly (glutamic acid) | Improved the solubility and cellular uptake | (Jeon et al., 2016) | |
| Resveratrol | Zeina | Improved bioavailability | (Jayan et al., 2019) | |
| Lutein | Chitosan/poly-glutamic acid | Improved the solubility | (Lee and Lee, 2016) | |
| Green tea catechins | Zeina | Improved cell uptake | (Bhushani et al., 2017) | |
| Carotenoids | Beta carotene | Starcha | Intestine specific release of beta carotene | (Santoyo-Aleman et al., 2019) |
| Lutein | Starcha | Improved solubility and stability of lutein | (Fu et al., 2019) | |
| Lycopene | Zeina | Improved antioxidant activity | (Horuz and Belibagli, 2019) | |
| Vitamins | Folate B9 | Whey protein isolate and resistant starch | Improved stability of folic acid | (Pérez-Masiá et al., 2015) |
| Cobalamin B12 | Soy protein | Improved intestinal transport | (Zhang et al., 2015) | |
| Vitamin D | Fish oil | Improved bioavailability | (Walia et al., 2017) |
Considered GRAS by FDA (21 CFR Ch. I [4–1–11 Edition]).
Starch is another nature-derived polymer that has been studied for nanoencapsulation. The natural amylose helices can serve to host the guest molecules, thereby improving their solubility and stability. A comprehensive review by Rosatmabadi et al. outlines the use of starch nanostructures produced from both top-down and bottom-up approaches for the encapsulation of nutraceuticals with proven health benefits. Given its Generally Recognized As Safe (GRAS) status and many sustainable techniques available for fabrication, starch serves as a good candidate for nanoencapsulation of various flavors, essential oils, vitamins, and carotenoids (Rostamabadi et al., 2019). Another important consideration is the ability of certain types of starch and processing conditions to moderate the release of the active ingredients within the gastrointestinal tract (GIT), thereby protecting some of the labile nutrients from degradation in the upper GIT (Sampathkumar and Loo, 2018).
β-Lactoglobulin, obtained from whey is a promising material for the preparation of nutraceutical carriers. Its high solubility, natural nutrient-binding capacity, and resistance against peptic digestion are advantageous for the encapsulation and complexation of various molecules, either by itself or in combination with other polymers (Teng et al., 2015). Zein, a protein extracted from maize, is another material used in the encapsulation of nutraceuticals (Horuz and Belibagli, 2019). Owing to its low cost and hydrophobic nature, it can be engineered to encapsulate hydrophobic nutrients (Pascoli et al., 2018).
Nutraceutical Encapsulation for Food Preservation
Certain substances produced naturally in foods have the potential to inhibit the growth of pathogenic organisms thereby offering food preservation. Essential oils obtained from a variety of food sources such as cinnamon oil, thymol, garlic oil, and citrus oil have been shown to be effective in retarding the growth of pathogenic bacteria (Pisoschi et al., 2018). These natural antimicrobials have been used advantageously in active food packaging by encapsulating them into a nano-delivery system, which can then be incorporated into packaging materials to provide a sustained release of the compounds, thereby extending the shelf-life of food substances. For example, edible films made of cellulose nanofibers incorporated with ginger oil and citric acid have been shown to impart antimicrobial and antioxidant properties to the films and also improved the shelf-life of ready-to-cook chicken by 6 days (Khaledian et al., 2019).
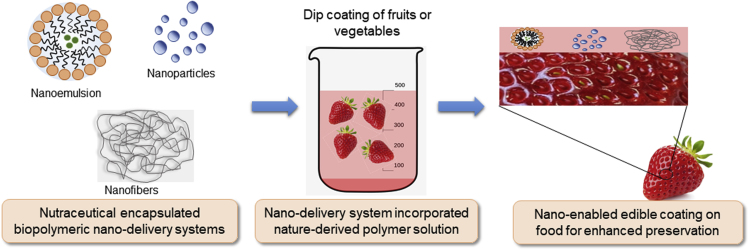
Nanoemulsions, formed by stabilizing two immiscible contents, are being studied extensively in the context of incorporating essential oils into edible coatings for food preservation (Aloui and Khwaldia, 2016). Salvia et al. used edible nano-coatings based on lemongrass essential oil (LEO) emulsified in alginate to improve the quality and safety parameters of freshly cut Fuji apples. It was observed that, during the study period of 2 weeks, the nano-coatings containing 0.5% or 1% LEO, inhibited the growth of Escherichia coli to undetectable amounts (2 log units), and minimized ethylene production (to nearly zero) and respiration rates in the apples. This study has shown the possibilities of using nanoemulsion-based coatings as delivery carriers of anti-microbial ingredients for protection of cut fruits (Salvia-Trujillo et al., 2015). Figure 3 shows a graphic of how such coatings can be achieved on food matrices. A recent study described the use of thymol nanoemulsion-loaded quinoa protein/chitosan edible coatings to preserve the quality and safety of refrigerated strawberries with reduced loads of yeast and fungus (2 log units) as well as lowered weight loss by 20% during the entire storage time (Robledo et al., 2018).
Figure 3.
Incorporation of Nano-Delivery Systems in Edible Coatings
Chitosan nanoparticles have also been widely studied for the encapsulation of essential oils that possess antibacterial properties. This, when combined with the inherent antibacterial property of chitosan, would provide a synergistic effect in killing the pathogens. Stoleto-Boyas et al. have encapsulated both LEO and thyme essential oil in chitosan nanoparticles and found that encapsulation increased the antibacterial activity (Sotelo-Boyás et al., 2017a, Sotelo-Boyás et al., 2017b). Mohammadi et al. have also shown that chitosan nanoparticle encapsulated with cinnamon essential oil (CEO) could provide a sustained release of the bioactive ingredient over a period of 21 days. These CEO-loaded particles were also tested on cucumbers in cold storage and were able to prevent the fungal decay of cucumbers for 21 days, with the coated ones having a firmer texture and brighter color, whereas the uncoated ones turned moldy (Mohammadi et al., 2015).
Another aspect of food preservation is the prevention of oxidation in foods. This has also been shown to be possible using biopolymeric nanoparticles. Liang et al. encapsulated the natural antioxidant epigallocatechin gallate (EGCG) extracted from green tea into zein/chitosan nanoparticles to provide a controlled release of the compound that helped to protect fatty foods from oxidation for longer periods. The free radical scavenging activity of EGCG in zein-coated chitosan nanoparticles was found to be four times higher than free EGCG (Liang et al., 2017). A CEO nano formulation made with chitosan has also been shown to retain the metmyoglobin content and color of beef patties in cold storage and increase their shelf life by 6 days compared with control (Ghaderi-Ghahfarokhi et al., 2017).
Nano-Delivery Systems Based on EPS
EPS materials are gaining interest to be engineered for encapsulating bioactive ingredients for food applications owing to their many beneficial properties (as illustrated in Figure 1), safety, and sustainability. These polymers have been studied for the encapsulation of probiotics as they would function as a prebiotic, resulting in a symbiotic relationship (Ispirli et al., 2018). In some cases, EPS has also been shown to increase survivability of the encapsulated probiotics by protecting them in the gastric environment (Çabuk and Harsa, 2015). Nanoparticles of EPS have only recently been explored for encapsulation of nutraceutical for food-related applications. There are previous reports of EPS materials being used for encapsulation of anticancer drugs, where Raveendran et al. developed composite EPS nanoparticles made of Mauran, an EPS from a halophilic bacterium Halomonas maura, together with chitosan for the sustained release of the anticancer drug, 5-fluorouracil (Raveendran et al., 2013). Nanoparticles of β-glucan, loaded with anticancer drug epirubicin, have been shown to provide a sustained release of the drug resulting in increased drug uptake in tumors and reduced tumor volume by 70% in mice (Gao et al., 2010). A nanohydrogel system based on gellan was developed for encapsulating hydrophobic paclitaxel and hydrophilic prednisolone to provide a synergistic effect. Promising synergistic effects were noticed on three different cancer cell lines in vitro (D'Arrigo et al., 2014). Given that the reports of EPS nanoparticles being used in agri-food applications are scarce, barring a few mentioned below, the above examples establish the possibility of using these nature-derive polymers for encapsulation and sustained release of active ingredients.
Semyonov et al. used an enzymatic method for the synthesis of dextran nanoparticles using the enzyme dextransucrase. The size of the particles could be tuned by varying the concentration of the enzyme. The particles were also shown to be able to host a hydrophobic nutraceutical, the isoflavone genistein and the loading could be increased by optimizing the synthesis conditions (Semyonov et al., 2014). This work serves as an example of how EPS materials could be used to encapsulate nutraceuticals for food applications through a non-toxic synthesis technique. Food packaging could be another application for EPS materials. Pullulan is a microbial EPS obtained from the fungus Aureobasidium pullulans. With its excellent film-forming properties, pullulan is a good example for non-toxic edible films obtained from a sustainable source (Kraśniewska et al., 2019). Morsy et al. had previously shown that essential oils and nanoparticles with antimicrobial properties could be incorporated into edible pullulan films to inhibit the growth of pathogenic microorganisms. In field studies, the pullulan incorporated films were able to inhibit pathogens in meat and poultry for up to 3 weeks when stored at 4°C (Morsy et al., 2014). Edible films with antioxidant and antimicrobial properties were developed by Silva et al. by incorporating lysozyme nanofibers into pullulan films. The homogeneous transparent films even showed augmented antibacterial efficacy against a lysozyme-resistant Staphylococcus aureus strain, providing promising example of how EPS could help create environment friendly active packaging materials (Silva et al., 2018).
Nano-Delivery Systems in Agriculture
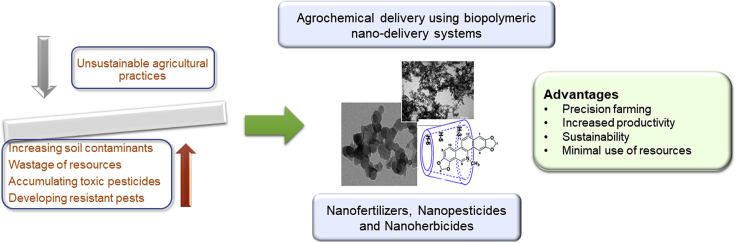
Conventional agricultural practices may no longer be able to sustain the food demands of a growing population without damaging the environment. Sustainable agricultural practices need to be adopted in every sector, as urgently as possible to solve some of the growing problems of toxic pesticides, resistant pests, and increasing soil contaminants (Lowry et al., 2019). Modern agriculture is seeking alternatives for the use of agrochemicals via green nanotechnology with renewable nanomaterials to implement precision farming that aims at increasing productivity sustainably with minimal resources (Figure 4) (Fraceto et al., 2016, Rai and Ingle, 2012). The two main fields where nanotechnology can contribute to agriculture are improving crop yields and increasing resource utilization efficiency, mainly by reducing excessive wastage. The fertilizers used currently have less than 50% use efficiencies, with only 45% of P and 30%–40% of N being absorbed from the applied fertilizers (Su et al., 2019). Nature-derived polymeric nanoparticles can be utilized in varying applications including nanoherbicides, nanodetectors, and nanofertilizers to resolve conventional challenges of agriculture including environmental contaminations and human health concerns. These engineered nanoproducts help enhance food production and nutritional value by improving the quality of pesticides, fertilizers, and growth regulators. For instance, nanocarriers are employed to carry and deliver pesticides in a more controlled and slow release profile to achieve “precision farming,” which targets only crop productions without affecting the water and soil (Duhan et al., 2017).
Figure 4.
Graphic Enunciating the Need for Nano-Delivery Systems in Agriculture
SEM micrographs adapted with permission from (Hazra and Kumar, 2015, Marchiol et al., 2019, Iswanti et al., 2019).
Controlled Release of Agrochemicals—Nanofertilizers
The main aim of encapsulation of fertilizers is to increase the nutrient conversion ratio and reduce wastage of the minerals. The polymer-coated controlled releasing fertilizers, loaded with N, P, K and other micro nutrients, as discussed in section Nature-Derived Polymers for Agriculture Application, are still being used extensively in agriculture. Nanoencapsulation is more prominently applied to pesticide encapsulation than fertilizers. With regards to nanotechnology in fertilizer applications, inorganic nanoparticles of zinc (Zn), copper (Cu), Fe, cerium (Ce), and titanium (Ti) are studied extensively both in laboratory and field conditions when compared with encapsulation of these nutrients into nanoparticulate systems (Raliya et al., 2018). A recent review on the use of engineered nanomaterials in different aspects of agriculture, such as delivery of nutrients to crops and insecticide and pesticide functions, discusses the whole range of ENMs, both organic and inorganic, that are being studied currently in this field (Adisa et al., 2019).
Chitosan is one of the nature-derived polymers that has many implications in agriculture. With its inherent growth enhancement and antimicrobial properties, chitosan could be exploited in agriculture, either stand alone or as an encapsulation matrix for nutrients (Kumaraswamy et al., 2018). Owing to the greater dispersibility of chitosan nanomaterials, it is preferred to be used in the nano from rather than in the macro form. Heba et al. have demonstrated the ability of chitosan nanoparticles loaded with nutrients N, P, and K, to accelerate plant growth, thereby shortening the life cycle of wheat plants they were tested on from 170 to 130 days (Abdel-Aziz et al., 2016). Barley field tests on using chitosan nanoparticles by itself have demonstrated their ability in improving grain yield through soil application to barley plants under drought stress (Behboudi et al., 2018).
Hydroxyapatite (HA), the mineral component of bone and teeth, is a naturally occurring mineral form of calcium apatite and has been applied for the delivery of macronutrients to soil. Kottegoda et al. used HA crystals modified with urea as a means of increasing the nitrogen utilization ratio of conventional fertilizers. The natural cellulose and lignin pores in wood stem were used as a reservoir for nano fertilizers, and HA urea-loaded wood chips were tested for N release under different soil conditions. This nano fertilizer provided a sustained release of N for more than 60 days compared with the initial burst and fluctuating N amounts releasing from conventional fertilizers (Kottegoda et al., 2011). The group also did a follow-up field study using the HA-based nanofertilizer on rice fields and showed an increase in yield compared with the conventional urea fertilizer, using only 50% of fertilizer used in the conventional treatment (Kottegoda et al., 2017).
Controlled Release of Agrochemicals—Nanopesticides
The main advantage of nanoencapsulation of pesticides is that encapsulation greatly reduces the amount of pesticides used, by solubilizing them and providing a controlled release of these chemicals at the target site. This in turn checks the development of resistant pest species that arise from overexposure and contamination of soil and water bodies with excess chemicals.
Nano complexation is an encapsulation technique using cyclodextrins, which are cyclic derivatives of starch containing a hydrophilic exterior and a hydrophobic interior, forming excellent complexes with molecules that have low solubility in aqueous media. Campos et al. have exploited this property of cyclodextrin to form inclusion complexes with two plant-derived compounds, carvacrol and linalool, that have insecticidal and repellent properties. The cyclodextrins were then used to functionalize chitosan before forming chitosan nanoparticles to facilitate easier application to crops. Given the high volatility of the natural compounds, nano complexation improved the lifetime of the compounds when exposed to the environment (Campos et al., 2018). Another work from the same group uses zein, a nature-derived protein, to form nanoencapsulates of plant-derived insecticides, namely, citronella oil (Oliveira et al., 2018), geraniol, eugenol, and cinnamaldehyde (de Oliveira et al., 2019). The use of such botanical extracts helps to check development of resistance in insects to commonly used insecticides as well. Encapsulation was shown to help prevent the degradation of the compounds, lower the IC50, and also provide prolonged release of compounds.
Biopolymeric nanocarrier made up of natural polymers, chitosan, and pectin was developed by Sandhya et al. to provide a sustained and controlled release of encapsulated carbendazim with good bio-efficacy and inhibition against fungi such as Fusarium oxysporus and Aspergillus parasiticus. Moreover, the phytotoxicity assessment showed that carbendazim-loaded polymeric nanoformulation provides better germination and root growth for the seeds of Zea mays, and Cucumis sativa (Sandhya et al., 2017). Encapsulation in chitosan alginate systems has shown to decrease the toxicity by 50% and improve the efficiency of two herbicides, imazapic and imazapyr, used to combat weeds in maize and peanut fields (Maruyama et al., 2016). Similar studies have also been done previously to demonstrate the ability of nanoencapsulation in chitosan nanoparticles to reduce the toxicity of the herbicide, Paraquat, by four folds, while preserving its activity (Grillo et al., 2014). Nanocapsules prepared by the polyelectrolyte complexation of chitosan and alginate were used to retard the fast release of the water-soluble insecticide acetamiprid. The carrier was able to slow down the release of the chemical, providing a controlled release of the chemical for 36 h (Kumar et al., 2015).
Chitosan nanoparticles have also been shown to boost the inherent immunity of plants thereby inducing disease resistance in them. Maize plants treated with Cu-chitosan nanoparticles were able to resist Curvularia leaf spot disease by boosting its antioxidant activities and defense enzymes (Choudhary et al., 2017). Similarly, in another study, chitosan nanoparticles were found to impart antifungal properties to the finger millet plants tested by inducing reactive oxygen species and activating peroxidases in the plant (Sathiyabama and Manikandan, 2016). The functional properties of such EPS-derived nanoparticles in plant protection have been demonstrated by Anusuya et al. The group prepared β-d-glucan nanoparticles that showed antifungal activity against Pythium aphanidermatum, a fungus affecting rhizome plants (Anusuya and Sathiyabama, 2014). As a follow-up field study, the group has also shown the ability of the biopolymeric nanoparticles in crop protection by application of the nanoparticles as foliar spray, which offered 77% protection to the turmeric plants tested (Anusuya and Sathiyabama, 2015). All these reported studies indicate the superior advantages of nanoformulations in minimizing the concentrations and amounts of herbicide or pesticide needed for effective treatments by providing a more targeted and precise delivery system with reduced adverse environmental effects. The quantitative benefits of the above-mentioned nano-delivery systems have been summarized in Table 3.
Table 3.
Beneficial Effects of Nanoencapsulation in Agri-Food Applications
| Nature-Derived Polymer Used | Delivery System or Technology | Active Ingredient | Beneficial Effect |
|---|---|---|---|
| Cellulose | Edible films with nanofibers | Ginger oil and citric acid | Improved shelf-life of ready-to-cook chicken by 6 days (Khaledian et al., 2019) |
| Alginate | Edible films containing nanoemulsions | LEO | Inhibited the growth of Escherichia coli to 2 log units and minimized ethylene production to nearly zero for 2 weeks on cut apples (Salvia-Trujillo et al., 2015) |
| Quinoa protein and chitosan | Nanoemulsion-loaded edible coatings | Thymol | Decreased yeast and fungal growths to 2 log units and reduced weight loss by 20% on refrigerated strawberries (Robledo et al., 2017) |
| Chitosan | Nanoparticles | CEO | Prevented fungal decay of cucumbers for 21 days (Mohammadi et al., 2015) |
| Zein-chitosan | Nanoparticles | EGCG | Prevented oxidation of fatty foods (Liang et al., 2017) |
| Chitosan | Nanoparticles | CEO | Increased shelf-life of beef patties by 6 days (Ghaderi-Ghahfarokhi et al., 2017) |
| Chitosan | Nanoparticles | Nutrients—N, P, and K | Shortened the life cycle of wheat plants from 170 to 130 days (Abdel-Aziz et al., 2016) |
| Hydroxyapatite | Nanofertilizer | Urea | Increase in yield with only 50% of the conventional fertilizer used (Kottegoda et al., 2017) |
| Chitosan-alginate | Nanoparticles | Imazapic and Imazapyr | Decreased the toxicity of pesticides by 50% (Maruyama et al., 2016) |
| Chitosan | Nanoparticles | Paraquat | Reduced the toxicity of the herbicide by 4-fold (Grillo et al., 2014) |
| Chitosan-alginate | Nanocapsules | Acetamiprid | Provided a controlled release of the insecticide for 36 h (Kumar et al., 2015) |
Public Receptivity—Reviewing Toxicity and Public Perceptions on Nanomaterials
The functionalities of nanomaterials, their properties, physiological interactions (pharmacokinetics and pharmacodynamics), toxicity, and interaction with other materials or chemicals under different environments are important considerations before they are commercialized for use in the market. Although some products, like the Smartcap by BASF and Amblyline cu by Syngenta for the controlled release of pesticides, and nano-enabled supplements like Nutralease, are already available in the market, it is important to consider the consequences and impact of these engineered nanomaterials (Grillo et al., 2016). An extensive review by McClements provides insights into the toxicity and fate of different organic and inorganic nanoparticles (McClements and Xiao, 2017). Cao et al. have studied the interactions of ENMs when ingested together with pesticides in foods. It was observed that the ENMs could increase the bioavailability of the pesticides when consumed together (Cao et al., 2019). Such unanticipated consequences require robust toxicological studies that better simulate the gastrointestinal environment, to understand how these nano-foods would be presented to and perceived by the GIT. One important consideration in the case of nano-fortified foods is that, because of their ability to improve the bioavailability of the nutrients, toxic effects may also arise owing to the accumulation of these extra amounts of nutrients in the body. A possible solution would be to redefine the daily requirements of the nanoencapsulated nutrients (Singh, 2016).
Although transport of nanomaterials in the GIT is being discussed widely, the transport of these materials through the plant system is also an important consideration and there seems to be a lack of knowledge in the understanding of these transport processes. Investigating these processes could provide better insights into these systems (Raliya et al., 2018). Joginder et al. in their perspectives article on using nanotechnology in agriculture have discussed some of the adverse effects observed in plants when nanoparticles of silver (Ag), aluminum (Al), Ti, Ce, and Zn were used on different plant varieties (Duhan et al., 2017).
Public awareness and acceptance are important for bringing such new technologies to the consumer. Public opinion surveys conducted around the world seem to concord that public are wary about nanotechnology and nano-enabled foods owing to the possible adverse health effects (Siegrist et al., 2007, George et al., 2014). Public awareness and acceptance toward nature-derived polymer nanoparticles tend to be more positive. This is mainly attributed to the natural ingredients or polymers used, which are GRAS-recognized by FDA (Duncan, 2011). Hence, using nature-derived polymers as delivery systems for nutrients, antimicrobials, pesticides, and fertilizers could be a favorable step in bringing these products to the market faster in the agri-food sector.
Nanotechnology stakeholders and companies have been engaging the public actively in promoting public awareness on the benefits as well as regulations of nanomaterials in both food and agricultural industries. The transparent bidirectional communications between the stakeholders and public on the risk assessment and management, as well as policy evaluation, have been carried out constantly to improve the health and environmental regulations for public acceptance (Kuzma et al., 2008). This has helped to promote public confidence and acceptance on the applications of nanotechnology in various sectors. According to a recent commentary published by Neena Mitter and Karen Hussey, there is a stark imbalance between the number of papers published, patents filed regarding nanotechnologies in food and agriculture, and the number of nano-enabled products being available in this sector. They attribute this difference to the lack of fit for purpose regulatory frameworks, as the existing frameworks cannot be used in context of nano-enabled foods (Mitter and Hussey, 2019).
Owing to the big impact of nanotechnology in many consumer products, there are currently efforts worldwide to address and regulate the safe production and handling of nanomaterials. This is especially important, considering the fact that nanomaterials can have very different properties from its larger sizes, whose impact on human health and environment is still yet to be well understood (Amenta et al., 2015). Current regulations and tests that are applied to chemicals may not be relevant for nanomaterials (Mitter and Hussey, 2019), and it is only time that framework and legislations be laid out to meet the growing number of nano-based products in the market. The European Union has taken the lead in this regard especially with European Food Safety Authority (EFSA) in charge, by establishing guidelines on the risk assessment of the application of nanoscience in the food and feed chain. It is also recommending revaluation of toxicity of the nanoform of the same materials previously approved (Rauscher et al., 2017). In terms of labeling, the most important consideration would be to list out specifically the ingredients present in nanoform, mainly to enable the consumer to make an informed choice of the product.
Conclusion and Perspective
The various advantages and applications of nano-delivery systems, outlined above, definitely holds promise that nanotechnology could potentially revolutionize the agri-food sector, while helping to solve major problems such as food scarcity, crop yield, and sustainability. An array of nature-derived polymers that can be engineered into nano-delivery systems for agri-food applications has been presented. A special mention on EPS materials throws light on these promising but less applied materials for encapsulation. The encapsulation capabilities of these biopolymers have been reviewed in the context of various nutraceuticals, plant-derived bioactive molecules, fertilizers, and pesticides that could be encapsulated into the biopolymeric nanoparticles for applications in food fortification, edible packaging, and agricultural practices. Although each of these materials has been shown to be useful in the various aspects of nutraceutical and agrochemical encapsulation and delivery, some materials are more widely used over others owing to their distinctive favorable properties. Starch and chitosan are two such examples. Starch, being a major component of food, can be engineered to possess different properties to suit the application based on its source and processing conditions, whereas chitosan has been the material of choice for agrochemical delivery, as the release can be triggered upon a stimulus such as change in soil pH. It should also be noted that the cost of the material and its processing into nano-delivery systems would be an important factor in the choice of the material and its use in any of the applications discussed above.
The concerns over the safety of the nanomaterials used directly affects the public acceptance of such new technologies especially in the agri-food sector. The reassurance, in the case of biopolymeric nanomaterials, comes from the fact that all of the materials discussed here are polymers that can be easily degraded in the body or soil, unlike inorganic nanoparticles, which continue to be cycled in the environment and be presented in different environments whereby the toxicity cannot be predicted. Also, showcasing the number of possibilities where nature-derived biopolymers can be used to replace inorganic nanoparticles in their existing roles in both food and agriculture serves as a step toward making nanotechnologies safer and more acceptable by the public enabling us to reap the full benefits of nanotechnology.
Given the promise of nature-derived polymers for encapsulation in agri-food applications, it is foreseeable that its usage will undoubtedly increase. In addition, the biodegradable and safe nature of such materials puts them in good stead for consumption (i.e., food) and environmental (i.e., agriculture) usage. The use of these materials would therefore be a sustainable approach to agri-food purposes. In a circular economy, the goal is to minimize waste by reusing and recycling materials. One excellent source of sustainable nature-derived polymers would be from food industry waste, such as bagasse and okara, given their high contents of polysaccharides. The successful use of food waste for bioplastic production (Tsang et al., 2019), which is now a burgeoning industry, is an example of how food wastes or even by-products of the agri-food industry could be put to useful applications. For instance, okara, the by-product of the tofu industry, is of immense interest for such applications, particularly in countries like Japan, Korea, China, and Singapore, where disposing off the tons of okara waste is a big challenge. There has been a recent breakthrough in this field with the making of okara-cellulose based packaging materials (Boh, 2017). Such developments are only promising that these materials have great potential and could definitely be engineered into safe nanomaterials for various applications in the agri-food sector.
Another potential source of biopolymers discussed in this review is that of microbial EPS. Microbes are seen as a sustainable and stable source of producing biopolymers with various advantageous properties. Polyhydroxyalkonates, biopolymer secreted from bacteria and used by companies successfully and cost-effectively to replace single-use plastic products such as straws and disposable cups, is a testimony to the many potentials that these microbe-derived polymers could offer. EPS materials especially from Lactobacillus bacteria is viewed with special interest because of its food origin and the numerous potential health-benefitting attributes (Oleksy and Klewicka, 2018). The successful engineering of these materials into nano-delivery systems would help us reap more benefits of their inherent beneficial properties.
In terms of production scale-up, all of the nature-derived polymer-based nano-delivery systems discussed above are still in their very early stages of development and considerable time and effort is required in order to commercialize them. Currently, large-scale production methods are only well established for inorganic nanoparticles and carbon-based nanomaterials. In order to successfully scale up some of the above-mentioned encapsulation and delivery systems, a great deal of technological and scientific investigation would be required, followed by a pilot plant setup before full-scale production. Quality control is an important factor to be considered. As the dimension becomes smaller, quality control becomes increasingly difficult. Cost would be another mitigating factor given that the presence of these new components should not lead to a huge increase in the price of the final product. Addressing some of these issues would help accelerate bringing these nano-based products to use.
Food fortification is seen as a means to provide better and balanced nutrition to children in developing countries to counter for any deficiencies in diet, and it is also viewed as a better source of offering care to the aging global population in order to keep them healthy. Encapsulation technology would complement food fortification, offering enhanced bioavailability while preserving the bioactivity of the active ingredients, by protecting them from any adverse exposure in the surrounding environments. Besides, encapsulation also helps to significantly lower the amount of active ingredients required, which would otherwise be lost due to incomplete absorption or degradation and hence be required to be added in large quantities. Since the materials used for encapsulation in food fortification applications would be considered a direct food additive, it is only justifiable why nature-derived materials such as polysaccharides and proteins, mainly obtained from food ingredients, are better candidates for this purpose. Having shown previously in our research as to how nature-derived polymers could be used as enteric coating materials over nano-delivery systems, it is another direction that nature-derived polymers could be used increasingly in the agri-food sector to maximize the nutrient value. In all, the sustainable approach of exploiting nature-derived polymers in the agro-food industry is expected to rise in the near future.
Acknowledgments
The authors would like to acknowledge the financial support from the Singapore Centre for Environmental Life Sciences Engineering (SCELSE) (MOE/RCE: M4330019.C70), Ministry of Education AcRF-Tier 1 grant (RG19/18), Agri-Food and Veterinary Authority of Singapore (APF LCK102), Biomedical Research Council (BMRC) – Therapeutics Development Review (TDR-G-004-001), NTU-HSPH grant (NTU-HSPH 17002), and the Bill and Melinda Gates Foundation (OPP1199116).
Author Contributions
K.S. conceptualized and drafted the manuscript with help from K.X.T. for certain sections. S.C.J.L. helped in conceptualizing and providing direction for the manuscript and edited and reviewed the manuscript drafts.
References
- Abdel-Aziz H.M.M., Hasaneen M.N.A., Omer A.M. Nano chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Span. J. Agric. Res. 2016;14:e0902. [Google Scholar]
- Adisa I.O., Pullagurala V.L.R., Peralta-Videa J.R., Dimkpa C.O., Elmer W.H., Gardea-Torresdey J.L., White J.C. Recent advances in nano-enabled fertilizers and pesticides: a critical review of mechanisms of action. Environ. Sci. Nano. 2019;6:2002–2030. [Google Scholar]
- Akalin G.O., Pulat M. Controlled release behavior of zinc-loaded carboxymethyl cellulose and carrageenan hydrogels and their effects on wheatgrass growth. J. Polym. Res. 2019;27:6. [Google Scholar]
- Akbari-Alavijeh S., Shaddel R., Jafari S.M. Encapsulation of food bioactives and nutraceuticals by various chitosan-based nanocarriers. Food Hydrocolloids. 2020:105774. [Google Scholar]
- Aloui H., Khwaldia K. Natural antimicrobial edible coatings for microbial safety and food quality enhancement. Compr. Rev. Food Sci. Food Saf. 2016;15:1080–1103. doi: 10.1111/1541-4337.12226. [DOI] [PubMed] [Google Scholar]
- Amenta V., Aschberger K., Arena M., Bouwmeester H., Botelho Moniz F., Brandhoff P., Gottardo S., Marvin H.J.P., Mech A., Quiros Pesudo L. Regulatory aspects of nanotechnology in the agri-feed-food sector in EU and non-EU countries. Regul. Toxicol. Pharmacol. 2015;73:463–476. doi: 10.1016/j.yrtph.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Anusuya S., Sathiyabama M. Preparation of β-d-glucan nanoparticles and its antifungal activity. Int. J. Biol. Macromol. 2014;70:440–443. doi: 10.1016/j.ijbiomac.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Anusuya S., Sathiyabama M. Foliar application of β-d-glucan nanoparticles to control rhizome rot disease of turmeric. Int. J. Biol. Macromol. 2015;72:1205–1212. doi: 10.1016/j.ijbiomac.2014.10.043. [DOI] [PubMed] [Google Scholar]
- Azevedo M.A., Bourbon A.I., Vicente A.A., Cerqueira M.A. Alginate/chitosan nanoparticles for encapsulation and controlled release of vitamin B2. Int. J. Biol. Macromol. 2014;71:141–146. doi: 10.1016/j.ijbiomac.2014.05.036. [DOI] [PubMed] [Google Scholar]
- Bajpai V.K., Kamle M., Shukla S., Mahato D.K., Chandra P., Hwang S.K., Kumar P., Huh Y.S., Han Y.-K. Prospects of using nanotechnology for food preservation, safety, and security. J. Food Drug Anal. 2018;26:1201–1214. doi: 10.1016/j.jfda.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandala E.R., Berli M. Engineered nanomaterials (ENMs) and their role at the nexus of Food, Energy, and Water. Mater. Sci. Energy Technol. 2019;2:29–40. [Google Scholar]
- Behboudi F., Tahmasebi Sarvestani Z., Kassaee M.Z., Modares Sanavi S.A.M., Sorooshzadeh A., Ahmadi S.B. Evaluation of chitosan nanoparticles effects on yield and yield components of barley (Hordeum vulgare L.) under late season drought stress. J. Water Environ. Nanotechnol. 2018;3:22–39. [Google Scholar]
- Bhushani J.A., Kurrey N.K., Anandharamakrishnan C. Nanoencapsulation of green tea catechins by electrospraying technique and its effect on controlled release and in-vitro permeability. J. Food Eng. 2017;199:82–92. [Google Scholar]
- Boh S. The Straits Times; 2017. Turning Soya Bean Waste into Packaging. [Google Scholar]
- Çabuk B., Harsa Ş. Whey protein-pullulan (WP/Pullulan) polymer blend for preservation of viability of Lactobacillus acidophilus. Dry. Technol. 2015;33:1223–1233. doi: 10.3109/02652048.2015.1017618. [DOI] [PubMed] [Google Scholar]
- Calvo P., Remunan-Lopez C., Vila-Jato J.L., Alonso M. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997;63:125–132. [Google Scholar]
- Campos E.V.R., De Oliveira J.L., Fraceto L.F., Singh B. Polysaccharides as safer release systems for agrochemicals. Agron. Sustain. Dev. 2015;35:47–66. [Google Scholar]
- Campos E.V.R., Proença P.L.F., Oliveira J.L., Melville C.C., Della Vechia J.F., De Andrade D.J., Fraceto L.F. Chitosan nanoparticles functionalized with β-cyclodextrin: a promising carrier for botanical pesticides. Sci. Rep. 2018;8:2067. doi: 10.1038/s41598-018-20602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Deloid G.M., Bitounis D., De La Torre-Roche R., White J.C., Zhang Z., Ho C.G., Ng K.W., Eitzer B.D., Demokritou P. Co-exposure to the food additives SiO2 (E551) or TiO2 (E171) and the pesticide boscalid increases cytotoxicity and bioavailability of the pesticide in a tri-culture small intestinal epithelium model: potential health implications. Environ. Sci. Nano. 2019;6:2786–2800. doi: 10.1039/c9en00676a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary R.C., Kumaraswamy R.V., Kumari S., Sharma S.S., Pal A., Raliya R., Biswas P., Saharan V. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.) Sci. Rep. 2017;7:9754. doi: 10.1038/s41598-017-08571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demitri C., Scalera F., Madaghiele M., Sannino A., Maffezzoli A. Potential of cellulose-based superabsorbent hydrogels as water reservoir in agriculture. Int. J. Polym. Sci. 2013;2013:6. [Google Scholar]
- Dorier M., Beal D., Marie-Desvergne C., Dubosson M., Barreau F., Houdeau E., Herlin-Boime N., Carriere M. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology. 2017;11:751–761. doi: 10.1080/17435390.2017.1349203. [DOI] [PubMed] [Google Scholar]
- Duhan J.S., Kumar R., Kumar N., Kaur P., Nehra K., Duhan S. Nanotechnology: the new perspective in precision agriculture. Biotechnol. Rep. (Amst) 2017;15:11–23. doi: 10.1016/j.btre.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan T.V. The communication challenges presented by nanofoods. Nat. Nanotechnol. 2011;6:683. doi: 10.1038/nnano.2011.193. [DOI] [PubMed] [Google Scholar]
- D’Arrigo G., Navarro G., Di Meo C., Matricardi P., Torchilin V. Gellan gum nanohydrogel containing anti-inflammatory and anti-cancer drugs: a multi-drug delivery system for a combination therapy in cancer treatment. Eur. J. Pharm. Biopharm. 2014;87:208–216. doi: 10.1016/j.ejpb.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Ekebafe L., Ogbeifun D., Okieimen F. Polymer applications in agriculture. Biokemistri. 2011;23 [Google Scholar]
- Fraceto L.F., Grillo R., De Medeiros G.A., Scognamiglio V., Rea G., Bartolucci C. Nanotechnology in agriculture: which innovation potential does it have? Front. Environ. Sci. 2016;4:20. [Google Scholar]
- Fu Y.J., Yang J.D., Jiang L.W., Ren L.L., Zhou J. Encapsulation of lutein into starch nanoparticles to improve its dispersity in water and enhance stability of chemical oxidation. Starch-Starke. 2019;71:7. [Google Scholar]
- Gao F., Li L., Zhang H., Yang W., chen H., Zhou J., Zhou Z., Wang Y., Cai Y., Li X. Deoxycholic acid modified-carboxymethyl curdlan conjugate as a novel carrier of epirubicin: in vitro and in vivo studies. Int. J. Pharm. 2010;392:254–260. doi: 10.1016/j.ijpharm.2010.03.044. [DOI] [PubMed] [Google Scholar]
- George S., Kaptan G., Lee J., Frewer L. Awareness on adverse effects of nanotechnology increases negative perception among public: survey study from Singapore. J. Nano. Res. 2014;16:2751. [Google Scholar]
- Ghaderi-Ghahfarokhi M., Barzegar M., Sahari M.A., Ahmadi Gavlighi H., Gardini F. Chitosan-cinnamon essential oil nano-formulation: application as a novel additive for controlled release and shelf life extension of beef patties. Int. J. Biol. Macromol. 2017;102:19–28. doi: 10.1016/j.ijbiomac.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Global Industry Analysts Inc . Allied Market Research; 2019. The Global Nanotechnology Market. [Google Scholar]
- Griffin S., Masood M.I., Nasim M.J., Sarfraz M., Ebokaiwe A.P., Schäfer K.-H., Keck C.M., Jacob C. Natural nanoparticles: a particular matter inspired by nature. Antioxidants (Basel) 2017;7:3. doi: 10.3390/antiox7010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo R., Pereira A.E., Nishisaka C.S., De Lima R., Oehlke K., Greiner R., Fraceto L.F. Chitosan/tripolyphosphate nanoparticles loaded with paraquat herbicide: an environmentally safer alternative for weed control. J. Hazard. Mater. 2014;278:163–171. doi: 10.1016/j.jhazmat.2014.05.079. [DOI] [PubMed] [Google Scholar]
- Grillo R., Abhilash P.C., Fraceto L.F. Nanotechnology applied to bio-encapsulation of pesticides. J. Nanosci. Nanotechnol. 2016;16:1231–1234. doi: 10.1166/jnn.2016.12332. [DOI] [PubMed] [Google Scholar]
- Grujić R., Vujadinović D., Savanović D. Biopolymers as food packaging materials. In: Pellicer E., Nikolic D., Sort J., Baró M., Zivic F., Grujovic N., Grujic R., Pelemis S., editors. Advances in Applications of Industrial Biomaterials. Springer International Publishing; 2017. pp. 139–160. [Google Scholar]
- Hazra S., Kumar G.S. Physicochemical properties of inclusion complexes of sanguinarine with natural cyclodextrins: spectroscopy, calorimetry and NMR studies. RSC Adv. 2015;5:1873–1882. [Google Scholar]
- He X., Fu P., Aker W.G., Hwang H.-M. Toxicity of engineered nanomaterials mediated by nano–bio–eco interactions. J. Environ. Sci. Health C. 2018;36:21–42. doi: 10.1080/10590501.2017.1418793. [DOI] [PubMed] [Google Scholar]
- He X., Deng H., Hwang H.-M. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019;27:1–21. doi: 10.1016/j.jfda.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochella M.F., Mogk D.W., Ranville J., Allen I.C., Luther G.W., marr L.C., Mcgrail B.P., Murayama M., Qafoku N.P., Rosso K.M. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science. 2019;363:eaau8299. doi: 10.1126/science.aau8299. [DOI] [PubMed] [Google Scholar]
- Horuz T.I., Belibagli K.B. Nanoencapsulation of carotenoids extracted from tomato peels into zein fibers by electrospinning. J. Sci. Food Agric. 2019;99:759–766. doi: 10.1002/jsfa.9244. [DOI] [PubMed] [Google Scholar]
- Huang B., Chen F., Shen Y., Qian K., Wang Y., Sun C., Zhao X., Cui B., Gao F., Zeng Z., Cui H. Advances in targeted pesticides with environmentally responsive controlled release by nanotechnology. Nanomaterials. 2018;8:102. doi: 10.3390/nano8020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inkwood Research Global food encapsulation market Forecast 2017-2025. 2019. https://www.inkwoodresearch.com/reports/global-food-encapsulation-market/
- Ispirli H., Demirbaş F., Dertli E. Glucan type exopolysaccharide (EPS) shows prebiotic effect and reduces syneresis in chocolate pudding. J. Food Sci. Technol. 2018;55:3821–3826. doi: 10.1007/s13197-018-3181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iswanti F.C., Nurulita I., Djauzi S., Sadikin M., Witarto A.B., Yamazaki T. Preparation, characterization, and evaluation of chitosan-based nanoparticles as CpG ODN carriers. Biotechnol. Biotechnol. Equip. 2019;33:390–396. [Google Scholar]
- Jafarizadeh H., Sayyar Z., Anarjan N., Berenjian A. Springer; 2019. Nanobiotechnology in Food: Concepts, Applications and Perspectives. [Google Scholar]
- Jayan H., Leena M.M., Sundari S.K.S., Moses J.A., anandharamakrishnan C. Improvement of bioavailability for resveratrol through encapsulation in zein using electrospraying technique. J. Funct. Foods. 2019;57:417–424. [Google Scholar]
- Jeon Y.O., Lee J.-S., Lee H.G. Improving solubility, stability, and cellular uptake of resveratrol by nanoencapsulation with chitosan and γ-poly (glutamic acid) Colloids Surf. B Biointerfaces. 2016;147:224–233. doi: 10.1016/j.colsurfb.2016.07.062. [DOI] [PubMed] [Google Scholar]
- Jiao G.-J., Xu Q., Cao S.-L., Peng P., She D. Controlled-release fertilizer with lignin used to trap urea/hydroxymethylurea/urea-formaldehyde polymers. BioResources. 2018;13:18. [Google Scholar]
- Jindal N., Khattar J.I.S. Academic Press; 2018. Microbial Polysaccharides in Food Industry. [Google Scholar]
- Khaledian Y., Pajohi-Alamoti M., Bazargani-Gilani B. Development of cellulose nanofibers coating incorporated with ginger essential oil and citric acid to extend the shelf life of ready-to-cook barbecue chicken. J. Food Process. Preserv. 2019;43:e14114. [Google Scholar]
- Kottegoda N., Munaweera I., Madusanka N., Karunaratne V. A green slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsulated wood. Curr. Sci. 2011;101:73–78. [Google Scholar]
- Kottegoda N., Sandaruwan C., Priyadarshana G., Siriwardhana A., Rathnayake U.A., Berugoda Arachchige D.M., Kumarasinghe A.R., dahanayake D., Karunaratne V., Amaratunga G.A.J. Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano. 2017;11:1214–1221. doi: 10.1021/acsnano.6b07781. [DOI] [PubMed] [Google Scholar]
- Kraśniewska K., Pobiega K., Gniewosz M. Pullulan – biopolymer with potential for use as food packaging. Int. J. Food Eng. 2019;15 [Google Scholar]
- Kumar S., Chauhan N., Gopal M., Kumar R., Dilbaghi N. Development and evaluation of alginate–chitosan nanocapsules for controlled release of acetamiprid. Int. J. Biol. Macromol. 2015;81:631–637. doi: 10.1016/j.ijbiomac.2015.08.062. [DOI] [PubMed] [Google Scholar]
- Kumaraswamy R.V., Kumari S., Choudhary R.C., Pal A., Raliya R., Biswas P., Saharan V. Engineered chitosan based nanomaterials: Bioactivities, mechanisms and perspectives in plant protection and growth. Int. J. Biol. Macromol. 2018;113:494–506. doi: 10.1016/j.ijbiomac.2018.02.130. [DOI] [PubMed] [Google Scholar]
- Kuzma J., Romanchek J., Kokotovich A. Upstream oversight assessment for agrifood nanotechnology: a case studies approach. Risk Anal. 2008;28:1081–1098. doi: 10.1111/j.1539-6924.2008.01071.x. [DOI] [PubMed] [Google Scholar]
- Lee J.-S., Lee H.G. Chitosan/poly-γ-glutamic acid nanoparticles improve the solubility of lutein. Int. J. Biol. Macromol. 2016;85:9–15. doi: 10.1016/j.ijbiomac.2015.12.044. [DOI] [PubMed] [Google Scholar]
- Liang J., Yan H., Wang X., Zhou Y., Gao X., Puligundla P., Wan X. Encapsulation of epigallocatechin gallate in zein/chitosan nanoparticles for controlled applications in food systems. Food Chem. 2017;231:19–24. doi: 10.1016/j.foodchem.2017.02.106. [DOI] [PubMed] [Google Scholar]
- Lovegrove A., Edwards C.H., De Noni I., Patel H., El S.N., Grassby T., Zielke C., Ulmius M., Nilsson L., Butterworth P.J. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017;57:237–253. doi: 10.1080/10408398.2014.939263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry G.V., Avellan A., Gilbertson L.M. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat. Nanotechnol. 2019;14:517–522. doi: 10.1038/s41565-019-0461-7. [DOI] [PubMed] [Google Scholar]
- Luo Y., Hu Q. 7 - food-derived biopolymers for nutrient delivery. In: Grumezescu A.M., editor. Nutrient Delivery. Academic Press; 2017. pp. 251–291. [Google Scholar]
- Lutz G., Maya H., Sanghvi S. McKinsey & Company; 2015. Global Agriculture’s Many Opportunities.https://www.mckinsey.com/industries/private-equity-and-principal-investors/our-insights/global-agricultures-many-opportunities [Google Scholar]
- Majeed K., Jawaid M., Hassan A., Abu Bakar A., Abdul Khalil H.P.S., Salema A.A., Inuwa I. Potential materials for food packaging from nanoclay/natural fibres filled hybrid composites. Mater. Des. 2013;46:391–410. [Google Scholar]
- Majeed Z., Ramli Nur K., Mansor N., Man Z. A comprehensive review on biodegradable polymers and their blends used in controlled-release fertilizer processes. Rev. Chem. Eng. 2015;31:69. [Google Scholar]
- Mangaraj S., Yadav A., Bal L.M., Dash S.K., Mahanti N.K. Application of biodegradable polymers in food packaging industry: a comprehensive review. J. Packag. Technol. Res. 2019;3:77–96. [Google Scholar]
- Marchiol L., Filippi A., Adamiano A., Degli Esposti L., Iafisco M., Mattiello A., Petrussa E., BRAIDOT E. Influence of hydroxyapatite nanoparticles on germination and plant metabolism of tomato (Solanum lycopersicum L.): preliminary evidence. Agronomy. 2019;9:161. [Google Scholar]
- Maruyama C.R., Guilger M., Pascoli M., Bileshy-José N., Abhilash P.C., Fraceto L.F., De Lima R. Nanoparticles based on chitosan as carriers for the combined herbicides imazapic and imazapyr. Sci. Rep. 2016;6:19768. doi: 10.1038/srep19768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max R., Hannah R., Ortiz-Ospina E. Our World in data; 2019. World Population Growth.https://ourworldindata.org/world-population-growth [Google Scholar]
- McClements D.J., Xiao H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci. Food. 2017;1:6. doi: 10.1038/s41538-017-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignon A., De Belie N., Dubruel P., Van Vlierberghe S. Superabsorbent polymers: a review on the characteristics and applications of synthetic, polysaccharide-based, semi-synthetic and ‘smart’ derivatives. Eur. Polym. J. 2019;117:165–178. [Google Scholar]
- Milani P., França D., Balieiro A.G., Faez R. Polymers and its applications in agriculture. Polímeros. 2017;27:256–266. [Google Scholar]
- Mitter N., Hussey K. Moving policy and regulation forward for nanotechnology applications in agriculture. Nat. Nanotechnol. 2019;14:508–510. doi: 10.1038/s41565-019-0464-4. [DOI] [PubMed] [Google Scholar]
- Mohammadi A., Hashemi M., Hosseini S.M. Chitosan nanoparticles loaded with Cinnamomum zeylanicum essential oil enhance the shelf life of cucumber during cold storage. Postharvest Biol. Technol. 2015;110:203–213. [Google Scholar]
- Momin J.K., Joshi B.H. Nanotechnology in foods. In: Rai M., Ribeiro C., Mattoso L., Duran N., editors. Nanotechnologies in Food and Agriculture. Springer International Publishing; 2015. pp. 3–24. [Google Scholar]
- Mondal K., Ghosh T., Bhagabati P., katiyar V. Chapter 8 - sustainable nanostructured materials in food packaging. In: Karak N., editor. Dynamics of Advanced Sustainable Nanomaterials and Their Related Nanocomposites at the Bio-Nano Interface. Elsevier; 2019. pp. 171–213. [Google Scholar]
- Morsy M.K., Khalaf H.H., Sharoba A.M., El-Tanahi H.H., Cutter C.N. Incorporation of essential oils and nanoparticles in pullulan films to control foodborne pathogens on meat and poultry products. J. Food Sci. 2014;79:M675–M684. doi: 10.1111/1750-3841.12400. [DOI] [PubMed] [Google Scholar]
- Moscovici M. Present and future medical applications of microbial exopolysaccharides. Front. Microbiol. 2015;6:1012. doi: 10.3389/fmicb.2015.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naz M.Y., Sulaiman S.A. Testing of starch-based carbohydrate polymer coatings for enhanced urea performance. J. Coat. Technol. Res. 2014;11:747–756. [Google Scholar]
- Neri-Badang M.C., Chakraborty S. Carbohydrate polymers as controlled release devices for pesticides. J. Carbohydr. Chem. 2019;38:67–85. [Google Scholar]
- Oleksy M., Klewicka E. Exopolysaccharides produced by Lactobacillus sp.: Biosynthesis and applications. Crit. Rev. Food Sci. Nutr. 2018;58:450–462. doi: 10.1080/10408398.2016.1187112. [DOI] [PubMed] [Google Scholar]
- Oliveira J.L.D., Campos E.V.R., Pereira A.E.S., Pasquoto T., Lima R., Grillo R., Andrade D.J.D., Santos F.A.D., Fraceto L.F. Zein nanoparticles as eco-friendly carrier systems for botanical repellents aiming sustainable agriculture. J. Agric. Food Chem. 2018;66:1330–1340. doi: 10.1021/acs.jafc.7b05552. [DOI] [PubMed] [Google Scholar]
- de Oliveira J.L., Campos E.V.R., Germano-Costa T., Lima R., Vechia J.F.D., Soares S.T., De Andrade D.J., Goncalves K.C., Do Nascimento J., Polanczyk R.A., Fraceto L.F. Association of zein nanoparticles with botanical compounds for effective pest control systems. Pest Manag. Sci. 2019;75:1855–1865. doi: 10.1002/ps.5338. [DOI] [PubMed] [Google Scholar]
- Özcan E., Toksoy Oner E. Springer; 2015. Microbial Production of Extracellular Polysaccharides from Biomass Sources. [Google Scholar]
- Pan K., Chen H., Baek S.J., Zhong Q. Self-assembled curcumin-soluble soybean polysaccharide nanoparticles: Physicochemical properties and in vitro anti-proliferation activity against cancer cells. Food Chem. 2018;246:82–89. doi: 10.1016/j.foodchem.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons R. What is the difference between controlled release and slow release fertiliser? 2017. https://fernland.com.au/blog/what-is-the-difference-between-controlled-release-and-slow-release-fertiliser/
- Pascoli M., De Lima R., Fraceto L.F. Zein nanoparticles and strategies to improve colloidal stability: a mini-review. Front. Chem. 2018;6:6. doi: 10.3389/fchem.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul F., Morin A., Monsan P. Microbial polysaccharides with actual potential industrial applications. Biotechnol. Adv. 1986;4:245–259. doi: 10.1016/0734-9750(86)90311-3. [DOI] [PubMed] [Google Scholar]
- Perez J.J., Francois N.J. Chitosan-starch beads prepared by ionotropic gelation as potential matrices for controlled release of fertilizers. Carbohydr. Polym. 2016;148:134–142. doi: 10.1016/j.carbpol.2016.04.054. [DOI] [PubMed] [Google Scholar]
- Pérez-Masiá R., López-nicolás R., Periago M.J., Ros G., Lagaron J.M., López-Rubio A. Encapsulation of folic acid in food hydrocolloids through nanospray drying and electrospraying for nutraceutical applications. Food Chem. 2015;168:124–133. doi: 10.1016/j.foodchem.2014.07.051. [DOI] [PubMed] [Google Scholar]
- Pisoschi A.M., Pop A., Georgescu C., Turcuş V., Olah N.K., Mathe E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018;143:922–935. doi: 10.1016/j.ejmech.2017.11.095. [DOI] [PubMed] [Google Scholar]
- Prasad R., Bhattacharyya A., Nguyen Q.D. Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives. Front. Microbiol. 2017;8:1014. doi: 10.3389/fmicb.2017.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puoci F., Iemma F., Spizzirri U.G., Cirillo G., Curcio M., Picci N. Polymer in agriculture: a review. Am. J. Agric. Biol. Sci. 2008;3:299–314. [Google Scholar]
- Rahbar Saadat Y., Yari Khosroushahi A., Pourghassem Gargari B. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019;217:79–89. doi: 10.1016/j.carbpol.2019.04.025. [DOI] [PubMed] [Google Scholar]
- Rai M., Ingle A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012;94:287–293. doi: 10.1007/s00253-012-3969-4. [DOI] [PubMed] [Google Scholar]
- Raliya R., Saharan V., Dimkpa C., Biswas P. Nanofertilizer for precision and sustainable agriculture: current state and future perspectives. J. Agric. Food Chem. 2018;66:6487–6503. doi: 10.1021/acs.jafc.7b02178. [DOI] [PubMed] [Google Scholar]
- Rauscher H., Rasmussen K., Sokull-Klüttgen B. Regulatory aspects of nanomaterials in the EU. Chem. Ing. Tech. 2017;89:224–231. [Google Scholar]
- Raveendran S., Poulose A.C., Yoshida Y., Maekawa T., Kumar D.S. Bacterial exopolysaccharide based nanoparticles for sustained drug delivery, cancer chemotherapy and bioimaging. Carbohydr. Polym. 2013;91:22–32. doi: 10.1016/j.carbpol.2012.07.079. [DOI] [PubMed] [Google Scholar]
- Rayner M., Östbring K., purhagen J. Application of natural polymers in food. In: Olatunji O., editor. Natural Polymers: Industry Techniques and Applications. Springer International Publishing; 2016. pp. 115–161. [Google Scholar]
- Ren X., Hou T., Liang Q., Zhang X., Hu D., Xu B., Chen X., Chalamaiah M., Ma H. Effects of frequency ultrasound on the properties of zein-chitosan complex coacervation for resveratrol encapsulation. Food Chem. 2019;279:223–230. doi: 10.1016/j.foodchem.2018.11.025. [DOI] [PubMed] [Google Scholar]
- Rivero P.J., Urrutia A., Goicoechea J., Arregui F.J. Nanomaterials for functional textiles and fibers. Nanoscale Res. Lett. 2015;10:501. doi: 10.1186/s11671-015-1195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo N., Vera P., Lopez L., Yazdani-Pedram M., Tapia C., Abugoch L. Thymol nanoemulsions incorporated in quinoa protein/chitosan edible films; antifungal effect in cherry tomatoes. Food Chem. 2017;246:211–219. doi: 10.1016/j.foodchem.2017.11.032. [DOI] [PubMed] [Google Scholar]
- Robledo N., López L., Bunger A., Tapia C., Abugoch L. Effects of antimicrobial edible coating of thymol nanoemulsion/quinoa protein/chitosan on the safety, sensorial properties, and quality of refrigerated strawberries (Fragaria × ananassa) under commercial storage environment. Food Bioproc. Technol. 2018;11:1566–1574. [Google Scholar]
- Rostamabadi H., Falsafi S.R., Jafari S.M. Starch-based nanocarriers as cutting-edge natural cargos for nutraceutical delivery. Trends Food Sci. Technol. 2019;88:397–415. [Google Scholar]
- Salvia-Trujillo L., Rojas-Graü M., Soliva-Fortuny R., Martin-Belloso O. Use of antimicrobial nanoemulsions as edible coatings: impact on safety and quality attributes of fresh-cut Fuji apples. Postharvest Biol. Technol. 2015;105:8–16. [Google Scholar]
- Sampathkumar K., Loo S.C.J. Targeted gastrointestinal delivery of nutraceuticals with polysaccharide-based coatings. Macromol. Biosci. 2018;18:1700363. doi: 10.1002/mabi.201700363. [DOI] [PubMed] [Google Scholar]
- Sandhya, Kumar S., Kumar D., Dilbaghi N. Preparation, characterization, and bio-efficacy evaluation of controlled release carbendazim-loaded polymeric nanoparticles. Environ. Sci. Pollut. Res. Int. 2017;24:926–937. doi: 10.1007/s11356-016-7774-y. [DOI] [PubMed] [Google Scholar]
- Santoyo-Aleman D., Sanchez L.T., Villa C.C. Citric-acid modified banana starch nanoparticles as a novel vehicle for beta-carotene delivery. J. Sci. Food Agric. 2019;99:6392–6399. doi: 10.1002/jsfa.9918. [DOI] [PubMed] [Google Scholar]
- Sardar U.R., Bhargavi E., Devi I., Bhunia B., Tiwari O.N. Advances in exopolysaccharides based bioremediation of heavy metals in soil and water: a critical review. Carbohydr. Polym. 2018;199:353–364. doi: 10.1016/j.carbpol.2018.07.037. [DOI] [PubMed] [Google Scholar]
- Sathiyabama M., Manikandan A. Chitosan nanoparticle induced defense responses in fingermillet plants against blast disease caused by Pyricularia grisea (Cke.) Sacc. Carbohydr. Polym. 2016;154:241–246. doi: 10.1016/j.carbpol.2016.06.089. [DOI] [PubMed] [Google Scholar]
- Semyonov D., Ramon O., Shoham Y., shimoni E. Enzymatically synthesized dextran nanoparticles and their use as carriers for nutraceuticals. Food Funct. 2014;5:2463–2474. doi: 10.1039/c4fo00103f. [DOI] [PubMed] [Google Scholar]
- Siegrist M., Keller C., Kastenholz H., Frey S., Wiek A. Lay people's and experts' perception of nanotechnology hazards. Risk Anal. 2007;27:59–69. doi: 10.1111/j.1539-6924.2006.00859.x. [DOI] [PubMed] [Google Scholar]
- Silva N.H.C.S., Vilela C., Almeida A., Marrucho I.M., Freire C.S.R. Pullulan-based nanocomposite films for functional food packaging: exploiting lysozyme nanofibers as antibacterial and antioxidant reinforcing additives. Food Hydrocoll. 2018;77:921–930. [Google Scholar]
- Singh H. Nanotechnology applications in functional foods; opportunities and challenges. Prev. Nutr. Food Sci. 2016;21:1–8. doi: 10.3746/pnf.2016.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha T., Bhagwatwar P., Krishnamoorthy C., Chidambaram R. Polymer based micro- and nanoencapsulation of agrochemicals. In: Gutiérrez T.J., editor. Polymers for Agri-Food Applications. Springer International Publishing; 2019. pp. 5–28. [Google Scholar]
- Siracusa V. Microbial degradation of synthetic biopolymers waste. Polymers. 2019;11:1066. doi: 10.3390/polym11061066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo-Boyás M., Correa-Pacheco Z., Bautista-Baños S., Corona-Rangel M. Physicochemical characterization of chitosan nanoparticles and nanocapsules incorporated with lime essential oil and their antibacterial activity against food-borne pathogens. LWT. 2017;77:15–20. [Google Scholar]
- Sotelo-Boyás M., Correa-Pacheco Z., Bautista-Baños S., Y Gómez Y.G. Release study and inhibitory activity of thyme essential oil-loaded chitosan nanoparticles and nanocapsules against foodborne bacteria. Int. J. Biol. Macromol. 2017;103:409–414. doi: 10.1016/j.ijbiomac.2017.05.063. [DOI] [PubMed] [Google Scholar]
- Steinmetz Z., Wollmann C., Schaefer M., Buchmann C., David J., Tröger J., Muñoz K., Frör O., Schaumann G. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016;550:690–705. doi: 10.1016/j.scitotenv.2016.01.153. [DOI] [PubMed] [Google Scholar]
- Su Y., Ashworth V., Kim C., Adeleye A.S., Rolshausen P., Roper C., White J., Jassby D. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: a critical review and data analysis. Environ. Sci. Nano. 2019;6:2311–2331. [Google Scholar]
- Tan C., Xie J., Zhang X., Cai J., Xia S. Polysaccharide-based nanoparticles by chitosan and gum Arabic polyelectrolyte complexation as carriers for curcumin. Food Hydrocoll. 2016;57:236–245. [Google Scholar]
- Tang J., Hong J., Liu Y., Wang B., Hua Q., Liu L., Ying D. Urea controlled-release fertilizer based on gelatin microspheres. J. Polym. Environ. 2017;26:1930–1939. [Google Scholar]
- Teng Z., Xu R., Wang Q. Beta-lactoglobulin-based encapsulating systems as emerging bioavailability enhancers for nutraceuticals: a review. RSC Adv. 2015;5:35138–35154. [Google Scholar]
- Thombare N., Mishra S., Siddiqui M.Z., Jha U., Singh D., Mahajan G.R. Design and development of guar gum based novel, superabsorbent and moisture retaining hydrogels for agricultural applications. Carbohydr. Polym. 2018;185:169–178. doi: 10.1016/j.carbpol.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Tsang Y.F., Kumar V., Samadar P., Yang Y., Lee J., Ok Y.S., Song H., Kim K.-H., Kwon E.E., Jeon Y.J. Production of bioplastic through food waste valorization. Environ. Int. 2019;127:625–644. doi: 10.1016/j.envint.2019.03.076. [DOI] [PubMed] [Google Scholar]
- Valencia G.A., Zare E.N., Makvandi P., gutiérrez T.J. Self-assembled carbohydrate polymers for food applications: a review. Compr. Rev. Food Sci. Food Saf. 2019;18:2009–2024. doi: 10.1111/1541-4337.12499. [DOI] [PubMed] [Google Scholar]
- Wales Maurya S. AZoNano; 2019. Is Nanotechnology Found in Food?https://www.azonano.com/article.aspx?ArticleID=4839 [Google Scholar]
- Walia N., Dasgupta N., Ranjan S., Chen L., Ramalingam C. Fish oil based vitamin D nanoencapsulation by ultrasonication and bioaccessibility analysis in simulated gastro-intestinal tract. Ultrason. Sonochem. 2017;39:623–635. doi: 10.1016/j.ultsonch.2017.05.021. [DOI] [PubMed] [Google Scholar]
- Xing Y., Li W., Wang Q., Li X., Xu Q., Guo X., Bi X., Liu X., Shui Y., Lin H., Yang H. Antimicrobial nanoparticles incorporated in edible coatings and films for the preservation of fruits and vegetables. Molecules. 2019;24 doi: 10.3390/molecules24091695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz M.T., Yilmaz A., Akman P.K., Bozkurt F., Dertli E., Basahel A., Al-Sasi B., Taylan O., Sagdic O. Electrospraying method for fabrication of essential oil loaded-chitosan nanoparticle delivery systems characterized by molecular, thermal, morphological and antifungal properties. Innov. Food Sci. Emerging Tech. 2019;52:166–178. [Google Scholar]
- Zhang J., Field C.J., Vine D., Chen L. Intestinal uptake and transport of vitamin B 12-loaded soy protein nanoparticles. Pharm. Res. 2015;32:1288–1303. doi: 10.1007/s11095-014-1533-x. [DOI] [PubMed] [Google Scholar]