Abstract
Background
Zika virus (ZIKV), an arbovirus capable of causing neurological abnormalities, is a recognised human pathogen, for which a vaccine is required. As ZIKV antibodies can mediate antibody-dependent enhancement (ADE) of dengue virus (DENV) infection, a ZIKV vaccine must not only protect against ZIKV but must also not sensitise vaccinees to severe dengue.
Methods
The N-terminal 80% of ZIKV envelope protein (80E) was expressed in Pichia pastoris and its capacity to self-assemble into particulate structures evaluated using dynamic light scattering and electron microscopy. Antigenic integrity of the 80E protein was evaluated using ZIKV-specific monoclonal antibodies. Its immunogenicity and protective efficacy were assessed in BALB/c and C57BL/6 Stat2−/− mice, respectively. Its capacity to enhance DENV and ZIKV infection was assessed in AG129 and C57BL/6 Stat2−/− mice, respectively.
Findings
ZIKV-80E protein self-assembled into discrete nanoparticles (NPs), which preserved the antigenic integrity of neutralising epitopes on E domain III (EDIII) and elicited potent ZIKV-neutralising antibodies predominantly against this domain in BALB/c mice. These antibodies conferred statistically significant protection in vivo (p = 0.01, Mantel–Cox test), and did not exacerbate sub-lethal DENV-2 or ZIKV challenges in vivo.
Interpretation
Yeast-expressed ZIKV-80E, which forms highly immunogenic EDIII-displaying NPs, elicits ZIKV EDIII-specific antibodies capable of offering significant protection in vivo, without the potential risk of ADE upon subsequent DENV-2 or ZIKV infection. This offers a promising vaccine candidate for further development.
Funding
This study was supported partly by ICGEB, India, and by NIAID, USA.
Keywords: Zika virus vaccine, VLPs, Nanoparticles, Pichia pastoris;Dengue virus, Antibody-dependent enhancement, AG129, C57BL/6 Stat2−/−
Research in context.
Evidence before this study
Currently there is no vaccine to prevent infection by Zika virus, a mosquito-borne flavivirus closely related to dengue viruses. The recent explosive Zika virus epidemics associated with severe neurological abnormalities, especially in infants, occurred in regions where dengue virus was prevalent. This observation, together with the known serological cross-reactivity that exists between Zika and dengue viruses, has led to the hypothesis that pre-existing dengue virus antibodies may have helped enhance the severity of Zika virus disease in these epidemics. Human sera from dengue patients can enhance Zika virus infection both in cultured cells and mice. Likewise, sera from Zika virus-infected patients can enhance dengue virus infection of cells in culture. The cross-reactivity underlying the infection enhancement phenomenon is linked to antibodies elicited by the pre-membrane protein and the fusion loop epitope of the envelope protein.
Added value of this study
Our study shows for the first time that it is possible to create a recombinant nanoparticle vaccine for Zika in the absence of the pre-membrane protein implicated in the induction of cross-reactive, infection-enhancing antibodies. A further advantage is that the fusion loop epitope is not exposed on these nanoparticles. This experimental vaccine, which can produce potent virus-blocking antibodies capable of conferring significant protection against lethal challenge, does not cause enhancement of either dengue or Zika virus infection in mice.
Implications of all the available evidence
None of the other Zika vaccine candidates being developed currently is designed to eliminate the induction of cross-reactive, infection-enhancing antibodies. An ideal Zika vaccine must induce antibodies which can prevent Zika virus infection, while at the same time being incapable of enhancing the severity of eventual dengue infection. As the incidence of new Zika infections is almost absent at the current time, clinical efficacy testing of vaccine candidates is not possible. Active pre-clinical development of Zika virus-blocking, infection enhancement-free vaccine candidates will ensure their availability for efficacy testing when Zika virus appears again.
Alt-text: Unlabelled box
1. Introduction
Zika virus (ZIKV) is a mosquito-borne flavivirus, phylogenetically related to West Nile virus, Yellow fever virus, Japanese encephalitis virus, tick-borne encephalitis virus and dengue viruses (DENV) [1]. It has a plus sense genomic RNA of ∼11 kb bases which contains a single open reading frame encoding three structural proteins, capsid, pre-membrane (prM) and envelope (E), and seven non-structural (NS) proteins, NS1, NS2a, NS2b, NS3, NS4a, NS4b and NS5 (Fig. 1a) [2]. ZIKV is spread to humans by Aedes mosquitoes and has been historically associated with mild and self-limiting fever, akin to dengue fever [3,4]. ZIKV may also be transmitted between humans sexually [5] or vertically [6,7]. ZIKV outbreaks have been documented in recent times starting from 2007 until about mid-2016, by which time it had spread to several countries in the Pacific as well as to countries in Asia, Africa and the Americas [3,8,9]. More severe clinical manifestations of ZIKV infection became apparent in the Brazilian outbreaks during 2015. Exposure of fetuses to ZIKV in the first trimester of pregnancy has been linked to neurodevelopmental malfunction resulting in congenital birth defects including microcephaly [7,[10], [11], [12]]. On the other hand, ZIKV infection in adults appears to lead to an autoimmune disease known as Guillain Barré syndrome [13]. These neurological abnormalities have been linked to the capacity of ZIKV to infect human neural progenitor cells [14]. ZIKV infections are beginning to be reported in India. It is currently estimated that more than 2 billion people live in areas considered suitable for ZIKV transmission [15]. The incidence of ZIKV infections has almost totally vanished as of now and it has been hypothesised that herd immunity resulting from these recent outbreaks will likely delay the occurrence of the next epidemic by about a decade [16]. It has been suggested that a preventive ZIKV vaccine is an urgent need [17] and that this lull period should be taken advantage of to develop ZIKV vaccine candidates for possible future use [18].
Fig. 1.
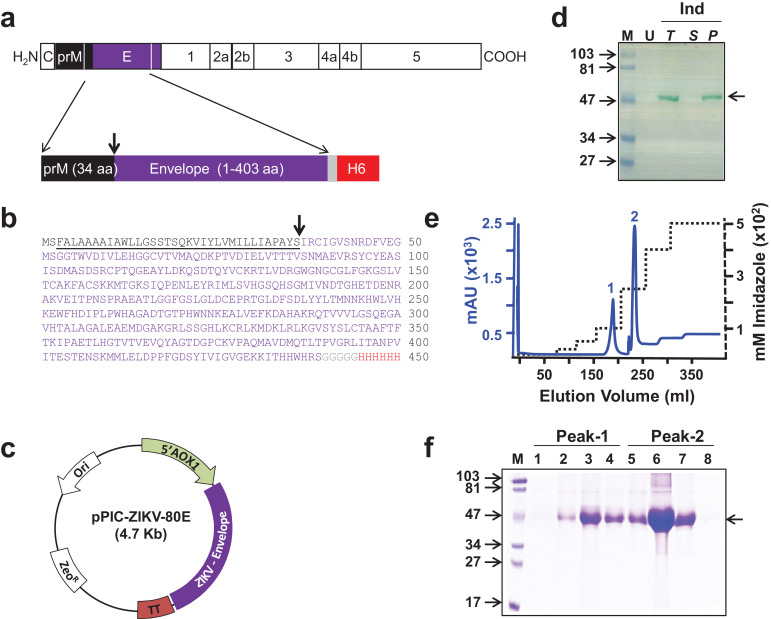
Design, expression, and purification of ZIKV-80E antigen. (a) Schematic representation of the ZIKV polyprotein. Proteins prM and E are indicated by black and purple boxes, respectively. The region of the polyprotein included in the antigen design is bounded by the two white lines in the C-terminal regions of prM and E. Shown below is the schematic representation of the design of the recombinant ZIKV-80E antigen consisting of the last 34 aa residues of prM (black box) and the first 403 aa residues of E (purple box). The C-terminally located grey and red boxes denote the pentaglycyl peptide linker and the hexa-histidine (H6) tag, respectively. (b) Predicted aa sequence of the recombinant ZIKV-80E antigen. The colour scheme corresponds to that shown in ‘a’. prM aa residues are underlined. The N-terminal dipeptide ‘MS’ was introduced during cloning. The downward arrow in panels ‘a’ and ‘b’ denotes the signal peptide cleavage site. (c) Map of the ZIKV-80E expression plasmid, pPIC-ZIKV-80E. The ZIKV-80E gene (ZIKV-Envelope) is inserted between the AOX1 promoter (5′ AOX1) on the 5′ side and the transcriptional terminator (TT) on the 3′ side. The vector carries the zeocin selection marker (ZeoR), which is functional in both E. coli and P. pastoris, and the E. coli origin of replication (Ori), for bacterial propagation. (d) Analysis of localisation of recombinant ZIKV-80E protein in P. pastoris. An aliquot of methanol-induced (Ind) culture of P. pastoris was lysed with glass beads and separated into supernatant (S) and pellet (P) fractions. Total (T) extract prepared from an equivalent aliquot of the induced culture, S, and urea-solubilised P fractions were run on SDS-polyacrylamide gel and subjected to Western blot analysis using mAb 24A12. T extract prepared from an equivalent aliquot of the un-induced (U) culture was analysed in parallel. Pre-stained protein markers were analysed in lane ‘M’. Their sizes (in KDa) are indicated to the left. The arrow on the right indicates the position of the recombinant ZIKV-80E protein. (e) Chromatographic profile of recombinant ZIKV-80E purification by Ni2+ affinity chromatography, starting from P fraction of induced cell lysate under denaturing conditions. The solid blue and the dotted black curves represent the profiles of UV absorbance (at 280 nm) and the imidazole step-gradient, respectively. Bound protein was eluted as two peaks (1 and 2). (f) Coomassie-stained SDS-polyacrylamide gel analysis of fractions corresponding to peaks 1 (lanes 1–4) and 2 (lanes 5–8) shown in panel ‘e’. Protein markers were analysed in lane ‘M’. Their sizes (in KDa) are indicated to the left. The arrow on the right indicates the position of the recombinant ZIKV E protein.
ZIKV vaccine development is complicated by the existence of the phenomenon of antibody-dependent enhancement (ADE) of infection. Antibodies to one virus can cross-react with and promote cellular entry of another phylogenetically related heterologous virus via Fc gamma receptors (Fcγ-R) and increase tissue virus load [19]. ZIKV is not only genetically and antigenically similar to DENVs, but also shares the same mosquito vector [20]. The high seroprevalence of DENV in areas [21], [22], [23] that witnessed the recent ZIKV epidemics, has raised speculation that pre-existing DENV antibodies may have been responsible for ADE of ZIKV infection, resulting in more severe clinical disease [24,25]. The corollary that ZIKV-induced antibodies may possess the potential to promote ADE of DENV infection, which in turn could lead to severe dengue disease, poses a significant hurdle to ZIKV vaccine development.
Numerous ZIKV vaccine candidates, employing multiple technologies and platforms, are being explored by various investigators. These include live attenuated and inactivated vaccines, as well as those based on recombinant virus vectors, nucleic acids, proteins and peptides [17,26,27]. Several of these, including an mRNA-based vaccine, a recombinant measles virus-vectored vaccine and several plasmid DNA-based and inactivated ZIKV candidates are in phase 1 trials, with the mRNA vaccine and one of the plasmid DNA-based vaccines in phase 2 [17,[27], [28], [29], [30]]. Apart from the live attenuated vaccines and purified inactivated vaccines, all nucleic acid-based and most protein-based vaccine candidates are designed to encode the two ZIKV structural proteins, prM and E [28], [29], [30]. The E protein is the major component involved in receptor binding, membrane fusion and in recognition by the host immune system, and contains epitopes recognised by potent murine [31] and human [32,33] neutralising antibodies (nAbs). Just like its other flaviviral counterparts, the ZIKV E protein is also organized into three distinct domains, I, II and III [34]. On the other hand, the prM protein appears to function as a chaperone of the E protein, preventing premature fusion of the immature virion, as it transits the trans-Golgi network within the infected cell. Cryo-electron microscopic analyses reveal that the overall structure of the mature ZIKV particle is similar to that of other flaviviruses [35,36]. Comparative sequence analysis of ancestral and contemporary ZIKV strains has led to the identification of a single mutation in the prM protein (S139N) [37]. This observation appears to link prM to the increased neurovirulence of the latter strains and has led to the hypothesis that the S139N prM mutation presumably confers tropism for neural progenitor cells [38].
Co-expression of ZIKV prM and E proteins in insect [39] and mammalian [40], [41], [42] cell-based expression systems has been shown to result in the production of virus-like particles (VLPs). From a vaccine perspective, these VLPs are highly immunogenic, presenting repetitive epitope arrays to the immune system, and inherently safe, as they lack the infectious viral genome. We recently found that the amino-terminal 80% of the DENV E molecule (DENV-80E), of all four serotypes, expressed in the methylotrophic yeast Pichia pastoris can self-assemble into 20–50 nm particles, in the absence of prM [43], [44], [45], [46]. Though the reports call these particles as VLPs, they are different from the regular ‘prM + E’ VLPs referred to above in certain important respects: they lack prM by intentional design and display the envelope domain III (EDIII) more efficiently (compared to the classic VLPs). Thus, we now refer to the P. pastoris-produced DENV-80E-based particles henceforth in this paper as nanoparticles (NPs), rather than as VLPs. Of note, we also observed that all four DENV-80E-based NPs were highly immunogenic, eliciting homotypic DENV-specific EDIII-focused nAbs. These studies strongly suggested that the DENV-80E NPs serve as platforms for the efficient display of EDIII. Like the DENV EDIIIs, ZIKV EDIII is also the target of potent nAbs. ZIKV EDIII-specific monoclonal antibodies (mAbs) from mice [31] as well as humans [32,47] have been documented to not only neutralise ZIKV in vitro, but also to confer significant protection against ZIKV challenge in vivo. Taken together, this prompted us to explore if the N-terminal 80% of ZIKV E protein (hereinafter referred to as ZIKV-80E), expressed using the P. pastoris host, could also self-assemble into NPs, in the absence of prM. We also tested if these putative NPs would display EDIII, which is documented to contain potent ZIKV-neutralising epitopes, effectively to the immune system. Further, we investigated if the antibodies elicited by such NPs would manifest ZIKV-neutralising potency. Importantly, we wanted to investigate whether such ZIKV-80E NP-induced antibodies would enhance DENV and ZIKV infections in vivo. This paper describes our efforts to create such ZIKV-80E NPs and characterise them functionally in terms of their protective and pathogenic potential.
2. Materials and methods
2.1. Ethics statement
Immunisation of BALB/c mice was conducted either at Advinus Therapeutics Limited, Bangalore, India (IAEC No. 008/Feb 2016) or the International Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi (ICGEB/IAEC/08/2016/RGP-14 and ICGEB/IAEC/02042019/RGP-2). DENV type 2 (DENV-2) challenge experiments using AG129 mice were carried out at ICGEB, New Delhi, India (ICGEB/IAEC/2017/01/RGP-16). BALB/c and AG129 mouse experiments were strictly compliant with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals of the Government of India. Experiments with C57BL/6 Stat2−/− mice were performed at Icahn School of Medicine at Mount Sinai (IACUC Approved Protocol #s 13-0193 and LA11-00147) in accordance with the US federal regulations and the guidelines contained in the National Research Council Guide for the Care and Use of Laboratory Animals.
2.2. ZIKV-80E gene, plasmids, cells, viruses and reagents
A synthetic gene ZIKV-80E, encoding amino acid (aa) residues 1-403 of the E protein of ZikaSPH2015, codon-optimised for P. pastoris expression, was custom-synthesised by GenScript (New Jersey, USA). P. pastoris expression host (strain KM71H) and the P. pastoris integrative, expression plasmid, pPICZ-A, were from Invitrogen Life Technologies (Thermo Fisher Scientific), Carlsbad, CA, USA. E. coli plasmids for expression of maltose-binding protein (MBP) fusions of ZIKV EDIII [48] and DENV EDIIIs [49] have been mentioned before. Vero cells (ATCC Cat# CCL-81; RRID: CVCL_0059), and K562 cells (ATCC Cat# CCL-243; RRID: CVCL_0004), from American Type Culture Collection (Manassas, VA, USA), were cultured in Dulbecco's Modified Eagle Medium supplemented with 10% heat-inactivated fetal bovine serum, at 37 °C in a humidified 10% CO2 incubator. ZIKV reporter virus particles (RVPs), containing the structural proteins (prM and E) of ZikaSPH2015, but encoding Renilla luciferase replicon, were procured from Integral Molecular, PA, USA. The Uganda 1947 (MR766) and Puerto Rico 2015 (PRVABC59) strains of ZIKV have been described before [50]. DENV-1 (WP 74), DENV-2 (S16803), DENV-2 S221, DENV-3 (CH53489), and DENV-4 (TVP-360) have been described earlier [49,51]. mAb 24A12, specific to DENVs 1–3 EDIIIs [52] and ZIKV EDIII [48], mAb 4G2 [53], specific to the flavivirus fusion loop epitope (FLE), the ZIKV EDIII-specific murine mAbs [31], ZV48 (RRID: AB_2725796), ZV-2, and ZV-67 (RRID: AB_2725795) and the human mAb ZKA64 [32] have also been described earlier. Anti-mouse immunoglobulin (Ig) G-horse radish peroxidase (HRPO) conjugate was from Calbiochem (San Diego, CA, USA) and the HRPO substrate 3, 3′, 5, 5′-Tetramethylbenzidine and acid-washed glass beads were obtained from Sigma-Aldrich (St. Louis, MO, USA). ViviRen Live Cell Substrate was purchased from Promega (WI, USA). Ni2+-NTA Super-flow resin was obtained from Qiagen (Hilden, Germany). Uranyl acetate and bicinchoninic acid protein assay reagent were from TAAB Laboratories Equipment Ltd (UK) and Thermo Scientific (Rockford, USA), respectively. Alhydrogel was purchased from Brenntag Biosector, Denmark. High binding polystyrene microtitre plates were from Corning Incorporated, NY, USA.
2.3. Creation of ZIKV-80E-expressing P. pastoris clone
The synthetic ZIKV-80E gene was cloned under the control of alcohol oxidase 1 (AOX1) promoter of pPICZ-A vector and electroporated into P. pastoris host (strain KM71H). Transformants were obtained through zeocin selection and screened for ZIKV-80E protein expression using mAb 24A12 in Western blot assays.
2.4. Methanol-induction of ZIKV-80E and its localisation
Cultures of P. pastoris harbouring the ZIKV-80E gene were grown to logarithmic phase, induced and the presence of the recombinant protein analysed, in the total extract (T), supernatant (S), and urea-solubilised pellet (P) fractions, as described (Methods S1).
2.5. Affinity purification of recombinant ZIKV-80E protein
Recombinant ZIKV-80E protein was purified starting from a 2 L culture using Ni2+-NTA affinity chromatography, under denaturing conditions essentially as reported earlier [43], with minor modifications. Details of purification are provided in Methods S1 (Supplementary File). Purity was assessed by image analysis (Bio Rad Gel Doc EZ imager). Yields were typically ∼1 mg ZIKV-80E protein of ∼95% purity, per g wet weight of induced cell pellet.
2.6. Characterisation of recombinant ZIKV-80E protein
Recombinant ZIKV-80E protein in T, S and P fractions, Ni2+-NTA column fractions and purified protein preparations was analysed by SDS-PAGE and Western blotting using mAb 24A12. The purified ZIKV-80E protein was also analysed by indirect enzyme-linked immunosorbent assay (ELISA) (Methods S1), using ZIKV mAbs ZV48, ZV-2, ZV-67 and ZKA-64 [31,32]. NP formation was assessed by dynamic light scattering (DLS) and electron microscopic (EM) analyses. DLS analysis was performed using Zetasizer Nano ZS90 (Malvern instruments, Malvern, UK) after adjusting the concentration of purified protein to 300 µg/ml in 20 mM Tris-HCl/50 mM NaCl (pH 8.5). For EM examination, the purified protein concentration was adjusted to 5–10 µg/ml, in 20 mM Tris-HCl/50 mM NaCl (pH 8.5), applied onto carbon-coated EM grid (1–2 min), negatively stained with 1% uranyl acetate (30 s ) and washed (using Milli-Q water), before visualisation under a Tecnai electron microscope.
2.7. Immunisation of BALB/c mice
A single immunisation dose consisted of 20 µg purified ZIKV-80E NPs adsorbed onto 500 µg alhydrogel in a volume of 100 µl 1× phosphate buffered saline (PBS). Groups of 4–6 week old BALB/c mice were either mock-immunised (500 µg alhydrogel in 100 µl 1× PBS), or immunised with alhydrogel-formulated ZIKV-80E NPs. Three doses were administered on days 0, 14 and 28, by intraperitoneal (i.p.) route. Sera were collected on day 38 for determination of total antibody titres by indirect ELISA and nAb titres by ZIKV and DENV virus neutralisation assays.
Polyclonal anti-DENV-2 antiserum as a control for ADE experiments was generated as follows. BALB/c mice (n = 5) were immunised (i.m.) with ∼105 Infectious Units (IU) of purified DENV-2 (S16803), twice on days 0 and 21. Immune sera were collected on day 28, pooled and nAb titres determined against all four DENV serotypes. Additional details are provided in Methods S2 (Supplementary File).
2.8. Determination of total antibody titres in immune sera
Total antibody titres in immune sera were estimated by indirect ELISA, using five different MBP-EDIII fusion proteins (in which the EDIII moieties were from ZIKV, DENV-1, DENV-2, DENV-3 and DENV-4) and five different 80E proteins (corresponding to ZIKV and DENV serotypes 1–4), as the capture antigens (Methods S1). Background ELISA absorbance values were ≤ 0.05. ELISA end-point titres, defined as the highest reciprocal serum dilution that yielded an absorbance ∼4-fold over background (that is, a cut-off absorbance value of 0.2), were determined from extrapolation of non-linear regression curves using GraphPad Prism (v7.0) software.
2.9. Determination of nAb titres in immune sera
ZIKV nAb titres in immune sera against two different ZIKV strains (MR766 and PRVABC59) were determined by using an appropriately modified fluorescence activated cell sorting (FACS)-based method, initially developed for determining DENV nAb titres [51]. Serial dilutions of heat-inactivated (56 °C/30 min), filter-sterilised immune sera were pre-incubated with ZIKVs (adequate to infect 10–15% cells) for 1 h at 37 °C and titrated on Vero cells as described (Methods S1). The ZIKV neutralisation titre, NT50, is defined as the reciprocal of the serum dilution which reduced the number of ZIKV-infected cells by 50%, with reference to the number of ZIKV-infected cells in the absence of serum taken as 100%.
ZIKV nAb titres were also determined using single-round infectious ZIKV RVPs, encoding Renilla luciferase reporter, as described earlier [48]. In this assay, the NT50 titre is defined as the serum dilution capable of causing a 50% reduction in ZIKV RVP-expressed luciferase activity, with reference to the luciferase activity expressed by ZIKV RVP in the absence of immune serum, taken as 100%. In some ZIKV RVP experiments, the immune serum was subjected to pre-depletion on a chromatographic matrix on which ZIKV EDIII was immobilised (Methods S1).
DENV-specific nAb titres were determined against the WHO reference DENV-1, DENV-2, DENV-3 and DENV-4 strains, using the FACS-based assay mentioned above, with mAb 2H2-Alexa 488 conjugate to detect DENV-infected cells, as before [49,51]. For DENV nAbs, NT50 titre of the immune serum is defined as the dilution corresponding to 50% reduction in the number of DENV-infected cells with reference to the number of cells infected by DENV in absence of immune serum, taken as 100%.
2.10. Determination of protective efficacy of the vaccine
Groups (n = 4–6) of 5–6 week old C57BL/6 Stat2−/− mice were injected i.p. with indicated amounts of pooled serum either from mock-immunised (200 µl/mouse) or ZIKV-80E NP-immunised (at two dose levels: 20 µl/mouse and 200 µl/mouse) BALB/c mice. Two hours following passive transfer, mice were challenged i.d. with 1 × 103 plaque forming units (PFUs) of the highly pathogenic ZIKV strain MR766. A group of mice that received neither immune plasma nor the ZIKV challenge was included for comparison. Mice were bled on day 4 to determine viremia by q-RTPCR (Methods S1). All mice were monitored for 15 days post-infection for survival, development of clinical symptoms and weight loss, as previously described [24,50].
2.11. ADE assays
The capacity of polyclonal anti-ZIKV-80E antisera to promote ADE of DENV in vitro was evaluated using the FcγII-R-expressing K562 cell line. The ability of these antibodies to promote ADE of DENV in vivo was assessed by monitoring survival of interferon α/β and γ receptor double knock-out AG129 mice [54] following challenge with a sub-lethal dose of DENV-2 S221 that had been pre-incubated with anti-ZIKV-80E antiserum (Methods S1). This antiserum was also assessed for its ability to mediate ADE of a non-pathogenic ZIKV infection in vivo, using C57BL/6 Stat2−/− mouse model [24,50] (Methods S1).
2.12. Statistical analyses
Unpaired two-tailed Student t-test, Mann–Whitney test and one way ANOVA (Tukey's test for multiple comparisons) were used to determine statistical significance of the difference between data sets. Kaplan–Meier survival curves were analysed by the Log-Rank test for significance. Probability (p) levels less than 0.05 were considered as significant. All statistical calculations were performed using GraphPad Prism (v7.0) software (RRID: SCR_002798).
3. Results
3.1. Design, expression and purification of ZIKV-80E protein
We created a synthetic ZIKV-80E gene based on the previous design of the DENV 80E genes. Thus, the ZIKV-80E gene (codon-optimised for expression in P. pastoris) encoded the last 34 aa residues of the ZIKV prM protein followed by the first 403 aa residues of the ZIKV E protein (Fig. 1a and b). These sequences were derived from the ZikaSPH2015 Brazilian strain. ZikaSPH2015 E protein displays at least 99% sequence identity with the E proteins of Asian ZIKV strains, at least 96% sequence identity with the E proteins of African ZIKV strains and 55–57% sequence identity with respect to E proteins of the four DENVs (Supplementary File, Fig. S1).
As done for the DENV-80E proteins, we also appended nucleotide sequences at the 3′ end of the ZIKV-80E gene encoding a penta-glycine peptide linker followed by a hexa-histidine tag (Fig. 1a and b). This gene is predicted to encode a protein of ∼49 KDa in its unprocessed form, which upon signal peptide cleavage exists as a ∼45 KDa protein (Supplementary File, Table S1). This gene, placed under the methanol-inducible AOX1 promoter of the pPICZ- A vector (Fig. 1c), was integrated into the genome of P. pastoris strain KM71H. The resultant transformants were subjected to an expression screening step to identify clones capable of expressing the ZIKV-80E protein. Recombinant ZIKV-80E protein was identified with an ‘in-house’ flavivirus E protein-specific mAb 24A12 in Western blots (Supplementary File, Fig. S2). Previously, we had observed that DENV-80E proteins expressed in P. pastoris are associated with the insoluble fraction of the cell lysate [43], [44], [45], [46]. We therefore examined the localisation of the induced ZIKV-80E protein, by analysing the S and denaturant-solubilised P fractions of the induced lysate using the same Western blot assay (Fig. 1d). This revealed that recombinant ZIKV-80E protein, like its DENV counterparts, is also exclusively present in the insoluble P fraction of the total lysate. Therefore, we purified it under denaturing conditions.
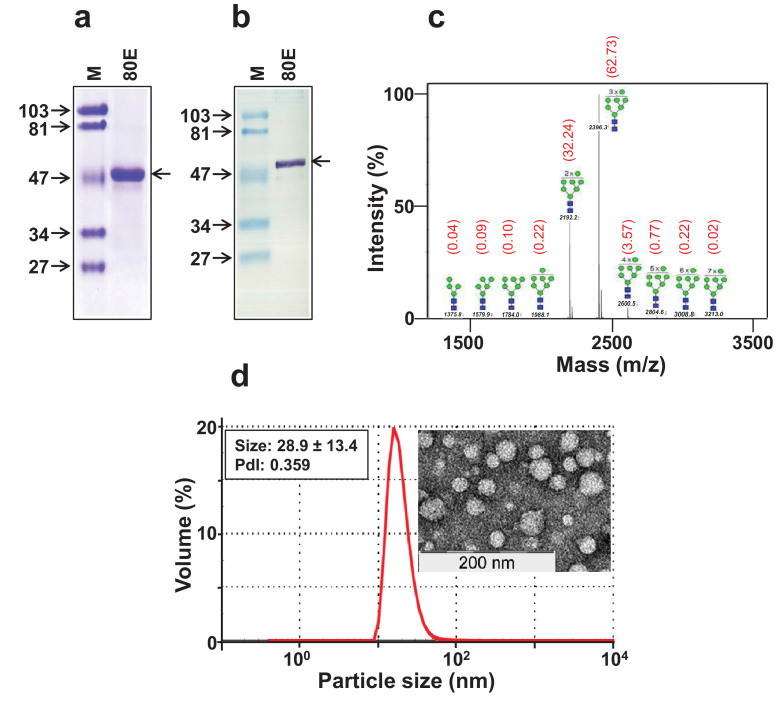
Recombinant ZIKV-80E protein was purified using Ni2+-NTA affinity chromatography, taking advantage of the C-terminally engineered 6x-His tag. The P fraction obtained after glass-bead lysis of induced cells in a Dynomill was solubilised in the presence of a denaturant. We used 6 M guanidinium hydrochloride (Gu-HCl) instead of 8 M urea as the extraction is twice as efficient with the former compared to the latter denaturant [43]. The solubilised P fraction was clarified by centrifugation and bound to Ni2+-NTA resin. As column fractions in 6 M Gu-HCl are not suitable for analysis on SDS-PAGE, the column was washed extensively with 8 M urea-containing buffer, followed by subsequent elution using an imidazole step-gradient in 8 M urea-buffer. This resulted in the emergence of two peaks, one at ∼100 mM and the other at ∼250 mM imidazole (Fig. 1e). SDS-PAGE analysis revealed the presence of a ∼45 kDa protein in all fractions across both peaks 1 and 2 (Fig. 1f). This essentially reflects that 100 mM imidazole was adequate to displace only a small fraction of the bound recombinant protein, with more effective elution occurring at 250 mM imidazole. Thus, both peaks were pooled together, dialysed to remove urea and imidazole and stored frozen in aliquots until use. An aliquot of the pooled material was analysed by SDS-PAGE and found to contain a single protein band of ∼45 kDa (Fig. 2a). This band was recognised by mAb 24A12, which recognises ZIKV EDIII [48] in a Western blot (Fig. 2b). As mentioned above, a prM signal peptide (34 aa) was included at the N-terminus of the recombinant protein to ensure proper processing. N-terminal sequence analysis revealed that the prM signal peptide had been cleaved off from the P. pastoris-expressed recombinant ZIKV-80E protein (data not shown), consistent with the observed size of the purified protein. N-linked glycan analysis of the purified recombinant ZIKV-80E protein was carried out using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Fig. 2c). Oligomannose structures up to Man13 were observed with Man9 being the most abundant species. Starting from a 2 L shake-flask culture (∼50 g induced biomass), the typical yield of purified recombinant protein was ∼50 mg (∼1 mg purified protein/gram wet weight of induced cell biomass). Purity was assessed to be >95%.
Fig. 2.
Physical characterisation of purified ZIKV-80E protein. (a) Coomassie-stained SDS-polyacrylamide gel analysis of pooled peaks 1 and 2 (from Fig. 1f) after dialysis. (b) Western blot analysis of the pooled peaks using mAb 24A12. In panels ‘a’ and ‘b’, protein markers were run in lanes ‘M’ and the purified ZIKV-80E protein was run in lanes marked ‘80E’. Their sizes (in KDa) are shown to the left of each panel. The arrow on the right, of both these panels, indicates the position of the ZIKV-80E protein. (c) The mass spectrometric N-glycan profile of recombinant ZIKV-80E protein, obtained using Bruker UltraFlex II MALDI-TOF mass spectrometer. The numbers in black above/across the peaks indicate the masses of the N-glycan species. The proposed structures of the N-glycan moieties, with the blue squares representing N-acetyl glucosamine and the green circles representing mannose residues, are shown for each of the peaks. The numbers in red shown in parentheses above the peaks denote the relative abundance (%) of the N-glycan moieties of the purified ZIKV-80E protein. (d) Analysis of volume distribution profile of particles in purified ZIKV-80E preparation. The left inset shows DLS parameter values, obtained based on size distribution by intensity, and the right inset depicts the transmission EM image of ZIKV-80E NPs (the scale is shown on the left lower edge).
3.2. ZIKV-80E protein self-assembles into NPs which preserve key EDIII-neutralising epitopes
We had previously observed that purified recombinant DENV-80E proteins, of all four DENV serotypes, when expressed using the P. pastoris host system self-assemble into unique NPs, in the absence of the cognate prM proteins [43], [44], [45], [46]. Therefore, we examined if ZIKV-80E expressed in P. pastoris in the absence of prM also would possess the ability to form higher order structures through self-assembly. Laser-based DLS analysis revealed a single major homogenous peak (PdI=0.359), representing particles of an average size of ∼29 nm. This suggests that the purified ZIKV-80E preparation does indeed self-assemble into discrete higher order structures (Fig. 2d). We corroborated this finding by analysis of the purified material by EM, which showed discrete particles of ∼25–35 nm (Fig. 2d, right inset). Collectively, these data demonstrate that purified ZIKV-80E protein prepared using the P. pastoris host system does self-assemble into discrete NPs, in the absence of any prM. The capacity of recombinant ZIKV-80E protein to self-assemble is interesting given that there are several reports which suggest that ZIKV VLPs are formed only when prM and E proteins are co-expressed together [39], [40], [41], [42]. This may reflect subtle differences in the disposition of the E protein molecules in the prM-lacking NPs and prM-containing VLPs, leading to the conclusion that the NPs may be structurally distinct from the VLPs.
We next asked the question: Do the prM-lacking ZIKV-80E NPs preserve the antigenic integrity of neutralising epitopes? To answer this question, we probed these NPs with a panel of ZIKV-specific human and murine conformational mAbs. Since potent ZIKV-specific neutralising epitopes map to EDIII, we focused on a set of ZIKV EDIII-specific mAbs and carried out indirect ELISAs using the purified ZIKV-80E NPs as the coating antigen. The data are summarised in Table 1. The ZIKV-80E protein NPs were recognised efficiently by the murine mAbs ZV-48 and ZV-67, but not by ZV-2. Their binding sites on the ZIKV E protein have been mapped using recombinant protein-binding studies in conjunction with X-ray crystallography [31]. ZV-2, ZV-48 and ZV-67 bind to the ABDE sheet, the C–C’ loop and the lateral ridge (LR), respectively of ZIKV EDIII. Of these, the LR epitope is quite complex in that it is comprised of several secondary structure elements that include the A-strand, the B–C loop, D–E loop and F–G loop involving 21 contact residues [31]. The efficient recognition by mAb ZV-67, a potent neutraliser of ZIKV infectivity, suggests that the antigenic integrity of the LR epitope is largely preserved in the ZIKV-80E NPs. The binding of mAb ZV-48 (also a strong neutraliser of ZIKV infectivity, but not as potent as ZV-67), which recognises another distinct secondary structural element, the C–C’ loop, suggest subtle difference(s) between ZIKV-80E NPs and the ZIKV particle, as the C–C’ loop is not predicted to be accessible on the virion surface [35,36]. On the other hand, ABDE sheet recognised by mAb ZV-2, a weak neutraliser, and also not predicted to be accessible on the ZIKV particle [35,36], was not detectable on the ZIKV-80E NPs as well. ZIKV-80E NPs were also recognised by a human mAb, ZKA-64, a highly potent nAb, specific to ZIKV EDIII [32]. Interestingly, none of these ZIKV mAbs recognised ZIKV EDIII displayed on HBV S-based VLPs, providing an explanation for why ZIKV EDIII displayed on HBV S VLPs elicits very modest ZIKV nAb titres [48]. As expected none of the ZIKV mAbs recognised DSV4 VLPs (which display the EDIIIs of all four DENV serotypes [49]) as well as DENV-2-80E NPs [43]. However, the cross-reactive mAb 24A12 recognised EDIII moieties of ZIKV and DENV-2, regardless of the particle platform. We also tested the ZIKV-80E NPs using the FLE-specific flavivirus cross-reactive mAb 4G2 (Table 1). Interestingly, this mAb did not manifest any discernible reactivity towards ZIKV-80E NPs, suggesting that in these NPs the ZIKV E FLE is not accessible. Taken collectively, the mAb probing data suggest that the ZIKV-80E NPs preserve the overall antigenic integrity of EDIII, but with subtle differences.
Table 1.
ZIKV-80E NPs display EDIII, but not FLE.
| mAb (binding site) | Coating antigena |
|||
|---|---|---|---|---|
| ZSVb | ZIKV-80E | DSV4c | DENV-2-80Ed | |
| ZV-48 (C–C’ loop) | 0.01 | 2.03 | 0.01 | 0.01 |
| ZV-2 (ABDE sheet) | 0.01 | 0.05 | 0.01 | 0.02 |
| ZV-67 (LR) | 0.01 | 1.59 | 0.01 | 0.01 |
| ZKA-64 (EDIII) | 0.01 | 2.50 | 0.01 | 0.01 |
| 24A12 (EDIII)e | 1.40 | 3.30 | 2.59 | 0.94 |
| 4G2 (FLE)f | 0.01 | 0.01 | 0.01 | – |
The coating antigens listed (all are P. pastoris-expressed, purified proteins, assembled into NPs) were probed with mAbs shown in an indirect ELISA format. The assay was performed twice independently. Data shown are average ELISA absorbance values from duplicate assays of one experiment.
Zika subunit vaccine, with ZIKV EDIII displayed on HBV S-based VLPs [48].
Dengue subunit vaccine, with dengue EDIIIs of all four serotypes linked in frame and displayed on HBV S-based VLPs [49].
The N-terminal 80% of DENV-2 E protein self-assembled into NPs [43], similar to the ZIKV-80E described in this manuscript.
This is a flavivirus cross-reactive mAb capable of recognising EDIIIs of ZIKV [48] as well as DENVs 1–3 [53].
This pan-flavivirus-specific mAb was confirmed to recognise DENV-2 virions (in a parallel control assay, data not shown).
3.3. ZIKV-80E NPs elicit potent ZIKV-specific nAbs
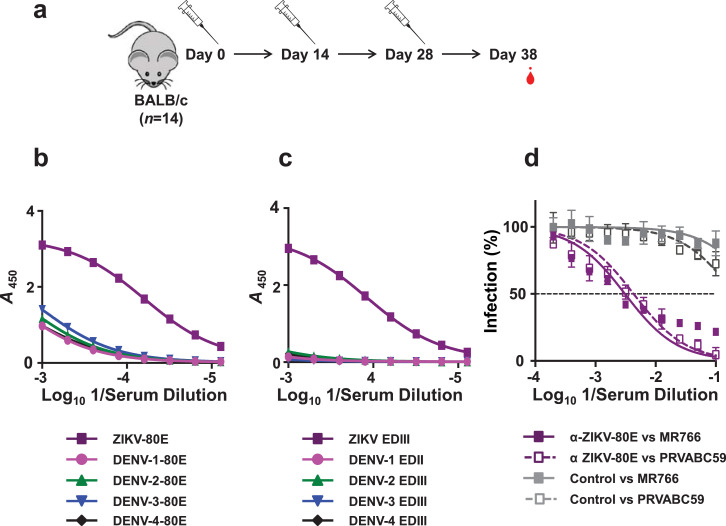
Having obtained ZIKV-80E NPs and found that they present EDIII on their surface, (particularly the LR epitope) we examined their immunogenicity in BALB/c mice. Mice were immunised with ZIKV-80E NPs as described in methods (Fig. 3a). Immune sera drawn 10 days after the final dose were analysed by indirect ELISA using different coating antigens, as depicted in Fig. 3b. Pooled immune sera displayed very high anti-ZIKV-80E NP antibody titres (serum log10 titres >105), using the purified ZIKV-80E NPs as the coating antigen. As this experiment used pooled immune sera, it is likely that differences in individual mice may be masked, with only a subset responding efficiently to the antigen. To address this concern, we analysed all 14 individual sera, at a single dilution, within the linear dose-response range of the pooled sera. These results confirmed that all mice respond quite efficiently and comparably to the ZIKV-80E NPs (Supplementary File, Fig. S3a). The difference in ELISA absorbance values between the highest (mouse #7) and lowest (mouse #10) responders was not significant (p = 0.33, Mann–Whitney test). Next, we examined the degree to which the anti-ZIKV-80E NP antisera can cross-react with DENV-80E NP antigens, in this indirect ELISA. Consistent with the antigenic similarity which exists between ZIKV E and the E proteins of the four DENV serotypes (Supplementary File, Fig. S1), we observed that the anti-ZIKV-80E NP antisera indeed manifested cross-reactivity against the DENV-80E NPs (Fig. 3b). However, the DENV-80E NP-cross-reactive end-point titres were more than an order of magnitude lower than those against ZIKV E NPs (Supplementary File, Fig. S3b).
Fig. 3.
Determination of ZIKV-80E NP-induced antibody titres in mice. (a) Schematic representation of the immunisation schedule. Mice (n = 14) were given i.p. injections on days 0, 14, and 28, and bled 10 days later for antibody titration. A single dose contained 20 µg NPs formulated with 500 µg alum. (b) Indirect ELISA titration curves obtained using pooled immune sera collected on day 38 using P. pastoris-produced purified 80E NPs of ZIKV (purple), DENV-1 (magenta), DENV-2 (green), DENV-3 (blue) and DENV-4 (black), as coating antigens. (c) Similar indirect ELISA titration curves of the same immune sera pool as in panel ‘b’, but using E. coli-expressed, purified EDIII (as MBP fusion) proteins, corresponding to the same set of five viruses listed above, as the coating antigens. Data in panels ‘b’ and ‘c’ are plotted as ELISA reactivity, in terms of absorbance at 450 nm (A450), as a function of serum dilution. Each data point represents the average of duplicates. The ELISA titration curves shown depict one of two independent experiments. (d) Determination of ZIKV nAb titres in ZIKV-80E NP-immunised mice using FACS neutralisation assay. Serial dilutions of pooled heat-inactivated immune sera collected on day 38 were titrated against ZIKV MR766 (solid purple curve) and ZIKV PRVABC59 (dashed purple curve). In parallel, mock-immune serum was similarly titrated against the same two ZIKV strains (MR766: solid grey curve; PRVABC59: dashed grey curve). Data depict ZIKV infection (%) as a function of serum dilution. The horizontal dashed line denotes 50% ZIKV infection.
Next, we carried out an indirect ELISA using recombinant EDIII proteins (as MBP fusions) corresponding to the five viruses, ZIKV and DENVs 1–4 (Fig. 3c). This revealed that the antibody titres against recombinant ZIKV EDIII protein were quite high (serum log10 titres were >5) and very similar to titres against recombinant ZIKV-80E protein. This raised the question: what proportion of these antibodies would manifest cross-reactivity towards DENV EDIIIs? We addressed this by replacing ZIKV EDIII with DENV EDIII proteins as the coating antigen in indirect ELISA (Fig. 3c). The data revealed that DENV EDIII cross-reactive titres were virtually indiscernible for DENV serotypes 1 and 3 and significantly lower (p<0.0001) for the remaining serotypes, compared to ZIKV EDIII-specific titres (Supplementary File, Fig. S3b). Collectively the data lead to the conclusion that ZIKV-80E NP-elicited antibodies are predominantly (≥90%) specific to ZIKV EDIII. We interpret this as a reflection of efficient display of EDIII by the ZIKV-80E NPs. This interpretation is also consistent with the efficient recognition of EDIII on ZIKV-80E NPs by ZIKV EDIII-specific mAbs above (Table 1). The low levels (≤10%) of cross-reactivity towards DENV-80E proteins are presumably directed towards epitopes outside of EDIII. Interestingly, an alternate route and schedule of immunisation also resulted in similar indirect ELISA titers against recombinant 80E and EDIII proteins corresponding to ZIKV and DENV (Supplementary File, Fig. S4).
We next evaluated if the EDIII-specific antibodies elicited by ZIKV-80E NPs would be able to block the infectivity of ZIKV. This was addressed using a FACS-based ZIKV neutralisation assay. In this experiment we tested the capacity of anti-ZIKV-80E NP antisera to block the infectivity of two different ZIKV strains: the highly pathogenic MR766 strain and the mildly pathogenic PRVABC59. Both these ZIKV strains were efficiently blocked by the antibodies induced by ZIKV-80E NPs (NT50 titres of 461 and 306, against MR766 and PRVABC59, respectively), as shown in Fig. 3d. Mock-immune sera was ineffective against either virus (NT50 <10). We also measured ZIKV nAb titres of these sera using ZIKV RVPs, which are identical to ZIKV on the outside and infect susceptible cells once, but do not produce infectious progeny, as they lack the structural genes. Instead they encode Renilla luciferase reporter whose activity provides a read-out of ZIKV RVP entry into cells [55,56]. Data from this experiment depicted in Fig. 4a revealed that the ZIKV-RVP assay resulted in quite similar nAb titre (NT50=335), consistent with earlier reports that NT50 titres obtained with recombinant ZIKV RVPs and wild-type ZIKVs are comparable [55,56]. Analysing single mouse sera using the ZIKV-RVP assay, we found that pooling the immune sera did not mask non-responders or low responders (Supplementary File, Fig. S5). All individual sera tested manifested comparable NT50 titres in the ZIKV RVP virus neutralisation assay, with the difference in maximal (mouse #1) and minimal (mouse #3) NT50 titres being insignificant (p = 0.33, Mann–Whitney test). In parallel, we also determined the nAb titres against each of the four DENV serotypes using a flow cytometry-based assay [51]. Interestingly, this experiment revealed that the antibodies elicited by ZIKV-80E NPs did not have any neutralising potency against any of the four DENVs (Supplementary File, Fig. S4d). Thus, the ZIKV-80E NPs elicit nAbs that are highly ZIKV-specific.
Fig. 4.
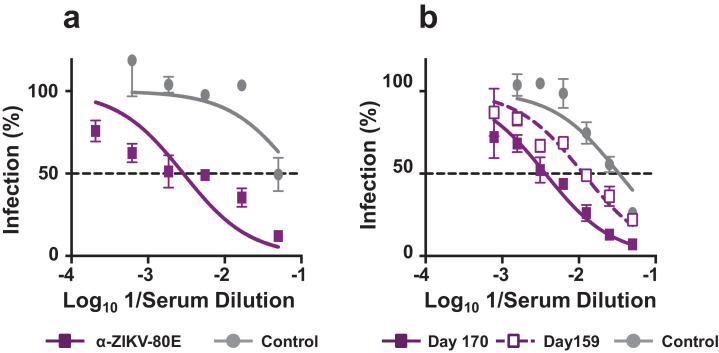
Determination of recall nAb response in immunised mice using the ZIKV luciferase reporter assay. (a) Determination of ZIKV nAb titre in the same pooled immune serum as in Fig. 3d, but determined using ZIKV RVPs. The data are represented as percent infection (in terms of Renilla luciferase activity of ZIKV RVPs) as a function of immune serum dilution, with luciferase activity of ZIKV RVP in absence of immune serum taken to represent 100% infection. Virus neutralisation curves obtained using pooled sera from mock-immunised and ZIKV-80E NP-immunised mice are shown using solid grey and solid purple curves, respectively. (b) A subset (n = 5) of mice was bled ∼4 months after the 3rd immunisation dose (on day 159) and given a booster dose of alum-formulated ZIKV-80E NPs (4th dose) the next day to assess recall response. Ten days later (day 170) the mice were bled again. Sera pools corresponding to day 159 (dashed purple curve) and 170 (solid purple curve) were assayed for nAb titres as described in panel ‘a’. Mock-immune serum analysed in parallel is shown by the solid grey curve. The dashed horizontal line in both panels denotes 50% infection based on ZIKV RVP luciferase activity.
Can this ZIKV-specific nAb response be re-called? To answer this, a subset of immunised mice (n = 5) were allowed to rest for >4 months after the 3rd immunisation, and then given a 4th dose (day 160). Mice were bled one day before (day 159) and 10 days after (day 170) the 4th dose (Supplementary File, Fig. S6), for nAb estimation using the ZIKV RVP luciferase assay. This experiment, the results of which are presented in Fig. 4b, demonstrated >4-fold increase in resting titres following recall (NT50 =55 before and 241 after recall, Supplementary File, Fig. S6, table on the left). This reflects a specific recall response, and is indicative of the generation of memory B cells as a result of the primary immunisation. Specific T-cell stimulation studies may shed further light on this. Interestingly, the day 170 immune serum also failed to neutralise any of the four DENV serotypes (Supplementary File, Fig. S6, table on the right). This suggests that memory recall preserves the absolute specificity for ZIKV and does not expand to DENVs.
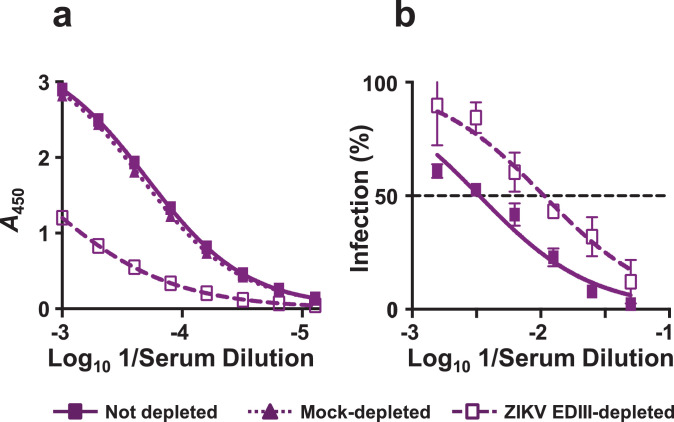
That the antibody response to ZIKV-80E NPs is predominantly directed to EDIII, prompted the question: what is the contribution of EDIII-specific antibodies to ZIKV neutralising activity? To address this, we depleted EDIII-specific antibodies from the anti-ZIKV-80E NP antiserum by pre-incubating it with amylose resin-bound MBP-ZIKV EDIII, and determined residual antibody titres, as shown in Fig. 5. In this experiment, we analysed immune sera from the memory recall experiment above. Total EDIII-specific antibody titres in the day 170 immune serum, which were virtually unaffected following mock depletion using immobilised MBP, underwent considerable decrease following depletion on immobilised MBP-ZIKV EDIII (Fig. 5a). For example at a 1000-fold dilution of serum, ELISA absorbance values decreased ∼60% following depletion on immobilised MBP-ZIKV EDIII. As a next step, nAb titres in the un-depleted and MBP-ZIKV EDIII-depleted immune sera were determined. This showed that nAb titres were also reduced by ∼70% following depletion of EDIII-specific Abs (NT50 titres before and after depletion were 301 and 91, respectively), as can be seen from the data in Fig. 5b. That the depletion of EDIII-specific antibody titres is paralleled by a corresponding reduction in nAb titres, strongly suggest a significant role for EDIII-specific antibodies in ZIKV neutralisation.
Fig. 5.
Depletion of EDIII-specific antibodies from anti-ZIKV-80E NP antiserum is accompanied by reduction in ZIKV nAb titres. (a) Pooled serum (n = 5) collected on day 170 from BALB/c mice immunised with 4 doses of ZIKV-80E NPs (on days 0, 14, 28, and 160) was analysed for ZIKV EDIII-specific antibody titres by indirect ELISA using MBP-ZIKV EDIII as the coating antigen. The pooled serum was tested either before (solid curve) or after depletion on amylose resin-bound MBP protein (dotted curve) or MBP-ZIKV EDIII (dashed curve). Data are plotted as ELISA reactivity, in terms of absorbance at 450 nm (A450), as a function of serum dilution. Each data point represents the average of duplicates. The ELISA titration curves shown depict one of two independent experiments. (b) The pooled serum described in panel ‘a’ was assayed for ZIKV nAb titres either before (solid curve) or after (dashed curve) depletion on amylose resin-bound MBP-ZIKV EDIII using ZIKV RVP neutralisation assay. Note that in this experiment, MBP-depleted serum was omitted as its total IgG titres did not change significantly after MBP depletion, as shown in panel ‘a’ (dotted curve). The data are represented as percent infection (in terms of Renilla luciferase activity of ZIKV RVPs) as a function of immune serum dilution, with luciferase activity of ZIKV RVP in absence of immune serum taken to represent 100% infection. The dashed horizontal line denotes 50% inhibition of ZIKV reporter activity.
3.4. Antibodies elicited by ZIKV-80E NPs offer significant protection in vivo
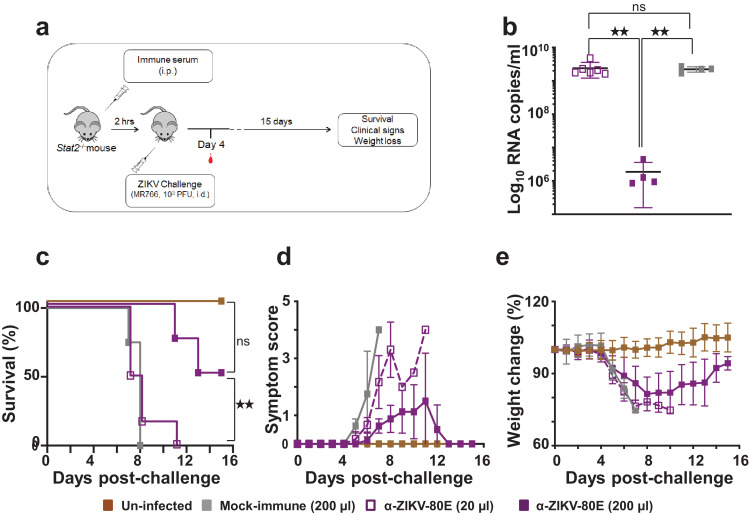
Can the anti-ZIKV-80E NP antibodies which block ZIKV infectivity in vitro be effective in countering lethal ZIKV infection in vivo? To address this question, we used C57BL/6 Stat2−/− mice, which are not only susceptible to ZIKV infection, but also display several key aspects of ZIKV pathogenesis. When challenged with ZIKV MR766 (103 PFU/mouse), these mice lose body weight starting on day 3, become morbid, show limited movement and completely succumb by the end of the week [50]. In this experiment, groups of C57BL/6 Stat2−/− mice were given i.p. injections of immune sera from BALB/c mice and then challenged 2 h later with a lethal dose of ZIKV MR766 strain (103 PFU/mouse), and monitored for 15 days (Fig. 6a). One group of mice received mock-immune serum (200 µl/mouse), while two groups received anti-ZIKV-80E NP antiserum, with one group receiving 20 µl/mouse [α-ZIKV-80E (20 µl) group] and the other, 200 µl/mouse [α-ZIKV-80E (200 µl) group]. All three groups were challenged at 2 h post serum transfer, as mentioned above. A fourth group comprised of mice which received neither serum transfer nor lethal ZIKV inoculation (‘Un-infected’). On day 4 post-challenge, mice were bled and ZIKV RNA levels determined by RT-qPCR. The resultant viremia data, presented in Fig. 6b, revealed that, the mean ZIKV RNA level in the α-ZIKV-80E (200 µl) group was ∼3 logs lower, compared to the mean ZIKV RNA levels in the groups which received 10-fold lower amount of anti-ZIKV-80E NP antiserum [α-ZIKV-80E (20 µl) group, p = 0.0044] and mock immune serum (p = 0.0017). In fact, viremia levels in the latter 2 groups were very similar to each other (p = 0.76). The viremia data closely reflected survival outcome shown in Fig. 6c. Passive transfer of mock immune serum at 200 µl/mouse failed to confer protection against lethal ZIKV challenge, with all mice succumbing by post-challenge day 8. Survival in the ‘α-ZIKV-80E (20 µl)’ group was slightly better, but there was 100% mortality by post-challenge day 11. In contrast, 50% mice receiving the higher dose of anti-ZIKV-80E NP antiserum [α-ZIKV-80E (200 µl) group] were protected when challenged with ZIKV MR766. This was statistically significant, compared to the survival of mice either in the ‘Mock-immune (200 µl)’ group (p = 0.01) or in the ‘α-ZIKV-80E (20 µl)’ group (p = 0.0074). The difference in survival between the mice of the ‘α-ZIKV-80E (200 µl)’ and ‘Un-infected’ groups was not significant (p = 0.0888). Mice in the ‘α-ZIKV-80E (200 µl)’ group showed milder clinical symptoms, which resolved after day 12 post-challenge, compared to mice in the ‘Mock-immune (200 µl)’and ‘α-ZIKV-80E (20 µl)’ groups. Body weight loss in the ‘α-ZIKV-80E (200 µl)’ group, which was similar to that in the other challenged groups during the early phase, tended to show a recovery in the later phase (Fig. 6e). Taken together, the data suggest that antibodies induced by the ZIKV-80E NPs manifest clear evidence of a dose-dependent protective effect against lethal ZIKV challenge.
Fig. 6.
Evaluation of the capacity of ZIKV-80E NP-induced antibodies to protect C57BL/6 Stat2−/− mice against lethal ZIKV MR766 challenge. (a) Schematic depiction of the efficacy test using C57BL/6 Stat2−/- mice. Mice were passively administered (i.p.) with 20 µl anti-ZIKV-80E NP antiserum [‘α-ZIKV-80E (20 µl)’ group, n = 6], 200 µl anti-ZIKV-80E NP antiserum [‘α-ZIKV-80E (200 µl)’ group, n = 4], or 200 µl mock-immune serum [‘Mock immune (200 µl)’ group, n = 4] and challenged i.d. with ZIKV MR766 (103 PFU/mouse). A fourth group of mice, which received neither passive serum transfer nor the challenge virus (‘Un-infected’ group) was included for comparison. Mice were bled on day 4 post-challenge for ZIKV RNA determination. All mice were monitored for the criteria listed for up to 15 days. (b) Blood plasma viral RNA levels (ZIKV genome copies/ml plasma) in ZIKV MR766-challenged mice, determined by q-RTPCR on day 4 post-challenge as described (Methods S1). The different groups are designated by the following symbols: empty purple squares, ‘α-ZIKV-80E (20 µl)’ group; solid purple squares, ‘α-ZIKV-80E (200 µl)’ group; and solid grey squares, ‘Mock immune (200 µl)’ group. Error bars represent mean ± standard deviation. The twin star symbol denotes significant difference between viremia in the ‘α-ZIKV-80E (200 µl)’ group, compared to either the ‘α-ZIKV-80E (20 µl)’ group (p = 0.0044) or the ‘Mock immune (200 µl)’ group (p = 0.0017), based on unpaired t-test with Welch's correction. (c) Kaplan–Meier survival curves of the different mice groups described in ‘a’. Survival of mice in the ‘α-ZIKV-80E (200 µl)’ group was significantly higher than that in the ‘α-ZIKV-80E (20 µl)’ group (p = 0.0074) and ‘Mock-immune (200 µl)’ group (p = 0.010), but comparable to that in the ‘Un-infected’ group (p = 0.0888), based on the Log-Rank (Mantel–Cox) test. (d) Clinical progression (using a slightly modified scoring system described earlier [24]) of the same mice groups in ‘c’. (e) Body weight loss profiles of the same groups of mice during the course of the experiment. In panels ‘c-e’ the different mouse groups are indicated by: dashed purple curve, ‘α-ZIKV-80E (20 µl)’ group; solid purple curve, ‘α-ZIKV-80E (200 µl)’ group; solid grey curve, ‘Mock immune (200 µl)’ group; and sold brown curve, ‘Un-infected’ group.
3.5. ZIKV-80E NP-induced antibodies do not manifest ADE
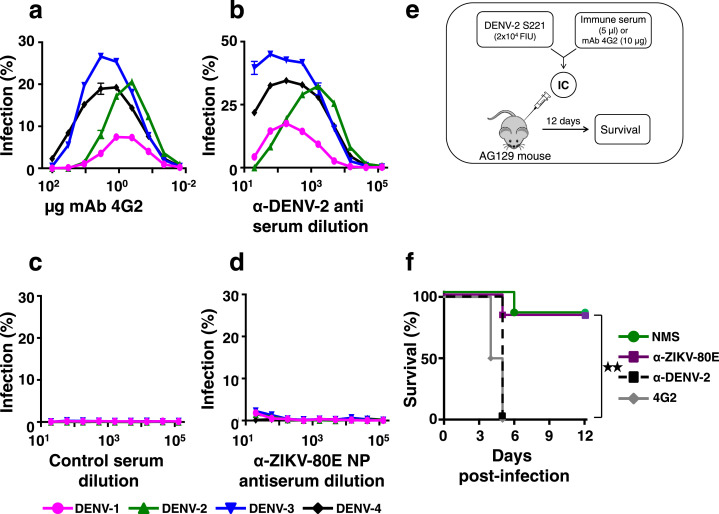
In the context of the existence of the phenomenon of ADE in flaviviral infections, it is important to evaluate if the ZIKV-80E NP-induced DENV-80E-cross-reactive antibodies observed (Fig. 3b, and Supplementary File Fig. S4b,c) possess infection-enhancing potential. To this end, we assessed the ADE potential of anti-ZIKV-80E NP antiserum using both in vitro and in vivo assays (Fig. 7). In the in vitro experiment, we examined the capacity of DENVs of each of the 4 serotypes to infect K562 cells expressing the FcγII-R, in the presence of cross-reactive anti-DENV antibodies or anti-ZIKV-80E NP antibodies (Fig. 7a–d). As a positive control in the in vitro ADE assay, we used the DENV cross-reactive pan-flavivirus-specific mAb 4G2. This mAb, specific to the highly conserved flaviviral FLE of EDI/EDII, is documented to manifest ADE activity [49,54,57,58]. As expected, pre-incubation of DENVs with mAb 4G2, followed by infection onto K562 cells, resulted in ADE of infection for each of the four DENV serotypes. This was evident from the typical bell-shaped infection profiles, as a function of mAb 4G2 concentration during the pre-incubation step, for each of the four DENV serotypes (Fig. 7a). Qualitatively similar, but relatively greater, levels of ADE behaviour was also observed when we used murine polyclonal anti-DENV-2 antiserum, instead of mAb 4G2, with peak enhancement occurring in the serum dilution range of 102–103 (Fig. 7b). It is interesting that this polyclonal anti-DENV-2 antiserum enhanced DENV-2, despite its DENV-2 specific NT50 titre being >2560. However, this antiserum also had the ability to cross-neutralise the remaining three DENV serotypes as well (NT50 titres against DENVs-1, −3 and −4 were, 300, 798, and 421, respectively). As expected, when the DENVs were pre-incubated with polyclonal control antisera from mock-immunised mice (DENV-naive), using the same dilution range tested for the polyclonal anti-DENV-2 antiserum, no ADE of infection was discernible in K562 cells for any of the four DENV serotypes (Fig. 7c). Interestingly, none of the DENVs could infect K562 cells over a wide range of anti-ZIKV-80E NP antisera dilution (102 to 105) tested (Fig. 7d).
Fig. 7.
Evaluation of DENV infection-enhancement by anti-ZIKV-80E NP antiserum. (a) Each of the four DENV serotypes was separately pre-incubated with different amounts of mAb 4G2 (0.01–100 µg), and then allowed to infect K562 cells for 24 h. The percentage of DENV-infected cells was determined by flow cytometry using mAb 2H2-Alexa 488 conjugate. Data depict infection profiles of DENV-1 (magenta), DENV-2 (green), DENV-3 (blue) or DENV-4 (black), as functions of mAb 4G2 concentration. (b) Similar experiment as in panel ‘a’, except that DENV pre-incubations were performed with pooled, serially diluted (10−1–10−5) immune serum, obtained from BALB/c mice immunised with DENV-2 S16803 strain (α-DENV-2 antiserum). (c) In vitro ADE experiment as in panel ‘b’, except that the murine polyclonal antiserum was from mock-immunised BALB/c mice (control serum); (d) Similar experiment as in panels ‘b’ and ‘c’, but using pooled immune serum from ZIKV-80E NP-immunised BALB/c mice (α-ZIKV-80E NP antiserum). (e) Schematic representation of the in vivo DENV-2 ADE experiment in AG129 mice (abbreviations: IC: immune complex; FIU: FACS infectious unit). (f) Kaplan–Meier survival curves for groups (n = 6) of AG129 mice which were intravenously administered with a sub-lethal dose of DENV-2 S221 (pre-incubated with NMS, green curve) or with ICs generated in vitro by pre-incubating the sub-lethal dose of DENV-2 S221 with mAb 4G2 (4G2, grey curve), murine anti-DENV-2 antiserum (α-DENV-2, black dashed curve) or with serum from BALB/c mice immunised with ZIKV-80E NPs (α-ZIKV-80E, purple curve). Survival was observed for 12 days post-challenge. Survival of mice in the α-ZIKV-80E and NMS groups were comparable to each other (p = 0.5282) and significantly higher (p = 0.0095) than that in the 4G2 or α-DENV-2 groups, based on the Log-Rank (Mantel–Cox) test. Statistical differences are indicated as not significant (ns) or as significant (twin star symbol).
That anti-ZIKV-80E NP antibodies did not enhance DENV infection is notable. How relevant are the data on ADE obtained with K562 cells to the in vivo situation? To address this, we used the interferon α/β and γ receptor double knock-out DENV-sensitive AG129 mouse-based ADE model (Fig. 7e). This mouse can sustain a sub-lethal DENV-2 S221 infection without succumbing to it. But in the presence of an enhancing antibody, the sub-lethal infection escalates to a lethal infection [49,54,57,58]. When these mice were injected with immune complexes (ICs) generated in vitro by pre-incubating a sub-lethal dose of the challenge strain DENV-2 S221, either with mAb 4G2 (4G2 group) or murine polyclonal anti-DENV-2 antiserum (α-DENV-2 group), there was 100% mortality by day 5 post-challenge, in contrast to control mice which received the same sub-lethal dose of DENV-2 S221, but which had been pre-incubated with naive mouse serum (NMS group) (Fig. 7f). It is noteworthy that in both these types of ICs (4G2 and α-DENV-2 groups), DENV-2 S221 was 100% neutralised, in terms of its ability to infect Vero cells. However, ICs generated by pre-incubating the sub-lethal dose of DENV-2 S221 with anti-ZIKV-80E NP antiserum (α-ZIKV-80E group) were largely non-lethal to the mice, with overall survival (∼70%) comparable to that of mice challenged with just the non-lethal dose of DENV-2 S221 (NMS group). This survival was despite the inability of the anti-ZIKV-80E NP antiserum to neutralise DENV-2 in vitro. We interpret this as clear evidence of lack of in vivo ADE of DENV-2 by polyclonal antibodies elicited by ZIKV-80E NPs. Survival of mice in the α-ZIKV-80E group was not significantly different (p = 0.5282) from that of the NMS group, but significantly higher (p = 0.0095) than those of the 4G2 group and α-DENV-2 group.
ADE can also ensue when neutralising antibodies are diluted down to sub-neutralising levels [59]. What if the ZIKV nAb titres were to wane to sub-neutralising level? Would it result in homotypic ADE of ZIKV infection? Thus, a final question we addressed was whether the polyclonal antibody repertoire induced by ZIKV-80E NPs could potentially cause enhancement of ZIKV infection in vivo, under conditions of sub-neutralising nAb levels. To this end, we used C57BL/6 Stat2−/− mouse model, in conjunction with ZIKV strain PRVABC59. Infection with this ZIKV strain, which is least pathogenic to this model, can be escalated to severe disease, in the presence of DENV immune serum, marked by significant weight loss, enhanced clinical symptoms and rapid mortality [24].
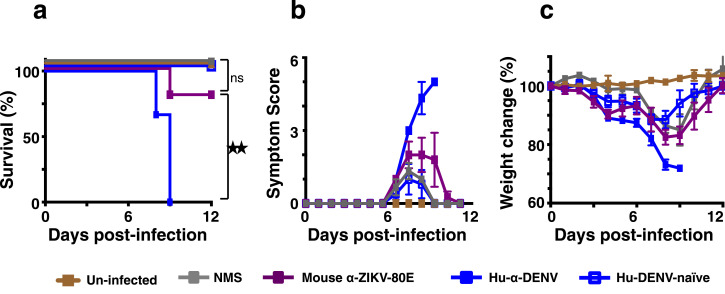
To test for possible homotypic ADE of ZIKV, we injected (i.p.) anti-ZIKV-80E NP antiserum (mouse α-ZIKV-80E) into Stat2−/− mice (to result in sub-neutralising circulating nAb titres, with expected NT50 <10) and challenged them 2 h later with ZIKV strain PRVABC59, administered i.d. For comparison, groups of mice which received i.p. injections of naïve mouse serum (NMS), DENV-positive human plasma (human α-DENV) and DENV-negative human plasma (Dengue-naïve human plasma), were also similarly challenged. These were monitored for survival, clinical symptoms and weight loss. The data are presented in Fig. 8. As expected, mice which received DENV-positive human plasma, but not those which received DENV-naïve human plasma, displayed severe clinical symptoms and weight loss, succumbing by day 8 post ZIKV challenge. On the other hand, survival of mice which had received anti-ZIKV-80E NP antiserum was ∼80%, manifesting similar symptoms and weight-loss profile, but recovering at the end of the experiment, as mice in the groups which received NMS or naïve human plasma, prior to challenge. A careful look at the data would seem to suggest that, despite a clear survival difference (Fig. 8a), administration of DENV-positive human plasma and mouse anti-ZIKV-80E NP antiserum may drive an ‘early weight loss phenotype’ (days 3 and 4) in Stat2−/− mice (Fig. 8c), although no statistically significant difference was observed. Overall, the data lead to the conclusion that antibodies elicited by ZIKV-80E NPs, at sub-neutralising levels, may not be capable of enhancing ZIKV infection. However, it is to be noted that the in vivo relevance of the FcR pathway, in the context of ZIKV infection is unknown at this time.
Fig. 8.
Evaluation of ZIKV infection-enhancement by anti-ZIKV-80E NP antiserum. C57BL/6 Stat2−/− mice were inoculated i.p. with 20 µl dengue-positive human plasma (Hu-α-DENV, solid blue squares), 20 µl dengue-negative human plasma (Hu-DENV-naïve, empty blue squares), 20 µl naïve mouse serum (NMS, solid grey squares) or 20 µl anti-ZIKV-80E NP antiserum (mouse α-ZIKV-80E, solid purple squares) and then challenged with live ZIKV (PRVABC59, 5 × 103 PFU/mouse, i.d.) two hours later. One group of mice which received neither immune serum nor ZIKV inoculation (Un-infected, solid brown squares) was included for comparison. Mice were monitored for survival (panel ‘a’), clinical symptoms (scored as described [24], panel ‘b’) and weight loss (panel ‘c’) for a period of 12 days following virus challenge. Differences between the weights of mice in the ‘Hu-α-DENV’, ‘Hu-DENV-naïve’ and ‘mouse α-ZIKV-80E’ groups, for days 3, 4, 5, and 6, analysed using a one way ANOVA (Tukey's test for multiple comparisons), were statistically not significant (p>0.05). Survival in the ‘mouse α-ZIKV-80E’ group was significantly higher than that in ‘Hu-α-DENV of mice’ group (p = 0.027) and comparable to ‘NMS’ group (p = 0.371) and ‘Un-infected’ group (p = 0.438), based on Mantel–Cox test. Statistical differences are indicated as not significant (ns) or as significant (twin star symbol).
4. Discussion
An effective ZIKV vaccine continues to be actively sought [17,[26], [27], [28], [29], [30]]. These initiatives exploit multiple strategies and platforms for ZIKV vaccine development. Of the many potential vaccine platforms available, we have been interested in particulate vaccines because of the advantages they offer in terms of safety and immunogenicity. In previous work, we found that DENV-80E proteins, when expressed in the absence of the prM proteins, in P. pastoris, exhibit the capacity to self-assemble into NPs [43], [44], [45], [46]. An interesting observation was that these DENV-80E NPs, which were highly immunogenic, displayed EDIII prominently, based on DENV EDIII-specific mAb-probing analyses, as well as the predominantly EDIII-focused nAbs they consistently elicited. From a vaccine perspective, this is important, as anti-EDIII antibodies are not only potent neutralisers of virus infectivity, but are also type-specific and inherently appear to lack ADE potential. This prompted us to question if ZIKV-80E when expressed in P. pastoris, would assemble into ZIKV EDIII-displaying NPs with similar attributes. The elimination of the prM protein from the vaccine candidate may also be advantageous. Though it is not known if ZIKV prM antibodies may have a role in ADE, like their DENV counterparts, this cannot be entirely ruled out given that pups born to ZIKV-immune mice [60] and macaques pre-infected with ZIKV [61] manifest signs of ADE of DENV infection. Thus, it is preferable to eliminate prM from vaccine design.
Accordingly, we expressed recombinant ZIKV-80E in P. pastoris and purified it to near-homogeneity and found that it does indeed self-assemble into NPs, like its P. pastoris-expressed DENV-80E counterparts. Similarly, mammalian cell-expressed DENV-4 80E, which tends to associate with host membranes, also forms particles [62]. It is significant that the particle formation in these instances occurs in the absence of prM. The mechanism of assembly of P. pastoris-expressed ZIKV-80E into NPs needs to be elucidated. However, it is interesting that this particle assembly occurred during renaturation following purification of recombinant ZIKV-80E under denaturing conditions, and is likely to be different from the regular VLP assembly that occurs in mammalian cells expressing both prM and E proteins.
The size diversity in the ZIKV-80E NPs, evidenced by DLS and EM data, may be a consequence of particle assembly taking place during renaturation by dialysis, after purification of the recombinant ZIKV-80E under denaturing conditions. As the primary purpose of this work was to assess if P. pastoris-expressed ZIKV-80E protein possessed inherent self-assembling property to make immunogenic NPs, the conditions under which renaturation was implemented were empirically determined. Elucidation of the NP assembly mechanism and more rigorous optimisation of the in vitro self-assembly step and/or ultra-sedimentation may help obtain a greater degree of size homogeneity.
It is conceivable that the lack of prM could adversely affect the structural integrity of key EDIII epitopes presented on the NPs. We carried out an EDIII-focused antigenic analysis using a small panel of six mAbs. Four of these were ZIKV EDIII-specific conformational mAbs, the fifth mAb specific to EDIIIs of ZIKV as well as DENVs, and the sixth mAb specific to the FLE of flaviviruses. Three out of four conformational mAbs recognised ZIKV-80E NPs efficiently, attesting to the preservation of the overall conformational integrity of EDIII epitopes. However, subtle differences between the prM-lacking ZIKV-80E NP and the prM-containing ZIKV particle could be inferred from the mAb probing analysis. For example, the EDIII C–C’ loop which is not accessible on the ZIKV particle, is accessible on the ZIKV-80E NP, as evidenced by its recognition by mAb ZV-48. Similarly, the pan-flavivirus cross-reactive mAb 4G2 specifc to the FLE, which can efficiently bind flavivirus particles, did not recognise the ZIKV-80E NPs. This has implications for vaccine safety as ZIKV FLE-specific antibodies have been reported to mediate ADE of DENV-2 infection in vivo using the AG129 mouse model [32].
Consistent with their ability to display EDIII, the ZIKV-80E NPs elicited predominantly ZIKV EDIII-specific total antibody titres (log10 serum titres, >5). Importantly, the ZIKV-80E NPs elicited high ZIKV-specific nAb titres (NT50≥300). This finding also implies that the ZIKV-80E NPs offer a more efficient EDIII display platform, in the light of recent findings that ZIKV EDIII displayed on Hepatitis B surface antigen VLP platform elicited much lower nAb titres, by comparison [48]. Many ZIKV vaccine development studies, investigating multiple delivery formats have consistently and compellingly demonstrated that protection against ZIKV challenge is dependent on ZIKV-specific nAbs [17,31,47,[63], [64], [65], [66]]. Experiments in mice with ZIKV prM+E DNA vaccine candidates have shown that log10 serum antibody titres ≥2.35, specific to recombinant ZIKV E, and ZIKV-specific nAb titres >10 can confer effective protection against live ZIKV challenge [65]. In contrast, another study using ZIKV prM+E VLPs found that NT50 ≥125 protects mice against lethal challenge [63]. A purified inactivated ZIKV vaccine that could elicit nAb titres of NT50 ∼100 was capable of conferring protection against ZIKV challenge in mice [66]. By these criteria, we surmise that the P. pastoris-expressed ZIKV-80E NPs, eliciting ZIKV-80E-specific log10 serum antibody titres and ZIKV-specific nAb titres far beyond these reported thresholds [63,65,66], should be capable of conferring protective immunity in mice. We observed that protection conferred on C57BL/6 Stat2−/− challenged with a lethal dose of ZIKV MR766 by passively transferred anti-ZIKV-80E NP antiserum was dose-dependent. While a dose of 20 µl immune serum was ineffective, 200 µl immune serum effectively suppressed viremia and conferred 50% protection. Paucity of immune serum precluded the testing of higher doses of immune serum. Additionally, the passive transfer strategy has an inherent limitation in that it only simulates waning ZIKV nAb titres. Assuming that the 200 µl immune serum (NT50 titre against ZIKV MR766=461), passive-transferred per mouse, undergoes ∼12-fold dilution (assuming a total blood volume of ∼2.5 ml/mouse), one may argue that the resulting circulating ZIKV NT50 titre of ∼36 affords 50% protection against lethal challenge. Comparison of the survival curves of the groups receiving mock-immune serum and anti-ZIKV-80E NP antiserum (both at 200 µl/mouse) showed the survival of the latter group was statistically significant (p = 0.01, Mantel–Cox test). Also, survival of mice in this anti-ZIKV-80E NP antiserum group was not statistically significant compared to survival of mice in the un-infected group (p = 0.0888, Mantel–Cox test). Thus, it is likely that active immunisation with ZIKV-80E NPs, which has the potential to result in much higher circulating nAb titres, can afford full protection. However, it is not possible to make an absolute comparison of our efficacy data with those published in the literature. This is because protective efficacy has been reported based on different criteria, under different experimental conditions, using different animal models and different ZIKV challenge viruses. For example, using either DNA-based ZIKV vaccine or a purified inactivated ZIKV vaccine, some investigators have actively immunised BALB/c or AG129 mice and assessed protective efficacy following challenge with Brazilian, Puerto Rican or African ZIKV strains, in terms of either suppression of viremia or survival [65,66]. One study which used passive transfer of immune sera from ZIKV prM+E VLP vaccinated CB6F1 mice, into AG129 mice as we did, however used a Nicaraguan strain of ZIKV for challenge [63]. Standardisation of the efficacy assay will help meaningful comparison of different vaccine candidates. As severe ZIKV infection is associated with fetal abnormalities, it would be relevant to test vaccine efficacy in a suitable pregnancy mouse model [67], with the caveat that this model has a genetic defect in the innate immune signalling pathway.
ZIKV vaccine development is complicated by the existence of the ADE phenomenon, stemming from the interaction between ZIKV on the one hand and DENVs on the other [67], [68], [69]. Not only do these viruses share the same mosquito vector [20], but also are genetically and antigenically similar. DENV-antibodies which cross-react with ZIKV can enhance its uptake via Fcγ-R pathway, to increase tissue ZIKV load. Likewise, anti-ZIKV antibodies from ZIKV-infected humans can cross-react with DENVs and promote their uptake into Fcγ-R-bearing cells, contributing to increased tissue DENV load. While epidemiological data in support of this is currently not available, recent experiments have demonstrated that ZIKV mAbs specific to EDI/II from ZIKV-infected patients can enhance DENV-2 infection both in vitro and in vivo [32]. Thus, a potential safety attribute of a future ZIKV vaccine is that, in addition to eliciting adequate levels of virus-nAbs, it must not contribute to ADE of DENV infection, especially in the current situation when DENV infections continue to occur in the absence of new ZIKV infections. Our data showed that ZIKV-80E NPs induced DENV cross-reactive, but non-neutralising antibodies. Would these antibodies enhance DENV infection? That they may not is evident from the failure of anti-ZIKV-80E NP antisera to escalate a sub-lethal DENV-2 S221 infection of AG129 mice into a lethal infection. It is to be emphasised that ADE in vivo was not evident despite the inability of these anti-ZIKV-80E NP-induced antibodies to neutralise DENV-2 in vitro. The precise mechanism underlying the lack of ADE needs to be understood. One possibility is that the cross-reactive epitopes on DENV-80E NPs recognised by anti-ZIKV-80E NP antibodies are not accessible on the DENV-2 S221 virion surface, and presumably this precludes ADE. Another likely explanation is that the EDIII-directed antibodies elicited by ZIKV-80E NPs, being highly type-specific, lack any inherent heterotypic ADE potential.
It is believed that neutralisation is not possible when the number of antibody molecules bound to their cognate docking sites on the virion is below a certain threshold [59]. In fact, it has been demonstrated that such non-neutralised virion-antibody complexes can enter cells via the Fcγ-R pathway in K562 cells. In the light of this, we also examined if anti-ZIKV-80E NP antibodies at low levels might possibly possess homotypic ZIKV infection-enhancing potential. However, passive transfer of anti-ZIKV-80E NP antiserum (20 µl) into ZIKV-susceptible Stat2−/− mice (resulting in ∼100-fold dilution, lowering nAb titres to sub-neutralising levels), followed by live ZIKV (strain PRVABC59) challenge, did not exacerbate morbidity and mortality in these mice. This leads to the obvious conclusion that the EDIII-focused anti-ZIKV antibodies do not possess any homotypic ZIKV infection-enhancing activity in vivo, even at very low concentrations. However, it is pertinent to point out that the hypothesis predicting the homotypic ADE potential of nAbs at sub-neutralising levels is based on in vitro observation [59]. Further support that in vitro ADE data may not mirror the true in vivo situation comes from recent data using the AG129 mouse ADE model [54]. This study showed that cross-reactive mAbs, 4G2 and 6B6C-1, cause ADE of DENV-2 even at neutralising concentrations, while the DENV-2 type-specific EDIII-directed mAb 3H5 did not cause ADE, even at sub-neutralising concentrations. Having shown that ZIKV-80E NP-induced antibodies lack ADE potential, but possess potent neutralising activity, it would be informative in future to examine the in vivo protective efficacy of this vaccine candidate in a pregnancy mouse model [67].
There are certain limitations to the work presented in this study. As the primary purpose was to evaluate the feasibility of ZIKV-80E as a putative vaccine candidate, an affinity tag was engineered into it, to facilitate purification. In a vaccine intended eventually for human trials, it is preferable to eliminate such tags and devise alternate purification strategies. Another potential shortcoming is that this study has carried out only a limited biophysical characterisation of the ZIKV-80E NPs. A more comprehensive biophysical characterisation including composition, conformation and stability, would be essential. Finally, vaccine efficacy data obtained is based on the use of the Stat2−/− mouse, which lacks types I and III interferon signalling. As this model is based on passive transfer of immune serum, it does not address a possible role for T cell activity in protective efficacy. Therefore, it is necessary to exercise caution while extrapolating vaccine efficacy in the mouse model to ZIKV infection in humans.
That P. pastoris expressed ZIKV-80E can self-assemble into immunogenic NPs is associated with some desirable features. The absence of prM eliminates any potential safety concern relating to ADE of both ZIKV as well as DENVs. Further, in the absence of prM, the E protein self-assembly process appears to favour efficient display of EDIII on the NP surface, while at the same time masking FLE in its interior. From a vaccine perspective this appears to be advantageous, as the data show that the antibodies elicited by these NPs are EDIII-directed and possess potent neutralising ability. Importantly, antibodies induced by ZIKV-80E NPs lack ADE potential, in vivo, both towards DENV as well as ZIKV. To our knowledge, this is the first ZIKV vaccine candidate to demonstrate lack of ADE in vivo. Finally, the high expression potential of the P. pastoris system [70,71] using inexpensive media is another advantage of this ZIKV vaccine candidate. Taken together, the data justify further research on this ZIKV-80E-based NP vaccine to evaluate its utility as a possible ZIKV subunit vaccine candidate.
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information file. The ZIKV-80E gene sequence is available in GenBank (MK890246).
Declaration of Competing Interest
Nothing to disclose: GB, JAA; Grant from University Grants Commission, RKS; Grant from ICGEB: RS, VR, SS, NK; Grant from NIAID: JKL, FK; Patent 201911014359 pending: RS, RKS, VR, UA, SS, NK.
Acknowledgments
Acknowledgements
The authors thank Ms. Tenzin Choedon for help with EM analysis.
Funding
This work was supported in part by intramural funds from ICGEB (NK) and NIAID grant 1R21AI129477 (FK). The funders had no role in any aspect of the performance of the study or in the decision to submit the work for publication.
Author Contributions
Literature search: SS; Figures: RS, RKS, SS, NK; Study design: SS, NK, JKL, FK; Data collection: RS, RKS, VR, UA, GB, JAA; Data analysis and interpretation: SS, NK, JKL, FK; Writing: SS and NK; Approval of final manuscript: all authors.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.102738.
Contributor Information
Sathyamangalam Swaminathan, Email: swami@icgeb.res.in.
Navin Khanna, Email: navin@icgeb.res.in.
Appendix. Supplementary materials
References
- 1.Pierson T.C., Diamond M.S. Flaviviruses. In: Knipe DM, Howley PM, editors. Fields virology. 6 ed. Wolters Kluwer and Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 747–794. [Google Scholar]
- 2.Pierson T.C., Diamond M.S. The emergence of Zika virus and its new clinical syndromes. Nature. 2018;560:573–581. doi: 10.1038/s41586-018-0446-y. [DOI] [PubMed] [Google Scholar]
- 3.Gatherer D., Kohl A. Zika virus: a previously slow pandemic spreads rapidly through the Americas. J Gen Virol. 2016;97:269–273. doi: 10.1099/jgv.0.000381. [DOI] [PubMed] [Google Scholar]
- 4.Lazear H.M., Diamond M.S. Zika virus: new clinical syndromes and its emergence in the Western hemisphere. J Virol. 2016;90:4864–4875. doi: 10.1128/JVI.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musso D., Roche C., Robin E., Nhan T., Teissier A., Cao-Lormeau V.M. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21:359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besnard M., Lastère S., Teissier A., Cao-Lormeau V.M., Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13) http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20751 pii=20751. Available online: [PubMed] [Google Scholar]
- 7.Mlakar J., Korva M., Tul N., Popović M. Zika virus associated with microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 8.Baud D., Gubler D.J., Schaub B., Lanteri M.C., Musso D. An update on Zika virus infection. Lancet. 2017;390:2099–2109. doi: 10.1016/S0140-6736(17)31450-2. [DOI] [PubMed] [Google Scholar]
- 9.Lessler J., Chaisson L.H., Kucrka L.M. Assessing the global threat from Zika virus. Science. 2016;353 doi: 10.1126/science.aaf8160. aaf8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasmussen S.A., Jamieson D.J., Honein M.A., Petersen L.R. Zika virus and birth defects-reviewing the evidence for causality. N Engl J Med. 2016;374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 11.Rubin E.J., Greene M.F., Baden L.R. Zika virus and microcephaly. N Engl J Med. 2016;374:984–985. doi: 10.1056/NEJMe1601862. [DOI] [PubMed] [Google Scholar]
- 12.Calvet G., Aguiar R.S., Melo A.S.O. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016;16:653–660. doi: 10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- 13.Cao-Lormeau V.M., Blake A., Mons S. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang H., Hammack C., Ogden S.C. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18:587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messina J.P., Kraemer M.U.G., Brady O.J. Mapping global environmental suitability for Zika virus. eLife. 2016;5:e15272. doi: 10.7554/eLife.15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson N.M., Cucunuba Z.M., Dorigatti I. Countering the Zika epidemic in Latin America. Science. 2016;353:353–354. doi: 10.1126/science.aag0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbink P., Stephenson K.E., Barouch D.H. Zika virus vaccines. Nature. 2018;16:594–600. doi: 10.1038/s41579-018-0039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Lancet Infectious Diseases Editorial Board Vaccine against Zika virus must remain a priority. Lancet Infect Dis. 2018;17:1003. doi: 10.1016/S1473-3099(17)30534-0. [DOI] [PubMed] [Google Scholar]
- 19.Taylor A., Foo S.S., Bruzzone R., Dinh L.V., King N.J.C., Mahalingam S. Immunol Rev. 2015;268:340–364. doi: 10.1111/imr.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver S.C., Costa F., Garcia-Blanco M.A. Zika virus: history, emergence, biology, and prospects for control. Antiviral Res. 2016;130:69–80. doi: 10.1016/j.antiviral.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brathwaite Dick O., San Martin J.L., Montoya R.H., del Deigo J., Zambrano Z., Dayan G.H. Review: the history of dengue outbreaks in the Americas. Am J Trop Med Hyg. 2012;87:584–593. doi: 10.4269/ajtmh.2012.11-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castanha P.M.S., Cordeiro M.T., Martelli C.M.T., Souza W.V., Marques E.T.A., Jr, Braga C. Force of infection of dengue serotypes in a population-based study in northeast Brazil. Epidemiol Infect. 2013;141:1080–1088. doi: 10.1017/S0950268812001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braga C., Luna C.F., Martelli C.M. Seroprevalence and risk factors for dengue infection in socio-economically distinct areas of Recife, Brazil. Acta Trop. 2010;113:234–240. doi: 10.1016/j.actatropica.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bardina S.V., Bunduc P., Tripathi S. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science. 2017;356:175–180. doi: 10.1126/science.aal4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dejnirattisai W., Supasa P., Wongwiwat W. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with Zika virus. Nat Immunol. 2016;17:1102–1108. doi: 10.1038/ni.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poland G.A., Kennedy R.B., Ovsyannikova I.G., Palacios R., Ho P.L., Kalil J. Development of vaccines against Zika virus. Lancet Infect Dis. 2018;18:e211–e219. doi: 10.1016/S1473-3099(18)30063-X. [DOI] [PubMed] [Google Scholar]
- 27.Wilder-Smith A., Vannice K., Durbin A. Zika vaccines and therapeutics: landscape analysis and challenges ahead. BMC Med. 2018;16:84. doi: 10.1186/s12916-018-1067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrett A.D.T. Current status of Zika vaccine development: Zika vaccines advance into clinical evaluation. npj Vaccines. 2018;3:24. doi: 10.1038/s41541-018-0061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richner J.M., Diamond M.S. Zika virus vaccines: immune response, current status, and future challenges. Current Opin Immunol. 2018;53:130–136. doi: 10.1016/j.coi.2018.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The World Health Organization. Vaccine pipeline tracker. 2020 https://docs.google.com/spreadsheets/d/19otvINcayJURCMg76xWO4KvuyedYbMZDcXqbyJGdcZM/pubhtml# Accessed 24st Feb 2020. [Google Scholar]
- 31.Zhao H., Fernandez E., Dowd K.A. Structural basis of Zika virus-specific antibody protection. Cell. 2016;166:1016–1027. doi: 10.1016/j.cell.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stettler K., Beltramello M., Espinosa D.A. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016;353:823–826. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q., Yang H., Liu X. Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus. Sci Transl Med. 2016;8 doi: 10.1126/scitranslmed.aai8336. 369ra179. [DOI] [PubMed] [Google Scholar]
- 34.Dai L., Song J., Lu X. Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microb. 2016;19:696–704. doi: 10.1016/j.chom.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Sirohi D., Chen Z., Sun L. The 3.8 Å resolution cryo-EM structure of Zika virus. Science. 2016;352:467–470. doi: 10.1126/science.aaf5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kostyuchenko V.A., Lim E.X.Y., Zhang S. Structure of the thermally stable Zika virus. Nature. 2016;533:425–428. doi: 10.1038/nature17994. [DOI] [PubMed] [Google Scholar]
- 37.Yuan L., Huang X.Y., Liu Z.Y. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science. 2017;358:933–936. doi: 10.1126/science.aam7120. [DOI] [PubMed] [Google Scholar]
- 38.Screaton G., Mongkolsapaya J. Evolution of neurovirulent Zika virus. Science. 2017;358:863–864. doi: 10.1126/science.aaq1297. [DOI] [PubMed] [Google Scholar]
- 39.Salvo M.A., Kingstad-Bakke B., Salas-Quinchucua C., Camacho E., Osorio J.E. Zika virus like particles elicit protective antibodies in mice. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boigard H., Alimova A., Martin G.R., Katz A., Gottlieb P., Galarza J.M. Zika virus-like particle (VLP) based vaccine. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garg H., Sedano M., Plata G., Punke E.B., Joshi A. Development of virus-like-particle vaccine and reporter assay for Zika virus. J Virol. 2017;91 doi: 10.1128/JVI.00834-17. e00834–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urakami A., Tun M.M.N., Moi M.L. An envelope-modified tetravalent dengue virus-like particle vaccine has implications for flavivirus vaccine design. J Virol. 2017;91 doi: 10.1128/JVI.01181-17. e01181–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mani S., Tripathi L., Raut R. Pichia pastoris-expressed Dengue 2 envelope forms virus-like particles without pre-membrane protein and induces high titer neutralizing antibodies. PLoS One. 2013;8:e64595. doi: 10.1371/journal.pone.0064595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tripathi L., Mani S., Raut R. Pichia pastoris-expressed Dengue 3 envelope-based virus-like particles elicit predominantly domain III-focused high titer neutralizing antibodies. Front Microbiol. 2015;6:1005. doi: 10.3389/fmicb.2015.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poddar A., Ramasamy V., Shukla R. Virus-like particles derived from Pichia pastoris-expressed Dengue virus type 1 glycoprotein elicit homotypic virus-neutralizing envelope domain III-directed antibodies. BMC Biotechnol. 2016;16:50. doi: 10.1186/s12896-016-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khetarpal N., Shukla R., Rajpoot R.K. Recombinant Dengue virus 4 envelope glycoprotein virus-like particles derived from Pichia pastoris are capable of eliciting homotypic domain III-directed neutralizing antibodies. Am J Trop Med Hyg. 2017;96:126–134. doi: 10.4269/ajtmh.16-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sapparapu G., Fernandez E., Kose N. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature. 2016;540:443–447. doi: 10.1038/nature20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shanmugam R.K., Ramasamy V., Shukla R., Arora U., Swaminathan S., Khanna N. Pichia pastoris-expressed Zika virus envelope domain III on a virus-like particle platform: design, production and immunological evaluation. Pathogens Dis. 2019;77 doi: 10.1093/femspd/ftz026. ftz026. [DOI] [PubMed] [Google Scholar]
- 49.Ramasamy V., Arora U., Shukla R. A tetravalent virus-like particle vaccine designed to display domain III of dengue envelope proteins induces multi-serotype neutralizing antibodies in mice and macaques which confer protection against antibody dependent enhancement in AG129 mice. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tripathi S., Balasubramaniam V.R.M.T., Brown J.A. A novel Zika virus mouse model reveals strain specific differences in virus pathogenesis and host inflammatory immune responses. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kraus A.A., Messer W., Haymore L.B., de Silva A.M. Comparison of plaque- and flow cytometry-based methods for measuring dengue virus neutralization. J Clin Microbiol. 2007;45:3777–3780. doi: 10.1128/JCM.00827-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batra G., Gurramkonda C., Nemani S.K., Jain S.K., Swaminathan S., Khanna N. Optimization of conditions for secretion of dengue virus type 2 envelope domain III using Pichia pastoris. J Biosci Bioengg. 2010;110:408–414. doi: 10.1016/j.jbiosc.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Henchal E.A., Gentry M.K., McCown J.M., Brandt W.E. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am J Trop Med Hyg. 1982;3:830–836. doi: 10.4269/ajtmh.1982.31.830. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe S.K., Chan W.K., Wang J., Rivino J.L., Lok S.M., Vasudevan S.G. Dengue virus infection with highly neutralizing levels of cross-reactive antibodies causes acute lethal small intestinal pathology without a high level of viremia in mice. J Virol. 2015;89:5847–5861. doi: 10.1128/JVI.00216-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shan C., Ortiz D.A., Yang Y. Evaluation of a novel reporter virus neutralization test for serological diagnosis of Zika and dengue virus infection. J Clin Microbiol. 2017;55:3028–3036. doi: 10.1128/JCM.00975-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shan C., Xie X., Ren P. A rapid Zika diagnostic assay to measure neutralizing antibodies in patients. EBioMed. 2017;17:157–162. doi: 10.1016/j.ebiom.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shukla R., Rajpoot R.K., Arora U., Poddar A., Swaminathan S., Khanna N. Pichia pastoris-expressed bivalent virus-like particulate vaccine induces domain III-focused bivalent neutralizing antibodies without antibody-dependent enhancement. Front Microbiol. 2018;8:2644. doi: 10.3389/fmicb.2017.02644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajpoot R.K., Shukla R., Arora U., Swaminathan S., Khanna N. Dengue envelope-based ‘four-in-one’ virus-like particles produced using Pichia pastoris induce enhancement-lacking, domain III-directed tetravalent neutralizing antibodies in mice. Sci Rep. 2018;8:8643. doi: 10.1038/s41598-018-26904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pierson T.C., Xu Q., Nelson S. The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell Host Microbe. 2007;1:135–145. doi: 10.1016/j.chom.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fowler A.M., Tang W.W., Young M.P. Maternally acquired Zika antibodies enhance Dengue disease severity in mice. Cell Host Microbe. 2018;24:743–750. doi: 10.1016/j.chom.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.George J., Valiant W.G., Mattapallil M.J. Prior exposure to Zika virus significantly enhances peak Dengue-2 viremia in Rhesus macaques. Sci Rep. 2017;7:10498. doi: 10.1038/s41598-017-10901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsieh S.C., Tsai W.Y., Nerurkar V.R., Wang W.K. Characterization of the ectodomain of the envelope protein of dengue virus type 4: expression, membrane association, secretion and particle formation in the absence of precursor membrane protein. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Espinosa D., Mendy J., Manayani D. Passive transfer of immune sera induced by a Zika virus-like particle vaccine protects AG129 mice against lethal Zika virus challenge. EBioMedicine. 2018;27:61–70. doi: 10.1016/j.ebiom.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abbink P., Larocca R.A., De La Barrera R.A. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353:1129–1132. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Larocca R.A., Abbink P., Peron J.P.S. Vaccine protection against Zika virus from Brazil. Nature. 2016;536:474–478. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sumathy K., Kulkarni B., Gondu R.K. Protective efficacy of Zika vaccine in AG129 mouse model. Sci Rep. 2017;7:46375. doi: 10.1038/srep46375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown J.A., Singh G., Acklin J.A. Dengue virus immunity increases Zika virus-induced damage during pregnancy. Immunity. 2019;50:1–12. doi: 10.1016/j.immuni.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Culshaw A., Mongkolsapaya J., Screaton G.R. The immunopathology of dengue and Zika virus infections. Current Opin Immunol. 2017;48:1–6. doi: 10.1016/j.coi.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 69.Harrison S.C. Immunogenic cross-talk between dengue and Zika viruses. Nature Immunol. 2016;17:1010–1012. doi: 10.1038/ni.3539. [DOI] [PubMed] [Google Scholar]
- 70.Vogl T., Hartner F.S., Glieder A. New opportunities by synthetic biology for biopharmaceutical production in Pichia pastoris. Current Opin Biotechnol. 2013;24:1094–1101. doi: 10.1016/j.copbio.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmad M., Hirz M., Pichler H., Schwab H. Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl Microbiol Biotechnol. 2014;98:5301–5317. doi: 10.1007/s00253-014-5732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Supplementary Information file. The ZIKV-80E gene sequence is available in GenBank (MK890246).