Abstract
PURPOSE
The Children’s Oncology Group trial ACNS0121 estimated event-free survival (EFS) and overall survival for children with intracranial ependymoma treated with surgery, radiation therapy, and—selectively—with chemotherapy. Treatment was administered according to tumor location, histologic grade, and extent of resection. The impacts of histologic grade, focal copy number gain on chromosome 1q, and DNA methylation profiles were studied for those undergoing surgery and immediate postoperative conformal radiation therapy (CRT).
METHODS
ACNS0121 included 356 newly diagnosed patients (ages 1 to 21 years). Patients with classic supratentorial ependymoma were observed after gross total resection (GTR). Those undergoing subtotal resection received chemotherapy, second surgery, and CRT. The remaining patients received immediate postoperative CRT after near-total resection or GTR. CRT was administered with a 1.0-cm clinical target volume margin. The cumulative total dose was 59.4 Gy, except for patients who underwent GTR and were younger than age 18 months (who received 54 Gy). Patients were enrolled between October 2003 and September 2007 and were observed for 5 years. Supratentorial tumors were evaluated for RELA fusion; infratentorial tumors, for chromosome 1q gain. Classification of posterior fossa groups A and B was made by methylation profiles.
RESULTS
The 5-year EFS rates were 61.4% (95% CI, 34.5% to 89.6%), 37.2% (95% CI, 24.8% to 49.6%), and 68.5% (95% CI, 62.8% to 74.2%) for observation, subtotal resection, and near-total resection/GTR groups given immediate postoperative CRT, respectively. The 5-year EFS rates differed significantly by tumor grade (P = .0044) but not by age, location, RELA fusion status, or posterior fossa A/posterior fossa B grouping. EFS was higher for patients with infratentorial tumors without 1q gain than with 1q gain (82.8% [95% CI, 74.4% to 91.2%] v 47.4% [95% CI, 26.0% to 68.8%]; P = .0013).
CONCLUSION
The EFS for patients with ependymoma younger than 3 years of age who received immediate postoperative CRT and for older patients is similar. Irradiation should remain the mainstay of care for most subtypes.
INTRODUCTION
Ependymomas vary in clinicopathologic features, molecular characteristics, and lethality. In children, ependymomas commonly arise at midline or lateral compartments of the posterior fossa.1 Incidence is highest in children younger than 5 years of age. Inconsistent manifestation of clinical symptoms can lead to diagnosis delay, extension of local tumor, and association with critical neural and vascular structures.2
Historically, age and tumor location were prognostic factors for treatment selection. Extent of tumor resection was limited to avoid morbidity and reduced the adjuvant radiation therapy (RT) and chemotherapy effectiveness. RT was avoided because of its somatic, endocrine, and cognitive impacts. Two age-based approaches were taken: (1) children age 3 years or younger were treated with surgery, multiagent conventional chemotherapy, and RT delayed by 1 to 2 years or until tumor progression occurred; and (2) children older than age 3 years were treated with combined modality therapy and postoperative conventional irradiation with or without combination chemotherapy.3-6
Use of immediate high-dose postoperative conformal RT (CRT) was investigated in children as young as 12 months of age.7 CRT showed promise to reduce radiation exposure to normal tissues and to limit complications.8 To better improve local tumor control, methods to optimize the extent of resection before irradiation, including second surgery, were implemented.9 The benefit was clearly demonstrated in a trial that showed increased tumor control rates and cognitive sparing in pediatric patients who received CRT.10,11 For patients who received immediate postoperative CRT, 7-year event-free survival (EFS) and overall survival (OS) were 76.9% and 85%, respectively. Subsequent adverse effects were reduced, and effects on hearing and the function of endocrine and other systems could be predicted by radiation dosimetry.12-14
The ACNS0121 trial was activated in 2003 by the Children’s Oncology Group to validate use of immediate postoperative CRT and an aggressive surgical approach in pediatric ependymoma. ACNS0121 was designed to estimate the local control and pattern of failure in children with completely resected, classic (differentiated), supratentorial localized ependymoma after surgery; to estimate the rate of complete resection with second surgery after chemotherapy for children; to estimate the local control and pattern of failure for children with localized ependymoma treated with three-dimensional CRT using an anatomically defined 1-cm clinical target volume (CTV); to determine the influence of histologic grade on time to progression after CRT; and to determine the effect of focal copy number gains or losses and genomic profiling on outcome.15
In a major departure from previous strategies, ACNS0121 was, to our knowledge, the first cooperative group trial to use immediate postoperative irradiation for newly diagnosed children age 3 years or younger with ependymoma. The trial was the first, also, to our knowledge, to investigate the rate and pattern of failure in patients with ependymoma treated according to rigorously defined target volume definitions and a minimal CTV margin. Herein, we report patient outcomes by treatment and stratum, RELA fusion and chromosome 1q status, and ependymoma molecular subgroup.
METHODS
Eligibility
Patients with histologically confirmed nonmetastatic intracranial ependymoma were eligible for enrollment within 56 days of initial surgical resection. Age at enrollment was older than 1 year and younger than 21 years. Magnetic resonance imaging of the brain and spine was required to confirm the extent of disease and resection. Patients previously treated with chemotherapy or RT were not eligible. Informed consent and assent were obtained according to institutional guidelines.
Treatment Regimens and Stratification
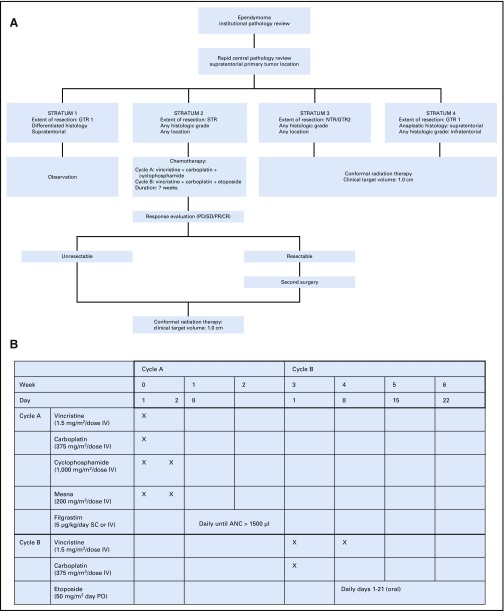
Protocol treatment was based on extent of resection, tumor location, and histologic grade. The protocol included three treatment regimens and four strata (Fig 1).
FIG 1.
(A) Protocol schema and (B) chemotherapy schema. ANC, absolute neutrophil count; CR, complete response; CTV, clinical target volume; GTR, gross total resection; IV, intravenous; NTR, near-total resection; PD, progressive disease; PO, oral; PR, partial response; SC, subcutaneous; SD, stable disease; STR, subtotal resection.
Surgery
Multiple resections before enrollment were permitted. Gross tumor resection 1 (GTR 1) was defined as no visible residual tumor identified under the operating microscope and no evidence of disease in postoperative neuroimaging. GTR 2 was defined as microscopically visible residual tumor identified under the operating microscope and no evidence of disease in postoperative neuroimaging. Near-total resection (NTR) was defined as residual tumor evident on postoperative neuroimaging with thickness or nodularity measuring 0.5 cm or smaller in the greatest dimension. Subtotal resection was defined as residual tumor measuring greater than 0.5 cm on postoperative imaging.
Pathology and Molecular Analyses
Enrollment was based on institutional diagnosis. Patients in stratum 1 underwent immediate pathology review. Other patients underwent central pathology review within 5 days of enrollment. Tumors were classified and graded according to the 2007 WHO criteria. Molecular analyses were performed as described in the Data Supplement.
Radiation Therapy and Chemotherapy
Conformal or intensity-modulated photon and proton therapy using passive scattering methods was allowed. Only 20 patients were treated with passively scattered proton therapy in this study. Gross tumor volume included the postoperative tumor bed and residual tumor; the CTV included an anatomically defined margin of 1.0 cm surrounding the gross tumor volume; and the planning target volume included a geometric margin of 0.3 to 0.5 cm surrounding the CTV. The CTV was meant to include subclinical microscopic disease. The planning target volume was meant to account for variation in daily treatment. The prescribed total dose was 59.4 Gy, using conventional fractionation of 1.8 Gy per day. Patients younger than 18 months with GTR 1 or GTR 2 received 54 Gy. Patients in stratum 2 received two cycles of chemotherapy, which began within 21 days of enrollment; included one cycle of vincristine, carboplatin, and cyclophosphamide and one cycle of vincristine, carboplatin, and oral etoposide; and lasted approximately 7 weeks.
Response and Evaluation
Required neuroimaging and follow-up information are described in the Data Supplement. A modified version of RECIST was used to evaluate responses. Additional information about response is in the Data Supplement. Common Toxicity Criteria, version 2.0, was used for toxicity and performance reporting, and adverse events of grades 3 and 4 were reported to the Children’s Oncology Group database. This study did not include uniform digital treatment planning data submission, which limited evaluation of radiation dose to the brainstem and other critical normal tissue volumes.
Statistical Analyses
EFS was defined as time to disease progression, disease relapse, occurrence of a second neoplasm, or death as a result of any cause measured from the time of study enrollment. The product-limit method estimated EFS and OS probability. The log-rank test compared survival curves. The McNemar test tested the agreement in pathology. Cumulative incidence of local failure was defined as the time to local failure measured from the date of patient enrollment. Distant failure, second malignancy, or death before local failure were considered as competing events. Cumulative incidence of distant failure was defined as time to distant failure measured from the date of patient enrollment. Local failure, second malignancy, or death before distant failure were considered as competing events.16
RESULTS
Patients and Eligibility
From October 2, 2003 to September 28, 2007, the study enrolled 378 pediatric patients from 115 institutions. The median age was 5.6 years (range, 1.01 to 21.01 years), and 108 (28.6%) were age 3 years or younger. The eligible study group had 356 patients: 150 (42.12%) were women or girls, and 206 (57.87%) were men or boys. Information about eligibility and misdiagnosis are in the Data Supplement. Diagnosis was classic ependymoma in 215 patients (60.39%) and was anaplastic ependymoma in 141 patients (39.61%). Tumor location was infratentorial in 258, supratentorial in 96, and transtentorial in two patients.
Outcomes by Treatment
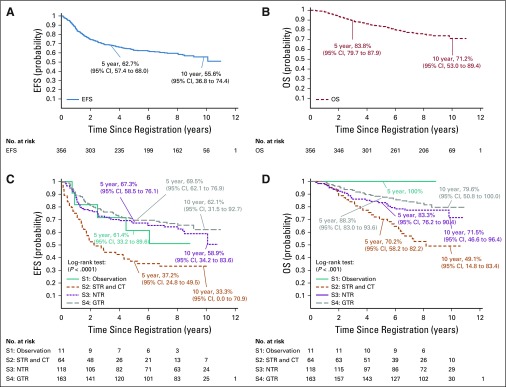
At a median follow-up time of 7.89 years (range, 0.09 to 11.00 years), the 5-year EFS and OS were 62.7% (95% CI, 57.4% to 70.0%) and 83.8% (95% CI, 79.7% to 87.9%), respectively (Fig 2). Overall responses and toxicities were reported by stratum 2 or combined strata 3 and 4 and are available in the Data Supplement.
FIG 2.
(A) Event-free survival (EFS) and (B) overall survival (OS) for study cohort and (C) EFS and (D) OS by stratum: S1, stratum 1; S2, stratum 2; S3, stratum 3; S4, stratum 4. CT, chemotherapy; GTR, gross total resection;; NTR, near-total resection; STR, subtotal resection.
Stratum 1 included 11 eligible patients. The 5-year EFS was 61.4% (95% CI, 33.2% to 89.6%). Local control was achieved in six patients (54.55%); local failure occurred in four patients (36.36%), and local and distant failure occurred in one patient (9.09%). The 5-year OS was 100%.
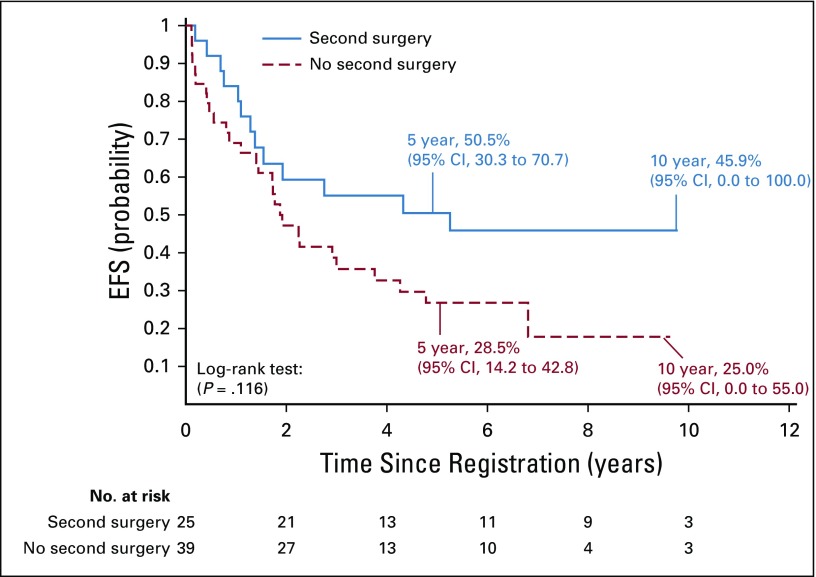
Stratum 2 included 64 eligible patients. The 5-year EFS was 37.2% (95% CI, 24.8% to 49.5%). Local control was achieved in 28 patients (47.46%); local failure occurred in 27 patients (45.76%). Local and distant failure occurred in four patients (6.78%), and distant failure occurred in five patients (8.47%). Second surgery occurred in 39% of patients. For those undergoing second surgery, the 5-year EFS was 50.5% (95% CI, 30.3% to 70.7%) compared with 28.5% (95% CI, 14.2% to 42.8%) for those not undergoing second surgery (P = .1163; Appendix Fig A1, online only). The 5-year OS was 70.2% (95% CI, 58.2% to 82.2%) for patients in stratum 2.
Stratum 3 included 118 eligible patients. The 5-year EFS was 67.3% (95% CI, 58.5% to 76.1%). Local control was achieved in 88 patients (74.58%); local failure occurred in 27 patients (22.88%). Local and distant failure occurred in three patients (2.54%), and distant failure occurred in 13 patients (11.02%). The 5-year OS was 83.3% (95% CI, 76.2% to 90.4%).
Stratum 4 included 163 eligible patients. The 5-year EFS was 69.5% (95% CI, 62.1% to 76.9%). Local control was achieved in 129 patients (79.14%) patients; local failure occurred in 30 patients (18.4%). Local and distant failure occurred in four patients (2.45%), and distant failure occurred in 13 patients (7.98%). The 5-year OS was 88.3% (95% CI, 83.0% to 93.6%). When data from strata 3 and 4 for those who received immediate postoperative irradiation (n = 281), were combined, the 5-year EFS and OS rates were 68.5% (95% CI, 62.8% to 74.2%) and 86.2% (95% CI, 81.9% to 90.6%), respectively.
Outcomes by Tumor Grade and Age
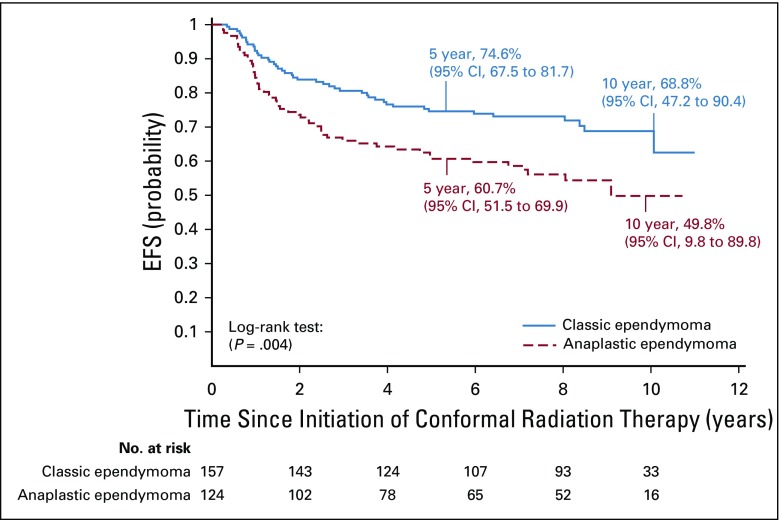
The difference in agreement of institutional and central review by the McNemar test was not significant (P = .6374). The 5-year EFS was 74.6% (95% CI, 67.5% to 81.7%) for patients with classic ependymoma and was 60.7% (95% CI, 51.5% to 69.9%) for those with anaplastic ependymoma treated with immediate postoperative irradiation (P = .0044; Fig 3).
FIG 3.
Event-free survival (EFS) for patients treated with immediate postoperative radiation therapy (strata 3 and 4) by ependymoma pathologic subtype.
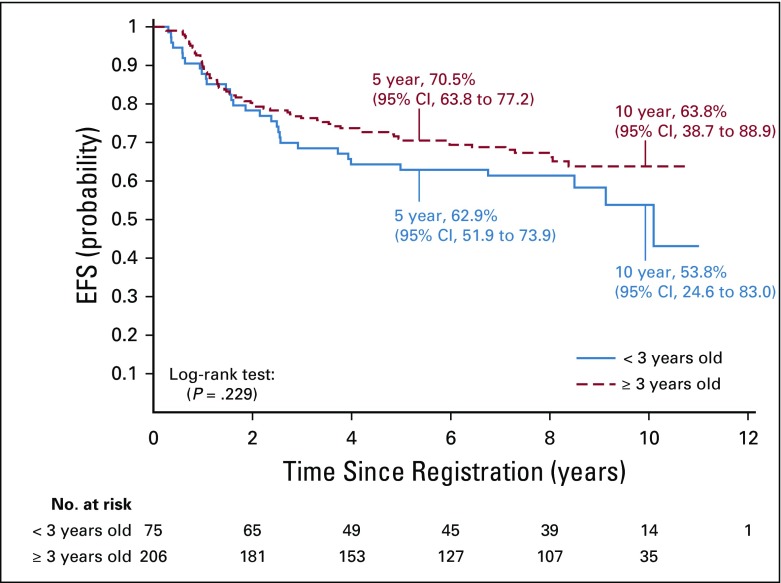
The 5-year EFS rates were 62.9% (95% CI, 51.9% to 73.9%) and 70.5% (95% CI, 63.8% to 77.2%) for patients younger than age 3 years versus age 3 years or older, respectively, at enrollment who were treated with immediate postoperative irradiation (P = .2295). The respective OS rates for those age groups were 87.4% (95% CI, 79.6% to 95.2%) and 85.8% (95% CI, 80.7% to 90.9%; P = .6904; Appendix Fig A2).
Outcomes by RELA Fusion, 1q Gain, and PFA/PFB Subgrouping
The interphase fluorescence in situ hybridization analysis identified RELA fusion in 30 (77%) of 39 supratentorial tumors. There were no significant differences in EFS, OS, or pattern of failure by fusion status. Also, there were no significant differences in EFS, OS, or pattern of failure between 23 patients with RELA fusion–positive tumors treated with surgery and immediate postoperative irradiation and five patients with non-RELA fusion–positive tumors who received similar treatment.
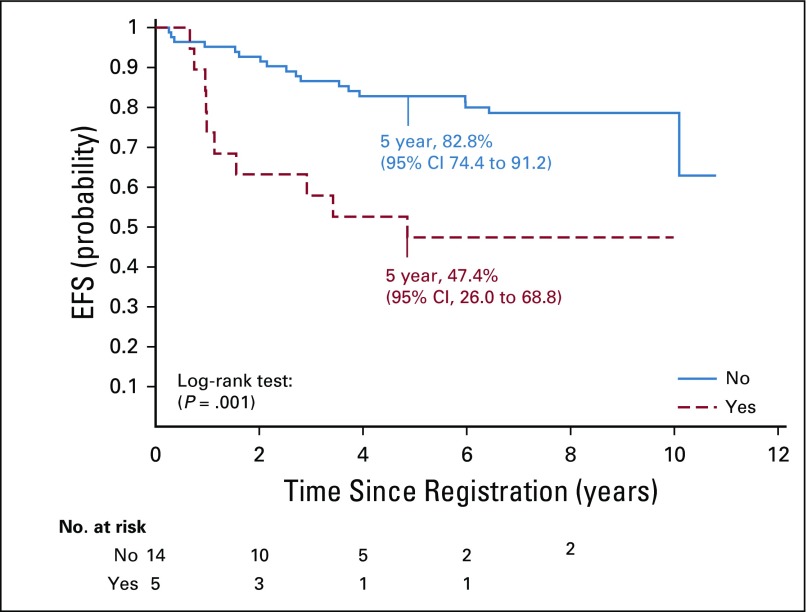
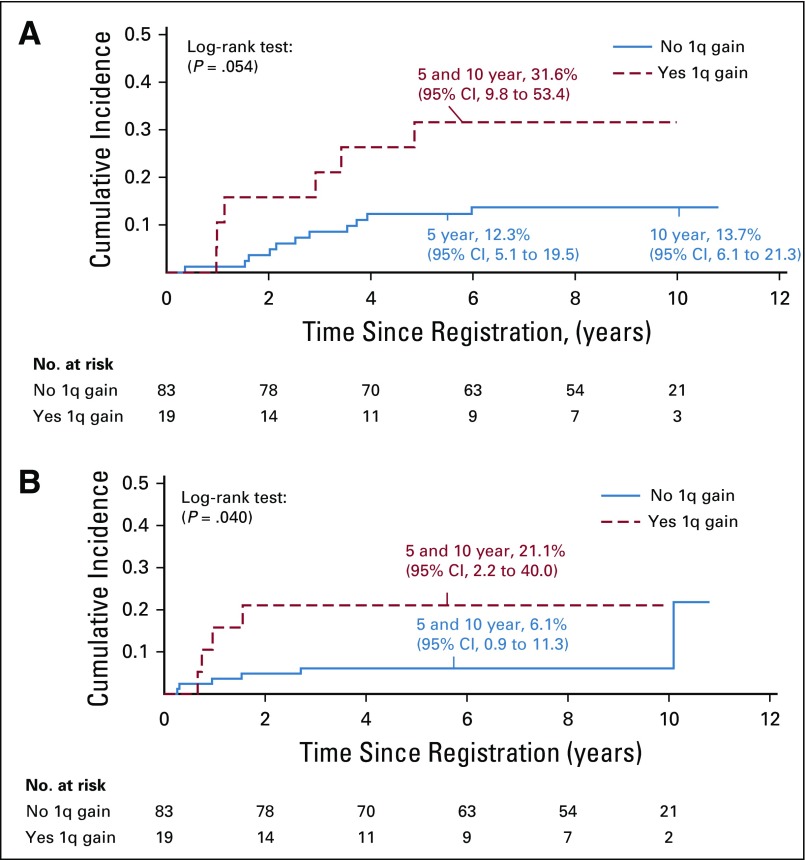
The interphase fluorescence in situ hybridization analysis detected gain of 1q in 24 (20%) of 121 patients with infratentorial tumors: five who received treatment on stratum 2, and 19 who received immediate postoperative irradiation. For patients in stratum 2, there was no association between 1q gain status and failure pattern or EFS; however, the 5-year OS was 20% (95% CI, 0.0% to 44.7%) for those with 1q gain versus 84.6% (95% CI, 65.8% to 100.0%) for those without 1q gain (P = .0189). For patients who received immediate postoperative irradiation, the 5-year EFS rates were 82.8% (95% CI, 74.4% to 91.2%) versus 47.4% (95% CI, 26.0% to 68.8%) for those without and with 1q gain, respectively (P = .0013; Fig 4). The respective OS rates were 91.3% (95% CI, 85.0% to 97.8%) versus 68.4% (95% CI, 48.2% to 88.6%; P = .0028). There was a significant association between local and distant failure patterns and 1q gain (P = .0101). The 5-year cumulative incidence of local failure was 31.6% (95% CI, 9.8% to 53.4%) versus 12.3% (95% CI, 5.1% to 19.5%) for those with and without 1q gain, respectively (P = .054); the cumulative incidence for distant failure was 21.1% (95% CI, 2.2% to 40.0%) versus 6.1% (95% CI, 0.9% to 11.3% for those with and without 1q gain, respectively (P = .040; Fig 5).
FIG 4.
Event-free survival (EFS) for patients treated with immediate postoperative radiation therapy (strata 3 and 4) according to 1q gain status.
FIG 5.
Cumulative incidence of (A) local and (B) distant failure for patients treated with immediate postoperative radiation therapy (strata 3 and 4).
The patients in stratum 2 (n = 18) and in the combined strata 3 and 4 (n = 100) were classified according to PFA (posterior fossa group A)/PFB (posterior fossa group B) subgrouping (Data Supplement). No significant associations between PFA/PFB subgrouping and failure pattern, EFS, or OS for either treatment existed. Outcomes of patients who received treatment on strata 3 and 4 were comparable with those of the subset who contributed to the PFA/PFB analysis. The 5-year EFS and OS rates were 76.6% (95% CI, 68.0% to 85.2%) and 87.8% (95% CI, 81.1% to 94.5%), respectively.
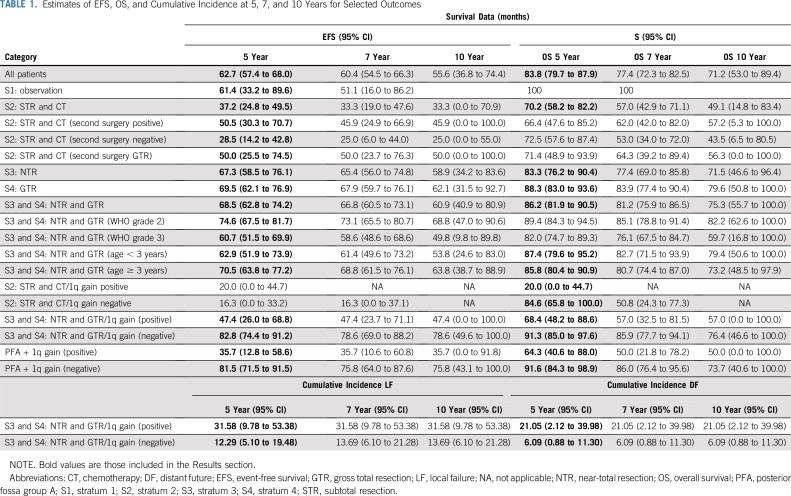
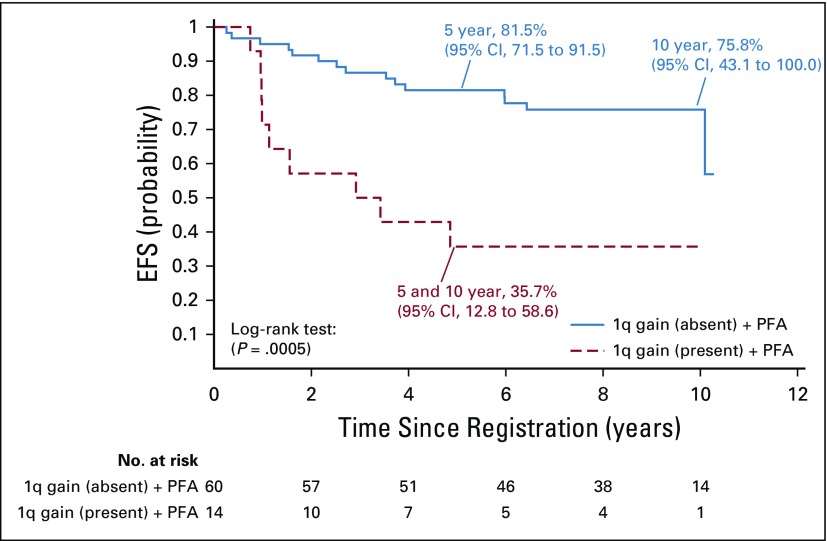
We evaluated 101 patients who received surgery and immediate postoperative CRT for combined effects of 1q gain and PFA/PFB grouping on EFS (P = .0011) and OS (P = .0119). Differences were most pronounced between those with the PFA subgroup and 1q gain versus those with the PFA subgroup and no 1q gain: The 5-year EFS was 35.7% (95% CI, 12.8% to 58.6%) versus 81.5% (95% CI, 71.5% to 91.5%; P = .0005; Appendix Fig A3), respectively; the 5-year OS was 64.3% (95% CI, 40.6% to 88.0%) versus 91.6% (95% CI, 84.3% to 98.9%; P = .0019), respectively. Table 1 lists estimates of EFS and OS at 5, 7, and 10 years for selected outcomes.
TABLE 1.
Estimates of EFS, OS, and Cumulative Incidence at 5, 7, and 10 Years for Selected Outcomes
Second Malignancies
Seven patients had second malignancies. Second malignancies were distributed by stratum across stratum 2 (n = 2), stratum 3 (n = 1), and stratum 4 (n = 4). The cumulative incidence of second malignancy was 3.43% (95% CI 0.4%-6.5%) when estimated at 10 years (Table 1). Second malignancy diagnosis information was not collected.
DISCUSSION
This is the first study, to our knowledge, to prospectively observe patients after complete resection of classic supratentorial ependymoma. Earlier, supratentorial anaplastic ependymoma was considered more aggressive than classic ependymoma and more common in older than younger children.
The rationale for using histologic grade in treatment stratification was based on a blinded pathology review of 50 patients, which showed that histologic grade affected progression-free survival (PFS; P < .001) after irradiation.17 Observation after resection was empirically recommended by some groups, which made it a controversial option that was not resolved by this prospective trial.18-20 Surgery alone was an effective strategy in some patients and continues to be used in the successor ACNS0831 trial.21
ACNS0121 is the first prospective trial, to our knowledge, to recommend the use of chemotherapy and second surgery before irradiation. Trial design was influenced by data that suggested easier tumor resection after chemotherapy and reduction in surgical morbidity by allowing patients to heal between resections. Chemotherapy provided time to improve surgical planning or access to expert teams.
Surgical resection is the most important prognostic factor for children with ependymoma. Children who achieve complete resection have the highest PFS and OS rates. An earlier series, in which second resection was systematically performed before CRT, reported the highest rates of local control, PFS, and OS.11 Second surgery was often feasible, depending on location of the residual tumor.
Although the main goal for patients in stratum 2 was to use chemotherapy to facilitate second surgery before CRT, only 39% of patients underwent second surgery, and only 56% achieved GTR. The ACNS0831 trial was activated in 2010.21 The study randomly assigned patients to post-CRT chemotherapy or to observation, a logical next step beyond surgery and immediate postoperative irradiation.
The SIOP-EP-II study is testing conventional and novel agents as front-line treatment in patients with ependymoma.22 Patients with no evidence of residual disease are randomly assigned to receive 16 weeks of multiagent chemotherapy or observation after irradiation. Patients with residual disease receive preirradiation conventional chemotherapy with or without methotrexate and post-CRT conventional chemotherapy. Patients not eligible to receive CRT because of age or other considerations receive conventional chemotherapy for 1 year with or without the histone deacetylase inhibitor valproate. To combat local failures, patients with residual disease receive additional irradiation, given data to suggest that escalation of the RT dose can improve outcomes.23
A groundbreaking aspect of this study was that immediate postoperative CRT was administered to very young children. Rates of disease control and survival of children age 3 years or younger who achieved NTR or GTR and who were receiving immediate postoperative CRT were similar to those of older children. The survival of very young children with newly diagnosed ependymoma was higher than previous studies using chemotherapy after surgery to delay or avoid irradiation. The landmark Pediatric Oncology Group (POG) study POG 9233 (conducted from 1992 to 1997) used standard or dose-intensified chemotherapy for children with ependymoma, with radiation therapy deferred to time of progression.6 The 5-year EFS and OS rates for the entire POG 9233 cohort were 24.4% (95% CI, 15.3% to 33.5%) and 42.7% (95% CI, 32.1% to 53.3%), respectively. In this ACNS0121 study, the 5-year EFS and OS rates for the most favorable cohort (combined strata 3 and 4) were 68.5% (95% CI, 62.8% to 74.2%) and 86.2% (95% CI, 81.9% to 90.5%), respectively. For the less favorable subtotal resection cohort, the 5-year EFS and OS rates were 37.2% (95% CI, 24.9% to 49.5%) and 70.2% (95% CI, 58.2% to 82.2%), respectively.
In ACNS0121, EFS and OS were better for older patients than they were for those patients in the CCG9942 study (conducted from 1995 to 1999), which included chemotherapy before RT for selected patients. The study reported 5-year EFS and OS rates of 57% and 71%, respectively.5 In this ACNS0121 study, 5-year EFS and OS rates were 65% and 83%, respectively, for the entire cohort older than age 3 years and were 71% and 85%, respectively, for those who received immediate postoperative CRT.
Intraobserver variability in the histopathologic assessment of ependymoma was less than expected. Survival outcomes by tumor grade were consistent. These findings are important because the histopathologic assessment of ependymoma has come into question, along with the need to incorporate and validate molecular markers.24,25
Nine distinct molecular groups of ependymoma have been identified across the neuraxis.26 Patients with RELA fusion–positive tumors were considered to have a poor prognosis.27 Our data confirm that most supratentorial tumors have RELA fusion. Contrary to previous reports, patients with RELA fusion–positive tumors do not have uniformly poor survival when treated with immediate postoperative irradiation.26 Prognostic significance of 1q gain in infratentorial ependymoma has been examined. Gain of 1q was observed at diagnosis in 16 (13%) of 119 patients with infratentorial ependymoma who received uniform CRT.28 The hazard ratio for tumor progression was 2.51, which was highly significant (P = .008), and confirmed that 1q gain is a marker for increased local and distant tumor progression.
Prognostic significance of the PFA and PFB distinction was not shown among patients who received uniform CRT. These results will serve as an important benchmark for future studies that propose treatment intensification or reduction in selected cohorts.29,30 Children with a PFA tumor who undergo GTR clearly benefit from CRT. Postoperative irradiation for PFA should remain the backbone of current treatment protocols. Children with PFA tumors without 1q gain fare well with surgery and irradiation.
In summary, EFS and OS rates of very young children treated with immediate postoperative CRT after GTR or NTR in this study were more than twice that of children treated using strategies that delayed the use of irradiation. Survival for young children was similar to those of older children who received front-line CRT. CRT is confirmed as an effective adjuvant therapy, irrespective of subgrouping. RELA fusion–positive tumors comprise the majority of supratentorial tumors, and patients with RELA fusion–positive tumors who receive CRT do not have a uniformly poorer survival. Patients with PFA tumors without 1q gain have excellent outcomes with surgery and CRT.
Appendix
FIG A1.
Event-free survival (EFS) for patients in stratum 2 who were undergoing versus not undergoing second surgery.
FIG A2.
Event-free survival (EFS) for patients treated with immediate postoperative radiation therapy (strata 3 and 4) by age at time of conformal radiation therapy.
FIG A3.
Event-free survival (EFS) for patients with infratentorial tumors and posterior fossa group A (PFA) classification who were treated with immediate postoperative radiation therapy (strata 3 and 4) according to 1q gain status.
Footnotes
Presented in part at the 57th Annual Meeting of the American Society for Radiation Oncology, San Antonio, TX, October, 18-21, 2015.
Supported by Children’s Oncology Group (COG) Operations Grant No. U10 CA098543, COG Statistics and Data Center Grant No. U10 CA098413, COG Operations Grant No. U10 CA180886, and COG Statistics and Data Center Grant No. U10 CA180899; by the Ontario Institute for Cancer Research through funding provided by the Government of Ontario, Ministry of Research, Innovation, and Science; and in part by American Lebanese Syrian and Associated Charities.
Research reported in this publication was supported by Cancer Oncology Group (COG), but COG did not have a role in study design, or in the collection, analysis, or interpretation of the data, in writing of the report, or in the decision to submit the paper for publication.
AUTHOR CONTRIBUTIONS
Conception and design: Thomas E. Merchant, Anne E. Bendel, Eric Chang, Tianni Zhou, Nicholas K. Foreman, Juliette Hukin, Ian F. Pollack, Fred H. Laningham, Robert H. Lustig, Floyd D. Armstrong, Chris Williams-Hughes, Mehmet Kocak
Collection and assembly of data: Thomas E. Merchant, Anne E. Bendel, Noah D. Sabin, Peter C. Burger, Tianni Zhou, David D. Eisenstat, Christine E. Fuller, Edwina Templeton Anderson, Ching C. Lau, Fred H. Laningham, Floyd D. Armstrong, Michael H. Handler, Sandra Kessel, David W. Ellison, Vijay Ramaswamy
Provision of study material or patients: Thomas E. Merchant, Anne E. Bendel, David D. Eisenstat, Ian F. Pollack, Michael H. Handler
Data analysis and interpretation: Thomas E. Merchant, Anne E. Bendel, Noah D. Sabin, Peter C. Burger, Dennis W. Shaw, Shengjie Wu, Tianni Zhou, David D. Eisenstat, Nicholas K. Foreman, Ching C. Lau, Robert H. Lustig, Mehmet Kocak, David W. Ellison, Vijay Ramaswamy
Administrative support: Thomas E. Merchant, Ian F. Pollack
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Conformal Radiation Therapy for Pediatric Ependymoma, Chemotherapy for Incompletely Resected Ependymoma, and Observation for Completely Resected, Supratentorial Ependymoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Thomas E. Merchant
Travel, Accommodations, Expenses: Philips Healthcare
Eric Chang
Honoraria: Brainlab
Consulting or Advisory Role: Toshiba
Research Funding: DFINE (Inst)
Travel, Accommodations, Expenses: Brainlab
Juliette Hukin
Stock and Other Ownership Interests: AbbVie (I)
Michael H. Handler
Travel, Accommodations, Expenses: Brainlab
No other potential conflicts of interest were reported.
REFERENCES
- 1.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro-oncol. 2017;19:v1–v88. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merchant TE, Gilbertson RJ. 2010. Ependymoma, in Estlin EJ, Gilbertson RJ, Wynn RF (eds): Pediatric Hematology and Oncology. Wiley-Blackwell, Oxford, United Kingdom, [Google Scholar]
- 3.Duffner PK, Horowitz ME, Krischer JP, et al. The treatment of malignant brain tumors in infants and very young children: An update of the Pediatric Oncology Group experience. Neuro-oncol. 1999;1:152–161. doi: 10.1093/neuonc/1.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans AE, Anderson JR, Lefkowitz-Boudreaux IB, et al. Adjuvant chemotherapy of childhood posterior fossa ependymoma: Cranio-spinal irradiation with or without adjuvant CCNU, vincristine, and prednisone—A Childrens Cancer Group study. Med Pediatr Oncol. 1996;27:8–14. doi: 10.1002/(SICI)1096-911X(199607)27:1<8::AID-MPO3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 5.Garvin JH, Jr, Selch MT, Holmes E, et al. Phase II study of pre-irradiation chemotherapy for childhood intracranial ependymoma: Children’s Cancer Group protocol 9942—A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2012;59:1183–1189. doi: 10.1002/pbc.24274. [DOI] [PubMed] [Google Scholar]
- 6.Strother DR, Lafay-Cousin L, Boyett JM, et al. Benefit from prolonged dose-intensive chemotherapy for infants with malignant brain tumors is restricted to patients with ependymoma: A report of the Pediatric Oncology Group randomized controlled trial 9233/34. Neuro-oncol. 2014;16:457–465. doi: 10.1093/neuonc/not163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merchant TE, Zhu Y, Thompson SJ, et al. Preliminary results from a phase II trial of conformal radiation therapy for pediatric patients with localised low-grade astrocytoma and ependymoma. Int J Radiat Oncol Biol Phys. 2002;52:325–332. doi: 10.1016/s0360-3016(01)01807-7. [DOI] [PubMed] [Google Scholar]
- 8.Merchant TE. Current management of childhood ependymoma. Oncology (Williston Park) 2002;16:629–642,644. [PubMed] [Google Scholar]
- 9.Morris EB, Li C, Khan RB, et al. Evolution of neurological impairment in pediatric infratentorial ependymoma patients. J Neurooncol. 2009;94:391–398. doi: 10.1007/s11060-009-9866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conklin HM, Li C, Xiong X, et al. Predicting change in academic abilities after conformal radiation therapy for localized ependymoma. J Clin Oncol. 2008;26:3965–3970. doi: 10.1200/JCO.2007.15.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merchant TE, Li C, Xiong X, et al. Conformal radiotherapy after surgery for paediatric ependymoma: A prospective study. Lancet Oncol. 2009;10:258–266. doi: 10.1016/S1470-2045(08)70342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merchant TE, Gould CJ, Xiong X, et al. Early neuro-otologic effects of three-dimensional irradiation in children with primary brain tumors. Int J Radiat Oncol Biol Phys. 2004;58:1194–1207. doi: 10.1016/j.ijrobp.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Merchant TE, Rose SR, Bosley C, et al. Growth hormone secretion after conformal radiation therapy in pediatric patients with localized brain tumors. J Clin Oncol. 2011;29:4776–4780. doi: 10.1200/JCO.2011.37.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merchant TE, Sharma S, Xiong X, et al. Effect of cerebellum radiation dosimetry on cognitive outcomes in children with infratentorial ependymoma. Int J Radiat Oncol Biol Phys. 2014;90:547–553. doi: 10.1016/j.ijrobp.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00027846?term=nct00027846&rank=1 NCT00027846: Observation or radiation therapy and/or chemotherapy and second surgery in treating children who have undergone surgery for ependymoma.
- 16.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing Risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 17.Merchant TE, Jenkins JJ, Burger PC, et al. Influence of tumor grade on time to progression after irradiation for localized ependymoma in children. Int J Radiat Oncol Biol Phys. 2002;53:52–57. doi: 10.1016/s0360-3016(01)02801-2. [DOI] [PubMed] [Google Scholar]
- 18.Rogers L, Pueschel J, Spetzler R, et al. Is gross-total resection sufficient treatment for posterior fossa ependymomas? J Neurosurg. 2005;102:629–636. doi: 10.3171/jns.2005.102.4.0629. [DOI] [PubMed] [Google Scholar]
- 19.Hukin J, Epstein F, Lefton D, et al. Treatment of intracranial ependymoma by surgery alone. Pediatr Neurosurg. 1998;29:40–45. doi: 10.1159/000028683. [DOI] [PubMed] [Google Scholar]
- 20.Ailon T, Dunham C, Carret AS, et al. The role of resection alone in select children with intracranial ependymoma: The Canadian Pediatric Brain Tumour Consortium experience. Childs Nerv Syst. 2015;31:57–65. doi: 10.1007/s00381-014-2575-4. [DOI] [PubMed] [Google Scholar]
- 21.ClinicalTrials.gov NCT01096368: Maintenance chemotherapy or observation following induction chemotherapy and radiation therapy in treating younger patients with newly diagnosed ependymoma. https://clinicaltrials.gov/ct2/show/NCT01096368?term=nct01096368&rank=1
- 22.ClinicalTrials.gov NCT02265770: An international clinical program for the diagnosis and treatment of children with ependymoma (SIOP-EP-II) https://clinicaltrials.gov/ct2/show/NCT02265770?term=nct02265770&rank=1
- 23.Massimino M, Miceli R, Giangaspero F, et al. Final results of the second prospective AIEOP protocol for pediatric intracranial ependymoma. Neuro-oncol. 2016;18:1451–1460. doi: 10.1093/neuonc/now108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pajtler KW, Mack SC, Ramaswamy V, et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 2017;133:5–12. doi: 10.1007/s00401-016-1643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellison DW, Kocak M, Figarella-Branger D, et al. Histopathological grading of pediatric ependymoma: Reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011;10:7. doi: 10.1186/1477-5751-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pajtler KW, Witt H, Sill M, et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27:728–743. doi: 10.1016/j.ccell.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramaswamy V, Hielscher T, Mack SC, et al. Therapeutic impact of cytoreductive surgery and irradiation of posterior fossa ependymoma in the molecular era: A retrospective multicohort analysis. J Clin Oncol. 2016;34:2468–2477. doi: 10.1200/JCO.2015.65.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godfraind C, Kaczmarska JM, Kocak M, et al. Distinct disease-risk groups in pediatric supratentorial and posterior fossa ependymomas. Acta Neuropathol. 2012;124:247–257. doi: 10.1007/s00401-012-0981-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khatua S, Ramaswamy V, Bouffet E. Current therapy and the evolving molecular landscape of paediatric ependymoma. Eur J Cancer. 2017;70:34–41. doi: 10.1016/j.ejca.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Thompson YY, Ramaswamy V, Diamandis P, et al. Posterior fossa ependymoma: Current insights. Childs Nerv Syst. 2015;31:1699–1706. doi: 10.1007/s00381-015-2823-2. [DOI] [PubMed] [Google Scholar]