Abstract
Mounting evidence indicates that the vagina can harbor uropathogenic bacteria. Here, we consider three roles played by the vagina and its bacterial inhabitants in urinary tract infection (UTI) and urinary health. First, the vagina can serve as a reservoir for Escherichia coli, the most common cause of UTI, and other recognized uropathogens. Second, several vaginal bacterial species are frequently detected upon urine culture but are underappreciated as uropathogens, and other vaginal species are likely under-reported because of their fastidious nature. Third, some vaginal bacteria that are not widely viewed as uropathogens can transit briefly in the urinary tract, cause injury or immunomodulation, and shift the balance of host-pathogen interactions to influence the outcomes of uropathogenesis. This chapter describes the current literature in these three areas and summarizes the impact of the vaginal microbiota on susceptibility to UTI and other urologic conditions.
Summary of findings
Data from clinical studies and model systems highlight the vaginal microbiota as a key factor impacting susceptibility to UTI and other urologic conditions.
The vagina can serve as a reservoir for uropathogen colonization.
Certain members of the vaginal microbiome are frequently detected in urine yet are underappreciated as uropathogens. Other vaginal bacterial species are likely underreported because they are difficult to culture or to identify.
The urinary tract can be transiently exposed to vaginal bacteria, some of which can cause injury to the bladder epithelium and impact pathogenesis of recognized uropathogens.
1 Introduction
It is well known, that women suffer from urinary tract infections (UTI) much more frequently than men. Mounting evidence indicates that the vagina can harbor uropathogenic bacteria. According to the current literature we consider the evidence from clinical correlations and experimental models that the vaginal microbiota impacts susceptibility to UTI and other urologic conditions.
2 Methods
We performed literature searches in PubMed. For our overview of the vaginal microbiome and its impact on the urinary tract, we searched “vaginal microbiome” OR “vaginal microbiota” AND “bacterial vaginosis” OR “BV” OR “dysbiosis”. To find articles relating to vaginal colonization by uropathogens, we searched “Escherichia coli” OR “Staphylococcus” OR “group B Streptococcus” OR “GBS” AND “vagina” OR “vaginal”. To identify articles relating to vaginal species that may be underappreciated uropathogens, we examined the list of organisms commonly detected in vaginal microbiome studies and searched these organisms along with “urinary tract” OR “urine” OR “UTI”.
3 Results and discussion
In order to better understand the role of the vaginal microbiota in UTI, we consider the “healthy” or “normal” state in reproductive age women and in pregnancy and three roles played by the bacterial inhabitants of the vagina in UTI and urinary health. First, the vagina can serve as a reservoir for Escherichia coli, the most common cause of UTI, and other recognized uropathogens. Second, several vaginal bacterial species are frequently detected upon urine culture but are underappreciated as uropathogens, and other vaginal species are likely under-reported because of their fastidious nature. Third, some vaginal bacteria that are not widely viewed as uropathogens can transit briefly in the urinary tract, cause injury or immunomodulation, and shift the balance of host-pathogen interactions to influence the outcomes of uropathogenesis.
3.1 Vaginal microbiota composition
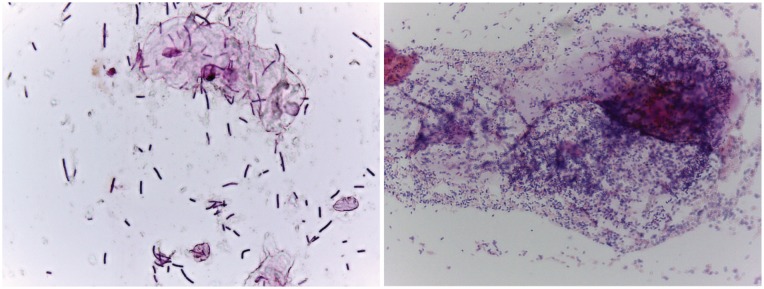
In reproductive age women, the vaginal microbiota is dominated by a few Lactobacillus species, including L. crispatus, L. gasseri, L. jensenii, and L. iners. These lactobacilli are thought to prevent growth of potential pathogens by maintaining the vagina’s characteristic low pH (by producing lactic acid [1]) and by producing antimicrobials such as hydrogen peroxide and bacteriocin-like substances [2]. Although a Lactobacillus-dominant vaginal microbiota is considered the “healthy” or “normal” state, a large proportion of reproductive age women (up to one-third in the United States) have a more diverse vaginal microbiota that contains no or low levels of Lactobacillus and a mixture of Gram-negative anaerobes, Actinobacteria, and other Firmicutes [3]. This apparent dysbiosis is called bacterial vaginosis (BV) or community state type IV [4]. While fewer studies have examined postmenopausal women, these echo what has been observed in younger cohorts, with some women having Lactobacillus-dominant and others having a more diverse polymicrobial vaginal microbiome [5], [6], [7]. A few studies have suggested that pregnancy may favor a stable Lactobacillus-dominant vaginal microbiome, however, a diverse vaginal microbiome is nonetheless present in some pregnant women [8]. In the clinic, a woman is diagnosed with BV if she has three of the four following signs (Amsel criteria): vaginal pH>4.5, ‘thin’ grayish homogenous vaginal fluid, vaginal fluid that produces a fishy odor when treated with potassium hydroxide, and the presence of epithelial cells studded with bacteria (i.e., ‘clue-cells’) in wet mount. In the laboratory, BV is diagnosed via the Nugent scoring system [9], which is based on morphotype assessment of Gram-stained slides; a score of 7 or higher on a 10-point scale indicates BV (see Figure 1 (Fig. 1) for examples of Gram-stained slides from women with and without BV). Women with BV are at higher risk of experiencing a wide array of negative health outcomes, including increased risk of a variety of secondary infections and adverse pregnancy outcomes.
Figure 1. Examples of Gram-stained slides from women with (right, Nugent score = 9) and without (left, Nugent score = 1) BV.
In women with a lactobacilli-dominated microbiome (left), long purple rods are the main morphotype. In contrast, women with BV tend to have a lot more bacteria overall, with a significant fraction that do not stain as Gram-positive (i.e. pink). Morphotypes in BV tend not to be long Gram-positive rods. Although not part of the Nugent scoring system, women with BV tend to have large conglomerations of bacteria associated with epithelial cells as seen in the upper right quadrant.
3.2 Relationships between the vaginal microbiota, UTI, and urinary health
Data from multiple clinical studies suggest that a woman’s vaginal microbiota affects her susceptibility to UTI [10]. For example, women with BV have higher UTI risk than women with lactobacilli-dominated vaginal microbiota [11], [12], [13]. Furthermore, clinical trials suggest that vaginal interventions that affect the microbiota (e.g., vaginal probiotic and estrogen treatments) can protect against additional episodes of recurrent UTI (rUTI) [14], [15]. As described later in this chapter, many of the bacterial genera found in the BV-associated vaginal environment have been detected, via both culture-dependent and -independent methods, in the urinary tract. Additionally, some of these bacteria have been implicated as causes of acute UTI or other urologic conditions. These findings suggest that fastidious BV-associated organisms may be important in the etiology of uropathology and uropathogenesis; however, in many cases additional studies are needed to ascribe causal roles for these organisms in the urinary tract.
Why women with a disrupted vaginal microbiota are at increased risk of UTI is unclear. Below, we describe three ways the vaginal microbiota could influence UTI and other urologic conditions. First, the vaginal introitus appears to be a key reservoir for uropathogenic E. coli. Second, other somewhat less common uropathogens can also be commonly harbored in the vagina (see Figure 2 (Fig. 2)). Finally, transient exposures of the urinary tract to certain vaginal bacteria may prime the urinary tract for uropathogens or trigger rUTI via other mechanisms.
Figure 2. Schematic illustrating vaginal bacteria with potential to impact the urinary tract.
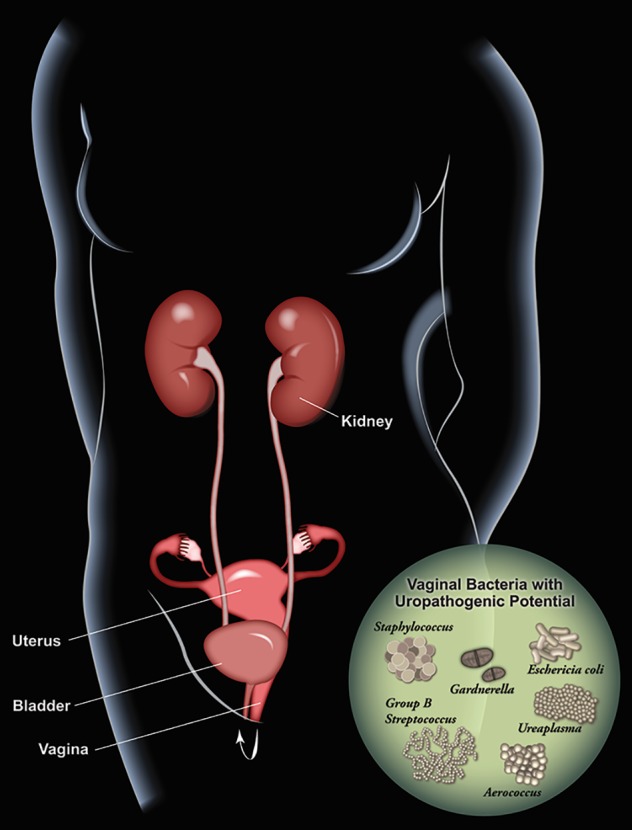
The vagina can serve as a reservoir for several bacterial species known to be causes of UTI (E. coli, GBS, Staphylococcus) as well as underappreciated potential uropathogens (G. vaginalis, Aerococcs Ureaplasma) that can cause UTI and have been associated with urological conditions such as urgency incontinence and “sterile” pyuria.
3.3 The vagina provides a reservoir for uropathogenic E. coli and other recognized uropathogens
3.3.1 E. coli
The most common cause of UTI in most patient populations is uropathogenic E. coli [16], [17]. The pathogenesis of E. coli UTI is often described as a series of colonization events, starting in the gastrointestinal tract, followed by the vaginal introitus and urethral meatus, and finally the bladder and possibly kidneys [10], [18]. This progression makes sense given the proximity of the urethra to the vaginal introitus and anus in females and that longitudinal examinations have indicated that introital and urethral colonization precedes UTI symptom onset [19], [20]. Moreover, multiple clinical studies have found that women with a history of rUTI more commonly have E. coli in the vaginal introitus or vagina than do healthy controls [19], [20], [21], [22]. One such study found that E. coli colonization of the vaginal introitus reached higher levels in women with a history of UTI (>105 colony forming units/mL) than in controls (<200 cfu/mL) [20]. In a separate study, concurrent colonization of the vaginal introitus and urinary tract by the same strain of E. coli (based on random amplified polymorphic DNA fingerprinting) occurred in 85% of paired isolates from women with a history of rUTI [20]. Together, these results demonstrate that the vagina provides a reservoir for uropathogenic E. coli.
As described above, Lactobacillus in the vagina may prevent colonization by potential pathogens such as E. coli. Indeed, women with low levels of lactobacilli more commonly carry vaginal E. coli than do those with lactobacilli-dominated microbiomes [13], [21]. In one study, E. coli vaginal (introital) colonization was more common among women whose vaginal introitus was negative for hydrogen peroxide-forming lactobacilli than among those whose introitus was positive for these ‘beneficial’ bacteria (35% vs. 11%, odds ratio [OR], 4.0; P=0.01) [21]. Multiple studies in both nonpregnant and pregnant women suggest that those with BV have increased risk for UTI (odds ratios from 2.21 to 13.75) [12], [23], [24]. In one study, BV was associated with both E. coli introital colonization and UTI [13]. In another study, over 50% of women with a history of UTI, but only 13% of those with no UTI history, had abnormal vaginal microbiota (P=0.03) as defined by a Nugent score greater than 3 (mean 4.6 vs. 1.7). Having a Nugent score of 7 or greater (indicative of BV) was also more common in UTI-prone women (6/22 [27%] vs. 1/17 [6%], P=0.095) [25]. This may be because lactic acid, hydrogen peroxide, and other small molecules produced by lactobacilli create a hostile environment for potential pathogens, including uropathogens, in the vagina [26]. Additional studies in experimental models are needed to define the effects of specific small molecules on the vaginal environment.
One intervention that appears to restore Lactobacillus colonization and may protect against rUTI in some patients is vaginal probiotics. A double-blind placebo-controlled trial studied 100 premenopausal women with at least one UTI in the past 12 months. Women received either intravaginal suppositories of powdered L. crispatus (Lactin-V) once daily for 5 days and then weekly for 10 weeks or placebo suppositories at the same interval. Fewer women who established high-level vaginal L. crispatus colonization (≥106 throughout follow-up) developed rUTI (relative risks were 0.07 for Lactin-V and 1.1 for placebo; P<0.01) [14]. In contrast, a smaller study found that vaginal application of other types of lactobacilli (L. casei and L. rhamnosus) twice weekly did not reduce the UTI incidence [27]. However, the latter study used a higher E. coli threshold (104 vs. 102) to diagnose an rUTI. Additionally, L. casei and L. rhamnosus, which are more common in the intestine than in the vagina, failed to colonize the vagina [27]. Future studies are needed to develop effective vaginal prebiotic and probiotic strategies to treat or prevent rUTI.
Another promising intervention is vaginal estrogen. This idea is based on the observation that high levels of vaginal lactobacilli occur only in 25% to 30% of postmenopausal women (who tend to have low estrogen levels) but in 60% to 70% of women who receive estrogen replacement therapy. In a placebo-controlled study of postmenopausal women with rUTI, vaginal estrogen restored vaginal Lactobacillus colonization and decreased vaginal Enterobacteriaceae colonization (67% of women pre-treatment vs. 31% post-treatment). Moreover, the UTI incidence was lower in the estrogen group than in the placebo group (0.5 vs. 5.9 episodes per patient year; P<0.001) [15]. In a separate study, women receiving vaginal estrogen had a 45% cumulative likelihood of remaining UTI-free over a 36-week follow up period, whereas those in the control group had only 20% likelihood of remaining UTI-free [28]. For a more detailed description of the clinical studies examining the role of Lactobacillus and E. coli vaginal colonization and vaginal interventions for UTI, please see this recent review [10].
3.3.2 Staphylococcus
The most frequent Gram-positive agent of community-acquired UTI is S. saprophyticus, but S. aureus and S. epidermidis can also cause UTI in certain settings (catheterization, pregnancy) [29], [30], [31]. Staphylococcus uropathogenesis was recently reviewed [32]. Data from in vitro studies and rat UTI models have revealed several factors required for S. saprophyticus virulence, including the secreted surface-associated proteins Aas (hemagglutinin) and Ssp (lipase); the cell wall proteins UafA, SdrI, SssF, and UafB, which mediate adherence; and a urease that is associated with urinary stone formation. Additionally, data from a mouse model suggest that the nickel ABC-transporters Opp2 and Opp5a contribute to S. aureus uropathogenesis.
Both S. aureus and S. epidermidis have been detected in vaginal or cervical samples [33], [34], [35]. Vaginal S. aureus has been implicated in toxic shock syndrome and aerobic vaginitis [36], [37], [38], a controversial inflammatory condition often mistaken for BV or Candida yeast infection. One study found that women with vaginal toxicogenic S. aureus were significantly more likely than those without S. aureus to harbor vaginal E. coli [38]. However, vaginal colonization by staphylococci has not been examined as a risk factor for UTI.
3.3.3 Group B Streptococcus
Streptococcus agalactiae, otherwise known as group B Streptococcus (GBS), is a Gram-positive β-hemolytic chain-forming coccus that commonly inhabits the lower gastrointestinal tract and the vagina. Although GBS colonization is often asymptomatic, GBS has been implicated in aerobic vaginitis [39]. GBS is also a recognized uropathogen, causing 2–3% (or ~160,000 cases annually in the U.S.) of all uncomplicated UTIs [17], [40]. GBS UTIs are more common in certain vulnerable populations. For example, among nursing home residents over 70 years of age, up to 39% of UTI cases involve GBS [41]. Additionally, GBS often causes asymptomatic bacteriuria and is found at significant titers in up to 7% of pregnant women [42], [43]. GBS is also frequently found in the urinary tract of people with diabetes, immunocompromised individuals, and those with pre-existing urologic abnormalities, all of whom are at increased risk of ascending pyelonephritis that can progress to bacteremia and/or urosepsis [44], [45]. Although GBS can inhabit both the vagina and the urinary tract, no studies have assessed the association between vaginal GBS and GBS UTI.
Mouse models of GBS UTI [46], [47], [48], [49] have mainly used serotype III GBS strains, which cause more symptomatic UTIs than most other serotypes [45]. The role of GBS virulence factors has not been as thoroughly examined in the urinary tract as in other niches, such as the bloodstream. However, available data suggest that the β-hemolysin/cytolysin is dispensable, whereas sialic acid residues of the GBS capsular polysaccharide are important for GBS survival in the urinary tract [47], [48]. Further studies are needed to define the bacterial and host mechanisms governing GBS disease in the urinary tract.
3.4 Other vaginal bacterial species that are underappreciated as uropathogens
3.4.1 Gardnerella vaginalis
Gardnerella vaginalis, a facultative Gram-variable Actinobacteria (a class frequently called ‘high-GC Gram-positives’), is best known as a frequent isolate and dominant member of the vaginal microbiota in BV. Although G. vaginalis is considered an unusual primary pathogen of the urinary tract, this bacterium can cause acute UTI [50]. The contribution of G. vaginalis to urinary tract pathology is likely underestimated for two reasons. First, G. vaginalis does not grow under the standard (aerobic) culture conditions that most clinical microbiology labs use. Second, when labs identify G. vaginalis in urine cultures, they do not report it as a potential uropathogen (even if present in pure culture at levels exceeding clinical thresholds for UTI diagnosis). Several studies suggest that G. vaginalis should be considered as a potential cause of urinary tract pathology. For example, in one study using appropriate culture conditions to detect it, G. vaginalis was isolated from 2.3% of urines from hospitalized patients, often in pure culture and >10,000 colony forming units per milliliter (cfu/ml). Compared to individuals in whom G. vaginalis was not detected, patients with G. vaginalis bacteriuria were more likely to have a history of rUTI or current pyelonephritis (kidney infection) [51]. These patients often reported symptoms, and 58% had pyuria (neutrophils in urine) [51]. Another study showed that the frequency of G. vaginalis in catheterized urine samples was higher in women with urgency urinary incontinence than in women with other urologic conditions [52]. Other studies have used suprapubic needle aspiration to collect urine from the bladder, bypassing possible vaginal contaminants, concluding that pregnancy increases women’s risk of harboring G. vaginalis in their bladders and that G. vaginalis was especially common in women with underlying renal disease [53], [54], [55], [56], [57]. In another study, G. vaginalis was commonly found in suprapubic aspirates from women with reflux scarring and “sterile pyelonephritis” [58]. Bladder washout studies strongly suggested that G. vaginalis was present in the kidneys of 75% of these patients. Finally, case reports implicate G. vaginalis in more serious diseases. For example, G. vaginalis has been isolated from women's bloodstreams during or after giving birth [59], and G. vaginalis bacteremia has been implicated in systemic diseases coinciding with urolithiasis (kidney stones) [60]. In short, G. vaginalis has been implicated in rUTI, urgency incontinence, kidney disease, and systemic infections originating in the genitourinary system.
Thus far, few reports have assessed G. vaginalis in both the vagina and the urinary tract. However, one recent study compared the microbiota of paired midstream urine and vaginal fluid from 42 women with BV [61] and found high levels of G. vaginalis in urine in a subset of women. The authors noted an association between urine and vaginal fluid communities in ~60% of women with urotypes dominated by G. vaginalis, Prevotella amni, Atopobium vaginae, or Sneathia amnii. In contrast, they reported only a 10–30% correlation among other urotypes (E. coli, Lactobacillus, etc.). The authors state that urine and vaginal microbiota patterns were not correlated in most women, and suggest that the two communities are therefore distinct in many women. However, despite the suggestion that some vaginal bacteria, including G. vaginalis, can exist concurrently in the vagina and urinary tract, this manuscript does not appear to have evaluated the co-occurrence of individual taxa per se in the two different compartments. The findings raise a number of interesting questions, including whether vaginal colonization with G. vaginalis a risk factor for G. vaginalis in urine or having concurrent or subsequent urologic pathologies. G. vaginalis isolates have highly diverse genomes and can be divided into at least four clades. In fact, some authors have suggested that G. vaginalis should be divided into multiple species or even genera [62], raising the question of whether certain G. vaginalis subtypes are more likely to be pathogenic in the urinary tract.
3.4.2 Aerococcus
Aerococcus is an α-hemolytic and microaerophilic or facultatively anaerobic Gram-positive coccus. Several Aerococcus species, including A. urinae, A. viridans, and A. sanguinicola, can cause UTI and urosepsis [63], [64], [65], [66], [67], [68], [69]. Patients with Aerococcus UTI often have underlying risk factors, such as urological abnormalities or older age [66], [67], [70]. Many case reports describing significant Aerococcus titers in urine also reported isolating the bacterium from blood [66], [71], [72]. Aerococcus must be properly identified and treated to avoid life-threatening systemic infection [68]. However, Aerococcus is difficult to distinguish from viridans-group streptococci with routine phenotypic tests and thus requires molecular tools such as amplification and 16S rRNA sequencing, a method not commonly employed in clinical microbiology labs. Additionally, most reported Aerococcus isolates are resistant to sulfonamides [69], [70]. Thus, when Aerococcus is not recognized, ineffective antibiotic treatment can be given, leading to rapid progression to systemic infection [67], [69], [71], [72], [73]. In addition to being implicated in UTI, Aerococcus was more frequently cultured from urine (collected by transurethral catheterization) from patients with urgency urinary incontinence than from women with other urinary tract conditions [52]. To our knowledge, Aerococcus pathogenesis in the urinary tract has not been examined with in vivo models.
Aerococcus sp. are commonly isolated from the human vagina and urinary tract and from air, dust, and vegetation. More studies are needed to understand how these natural Aerococcus niches affect pathogenesis and the clinical course of infections [74]. Aerococcus vaginal colonization is more common and abundant in women with BV [75], [76], but no studies have examined whether women with BV, or their sexual partners, are at increased risk for Aerococcus UTI.
3.4.3 Ureaplasma
Ureaplasma species, including U. urealyticum and U. parvum, are characterized by their ability to hydrolyze urea. U. parvum UTI pathogenesis was examined in Fischer 344 rats, revealing two distinct outcomes. Some animals developed a minimal immune response with limited monocytic and lymphocytic lesions and elevated urinary interferon (IFN)-γ, interleukin (IL)-18, and monocyte chemoattractant protein-1. Other animals developed an exaggerated pro-inflammatory immune response with elevated urinary IL-1α, IL-1β, CXCL1 (a.k.a. KC), neutrophilic lesions with extensive uroepithelial hyperplasia, and struvite (stone) formation [77]. A separate study demonstrated that rat strains differed in their susceptibility to U. parvum UTI and struvite formation [78]. Together, these studies suggest that host factors influence U. parvum UTI outcomes.
Between 40% and 80% of sexually active women have vaginal/cervical ureaplasmas [79]. Some studies indicate that Ureaplasma vaginal colonization increases during BV, but others have failed to find associations with BV [80] or vaginal symptoms in general [81]. However, ureaplasmas are reproducibly the most common cause of amniotic fluid infection in pregnant women, suggesting the bacteria can invade urogenital tissues and cause serious infection [82], [83]. Although acute cystitis by Ureaplasma is rare, the genus has been implicated in lower urinary tract symptoms [84], [85], including overactive bladder [86], “sterile pyuria” [87], and unexplained chronic voiding symptoms [85]. Further studies are needed to determine whether vaginal Ureaplasma is a risk factor for UTI or other lower urinary tract symptoms.
3.5 Covert pathogenesis
Although many consider the bladder to be sterile in the absence of UTI, clinical microbiology laboratories often find that urines have “insignificant” titers of bacteria (the “significant” threshold is usually ~100,000 cfu/ml). Additionally, numerous reports have described the composition of bacteria in urine (the urinary microbiome), further suggesting that bacteria can exist, at least transiently, in the urinary tract. The urinary microbiome is covered in greater detail elsewhere. Here, it is relevant to note that many of these studies have detected bacterial genera that have been independently found as part of the vaginal microbiota, including Lactobacillus, Streptococcus, and the BV-associated organisms Gardnerella, Prevotella, Bacteroides, and others [88]. Several studies classified urine samples into “urotypes” based on the dominant organisms, L. crispatus and G. vaginalis often being the most frequent [52]. Although the presence of these bacteria in urine is often attributed to contamination of urine by vaginal fluid, several studies have identified Gardnerella and lactobacilli in urine collected by transurethral catheterization or suprapubic aspiration, which completely rules out the possibility of contamination by periurethral or vaginal bacteria [89].
Vaginal bacteria can enter the urinary tract by mechanical transfer from nearby sites [10], [18], such as during sexual activity. In support of this idea, sexual activity (and its frequency) is one of the strongest risk factors for UTI and rUTI [15], [16], [90], [91], [92], [93], [94]. In addition to uropathogens such as E. coli, sexual activity likely also transfers numerous other vaginal bacteria. This possibility points to a new idea: that transient urinary tract exposure to certain vaginal bacteria can directly influence UTI pathophysiology even if these vaginal bacteria do not colonize the bladder or are cleared by the host before UTI diagnosis. This idea has been referred to as “covert pathogenesis” and is supported by findings from mouse models in which the urinary tract was exposed to different common vaginal bacteria within the context of E. coli UTI. For example, in one model, mice were exposed to group B Streptococcus (GBS, see above), a known immunomodulatory bacterium during acute or chronic bladder lumen infection by E. coli in C3H/HeN mice. The studies demonstrated that the presence of GBS enhanced E. coli survival in the bladder lumen in the early hours of acute infection, despite the fact that GBS was more rapidly cleared by the host during E. coli infection. In fact, even attenuated strains of E.c. lacking the ability to adhere to the bladder epithelium due to mutation of the type I pilus, had higher E. coli bladder lumen titers when GBS was present [11]. The presence of GBS also had effects on E. coli infection during chronic inflammatory infection of the bladder lumen by E. coli. This study provided an initial proof of principle that the composition of bacterial exposures to the urinary tract (containing E. coli) may influence initial host-E. coli interactions and therefore help determine whether E. coli causes UTI.
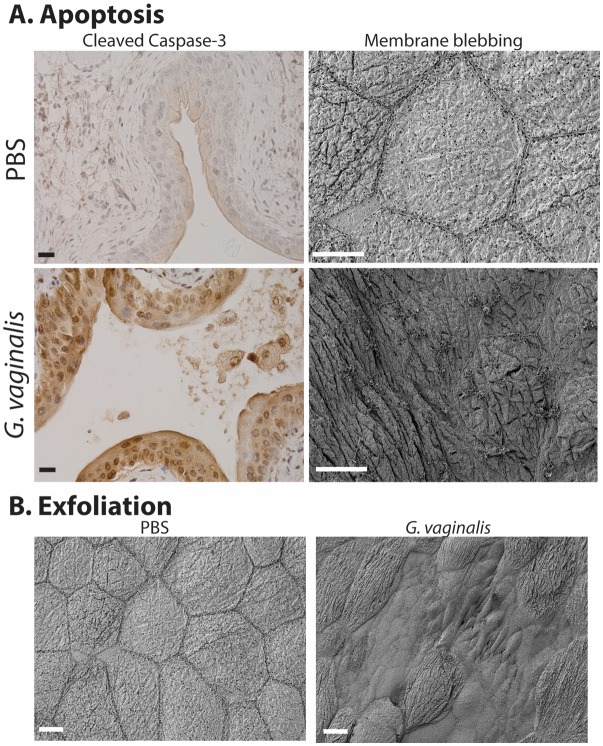
In another model, effects of another vaginal bacterium were studied during latent E. coli infection within intracellular epithelial reservoirs in C57/Bl6 mice (from a previous experimental infection), to investigate possible triggers of recurrent E. coli UTI arising from such reservoirs [95]. In these mice, two exposures to G. vaginalis triggered E. coli emergence into the bladder lumen, resulting in rUTI. G. vaginalis exposure also increased the incidence of severe E. coli kidney infections. G. vaginalis caused these effects despite being rapidly cleared from the urinary tract (by 12 hours in most mice). Even in the absence of latent E. coli (but also in the rUTI model), G. vaginalis caused apoptosis and exfoliation of the bladder epithelium (see Figure 3 (Fig. 3)) and also caused kidney damage by an IL-1-receptor-mediated mechanism. These findings suggest that G. vaginalis could be an important trigger of rUTI and a risk factor for pyelonephritis in women. If data from clinical studies support this idea, then new treatment options (antibiotics to limit G. vaginalis colonization) could be tested to help prevent rUTI, especially among patients with BV.
Figure 3. Gardnerella vaginalis induces apoptosis (A) and exfoliation (B) of the mouse bladder epithelium following two transurethral exposures, using our previously reported model [95].
Our blinded observation showed that ~75% of animals exposed to Gardnerella (compared to only ~25% of controls) exhibited staining for cleaved caspase-3, a marker of apoptotic cell death, in the outermost epithelium (A, left). Membranous protrusions were another feature we observed by scanning electron microscopy that is consistent with apoptosis (A, right). We also observed that G. vaginalis triggers exfoliation of superficial umbrella cells, revealing smaller underlying cells of the transitional epithelium (B). Scale bars = 20 μm.
4 Further research
We have described multiple mechanisms by which vaginal bacteria can impact UTI incidence or pathogenesis. Vaginal interventions, whether to eliminate the uropathogen reservoir or to treat associated vaginal conditions (e.g., BV), should be investigated as means to improve UTI outcomes, particularly in patients with rUTI for whom chronic antibiotic treatment is the only other option.
5 Conclusions
Although E. coli is unquestionably the dominant cause of UTI in young, sexually active women, it is clear that UTI and recurrent UTI stem from a wide array of etiologies. As outlined here, vaginal bacteria may cause UTI, either themselves (i.e. a traditional uropathogen using the vagina as a reservoir) or by acting as a “covert pathogens” to facilitate pathogenesis of another organism. Clinical microbiology labs commonly designate vaginal bacteria – whether identified in pure culture or detected alongside an accepted uropathogen that is present at levels above the usual clinical threshold for UTI – as being of “questionable clinical significance”. We emphasize that, rather than meaning that vaginal bacteria in urine are not important, this simply means that we do not fully understand their effects. UTI caused or encouraged by vaginal bacteria represent significant etiologies that should not be overlooked, as evidenced by findings that vaginal interventions to increase lactobacilli colonization have promising effects in certain patient subsets, as well as experimental models showing that certain vaginal bacteria can be “covert pathogens” (i.e. encourage virulence by recognized uropathogens despite their own rapid clearance).
Note
This article is also to be published as a chapter of the Living Handbook “Urogenital Infections and Inflammations“ [96].
Acknowledgements
We thank Deborah Frank for editorial assistance and thoughtful feedback on the manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tachedjian G, Aldunate M, Bradshaw CS, Cone RA. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol. 2017 Nov-Dec;168(9-10):782–792. doi: 10.1016/j.resmic.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Borges S, Silva J, Teixeira P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch Gynecol Obstet. 2014 Mar;289(3):479–489. doi: 10.1007/s00404-013-3064-9. [DOI] [PubMed] [Google Scholar]
- 3.Allsworth JE, Lewis VA, Peipert JF. Viral sexually transmitted infections and bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey data. Sex Transm Dis. 2008 Sep;35(9):791–796. doi: 10.1097/OLQ.0b013e3181788301. [DOI] [PubMed] [Google Scholar]
- 4.Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UM, Zhong X, Koenig SS, Fu L, Ma ZS, Zhou X, Abdo Z, Forney LJ, Ravel J. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012 May;4(132):132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hummelen R, Macklaim JM, Bisanz JE, Hammond JA, McMillan A, Vongsa R, Koenig D, Gloor GB, Reid G. Vaginal microbiome and epithelial gene array in post-menopausal women with moderate to severe dryness. PLoS ONE. 2011;6(11):e26602. doi: 10.1371/journal.pone.0026602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brotman RM, Shardell MD, Gajer P, Fadrosh D, Chang K, Silver MI, Viscidi RP, Burke AE, Ravel J, Gravitt PE. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2018 Nov;25(11):1321–1330. doi: 10.1097/GME.0000000000001236. [DOI] [PubMed] [Google Scholar]
- 7.Burton JP, Reid G. Evaluation of the bacterial vaginal flora of 20 postmenopausal women by direct (Nugent score) and molecular (polymerase chain reaction and denaturing gradient gel electrophoresis) techniques. J Infect Dis. 2002 Dec;186(12):1770–1780. doi: 10.1086/345761. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, Galuppi M, Lamont RF, Chaemsaithong P, Miranda J, Chaiworapongsa T, Ravel J. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014 Feb;2(1):4. doi: 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991 Feb;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stapleton AE. The Vaginal Microbiota and Urinary Tract Infection. Microbiol Spectr. 2016 Dec;4(6) doi: 10.1128/microbiolspec.UTI-0025-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumati AH, Saritha NK. Association of urinary tract infection in women with bacterial vaginosis. J Glob Infect Dis. 2009 Jul;1(2):151–152. doi: 10.4103/0974-777X.56254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillebrand L, Harmanli OH, Whiteman V, Khandelwal M. Urinary tract infections in pregnant women with bacterial vaginosis. Am J Obstet Gynecol. 2002 May;186(5):916–917. doi: 10.1067/mob.2002.123987. [DOI] [PubMed] [Google Scholar]
- 13.Hooton TM, Fihn SD, Johnson C, Roberts PL, Stamm WE. Association between bacterial vaginosis and acute cystitis in women using diaphragms. Arch Intern Med. 1989 Sep;149(9):1932–1936. [PubMed] [Google Scholar]
- 14.Stapleton AE, Au-Yeung M, Hooton TM, Fredricks DN, Roberts PL, Czaja CA, Yarova-Yarovaya Y, Fiedler T, Cox M, Stamm WE. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011 May;52(10):1212–1217. doi: 10.1093/cid/cir183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993 Sep;329(11):753–756. doi: 10.1056/NEJM199309093291102. [DOI] [PubMed] [Google Scholar]
- 16.Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am. 2014 Mar;28(1):1–13. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010 Dec;7(12):653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 18.Beerepoot M, Geerlings S. Non-Antibiotic Prophylaxis for Urinary Tract Infections. Pathogens. 2016 Apr;5(2) doi: 10.3390/pathogens5020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfau A, Sacks T. The bacterial flora of the vaginal vestibule, urethra and vagina in premenopausal women with recurrent urinary tract infections. J Urol. 1981 Nov;126(5):630–634. doi: 10.1016/s0022-5347(17)54661-3. [DOI] [PubMed] [Google Scholar]
- 20.Navas-Nacher EL, Dardick F, Venegas MF, Anderson BE, Schaeffer AJ, Duncan JL. Relatedness of Escherichia coli colonizing women longitudinally. Mol Urol. 2001;5(1):31–36. doi: 10.1089/109153601750124285. [DOI] [PubMed] [Google Scholar]
- 21.Gupta K, Stapleton AE, Hooton TM, Roberts PL, Fennell CL, Stamm WE. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli colonization in women with recurrent urinary tract infections. J Infect Dis. 1998 Aug;178(2):446–450. doi: 10.1086/515635. [DOI] [PubMed] [Google Scholar]
- 22.Stamey TA, Sexton CC. The role of vaginal colonization with enterobacteriaceae in recurrent urinary infections. J Urol. 1975 Feb;113(2):214–217. doi: 10.1016/s0022-5347(17)59447-1. [DOI] [PubMed] [Google Scholar]
- 23.Amatya R, Bhattarai S, Mandal PK, Tuladhar H, Karki BM. Urinary tract infection in vaginitis: a condition often overlooked. Nepal Med Coll J. 2013 Mar;15(1):65–67. [PubMed] [Google Scholar]
- 24.Harmanli OH, Cheng GY, Nyirjesy P, Chatwani A, Gaughan JP. Urinary tract infections in women with bacterial vaginosis. Obstet Gynecol. 2000 May;95(5):710–712. doi: 10.1016/s0029-7844(99)00632-8. [DOI] [PubMed] [Google Scholar]
- 25.Kirjavainen PV, Pautler S, Baroja ML, Anukam K, Crowley K, Carter K, Reid G. Abnormal immunological profile and vaginal microbiota in women prone to urinary tract infections. Clin Vaccine Immunol. 2009 Jan;16(1):29–36. doi: 10.1128/CVI.00323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovachev S. Defence factors of vaginal lactobacilli. Crit Rev Microbiol. 2018 Feb;44(1):31–39. doi: 10.1080/1040841X.2017.1306688. [DOI] [PubMed] [Google Scholar]
- 27.Baerheim A, Larsen E, Digranes A. Vaginal application of lactobacilli in the prophylaxis of recurrent lower urinary tract infection in women. Scand J Prim Health Care. 1994 Dec;12(4):239–243. doi: 10.3109/02813439409029247. [DOI] [PubMed] [Google Scholar]
- 28.Eriksen B. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am J Obstet Gynecol. 1999 May;180(5):1072–1079. doi: 10.1016/s0002-9378(99)70597-1. [DOI] [PubMed] [Google Scholar]
- 29.Muder RR, Brennen C, Rihs JD, Wagener MM, Obman A, Stout JE, Yu VL. Isolation of Staphylococcus aureus from the urinary tract: association of isolation with symptomatic urinary tract infection and subsequent staphylococcal bacteremia. Clin Infect Dis. 2006 Jan;42(1):46–50. doi: 10.1086/498518. [DOI] [PubMed] [Google Scholar]
- 30.Baraboutis IG, Tsagalou EP, Lepinski JL, Papakonstantinou I, Papastamopoulos V, Skoutelis AT, Johnson S. Primary Staphylococcus aureus urinary tract infection: the role of undetected hematogenous seeding of the urinary tract. Eur J Clin Microbiol Infect Dis. 2010 Sep;29(9):1095–1101. doi: 10.1007/s10096-010-0967-2. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert NM, O'Brien VP, Hultgren S, Macones G, Lewis WG, Lewis AL. Urinary tract infection as a preventable cause of pregnancy complications: opportunities, challenges, and a global call to action. Glob Adv Health Med. 2013 Sep;2(5):59–69. doi: 10.7453/gahmj.2013.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kline KA, Lewis AL. Gram-Positive Uropathogens, Polymicrobial Urinary Tract Infection, and the Emerging Microbiota of the Urinary Tract. Microbiol Spectr. 2016 Apr;4(2) doi: 10.1128/microbiolspec.UTI-0012-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kazi YF, Saleem S, Kazi N. Investigation of vaginal microbiota in sexually active women using hormonal contraceptives in Pakistan. BMC Urol. 2012 Aug;12:22. doi: 10.1186/1471-2490-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Y, Merkatz RB, Hillier SL, Roberts K, Blithe DL, Sitruk-Ware R, Creinin MD. Effects of a One Year Reusable Contraceptive Vaginal Ring on Vaginal Microflora and the Risk of Vaginal Infection: An Open-Label Prospective Evaluation. PLoS ONE. 2015;10(8):e0134460. doi: 10.1371/journal.pone.0134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akhi MT, Esmailkhani A, Sadeghi J, Niknafs B, Farzadi L, Akhi A, Nasab EN. The Frequency of Isolated from Endocervix of Infertile Women in Northwest Iran. Int J Fertil Steril. 2017 Apr-Jun;11(1):28–32. doi: 10.22074/ijfs.2016.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacPhee RA, Miller WL, Gloor GB, McCormick JK, Hammond JA, Burton JP, Reid G. Influence of the vaginal microbiota on toxic shock syndrome toxin 1 production by Staphylococcus aureus. Appl Environ Microbiol. 2013 Mar;79(6):1835–1842. doi: 10.1128/AEM.02908-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tansarli GS, Kostaras EK, Athanasiou S, Falagas ME. Prevalence and treatment of aerobic vaginitis among non-pregnant women: evaluation of the evidence for an underestimated clinical entity. Eur J Clin Microbiol Infect Dis. 2013 Aug;32(8):977–984. doi: 10.1007/s10096-013-1846-4. [DOI] [PubMed] [Google Scholar]
- 38.Chow AW, Bartlett KH, Percival-Smith R, Morrison BJ. Vaginal colonization with Staphylococcus aureus, positive for toxic-shock marker protein, and Escherichia coli in healthy women. J Infect Dis. 1984 Jul;150(1):80–84. doi: 10.1093/infdis/150.1.80. [DOI] [PubMed] [Google Scholar]
- 39.Donders GG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G, Spitz B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. BJOG. 2002 Jan;109(1):34–43. doi: 10.1111/j.1471-0528.2002.00432.x. [DOI] [PubMed] [Google Scholar]
- 40.Tan CK, Ulett KB, Steele M, Benjamin WH, Jr, Ulett GC. Prognostic value of semi-quantitative bacteruria counts in the diagnosis of group B streptococcus urinary tract infection: a 4-year retrospective study in adult patients. BMC Infect Dis. 2012 Oct;12:273. doi: 10.1186/1471-2334-12-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beyer I, Mergam A, Benoit F, Theunissen C, Pepersack T. Management of urinary tract infections in the elderly. Z Gerontol Geriatr. 2001 Apr;34(2):153–157. doi: 10.1007/s003910170080. [DOI] [PubMed] [Google Scholar]
- 42.Muller AE, Oostvogel PM, Steegers EA, Dörr PJ. Morbidity related to maternal group B streptococcal infections. Acta Obstet Gynecol Scand. 2006;85(9):1027–1037. doi: 10.1080/00016340600780508. [DOI] [PubMed] [Google Scholar]
- 43.Persson K, Bjerre B, Elfström L, Polberger S, Forsgren A. Group B streptococci at delivery: high count in urine increases risk for neonatal colonization. Scand J Infect Dis. 1986;18(6):525–531. doi: 10.3109/00365548609021657. [DOI] [PubMed] [Google Scholar]
- 44.Edwards MS, Baker CJ. Group B streptococcal infections in elderly adults. Clin Infect Dis. 2005 Sep;41(6):839–847. doi: 10.1086/432804. [DOI] [PubMed] [Google Scholar]
- 45.Ulett KB, Benjamin WH, Jr, Zhuo F, Xiao M, Kong F, Gilbert GL, Schembri MA, Ulett GC. Diversity of group B streptococcus serotypes causing urinary tract infection in adults. J Clin Microbiol. 2009 Jul;47(7):2055–2060. doi: 10.1128/JCM.00154-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kline KA, Schwartz DJ, Gilbert NM, Lewis AL. Impact of host age and parity on susceptibility to severe urinary tract infection in a murine model. PLoS ONE. 2014;9(5):e97798. doi: 10.1371/journal.pone.0097798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulkarni R, Randis TM, Antala S, Wang A, Amaral FE, Ratner AJ. β-Hemolysin/cytolysin of Group B Streptococcus enhances host inflammation but is dispensable for establishment of urinary tract infection. PLoS ONE. 2013;8(3):e59091. doi: 10.1371/journal.pone.0059091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kline KA, Schwartz DJ, Gilbert NM, Hultgren SJ, Lewis AL. Immune modulation by group B Streptococcus influences host susceptibility to urinary tract infection by uropathogenic Escherichia coli. Infect Immun. 2012 Dec;80(12):4186–4194. doi: 10.1128/IAI.00684-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan MJ, Carey AJ, Leclercq SY, Tan CK, Ulett GC. Increased Age, but Not Parity Predisposes to Higher Bacteriuria Burdens Due to Streptococcus Urinary Tract Infection and Influences Bladder Cytokine Responses, Which Develop Independent of Tissue Bacterial Loads. PLoS ONE. 2016;11(12):e0167732. doi: 10.1371/journal.pone.0167732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pedraza-Avilés AG, Zaragoza MC, Mota-Vázquez R, Hernández-Soto C, Ramírez-Santana M, Terrazas-Maldonado ML. Treatment of urinary tract infection by Gardnerella vaginalis: a comparison of oral metronidazole versus ampicillin. Rev Latinoam Microbiol. 2001 Apr-Jun;43(2):65–69. [PubMed] [Google Scholar]
- 51.Josephson S, Thomason J, Sturino K, Zabransky R, Williams J. Gardnerella vaginalis in the urinary tract: incidence and significance in a hospital population. Obstet Gynecol. 1988 Feb;71(2):245–250. [PubMed] [Google Scholar]
- 52.Pearce MM, Hilt EE, Rosenfeld AB, Zilliox MJ, Thomas-White K, Fok C, Kliethermes S, Schreckenberger PC, Brubaker L, Gai X, Wolfe AJ. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014 Jul;5(4):e01283–e01214. doi: 10.1128/mBio.01283-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lam MH, Birch DF, Fairley KF. Prevalence of Gardnerella vaginalis in the urinary tract. J Clin Microbiol. 1988 Jun;26(6):1130–1133. doi: 10.1128/jcm.26.6.1130-1133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilbert GL, Garland SM, Fairley KF, McDowall DM. Bacteriuria due to ureaplasmas and other fastidious organisms during pregnancy: prevalence and significance. Pediatr Infect Dis. 1986 Nov-Dec;5(6 Suppl):S239–S243. doi: 10.1097/00006454-198611010-00007. [DOI] [PubMed] [Google Scholar]
- 55.Savige JA, Gilbert GL, Fairley KF, McDowall DR. Bacteriuria due to Ureaplasma urealyticum and Gardnerella vaginalis in women with preeclampsia. J Infect Dis. 1983 Sep;148(3):605. doi: 10.1093/infdis/148.3.605. [DOI] [PubMed] [Google Scholar]
- 56.McDowall DR, Buchanan JD, Fairley KF, Gilbert GL. Anaerobic and other fastidious microorganisms in asymptomatic bacteriuria in pregnant women. J Infect Dis. 1981 Aug;144(2):114–122. doi: 10.1093/infdis/144.2.114. [DOI] [PubMed] [Google Scholar]
- 57.McFadyen IR, Eykyn SJ. Suprapubic aspiration of urine in pregnancy. Lancet. 1968 May;1(7552):1112–1114. doi: 10.1016/s0140-6736(68)90185-2. [DOI] [PubMed] [Google Scholar]
- 58.Fairley KF, Birch DF. Unconventional bacteria in urinary tract disease: Gardnerella vaginalis. Kidney Int. 1983 Jun;23(6):862–865. doi: 10.1038/ki.1983.107. [DOI] [PubMed] [Google Scholar]
- 59.Boggess KA, Watts DH, Hillier SL, Krohn MA, Benedetti TJ, Eschenbach DA. Bacteremia shortly after placental separation during cesarean delivery. Obstet Gynecol. 1996 May;87(5 Pt 1):779–784. doi: 10.1016/0029-7844(96)00037-3. [DOI] [PubMed] [Google Scholar]
- 60.Babics A, Roussellier P. Gardnerella vaginalis: An overlooked pathogen in male patients? Med Mal Infect. 2015 Oct;45(10):423–424. doi: 10.1016/j.medmal.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 61.Gottschick C, Deng ZL, Vital M, Masur C, Abels C, Pieper DH, Wagner-Döbler I. The urinary microbiota of men and women and its changes in women during bacterial vaginosis and antibiotic treatment. Microbiome. 2017 Aug;5(1):99. doi: 10.1186/s40168-017-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahmed A, Earl J, Retchless A, Hillier SL, Rabe LK, Cherpes TL, Powell E, Janto B, Eutsey R, Hiller NL, Boissy R, Dahlgren ME, Hall BG, Costerton JW, Post JC, Hu FZ, Ehrlich GD. Comparative genomic analyses of 17 clinical isolates of Gardnerella vaginalis provide evidence of multiple genetically isolated clades consistent with subspeciation into genovars. J Bacteriol. 2012 Aug;194(15):3922–3937. doi: 10.1128/JB.00056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL. Clinical significance of Aerococcus urinae: a retrospective review. Diagn Microbiol Infect Dis. 2005 Dec;53(4):289–292. doi: 10.1016/j.diagmicrobio.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 64.Senneby E, Petersson AC, Rasmussen M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin Microbiol Infect. 2012 Jun;18(6):546–550. doi: 10.1111/j.1469-0691.2011.03609.x. [DOI] [PubMed] [Google Scholar]
- 65.Murray TS, Muldrew KL, Finkelstein R, Hampton L, Edberg SC, Cappello M. Acute pyelonephritis caused by Aerococcus urinae in a 12-year-old boy. Pediatr Infect Dis J. 2008 Aug;27(8):760–762. doi: 10.1097/INF.0b013e318170af46. [DOI] [PubMed] [Google Scholar]
- 66.Ibler K, Truberg Jensen K, Ostergaard C, Sönksen UW, Bruun B, Schønheyder HC, Kemp M, Dargis R, Andresen K, Christensen JJ. Six cases of Aerococcus sanguinicola infection: clinical relevance and bacterial identification. Scand J Infect Dis. 2008;40(9):761–765. doi: 10.1080/00365540802078059. [DOI] [PubMed] [Google Scholar]
- 67.de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010 Sep;48(9):3445–3447. doi: 10.1128/JCM.00835-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cattoir V, Kobal A, Legrand P. Aerococcus urinae and Aerococcus sanguinicola, two frequently misidentified uropathogens. Scand J Infect Dis. 2010 Oct;42(10):775–780. doi: 10.3109/00365548.2010.485576. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Q, Kwoh C, Attorri S, Clarridge JE., 3rd Aerococcus urinae in urinary tract infections. J Clin Microbiol. 2000 Apr;38(4):1703–1705. doi: 10.1128/jcm.38.4.1703-1705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997 Dec;16(12):871–875. doi: 10.1007/bf01700552. [DOI] [PubMed] [Google Scholar]
- 71.Gritsch W, Nagl M, Hausdorfer J, Gschwendtner A, Pechlaner C, Wiedermann CJ. Septicaemia and endomyocarditis caused by Aerococcus urinae. Wien Klin Wochenschr. 1999 Jun;111(11):446–447. [PubMed] [Google Scholar]
- 72.Christensen JJ, Jensen IP, Faerk J, Kristensen B, Skov R, Korner B. Bacteremia/septicemia due to Aerococcus-like organisms: report of seventeen cases. Danish ALO Study Group. Clin Infect Dis. 1995 Oct;21(4):943–947. doi: 10.1093/clinids/21.4.943. [DOI] [PubMed] [Google Scholar]
- 73.Heilesen AM. Septicaemia due to Aerococcus urinae. Scand J Infect Dis. 1994;26(6):759–760. doi: 10.3109/00365549409008648. [DOI] [PubMed] [Google Scholar]
- 74.Rasmussen M. Aerococcus: an increasingly acknowledged human pathogen. Clin Microbiol Infect. 2016 Jan;22(1):22–27. doi: 10.1016/j.cmi.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 75.Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, Ross FJ, McCoy CO, Bumgarner R, Marrazzo JM, Fredricks DN. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS ONE. 2012;7(6):e37818. doi: 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ling Z, Kong J, Liu F, Zhu H, Chen X, Wang Y, Li L, Nelson KE, Xia Y, Xiang C. Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC Genomics. 2010 Sep;11:488. doi: 10.1186/1471-2164-11-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reyes L, Reinhard M, Brown MB. Different inflammatory responses are associated with Ureaplasma parvum-induced UTI and urolith formation. BMC Infect Dis. 2009 Jan;9:9. doi: 10.1186/1471-2334-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reyes L, Reinhard M, O’donell LJ, Stevens J, Brown MB. Rat strains differ in susceptibility to Ureaplasma parvum-induced urinary tract infection and struvite stone formation. Infect Immun. 2006 Dec;74(12):6656–6664. doi: 10.1128/IAI.00984-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taylor-Robinson D. Mollicutes in vaginal microbiology: Mycoplasma hominis, Ureaplasma urealyticum, Ureaplasma parvum and Mycoplasma genitalium. Res Microbiol 2017 Nov -;168(9-10):875–881. doi: 10.1016/j.resmic.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 80.Cox C, Watt AP, McKenna JP, Coyle PV. Mycoplasma hominis and Gardnerella vaginalis display a significant synergistic relationship in bacterial vaginosis. Eur J Clin Microbiol Infect Dis. 2016 Mar;35(3):481–487. doi: 10.1007/s10096-015-2564-x. [DOI] [PubMed] [Google Scholar]
- 81.Marovt M, Keše D, Kotar T, Kmet N, Miljković J, Šoba B, Matičič M. Ureaplasma parvum and Ureaplasma urealyticum detected with the same frequency among women with and without symptoms of urogenital tract infection. Eur J Clin Microbiol Infect Dis. 2015 Jun;34(6):1237–1245. doi: 10.1007/s10096-015-2351-8. [DOI] [PubMed] [Google Scholar]
- 82.Sweeney EL, Dando SJ, Kallapur SG, Knox CL. The Human Ureaplasma Species as Causative Agents of Chorioamnionitis. Clin Microbiol Rev. 2017 Jan;30(1):349–379. doi: 10.1128/CMR.00091-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Revello R, Alcaide MJ, Abehsera D, Martin-Camean M, Sousa E Faro Gomes M, Alonso-Luque B, Bartha JL. Prediction of chorioamnionitis in cases of intraamniotic infection by ureaplasma urealyticum in women with very preterm premature rupture of membranes or preterm labour. J Matern Fetal Neonatal Med. 2018 Jul;31(14):1839–1844. doi: 10.1080/14767058.2017.1330407. [DOI] [PubMed] [Google Scholar]
- 84.Humburg J, Frei R, Wight E, Troeger C. Accuracy of urethral swab and urine analysis for the detection of Mycoplasma hominis and Ureaplasma urealyticum in women with lower urinary tract symptoms. Arch Gynecol Obstet. 2012 Apr;285(4):1049–1053. doi: 10.1007/s00404-011-2109-1. [DOI] [PubMed] [Google Scholar]
- 85.Baka S, Kouskouni E, Antonopoulou S, Sioutis D, Papakonstantinou M, Hassiakos D, Logothetis E, Liapis A. Prevalence of Ureaplasma urealyticum and Mycoplasma hominis in women with chronic urinary symptoms. Urology. 2009 Jul;74(1):62–66. doi: 10.1016/j.urology.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 86.Lee YS, Kim JY, Kim JC, Park WH, Choo MS, Lee KS. Prevalence and treatment efficacy of genitourinary mycoplasmas in women with overactive bladder symptoms. Korean J Urol. 2010 Sep;51(9):625–630. doi: 10.4111/kju.2010.51.9.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nassar FA, Abu-Elamreen FH, Shubair ME, Sharif FA. Detection of Chlamydia trachomatis and Mycoplasma hominis, genitalium and Ureaplasma urealyticum by polymerase chain reaction in patients with sterile pyuria. Adv Med Sci. 2008;53(1):80–86. doi: 10.2478/v10039-008-0020-1. [DOI] [PubMed] [Google Scholar]
- 88.Domann E, Hong G, Imirzalioglu C, Turschner S, Kühle J, Watzel C, Hain T, Hossain H, Chakraborty T. Culture-independent identification of pathogenic bacteria and polymicrobial infections in the genitourinary tract of renal transplant recipients. J Clin Microbiol. 2003 Dec;41(12):5500–5510. doi: 10.1128/jcm.41.12.5500-5510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wolfe AJ, Toh E, Shibata N, Rong R, Kenton K, Fitzgerald M, Mueller ER, Schreckenberger P, Dong Q, Nelson DE, Brubaker L. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol. 2012 Apr;50(4):1376–1383. doi: 10.1128/JCM.05852-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nicolle LE, Harding GK, Preiksaitis J, Ronald AR. The association of urinary tract infection with sexual intercourse. J Infect Dis. 1982 Nov;146(5):579–583. doi: 10.1093/infdis/146.5.579. [DOI] [PubMed] [Google Scholar]
- 91.Stapleton A, Latham RH, Johnson C, Stamm WE. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection. A randomized, double-blind, placebo-controlled trial. JAMA. 1990 Aug;264(6):703–706. [PubMed] [Google Scholar]
- 92.Stamatiou C, Bovis C, Panagopoulos P, Petrakos G, Economou A, Lycoudt A. Sex-induced cystitis – patient burden and other epidemiological features. Clin Exp Obstet Gynecol. 2005;32(3):180–182. [PubMed] [Google Scholar]
- 93.Hooton TM, Scholes D, Hughes JP, Winter C, Roberts PL, Stapleton AE, Stergachis A, Stamm WE. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996 Aug;335(7):468–474. doi: 10.1056/NEJM199608153350703. [DOI] [PubMed] [Google Scholar]
- 94.Scholes D, Hooton TM, Roberts PL, Stapleton AE, Gupta K, Stamm WE. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000 Oct;182(4):1177–1182. doi: 10.1086/315827. [DOI] [PubMed] [Google Scholar]
- 95.Gilbert NM, O’Brien VP, Lewis AL. Transient microbiota exposures activate dormant Escherichia coli infection in the bladder and drive severe outcomes of recurrent disease. PLoS Pathog. 2017 Mar;13(3):e1006238. doi: 10.1371/journal.ppat.1006238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lewis AL, Gilbert NM. Roles of the vagina and the vaginal microbiota in urinary tract infection: Evidence from clinical correlations and experimental models. In: Bjerklund Johansen TE, Wagenlehner FME, Cho YH, Matsumoto T, Krieger JN, Shoskes D, Naber KG, editors. Urogenital Infections and Inflammations. Duesseldorf: GMS; 2017-. [DOI] [PMC free article] [PubMed] [Google Scholar]