Abstract
Background
Early tuberculosis (TB) diagnostic is one of the critical steps to TB control. GeneXpert MTB/RIF has been widely proven for a prompt TB diagnosis. The use of GeneXpert MTB/RIF assay with transbronchial lung cryobiopsy samples may increase diagnostic accuracy. We aim to assess the diagnostic of TB with GeneXpert MTB/RIF assay with transbronchial lung cryobiopsy.
Methods
Patients with suspected diagnosis of TB and negative smear microscopies, with TB culture and GeneXpert MTB/RIF assay with transbronchial lung cryobiopsy were included in this cross-sectional study. Participants were enrolled from 2016 to 2018 at National Institute of Respiratory Diseases, Mexico.
Results
We included 54 patients (77.8% males) aged 30 to 65 years. The sensitivity of the GeneXpert MTB/RIF assay with transbronchial lung cryobiopsy was 81.3% (95% CI, 62.1–100%), with a specificity of 100% (95% CI, 100–100%) and a negative predictive value of 92.7% (95% CI, 84.7–100%). Twenty-two patients of the total population have HIV, the sensitivity of the test in these patients was 87.5% (95% CI, 64.6–100%). Also, 28 patients with a peripheral localized lesion which had a solid pattern were identified (51.9%). The sensitivity in patients with peripheral localized lesions was 88.9% (68.4–100%).
Conclusions
The GeneXpert MTB/RIF assay with transbronchial lung cryobiopsy test is as efficient as broncho alveolar lavage for TB diagnosis. Transbronchial lung cryobiopsy increases a major diagnostic opportunity when the nature of illness is malignant. Transbronchial lung cryobiopsy is efficient in HIV patients, especially in patients with peripheral localized lesion.
Keywords: Transbronchial lung cryobiopsy, GeneXpert MTB/RIF, Mycobacterium tuberculosis (MTB)
Introduction
Tuberculosis (TB) continues to be one of the most contagious diseases with one of the highest mortality rates. TB is caused by the Mycobacterium tuberculosis (MTB) bacteria. Early TB diagnostic is one of the critical steps to TB control and the sputum smears microscopy for acid fast bacilli alcohol resistance continues to be an important starting step for the diagnosis. However, its sensitivity could vary from 20% to 80% based on the mycobacteria load, as well as the trained employees who perform the test and the fact that the microscopy does not distinguish between a MTB and other mycobacteria species.
The culture of mycobacteria is the conventionally gold standard for the diagnosis; its sensitivity and specificity is of 80% and 99% respectively, however, the results could take weeks to be confirmed (1-6).
GeneXpert® MTB/RIF (Cepheid, Sunnyvale, CA, EE. UU.) is a total authorized Nucleic Acid Amplification Test (NNAT), which has been widely proven in multiple studies and has been approved by the WHO since 2010, for a prompt TB diagnosis on sputum and other non-respiratory samples. This allows the diagnosis to be performed within a few hours. It even detects low genomics MTB copies in different samples and could identify mutations related with rifampin, as well (7-13).
Confirming the diagnosis poses a clinical challenge for patients who are suspected to have clinical TB but cannot sputum; among those patients are those infected with HIV. These patients normally have negative microscopies. There are few studies regarding the use of GeneXpert® in Broncho Alveolar Lavage samples (BAL) (14-18) and even less studies with Lung Biopsy or Mediastinal Nodes samples (19-22), this is due to the fact that the procedure requires expert staff to perform the analysis. The test is performed in specialized or reference centers. Even when the latest has proven to increase the diagnostic performance. There is evidence over GeneXpert® studies on different respiratory samples of patients infected with HIV, as well, where the performance has been proven (19,22,23).
The purpose of the instant study is to evaluate the GeneXpert® MTB/RIF test in transbronchial lung cryobiopsy (TLCB).
Methods
This is a diagnostic test. An observational, retrospective, analytical study that includes data collected between June 2016 and August 2018 from the Interventional Pulmonology Unit. Patients were considered eligible if they were over 18 years of age with suspected pulmonary TB diagnosis and negative smear microscopies. Bronchoscopy with TLCB samples was performed on those patients, and samples were sent simultaneously for analysis of both TB bacterial culture and at GeneXpert® MTB/RIF. The study was approved (C13-19) by the ethics committee of the National Institute of Respiratory Diseases, Ismael Cosío Villegas (INER).
To identify the type of lesion, helical chest computed tomography (Chest CT) was performed. The lung tomographic patterns were differentiated by peripheral localized lesion and diffuse parenchymal lung lesion. A diffuse parenchymal lung lesion is analyzed by TLCB. It is performed using a technique known as bronchoscopy cryobiopsy sampling taken from the most affected area (24,25). The TLCB was performed under radial endobronchial ultrasound (R-EBUS) at the peripheral localized lesion. Each and all of the procedures were executed with a STORZ Num, 12- or 14-mm rigid bronchoscope guided by Fluoroscopy. Moreover, the rest of the procedures were performed or supervised by an expert interventional pulmonologist.
Samples were sent to be cultured in liquid (BACTEC MGIT 960) and solid (Löwenstein–Jensen) media, the species were identified using the line probe assay (HAIN) test. For drug susceptibility testing in MTB, The BACTEC MGIT 960 SIRE kit was used. Samples were also sent for GeneXpert® MTB/RIF testing.
The culture was considered as the gold standard to obtain the real prevalence TB rates, as well as to assess the GeneXpert® MTB/RIF; the TB calculated prevalence was obtained by identification through GeneXpert® MTB/RIF cases on TLCB or the BAL.
The GeneXpert® MTB/RIF results were reviewed by TLCB and BAL samples and compared with culture results of each corresponding sample; giving as a result an accurate diagnosis and performance.
Statistical analysis
A descriptive statistic was used for the study population characteristics. Categorical variables were reported as absolute and relative frequencies (percentages) and continuous variables were reported as mean (± SD) or median (interpercentile ranges) according to distribution. The sensitivity, specificity, and the negative and positive predictive values, with a 95% confidence interval were reviewed. To identify the differences among patients, with or without a TB diagnosis, the t-Student or Mann-Whitney tests were used for the independent samples, based on the data distribution. The results were stratified on patients with HIV or without it. Based on the tomographic image (diffuse or localized lesion), the results were compared throughout the χ2 or Fisher’s exact test; the Kappa index was calculated as well, in order to know the GeneXpert® MTB/RIF consistency in TLCB and BAL at the abovementioned strata. Statistical significance was established for the P<0.05 values. The Statistical analysis was carried out with IBM SPSS V.23 software. To evaluate the GeneXpert® MTB/RIF test the Win Epi calculator was utilized.
Results
Baseline characteristics
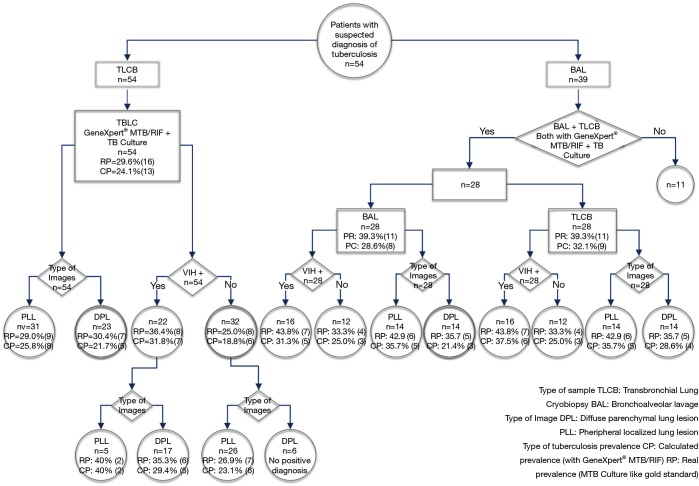
Study population: 54 patients that met the selection criteria were selected, from which 39 patients had also given samples in BAL even when only 28 of those patients had TB cultivated samples and GeneXpert® MTB/RIF and in BAL (Figure 1).
Figure 1.
Flow diagram.
From the total population assessed 77.8% (42 were male), with a mean (± SD) age of 47.8±17.6 years old. The patients average age with TB was 42.6±16.3 years old. The most frequent shown symptoms before the performance of bronchoscopy were: cough (64.8%), dyspnoea (55.6%) and weight loss (35.2%). The cough frequency was statistically greater in the TB patients’ group compared with those patients that were not diagnosed [21 (55.3%) vs. 14 (87.5%), P=0.03]. About 42.2% of patients had a background of previous antibiotics use. Being clarithromycin the most frequently used. From the total study population 40.7% [22] had a HIV infection diagnosis (Table 1).
Table 1. General characteristics of patients.
| Variable | All patients, n=54 | Patients without TB, n=38 | Patients with TB, n=16 | P |
|---|---|---|---|---|
| Age, yr, mean ± SD | 47.8 ±17.6 | 50±17.9 | 42.6±16.3 | 0.15 |
| Male, % [n] | 77.8 [42] | 84.2 [32] | 62.5 [10] | 0.15 |
| Smoking, % [n] | 50.0 [27] | 52.6 [20] | 43.8 [7] | 0.77 |
| Tobacco index (cigars/year) | 7.6 (1.85–14.25) | 8 (2.5–14.75) | 3.4 (0.6–20.8) | |
| Smoke wood exposure, % [n] | 5.6 [3] | 5.3 [2] | 6.3 [1] | 0.63 |
| Symptoms, % [n] | ||||
| Dyspnea | 55.6 [30] | 57.9 [22] | 50 [8] | 0.77 |
| Cough | 64.8 [35] | 55.3 [21] | 87.5 [14] | 0.03 |
| Weight loss | 35.2 [19] | 34.2 [13] | 37.5 [6] | 0.82 |
| Fever | 22.2 [12] | 15.8 [6] | 37.5 [6] | 0.15 |
| Hemoptysis | 7.4 [4] | 10.5 [4] | 0 | 0.31 |
| Antibiotic, % [n] | 46.3 [25] | 44.7 [17] | 50.0 [8] | 0.49 |
| Clarithromycin | 24.0 [6] | 13.2 [5] | 6.3 [1] | |
| Antituberculosis treatment | 8.0 [2] | 0 | 12.5 [2] | |
| Characteristics of the lesion identified by tomographic image | ||||
| Pheripheral localized lesion, % [n] | 57.4 [31] | 57.9 [22] | 56.3 [9] | 0.91 |
| Type of pattern, % [n] | 0.35 | |||
| Solid | 51.9 [28] | 52.6 [20] | 50.0 [8] | |
| Mix | 3.7 [2] | 0 | 12.5 [2] | |
| Subsolid | 1.9 [1] | 2.6 [1] | 0 | |
| Bronchus sign, % [n] | 50.0 [27] | 47.4 [18] | 56.3 [9] | 0.45 |
| Diameter (mm) | 35.8 (27–49.9) | 35.2 (25.3–38.3) | 44.6±15 | 0.18 |
| Diffuse parenchymal lung lesion, % [n] | 42.6 [23] | 42.1 [16] | 43.8 [7] | 0.91 |
| Type of pattern, % [n] | ||||
| Consolidation | 18.5 [10] | 23.7 [9] | 6.3 [1] | 0.28 |
| Micronodules | 13.0 [7] | 10.5 [4] | 18.8 [3] | |
| Ground glass | 7.4 [4] | 5.3 [2] | 12.5 [2] | |
| Cavitated disease | 3.7 [2] | 2.6 [1] | 6.3 [1] | |
| Patients with HIV infection, % [n] | 40.7 [22] | 36.8 [14] | 50.0 [8] | 0.55 |
| Lymphocytes (cells/µL) | 650 (325–1,450) | 700 (300–1,525) | 450 (325–1,325) | 0.11 |
| CD4 cell count (cells/µL) | 106 (29–210) | 68 (22.3–197.5) | 201 (36–264) | 0.2 |
| CD4 cell count % [n] | ||||
| >500 [State 1] | 0 | 0 | 0 | |
| 200–499 [State 2] | 36.8 [7] | 21.4 [3] | 50 [4] | 0.33 |
| <200 [State 3] | 63.2 [12] | 64.3 [9] | 37.5 [3] | |
| Viral load | ||||
| Indetectable, % [n] | 13.63 [3] | 21.4 [3] | 0 | – |
| Detectable (copies/mL) | 247,289.50 (590.25–345,000) | 252,620 (638.5–845,836.5) | 241,959 (40–255,338) | 0.45 |
Data is presented as % [n] or mean (± SD) or median (P25–P75). *, anti-TB: isoniazide, rifampicin, pyrazinamide, ethambutol.
Localized injuries were shown at 57.4% [31] of patients according to Chest CT image characteristics with a solid pattern on 51.9%, bronchus sign was shown at 87.1% of those localized injuries. The median diameter of the lesions was 35.8 (27–49.9) mm; the diameter was bigger in the TB patients’ group (44.6±15). The diffuse parenchymal lung lesions frequency was 42.6% [23] and the main lesion pattern was consolidated in 18.5% of patients (Table 1).
The Bronchoscopy diagnostic performance in the study population was 90.7%, in BAL 51.3% and 87% in TLCB.
Among the diagnosis obtained by BAL and TLCB, 51.9% had benign infectious disease origins, 24.1% were benign, non-infectious, while 14.8% were from malignant origins (Table 2).
Table 2. Final diagnosis.
| Diagnostic groups | Disease | BAL (n=39) | TLCB (n=54) | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||||
| Benign Infectious | Mycobacterium tuberculosis | 12/12 | 100 | 16/16 | 100 | 16 | 51.9 | ||
| Pneumocystis jirovecii | 3/5 | 60 | 5/5 | 100 | 5 | ||||
| Streptococcus pneumoniae | 1/1 | 100 | 1/1 | 100 | 1 | ||||
| Aspergillus flavus | 1/1 | 100 | 0/1 | – | 1 | ||||
| Staphylococcus aureus | 0/1 | – | 1/1 | 100 | 1 | ||||
| Klebsiella pneumoniae | 0/1 | – | 1/1 | 100 | 1 | ||||
| Mycobacterium avium complex | 1/1 | 100 | 1/1 | 100 | 1 | ||||
| Moraxella catarrhalis | 1/1 | 100 | 1/1 | 100 | 1 | ||||
| Paracoccidioides brasilensis | 1/1 | 100 | 0/1 | – | 1 | ||||
| Benign, non-infectious | Pneumonia | 0/6 | – | 6/6 | 100 | 6 | 24.1 | ||
| Anthracosis | 0/2 | – | 3/3 | 100 | 3 | ||||
| Granulomatous disease | 0/1 | – | 2/2 | 100 | 2 | ||||
| Interstitial lung disease | 0/1 | – | 1/1 | 100 | 1 | ||||
| Calcified nodules | 0/1 | – | 1/1 | 100 | 1 | ||||
| Malignant | Adenocarcinoma | 0/1 | – | 6/6 | 100 | 6 | 14.8 | ||
| Melanoma | – | – | 1/1 | 100 | 1 | ||||
| Poorly differentiated carcinoma | – | – | 1/1 | 100 | 1 | ||||
| No diagnostics* | 0/3 | – | 0/5 | – | 5 | 9.3 | |||
| Diagnostic yield | 20/39 | 51.3 | 47/54 | 87.0 | 49/54 | 90.7 | |||
*, three patients had diagnosis by surgical biopsy, biopsy guided by ultrasound and cervical biopsy, respectively (carcinoma, metastasic adenocarcinoma and linfoma Hodking). BAL, bronchoalveolar lavage; TLCB, transbronchial lung cryobiopsy.
Results of Xpert on TLCB
From all the 54 patients, 29.6% (Gold Standard) TB real prevalence was obtained by TLCB, while 24.1% of TB calculated prevalence was obtained by TLCB (GeneXpert®MTB/RIF). In the group of patients, with and without HIV infection, the real TB prevalence was 36.4% and 25.0%, respectively. It was shown that in patients with and without HIV infection, the real TB prevalence showing localized lesions was 40.0% and 26.9% respectively (Figure 1).
At the GeneXpert® MTB/RIF test in TLCB were identified 13 patients out of 16 were identified with TB by culture. The sensitivity test was 81.3% (95% CI, 62.1–100%), the specificity 100% (95% CI, 100–100%) and a predictive negative value of 92.7% (95% CI, 84.7–100%). The sensitivity in HIV patients was 87.5% (95% CI, 64.6–100%) and the specificity 100% (95% CI, 100–100%).
Based on the kind of lesion, it shows that the test sensitivity in patients with localized injuries was 88.9% (95% CI, 68.4–100%), the specificity 100% (95% CI, 100–100%) and the negative predictive value 95.7% (95% CI, 87.3 to 100%). At diffuse parenchymal lung lesion the sensitivity and specificity values were 71.4% (95% CI, 38–100%) and 100% (95% CI, 100–100%), respectively (Table 3).
Table 3. GeneXpert MTB/RIF assay in TLCB.
| Sample | N | Diagnostic yield, % | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Positive predictive value, % (95% CI) | Negative predictive value, % (95% CI) | Diagnostic accuracy | P |
|---|---|---|---|---|---|---|---|---|
| TLCB, global | 22 | 24.1 | 81.3 (62.1 to 100) | 100 (100 to 100) | 100 (100 to 100) | 92.7 (84.7 to 100) | 81.3 (62.1 to 100) | <0.001 |
| Patients with HIV and no HIV infection | ||||||||
| In no HIV patients | 6/32 | 18.8 | 75 (45 to 100) | 100 (100 to 100) | 100 (100 to 100) | 92.3 (82.1 to 100) | 75 (45 to 100) | <0.001 |
| In HIV patients | 7/22 | 31.8 | 87.5 (64.6 to 100) | 100 (100 to 100) | 100 (100 to 100) | 93.3 (80.7 to 100) | 87.5 (64.6 to 100) | <0.001 |
| With peripheral localized lesion | ||||||||
| TLCB | 8/31 | 25.8 | 88.9 (68.4 to 100) | 100 (100 to 100) | 100 (100 to 100) | 95.7 (87.3 to 100) | 88.9 (68.4 to 100) | <0.001 |
| In HIV patients | 2/5 | 40.0 | 100 (100 to 100) | 100 (100 to 100) | 100 (100 to 100) | 100 (100 to 100) | 100 (100 to 100) | 0.1 |
| In no HIV patients | 6/26 | 23.1 | 85.7 (59.8 to 100) | 100 (100 to 100) | 100 (100 to 100) | 95 (85.4 to 100) | 85.7 (59.8 to 100) | <0.001 |
| With diffuse parenchymal lung lesion | ||||||||
| TLCB | 5/23 | 21.7 | 71.4 (38 to 100) | 100 (100 to 100) | 100 (100 to 100) | 88.9 (74.4 to 100) | 71.4 (38 to 100) | 0.001 |
| In HIV patients | 5/17 | 29.4 | 83.3 (53.5 to 100) | 100 (100 to 100) | 100 (100 to 100) | 91.7 (76 to 100) | 83.3 (53.5 to 100) | 0.001 |
TLCB, transbronchial lung cryobiopsy.
Results of Xpert BAL
The TB real prevalence in BAL as well as in TLCB was 39.3%, while the calculated prevalence in BAL and TLCB was 28.6% and 32.1%, respectively. Within the HIV population the real prevalence of TB was 43.8%, whereas the calculated prevalence of TB in BAL and TLCB was 31.3% and 37.5%, respectively. With respect to patients with diffuse parenchymal lung lesion by Chest CT, the TB prevalence by BAL and TLCB was 21.4% and 28.6%, respectively (Figure 1).
Comparison of the Xpert between BAL and TLCB
The sensitivity and specificity of GeneXpert® MTB/RIF at BAL was 72.7% (95% CI, 46.4 to 99) and 100% (95% CI, 100–100%), respectively, and the Kappa index between the BAL and the TLCBs was 0.92 (P<0.001). In HIV patients, the sensitivity and specificity was 71.4% (95% CI, 38–100%) and 100% (95% CI, 100–100%), respectively, and the Kappa Index between the BAL and the TLCBs was 0.86 (P=0.001). On the other hand, in patients without HIV with peripheral localized lesions, The Kappa index obtained among BAL and TLCB of 1.0, at (P<0.001 and P=0.005, respectively). While in patients with diffuse parenchymal lung lesion showed a sensitivity and specificity in BAL of 60% (95% CI, 17.1–100%) and 100% (95% CI, 100–100%) respectively; and, the sensitivity and specificity in TLCB was 80% (95% CI, 44.9–100%) and 100% (95% CI, 100–100%), respectively (Table 4).
Table 4. Assessment of GeneXpert MTB/RIF assay and Kappa index in BAL and TLCB.
| Sample | n | Diagnostic yield, % | Sensitivity, % (95% CI) | Specificity | Positive predictive value, % (95% CI) | Negative predictive value, % (95% CI) | Diagnostic accuracy, % (95% CI) | P | Kappa index | P |
|---|---|---|---|---|---|---|---|---|---|---|
| TLCB | 9/28 | 32.1 | 81.8 (59 to 100) | 100 (100 to 100) | 100 (100 to 100) | 89.5 (75.7 to 100) | 81.8 (59 to 100) | <0.001 | 0.92 | <0.001 |
| BAL | 8/28 | 28.6 | 72.7 (46.4 to 99) | 100 (100 to 100) | 100 (100 to 100) | 85 (69.4 to 100) | 72.7 (46.4 to 99) | <0.001 | ||
| Patients with HIV infection | ||||||||||
| TLCB | 6/16 | 37.5 | 85.7 (59.8 to 100) | 100 (100 to 100) | 100 (100 to 100) | 90 (71.4 to 100) | 85.7 (59.8 to 100) | <0.001 | 0.86 | 0.001 |
| BAL | 5/16 | 31.3 | 71.4 (38 to 100) | 100 (100 to 100) | 100 (100 to 100) | 81.8 (59 to 100) | 71.4 (38 to100) | 0.005 | ||
| Patients without HIV infection | ||||||||||
| TLCB | 3/12 | 25.0 | 75 (32.6 to 100) | 100 (100 to 100) | 100 (100 to 100) | 88.9 (68.4 to 100) | 75 (32.6 to 100) | 0.018 | 1.0 | 0.005 |
| BAL | 3/12 | 25.0 | 75 (32.6 to 100) | 100 (100 to 100) | 100 (100 to 100) | 88.9 (68.4 to 100) | 75 (32.6 to 100) | 0.018 | ||
| With peripheral localized lesion | ||||||||||
| TLCB | 5/14 | 35.7 | 83.3 (53.5 to 100) | 100 (100 to 100) | 100 (100 to 100) | 88.9 (68.4 to 100) | 83.3 (53.5 to 100) | 0.003 | 1.0 | <0.001 |
| BAL | 5/14 | 35.7 | 83.3 (53.5 to 100) | 100 (100 to 100) | 100 (100 to 100) | 88.9 (68.4 to 100) | 83.3 (53.5 to 100) | 0.003 | ||
| With diffuse parenchymal lung lesion | ||||||||||
| TLCB | 4/14 | 28.6 | 80 (44.9 to 100) | 100 (100 to 100) | 100 (100 to 100) | 90 (71.4 to 100) | 80 (44.9 to 100) | 0.005 | 0.81 | 0.011 |
| BAL | 3/14 | 21.4 | 60 (17.1 to 100) | 100 (100 to 100) | 100 (100 to 100) | 81.8 (59 to 100) | 60 (17.1 to 100) | 0.027 | ||
BAL, bronchoalveolar lavage; TLCB, transbronchial lung cryobiopsy.
Five complications during the procedures were encountered, mainly moderate bleeding. Bleeding was controlled by adrenaline administration (Table 5).
Table 5. Complications occurred during procedures.
| No. | Diagnosis | Complication | Control |
|---|---|---|---|
| 1 | Adenocarcinoma | Moderate bleeding | adrenaline (1 mg) |
| 2 | Anthracosis | Moderate bleeding | adrenaline (1 mg) |
| 3 | No diagnosis | Moderate bleeding | adrenaline (2 mg) |
| 4 | Adenocarcinoma | Moderate bleeding | adrenaline (3 mg) |
| 5 | Pneumonia | Bronchospasm | Short-acting bronchodilator |
| Noninvasive mechanical ventilation |
Discussion
The Bronchoscopy diagnostic performance in our study population was of 90.7%, while the performance in BAL and TLCB was 51.3% and 87%, respectively; which is similar with Sánchez-Cabral et al. published paper (19). The latter obtained a Bronchoscopy diagnostic performance of 92.2%, whereas the performance in BAL and TLCB was 39.8% and 75.7%, respectively. However, our population sample consisted of patients with a high clinical suspicion of TB, while at Sánchez-Cabral et al. (19) population sample, patients had a treatment of non-interstitial pulmonary disease.
The majority of images in this population sample showed localized lesions which corresponded to the early stages of the disease. This is consistent since none of samples from TB diagnosed patients, showed, either by culture or by GeneXpert® MTB/RIF, any resistance to rifampicin. Those patents were new TB cases, which clinical file, did not allow sputum samples. They were patients without previous or recent treatment, being a protection factor for rifampicin resistance (1). While some of the diffuse parenchymal lung lesions were due mainly to a consolidation, that in addition to the localized injuries, were diagnosed as pneumonia with an identified etiologic agent or due to its histology in 33% of our population.
The TLCB results in HIV patients, with localized lesions, had a greater TB prevalence than those without HIV. Localized injuries had a major TB prevalence among patients without HIV. There was a higher TB prevalence among HIV patients with diffuse parenchymal lung lesion than in patients without HIV with the same tomographic pattern.
With respect to diagnosis obtained by BAL and TLCB within our population, 76.0% were benign in origin, among those, 68% had infectious origin. It is important to point out that the population had a high suspicion of TB. Regarding the diagnosis of benign noninfectious origin and those with malignant origin, all of those diagnosis were possible to reach by TLCB and none of them were reached by BAL. This is important due to the well-known discrepancies in diagnostics among those two different type of samples obtained by bronchoscopy, as it is described by Sánchez-Cabral et al. (20) A high percentage of our patients had localized lesions, with some a bronchus sign and a lesion with a diameter >2 cm, which together contributed to a better diagnostic performance in TLCB cases (26-29).
The INER is a national reference hospital for respiratory disease. Among those respiratory diseases TB is the most important. Our TB real prevalence on TLCB was of 29.6% (Gold standard), while the TB calculated prevalence at TLCB (GeneXpert® MTB/RIF) was 24.1%, similar to the TB prevalence published by Lu et al. (18) of 32.9%, however, Their study population was diagnosed not only by its microbiology, but different aspects were considered such as histology, clinical, images, and treatment response. If not, the microbiology would have reported a prevalence of 22.5%. similar to the TB prevalence reported by Rakotoarivelo et al. (30) who obtained a global prevalence of 32.8%, 31.7% with culture and 31.8% with GeneXpert 31.8%. However, Madagascar it considered as a high TB prevalent country while Mexico is considered as a medium TB prevalent country. The latter shows a high concentration of TB Patients treated by the INER in Mexico and not by its prevalence as stated by WHO (1). The prevalence reported by Theron et al. was 29%, similar to their study performed in South Africa, which is considered a region with a high TB prevalence (23). In the study carried out by Sánchez-Cabral et al., and performed at the INER, the reported TB prevalence was 11.7%. However, the population suffered a general pulmonary pathology not interstitial (19). While in our study each and all the patients had a high TB suspicion.
The real TB prevalence in the patients’ group, with and without HIV, was 36.4% and 25.0%, respectively, The TB/HIV association was widely addressed (31).
It this study population, no statistically significant differences were observed with respect to the prevalence in either of the groups. This is probably due to the sample size. In addition, the TB real prevalence in localized lesions in patients with and without HIV infection was 40.0% and 26.9%, respectively. This comparison in future studies requires a mayor sample scope. Nevertheless, in our study population, a pulmonary localized lesion in one of our patients with TB would allow us to increase the TB suspicions and to perform a guided MTB search in the lesion.
Our Study is different from others. In the other studies different respiratory samples were analyzed addressing the GeneXpert® MTB/RIF test at TLCB. However, due to the fact that within our population there was a subgroup with samples showed by BAL as well as by TLCB; the samples were analyzed simultaneously by culture and by GeneXpert® MTB/RIF sample. This analysis revealed the same TB real prevalence through BAL than by TLCB, but a higher calculated prevalence by TLCB than by BAL (non-significant P value); none of our patients with positive GeneXpert had a negative culture, unlike other authors that had a negative result in culture and positive in GeneXpert (1,18,30). In the same vein, it was observed that within the HIV population the real TB prevalence was the same by BAL than by TLCB, while the TB calculated prevalence was greater in TLCB than in BAL (non-significant P value). Finally, patients with a diffuse parenchymal lung lesion in Chest CT, the calculated TB prevalence through TLCB was greater than in BAL (non-significant P value). Each and all of these differences were not statistically significant, probably due to the sample size.
With respect to the GeneXpert® MTB/RIF sensitivity and specificity by BAL was 72.7% (95% CI, 46.4–99%) and 100% (95% CI, 100–100%), respectively. Inferior to the sensibilities of GeneXpert® MTB/RIF at BAL reported by Lu et al, which were 84.5% (95% CI, 72.1–92.2%) (18) and those reported by To KW. et al. 90.0% (95% CI, 69.9–97.2%) (16).
Even though there is no existent precedent regarding the sensitivity and specificity of the GeneXpert® MTB/RIF at the biopsy’s nor in TLCB. Sensitivity and a specificity was observed through TLCB’s was 81.3% and 100%, respectively, greater than those shown via BAL. On the other hand, in HIV patients the specificity and sensitivity was 87.5% and 100%, respectively. According to other type of lesions, patients with peripheral localized lesions showed a sensitivity, specificity and a negative predictive value of 88.9%, 100% and 95.7% respectively. The sensitivity and specificity of the GeneXpert® MTB/RIF in TLCB, HIV patients and in patients with peripheral localized lesions are greater than those reported by different authors even in expectoration samples (12,16,23,30). In our study we encountered 9.3% complications, none of them showed pneumothorax, which is less than those reported by Ravaglia et al. (32). They reported 19.2% and 0.7% serious bleeding, compared to our population which showed moderate bleeding (7.4%), and none of them showed serious bleeding. The procedures’ safety in pulmonary cryobiopsies has been studied mainly in interstitial pulmonary diseases, even so, for in a pneumothorax case, it has been inferior to what has been reported and the bleeding is within the rates obtained in other series [4.9% (2.2–10.7%)] (19,33-35). It is important to point out that our proceedings were all guided by fluoroscopy and performed or supervised by highly trained staff.
Conclusions
Patients with a suspected TB diagnosis, with negative sputum smears, were subject to bronchoscopy, The GeneXpert test in TLCB is as efficient as in BAL. However, TLCB adds a major diagnostic opportunity when the nature of the illness is benign as well as malignant. Furthermore, through TLCB the totality of benign and malignant non infections diagnosis were obtained, while through BAL none of the diagnosis were obtained. Finally, TLCB has been proven to be safer when used by expert staff, and with a low complication percentage.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved (C13-19) by the ethics committee of the National Institute of Respiratory Diseases, Ismael Cosío Villegas.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.World Health Organization. Global Tuberculosis Report 2018.
- 2.World Health Organization. Global tuberculosis Report 2014.
- 3.Steingart KR, Ramsay A. Optimizing sputum smear microscopy for the diagnosis of pulmonary tuberculosis. Expert Rev Anti Infect Ther 2007;5:327-31. 10.1586/14787210.5.3.327 [DOI] [PubMed] [Google Scholar]
- 4.Davis JL, Cattamanchi A, Cuevas LE, et al. Diagnostic accuracy of same-day microscopy versus standard microscopy for pulmonary tuberculosis: a systemic review and meta-analysis. Lancet Infect Dis 2013;13:147-54. 10.1016/S1473-3099(12)70232-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganeswrie R, Chui CS, Balan S, et al. Comparison of BACTEC MGIT 960 system and BACTEC 460 TB system for growth and detection of Mycobacteria from clinical specimens. Malays J Pathol 2004;26:99-103. [PubMed] [Google Scholar]
- 6.Phyu MH, Kyaw KWY, Myint Z, et al. Sputum smear-positive, Xpert® MTB/RIF-negative results: magnitude and treatment outcomes of patients in Myanmar. Public Heal action 2018;8:181-6. [DOI] [PMC free article] [PubMed]
- 7.Organización Mundial de la Salud. Informe mundial sobre la tuberculosis 2017.
- 8.Armand S, Vanhuls P, Delcroix G, et al. Comparison of the Xpert MTB/RIF test with an IS6110-TaqMan real-time PCR assay for direct detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. J Clin Microbiol 2011;49:1772-6. 10.1128/JCM.02157-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luca G, Tanna D. Effect of Xpert MTB/RIF on clinical outcomes in routine care settings: individual patient data meta-analysis. Lancet Glob Heal 2019;7:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jafari C, Ernst M, Kalsdorf B, et al. Comparison of molecular and immunological methods for the rapid diagnosis of. Int J Tuberc Lung Dis 2013;17:1459-65. 10.5588/ijtld.13.0108 [DOI] [PubMed] [Google Scholar]
- 11.Zawedde-Muyanja S, Manabe YC, Sewankambo NK, et al. Xpert® MTB/RIF associated with improved treatment initiation among patients with smear-negative tuberculosis. Int J Tuberc lung Dis 2018;22:1475-80. 10.5588/ijtld.17.0460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steingart KR, Schiller I, Horne DJ, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014;(1):CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridzon R. The tip of the iceberg. Int J Tuberc Lung Dis 2018;22:237. 10.5588/ijtld.17.0865 [DOI] [PubMed] [Google Scholar]
- 14.Walters E, Goussard P, Bosch C, et al. GeneXpert MTB/RIF on bronchoalveolar lavage samples in children with suspected complicated intrathoracic tuberculosis: A pilot study. Pediatr Pulmonol 2014;49:1133-7. 10.1002/ppul.22970 [DOI] [PubMed] [Google Scholar]
- 15.Iqbal M, Jamil B, Mukhtar Bukhari MT, et al. Mycobacterium tuberculosis infection and resistance to rifampicin with GeneXpert®MTB/RIF: a single-center experience on bronchoalveolar lavage samples in renal failure patients. J Pak Med Assoc 2019;69:244-5. [PubMed] [Google Scholar]
- 16.To KW, Kam KM, Chan DPC, et al. Utility of GeneXpert in analysis of bronchoalveolar lavage samples from patients with suspected tuberculosis in an intermediate-burden setting. J Infect 2018;77:296-301. 10.1016/j.jinf.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 17.Akkerman OW, Van Der Werf TS, De Boer M, et al. Comparison of 14 molecular assays for detection of mycobacterium tuberculosis complex in Bronchoalveolar Lavage fluid. J Clin Microbiol 2013;51:3505-11. 10.1128/JCM.00843-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Y, Zhu Y, Shen N, et al. Evaluating the diagnostic accuracy of the Xpert MTB/RIF assay on bronchoalveolar lavage fluid: A retrospective study. Int J Infect Dis 2018;71:14-9. 10.1016/j.ijid.2018.01.030 [DOI] [PubMed] [Google Scholar]
- 19.Sánchez-Cabral O, Martínez-Mendoza D, Fernandez-Bussy S, et al. Utility of Transbronchial Lung Cryobiopsy in Non-Interstitial Diseases. Respiration 2017;94:285-92. 10.1159/000478786 [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-Cabral O, Martínez-Mendoza D, Flores-Bello ÁP, et al. Diagnostic discrepancy between bronchoalveolar lavage and transbronchial biopsy from bronchoscopies of HIV patients with pneumonia: toward an integral diagnosis. HIV AIDS (Auckl) 2018;10:115-23. 10.2147/HIV.S161899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhasmana DJ, Ross C, Bradley CJ, et al. Performance of Xpert MTB/RIF in the diagnosis of tuberculous mediastinal lymphadenopathy by endobronchial ultrasound. Ann Am Thorac Soc 2014;11:392-6. 10.1513/AnnalsATS.201308-250OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sánchez-Cabral O, Martínez-Mendoza D, Fernandez-Bussy S, et al. Usefulness of Endobronchial Ultrasound in Patients with Human Immunodeficiency Virus Infection and Mediastinal Lymphadenopathy. Respiration 2017;93:424-9. 10.1159/000465527 [DOI] [PubMed] [Google Scholar]
- 23.Theron G, Peter J, van Zyl-Smit R, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med 2011;184:132-40. 10.1164/rccm.201101-0056OC [DOI] [PubMed] [Google Scholar]
- 24.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-68. 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 25.Barisione E, Salio M, Romagnoli M, et al. Competence in transbronchial cryobiopsy. Panminerva Med 2019;61:290-7. 10.23736/S0031-0808.18.03567-X [DOI] [PubMed] [Google Scholar]
- 26.Han Y, Kim HJ, Kong KA, et al. Diagnosis of small pulmonary lesions by transbronchial lung biopsy with radial endobronchial ultrasound and virtual bronchoscopic navigation versus CT-guided transthoracic needle biopsy: A systematic review and meta-analysis. PLoS One 2018;13:e0191590. 10.1371/journal.pone.0191590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali MS, Trick W, Mba BI. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: A systematic review and meta-analysis. Respirology 2017;22:443-53. 10.1111/resp.12980 [DOI] [PubMed] [Google Scholar]
- 28.Chen A, Chenna P, Loiselle A, et al. Radial Probe Endobronchial Ultrasound for Peripheral Pulmonary Lesions A 5-Year Institutional Experience. Ann Am Thorac Soc 2014;11:578-82. 10.1513/AnnalsATS.201311-384OC [DOI] [PubMed] [Google Scholar]
- 29.Jacomelli M, Demarzo SE, Cardoso PFG. Radial-probe EBUS for the diagnosis of peripheral pulmonary lesions. J Bras Pneumol 2016;42:248-53. 10.1590/s1806-37562015000000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rakotoarivelo R, Ambrosioni J, Rasolofo V, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of smear-negative pulmonary and extrapulmonary tuberculosis in Madagascar. Int J Infect Dis 2018;69:20-5. 10.1016/j.ijid.2018.01.017 [DOI] [PubMed] [Google Scholar]
- 31.Unaids. UNAIDS DATA 2018.
- 32.Ravaglia C, Wells AU, Tomassetti S, et al. Diagnostic yield and risk/benefit analysis of trans-bronchial lung cryobiopsy in diffuse parenchymal lung diseases: a large cohort of 699 patients. BMC Pulm Med 2019;19:16. 10.1186/s12890-019-0780-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhooria S, Agarwal R, Sehgal IS, et al. Bronchoscopic lung cryobiopsy: An Indian Association for Bronchology position statement. Lung India 2019;36:48. 10.4103/lungindia.lungindia_75_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iftikhar IH, Alghothani L, Sardi A. Transbronchial Lung Cryobiopsy and Video-assisted Thoracoscopic Lung Biopsy in the Diagnosis of Diffuse Parenchymal Lung Disease A Meta-analysis of Diagnostic Test Accuracy. Ann Am Thorac Soc 2017;14:1197-211. [DOI] [PubMed] [Google Scholar]
- 35.Johannson KA, Marcoux VS, Ronksley PE, et al. SYSTEMATIC REVIEW Diagnostic Yield and Complications of Transbronchial Lung Cryobiopsy for Interstitial Lung Disease A Systematic Review and Metaanalysis. Ann Am Thorac Soc 2016;13:1828-38. [DOI] [PubMed] [Google Scholar]