Abstract
Purpose
The mammalian target of rapamycin inhibitor everolimus targets aberrant signaling through the PI3K/AKT/mammalian target of rapamycin pathway, a mechanism of resistance to anti-estrogen therapy in estrogen receptor (ER)–positive breast cancer. We hypothesized that everolimus plus the selective ER downregulator fulvestrant would be more efficacious than fulvestrant alone in ER-positive metastatic breast cancer resistant to aromatase inhibitor (AI) therapy.
Patients and Methods
This randomized, double-blind, placebo-controlled, phase II study included 131 postmenopausal women with ER-positive, human epidermal growth factor receptor 2–negative, AI-resistant metastatic breast cancer randomly assigned to fulvestrant (500 mg days 1 and 15 of cycle 1, then day 1 of cycles 2 and beyond) plus everolimus or placebo. The study was designed to have 90% power to detect a 70% improvement in median progression-free survival from 5.4 months to 9.2 months. Secondary end points included objective response and clinical benefit rate (response or stable disease for at least 24 weeks). Prophylactic corticosteroid mouth rinses were not used.
Results
The addition of everolimus to fulvestrant improved the median progression-free survival from 5.1 to 10.3 months (hazard ratio, 0.61 [95% CI, 0.40 to 0.92]; stratified log-rank P = .02), indicating that the primary trial end point was met. Objective response rates were similar (18.2% v 12.3%; P = .47), but the clinical benefit rate was significantly higher in the everolimus arm (63.6% v 41.5%; P = .01). Adverse events of all grades occurred more often in the everolimus arm, including oral mucositis (53% v 12%), fatigue (42% v 22%), rash (38% v 5%), anemia (31% v. 6%), diarrhea (23% v 8%), hyperglycemia (19% v 5%), hypertriglyceridemia (17% v 3%), and pneumonitis (17% v 0%), although grade 3 to 4 events were uncommon.
Conclusion
Everolimus enhances the efficacy of fulvestrant in AI-resistant, ER-positive metastatic breast cancer.
INTRODUCTION
Breast cancer is the most common cancer in women globally and in the United States.1 Although metastatic breast cancer is incurable, endocrine therapy prolongs survival and is an effective therapy for the 70% of women who have hormone receptor–positive disease. Current endocrine therapy treatment options include selective estrogen receptor (ER) modulators (eg, tamoxifen), aromatase inhibitors (AIs; eg, anastrozole, letrozole, exemestane), and selective ER downregulators (SERD; eg, fulvestrant).2 Several agents enhance the efficacy of AIs, including the mammalian target of rapamycin (mTOR) inhibitor everolimus and the CDK4/6 inhibitors (eg, palbociclib, ribociclib, abemaciclib).3 Although the mechanisms of AI resistance are incompletely understood, altered ER signaling and upregulation of the PI3K-Akt-mTOR pathway are contributing factors.4,5
Fulvestrant is a SERD that binds, inhibits, and degrades the ER. It binds with 100-fold greater affinity than does tamoxifen, and inhibits estrogen signaling more effectively than either tamoxifen or AIs,6-8 suggesting that it may be a better platform for combining with agents targeting other pathways. Everolimus is an orally bioavailable first-generation mTOR inhibitor that binds with high affinity to its intracellular receptor FKBP12. The everolimus-FKBP12 complex interacts with mTOR to inhibit downstream signaling events.9 The addition of everolimus to the corticosteroidal AI exemestane improved median progression-free survival (PFS; 3.2 v 7.8 months; P < .0001) in patients with disease that was resistant to noncorticosteroidal AIs (ie, anastrozole, letrozole) in the phase III BOLERO-2 trial10-12 and also improved clinical outcomes when added to tamoxifen in AI-resistant breast cancer in the randomized phase II Tamoxifen and RAD001 Study (TAMRAD) trial.13 On the basis of these considerations, we hypothesized that the addition of everolimus to fulvestrant would be more efficacious than the use of fulvestrant alone, and that fulvestrant might represent a potentially more efficacious endocrine therapy platform for combination with everolimus than either exemestane or tamoxifen. We therefore performed a randomized, double-blind phase II trial comparing fulvestrant with everolimus or placebo in patients with hormone receptor–positive metastatic breast cancer who had disease that was resistant to AIs, a common indication for fulvestrant therapy.14
PATIENTS AND METHODS
Patient Population
Eligibility included postmenopausal women with histologically or cytologically confirmed unresectable locally advanced or metastatic, ER-positive, human epidermal growth factor receptor 2/neu negative breast cancer (as defined by local institutional laboratories using ASCO—College of American Pathologists guidlelines15,16), measurable and/or nonmeasurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria,17 AI-resistant disease (defined either as relapse while receiving adjuvant AI therapy or disease progression while receiving an AI for metastatic disease), and no more than one prior chemotherapy regimen for metastatic disease. Other eligibility criteria included age ≥ 18 years, Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1, and adequate organ and marrow function (leukocytes ≥ 3,000/µL, absolute neutrophil count ≥ 1,500/μL, platelet count ≥ 100,000/µL, total bilirubin ≤ 2.0 mg/dL, AST and/or ALT ≤ 2.5× institutional upper limit of normal, serum creatinine ≤ 1.5 mg/dL). Patients were allowed to receive up to two doses of fulvestrant administered within a 4-week period before trial consent and registration.
Study Design and Treatment
This was a randomized, double-blind, placebo-controlled phase II trial. Patients were randomly assigned in a 1:1 fashion to fulvestrant plus everolimus or a matching placebo. Random assignment was conducted centrally using permuted blocks within strata. Stratification factors for random assignment included ECOG performance status (0 v 1), measurable disease (yes v no), and prior chemotherapy for metastatic disease (yes v no). The trial was coordinated by PrECOG (Philadelphia, PA). The local institutional review board at each participating institution approved the protocol. All patients provided written informed consent.
All patients received fulvestrant (AstraZeneca, Wilmington, DE) at a standard dose and schedule (500 mg intramuscularly days 1, 15 in cycle 1, and day 1 of each subsequent 28-day cycle). For those randomly assigned to everolimus (Novartis, New York, NY), the dose was 10 mg orally once per day. Treatment continued until evidence of disease progression, unacceptable toxicity, or withdrawal of consent, for a maximum of 12 cycles (48 weeks). Patients who did not experience progression by week 48 underwent unblinding of the treatment arm because it was believed that sufficient follow-up would have elapsed to detect the difference being sought in median PFS, the primary trial end point. Patients randomly assigned initially to placebo continued fulvestrant alone, whereas those randomly assigned initially to everolimus continued fulvestrant plus open label everolimus. Patients without evidence of disease progression before week 48 could continue fulvestrant and discontinue everolimus or placebo for excessive toxicity attributed to everolimus or placebo. Concurrent treatment with bone antiresorptive agents (eg, bisphosphonates, denosumab) was permitted for patients with bone metastases. Dose reduction for fulvestrant was not allowed. Dose reductions for everolimus and placebo were permitted according to guidelines in the product information brochure, and as described in the Data Supplement. Prophylactic corticosteroid mouth rinses were not specified in the protocol.
Tumor Assessments and Clinical Evaluations
All patients underwent computed tomography of the chest and abdomen and a bone scan within 4 weeks of registration. Tumor response was assessed every 12 weeks (± 1 week) after cycle 1, day 1 by computed tomography using RECIST criteria version 1.1, and bone scans were repeated every 24 weeks (± 2 weeks).17 If the baseline bone scan showed metastases, and if the bones were the only site of nonmeasurable disease, then the bone scan was repeated every 12 weeks (± 1 week). Response assessment for the primary and secondary trial end points was performed by the treating physician. Physician visits occurred on day 1 of each fulvestrant cycle. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology for Adverse Events (version 4.0).
Study End Points
The primary end point for the trial was investigator-assessed PFS, defined as the time from random assignment to disease progression or death from any cause, whichever occurred first. Patients who were still alive and free of disease progression were censored at last disease assessment date. Patients without any disease assessment after random assignment were censored at time of random assignment. Secondary end points included objective response rate (complete or partial response) as defined by RECIST criteria,17 clinical benefit rate (objective response or stable disease for at least 24 weeks), and overall survival (OS), which was defined as the time from random assignment to death from any cause. Patients who were still alive were censored at the last date known to be alive. For the 11 patients who received up to two fulvestrant doses within 4 weeks of registration and random assignment, time to event analysis was measured from random assignment.
Statistical Consideration
On the basis of the subgroup of patients with AI-resistant disease treated with 500 mg fulvestrant in the first report of the Comparison of Faslodex in Recurrent or Metastatic Breast Cancer (CONFIRM) trial, it was projected that the median PFS among patients with AI-resistant disease who were receiving fulvestrant alone would be approximately 5.4 months.18 We hypothesized that the addition of everolimus to fulvestrant would result in a 70% improvement in median PFS, to 9.2 months. To have 90% power to detect the hypothesized improvement with a one-sided type I error rate of 10% using a stratified log-rank test, approximately 120 eligible patients (60 per arm) would be required with full information on 98 events (progressions or deaths). Allowing up to 10 patients (approximately 10%) to be ineligible or to not start study treatment, approximately 130 patients were targeted for enrollment. PFS and OS were estimated using the Kaplan-Meier method and were compared between treatment arms using the stratified log-rank test. Clinical benefit rate, objective response rate, and incidence of treatment-related grade 3 or higher AEs were estimated using binomial proportions and 95% exact CIs and were compared between arms using Fisher’s exact test. All tests were two sided, and the significance level was set at 0.1 for the primary end point and 0.05 for all other end points. All analyses were conducted using STATA 13.0 (STATA, College Station, TX). The primary analysis for PFS was based on data as of December 2016, when a sufficient number of events to trigger the primary analysis had occurred; at that time, 101 PFS events (96 disease progression plus five deaths without disease progression) had occurred. Information on treatment, AEs, and OS were based on updated data using a cutoff date of March 28, 2017, at which point six patients were still receiving protocol therapy, including four receiving fulvestrant alone, and two receiving fulvestrant plus everolimus. AEs were coded and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). The intent-to-treat population was used for all efficacy analyses.
RESULTS
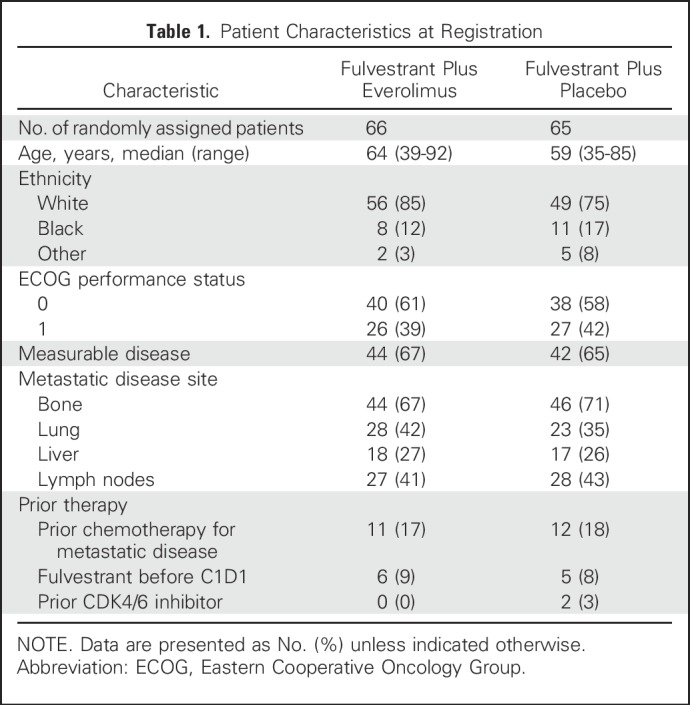
Patient Characteristics
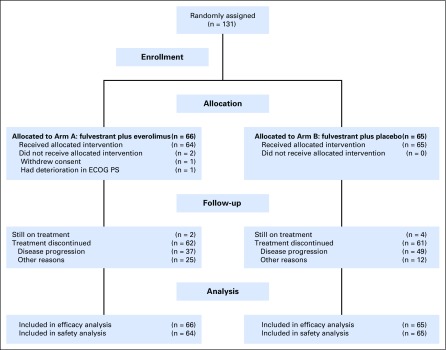
Between May 2013 and November 2015, a total of 131 patients from 23 institutions were randomly assigned, 66 to the everolimus arm and 65 to the placebo arm (Fig 1). Two patients randomly assigned to everolimus did not receive any protocol therapy (one patient withdrew consent and one patient was deemed ineligible because of performance status deterioration). The characteristics of the 131 enrolled patients are listed in Table 1. There were no significant differences between arms with regard to age (median, 64 v 59 years), ECOG performance status (0/1, 61%/39% v 58%/42%), prior chemotherapy for metastasis (17% v 18%), fulvestrant therapy before registration (9% v 8%), prior CDK4/6 inhibitor therapy (0% v 3%), or presence of liver metastases (27% v 26%).
Fig 1.
CONSORT diagram. ECOG PS, Eastern Cooperative Oncology Group performance status.
Table 1.
Patient Characteristics at Registration
PFS
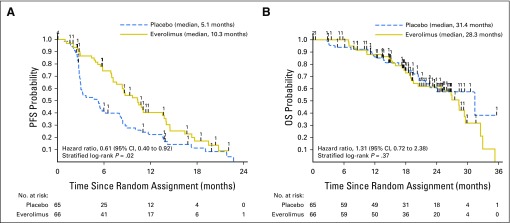
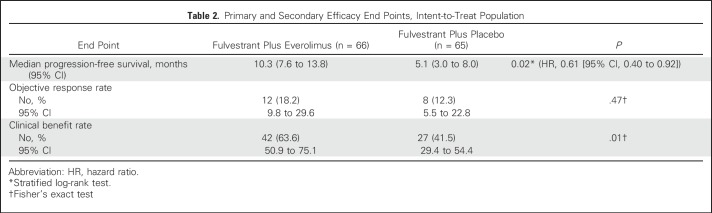
The addition of everolimus significantly improved the median PFS from 5.1 months (95% CI, 3.0 to 8.0 months) to 10.3 months (95% CI, 7.6 to13.8 months; stratified log-rank P value = .02; hazard ratio, 0.61 [95% CI, 0.40 to 0.92]), indicating that the end point was met (Fig 2A; Table 2).
Fig 2.
Kaplan-Meier estimates of investigator-assessed (A) progression-free survival (PFS) and (B) overall survival (OS) in the intent-to-treat (ITT) population.
Table 2.
Primary and Secondary Efficacy End Points, Intent-to-Treat Population
Objective Response and Clinical Benefit Rate
Secondary efficacy end points included objective response and clinical benefit rate (Table 2). Objective response occurred in 12 patients in the everolimus arm (18.2% [95% CI, 9.8% to 29.6%]) and eight patients in the placebo arm (12.3% [95% CI, 5.5% to 22.8%]), which was not significantly different (P = .47). The clinical benefit rate was 63.6% (95% CI, 50.9% to 75.1%) in the everolimus arm and 41.5% (95% CI, 29.4% to 54.4%) in the placebo arm, which was significantly different (P = .01).
OS
There were 51 deaths at the time of the analysis, including 30 in the everolimus arm and 21 in the placebo arm. The median follow-up was 19.3 months (range, 0 to 36.3 months) for the 80 surviving patients. The estimated median OS was 28.3 months (95% CI, 19.5 to 29.6 months) in the everolimus arm and 31.4 months (95% CI, 21.8 to month not reached) in the placebo arm (stratified log-rank test P value = .37; HR=1.31 [95% CI, 0.72 to 2.38; Fig 2B), indicating no significant difference between the everolimus arm and the placebo arm.
Treatment Administered and Reasons for Treatment Discontinuation
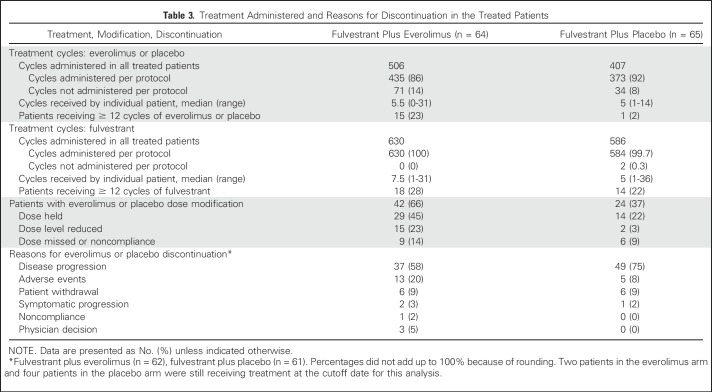
Information regarding treatment administered and reasons for treatment discontinuation are summarized in Table 3. A total of 1,102 cycles were administered in 129 treated patients (567 cycles in the everolimus arm and 535 cycles in the placebo arm) by the cutoff date. The median number of treatment cycles was 5.5 (range, 0 to 31) for everolimus and 7.5 (range, 1 to 31) for fulvestrant for patients in the everolimus arm, and five (range, one to14) for placebo and five (range, one to 36) for fulvestrant for patients in the placebo arm. The median duration of treatment was 5.1 months (range, zero to 28.6 months) for everolimus and 6.9 months (range, 1.4 to 29.8 months) for fulvestrant for patients in the everolimus arm, and 4.6 months (range, 0.03 to 12.4 months) for placebo and 4.6 months (range, 0.5 to 33.8 months) for fulvestrant for patients in the placebo arm. Dose modification of everolimus or placebo was higher in the everolimus arm than in the placebo arm, including any modification (66% v 37%), dose held (45% v 22%), dose reduced (23% v 3%), and dose missed because of noncompliance (14% v 9%). The most common reason for treatment discontinuation was disease progression, occurring in 37 patients (58%) in the everolimus arm and 49 patients (75%) in the placebo arm. Everolimus and placebo were discontinued because of AEs in 13 patients (20%) and five patients, (8%), respectively. Other reasons included patient withdrawal, symptomatic disease progression (before RECIST progression), and noncompliance, which were comparable in the two arms. At the time of unblinding at week 48, all patients assigned originally to everolimus during the blinded portion of the study before week 48 continued open-label everolimus after week 48.
Table 3.
Treatment Administered and Reasons for Discontinuation in the Treated Patients
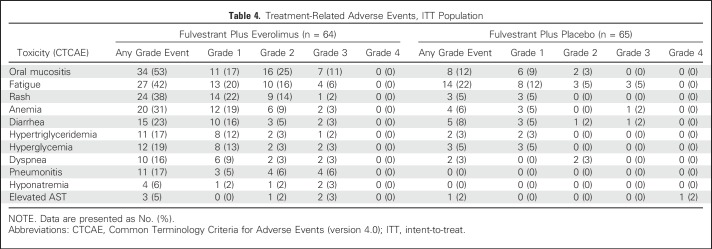
AEs
Worst grade treatment-related AEs (all grades) are summarized in Table 4. The most common AEs of any grade (occurring in at least 5% of patients) in the everolimus arm compared with the placebo arm included oral mucositis (53% v 12%), fatigue (42% v 22%), rash (38% v 5%), anemia (31% v 6%), diarrhea (23% v 8%), hyperglycemia (19% v 5%), hypertriglyceridemia (17% v 3%), pneumonitis (17% v 0%), dyspnea (16% v 3%), hyponatremia (6% v 0%), and elevated transaminase (5% v 2%). The most common grade 3 or higher treatment-related AEs (occurring in at least 5% of patients) in the everolimus arm compared with the placebo arm included oral mucositis (11% v 0%), fatigue (6% v 5%), and pneumonitis (6% v 0%).There was one grade 4 AE (elevated AST) in the placebo arm, and none in the everolimus arm. Three deaths occurred during, or within 30 days of completing, protocol therapy, including two in the everolimus arm (one sepsis and one cardiac arrest) and one in the placebo arm (one cardiac arrest), none of which were attributed to study treatment by the local treating physician.
Table 4.
Treatment-Related Adverse Events, ITT Population
DISCUSSION
We performed a randomized phase II trial of the SERD fulvestrant in combination with everolimus or placebo in 131 postmenopausal women with ER-positive metastatic breast cancer who had progressive disease after prior AI therapy. The addition of everolimus to fulvestrant significantly improved median PFS from 5.1 to 10.4 months (hazard ratio, 0.6; P = .02), meeting the prespecified primary end point. The median PFS for fulvestrant alone was comparable to the 5.4-month median PFS observed for fulvestrant using the same dose and schedule as in an AI-resistant population in the CONFIRM trial.17 Although there were numerically more deaths in the everolimus arm (30 everolimus v 21 placebo) at the time of primary PFS analysis, there was no statistically significant difference in median OS between the everolimus and placebo arms (28.3 v 31.4 months, P = .37), and the trial was not designed to demonstrate improved survival for the everolimus arm. This study provides additional evidence that adding everolimus to anti-estrogen therapy in AI-resistant disease improves PFS, as noted previously in the BOLERO-2 trial when everolimus was combined with exemestane,10 and in the TAMRAD trial when everolimus was combined with tamoxifen.13 In contrast, the mTOR inhibitor temsirolimus did not improve clinical outcomes when added to an AI as -line therapy; one possible explanation among others is that the development of AI resistance may select for tumors that are more dependent on PI3K/AKT/mTOR signaling and hence sensitive to mTOR inhibition.19
The addition of everolimus to fulvestrant resulted in more AEs and higher rates of treatment discontinuation as a result of AEs, when compared with placebo (20% v 8%). The most common grade 3 AEs occurring in > 5% of patients in the everolimus arm included oral mucositis (11%), pneumonitis (6%), fatigue (5%), and hyperglycemia (6%). The most common AE of all grades was oral mucositis, which occurred in 52% of patients and was grade 1 to 2 in 42%. This safety profile is similar to that of everolimus combined with either exemestane or tamoxifen in the BOLERO-2 trial10 and TAMRAD trials,13 respectively. Prophylactic corticosteroid mouthwash was not used in any of these trials, but it has been shown to reduce the risk of grade 1 to 3 oral mucositis (also commonly referred to as stomatitis) to approximately 20%, with the vast majority being grade 1.20 This may prove to be a helpful strategy to reduce the risk of stomatitis, one of the more common and troublesome adverse effects associated with everolimus that typically manifests within 8 weeks of initiating therapy.21
This study was completed before the availability of the CDK 4/6 inhibitors, which are effective when added to both first-line AI therapy and second-line fulvestrant in AI-resistant disease. The PALOMA3 trial demonstrated that the combination of the CDK4/6 inhibitor palbociclib with fulvestrant significantly prolonged median PFS compared with fulvestrant alone (median, 9.5 v 4.6 months; P < .0001).22,23 The addition of abemaciclib also improved median PFS when add to fulvestrant in the MONARCH2 trial (median, 16.4 v 9.3 months; P < .001) in a population of patients resistant to prior endocrine therapy including an AI (approximately 70%) or tamoxifen, with diarrhea being the most common AE.24 Given the activity of the CDK4/6 inhibitors such as palbociclib, ribociclib, and abemaciclib in combination with AIs as first-line endocrine therapy,25-28 the use of everolimus may represent an option for combination with fulvestrant as second-line therapy in AI-resistant disease. The findings of our trial were confirmed by the Fulvestrant With AZD2014 or Everolimus for Advanced Breast Cancer (MANTA) trial, in which 333 patients with AI-resistant, ER-positive metastatic breast cancer were randomly assigned to receive fulvestrant alone or in combination with everolimus or the dual target of rapamycin complex 1-2 (TORC 1-2) inhibitor vistusertib; PFS was significantly improved in the fulvestrant plus everolimus arm compared with fulvestrant alone (median PFS, 12.2 v 4.6 months; HR, 0.64 [95% CI, 0.43 to 0.94]; P = .02), but not in either of the arms that included fulvestrant plus two different vistusertib dosing regimens.29 Few patients in our trial or the MANTA trial received prior CDK4/6 inhibitor therapy, so the impact of such prior therapy on the efficacy of the fulvestrant-everolimus combination is unknown. However, upregulation of the PI3K-PDK1 pathway has emerged as an important mechanism of resistance to CDK4/6 inhibitor therapy, suggesting that mTOR blockade with everolimus plus fulvestrant may represent a viable strategy for the treatment of patients who have developed resistance to prior treatment including an AI plus a CDK4/6 inhibitor.30
In conclusion, our findings provide additional evidence that everolimus plus anti-estrogen therapy is more efficacious than is anti-estrogen therapy alone in patients with metastatic, hormone receptor–positive, human epidermal growth factor receptor 2–negative breast cancer resistant to AI therapy, and that the fulvestrant-everolimus combination represents a new therapeutic option for AI-resistant disease.
Footnotes
Supported by Novartis.
Presented in part at the San Antonio Breast Cancer Symposium, December 7, 2016.
Clinical trial information: NCT01797120.
Listen to the podcast by Dr Rugo at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Noah Kornblum, Adam Brufsky, Brian Burnette, Lori J. Goldstein, Joseph A. Sparano
Administrative support: Joseph A. Sparano
Provision of study materials or patients: Noah Kornblum, Paula Klein, Philip J. Stella, Della F. Makower, Joseph A. Sparano
Collection and assembly of data: Noah Kornblum, Paula Klein, Adam Brufsky, Philip J. Stella, Melinda Telli, Della F. Makower, Puneet Cheema, Cristina I. Truica, Antonio C. Wolff, Gamini S. Soori, Barbara Haley, Timothy R. Wassenaar, Kathy D. Miller, Joseph A. Sparano
Data analysis and interpretation: Noah Kornblum, Fengmin Zhao, Judith Manola, Bhuvaneswari Ramaswamy, Adam Brufsky, Cristina I. Truica, Antonio C. Wolff, Kathy D. Miller, Joseph A. Sparano
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase II Trial of Fulvestrant Plus Everolimus or Placebo in Postmenopausal Women With Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer Resistant to Aromatase Inhibitor Therapy: Results of PrE0102
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Noah Kornblum
No relationship to disclose
Fengmin Zhao
No relationship to disclose
Judith Manola
No relationship to disclose
Paula Klein
No relationship to disclose
Bhuvaneswari Ramaswamy
Consulting or Advisory Role: Pfizer
Adam Brufsky
Consulting or Advisory Role: Pfizer, Genentech, Agendia NV, Celgene, Novartis, Bayer AG, Eli Lilly, bioTheranostics, NanoString, chnologies, Genomic Health, Puma Biotechnology
Philip J. Stella
Employment: Physician Resource Management, CARET
Leadership: Physician Resource Management
Stock or Other Ownership: Physician Resource Management
Brian Burnette
No relationship to disclose
Melinda Telli
Consulting or Advisory Role: Vertex, AstraZeneca, Tesaro, PharmaMar, Pfizer, Celldex
Research Funding: Novartis (Inst), PharmaMar (Inst), Biomarin (Inst), Abbvie (Inst), Calithera Biosciences (Inst), Genentech (Inst), Medivation (Inst), OncoSec (Inst), Vertex (Inst), Biothera (Inst), Tesaro (Inst), Pfizer (Inst)
Della F. Makower
No relationship to disclose
Puneet Cheema
Stock or Other Ownership: Abbvie, Gilead Sciences, Bristol-Myers Squibb, Pfizer, Merck
Consulting or Advisory Role: Gilead Sciences
Travel, Accommodations, Expenses: Gilead Sciences
Cristina I. Truica
Honoraria: Novartis
Consulting or Advisory Role: Novartis
Research Funding: Novartis
Travel, Accommodations, Expenses: Novartis
Antonio C. Wolff
Research Funding: Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: Antonio Wolff has been named as inventor on one or more issued patents or pending patent applications relating to methylation in breast cancer, and has assigned his rights to Johns Hopkins University, and participates in a royalty sharing agreement with Johns Hopkins University.
Gamini S. Soori
Stock or Other Ownership: AstraZeneca, Eli Lilly, GlaxoSmithKline, Pfizer, Sanofi, Takdam, Teva Neuroscience, Merck
Barbara Haley
No relationship to disclose
Timothy R. Wassenaar
No relationship to disclose
Lori J. Goldstein
Honoraria: Roche Pharma AG
Consulting or Advisory Role: Dompè Farmaceutici SPA
Research Funding: Genentech (Inst)
Kathy D. Miller
No relationship to disclose
Joseph A. Sparano
Stock or Other Ownership: Metastatix
Consulting or Advisory Role: Genentech, Novartis, AstraZeneca, Celgene, Eli Lilly, Celldex, Pfizer, Prescient Therapeutics, Juno Therapeutics, Merrimack
Research Funding: Prescient Therapeutics (Inst), Deciphera (Inst), Genentech (Inst), Merck (Inst), Novartis (Inst), Merrimack (Inst)
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2017. CA Cancer J Clin 67:7-30, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Rugo HS, Rumble RB, Macrae E, et al. : Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol 34:3069-3103, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Turner NC, Neven P, Loibl S, et al. : Advances in the treatment of advanced oestrogen-receptor-positive breast cancer. Lancet 389:2403-2414, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Miller TW, Balko JM, Arteaga CL: Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol 29:4452-4461, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma CX, Reinert T, Chmielewska I, et al. : Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer 15:261-275, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Howell A, Robertson JF, Quaresma Albano J, et al. : Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol 20:3396-3403, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Osborne CK, Pippen J, Jones SE, et al. : Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: Results of a North American trial. J Clin Oncol 20:3386-3395, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Howell A, Robertson JF, Abram P, et al. : Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: A multinational, double-blind, randomized trial. J Clin Oncol 22:1605-1613, 2004 [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell A, Faivre S, Burris HA, III, et al. : Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol 26:1588-1595, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Baselga J, Campone M, Piccart M, et al. : Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366:520-529, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yardley DA, Noguchi S, Pritchard KI, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther 30:870-884, 2013 [Erratum: Adv Ther 31:1008-1009, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piccart M, Hortobagyi GN, Campone M, et al. : Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Overall survival results from BOLERO-2. Ann Oncol 25:2357-2362, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachelot T, Bourgier C, Cropet C, et al. : Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: A GINECO study. J Clin Oncol 30:2718-2724, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Di Leo A, Jerusalem G, Petruzelka L, et al. : Final overall survival: Fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst 106:djt337, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond ME, Hayes DF, Dowsett M, et al. : American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28:2784-2795, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff AC, Hammond ME, Hicks DG, et al. : Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997-4013, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Di Leo A, Jerusalem G, Petruzelka L, et al. : CONFIRM: A phase III, randomized, parallel-group trial comparing fulvestrant 250 mg vs fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. Cancer Res 69, 2009. (suppl 24; abstr 25) [DOI] [PubMed] [Google Scholar]
- 19.Wolff AC, Lazar AA, Bondarenko I, et al. : Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol 31:195-202, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rugo HS, Seneviratne L, Beck JT, et al. : Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): A single-arm, phase 2 trial. Lancet Oncol 18:654-662, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Rugo HS, Hortobagyi GN, Yao J, et al. : Meta-analysis of stomatitis in clinical studies of everolimus: incidence and relationship with efficacy. Ann Oncol 27:519-525, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner NC, Ro J, André F, et al. : Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 373:209-219, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Cristofanilli M, Turner NC, Bondarenko I, et al. : Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17:425-439, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Sledge GW, Jr, Toi M, Neven P, et al. : MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35:2875-2884, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Finn RS, Crown JP, Lang I, et al. : The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol 16:25-35, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Finn RS, Martin M, Rugo HS, et al. : Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375:1925-1936, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Hortobagyi GN, Stemmer SM, Burris HA, et al. : Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375:1738-1748, 2016 [DOI] [PubMed] [Google Scholar]
- 28.First-line abemaciclib effective in ER+ breast cancer Cancer Discov 7:OF6, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Schmid P, Zaiss M, Harper-Wynne C, et al. MANTA - a randomized phase II study of fulvestrant in combination with the dual mTOR inhibitor AZD2014 or everolimus or fulvestrant alone in estrogen receptor-positive advanced or metastatic breast cancer. Clin Cancer Res 76, 2017. (suppl 4; abstr OT1-03-12) [Google Scholar]
- 30.Jansen VM, Bhola NE, Bauer JA, et al. : Kinome-wide RNA interference screen reveals a role for PDK1 in acquired resistance to CDK4/6 inhibition in ER-positive breast cancer. Cancer Res 77:2488-2499, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]