Abstract
Cystic neoplasms arising from the prostate are rare, and stromal tumours of uncertain malignant potential and the spectrum of cystic epithelial tumours of the prostate are the major differential diagnoses of a cystic prostatic neoplasm. We report a case of a stromal tumour of uncertain malignant potential, which showed a multilocular cystic mass with some solid components. The solid component of the tumour did not show substantial diffusion restriction and uptake of 18F-FDG-PET, and this could be the critical finding suggesting a stromal tumour of uncertain malignant potential rather than a malignant cystic epithelial tumour.
Abbreviations: STUMP, stromal tumour of uncertain malignant potential of the prostate; PSA, prostate-specific antigen; BPH, benign prostate hyperplasia
Keywords: Stromal tumour of uncertain malignant potential, prostate, multilocular cysts, differential diagnosis
1. Introduction
Prostatic and periprostatic cystic lesions are a characteristic of various diseases of both neoplastic and non-neoplastic lesions. Non-neoplastic lesions include Müllerian duct cysts, prostatic utricle cysts, retention cysts, paramedian cysts, infectious cysts, and seminal vesicle cysts [1]. Although non-neoplastic cystic lesions are common, neoplastic cystic lesions arising from the prostate are rarely reported [2,3]. Recent studies have proposed that a spectrum of cystic epithelial tumours arise from the prostate glandular epithelium with multilocular cysts macroscopically and with malignant potential [4]. On the other hand, the stromal tumour of uncertain malignant potential of the prostate (STUMP) is a known prostatic mesenchymal tumour with multilocular cyst characteristics [5]. Previously, cystic epithelial tumours and STUMPs have been confused. However, current evidence shows that the diseases differ pathologically. Radiological reports that discuss these diseases are rare, and differentiating between the diseases is challenging. Here, we report a case of STUMP demonstrating a multilocular cystic prostatic mass radiologically.
2. Case report
A 68-year-old man complained of acute urinary retention after urethral catheter removal following an endoscopic submucosal dissection of early gastric cancer. Laboratory tests showed an elevated prostate-specific antigen (PSA) level with a peak of 6.25 ng/mL and carbohydrate antigen 19-9 up to 239.9 U/ml. He was non-febrile and had a normal C-reactive protein level and white blood cell count.
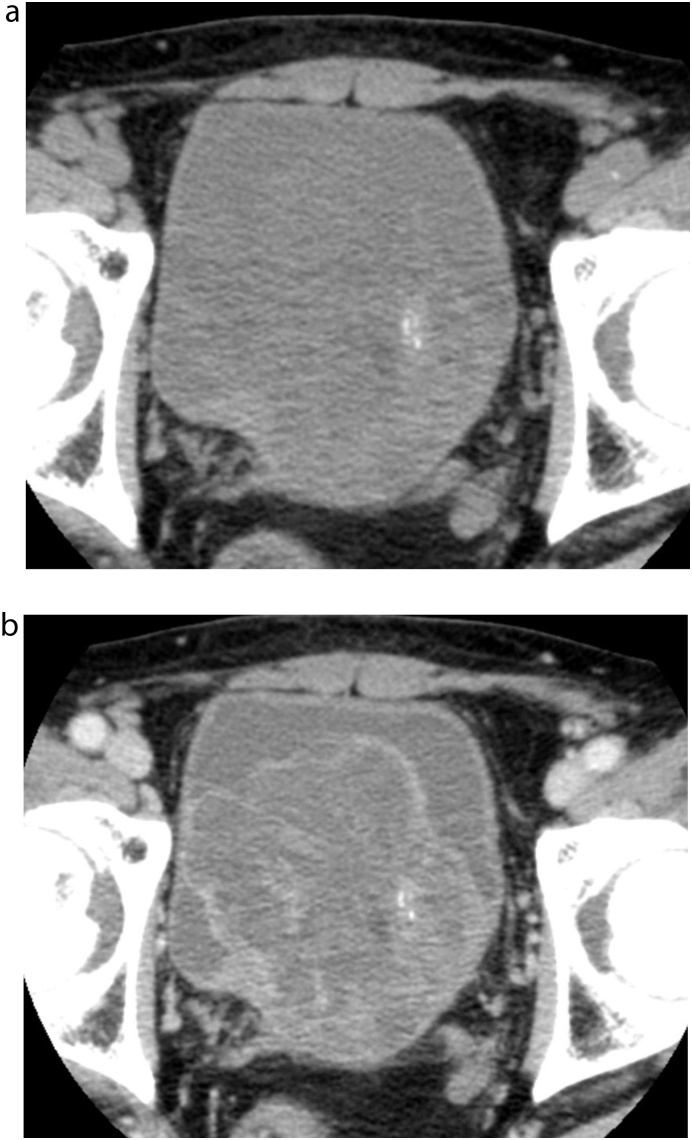
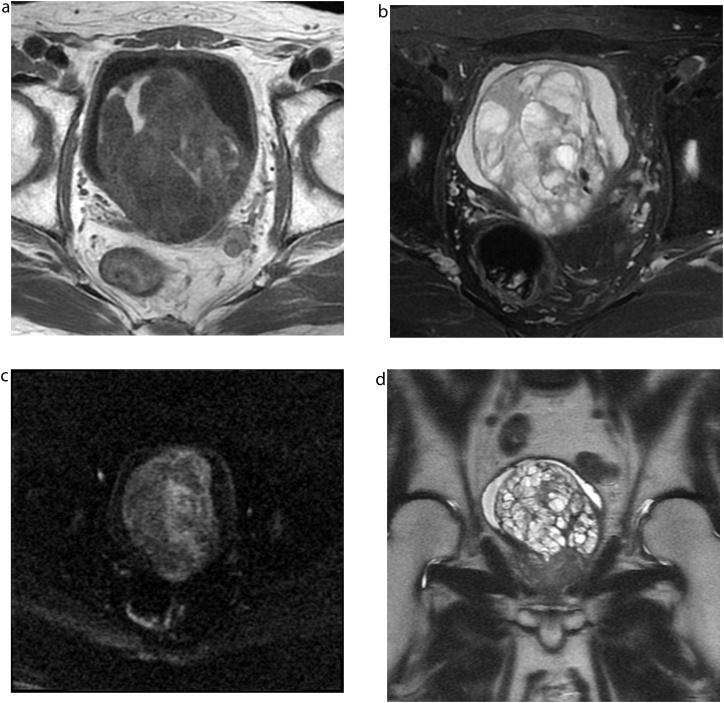
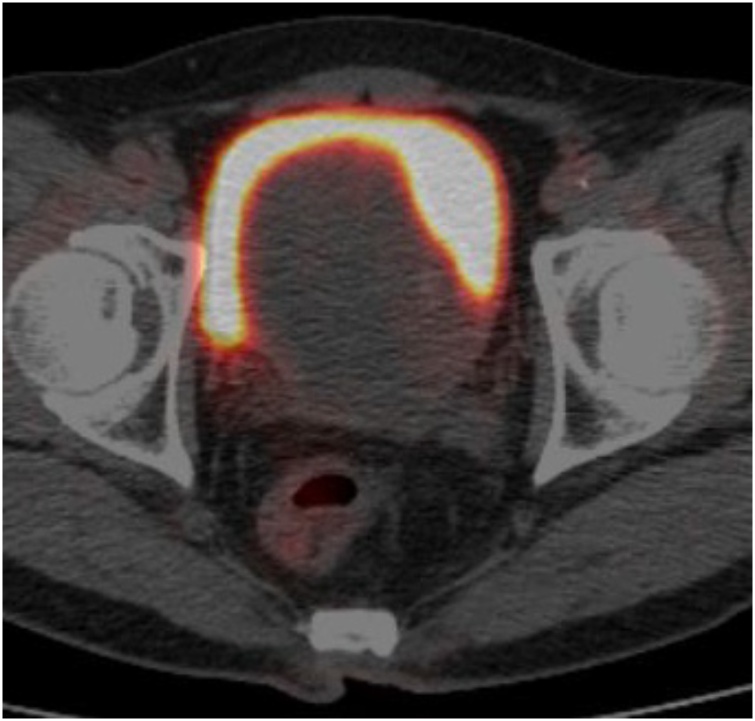
CT revealed a well-defined mass measuring approximately 10 cm in diameter behind the bladder. The mass was heterogeneously hypodense on unenhanced CT. On contrast-enhanced CT, the cyst walls and septa were enhanced (Fig. 1). Some solid parts were visualized with moderate enhancement. Most of the cyst contents showed iso- to slight low-attenuation on unenhanced CT. A part of the septa or solid portion had tiny calcification (Fig. 1a). On MRI, most cysts showed high signal intensity on T2-weighted images. Others had slightly high signal intensity and intermediate signal intensity on T1 and T2-weighted images, respectively (Fig. 2a and b). The high signal intensity area on T1-weighted images suggested a haemorrhagic change. The tumour showed a slightly high signal intensity on diffusion-weighted images (Fig. 2c). On the coronal T2-weighted image, the tumour was located above the prostate and protruded into the bladder (Fig. 2d). The tumour showed a positive embedded organ sign, suggesting the tumour originated from the prostate. 18F-FDG-PET did not show abnormal uptake in the tumour (Fig. 3). The systemic prostate biopsy revealed no malignant cells. A tumour resection with partial prostatectomy was performed.
Fig. 1.
Unenhanced and contrast-enhanced CT a) Unenhanced CT showed a heterogeneous mass behind the bladder. Small calcification was noted. The border between the tumour and the bladder was unclear. b) Contrast-enhanced CT demonstrated multilocular cysts and enhanced solid portions.
Fig. 2.
MRI a) Most of the mass showed heterogeneous hypointensity on T1-weighted MR images (repetition time [TR]/effective echo time [TE], 450/6.5 ms), whereas some parts showed hyperintensity. b) T2-weighted images with fat-saturation showed a heterogeneous hyperintense mass with multiple septations (TR/TE, 4517/85 ms). c) Diffusion-weighted images showed slightly high intensity (TR/TE, 8000/82 ms, b-value = 1000s/mm2). d) On the coronal T2-weighted image, the tumour was located above the prostate and protruded into the bladder (TR/TE, 2000/121 ms).
Fig. 3.
18F-FDG-PET. 18F-FDG-PET did not show abnormal uptake in the tumour.
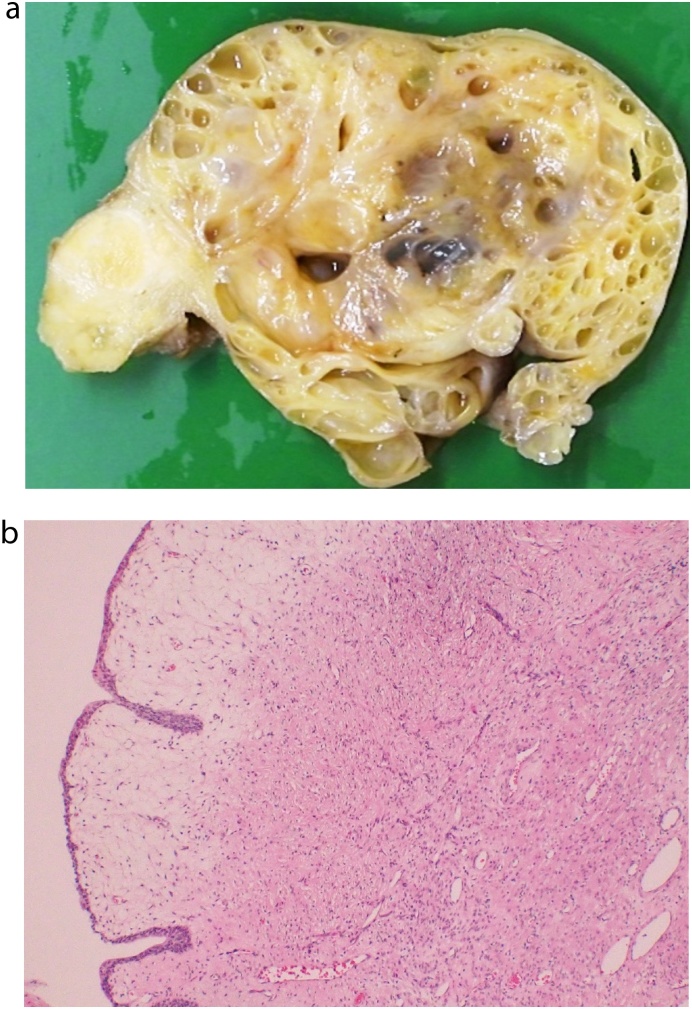
Histologically, the tumour consisted of cystically dilated glandular structures and a slightly hypercellular stroma with scattered cytologically atypical spindle cells with enlarged nuclei (Fig. 4). Necrosis was not found in the stroma. Some cysts had haemorrhagic changes. The cysts were lined with a flattened luminal cell layer and some basal cells without nuclear atypia. The epithelial lining of these cysts was positive for PSA staining. The tumour was finally diagnosed as STUMP after consultation with a pathologist. The blood PSA level decreased to the normal range, and there was no recurrence for more than four years after the operation.
Fig. 4.
Pathologic findings a) Photograph of the cut surface of the prostate tumour showed a multilocular cystic mass. b) On the macrograph of the resected specimen, cysts were lined with a flattened luminal cell layer and some basal cells without nuclear atypia. The tumour stroma showed slight hypercellularity with scattered atypical spindle cells.
3. Discussion
The most common prostatic diseases are prostate cancer and benign prostate hyperplasia (BPH). Prostate cancer usually shows a homogenous or heterogeneous solid mass in the peripheral zone. In contrast, BPH shows heterogeneous mass composed of multiple BPH nodules in the transitional zone. Most cystic lesions are BPH or other non-neoplastic lesions, such as Müllerian duct cyst, prostatic utricle cyst, retention cyst, paramedian cyst, infectious cyst, and seminal vesicle cyst [1]. Neoplastic prostatic lesions showing a cystic appearance radiologically are rare, and cystic epithelial tumours of the prostate and stromal tumour of the prostate correspond [1,6].
Prostatic stromal neoplasms are classified by the 2004 WHO classification into two groups: STUMP and stromal sarcoma. STUMP often occurs in patients between the ages of 50 and 60 [7]. The major clinical symptoms at presentation are an obstructive void and haematuria. Tumour sizes vary from 0.7 cm to 18 cm. The tumour originates in both the peripheral and transitional zones. The STUMP, having the potential for stromal proliferation, often shows multilocular cysts with thickened septa and solid components. A previous case report and pictorial review reported a solid mass with a multilocular cystic component that was similar to a cystadenoma, mucinous adenocarcinoma, and prostate sarcoma [8].
In 2016, cystic epithelial tumours of the prostate were becoming a new spectrum disease of the prostate [4]. This disease was formerly called cystadenoma or giant multilocular cyst of the prostate. Cystic epithelial tumours of the prostate show multilocular cyst macroscopically. There are several case reports of cystic epithelial tumours diagnosed as giant multilocular cystadenoma, including by ultrasound, CT, and MRI findings [2,3]. These tumours often present as a large multilocular cystic mass composed of variable-sized cysts, usually with some thin enhancing septations and an outer, thin, enhancing wall. Few enhanced solid components can be found within the lesion.
Differentiation between cystic degeneration of STUMP and cystic epithelial tumours of the prostate is challenging. Both tumours could show multiloculated cyst-like structures. In our case, the stromal proliferation of the tumour was clearly identified pathologically, and the CT and MRI findings showed thickened cyst walls and solid components. However, these lesions did not show invasion extending beyond the well-defined margin or other signs of malignancy, such as strongly restricted diffusion or FDG accumulation on 18F-FDG-PET. These findings do not correspond to solid lesions in cystic epithelial tumours that are thought to be invasive adenocarcinoma. These findings may have suggested the possibility of STUMP in the past, although it is uncertain whether the thickened cyst walls or solid components are the keys to differentiation.
In conclusion, STUMP can show a multiloculated cystic appearance and can be challenging to differentiate from cystic epithelial tumours. Imaging findings of thickened cyst walls and solid components without restricted diffusion or FDG accumulation in 18F-FDG-PET might be differentiation factors distinguishing STUMP from cystic epithelial tumours.
Declaration of Competing Interest
None of the authors have any relevant conflict of interest or industry support related to this report.
Acknowledgement
We would like to thank Dr. Toyonori Tsuzuki for his consultation on pathology.
This study does not require institutional review board approval. Informed consent was obtained for the case report to be published.
References
- 1.Shebel H.M., Farg H.M., Kolokythas O., El-Diasty T. Cysts of the lower male genitourinary tract: Embryologic and anatomic considerations and differential diagnosis. Radiographics. 2013;33:1125–1143. doi: 10.1148/rg.334125129. [DOI] [PubMed] [Google Scholar]
- 2.Portugal Teixeira I., Pereira P.R., Silva A., Castro M. Giant multilocular prostatic cystadenoma, a diagnosis to consider in large pelvic male masses. Radiol. Case Reports. 2019;14:1473–1477. doi: 10.1016/j.radcr.2019.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura Y., Shida D., Shibayama T., Yoshida A., Matsui Y., Shinoda Y., Iwata S., Kanemitsu Y. Giant multilocular prostatic cystadenoma. World J. Surg. Oncol. 2019;17:1–9. doi: 10.1186/s12957-019-1579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paner G.P., Lopez-Beltran A., So J.S., Antic T., Tsuzuki T., Mckenney J.K. Spectrum of cystic epithelial tumors of the prostate. Am. J. Surg. Pathol. 2016;40:886–895. doi: 10.1097/PAS.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 5.Humphrey P.A., Moch H., Cubilla A.L., Ulbright T.M., Reuter V.E. The 2016 WHO classification of tumours of the urinary system and male genital organs—part B: Prostate and bladder tumours. Eur. Urol. 2016;70:106–119. doi: 10.1016/j.eururo.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 6.Li Y., Mongan J., Behr S.C., Sud S., Coakley F.V., Simko J., Westphalen A.C. Beyond prostate adenocarcinoma: Expanding the differential diagnosis in prostate pathologic conditions. Radiographics. 2016;36:1055–1075. doi: 10.1148/rg.2016150226. [DOI] [PubMed] [Google Scholar]
- 7.Herawi M., Epstein J.I. Specialized stromal tumors of the prostate: A clinicopathologic study of 50 cases. Am. J. Surg. Pathol. 2006;30:694–704. doi: 10.1097/00000478-200606000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Muglia V.F., Saber G., Maggioni G., Monteiro A.J.C. MRI findings of prostate stromal tumour of uncertain malignant potential: A case report. Br. J. Radiol. 2011;84:194–196. doi: 10.1259/bjr/67699443. [DOI] [PMC free article] [PubMed] [Google Scholar]