Abstract
Preeclampsia is a serious hypertensive disorder of pregnancy, which is only cured with delivery of the placenta, thereby commonly necessitating preterm birth of the fetus. Low-molecular-weight heparin (LMWH) has demonstrated potential to reduce the incidence of preeclampsia in high-risk pregnant women, although the underlying mechanism by which LMWH protects against preeclampsia is unknown. Given the complex structure and biologic actions of heparin, we tested the hypothesis that heparin can mediate preeclampsia prevention via nonanticoagulant pathways. We compared the effects of a nonanticoagulant, glycol-split LMWH (gsHep)—rendered nonanticoagulant through disruption of the antithrombin binding regions—with the LMWH dalteparin in the rat reduced uterine perfusion pressure (RUPP) surgical model of preeclampsia. Although RUPP animals exhibit significantly elevated blood pressure and reduced plasma levels of placental growth factor (PGF) compared to sham, neither dalteparin nor gsHep treatment significantly impacted these parameters. However, the observed positive correlation between PGF levels and number of viable fetuses in RUPP-induced animals suggests that reduced PGF levels were predominately due to placental loss. Daily subcutaneous injections of low-dose dalteparin but not gsHep significantly restored fetal growth that was impaired by RUPP surgery. Placentas from RUPP animals exhibited an abnormal labyrinth structure, characterized by expanded sinusoidal blood spaces, relative to sham-operated animals. Morphometric analysis demonstrated that dalteparin but not gsHep treatment normalized development of the labyrinth in RUPP-exposed conceptuses. These data suggest that the antithrombin-binding regions of LMWH are required to confer its protective effects on fetal growth and placental development.
Keywords: preeclampsia, placenta, fetal development, intrauterine growth restriction, rodents
Low-molecular-weight heparin promotes placental development and fetal growth, thereby providing a mechanism by which heparin therapy could benefit pregnant women at risk of preeclampsia with growth restricted fetuses.
Introduction
Preeclampsia is a serious hypertensive disorder of pregnancy that is characterized by new onset hypertension and organ injury. The only definitive cure for preeclampsia is removal of the placenta through delivery. Preeclampsia is a heterogeneous syndrome, with symptoms ranging from mild to severe and clinical presentation from early in the third trimester (< 34 weeks) to near-term. Severe, early-onset preeclampsia is associated with maternal vascular malperfusion (MVM) disease of the placenta [1]. MVM disease is characterized by poor spiral artery remodeling, which results in impaired utero-placental perfusion and damage to the placental villi, ultimately disrupting placental function [2]. Severe cases of preeclampsia are typically associated with adverse fetal outcomes mediated by the associated growth restriction [3]. The development of effective therapies that can restrict placental damage in the face of impaired utero-placental blood flow offer the potential to improve perinatal outcomes by extending the duration of pregnancy and promoting greater fetal growth.
Prophylactic low-molecular-weight heparin (LMWH) may augment the effects of low-dose aspirin for preventing preeclampsia in high-risk pregnant women and improving fetal outcomes [4]. Although the beneficial effects of LMWH were hypothesized to be due to its anticoagulant actions on coagulation proteases via antithrombin, in vitro studies suggest that LMWH can also exert a range of nonanticoagulant effects that can directly modulate placental function. These effects include stimulation of extravillous trophoblast invasion into the decidua, promotion of healthy turnover of the placental villous trophoblast, and enhancement of placental growth factor (PGF) secretion by placental villous explants (reviewed in [5]). Our group recently demonstrated that glycol-split heparin (gsHep), a derivative of heparin that has chemically disrupted antithrombin-binding regions (ATBRs) and therefore minimal anticoagulant properties, retains its ability to promote PGF release from 1st trimester placental villous explants, promote endothelial tube formation in the presence of placenta conditioned media, inhibit complement activation, and prevent endothelial activation by preeclamptic serum relative to dalteparin, a clinically used anticoagulant LMWH [6]. Since these drug effects could in theory prevent or ameliorate progression of preeclampsia in high-risk pregnant women, gsHep therefore may confer the important effects of heparin without the attendant risks of anticoagulation in pregnancy. Presently, however, in vivo studies aimed at elucidating mechanisms by which LMWH may protect against severe preeclampsia with impaired fetal growth are limited, none of which have included evaluating the effects of gsHep derivatives.
Of the animal models of preeclampsia available, the reduced uterine perfusion pressure (RUPP) surgical model of preeclampsia in the pregnant rat is one of the most reproducible and well-characterized models [7]. In this model, perfusion into the uteroplacental circulation of the pregnant rat is partially occluded using silver clips, thereby mimicking the reduced perfusion due to impaired spiral artery remodeling. By late gestation, the pregnant dams show elevated blood pressure and fetal growth restriction, among other effects. RUPP reduces placental blood flow by approximately 60%, resulting in reduced placental weight and expression of the stress-related protein JNK [8, 9]. A recent study in the mouse showed that the RUPP procedure can also impair placental junctional zone and labyrinth development and structure, thereby providing a potential mechanism by which fetal growth could be impacted in this model [10].
The purpose of this study was to compare the effects of dalteparin and gsHep in the RUPP model of preeclampsia, with the hypothesis that both dalteparin and gsHep will ameliorate the symptoms of preeclampsia induced by the model via nonanticoagulant actions. Our results show that, contrary to our hypothesis, neither dalteparin nor gsHep treatment significantly lowered blood pressure or increased plasma levels of PGF in RUPP rats; however, dalteparin significantly promoted fetal growth in RUPP rats, which was associated with normalized placental histological features, while gsHep had no impact on fetal growth.
Materials and methods
RUPP model
All animal procedures performed were in accordance with the Canadian Animal Care Council guidelines, and the protocol was approved by the University Health Network Animal Care Committee. Animals were housed two to a cage in a temperature-controlled room set to 23 °C with a 12:12 h light/dark cycle. Animals were given standard chow and water ad libitum.
The surgical procedure was performed as previously described with modifications [11], and a schematic representation of the experimental setup is provided in Supplementary Figure S1. In brief, virgin Sprague Dawley female rats (200-275 g; from Charles River, Saint Constant, Canada) were time-mated to male Sprague Dawley rats. Gestational day (GD) 0 was designated by the presence of a vaginal plug. On GD14, the pregnant rat was anesthetized and maintained with 2% isoflurane. A pre-operative dose of 0.05 mg/kg buprenorphine was subcutaneously injected for analgesic purposes. A midline incision was performed to expose the abdominal cavity and exteriorize the uterine horn. A silver clip with 0.200 mm gap width was inserted over the abdominal aorta above the iliac bifurcation, and 0.100 mm silver clips were also placed on the left and right uterine arcades between the ovarian branch and the first segmental artery. The incision was then closed, and the animal was monitored for recovery. A single subcutaneous dose of 1 mg/kg meloxicam was given post-operatively for analgesic purposes.
Daily subcutaneous injections of dalteparin (Fragmin) or gsHep (kind gift from Dilafor, Sweden) were administered beginning 24 h after surgery with the last dose injected on GD18. Low- (67 IU/kg dalteparin or 0.42 mg/kg gsHep) and high- (300 IU/kg dalteparin or 1.92 mg/kg gsHep) doses were selected to reflect clinically relevant prophylactic and therapeutic doses (peak plasma levels approximately 0.2 and 1.0 IU/mL anti-Factor Xa activity, respectively; please refer to Supplementary Materials for methods and Supplementary Figure S2) [12, 13]. gsHep doses were calculated as previously described and based on the specific activity of dalteparin (approximately 150 IU/mg) [6, 13]. Saline of equivalent volume to treatment doses was used as the vehicle control.
Sample size was calculated using the values 1-β = 0.2 and a two-sided α = 0.05. Effect size was estimated based on preliminary blood pressure data obtained from sham-operated animals (mean = 80 ± 7.8 mmHg, n = 3), a 25% increase in blood pressure in vehicle RUPP animals based on published findings [11], and a 10% difference in blood pressure in treated RUPP animals compared to vehicle-treated animals. Using G*Power Statistical Power software [14], we estimated the required sample size to be n = 12 animals in each group.
Blood pressure measurements and sample collection
Although previous studies perform blood pressure measurements via an implanted catheter in the conscious animal, we opted to implant the catheter and perform blood pressure measurements immediately prior to sacrifice on GD19 to eliminate the need for anticoagulants to keep the catheter line patent, which could otherwise complicate interpretation of results. On GD19, the animal was anesthetized with 2% isoflurane, and the right carotid artery was catheterized with a Becton Dickinson 24G 0.75 inch Insyte Autoguard catheter (Franklin Lakes, NJ, USA) connected to a PendoTech pressure transducer (cat no.: PRESS-S-000; Princeton, NJ, USA). The pressure transducer was also connected to an in-house assembled data acquisition device (National Instruments, Austin, TX, USA; model USB-6003) that was calibrated with a medical-grade sphygmomanometer before each use. Measurements were recorded at 100 Hz over 30 s, and mean arterial pressure (MAP) was derived from averaging all data points over 5 s of stable readings. The surgical procedure and measurements were completed within 15 min to reduce the impact of anesthesia on blood pressure.
Following blood pressure measurements, Sarstedt S-monovettes (Numbrecht, Germany) were connected to the catheter to collect serum and plasma-EDTA samples. Serum blood samples were allowed to clot for 20 min at room temperature before centrifuging at 2000 x g for 10 min to collect serum. Samples were stored at −80 °C until further analyses. PGF levels in the plasma were measured using a commercially available ELISA kit from R&D Systems (Minneapolis, MN, USA; cat no.: MP200).
To perform fetal measurements and collect placenta samples, viable fetuses and placentas were dissected out of the uterus. Amniotic fluid was drained, and the fetuses and placentas were blotted on sterile gauze before weighing. Weights were averaged within the litter per data point. Placentas were bisected at the mid-point and then formalin-fixed overnight and embedded into paraffin blocks for histological analyses.
Placental morphometry analysis
Paraffin-embedded sections of placentas were stained with hematoxylin/eosin and analyzed by morphometry to semi-quantify placental structural features. Using Visiopharm Stereology Software (Hoersholm, Denmark), the labyrinth of each placenta was selected as the region of interest, and 12.5% of the labyrinth area was quantified at 40x magnification, evaluating approximately 50–70 randomly sampled frames per placenta. In each frame, six points marked by the software were manually categorized by a blinded investigator as either maternal blood space, fetal blood space, or placental tissue, which included syncytiotrophoblast, sinusoidal trophoblast giant cells, and fetal vascular endothelial cells. Frames and points that sampled nonregions of interest, such as the junctional zone, the central canal, and trophoblast giant cells were excluded. One section from an average of four placentas from each litter was quantified and averaged as one biological replicate per dam. Six animals in each of sham, RUPP, RUPP + dalteparinlow, and RUPP + gsHeplow were included in this analysis. For both vehicle and treated RUPP samples, animals were chosen based on a resorption rate of approximately 50% to minimize effects of compensatory mechanisms or poor model induction on analysis. Data are expressed relative to the total number of counts as an estimation of area. For junctional zone and labyrinth area quantification, measurements were taken with Visiopharm Stereology Software by demarcating the boundaries of the junctional zone and labyrinth at 10x magnification.
Fetoplacental arterial vasculature and placental gene expression were also quantified by micro-computed tomography and quantitative reverse transcription polymerase chain reaction, respectively. Please refer to Supplementary Materials for methods and figures.
Statistical analyses
All measurements were performed as technical triplicates. Data are presented as median unless otherwise stated. Multiple comparisons used RUPP (vehicle treated) as the comparison group unless otherwise stated. Parametric (one-way ANOVA with Dunnett’s post-hoc) or nonparametric (Kruskal-Wallis test with Dunn’s post-hoc) analyses were used where appropriate, based on the criteria of equal variance (Bartlett’s test). GraphPad Prism 8.0 software (La Jolla, CA, USA) was used for analyses and P ≤ 0.05 was considered statistically significant. Animals were excluded from analyses in cases of 100% fetal resorption and significant morbidity from surgery (e.g., lethargy and significant bleeding). Individual samples were also excluded when samples were not acquired (e.g., failed access to the carotid for blood pressure and plasma collection) or compromised (e.g., plasma turbidity for aPTT measurements).
Results
RUPP-induced hypertension was not reversed by dalteparin or gsHep
As previously described [15], reducing uterine perfusion pressure in the RUPP animals significantly elevated mean arterial blood pressure compared to the sham-operated group by 21% (n = 9 vs 11 in RUPP-vehicle vs. sham, respectively; P < 0.01; Figure 1A). However, neither dalteparin nor gsHep, at low or high doses, had a significant impact on mean arterial blood pressure compared to the vehicle-treated group (n = 8–10 per group; P > 0.05).
Figure 1.
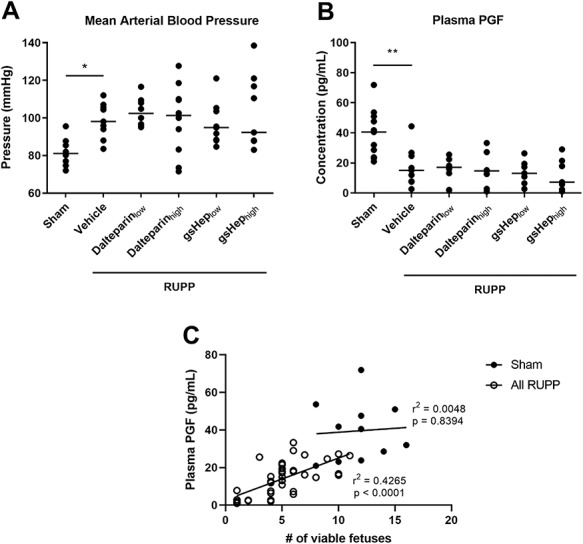
Effects of RUPP and treatments on maternal (A) mean arterial blood pressure (Kruskal-Wallis test with Dunn’s multiple comparison, *P < 0.05 compared to RUPP-vehicle group, N = 8–11) and (B) plasma PGF levels (one-way ANOVA with Dunnett’s multiple comparison, **P < 0.01 compared to RUPP-vehicle group, N = 7–11 rats in each group). Linear regression analysis determining the correlation between PlGF levels and number of viable fetuses in the sham and pooled RUPP groups (N = 11 vs. 39 samples).
Plasma PGF levels in RUPP rats were not impacted by dalteparin or gsHep treatments
Because PGF is emerging as an important angiogenic protein that negatively predicts the development of preeclampsia [16], we assessed whether the treatments altered plasma PGF levels. Consistent with previous findings [17], levels of PGF in the plasma of RUPP animals were 63% lower compared to sham (n = 9 vs 11 in RUPP-vehicle vs. sham; P < 0.01; Figure 1B). There was no significant difference in plasma PGF levels in RUPP rats treated with dalteparin or gsHep compared to control RUPP animals (n = 7–10 per group; P > 0.05).
We considered the possibility that levels of PGF in the plasma could be influenced by the number of viable fetoplacental units. For this analysis, we pooled together all RUPP-induced animals (i.e., including vehicle- and heparin-treated animals) to determine if PGF levels were affected by RUPP-induced fetal loss irrespective of treatment. Using linear regression analysis, we observed a significant positive correlation between PGF levels and number of viable fetuses in RUPP-induced animals (n = 39; P < 0.0001; Figure 1C), while there was no relationship between these two variables in the sham group (n = 11; P > 0.05).
Low-dose dalteparin improved fetal growth stunted by RUPP
As previously described [15], we found that reduction in uterine perfusion pressure significantly impaired fetal growth; fetuses from sham animals were 17% larger than the viable fetuses from RUPP animals (n = 12 per group; P < 0.001; Figure 2A). Low-dose dalteparin treatment significantly improved the growth of viable fetuses compared to the RUPP-vehicle group (n = 12; P < 0.05), while none of gsHep or high-dose dalteparin treatments elicited a similar effect (n = 11 per group; P > 0.05). There was no statistically significant difference in placental weight between the groups (n = 12 per group; P > 0.05; Figure 2B), although changes in placental weights trended in the same directions as fetal weight changes. In addition, while RUPP induced on average 60% fetal resorption rate compared to sham (n = 12 per group; P < 0.0001; Supplementary Figure S3), there was no significant difference in fetal loss between the RUPP-vehicle and treatment groups (n = 12 per group; P > 0.05). Adjusting for litter size as a covariate also did not significantly impact fetal and placental weight comparisons (data not shown). This suggests that improved fetal growth in the low-dose dalteparin group cannot be attributed to compensatory blood flow to fewer viable pups.
Figure 2.
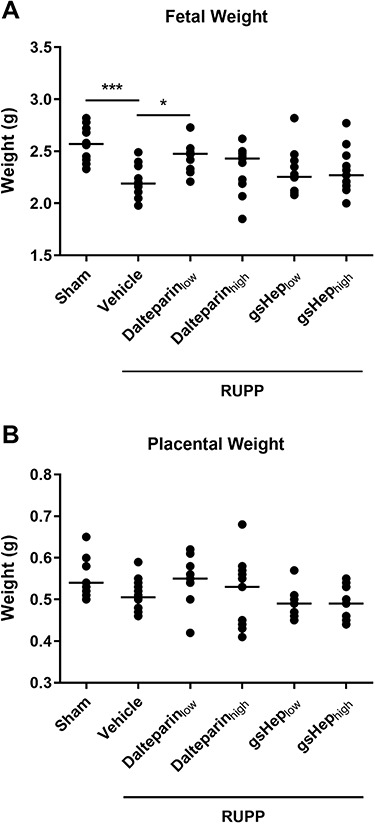
Effects of RUPP and treatments on (A) average viable fetal weight and (B) average viable placental weight. One-way ANOVA with Dunnett’s multiple comparison test, *P < 0.05, ***P < 0.001 compared to RUPP-vehicle group. N = 11–12 rats in each group.
Due to the lack of effect of high dose heparin treatments in our RUPP model, subsequent analyses were focused only on the low dose heparin treatments.
RUPP placentas were characterized by an abnormal histological labyrinth structure that was restored with dalteparin treatment
Since fetal growth was affected in this model and by our treatment, we examined histological sections of the placenta under the light microscope for structural changes. We observed that, compared to placentas from sham animals, those from RUPP animals showed perturbed features, especially in the labyrinth where maternal sinusoidal blood spaces appeared abnormally expanded (Figure 3). Morphometric analyses revealed that the relative area occupied by sinusoidal blood spaces in RUPP placentas was greater than in sham placentas (n = 5 vs. 6 in RUPP-vehicle vs. sham; P < 0.01; Figure 4A); correspondingly, placental tissue area was reduced in RUPP placentas compared to sham placentas (P < 0.01; Figure 4B). Placentas from RUPP animals treated with dalteparinlow showed a restorative effect, with reduced sinusoidal area (n = 6; P < 0.05) and increased placental tissue area (P < 0.01) resembling those of sham placentas. RUPP placentas treated with gsHeplow showed a mixed phenotype that was not statistically significant (n = 6; P > 0.05).
Figure 3.
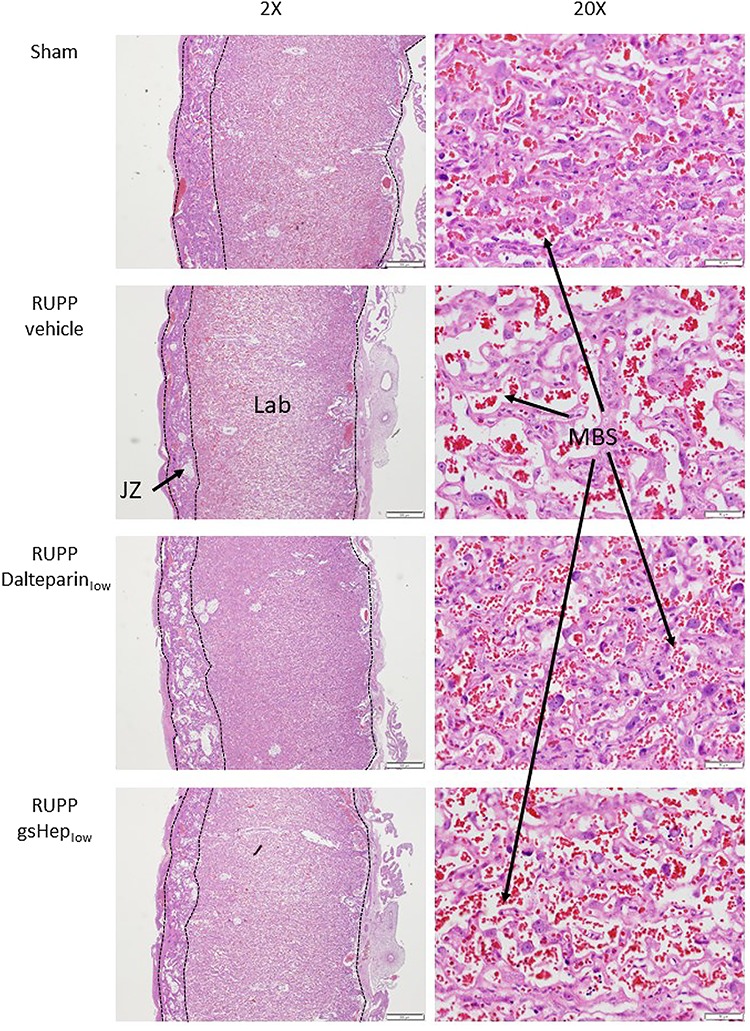
Representative images of H&E stained sections of rat placentas. 20x magnification sections show placental labyrinth structure. JZ: junctional zone; Lab: labyrinth; MBS: maternal blood space. Scale bars: 2X = 500 μm; 20X = 50 μm.
Figure 4.
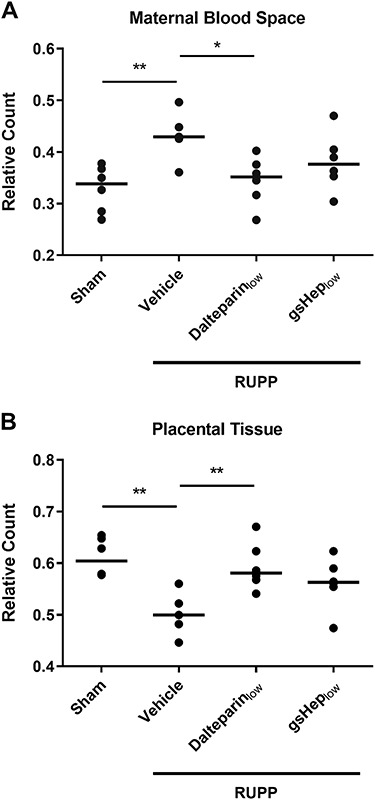
Results from morphometric relative count quantification of (A) maternal sinusoidal blood space and (B) placental tissue in the rat placental labyrinth. One-way ANOVA with Dunnett’s multiple comparison test, *P < 0.05, **P < 0.01 compared to RUPP-vehicle group. Each data point is derived from the average of 2–6 viable placentas from N = 5–6 rats in each group.
In addition, we observed that the junctional zone area of the RUPP placenta, normalized to the area of the labyrinth, was significantly smaller than that of sham placentas (n = 6 per group; P < 0.001; Figure 5). Treatment of RUPP animals with low-dose dalteparin appeared to have an effect on restoring junctional zone size, although this was not statistically significant (n = 6; P > 0.05). However, we note that the variation in the dalteparinlow group was mainly contributed by one of six data points, which may contribute to type II statistical error. By contrast, placentas from gsHep low-treated animals did not appear to be different from vehicle-treated RUPP animals (n = 6; P > 0.05).
Figure 5.
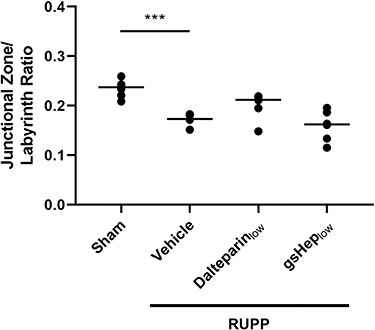
Area quantification of the junctional zone of rat placentas as a ratio of junctional zone to labyrinth area. One-way ANOVA with Dunnett’s multiple comparison test, ***P < 0.001 compared to RUPP-vehicle group. N = 6 rats in each group.
We isolated mRNA from whole placental tissue and perform quantitative reverse-transcription PCR to assess the expression of placental development and function genes, including GCM1, PGF, SFLT1, and SYN (Supplementary Table S1 and Supplementary Figure S4). However, we were unable to detect a statistically significant change in steady-state mRNA expression for any of the genes tested between any groups (n = 6–8 per group; P > 0.05).
Fetoplacental arterial vasculature development was not affected by either RUPP induction or heparin exposure
We were unable to detect changes to fetal blood space area through point counting morphometry since this technique was limited in sensitivity for such small structures. We therefore utilized micro-computed tomography to visualize the fetal arterial vasculature in the placenta in three dimension and assessed whether changes to fetoplacental arterial vasculature could explain changes in fetal development. We found no evidence that fetoplacental arterial vasculature development was affected by the RUPP procedure (n = 4–6 per group; P > 0.05; Supplementary Figures S5 and S6), suggesting that fetal growth restriction was mediated by dysregulation within the maternal placental compartment. We observed a small but statistically significant reduction in arterial lengths in dalteparinlow and gsHeplow-treated RUPP animals compared to vehicle- treated RUPP animals, but the functional implication of this effect is unclear. Since we observed that fetal growth was improved with low dose dalteparin treatment, the treatment-induced reduction in arterial network length does not result in impaired fetal development.
RUPP animals exhibit a thrombophilic phenotype that was not reversed by treatment
It has been hypothesized that the potential effects of LMWH in preventing preeclampsia include prevention of placental thrombotic lesions from forming [18]. We investigated whether dalteparin treatment chronically altered the hemostatic state of animals that had underwent the RUPP procedure. We performed an activated partial thromboplastin time (aPTT) assay on plasma collected at sacrifice. Plasma from RUPP animals exhibited a 34% reduction in aPTT clotting time compared to plasma from sham animals (n = 6 per group; P < 0.001), suggesting a thrombophilic state in RUPP animals. However, neither low nor high doses of dalteparin or gsHep had a significant impact on aPTT clotting time by the time of plasma collection (n = 6 per group; P > 0.05; Figure 6). In addition, we stained paraffin-embedded sections of placenta with Martius scarlet blue stain, which allows visualization of fibrin deposition (Supplementary Figure S7). The absence of red, acellular fibrin staining suggests that placental thrombosis is not relevant in this model of preeclampsia.
Figure 6.
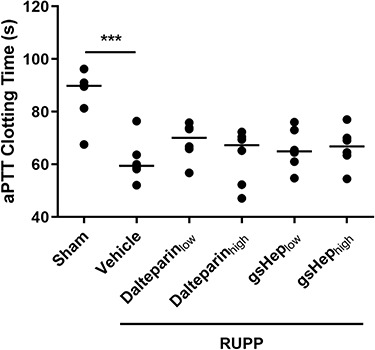
Hemostatic state of sham and RUPP placentas as measured by the activated partial thromboplastin time assay. One-way ANOVA with Dunnett’s multiple comparison test, ***P < 0.001 compared to RUPP-vehicle group. N = 6 rats in each group.
Discussion
In this study, we sought to determine whether dalteparin, an anticoagulant low molecular weight heparin, can reverse the adverse fetal and placental effects of preeclampsia induced in the RUPP model of this disease in the rat. We also assessed whether gsHep, a heparin derivative rendered nonanticoagulant due to chemical disruptions to the antithrombin binding regions, can elicit similar effects as dalteparin. Although neither heparin treatment reduced mean arterial blood pressure in this animal model of preeclampsia, low dose dalteparin but not gsHep significantly improved fetal growth; this selective effect of dalteparin was associated with preserved placental structure.
LMWH is commonly used in patients at risk of venous thromboembolism during pregnancy. Small clinical trials have identified its potential to also reduce recurrent preeclampsia [4], notably of the severe early-onset phenotype with associated fetal growth restriction, but its mechanism has not been elucidated to date. We have recently published a review article highlighting some of the potential nonanticoagulant mechanisms that may be involved [5], but to our knowledge, there have only been two studies exploring therapeutic potential of LMWH in animal models of preeclampsia. In these studies utilizing the L-NAME model of preeclampsia in the rat, LMWH reduced L-NAME-induced gestational hypertension by GD20 and GD21 [19, 20]. One of these studies also showed that LMWH increased the number of viable fetuses and improved fetal growth [19], while the other did not. Our results are at odds with these previous studies, as we found that dalteparin did not significantly reduce RUPP-induced gestational hypertension nor improve fetal viability, but significantly improved fetal growth in the RUPP model of preeclampsia. An important difference between the L-NAME and the RUPP models of preeclampsia is that the initial insult in the RUPP model is reduced uterine perfusion resulting in placental dysfunction which then triggers endothelial dysfunction, while the stimulus for the L-NAME model is direct endothelial dysfunction induced by inhibition of endothelial nitric oxide synthesis; it is possible that the pathways to endothelial dysfunction differ between these two models and dalteparin benefits one but not the other. In addition, we observed a positive correlation between PGF levels and the number of viable fetuses in the RUPP groups (Figure 1C). The reduced number of fetoplacental units was correlated with lower maternal PGF levels, suggesting that RUPP-induced reduction in plasma PGF could be due to loss of total placental mass. Since most cases of severe early-onset preeclampsia occur in singleton pregnancies, the clinical relevance of this finding is unclear.
Although dalteparin did not prevent the hypertensive phenotype in our study, this therapy restored the abnormal placental structure that was disrupted by the RUPP procedure. These results support the notion that LMWH therapy may be most beneficial for patients at high risk of developing preeclampsia characterized by severe placental dysfunction and fetal growth restriction, rather than the milder type of preeclampsia caused by maternal constitutive factors alone [21–23]. We speculate that LMWH-mediated normalization of placental structure can lead to improved fetal growth, which is critical in the context of severe early-onset preeclampsia, where the severity of the associated fetal growth restriction dictates the timing of iatrogenic preterm delivery [24]. The clinical implication of our findings is therefore that LMWH prophylaxis may be most relevant in those pregnancies at highest risk of early severe preeclampsia with fetal growth restriction; such women can be identified through maternal clinical characteristics and low circulating levels of PGF in the early 2nd trimester [25]. Normalization of placental function may also potentially prevent or delay the onset of preeclampsia-associated hypertension that is associated with severe preeclampsia, although this was not reflected in our study. It is possible that the irreversible and exaggerated stimulus of mechanically induced RUPP is too robust to observe such an indirect effect of LMWH on blood pressure in the RUPP model of preeclampsia.
Interestingly, we could not demonstrate any clear dose-dependent effect of dalteparin in our study, where low dose dalteparin significantly improved fetal growth but the higher dose did not. This observation lends support to the concept that nonanticoagulant mechanisms of LMWH do indeed confer the positive effects on placental development and fetal growth; if anticoagulation was mechanistically important, we would expect that higher doses with longer-lasting anticoagulant effects would confer greater protection. We also found that neither low nor high clinically relevant doses of dalteparin, administered on a once-a-day schedule applicable for prophylaxis, chronically altered hemostasis in RUPP rats, further suggesting a mechanism independent of coagulation.
Conceivably, one would expect that the mechanism responsible for improved fetal growth, anticoagulant or not, would also be more pronounced at higher concentrations. However, LMWH can elicit actions that are inversely dose-dependent. For example, longer unfractionated heparin chains can augment growth factor signaling by stabilizing ligand-heparin-receptor ternary complex formation, such as vascular endothelial growth factor (VEGF) interactions with VEGF receptor-2 [26]. However, shorter low molecular weight chains are insufficient to facilitate the interactions and can instead interfere with ligand-receptor interactions. For VEGF-VEGF receptor-2 interactions, the critical length is approximately 20 residues, approximately 6 kDa [26]. The dominance of inhibitory shorter chains at higher concentrations of LMWH could explain our previous observation that low concentrations of LMWH mildly stimulated angiogenesis, whereas higher concentrations did not [6]. This has potential clinical safety implications during pregnancy, since it is thought that proper angiogenic signaling is required for maintenance of normal maternal cardiovascular health [16].
Despite these speculations, it is not clear why the nonanticoagulant heparin derivative gsHep did not confer the same effects as dalteparin in the RUPP model. In this regard, we consider the possibility that the ATBRs of the molecule, which are intact and important for coagulation in dalteparin but chemically disrupted in gsHep, may also be involved in anticoagulation-independent mechanisms that could be relevant to placental development. The ATBRs of heparin interact with the serine protease inhibitor antithrombin and catalyze the irreversible inactivation of coagulation factors such as factor Xa and thrombin. Although primarily recognized for their role in coagulation, Factor Xa and thrombin can both directly participate in cell signaling, most notably in protease-activated receptor (PAR) signaling. The G-protein coupled PARs are activated via cleavage of a tethered ligand on the extracellular N-terminus and have been characterized to be important for signal transduction, including processes related to cell metabolism and growth [27]. In preeclampsia, placental PAR-1 is overexpressed [28], and suppression of its activation by thrombin reduces expression of the antiangiogenic protein soluble fms-like tyrosine kinase-1 (sFlt1) [29, 30]. PAR-2 activation also resulted in elevated sFlt1 secretion by an immortalized human trophoblast cell line [31]. A recent study demonstrated that inhibition of PAR-1 overexpression in the L-NAME model of preeclampsia improved placental vascular development, reduced circulating sFlt1, and reduced blood pressure [30]. Hence, PAR signaling could contribute to disrupted placental development, which could in turn impact fetal growth, and dalteparin could potentially reduce this impact of dysregulated PAR signaling. In addition, since the ATBRs have been disrupted in gsHep, gsHep cannot catalyze inactivation of Factor Xa or thrombin and prevent their signaling through PARs. It is possible that this difference between dalteparin and gsHep could explain the dissimilar effects observed in this study.
To our knowledge, our study is the first to characterize the effects of LMWH in the RUPP model of preeclampsia, currently regarded as one of the most relevant models of preeclampsia for its recapitulation of impaired placental perfusion. However, this study is not without limitations. There are several key limitations to the RUPP model of preeclampsia, including: a short study duration, compared to prophylaxis therapy beginning early 2nd trimester in clinical trials; small sample sizes, especially for placental morphometry analyses, which is likely underpowered to detect small differences in the fetoplacental parameters addressed in this study; absence of abnormal placentation or spiral artery remodeling, which LMWH could impact [32]; and absence of overt renal dysfunction, where LMWH can exert anti-inflammatory effects [5, 33]. All of these are mechanisms by which LMWH therapy may impact the clinical progression of preeclampsia but could not be assessed by the RUPP model. Another key limitation of our findings as they relate to human pregnancy is that the RUPP model is multi-fetal and includes fetal resorptions, whereas most women at risk of severe preeclampsia and associated fetal growth restriction have singleton pregnancies. The RUPP model pathology may therefore obscure the potential for dalteparin to prevent placenta-mediated severe preeclampsia. For this reason, we believe our data are sufficiently encouraging to support a pilot trial of dalteparin (or a similar LMWH) for the prevention of severe placenta-mediated pregnancy complications in women who remain vulnerable to these problems despite prophylactic low dose aspirin therapy [34].
In summary, prophylactic dalteparin significantly promoted fetal growth in the RUPP model of preeclampsia, while neither therapeutically dosed dalteparin nor gsHep exerted the same effects. Based on these findings, future clinical trials evaluating the effects of LMWH for the prevention of preeclampsia should focus on using prophylactic doses, and that anti-Factor Xa activity assays be used to monitor for appropriate peak plasma heparin activity. By doing so, heterogeneity surrounding heparin dosing between trials will be reduced. We believe that LMWH therapy can be a safe experimental therapy that does not cross the placental barrier and can be used at sub-anticoagulant levels that does not incur bleeding risks. The results from this study continue to demonstrate the therapeutic potential of LMWH for the prevention of preeclampsia but highlights the need for further molecular characterization of the effects of LMWH and robustly controlled clinical trials to evaluate its efficacy.
Supplementary Material
Acknowledgments
The authors thank Dilafor AB for providing gsHep for use in our studies and the University Health Network Animal Resource Centre for providing animal husbandry services.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1. Wright E, Audette MC, Ye XY, Keating S, Hoffman B, Lye SJ, Shah PS, Kingdom JC. Maternal vascular malperfusion and adverse perinatal outcomes in low-risk nulliparous women. Obstet Gynecol 2017; 130:1. [DOI] [PubMed] [Google Scholar]
- 2. Burton GJ, Woods AW, Jauniaux E, Kingdom JCP. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009; 30:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Redman CW, Sargent IL, Staff AC. IFPA senior award lecture: making sense of pre-eclampsia—two placental causes of preeclampsia? Placenta 2014; 35Suppl:S20–S25. [DOI] [PubMed] [Google Scholar]
- 4. Roberge S, Demers S, Nicolaides KH, Bureau M, Côté S, Bujold E. Prevention of pre-eclampsia by low-molecular-weight heparin in addition to aspirin: a meta-analysis. Ultrasound Obstet Gynecol 2016; 47:548–553. [DOI] [PubMed] [Google Scholar]
- 5. Wat JM, Audette MC, Kingdom JC. Molecular actions of heparin and their implications in preventing pre-eclampsia. J Thromb Haemost 2018; 16:1510–1522. [DOI] [PubMed] [Google Scholar]
- 6. Wat JM, Hawrylyshyn K, Baczyk D, Greig IR, Kingdom JC. Effects of glycol-split low molecular weight heparin on placental, endothelial, and anti-inflammatory pathways relevant to preeclampsia. Biol Reprod 2018; 99:1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol 2012; 303:H1–H8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. George EM, Arany I. Induction of heme oxygenase-1 shifts the balance from proinjury to prosurvival in the placentas of pregnant rats with reduced uterine perfusion pressure. Am J Physiol Integr Comp Physiol 2012; 302:R620–R626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP. Systemic hemodynamic and regional blood flow changes in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Physiol Circ Physiol 2007; 293:H2080–H2084. [DOI] [PubMed] [Google Scholar]
- 10. Natale BV, Mehta P, Vu P, Schweitzer C, Gustin K, Kotadia R, Natale DRC. Reduced Uteroplacental perfusion pressure (RUPP) causes altered trophoblast differentiation and pericyte reduction in the mouse placenta labyrinth. Sci Rep 2018; 8:17162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension 2000; 35:367–372. [DOI] [PubMed] [Google Scholar]
- 12. Wei MY, Ward SM. The anti-factor Xa range for low molecular weight heparin thromboprophylaxis. Hematol Rep 2015; 7:5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fragmin (dalteparin sodium) [product monograph]. Kirkland, Canada: Pfizer Canada Inc.; 2017. [Google Scholar]
- 14. Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009; 41:1149–1160. [DOI] [PubMed] [Google Scholar]
- 15. LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension 2008; 52:1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chappell LC, Duckworth S, Seed PT, Griffin M, Myers J, Mackillop L, Simpson N, Waugh J, Anumba D, Kenny LC, Redman CWG, Shennan AH. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation 2013; 128:2121–2131. [DOI] [PubMed] [Google Scholar]
- 17. Spradley FT, Ge Y, Haynes BP, Granger JP, Anderson CD. Adrenergic receptor blockade attenuates placental ischemia-induced hypertension. Physiol Rep 2018; 6:e13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arias F, Romero R, Joist H, Kraus FT. Thrombophilia: a mechanism of disease in women with adverse pregnancy outcome and thrombotic lesions in the placenta. J Matern Fetal Med 1998; 7:277–286. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Y, Liu F, Chen S, Zhong M. Low-molecular-weight heparin protects kidney through an anti-apoptotic mechanism in a rat pre-eclamptic model. Eur J Obstet Gynecol Reprod Biol 2015; 188:51–55. [DOI] [PubMed] [Google Scholar]
- 20. Liu W, Qiao F, Liu H, Gong X, Shi X, Li Y, Wu Y. Low molecular weight heparin improves proteinuria in rats with L-NAME induced preeclampsia by decreasing the expression of nephrin, but not podocin. Hypertens Pregnancy 2015; 34:24–35. [DOI] [PubMed] [Google Scholar]
- 21. Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension 2008; 52:873–880. [DOI] [PubMed] [Google Scholar]
- 22. McLaughlin K, Zhang J, Lye SJ, Parker JD, Kingdom JC. Phenotypes of pregnant women who subsequently develop hypertension in pregnancy. J Am Heart Assoc 2018; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McLaughlin K, Baczyk D, Potts A, Hladunewich M, Parker JD, Kingdom JCP. Low molecular weight heparin improves endothelial function in pregnant women at high risk of preeclampsia. Hypertension 2017; 69:180–188. [DOI] [PubMed] [Google Scholar]
- 24. Levytska K, Higgins M, Keating S, Melamed N, Walker M, Sebire NJ, Kingdom JCP. Placental pathology in relation to uterine artery Doppler findings in pregnancies with severe intrauterine growth restriction and abnormal umbilical artery Doppler changes. Am J Perinatol 2017; 34:451–457. [DOI] [PubMed] [Google Scholar]
- 25. Myers JE, Kenny LC, McCowan LME, Chan EHY, Dekker GA, Poston L, Simpson NAB, North RA. SCOPE consortium. Angiogenic factors combined with clinical risk factors to predict preterm pre-eclampsia in nulliparous women: a predictive test accuracy study. BJOG 2013; 120:1215–1223. [DOI] [PubMed] [Google Scholar]
- 26. Soker S, Goldstaub D, Svahn CM, Vlodavsky I, Levi BZ, Neufeld G. Variations in the size and sulfation of heparin modulate the effect of heparin on the binding of VEGF165 to its receptors. Biochem Biophys Res Commun 1994; 203:1339–1347. [DOI] [PubMed] [Google Scholar]
- 27. Rezaie AR. Protease-activated receptor signalling by coagulation proteases in endothelial cells. Thromb Haemost 2014; 112:876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Erez O, Romero R, Kim S-S, Kim J-S, Kim YM, Wildman DE, Than NG, Mazaki-Tovi S, Gotsch F, Pineles B, Kusanovic JP, Espinoza J et al. . Over-expression of the thrombin receptor (PAR-1) in the placenta in preeclampsia: a mechanism for the intersection of coagulation and inflammation. J Matern Fetal Neonatal Med 2008; 21:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao Y, Koga K, Osuga Y, Nagai M, Izumi G, Takamura M, Harada M, Hirota Y, Yoshino O, Taketani Y. Thrombin enhances soluble Fms-like tyrosine kinase 1 expression in trophoblasts; possible involvement in the pathogenesis of preeclampsia. Fertil Steril 2012; 98:917–921. [DOI] [PubMed] [Google Scholar]
- 30. Zhao Y, Zheng Y, Liu X, Luo Q, Wu D, Liu X, Zou L. Inhibiting trophoblast PAR-1 overexpression suppresses sFlt-1-induced anti-angiogenesis and abnormal vascular remodeling: a possible therapeutic approach for preeclampsia. Mol Hum Reprod 2018; 24:158–169. [DOI] [PubMed] [Google Scholar]
- 31. Hirai C, Sugimura M, Makino S, Takeda S. Chymotrypsin enhances soluble Fms-like tyrosine kinase 1 production through protease-activated receptor 2 in placenta-derived immortalized human trophoblast cells. Reprod Sci 2016; 23:1542–1550. [DOI] [PubMed] [Google Scholar]
- 32. Di Simone N, Di Nicuolo F, Sanguinetti M, Ferrazzani S, D’Alessio MC, Castellani R, Bompiani A, Caruso A. Low-molecular weight heparin induces in vitro trophoblast invasiveness: role of matrix metalloproteinases and tissue inhibitors. Placenta 2007; 28:298–304. [DOI] [PubMed] [Google Scholar]
- 33. Penning M, Chua JS, Kooten C, Zandbergen M, Buurma A, Schutte J, Bruijn JA, Khankin EV, Bloemenkamp K, Karumanchi SA, Baelde H. Classical complement pathway activation in the kidneys of women with preeclampsia. Hypertension 2015; 66:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, Paco Matallana C, Akolekar R, Cicero S, Janga D, Singh M, Molina FS, Persico N et al. . Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 2017; 377:613–622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


