Abstract
Background
Benzodiazepine medications can be used to treat anxiety, a condition affecting 15% of women of childbearing age in the United States. Studies have shown conflicting results for the association between benzodiazepine use during pregnancy and birth defects.
Methods
We analyzed 1997–2011 data from the National Birth Defects Prevention Study, a multisite, population-based case–control study. We assessed the prevalence of and factors associated with benzodiazepine use in pregnancy among mothers of live-born infants without a birth defect (control mothers). We used logistic regression to estimate adjusted odds ratios (aORs) and 95% confidence intervals (CIs) for the association between specific birth defects and benzodiazepine use; we estimated crude odds ratios (cORs) for defect categories with 3–4 exposed cases.
Results
Exposure to benzodiazepines during pregnancy was rare (N = 93/11,614; 0.8%). Benzodiazepine use was more common among control mothers who were ≥30 years, non-Hispanic white, had more education, smoked, and took antidepressant medication. We observed significantly elevated ORs for any benzodiazepine and Dandy–Walker malformation (cOR: 3.1; 95% CI: 1.1, 8.6); for alprazolam and anophthalmia or microphthalmia (cOR: 4.0; 95% CI: 1.2, 13.1) and esophageal atresia or stenosis (aOR: 2.7; 95% CI: 1.2, 5.9); and lorazepam and pulmonary valve stenosis (cOR: 4.1; 95% CI: 1.2, 14.2), but sample sizes were limited and therefore CIs were wide.
Conclusions
Our findings suggest that benzodiazepines use is rare and may be associated with risk for certain birth defects. However, these results need replication and should be interpreted with caution.
Keywords: benzodiazepine, birth defect, medication, pregnancy
1 |. BACKGROUND
Anxiety disorders comprise a spectrum of conditions, including panic disorder, generalized anxiety disorder, obsessive–compulsive disorder, posttraumatic stress disorder, social anxiety disorder, and phobias. (ACOG Committee on Practice Bulletins—Obstetrics, 2008; American Psychiatric Association, 2000; National Institute of Mental Health, 2010). Anxiety disorders are common among women of childbearing age; in one study, the 12-month prevalence among nonpregnant women aged 18–50 years was ~15%. (Vesga-Lopez et al., 2008) Maternal anxiety disorders have been associated with adverse pregnancy outcomes, including spontaneous abortion, small for gestational age, preterm delivery, prolonged or precipitate labor, fetal distress, forceps delivery, and postpartum depression.(ACOG Committee on Practice Bulletins—Obstetrics, 2008; Chen, Lin, & Lee, 2010).
Anxiety disorders can be treated with several different types of medications, including benzodiazepines, (National Institute of Mental Health, 2010) which cross the placenta and are present in amniotic fluid. (McElhatton, 1994) Benzodiazepine use during pregnancy, while rare in recent years, (Hanley & Mintzes, 2014) has been associated with increased risk for Cesarean delivery, low birth weight, floppy infant syndrome, and neonatal abstinence syndrome. (ACOG Committee on Practice Bulletins—Obstetrics, 2008) Early studies in mice (Miller & Becker, 1975) and observational human studies in the 1970s (Safra & Oakley, 1975; Saxen & Saxen, 1975) suggested an increased risk for orofacial clefts with in-utero diazepam exposure. A subsequent case–control study designed to test the hypothesis did not find an increase in risks with narrow confidence bounds, (Rosenberg et al., 1983) nor did most subsequent studies in humans. (REPROTOX, 2017) However, there are few studies focused on birth defect risks associated with benzodiazepines in general and specific benzodiazepines in particular.
We analyzed data from the National Birth Defects Prevention Study (NBDPS) to assess the prevalence of benzodiazepine use during pregnancy and factors associated with its use. We also assessed the possible association between use of these medications in pregnancy and risk for birth defects.
2 |. METHODS
NBDPS is a population-based multisite case–control study of selected major structural birth defects. (Reefhuis et al., 2015) The analysis included data from pregnancies ending on or after October 1, 1997 and with estimated dates of delivery (EDDs) on or before December 31, 2011. Cases were ascertained from existing population-based birth defects surveillance systems at 10 sites. Four surveillance catchment areas included the entire state: Arkansas (1998–2011), Iowa (1997–2011), New Jersey (1998–2002), and Utah (2003–2011); six included selected counties: California (1997–2011), Georgia (1997–2011), Massachusetts (1997–2011), New York (1997–2002; 2004–2011), North Carolina (2003–2011), and Texas (1997–2011). Cases included pregnancies ending in live birth, fetal death, or induced termination, although not all pregnancy outcomes were ascertained by all sites throughout the study period. (Reefhuis et al., 2015) Eligible cases were identified by means of detailed, standardized case definitions; in addition, a clinical geneticist and/or an expert in pediatric cardiology reviewed the abstracted clinical information. (Rasmussen et al., 2003) Controls were live-born infants without a major birth defect identified from vital records or hospital birth logs from the same catchment area and time period. Institutional Review Boards at each study site approved the study and all participants provided informed consent.
Mothers of eligible cases and controls were asked to complete a computer-assisted telephone interview on a variety of topics relevant to exposures before and during pregnancy. As part of this interview, mothers were asked about medication use in the 3 months before pregnancy through the end of pregnancy. They were asked about several medical conditions (e.g., epilepsy) with follow-up questions about medications used to treat those conditions, which could have included benzodiazepines. However, mothers were not specifically asked whether they had anxiety disorders, nor were they specifically asked whether they used benzodiazepines. Benzodiazepine use could have been reported in response to a medication “catch all” question: “Between [date 3 months before conception] and [date of end of pregnancy] did you take any medications, remedies, or treatments that we have not already talked about?” If a mother reported use of a medication, she was asked to specify the start date and either the stop date or the duration of use, as well as the frequency.
In this analysis, we first assessed the prevalence of benzodiazepine use among control mothers any time in the month before through the end of pregnancy. We included the month prior to pregnancy in our exposure windows to account for potential mistiming in pregnancy date estimates. To identify associations with selected birth defects, we considered benzodiazepine use any time in the month before through the third month of pregnancy (hereafter, “first trimester”), the exposure window most relevant to teratogenesis. We excluded data from mothers if they reported taking a benzodiazepine but did not provide information allowing us to determine the timing of use (N = 6). We assessed exposure to any benzodiazepine as well as to the most-commonly reported specific benzodiazepines: alprazolam, clonazepam, diazepam, and lorazepam. For the birth defect association analysis, we further excluded data from mothers who reported exposure to a benzodiazepine medication only outside the exposure window of interest; the referent group included only mothers who had no exposure to any benzodiazepine medication in the 3 months before through the end of pregnancy. In this part of the analysis, we also excluded data from mothers who reported use of an antiepileptic medication other than a benzodiazepine in the month before through the third month of pregnancy to control for potential confounding since antiepileptic medications are strongly associated with birth defects. (Gilboa et al., 2011) For analyses of hypospadias, we included only male control infants.
We assessed the distribution of several variables in relation to benzodiazepine use among control mothers: maternal age at conception (12–17 years, 18–29 years, ≥30 years), maternal race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other or more than one race/ethnicity), maternal education (<12 years, 12 years, >12 years), category of maternal prepregnancy body mass index (BMI; kg/m2; <18.5, 18.5–24.9, 25.0–29.9, ≥30), year of EDD, and any maternal cigarette smoking, antidepressant medication use, or antiepileptic medication use (excluding benzodiazepines) in the month before through the end of pregnancy. We used Chi-square tests to obtain p-values for the comparisons. We also assessed prevalence of use of any benzodiazepine and specific benzodiazepines by month of pregnancy.
We calculated odds ratios (ORs) and 95% confidence intervals (CIs) for the association between use of any benzodiazepine and specific benzodiazepines and risks for specific birth defects included in NBDPS for which there were at least three exposed case mothers. For associations with ≥5 exposed cases we adjusted for variables selected a priori as those most likely to be confounders: maternal age at conception (continuous), race/ethnicity (non-Hispanic white vs. any other race/ethnicity), smoking during the month before through the third month of pregnancy (any vs. none), and antidepressant medication use in the month before through the third month of pregnancy (any vs. none). For associations for which there were three or four exposed cases, we report only unadjusted (crude) ORs. We conducted a sensitivity analysis in which we included only isolated defects (i.e., defects that occurred in the absence of any other major defects; Rasmussen et al., 2003).
All analyses were conducted with SAS 9.4 (SAS Institute, Cary, NC).
3 |. RESULTS
Exposure to a benzodiazepine at any time during the month before through the end of pregnancy was rare, reported by only 93 (0.8%) of 11,614 control mothers in NBDPS (Table 1). Alprazolam was the most commonly reported benzodiazepine, reported by almost half of control mothers who reported any benzodiazepine use (N = 43), followed by clonazepam (N = 22), diazepam (N = 21), and lorazepam (N = 10). The only other benzodiazepines reported by controls mothers were chlorazepate dipotassium, triazolam, and oxazepam, each reported only once.
TABLE 1.
Characteristics of control mothers by report of benzodiazepine medication use any time during the month before through the end of pregnancy, National Birth Defects Prevention Study, 1997–2011
| No benzodiazepine exposure |
Any benzodiazepinea |
Alprazolam |
Clonazepam |
Diazepam |
Lorazepam |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | p-value | N | % | p-value | N | % | p-value | N | % | p-value | N | % | p-value | |
| Totalb | 11,521 | 93 | 0.8 | 43 | 0.4 | 22 | 0.2 | 21 | 0.2 | 10 | 0.1 | ||||||
| Age group (years) | |||||||||||||||||
| 12–17 | 584 | 5.1 | 2 | 2.2 | 0.004 | 1 | 2.3 | 0.416 | 1 | 4.5 | 0.069 | 1 | 4.8 | 0.272 | 0 | 0.0 | 0.274 |
| 18–29 | 6,842 | 59.4 | 43 | 46.2 | 23 | 53.5 | 8 | 36.4 | 9 | 42.9 | 4 | 40.0 | |||||
| ≥30 | 4,095 | 35.5 | 48 | 51.6 | 19 | 44.2 | 13 | 59.1 | 11 | 52.4 | 6 | 60.0 | |||||
| Race/ethnicity | |||||||||||||||||
| Non-Hispanic white | 6,676 | 58.0 | 77 | 82.8 | <0.001 | 38 | 88.4 | <0.001 | 19 | 86.4 | 0.061 | 17 | 81.0 | 0.182 | 6 | 60.0 | 0.112 |
| Non-Hispanic black | 1,260 | 10.9 | 4 | 4.3 | 0 | 0.0 | 1 | 4.5 | 1 | 4.8 | 2 | 20.0 | |||||
| Hispanic | 2,825 | 24.5 | 7 | 7.5 | 2 | 4.7 | 2 | 9.1 | 3 | 14.3 | 0 | 0.0 | |||||
| Other race/ethnicity | 754 | 6.6 | 5 | 5.4 | 3 | 7.0 | 0 | 0.0 | 0 | 0.0 | 2 | 20.0 | |||||
| Maternal education (years) | |||||||||||||||||
| <12 | 1,899 | 16.7 | 5 | 5.4 | <0.001 | 4 | 9.5 | 0.092 | 1 | 4.5 | 0.187 | 0 | 0.0 | 0.018 | 0 | 0.0 | 0.300 |
| 12 | 2,711 | 23.8 | 14 | 15.2 | 6 | 14.3 | 4 | 18.2 | 2 | 10.0 | 2 | 20.0 | |||||
| >12 | 6,781 | 59.5 | 73 | 79.3 | 32 | 76.2 | 17 | 77.3 | 18 | 90.0 | 8 | 80.0 | |||||
| Body mass index category | |||||||||||||||||
| Underweight (<18.5 kg/m2) | 586 | 5.3 | 7 | 7.6 | 0.434 | 2 | 4.7 | 0.700 | 3 | 13.6 | 0.058 | 1 | 5.0 | 0.797 | 1 | 10.0 | 0.426 |
| Normal weight (18.5–24.9 kg/m2) | 5,925 | 53.7 | 43 | 46.7 | 24 | 55.8 | 6 | 27.3 | 11 | 55.0 | 3 | 30.0 | |||||
| Overweight (25.0–29.9 kg/m2) | 2,506 | 22.7 | 21 | 22.8 | 7 | 16.3 | 7 | 31.8 | 3 | 15.0 | 4 | 40.0 | |||||
| Obese (≥30 kg/m2) | 2,022 | 18.3 | 21 | 22.8 | 10 | 23.3 | 6 | 27.3 | 5 | 25.0 | 2 | 20.0 | |||||
| Smoking during pregnancyc | |||||||||||||||||
| Any | 2,064 | 18.1 | 30 | 32.6 | <0.001 | 20 | 47.6 | <0.001 | 4 | 18.2 | 0.999 | 4 | 20.0 | 0.832 | 3 | 30.0 | 0.332 |
| None | 9,368 | 82.0 | 62 | 67.4 | 22 | 52.4 | 18 | 81.8 | 16 | 80.0 | 7 | 70.0 | |||||
| Year of estimated date of delivery | |||||||||||||||||
| 1997–1999d | 1,696 | 14.7 | 15 | 16.1 | 0.685 | 5 | 11.6 | 0.571 | 2 | 9.1 | 0.113 | 6 | 28.6 | 0.409 | 2 | 20.0 | 0.378 |
| 2000–2001 | 1,680 | 14.6 | 8 | 8.6 | 4 | 9.3 | 2 | 9.1 | 1 | 4.8 | 0 | 0.0 | |||||
| 2002–2003 | 1,572 | 13.6 | 12 | 12.9 | 8 | 18.6 | 3 | 13.6 | 1 | 4.8 | 0 | 0.0 | |||||
| 2004–2005 | 1,722 | 15.0 | 18 | 19.4 | 9 | 20.9 | 3 | 13.6 | 4 | 19.0 | 3 | 30.0 | |||||
| 2006–2007 | 1,659 | 14.4 | 15 | 16.1 | 8 | 18.6 | 2 | 9.1 | 4 | 19.0 | 3 | 30.0 | |||||
| 2008–2009 | 1,634 | 14.2 | 12 | 12.9 | 6 | 14.0 | 2 | 9.1 | 3 | 14.3 | 1 | 10.0 | |||||
| 2010–2011 | 1,558 | 13.5 | 13 | 14.0 | 3 | 7.0 | 8 | 36.4 | 2 | 9.5 | 1 | 10.0 | |||||
| Antidepressant medication use during pregnancyc | |||||||||||||||||
| Any | 528 | 4.6 | 38 | 40.9 | <0.001 | 17 | 39.5 | <0.001 | 14 | 63.6 | <0.001 | 6 | 28.6 | <0.001 | 5 | 50.0 | <0.001 |
| None | 10,985 | 95.4 | 55 | 59.1 | 26 | 60.5 | 8 | 36.4 | 15 | 71.4 | 5 | 50.0 | |||||
| Antiepileptic medication use during pregnancyc,e | |||||||||||||||||
| Any | 67 | 0.6 | 3 | 3.2 | 0.005 | 1 | 2.3 | 0.343 | 0 | 0.0 | 0.935 | 1 | 4.8 | 0.048 | 1 | 10.0 | <0.001 |
| None | 11,453 | 99.4 | 90 | 96.8 | 42 | 97.7 | 22 | 100.0 | 20 | 95.2 | 9 | 90.0 | |||||
Six mothers reported use of two different benzodiazepines during the month before pregnancy; this category includes exposures to chlorazepate dipotassium, triazolam, and oxazepam, each reported only once.
Row percentages are presented for the total; otherwise, column percentages are presented. Numbers may not add up to the total due to missing values.
Defined as the month before through the end of pregnancy.
1997 was a partial year of eligibility corresponding to the beginning of the study period (October 1).
Excluding benzodiazepines.
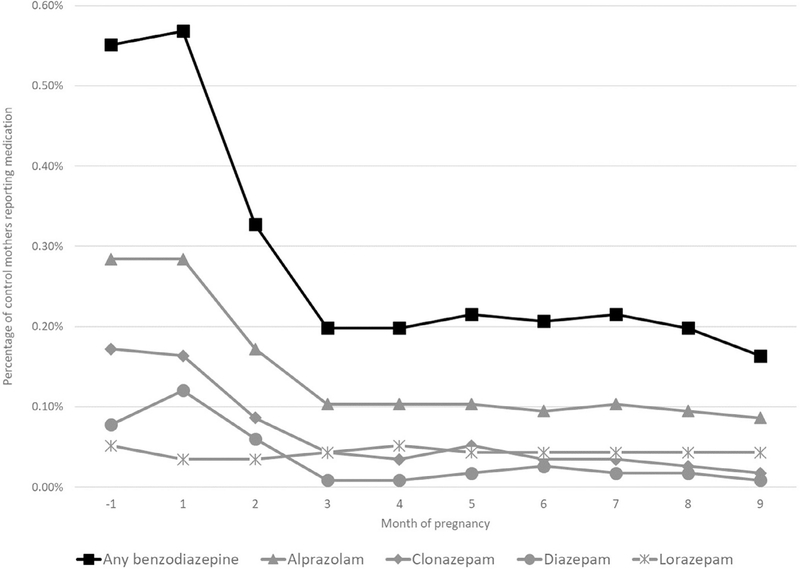
Use of any benzodiazepine among control mothers was highest during the month before (N = 64; 0.6%) and the first month (N = 66; 0.6%) of pregnancy (Figure 1), declining to 0.2% during the third month, where it remained for the duration of pregnancy. This pattern was observed for each of the specific benzodiazepines examined except for lorazepam, which had consistently low prevalence of use for all months of pregnancy.
FIGURE 1.
Prevalence of reported use of any benzodiazepine by month of pregnancy among control mothers in the National Birth Defects Prevention Study, 1997–2011
Relative to unexposed control mothers, control mothers who reported use of any benzodiazepine were more likely to be older (≥30 years), non-Hispanic white, have more than 12 years of education, and to have smoked during pregnancy. Among control mothers who reported benzodiazepine use, 38 (40.9%) reported use of an antidepressant, the majority of which were selective serotonin reuptake inhibitors (SSRIs; n = 33, data not shown). Benzodiazepines can be used to treat epilepsy; however, of the 93 control mothers who reported benzodiazepine medication use, only three also reported use of other antiepileptic medications.
We assessed 18 nonmutually exclusive categories of noncardiac birth defects (Table 2). Compared to mothers who reported no use in pregnancy, reported benzodiazepine use during the first trimester was associated with an elevated risk for Dandy–Walker malformation (crude OR [cOR]: 3.1; 95% CI: 1.1, 8.6), anophthalmia or microphthalmia (cOR: 2.5; 95% CI: 0.9, 6.9), and esophageal atresia or stenosis (adjusted OR [aOR]: 1.7; 95% CI: 0.9, 3.3). We observed inverse associations of first-trimester benzodiazepine use with hypospadias (aOR: 0.4; 95% CI: 0.2, 0.8) and craniosynostosis (aOR: 0.6; 95% CI: 0.3, 1.1). Exposure to alprazolam appeared to drive the observed associations with anophthalmia or microphthalmia (cOR: 4.0; 95% CI: 1.2, 13.1), esophageal atresia or stenosis (aOR: 2.7; 95% CI: 1.2, 5.9), and hypospadias (aOR: 0.3; 95% CI: 0.1, 0.9). We also observed an elevated OR for lorazepam exposure and risk for gastroschisis (cOR: 3.5; 95% CI: 0.9, 13.7).
TABLE 2.
Odds ratiosa for exposure to benzodiazepine medication in the month before through the third month of pregnancy and risk for specific noncardiac birth defects, National Birth Defects Prevention Study, 1997–2011
| Any benzodiazepine |
Alprazolam |
Clonazepam |
Diazepam |
Lorazepam |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Defect category | Total N | N | OR [95% CI] | N | OR [95% CI] | N | OR [95%CI] | N | OR [95% CI] | N | OR [95%CI] |
| Controls | 11,533 | 81 | Reference | 38 | Reference | 21 | Reference | 18 | Reference | 7 | Reference |
| Any neural tube defect | 2,105 | 14 | 1.0 [0.6, 1.8] | 8 | 1.3 [0.6, 2.9] | 5 | 1.3 [0.5, 3.6] | 1 | – | 2 | – |
| Anencephaly | 638 | 5 | 1.3 [0.5, 3.2] | 2 | – | 2 | – | 1 | – | 2 | – |
| Spina bifida | 1,241 | 7 | 0.8 [0.4, 1.8] | 4 | 1.0 [0.3, 2.7] | 3 | 1.3 [0.4, 4.4] | 0 | – | 0 | – |
| Hydrocephaly | 513 | 3 | 0.8 [0.3, 2.6] | 2 | – | 1 | – | 1 | – | 0 | – |
| Dandy–Walker malformation | 185 | 4 | 3.1 [1.1, 8.6] | 2 | – | 1 | – | 0 | – | 1 | – |
| Anophthalmia/microphthalmia | 230 | 4 | 2.5 [0.9, 6.9] | 3 | 4.0 [1.2, 13.1] | 0 | – | 0 | – | 1 | – |
| Anotia/microtia | 685 | 6 | 1.6 [0.7, 3.8] | 1 | – | 3 | 2.4 [0.7, 8.1] | 1 | – | 1 | – |
| Cleft lip with or without cleft palate | 3,086 | 27 | 1.2 [0.7, 1.8] | 11 | 1.0 [0.5, 1.9] | 6 | 1.0 [0.4, 2.6] | 5 | 1.1 [0.4, 2.9] | 4 | 2.1 [0.6, 7.2] |
| Cleft palate alone | 1,578 | 15 | 1.2 [0.7, 2.1] | 9 | 1.5 [0.7, 3.1] | 5 | 1.6 [0.6, 4.2] | 0 | – | 1 | – |
| Esophageal atresia/stenosis | 753 | 11 | 1.7 [0.9, 3.3] | 8 | 2.7 [1.2, 5.9] | 1 | – | 1 | – | 1 | – |
| Anorectal atresia/stenosis | 1,066 | 6 | 0.7 [0.3, 1.7] | 4 | 1.1 [0.4, 3.2] | 2 | – | 0 | – | 0 | – |
| Hypospadias | 2,540 | 12 | 0.4 [0.2, 0.8] | 5 | 0.3 [0.1, 0.9] | 5 | 0.8 [0.2, 2.7] | 0 | – | 2 | – |
| Preaxial longitudinal limb deficiency | 273 | 3 | 1.6 [0.5, 5.0] | 1 | – | 0 | – | 1 | – | 1 | – |
| Transverse limb deficiency | 710 | 4 | 0.8 [0.3, 2.2] | 1 | – | 2 | – | 0 | – | 1 | – |
| Craniosynostosis | 1,579 | 9 | 0.6 [0.3, 1.1] | 2 | – | 3 | 1.0 [0.3, 3.5] | 1 | – | 2 | – |
| Diaphragmatic hernia | 867 | 4 | 0.7 [0.2, 1.8] | 0 | – | 1 | – | 2 | – | 0 | – |
| Omphalocele | 434 | 3 | 1.0 [0.3, 3.1] | 2 | – | 0 | – | 1 | – | 0 | – |
| Gastroschisis | 1,400 | 10 | 1.3 [0.7, 2.7] | 4 | 0.9 [0.3, 2.4] | 2 | – | 1 | – | 3 | 3.5 [0.9, 13.7] |
Abbreviations. CI: confidence interval; OR: odds ratio; Ref: reference category.
Odds ratios for case groups for which there were at least five exposed cases were adjusted for maternal age at conception (continuous), race/ethnicity (non-Hispanic white vs. any other race/ethnicity), smoking during the month before through the third month of pregnancy (any vs. none), and antidepressant medication use in the month before through the third month of pregnancy (any vs. none). Crude odds ratios (italicized) were estimated for case groups for which there were three or four exposed cases. Odds ratios were not estimated for case groups for which there were fewer than three exposed cases. Women may have reported taking ≥1 type of benzodiazepine medication.
Similarly, we assessed 15 nonmutually exclusive categories of congenital heart defects (CHDs; Table 3). ORs for any benzodiazepine use during the first trimester were largely consistent with no association, except for an increased risk for pulmonary valve stenosis with atrial septal defect (cOR: 2.2; 95% CI: 0.8, 6.1), for which the estimate was relatively unstable. Among specific benzodiazepines, alprazolam use was associated with higher odds of atrioventricular septal defect (AVSD; cOR: 2.5; 95% CI: 0.8, 8.1), and lorazepam use was associated with higher odds of right ventricular outflow obstruction defects (cOR: 3.2; 95% CI: 0.9, 10.9), specifically pulmonary valve stenosis (cOR: 4.1; 95% CI: 1.2, 14.2).
TABLE 3.
Odds ratiosa for exposure to benzodiazepine medication in the month before through the third month of pregnancy and risk for specific cardiac birth defects, National Birth Defects Prevention Study, 1997–2011
| Defect category | Total N | Any benzodiazepine |
Alprazolam |
Clonazepam |
Diazepam |
Lorazepam |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | OR [95% CI] | N | OR [95% CI] | N | OR [95%CI] | N | OR [95% CI] | N | OR [95%CI] | ||
| Controls | 11,533 | 81 | Reference | 38 | Reference | 21 | Reference | 18 | Reference | 7 | Reference |
| Any NBDPS congenital heart defect [CHD] | 12,198 | 90 | 0.9 [0.6, 1.2] | 32 | 0.7 [0.4, 1.1] | 23 | 0.8 [0.5, 1.5] | 17 | 0.8 [0.4, 1.6] | 14 | 1.4 [0.6, 3.7] |
| Conotruncal CHD | 2,593 | 23 | 1.0 [0.6, 1.6] | 5 | 0.5 [0.2, 1.3] | 8 | 1.3 [0.6, 2.9] | 5 | 1.1 [0.4, 3.1] | 5 | 1.8 [0.5, 6.4] |
| D-transposition of the great arteries | 765 | 6 | 0.9 [0.4, 2.2] | 0 | – | 2 | – | 2 | – | 2 | – |
| Tetralogy of Fallot | 1,196 | 14 | 1.3 [0.7, 2.4] | 5 | 1.2 [0.4, 3.0] | 5 | 1.8 [0.7, 4.8] | 2 | – | 2 | – |
| Septal CHD | 4,673 | 34 | 1.0 [0.6, 1.5] | 13 | 0.8 [0.4, 1.5] | 10 | 1.1 [0.5, 2.3] | 5 | 0.7 [0.3, 1.9] | 3 | 1.1 [0.3, 4.1] |
| Perimembranous ventricular septal defect | 1,648 | 11 | 0.9 [0.5, 1.7] | 6 | 1.0 [0.4, 2.5] | 4 | 1.3 [0.5, 3.9] | 1 | – | 0 | – |
| Atrial septal defect [ASD] secundum or NOS | 3,025 | 26 | 1.2 [0.7, 1.8] | 12 | 1.1 [0.6, 2.1] | 4 | 0.7 [0.2, 2.1] | 4 | 0.8 [0.3, 2.5] | 3 | 1.6 [0.4, 6.3] |
| Atrioventricular septal defect | 369 | 6 | 1.9 [0.8, 4.5] | 3 | 2.5 [0.8, 8.1] | 2 | – | 2 | – | 0 | – |
| Right ventricular outflow tract obstruction | 2,080 | 19 | 1.0 [0.6, 1.7] | 6 | 0.7 [0.3, 1.6] | 5 | 1.0 [0.4, 2.7] | 3 | 0.9 [0.3, 3.1] | 4 | 3.2 [0.9, 10.9] |
| Pulmonary valve stenosis [PVS] | 1,532 | 17 | 1.1 [0.7, 1.9] | 4 | 0.8 [0.3, 2.1] | 5 | 1.2 [0.5, 3.4] | 3 | 1.2 [0.4, 4.1] | 4 | 4.1 [1.2, 14.2] |
| Left ventricular outflow tract obstruction | 2,216 | 12 | 0.6 [0.3, 1.1] | 5 | 0.6 [0.2, 1.4] | 3 | 0.7 [0.2, 2.5] | 2 | – | 2 | – |
| Coarctation of the aorta | 1,153 | 6 | 0.6 [0.3, 1.5] | 2 | – | 1 | – | 1 | – | 2 | – |
| Aortic stenosis | 510 | 4 | 1.1 [0.4, 3.1] | 2 | – | 1 | – | 0 | – | 1 | – |
| Ventricular septal defect and ASD | 757 | 7 | 1.3 [0.6, 2.9] | 4 | 1.6 [0.6, 4.5] | 1 | – | 2 | – | 0 | – |
| PVS and ASD | 258 | 4 | 2.2 [0.8, 6.1] | 1 | – | 2 | – | 0 | – | 1 | – |
Abbreviations. CI: confidence interval; NOS: not otherwise specified; OR: odds ratio; Ref: reference category.
Odds ratios for case groups for which there were at least five exposed cases were adjusted for maternal age at conception (continuous), race/ethnicity (non-Hispanic white vs. any other race/ethnicity), smoking during the month before through the third month of pregnancy (any vs. none), and antidepressant medication use in the month before through the third month of pregnancy (any vs. none). Crude odds ratios (italicized) were estimated for case groups for which there were three or four exposed cases. Odds ratios were not estimated for case groups for which there were fewer than three exposed cases. Women may have reported taking ≥1 type of benzodiazepine medication.
Associations between maternal benzodiazepine exposures and risk for isolated defects were generally similar to those observed for all defects, although for a few of the associations examined, the point estimate for isolated defects was markedly further from the null (Supporting Information Table S1). However, these estimates had wider CIs due to smaller sample size.
4 |. DISCUSSION
Benzodiazepine medication use during pregnancy was rare during the years of our study, reported by less than 1 % of mothers of control infants. Although anxiety disorders are common, benzodiazepines are only one type of medication that can be used to treat these conditions. The prevalence of use for alprazolam (0.4%), diazepam (0.2%), and lorazepam (0.1%) observed in our analysis were generally consistent with those observed in a study of United States pregnant women with private insurance for the years 2006–2011 (Hanley & Mintzes, 2014).
The American College of Obstetricians and Gynecologists recommends that decisions regarding mental health treatment during pregnancy be made jointly between a woman and her mental and obstetrical health providers prior to pregnancy. (ACOG Committee on Practice Bulletins—Obstetrics, 2008) The drop in benzodiazepine use that we observed for the second and third months of pregnancy, corresponding to the common timing of pregnancy recognition, (Branum & Ahrens, 2017) suggests that there is a reduction in benzodiazepine use upon pregnancy recognition; however we have no data regarding provider engagement in informing these changes. Factors potentially influencing this reduction in use include independent decision-making by the woman upon realizing that she is pregnant and high rates of unintended pregnancy, which accounts for almost half of pregnancies in the United States, that may not allow for preconception counseling in regards to benzodiazepine use (Finer & Zolna, 2016). Abrupt discontinuation of benzodiazepine use in general, (Rickels, Schweizer, Case, & Greenblatt, 1990) and specifically during pregnancy, (Einarson, Selby, & Koren, 2001) has been associated with adverse psychological effects for the user.
Our data do not support an association between use of benzodiazepines and increased risk for orofacial clefts, although we lacked statistical power to assess the relationship between diazepam or lorazepam and risk for cleft palate alone specifically. The most frequently studied association in the literature is for diazepam in relation to oral clefts; we observed an OR of 1.1 (95% CI: 0.6, 7.2) for the association between diazepam exposure and risk for cleft lip with or without cleft palate, which is similar to the estimate of 1.0 (95% CI: 0.5, 2.1) observed in another large case–control study designed specifically to test this association (Rosenberg et al., 1983). While we had insufficient data to assess the association of diazepam with cleft palate alone, that same study reported an OR of 0.8 (95% CI: 0.3, 2.7). For alprazolam and clonazepam, we observed slightly elevated OR point estimates for cleft palate alone, as we did for lorazepam in relation to cleft lip with or without cleft palate, but the CIs were wide and consistent with the null.
Previous studies have generally lacked sufficient statistical power to assess associations between specific benzodiazepine medications and specific birth defects other than oral clefts. Data from the Swedish Medical Birth Register from 1996 to 2011 showed an association between alprazolam use and the broad category of all cardiac defects (OR: 2.4; 95% CI: 1.4, 4.2; Kallen, Borg, & Reis, 2013). The only modestly increased risk for alprazolam we observed for CHDs was for AVSD. We did observe a statistically increased OR for pulmonary valve stenosis for mothers who reported lorazepam use. Data from a UK cohort showed no association between maternal first trimester prescriptions for diazepam and the broad categories of any birth defect, heart defects, limb defects, or genital system defects (Ban et al., 2014).
Healthcare utilization data in British Columbia were used to assess individual and joint associations of benzodiazepines and antidepressant medications (specifically serotonin reuptake inhibitors) and risk for birth defects (Oberlander et al., 2008). That study found no associations for benzodiazepine use alone; however, an increased risk for the broad category of cardiac malformation was observed for combined use of both medications (risk ratio ~3.5). Another study, using 1995–2008 data from the Swedish Medical Birth Register, reported no associations for individual or joint exposure to SSRIs and benzodiazepines and the broad categories of any major birth defect or any heart defect (Reis & Kallen, 2013). We observed no difference in OR estimates for benzodiazepine use and risk for the combined category of all NBDPS CHDs stratified by antidepressant medication use (both ORs = 0.9), although our sample size was small, leading to imprecise estimates (Supporting Information Table S2).
Our results should be interpreted within the context of certain limitations. Although collectively major birth defects are common, affecting ~3% of births in the United States, (Centers for Disease Control and Prevention, 2008) specific birth defects are rare. Benzodiazepine medication exposure was also relatively rare in this population. We therefore had limited statistical power to assess many of the associations with specific birth defects, resulting in unstable OR estimates with wide CIs for some associations and an inability to estimate ORs for all associations of interest.
We assessed many possible associations and some of those we observed may be attributable to chance. In addition, because we required a minimum number of exposed cases in order to promote more stable OR estimation, we were more likely to observe OR point estimates above the null based on random variation. Although we were able to adjust for important confounders in some of our models, we lacked sufficient statistical power to use multivariable models for all defect categories. In addition, there may be residual confounding due to variables not included in the model, including confounding by indication.
While some of our analyses had limited statistical power, NBDPS is one of the largest studies of its kind and many of the associations we assessed have not previously been investigated. When possible, it is important to assess specific birth defects, rather than large heterogeneous groupings, because teratogens rarely increase risk for all congenital malformations and associations with specific defects can be obscured. (Tinker et al., 2015) An additional strength of NBDPS data is rigorous clinical review of birth defect cases, (Rasmussen et al., 2003) which decreases the chance for outcome misclassification that can occur when case definitions use only diagnosis codes, as might occur in administrative healthcare databases.
We were not able to assess the potential impact of different benzodiazepine doses or indications for use, as NBDPS did not collect this information. However, our exposure assessment benefitted from maternal report of benzodiazepine use, rather than relying on prescription records. Particularly for medications that can be taken episodically, such as benzodiazepines, the presence of a prescription does not indicate whether someone took the prescribed medication or when they took it. Differences in the accuracy of exposure assessment and outcome definition may explain the disparate results with previous studies described above. Although information about the frequency of benzodiazepine use was available, we were not able to assess whether associations differed based on frequency of use due to limited sample size.
Our findings suggest that use of some specific benzodiazepines may be associated with modestly increased risks for certain birth defects, but these results need to be replicated in other studies. Even if the observed associations were causal, the absolute risk for individual defects is low, and the absolute risk for these defects would still be low among mothers with benzodiazepine exposure. Additional research is needed on the potential impact of both benzodiazepine exposure and untreated anxiety disorder during pregnancy on the outcomes for the infant and mother.
Supplementary Material
ACKNOWLEDGMENT
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Coding of drug information in the National Birth Defects Prevention Study used the Slone Drug Dictionary under license from the Slone Epidemiology Center of Boston University.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- ACOG Committee on Practice Bulletins—Obstetrics. (2008). ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstetrics and Gynecology, 111(4), 1001–1020. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: Author. [Google Scholar]
- Ban L, West J, Gibson JE, Fiaschi L, Sokal R, Doyle P, … Tata LJ (2014). First trimester exposure to anxiolytic and hypnotic drugs and the risks of major congenital anomalies: A United Kingdom population-based cohort study. PLoS One, 9(6), e100996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branum AM, & Ahrens KA (2017). Trends in timing of pregnancy awareness among US women. Maternal and Child Health Journal, 21(4), 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2008). Update on overall prevalence of major birth defects—Atlanta, Georgia, 1978–2005. MMWR. Morbidity and Mortality Weekly Report, 57(1), 1–5. [PubMed] [Google Scholar]
- Chen YH, Lin HC, & Lee HC (2010). Pregnancy outcomes among women with panic disorder—Do panic attacks during pregnancy matter? Journal of Affective Disorders, 120(1–3), 258–262. [DOI] [PubMed] [Google Scholar]
- Einarson A, Selby P, & Koren G (2001). Abrupt discontinuation of psychotropic drugs during pregnancy: Fear of teratogenic risk and impact of counselling. Journal of Psychiatry & Neuroscience, 26(1), 44–48. [PMC free article] [PubMed] [Google Scholar]
- Finer LB, & Zolna MR (2016). Declines in unintended pregnancy in the United States, 2008–2011. The New England Journal of Medicine, 374(9), 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa SM, Broussard CS, Devine OJ, Duwe KN, Flak AL, Boulet SL, … Honein MA (2011). Influencing clinical practice regarding the use of antiepileptic medications during pregnancy: Modeling the potential impact on the prevalences of spina bifida and cleft palate in the United States. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics, 157C(3), 234–246 157. [DOI] [PubMed] [Google Scholar]
- Hanley GE, & Mintzes B (2014). Patterns of psychotropic medicine use in pregnancy in the United States from 2006 to 2011 among women with private insurance. BMC Pregnancy and Childbirth, 14, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen B, Borg N, & Reis M (2013). The use of central nervous system active drugs during pregnancy. Pharmaceuticals (Basel), 6(10), 1221–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhatton PR (1994). The effects of benzodiazepine use during pregnancy and lactation. Reproductive Toxicology, 8(6), 461–475. [DOI] [PubMed] [Google Scholar]
- Miller RP, & Becker BA (1975). Teratogenicity of oral diazepam and diphenylhydantoin in mice. Toxicology and Applied Pharmacology, 32(1), 53–61. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health. (2010). Mental Health Medications. Bethesda, MD: Author. [Google Scholar]
- Oberlander TF, Warburton W, Misri S, Riggs W, Aghajanian J, & Hertzman C (2008). Major congenital malformations following prenatal exposure to serotonin reuptake inhibitors and benzodiazepines using population-based health data. Birth Defects Research. Part B, Developmental and Reproductive Toxicology, 83(1), 68–76. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, Moore CA, & National Birth Defects Prevention Study. (2003). Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Research Part A, Clinical and Molecular Teratology, 67(3), 193–201. [DOI] [PubMed] [Google Scholar]
- Reefhuis J, Gilboa SM, Anderka M, Browne ML, Feldkamp ML, Hobbs CA, … National Birth Defects Prevention Study. (2015). The National Birth Defects Prevention Study: A review of the methods. Birth Defects Research Part A, Clinical and Molecular Teratology, 103(8), 656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis M, & Kallen B (2013). Combined use of selective serotonin reuptake inhibitors and sedatives/hypnotics during pregnancy: Risk of relatively severe congenital malformations or cardiac defects. A register study. BMJ Open, 3(2), e002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REPROTOX Reproductive Hazard Information Database provider: RightAnswer. 2017. Diazepam; http://www.rightanswerknowledge.com/N1serveFile_new.asp?l=D&searchDB=RX&url=1138. [Google Scholar]
- Rickels K, Schweizer E, Case WG, & Greenblatt DJ (1990). Long-term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Archives of General Psychiatry, 47(10), 899–907. [DOI] [PubMed] [Google Scholar]
- Rosenberg L, Mitchell AA, Parsells JL, Pashayan H, Louik C, & Shapiro S (1983). Lack of relation of oral clefts to diazepam use during pregnancy. The New England Journal of Medicine, 309(21), 1282–1285. [DOI] [PubMed] [Google Scholar]
- Safra MJ, & Oakley GP Jr. (1975). Association between cleft lip with or without cleft palate and prenatal exposure to diazepam. Lancet, 2(7933), 478–480. [DOI] [PubMed] [Google Scholar]
- Saxen I, & Saxen L (1975). Letter: Association between maternal intake of diazepam and oral clefts. Lancet, 2(7933), 498. [DOI] [PubMed] [Google Scholar]
- Tinker SC, Gilboa S, Reefhuis J, Jenkins MM, Schaeffer M, & Moore CA (2015). Challenges in studying modifiable risk factors for birth defects. Current Epidemiology Reports, 2(1), 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesga-Lopez O, Blanco C, Keyes K, Olfson M, Grant BF, & Hasin DS (2008). Psychiatric disorders in pregnant and postpartum women in the United States. Archives of General Psychiatry, 65(7), 805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.