Abstract
PURPOSE
Dose-escalated radiotherapy (RT) with androgen-deprivation therapy (ADT) is a standard definitive treatment of localized prostate cancer (LPCa). The optimal sequencing of these therapies is unclear. Our phase III trial compared neoadjuvant versus concurrent initiation of ADT in combination with dose-escalated prostate RT (PRT).
PATIENTS AND METHODS
Patients with newly diagnosed LPCa with Gleason score ≤ 7, clinical stage T1b to T3a, and prostate-specific antigen < 30 ng/mL were randomly allocated to neoadjuvant and concurrent ADT for 6 months starting 4 months before RT (neoadjuvant group) or concurrent and adjuvant ADT for 6 months starting simultaneously with RT (concurrent group). The primary end point was biochemical relapse-free survival (bRFS). Stratified log-rank test was used to compare bRFS and overall survival (OS). Incidence of grade ≥ 3 late RT-related toxicities was compared by log-rank test.
RESULTS
Overall, 432 patients were randomly assigned to the neoadjuvant (n = 215) or concurrent group (n = 217). At 10 years, bRFS rates for the two groups were 80.5% and 87.4%, respectively. Ten-year OS rates were 76.4% and 73.7%, respectively. There was no significant difference in bRFS (P = .10) or OS (P = .70) between the two groups. Relative to the neoadjuvant group, the hazard ratio for the concurrent group was 0.66 (95% CI, 0.41 to 1.07) for bRFS and 0.94 (95% CI, 0.68 to 1.30) for OS. No significant difference was observed in the 3-year incidence of late RT-related grade ≥ 3 GI (2.5% v 3.9%) or genitourinary toxicity (2.9% v 2.9%).
CONCLUSION
In our study, there was no statistically significant difference in bRFS between the two treatment groups. Similarly, no difference was seen in OS or late RT-related toxicities. On the basis of these results, both neoadjuvant and concurrent initiations of short-term ADT with dose-escalated PRT are reasonable standards of care for LPCa.
INTRODUCTION
External-beam radiotherapy (RT) is an established modality for definitive management of localized prostate cancer (LPCa).1 Despite promising results with dose-escalated RT, a substantial proportion of patients develop recurrence with prolonged follow-up.2-5 This remains a major reason for addition of androgen-deprivation therapy (ADT) to RT. However, the majority of the evidence favoring the addition of ADT to RT is from trials using conventional-dose RT (66-70 Gy).6-9 ADT was tested before RT (neoadjuvant ADT or neoadjuvant hormone therapy [NAHT]) or as concurrent or adjuvant therapy in these trials. Overall, regardless of sequencing, addition of ADT has been shown to provide superior biochemical control and cancer-specific and overall survival (OS) compared with RT alone in men with LPCa or locally advanced PCa.6-8,10,11 The superior results obtained with this combination have been attributed to the codependent biologic interactions of ADT and conventional-dose RT involving several interfaces.12-14 However, because of the heterogeneity in patient population and difference in the timing, duration, and regimen of ADT, these studies did not allow for a comparative evaluation of sequencing of ADT and RT.
Results from preclinical studies tend to favor neoadjuvant initiation of ADT. In an athymic mouse model, Zietman et al15 showed neoadjuvant orchiectomy led to a significant reduction in the RT dose required to control 50% of the androgen-dependent Shionogi adenocarcinoma compared with adjuvant orchiectomy. Similar results were obtained by Granfors et al16; they showed that castration before RT forestalled tumor growth more efficiently than adjuvant castration. Finally, another study by Kaminski et al17 reproduced similar results favoring NAHT. In contrast, contemporary studies have raised the possibility of RT-mediated upregulation of androgen receptor expression and neoangiogenesis in PCa, which portend an increased risk of relapse after RT. On these grounds, use of concomitant and adjuvant ADT (AHT) may offer theoretic advantages.18,19 A retrospective study comparing neoadjuvant and adjuvant ADT showed no difference in 10-year survival, although caution is required in interpretation because of several confounding factors.20
In summary, there remains clinical equipoise in the preferred sequencing of ADT and RT in LPCa because of a lack of robust prospective clinical data. Our study aimed to compare two strategies of sequencing of short-term ADT and dose-escalated prostate RT (PRT) with respect to oncologic outcomes, toxicity, and health-related quality of life (HRQOL).
PATIENTS AND METHODS
Study Design and Participants
This was an open-label, parallel, two-arm phase III randomized controlled study approved by the research ethics committees of the two participating institutions. Written informed consent was obtained from all patients before enrollment.
Eligible patients were men age > 18 years with Eastern Cooperative Oncology Group performance status < 2 and histologically confirmed diagnosis of adenocarcinoma of the prostate, with Gleason score ≤ 7, clinical tumor stage (Tstage) of T1b to T3a, and serum prostate-specific antigen (PSA) < 30 ng/mL ≤ 4 weeks before enrollment. Initial evaluation of patients consisted of history and physical examination, including digital rectal examination (DRE) and laboratory investigations: complete blood count (CBC), PSA, total testosterone, liver function tests (LFTs), and serum creatinine. Patients with baseline PSA ≥ 10 ng/mL underwent a whole-body bone scan ≤ 12 weeks before study entry, whereas those with PSA ≥ 20 ng/mL underwent a contrast-enhanced computed tomography scan of the abdomen and pelvis performed ≤ 12 weeks before study entry. Patients with low-risk PCa (Gleason score ≤ 6, T1-T2a, and PSA ≤ 10 ng/mL) or radiologic evidence of nodal or distant metastasis were excluded. Also excluded were patients with active or prior malignancies, except for nonmelanoma skin carcinoma within 5 years of the diagnosis of PCa; those with contraindications to RT, including inflammatory bowel disease; and those who had received prior pelvic RT, cytotoxic chemotherapy, or ADT.
Random Assignment
Patients were centrally randomly assigned to one of the two treatment arms using a computer-generated randomization schedule. Random assignment was stratified by T stage (T1b-T2a v T2b-T3a), Gleason score (< 7 v 7), and PSA level (≤ 10 v > 10 ng/mL).
Treatment
In both arms, patients received a total of 6 months of ADT, which was commenced within 6 weeks of random assignment. In one group, patients received 4 months of NAHT followed by RT in conjunction with the last 2 months of ADT (arm A). In the other group, patients received RT concomitantly with the first 2 months of ADT followed by 4 months of AHT (arm B; Appendix Fig A1, online only). ADT comprised of an oral antiandrogen (bicalutamide 50 mg once daily) plus goserelin (10.8 mg subcutaneously starting 7 days after bicalutamide with a second injection administered 3 months thereafter). While receiving ADT, patients had monthly laboratory investigations, including CBC, LFTs, PSA, and total testosterone.
All patients received a total RT dose of 76 Gy in 38 fractions over 7.5 weeks. This was delivered in 2 phases using an image-guided 3-dimensional conformal technique. In the first phase, 56 Gy was delivered to the prostate and proximal 10 mm of seminal vesicles in 28 fractions over 5.5 weeks. An additional boost of 20 Gy in 10 fractions was subsequently delivered to the prostate alone over 2 weeks. Additional details of RT are described in the Appendix (online only).
Follow-Up
Patients were observed according to a prescribed schedule after treatment completion. Clinical assessment, including DRE and laboratory investigations, were repeated 1 month after completion of ADT, every 4 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter. Patients were restaged radiologically upon biochemical progression or clinical suspicion of progression. Toxicity assessments were performed weekly during RT, monthly during ADT, 1 month after RT, and at each follow-up visit. ADT-related toxicity was scored using the Common Toxicity Criteria (version 2.0).21 RT-related acute and late toxicities were scored using the Radiation Therapy Oncology Group (RTOG) scale and European Organisation for Research and Treatment of Cancer (EORTC)/RTOG scale, respectively.22 HRQOL was assessed at random assignment, every 2 months during ADT, every 4 months for the first year after completion of ADT, every 6 months during years 2 to 5, and annually thereafter using the EORTC questionnaires (QLQ-C30 and PR.25).
Outcomes
The primary end point of this study was biochemical relapse-free survival (bRFS), defined as time from random assignment to any of the following events: biochemical progression, as defined by RTOG–American Society for Radiation Oncology Phoenix criteria23; clinical evidence of recurrence in the absence of biochemical progression; or initiation of salvage ADT, despite lack of per-protocol clinical or biochemical progression. Secondary end points included OS, metastasis-free survival (MFS), local progression-free survival (LPFS), cumulative incidence of relapse, treatment-related toxicity, and HRQOL. Results of the comparison of HRQOL between treatment groups will be reported separately.
Statistical Methods
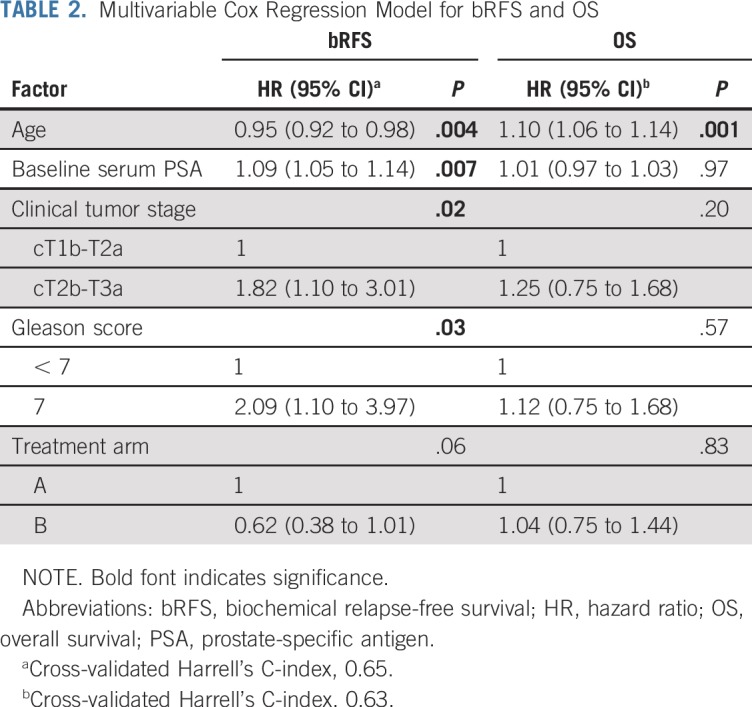
On the basis of previous studies, we postulated that 5-year bRFS would be 65% for the arm with inferior efficacy.10,24 We designed the trial to detect a 15% absolute difference in bRFS between the two arms, with 90% power and a 2-sided α of 5%. A 2-sided significance test was justified, because either arm could potentially have a better outcome. The estimated sample size with this statistical design was 394, and with allowance for 10% dropout, the overall sample size was set at 434. The Kaplan-Meier method was used to estimate bRFS, LPFS, MFS, and OS in the intention-to-treat population. For bRFS, LPFS, and MFS, patients who were event free at the time of analysis were censored at the date of last follow-up with known biochemical or clinical status. For OS, patients were censored at the date they were last known to be alive. Median follow-up was estimated using the reverse Kaplan-Meier method. The primary statistical method of comparison for time-to-event end points was the log-rank test, with stratification by 3 prespecified factors: T stage, Gleason score, and baseline PSA. Univariable Cox regression (UVR) was applied to obtain the treatment effect (hazard ratio [HR] with 95% CI) on bRFS and OS. HRs were defined such that values < 1 favored arm B relative to arm A. Secondary multivariable Cox regression (MVR) was performed for estimation of adjusted treatment effect (HR) on bRFS and OS. Covariables included Gleason score, T stage, PSA, and age at random assignment (post hoc inclusion); age and PSA were incorporated as continuous variables. Efron’s method was used for handling ties. Proportionality assumptions of the multivariable models were tested using the Grambsch-Therneau test.25 A competing-risk analysis was performed for estimating cumulative incidence of relapse using Fine and Gray’s methods. Death resulting from any cause was considered a competing event. The adjusted subdistribution HR (SHR) was estimated using competing-risk regression. Incidence of acute grade ≥ 2 GI and genitourinary toxicities was compared between the two arms using the weighted Mantel-Haenszel test. For late RT-related GI and genitourinary toxicities, overall (cumulative) toxicity scores were taken as the highest of the 2 scores. The earliest date from either source on which a toxicity grade ≥ 3 was reported was defined as the date of incidence of grade ≥ 3 late toxicity. Incidence probabilities of grade ≥ 3 late toxicity were estimated by the Kaplan-Meier method and compared between the 2 arms by the log-rank test. Patients without toxicity were censored at their last follow-up or death. Statistical calculations were performed using SPSS software (version 21; IBM Corp, Armonk, NY) and R software (version 3.5.3; R Foundation, Vienna, Austria).26
RESULTS
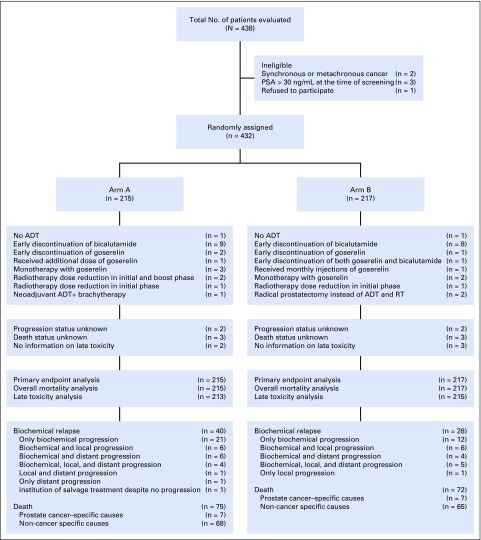
Between July 12, 2002, and March 28, 2012, 438 patients were evaluated for this study. Overall, 432 patients were randomly assigned: 215 to arm A and 217 to arm B. Baseline characteristics of randomly assigned patients were well balanced between the 2 arms (Table 1). One patient in arm A underwent brachytherapy, and 2 patients in arm B underwent radical prostatectomy. Median duration of RT in arms A and B was 56 days (interquartile range [IQR], 55-59) and 57 days (IQR: 55-59), respectively. RT dose was reduced in 3 patients in arm A and 1 patient in arm B. Median duration of ADT was 5.6 months in both arms. Bicalutamide was discontinued early in 9 and 8 patients and goserelin monotherapy was offered in 3 and 2 patients in arms A and B, respectively. One patient in arm B had early discontinuation of both bicalutamide and goserelin. One patient in each arm did not receive ADT (Fig 1).
TABLE 1.
Patient Characteristics
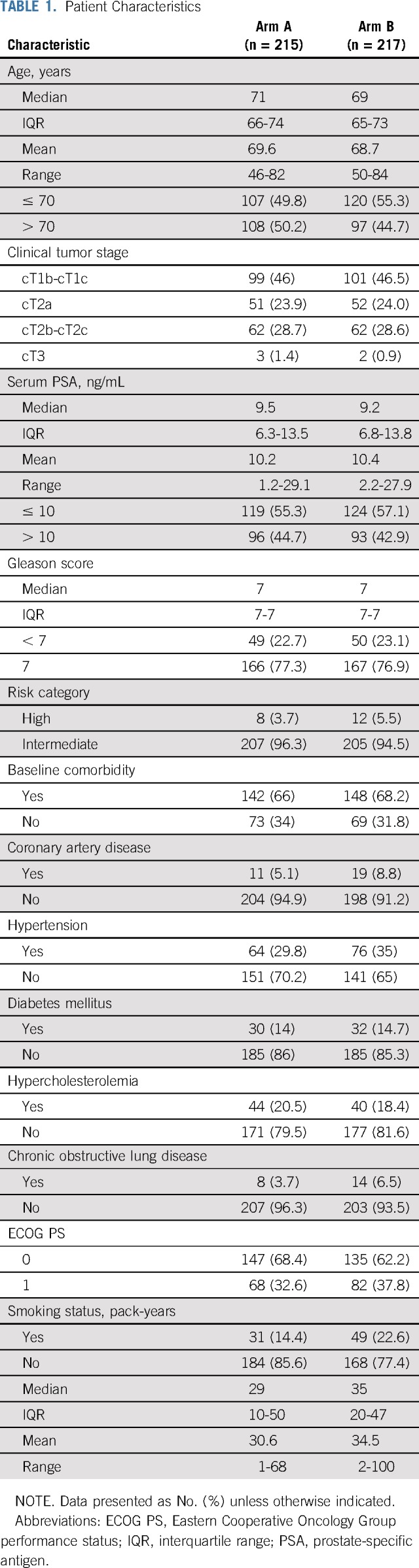
FIG 1.
CONSORT diagram. ADT, androgen-deprivation therapy; PSA, prostate-specific antigen; RT, radiotherapy.
As of the data cutoff date (May 9, 2019), median follow-up of the surviving patients in the entire cohort was 146 months (arm A, 148.2 months; arm B, 143.2 months). A total of 40 and 28 patients experienced relapse in arms A and B, respectively. Sensitivity analysis was performed to identify any systematic imbalance in the response-assessment schedules between the two groups (Appendix Table A1, online only). No significant difference was found in median time to assessments.
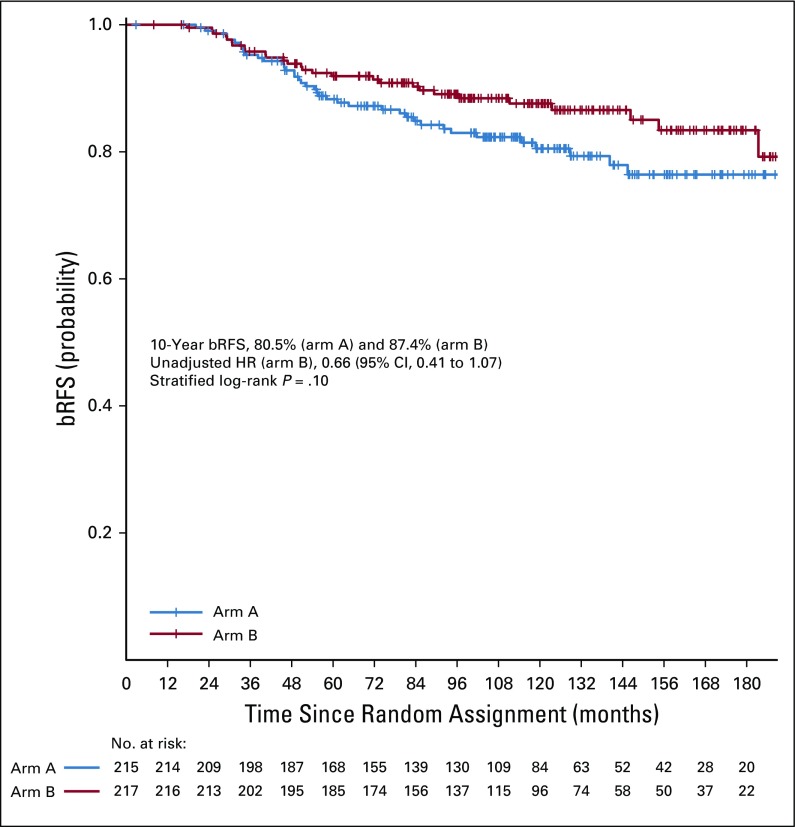
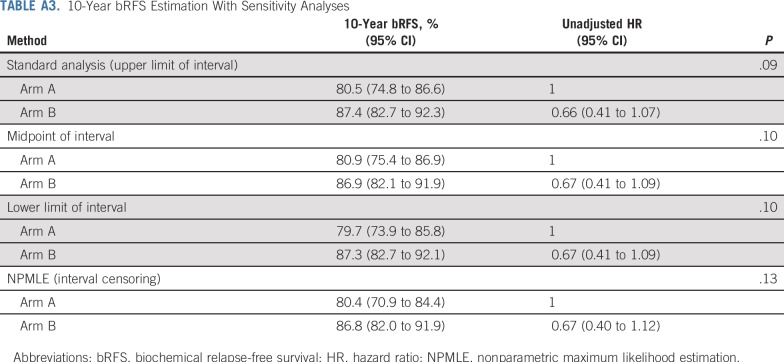
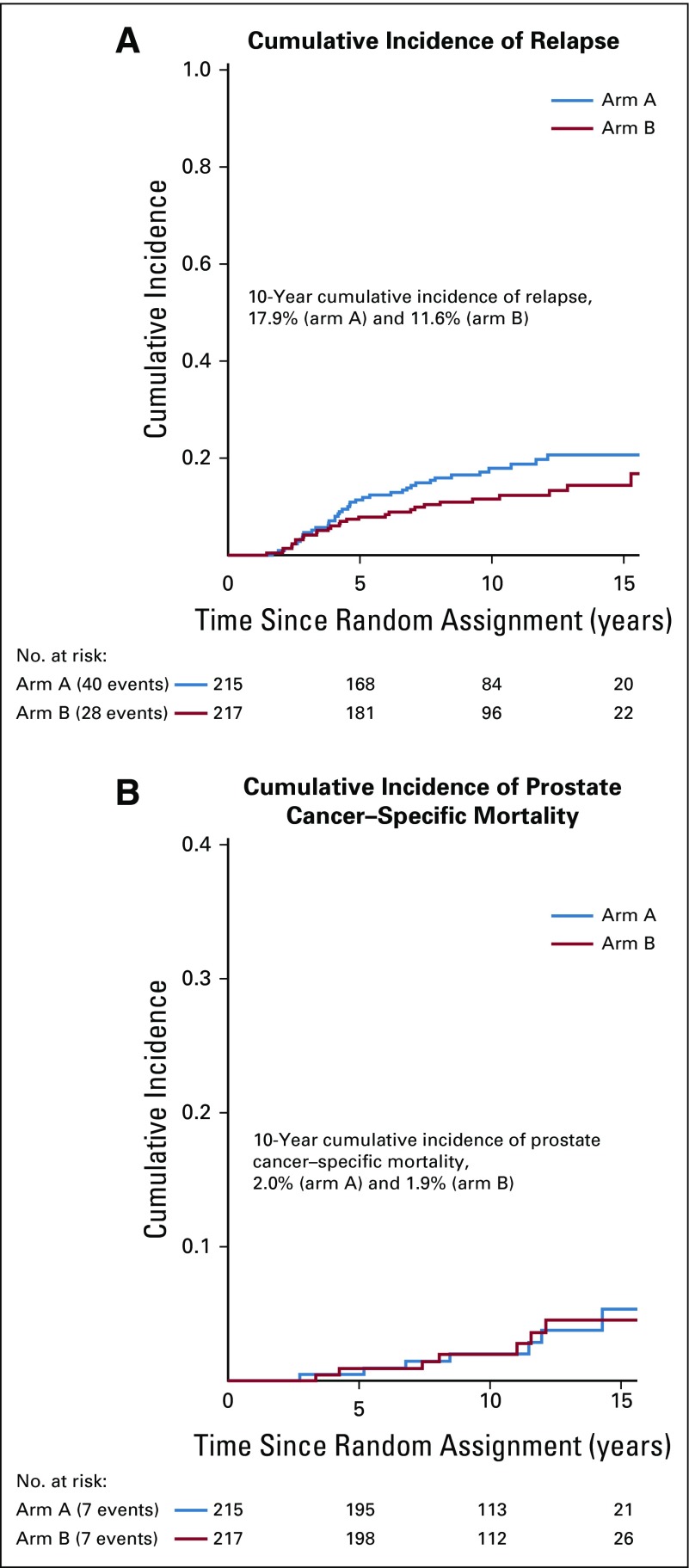
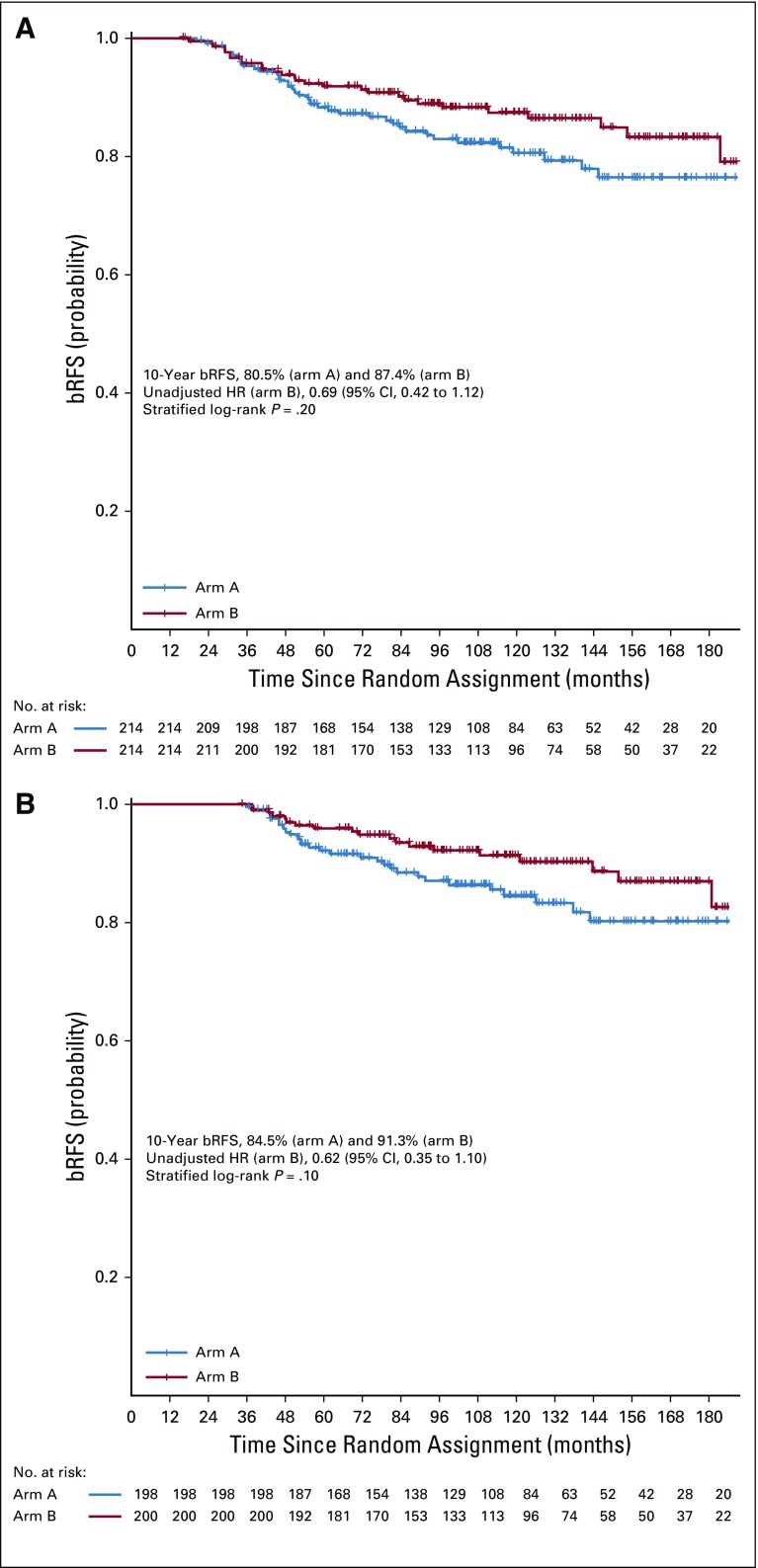
Ten-year bRFS for the entire cohort was 83.6% (95% CI, 79.8% to 87.6%). Five- and 10-year bRFS rates were 88.3% (95% CI, 84.0% to 92.8%) and 80.5% (95% CI, 74.8% to 86.6%), respectively, in arm A and 91.8% (95% CI, 88.1% to 95.6%) and 87.4% (95% CI, 82.7% to 92.3%), respectively, in arm B. There was no significant difference between the 2 arms on stratified log-rank test (P = .10). The HR of bRFS for arm B, relative to arm A, was 0.66 (95% CI, 0.41 to 1.07; P = .09; Fig 2). On MVR, arm B was associated with 38% relative reduction in the risk of relapse (HR, 0.62; 95% CI, 0.38 to 1.01; P = .06). Cumulative incidence of relapse at 10 years was 17.9% in arm A and 11.6% in arm B (SHR for arm B, 0.63; 95% CI, 0.38 to 1.07; P = .07; Appendix Fig A3). Post hoc landmark analyses (Appendix Table A2) and analysis to evaluate the impact of possible difference in interval assessment (Appendix Table A3) on bRFS are summarized in the Appendix (Appendix Fig A4, online only).27,28
FIG 2.
Kaplan-Meier graph of biochemical relapse-free survival (bRFS). HR, hazard ratio.
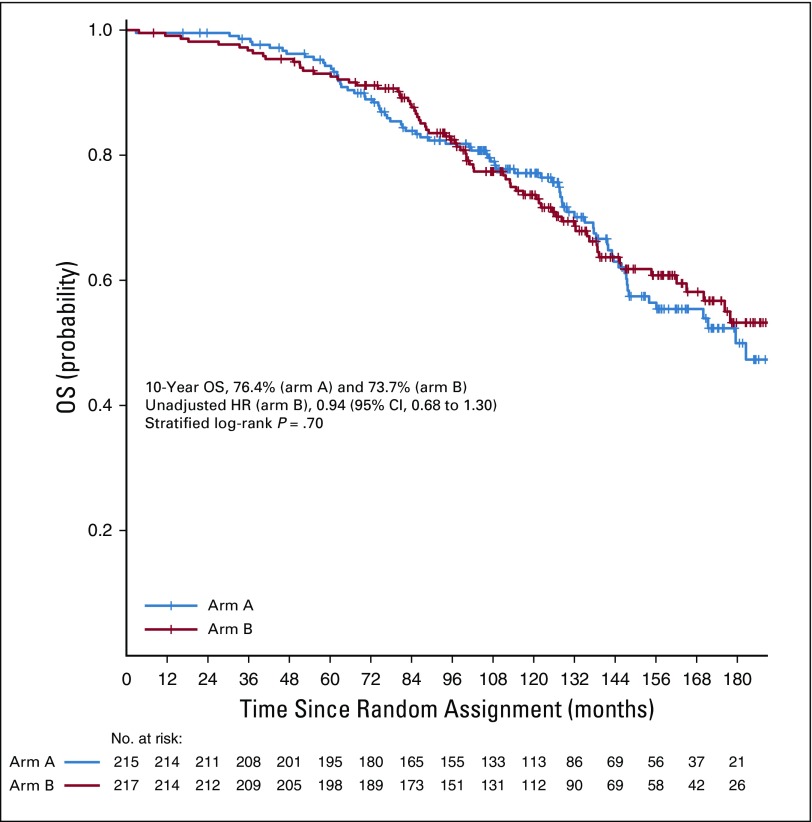
A total of 147 deaths were recorded in the entire cohort (arm A, n = 75; arm B, n = 72). OS rates at 10 years for arm A and arm B were 76.4% (95% CI, 70.6% to 82.7%) and 73.7% (95% CI, 67.6% to 80.2%), respectively (Fig 3). There was no significant difference between the arms on stratified log-rank test (P = .70), UVR (HR, 0.94; 95% CI, 0.68 to 1.30; P = .7), or MVR (Table 2). Ten-year cancer-specific mortality rates were 2% and 1.9% in arms A and B, respectively (P = .98). Overall, 12 and 9 distant-progression and 11 and 12 local-progression events were recorded in arms A and B, respectively. Ten-year LPFS rates for arms A and B were 92.2% (95% CI, 86.5% to 98.3%) and 90.4% (95% CI, 83.1% to 98.2%), respectively. Ten-year MFS was 94% (95% CI, 90.0% to 98.3%) for arm A and 95.1% (95% CI, 91.5% to 98.9%) for arm B. There was no significant difference between the treatment groups on log-rank test (P = .60 for both LPFS and MFS).
FIG 3.
Kaplan-Meier graph of overall survival (OS).
TABLE 2.
Multivariable Cox Regression Model for bRFS and OS
Median time to onset of acute toxicity was 41 days (IQR, 28-53) from the initiation of RT. There was no difference in the incidence of grade ≥ 2 RT-related GI (odds ratio for arm B, 0.79; 95% CI, 0.29 to 2.10; P = .82) or genitourinary toxicity (odds ratio for arm B, 1.11; 95% CI, 0.42 to 2.90; P = .83) between the two groups. The distribution of acute grade ≥ 2 symptoms at 6, 12, and 18 weeks after the start of RT is summarized in Appendix Table A4 (online only).
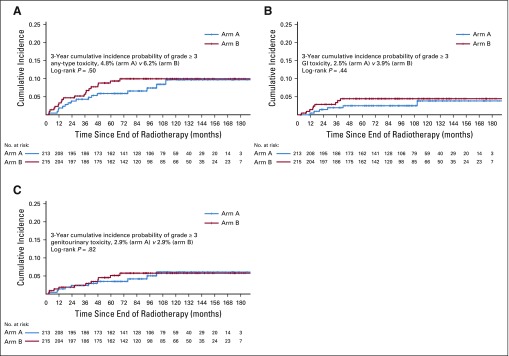
Three-year cumulative incidences of late RT-related grade ≥ 3 GI, genitourinary, and any-type toxicities were 2.5%, 2.9%, and 4.8%, respectively, in arm A and 3.9%, 2.9%, and 6.2%, respectively, in arm B, with no significant difference on log-rank test (Fig 4).
FIG 4.
Kaplan-Meier graph of cumulative incidence of late grade ≥ 3 radiotherapy-related (A) any-type, (B) GI, and (C) genitourinary toxicities.
DISCUSSION
In this phase III randomized study, we did not find any statistically significant difference in bRFS between patients who received concomitant and adjuvant ADT combined with dose-escalated PRT compared with those who received neoadjuvant and concurrent ADT with PRT. The long-term relapse rates in our study were much lower than anticipated because of limited information on the long-term efficacy of dose-escalated RT at the time of study design. Consequently, our study lacked sufficient power to achieve conventional levels of statistical significance, despite showing some evidence of improved bRFS with use of concomitant and AHT. No difference was identified between arms in the secondary end points of OS, MFS, LPFS, or RT-related morbidities.
Preclinical studies raised the hypothesis that NAHT potentiates the action of RT by allowing cytoreduction and reducing intratumoral hypoxia and may be more effective than AHT.15-17 However, a randomized trial comparing 8 with 28 weeks of NAHT followed by RT found no decrease in mortality or relapse with the longer course of ADT.29 An update from the 4-arm RTOG 9413 study showed 10-year PFS of 28.4% with NAHT followed by prostate and pelvic nodal RT (P+PNRT), 23.5% with NAHT followed by PRT, 19.4% with P+PNRT followed by AHT, and 30.2% with PRT and AHT.30 Overall, the findings suggested a sequence-dependent interaction between ADT and RT favoring NAHT in men who undergo P+PNRT and favoring the adjuvant approach in those who undergo PRT. The mechanism underlying such an interaction is unclear. In our study, patients were uniformly treated with PRT, and the findings allude to a possible improvement in bRFS with concomitant and AHT in these patients.
It is assumed that NAHT-induced tumor involution allows for effective sparing of critical structures and therefore minimizes treatment-related morbidities.31 Conversely, concern remains with commencing ADT concurrently with RT, because this might cause inconsistency in dose to critical structures as a result of dynamic changes in the size and shape of the prostate over the course of RT. We did not find any differences in RT-induced toxicity between the arms. Given the similar oncologic and toxicity outcomes, the results of our study support greater flexibility in sequencing short-term ADT with PRT in LPCa at the convenience of patients. These factors, taken together, could potentially improve adherence to treatment and thereby long-term outcomes.
We observed excellent bRFS and OS in both arms of our study. This is consistent with the results of the DART01/GICOR study, which showed no additional benefit of prolongation of ADT beyond 4 months in intermediate-risk PCa treated with dose-escalated RT.32 Bolla et al33 showed superior bRFS with addition of 6 months of ADT to RT in a study that predominantly consisted of men with intermediate-risk PCa, regardless of dose escalation from 70 to 78 Gy. Considering all this evidence, short-term ADT for 4 to 6 months remains standard systemic treatment for intermediate-risk PCa until prospective studies show a differential impact of ADT and RT combination on favorable and unfavorable intermediate-risk subgroups and provide clear evidence for altering treatment in these subsets.
Our study is subject to several limitations. Information on primary and secondary Gleason scores was incomplete. This precluded stratification of patients with Gleason score of 7 to grade groups 2 and 3 to assess differential treatment effect.34 Histopathologic confirmation of relapse was not mandatory, and this could have resulted in underestimation of clinical events in both arms. Finally, the results must be interpreted in light of the advancement in molecular imaging, which has substantially increased the accuracy and localization of recurrence after first-line treatment in PCa.35,36 Strengths of the trial include long-term follow-up (median of approximately 12 years), nearly universal adherence to protocol-specified therapy, uniform use of high-quality image-guided RT, and high rates of collection of toxicity and QOL data. Open-label studies with PFS as an end point are often susceptible to asymmetry in assessment schedules between control and study groups. Our sensitivity analysis showed a small, statistically nonsignificant difference in the time to sequential evaluations between the treatment groups; however, the difference was not directionally consistent over time.
To summarize, our study raises the possibility of a modest improvement in biochemical or clinical relapse with concomitant and adjuvant ADT rather than neoadjuvant and concurrent ADT combined with dose-escalated PRT in LPCa. This difference, however, did not reach statistical significance, possibly because of a lower-than-anticipated event rate with a corresponding loss of statistical power. No difference in late RT-related toxicity was observed between the 2 regimens. Irrespective of the sequencing strategy chosen, these results demonstrate encouraging long-term oncologic outcomes with the combination of short-term ADT and dose-escalated RT in intermediate-risk PCa. The results of our study support flexibility in tailoring the sequence of ADT and RT to optimize treatment adherence and convenience for patients with LPCa.
Appendix
Details of Radiotherapy
Patients were treated with image-guided coplanar 3-dimensional conformal radiotherapy (RT) using a 6-field isocentric beam arrangement with 18-megavolt photons. A minimum of three gold fiducials were transrectally inserted into the prostate to facilitate image guidance. The target volumes were delineated using International Commission on Radiation Units & Measurements Report 50. Clinical target volume 1 (CTV-1) encompassed the prostate and proximal 10 mm of the seminal vesicles. CTV-2 was limited to the prostate alone. Planning target volume 1 (PTV-1) was generated by expansion of CTV-1 by 10 mm in all directions except 7 mm posteriorly. PTV-2 was generated by expansion of CTV-2 by 10 mm in all directions except 5 mm in the posterior and superior directions, respectively. PTV-1 was treated to 56 Gy in 28 daily fractions over 5.5 weeks. An additional boost of 20 Gy in 10 fractions was subsequently delivered to PTV-2 over 2 weeks. Patient cases were reviewed once per week by the treating physician during RT.
Methodology for Detecting Systematic Imbalance in Response Assessment
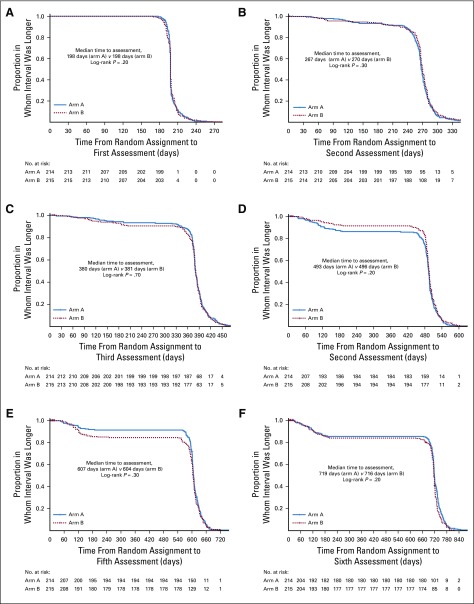
Clinical and laboratory assessments were repeated at 1 month after completion of androgen-deprivation therapy, every 4 months in the first 2 years, every 6 months for the next 3 years, and annually thereafter. We applied time-to-event analyses to rule out any systematic imbalance in the timing of the assessments between the arms. Median time to first six observed assessments was estimated in days for the two arms, respectively. Patients with clinical events or who were lost to follow-up were censored at the date of last follow-up. The log-rank test was applied for the comparison of the time to evaluation between the two treatment groups.
Results
Although a maximum 3-day gap was noted between the two groups with respect to time to second, fourth, fifth, and sixth assessments, the difference was not statistically significant. The direction of difference was not consistent across the first six observed assessments. The results are summarized in Appendix Table A1 and Figure A2, respectively.
Additional Landmark Analyses and Interval Censoring Based Analyses
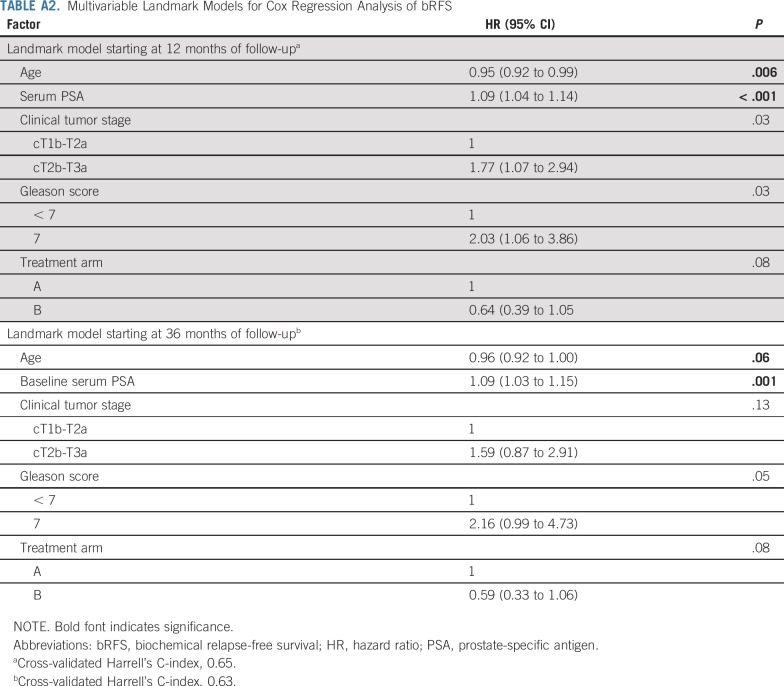
We performed two post hoc analyses to evaluate the treatment effect on biochemical relapse-free survival (bRFS) in two landmark populations starting at 12 and 36 months of follow-up, respectively. The 12-month landmark population included patients who were still alive and being observed at 12 months after random assignment, whereas the 36-month landmark population included patients who were alive and being observed at 36 months after random assignment. Univariable and multivariable Cox regressions were used to determine unadjusted and adjusted treatment effects, respectively. Multiple sensitivity analyses were carried out to evaluate the impact of possible difference in interval assessment on bRFS. To assess the bRFS precisely over time, we used a nonparametric maximum likelihood estimation of the survival curve using an expectation maximization and iterative convex minorant algorithm (interval-censored procedure). Additionally, we performed sensitivity analyses based on standard time-to-event estimation (Kaplan-Meier product limit method) assuming the progression time was in the middle of the interval or at the lower limit of the interval compared with the upper limit of the interval, which corresponded to the primary method of bRFS estimation.
At 12 months.
For the landmark analysis of bRFS at 12 months, a total of 428 patients were alive, still being observed, and free from biochemical relapse. Among the 4 patients excluded, 3 were dead and 1 ended follow-up before the landmark. Ten-year bRFS rates for arm A and arm B were 80.5% (95% CI, 74.8% to 86.6%) and 87.4% (95% CI, 82.7% to 92.3%), respectively (Appendix Fig A4A). The unadjusted hazard ratio (HR) for arm B, relative to arm A, was 0.69 (95% CI, 0.42 to 1.12; P = .13). On multivariable Cox regression, arm B was associated with a 36% relative reduction in risk of biochemical relapse (HR, 0.64; 95% CI, 0.39 to 1.05; P = .08; Appendix Table A2).
At 36 months.
For the landmark analysis at 36 months, 398 patients were eligible. Among the 34 patients who were excluded, 10 patients were dead and 24 ended follow-up assessments before the landmark. bRFS rates at 10 years for arm A and arm B were 84.5% (95% CI, 79.0% to 90.3%) and 91.3% (95% CI, 87.1% to 95.7%), respectively (Appendix Fig A4B). The unadjusted HR for arm A, relative to arm B, was 0.62 (95% CI, 0.35 to 1.10; P = .10). After adjustment for covariables, arm A was associated with a 41% relative reduction in risk of biochemical relapse (HR, 0.59; 95% CI, 0.33 to 1.06; P = .08; Appendix Table A2).
Interval Censoring Based Analyses
By the interval-censored procedure, 10-year bRFS rates for arm A and arm B were 80.4% (95% CI, 70.9% to 84.4%) and 86.8% (95% CI, 82.0% to 91.9%), respectively. Using the lower limit of the interval, 10-year bRFS rates in arm A and B were 79.7% (95% CI, 73.9% to 85.8%) and 87.3% (95% CI, 82.7% to 92.1%), respectively, whereas using the midpoint of the interval, 10-year bRFS rates were 80.9% (95% CI, 75.4% to 86.9%) and 86.9% (95% CI, 82.1% to 91.9%), respectively. The results of the additional sensitivity analyses of bRFS are summarized in Appendix Table A3.
TABLE A1.
Time to Assessment for Two Treatment Regimens
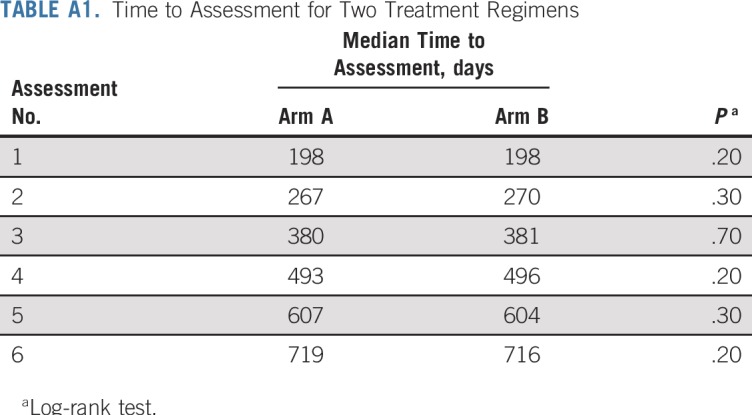
TABLE A2.
Multivariable Landmark Models for Cox Regression Analysis of bRFS
TABLE A3.
10-Year bRFS Estimation With Sensitivity Analyses
TABLE A4.
Distribution of Acute Treatment-Related Toxicity at 6 and 18 Weeks After Completion of RT
FIG A1.
Study design. ADT, androgen-deprivation therapy; EBRT, external-beam radiotherapy.
FIG A2.
Kaplan-Meier graph of time to (A) first, (B) second, (C) third, (D) fourth, (E) fifth, and (F) sixth post-treatment response assessments in the two treatment groups.
FIG A3.
Cumulative incidence of (A) relapse, (B) prostate cancer specific mortality using competing risk based analysis; Kaplan-Meier graph of (C) metastasis-free survival (MFS), and (D) local progression-free survival (LPFS). HR, hazard ratio.
FIG A4.
Kaplan-Meier graph of biochemical relapse-free survival (bRFS) from (A) 12- and (B) 36-month landmark analyses. HR, hazard ratio.
The fund provider played no role in study design, data collection, analysis, interpretation, or writing of the manuscript. The corresponding author had full access to all the data and had final responsibility for the submission.
PRIOR PRESENTATION
Presented in part at the 60th Annual Meeting of the American Society for Radiation Oncology, San Antonio, TX, Oct 21-24, 2018.
SUPPORT
Supported by AstraZeneca, which provided a grant-in-aid.
AUTHOR CONTRIBUTIONS
Conception and design: Shawn Malone, Choan E
Administrative support: Julia Craig, Scott Grimes, Kyle Malone
Provision of study material or patients: Julie Bowen, Rajiv Samant, Scott Morgan, Gad Perry, Libni Eapen, Choan E, Robert MacRae
Collection and assembly of data: Shawn Malone, Soumyajit Roy, Robert MacRae, Gad Perry, Julie Bowen, Rajiv Samant, Scott Morgan, Julia Craig, Kyle Malone, Scott Grimes
Data analysis and interpretation: Shawn Malone, Soumyajit Roy, Libni Eapen, Robert MacRae, Rajiv Samant, Scott Morgan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Sequencing of Androgen-Deprivation Therapy With External-Beam Radiotherapy in Localized Prostate Cancer: A Phase III Randomized Controlled Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Shawn Malone
Honoraria: Astellas, Janssen, Sanofi, Bayer
Travel, Accommodations, Expenses: Tersera, Sanofi
Choan E
Travel, Accommodations, Expenses: Sanofi Canada
Julie Bowen
Honoraria: Janssen Oncology
Speakers’ Bureau: Janssen Oncology
Scott Morgan
Consulting or Advisory Role: Janssen, Bayer, Astellas Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 2.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: Long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. doi: 10.1016/j.ijrobp.2007.06.054. Kuban DA, Tucker SL, Dong L, et al: Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 70:67-74, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Michalski JM, Moughan J, Purdy J, et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: The NRG Oncology RTOG 0126 randomized clinical trial. JAMA Oncol. 2018;4:e180039. doi: 10.1001/jamaoncol.2018.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. doi: 10.1016/S1470-2045(14)70040-3. Dearnaley DP, Jovic G, Syndikus I, et al: Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol 15:464-473, 2014. [DOI] [PubMed] [Google Scholar]
- 6. doi: 10.1200/JCO.2007.13.9881. Roach M III, Bae K, Speight J, et al: Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol 26:585-591, 2008. [DOI] [PubMed] [Google Scholar]
- 7. doi: 10.1016/S1470-2045(11)70063-8. Denham JW, Steigler A, Lamb DS, et al: Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol 12:451459, 2011. [DOI] [PubMed] [Google Scholar]
- 8. doi: 10.1016/S1470-2045(10)70223-0. Bolla M, Van Tienhoven G, Warde P, et al: External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 11:1066-1073, 2010. [DOI] [PubMed] [Google Scholar]
- 9. doi: 10.1056/NEJMoa1012348. Jones CU, Hunt D, McGowan DG, et al: Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med 365:107-118, 2011. [DOI] [PubMed] [Google Scholar]
- 10. doi: 10.1200/JCO.1997.15.3.1013. Pilepich MV, Caplan R, Byhardt RW, et al: Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: Report of Radiation Therapy Oncology Group Protocol 85-31. J Clin Oncol 15:1013-1021, 1997. [DOI] [PubMed] [Google Scholar]
- 11. doi: 10.1016/j.ijrobp.2004.08.047. Pilepich MV, Winter K, Lawton CA, et al: Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma: Long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys 61:1285-1290, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Spina CS: Androgen deprivation therapy and radiation therapy for prostate cancer: The mechanism underlying therapeutic synergy. Transl Cancer Res 7:S695-S703, 2018. [Google Scholar]
- 13. doi: 10.1038/nrurol.2015.50. Locke JA, Pra AD, Supiot S, et al: Synergistic action of image-guided radiotherapy and androgen deprivation therapy. Nat Rev Urol 12:193-204, 2015. [DOI] [PubMed] [Google Scholar]
- 14. doi: 10.1016/j.brachy.2016.11.017. Keyes M, Merrick G, Frank SJ, et al: American Brachytherapy Society Task Group report: Use of androgen deprivation therapy with prostate brachytherapy—A systematic literature review. Brachytherapy 16:245-265, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zietman AL, Nakfoor BM, Prince EA, et al: The effect of androgen deprivation and radiation therapy on an androgen-sensitive murine tumor: An in vitro and in vivo study. Cancer J Sci Am 3:3136, 1997. [PubMed] [Google Scholar]
- 16. doi: 10.1016/s0360-3016(97)00559-2. Granfors T, Damber JE, Bergh A, et al: Combined castration and fractionated radiotherapy in an experimental prostatic adenocarcinoma. Int J Radiat Oncol Biol Phys 39:1031-1036, 1997. [DOI] [PubMed] [Google Scholar]
- 17. doi: 10.1016/s0360-3016(03)00539-x. Kaminski JML, Hanlon AL, Joon DL, et al: Effect of sequencing of androgen deprivation and radiotherapy on prostate cancer growth. Int J Radiat Oncol Biol Phys 57:24-28, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Spratt DE, Evans MJ, Davis BJ, et al. Androgen receptor upregulation mediates radioresistance after ionizing radiation. Cancer Res. 2015;75:4688–4696. doi: 10.1158/0008-5472.CAN-15-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. doi: 10.1093/jnci/djs239. Kozin SV, Duda DG, Munn LL, et al: Neovascularization after irradiation: What is the source of newly formed vessels in recurring tumors? J Natl Cancer Inst 104:899-905, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. doi: 10.1016/j.clgc.2014.12.009. Weller MA, Kupelian PA, Reddy CA, et al: Adjuvant versus neoadjuvant androgen deprivation with radiotherapy for prostate cancer: Does sequencing matter? Clin Genitourin Cancer 13:e183-e189, 2015. [DOI] [PubMed] [Google Scholar]
- 21. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv2nom-4-30-99-final3.pdf Cancer Therapy Evaluation Program: Common Toxicity Criteria, Version 2.0: Notice of Modifications.
- 22. doi: 10.1016/0360-3016(95)00060-C. Cox JD, Stetz J, Pajak TF: Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol 31:1341-1346, 1995. [DOI] [PubMed] [Google Scholar]
- 23. doi: 10.1016/j.ijrobp.2006.04.029. Roach M III, Hanks G, Thames H Jr, et al: Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 65:965-974, 2006. [DOI] [PubMed] [Google Scholar]
- 24. doi: 10.1016/s0360-3016(98)00089-3. Hanks GE, Hanlon AL, Schultheiss TE, et al: Dose escalation with 3D conformal treatment: five year outcomes, treatment optimization, and future directions. [Internet]. Int J Radiat Oncol Biol Phys 41:501-510, 1998. [DOI] [PubMed] [Google Scholar]
- 25. Therneau TM, Grambsch PM: Modeling Survival Data: Extending the Cox Model. New York, NY, Springer-Verlag, 2000. [Google Scholar]
- 26.The R Project for Statistical Computing https://www.r-project.org/
- 27. Turnbull BW: The empirical distribution function with arbitrarily grouped, censored and truncated data. J R Stat Soc Ser B 38:290-295, 1976. [Google Scholar]
- 28. doi: 10.1093/jnci/djk091. Panageas KS, Ben-Porat L, Dickler MN, et al: When you look matters: The effect of assessment schedule on progression-free survival. J Natl Cancer Inst 99:428-432, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Pisansky TM, Hunt D, Gomella LG, et al. Duration of androgen suppression before radiotherapy for localized prostate cancer: Radiation Therapy Oncology Group randomized clinical trial 9910. J Clin Oncol. 2015;33:332–339. doi: 10.1200/JCO.2014.58.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roach M, Moughan J, Lawton CAF, et al. Sequence of hormonal therapy and radiotherapy field size in unfavourable, localised prostate cancer (NRG/RTOG 9413): Long-term results of a randomised, phase 3 trial. Lancet Oncol. 2018;19:1504–1515. doi: 10.1016/S1470-2045(18)30528-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. doi: 10.1111/luts.12142. Washino S, Hirai M, Saito K, et al: Impact of androgen deprivation therapy on volume reduction and lower urinary tract symptoms in patients with prostate cancer. Low Urine Tract Symptoms 10:57-63, 2018. [DOI] [PubMed] [Google Scholar]
- 32. doi: 10.1016/S1470-2045(15)70045-8. Zapatero A, Guerrero A, Maldonado X, et al: High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): A randomised, controlled, phase 3 trial. Lancet Oncol 16:320-327, 2015. [DOI] [PubMed] [Google Scholar]
- 33. doi: 10.1200/JCO.2015.64.8055. Bolla M, Maingon P, Carrie C, et al: Short androgen suppression and radiation dose escalation for intermediate- and high-risk localized prostate cancer: Results of EORTC trial 22991. J Clin Oncol 34:1748-1756, 2016. [DOI] [PubMed] [Google Scholar]
- 34. doi: 10.1016/j.eururo.2015.06.046. Epstein JI, Zelefsky MJ, Sjoberg DD, et al: A contemporary prostate cancer grading system: A validated alternative to the Gleason score. Eur Urol 69:428-435, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dietlein F, Kobe C, Neubauer S, et al. PSA-stratified performance of 18F- and 68Ga-PSMA PET in patients with biochemical recurrence of prostate cancer. J Nucl Med. 2017;58:947–952. doi: 10.2967/jnumed.116.185538. [DOI] [PubMed] [Google Scholar]
- 36.Mena E, Lindenberg ML, Shih JH, et al. Clinical impact of PSMA-based 18F-DCFBC PET/CT imaging in patients with biochemically recurrent prostate cancer after primary local therapy. Eur J Nucl Med Mol Imaging. 2018;45:4–11. doi: 10.1007/s00259-017-3818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]