Abstract
Aim
An easily performed method for examination of muscle abnormalities is anticipated. We aimed to elucidate the clinical usefulness of simple assessments for muscle abnormality including a simple five‐item questionnaire (SARC‐F) in chronic liver disease patients.
Methods
From February to July 2019, 383 outpatients (median age 71 years, 259 men; chronic hepatitis (CH) : liver cirrhosis Child–Pugh A : liver cirrhosis Child–Pugh B : liver cirrhosis Child–Pugh C = 157:176:39:11) who underwent a computed tomography examination were enrolled. SARC‐F, previously reported cut‐off values for muscle strength decline (MSD; handgrip), pre‐muscle volume loss (pre‐MVL), calf circumference and finger‐circle test results were used, and these results were analyzed retrospectively.
Results
A high SARC‐F score (≥4) was observed in 25 patients, and a low score (<4) in 358 patients. The frequency of high SARC‐F increased significantly with progression of chronic liver disease (chronic hepatitis : liver cirrhosis Child–Pugh A : liver cirrhosis Child–Pugh B/C = 2.5%:8.0%:14.0%, P=0.010). MSD frequency was 22.4% in men and 41.1% in women. Muscle volume loss and pre‐MVL were noted in 22% and 30.5%, respectively, of the male patients, and 9.7% and 32.3%, respectively, of the female patients. In cases with high SARC‐F and MSD, calf circumference and finger‐circle abnormalities were found in 56% and 40.0% of patients, respectively, whereas those values for patients with low SARC‐F and MSD were 14.5% and 10.6%, respectively (P < 0.001, for each; positive/negative predictive values: 0.560/0.855 and 0.400/0.894, respectively). Each SARC‐F item showed a good area under the curve for MSD, but not pre‐MVL.
Conclusion
SARC‐F score in combination with MSD and calf circumference or finger‐circle test results may be an easy and simple method for surveillance of chronic liver disease patients with a high risk of sarcopenia and decline of quality of life.
Keywords: chronic liver disease, muscle function, muscle volume, quality of life, SARC‐F, sarcopenia
Introduction
The idea of sarcopenia was originally proposed by Rosenberg,1 after which the European Working Group on Sarcopenia in Older People (EWGSOP)2 and Asian Working Group for Sarcopenia3 for elderly people developed diagnostic trees. Sarcopenia has been known to be an important prognostic factor of survival and quality of life (QOL). It has become well understood that sarcopenia is not rare in aged individuals, similar to such conditions as chronic heart failure, chronic renal failure, and cancer.2 The Japan Society of Hepatology (JSH) proposed criteria for determining sarcopenia in chronic liver disease (CLD) patients,4 after which physicians have recognized that muscle abnormalities (function and volume) in affected patients are not uncommon.5, 6, 7
For assessment of muscle volume, computed tomography (CT), dual‐energy X‐ray absorptiometry, and bioelectrical impedance analysis (BIA) are recommended modalities, although those are difficult for general practice physicians to perform for surveillance of their CLD patients. In an evaluation of muscle volume without use of special equipment, Kawakami reported the usefulness of calf circumference (CC) for assessment of muscle volume loss (cut‐off values indicating abnormality: men 34 cm, women 33 cm), while a yubi‐wakka (finger‐circle) test has also been developed to determine whether the maximum non‐dominant calf circumference is larger than finger‐circle circumference, formed with the index finger and thumbs of both hands, in affected patients.8, 9
In addition, an easy assessment tool for the evaluation of the decline of QOL in CLD patients is required in clinical practice. It is also a significant issue that many CLD patients with muscle abnormalities do not notice their decline of physical function, because it is a slow progression over a long period. Recently, the second version of the EWGSOP diagnostic tree (EWGSOP‐2) was developed,10 in which a simple five‐item questionnaire (SARC‐F),11 which consists of questions for QOL decline in daily life, is used as the first step for surveillance of sarcopenia. The present study aimed to elucidate whether SARC‐F test findings combined with simple assessment methods for determining muscle volume would be useful for screening high‐risk CLD patients for muscle abnormalities and QOL.
Methods
Clinical records of 383 enrolled outpatients who underwent a CT examination for screening of hepatocellular carcinoma (HCC) at Ehime Prefectural Central Hospital from February to July 2019 were examined. All patients were self‐reliant in regard to activities of daily living. Those with esophageal‐gastric varices, collateral vessel formation, pathological findings, and/or a platelet count <10 × 104 cells/μL were considered to have liver cirrhosis, and were classified using the Child–Pugh (CP) classification. Patients with a past history of treatment for chronic heart failure and/or chronic renal failure, which can result in extreme lower body edema, were excluded from this study. Tumor–node–metastasis stage was determined based on the criteria of the Liver Cancer Study Group of Japan 6th edition and used for evaluation of HCC.12 Patients positive for anti‐hepatitis C virus (HCV) were judged to have HCC due to HCV, whereas those positive for hepatitis B virus (HBV) surface antigen were judged to have HCC due to HBV.
Surveillance of muscle abnormalities
We performed surveillance of muscle abnormalities in the present CLD outpatients using data from their clinical records, each of whom were subjected to SARC‐F testing,11 determination of handgrip strength for assessment of muscle strength, and CC and finger‐circle testing for muscle quantity following the EWGSOP‐2 diagnostic flow‐chart.10 The study was conducted in compliance with the Helsinki Declaration, and the protocol was approved by the institutional ethics committee of Ehime Prefectural Central Hospital (No. 27–26).
Evaluation of muscle strength
Handgrip strength was measured using a hand dynamometer (TL110; TOEI LIGHT, Saitama, Japan) with the participant in a standing position. The highest values for both right and left handgrip strength from two measurements were averaged, and then used for analysis. For assessment of muscle strength decline (MSD), the JSH criteria for handgrip strength decline (cut‐off value in men and women, 26 and 18 kg, respectively) were assessed.4
Muscle volume assessment
Evaluation of muscle volume with CT images: Software capable of calculating total muscle area in CT images for muscle assessment is not available at our institution, thus we used a previously reported psoas index (PI) for objective evaluation of muscle area in individuals. PI was calculated based on the psoas muscle area at the middle of the third lumbar vertebra (L3) level (cm2), shown by CT, and height (m; total bilateral psoas muscle area/height2: cm2/m2).13 Values for the bilateral psoas muscles at the middle of the L3 level obtained with CT were evaluated, as noted in a previous report. The psoas muscle area was manually traced and calculated on CT images using personal computer software (Centricity Web DX, ver. 3.7.3.6417: GE Healthcare Japan, Tokyo, Japan). For this retrospective study, we used results from screening or follow‐up enhanced CT examinations performed for HCC. The PI values used for healthy young men (aged 45.6 ± 5.7 years, body mass index 25.3 ± 3.1 kg/m2) and women (47.0 ± 6.1 years, body mass index 21.7 ± 3.0 kg/m2) were 6.50 ± 1.13 and 4.30 ± 0.90 cm2/m2, respectively.13 Those were calculated from previous findings of CT examinations of young normal control individuals proven to have no obvious disease (e.g. chronic renal failure, chronic heart failure, CLD, diabetes mellitus), as well as previous results obtained in medical checkup procedures, including interview, electrocardiogram, urine, and blood examinations, with positron emission tomography/CT a part of the medical checkup procedures, and mean PI values of the healthy young cohort −2 standard deviation (SD) was defined as muscle volume loss (MVL).13 In the present study, a mean PI value lower than −1 SD was defined as pre‐MVL (men 5.37 cm2/m2, women 3.4 cm2/m2). Pre‐MVL was set as an abnormal line of muscle volume to investigate with SARC‐F, because the finger‐circle test, which has a predictive value for pre‐MVL in CLD, was used as one of the muscle volume assessment tools in the present study.9
Calf circumference
The cut‐off values for CC used for determining muscle volume decline were 34 cm in men and 33 cm in women, as previously described by Kawakami.14 CC values were recorded as the average measurements of each leg.
Finger‐circle test
The finger‐circle (yubi‐wakka) test was originally developed to determine whether the maximum non‐dominant CC value is greater than the individual participant's finger‐circle circumference, formed with the index finger and thumbs of both hands.8 With the patient in a seated position, finger‐circle testing was performed by turning up a hem. The dominant foot was determined as the usual first step side when beginning to walk. Based on the results of the finger‐circle test, the present patients were divided into three groups: bigger, just‐fits, and smaller. The results of previous reports (prediction for pre‐MVL: men just‐fits, women smaller) were used to determine the presence of muscle volume abnormality in the present study.9
SARC‐F questionnaire for surveillance of sarcopenia
The Japanese translated SARC‐F questionnaire was used for assessment of QOL of the present CLD outpatients.11 The following questions were used after translation: (i) “Strength”: How much difficulty do you have in lifting and carrying 10 pounds? (“10 pounds” was translated into “4.5 kg; for example, two water bottles holding 2 L and one bottle holding 0.5 L”; none = 0, some = 1, a lot or unable = 2); (ii) “Assistance with walking”: How much difficulty do you have walking across a room? (none = 0, some = 1, a lot or unable = 2); (iii) “Rising from a chair”: How much difficulty do you have transferring from a chair or bed? (none = 0, some = 1, a lot or unable = 2); (iv) “Climbing stairs”: How much difficulty do you have climbing a flight of 10 stairs? (none = 0, some = 1, a lot or unable = 2); and (v) “Falls”: How many times have you fallen in the past year? (none = 0, 1–3 falls = 1, 4 or more falls = 2). A SARC‐F score of ≥4 was defined as a high score and high risk of muscle abnormalities, including sarcopenia.
Statistical analysis
Values are expressed as the mean ± standard deviation (SD) or median (interquartile range). Statistical analyses were performed using a Kruskal–Wallis test, Fischer's exact test, receiver operator characteristic curve analysis, or area under the curve (AUC) analysis, as appropriate, with ezr version 1.29, a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).15 Holm's method was used in multiple comparison. P‐values <0.05 were considered to show statistical significance.
Results
The clinical features of the present cohort are shown in Table 1. The median age was 71 years (interquartile range 65–78 years). There were 259 men and 124 women. Chronic hepatitis was noted in 157 patients, liver cirrhosis with CP‐A in 176 patients, CP‐B in 39 patients, and CP‐C in 11 patients, whereas the CP score was 5, 6, 7, 8, 9, or ≥10 in 295, 38, 11, 1, 7, 11, and 11 patients, respectively. The etiological factors were HCV, HBV, HBV&HCV, alcohol, or others in 187, 49, 1, 63, and 83 patients, respectively.
Table 1.
Clinical features of present cohort
| Age, years (IQR) | 71 (65–78) |
|---|---|
| Sex (male : female) | 259:124 |
| Etiology (HCV : HBV : HBV&HCV : alcohol : others) | 187:49:1:63:83 |
| BMI, kg/m2 (IQR) | 23.2 (21.1–25.3) |
| CH : LC CP‐A : LC CP‐B : LC CP‐C | 157:176:39:11 |
| CP score (5:6:7:8:9:10:>10) | 295:38:11:17:11:11 |
| SARC‐F score (0:1:2:3:4:5:6:7:8:9:10) | 252:59:32:15:5:5:7:4:1:2:1 |
| SARC‐F (i) | 341:29:13 |
| SARC‐F (ii) | 306:56:21 |
| SARC‐F (iii) | 339:39:5 |
| SARC‐F (iv) | 351:22:10 |
| SARC‐F (v) | 316:59:8 |
| Muscle strength decline (handgrip strength) | 109 (28.5%) |
| Pre‐MVL | 184 (48.0%) |
Total n = 383. BMI, body mass index; CH, chronic hepatitis; CP, Child–Pugh; HBV, hepatitis B virus; HCV, hepatitis C virus; IQR, interquartile range; LC, liver cirrhosis; pre‐MVL, pre‐muscle volume loss.
MSD was observed in 109 patients (28.5%) and pre‐MVL in 184 patients (48.0%). A SARC‐F score of 0 was noted in 252 patients, one in 59, two in 32, three in 15, and four or more in 25 patients, with a high score (≥4) seen in 6.5% of the present cohort. The distributions of SARC‐F scores according to progression of CLD and muscle abnormalities are shown in Figure 1. The percentages of high SARC‐F scores (≥4) became significantly larger with progression of CLD (P = 0.010; Fig. 1a), whereas the frequency of high SARC‐F scores (≥4) was smaller (2.4%) in patients only with pre‐MVL, as well as those without MSD and pre‐MVL (2.5%), and patients with MSD showed significant elevation of SARC‐F score as compared with those without (P < 0.001, respectively; Fig. 1b). Nine of 58 patients both with MSD and pre‐MVL showed high SARC‐F scores (≥4; 15.5%), and three of the nine had sarcopenia (both with MSD and MVL). Among the patients in the high SARC‐F score group (n = 25), 76% had MSD, whereas that was seen in 25% of those in the other SARC‐F score groups (n = 358).
Figure 1.
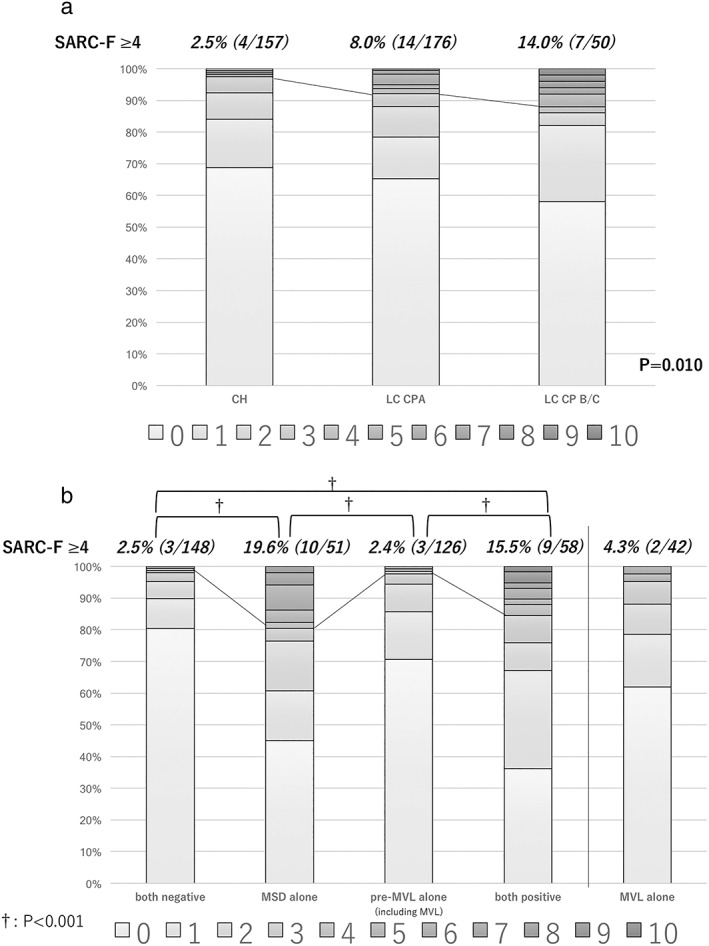
(a) Distribution of SARC‐F scores in each stage of chronic liver disease. Frequency of high SARC‐F score (≥4) related to chronic liver disease progression (P = 0.01). (b) In contrast, the distribution of the SARC‐F score in each situation of muscle (both negative for muscle strength decline [MSD] and pre‐muscle volume loss [pre‐MVL], MSD alone, pre‐MVL alone [including MVL], and both positive for them). The frequency of high SARC‐F score (≥4) was smaller (2.4%) for patients only with pre‐MVL, as well as those without MSD and pre‐MVL (2.5%). In addition, the distribution of SARC‐F in MVL alone was also shown (4.3%). Patients with MSD showed a significant larger number of high SARC‐F scores, as compared with those without (P < 0.001 in Holm's method, respectively). †P < 0.001
The frequency of high SARC‐F score was significantly increased with progression of CLD (CH : LC CP‐A : LC CP‐B/C = 2.5%:8.0%:14.0%, P = 0.010). When muscle volume was evaluated based on CC, 56% (14/25) of the patients in the high SARC‐F group showed both MSD and CC abnormalities, whereas those were seen in 14.5% (52/358) of the other patients (P < 0.001). Finger‐circle test analysis also showed that 40.0% (10/25) in the high SARC‐F group had both MSD and finger‐circle test abnormalities, whereas that value was just 10.6% (38/358) for the other patients (38/358; P < 0.001; Fig. 2). In additional analysis of MSD and pre‐MVL using PI, patients with both of those muscle abnormalities comprised 36.6% of the high SARC‐F group (9/25) and 10.6% of the other groups (49/358; P = 0.006). Each sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for muscle abnormalities by high SARC‐F score are shown in Table 2. Although sensitivity and PPV for MSD, and double positive for MSD and abnormalities of CC, finger‐circle test, or pre‐MVL were low, each specificity and NPV was good (Table 2). Sensitivity and PPV of double positive for MSD and abnormalities of CC or finger‐circle test were similar to those of combination of MSD and pre‐MVL (0.965/0.855, 0.955/0.894, and 0.951/0.863, respectively).
Figure 2.
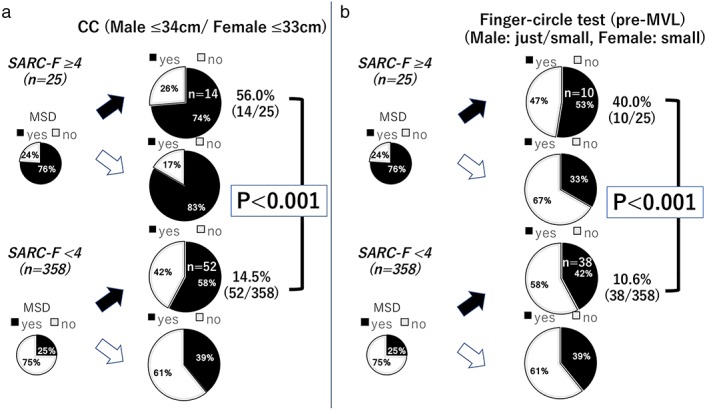
Percentage of high‐risk chronic liver disease patients with muscle abnormalities based on SARC‐F score. There were significant differences between the frequency of muscle strength decline (MSD) and abnormalities shown by (a) calf circumference (CC) and (b) finger‐circle test results in patients with and without a high SARC‐F score (≥4; P < 0.001). [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Sensitivity, specificity, positive predictive value and negative predictive value for muscle abnormalities by high SARC‐F score
| MSD | Double positive for MSD and CC abnormality | Double positive for MSD and finger‐circle test abnormality | Double positive for MSD and pre‐MVL | |
|---|---|---|---|---|
| Sensitivity | 0.176 | 0.212 | 0.208 | 0.151 |
| Specificity | 0.978 | 0.965 | 0.955 | 0.951 |
| PPV | 0.760 | 0.560 | 0.400 | 0.360 |
| NPV | 0.751 | 0.855 | 0.894 | 0.863 |
CC, calf circumference; MSD, muscle strength decline; NPV, negative predictive value; PPV, positive predictive value; pre‐MVL: pre‐muscle volume loss.
The cut‐off values for handgrip strength divided by SARC‐F classification i, ii, iii, iv, and v (each classification: score ≥1) (Figure 3a and 3b) in men were 24.0 kg (AUC 0.818, 95% CI 0.700–0.937), 30.0 kg (AUC 0.769, 95% CI 0.698–0.840), 30.3 kg (AUC 0.736, 95% CI 0.636–0.836), 18.2 k g (AUC 0.827, 95% CI 0.694–0.960), and 28.8 kg (AUC 0.756, 95% CI 0.669–0.842), respectively, whereas those in women were 15.8 kg (AUC 0.785, 95% CI 0.680–0.889), 19.6 kg (AUC 0.687, 95% CI 0.576–0.798), 15.6 kg (AUC 0.723, 95% CI 0.594–0.852), 14.4 kg (AUC 0.743, 95% CI 0.578–0.907), and 20.0 kg (AUC 0.613, 95% CI 0.496–0.729), respectively. As for cut‐off values of PI divided by SARC‐F classification (score ≥1), those in men were 4.992 cm2/m2 (AUC 0.671, 95% CI 0.528–0.815), 5.605 cm2/m2 (AUC 0.560, 95% CI 0.464–0.656), 4.219 cm2/m2 (AUC 0.660, 95% CI 0.528–0.793), 3.85 cm2/m2 (AUC 0.549, 95% CI 0.386–0.713), and 5.152 cm2/m2 (AUC 0.589, 95% CI 0.489–0.688), respectively, and in women were 3.331 cm2/m2 (AUC 0.549, 95% CI 0.420–0.677), 4.052 cm2/m2 (AUC 0.550, 95% CI 0.435–0.666), 4.004 cm2/m2 (AUC 0.546, 95% CI 0.407–0.684), 4.032 cm2/m2 (AUC 0.577, 95% CI 0.426–0.729), and 4.052cm2/m2 (AUC 0.623, 95% CI 0.510–0.736), respectively (Fig. 3c,d). Also, the cut‐off value of CC for prediction of pre‐MVL in CLD patients was 35.2 cm in men (sensitivity/specificity=0.743/0.667) (AUC 0.745, 95% CI 0.685–0.806) and 31.0 cm in women (sensitivity/specificity=0.561/0.794) (AUC 0.711, 95% CI 0.608–0.814).
Figure 3.
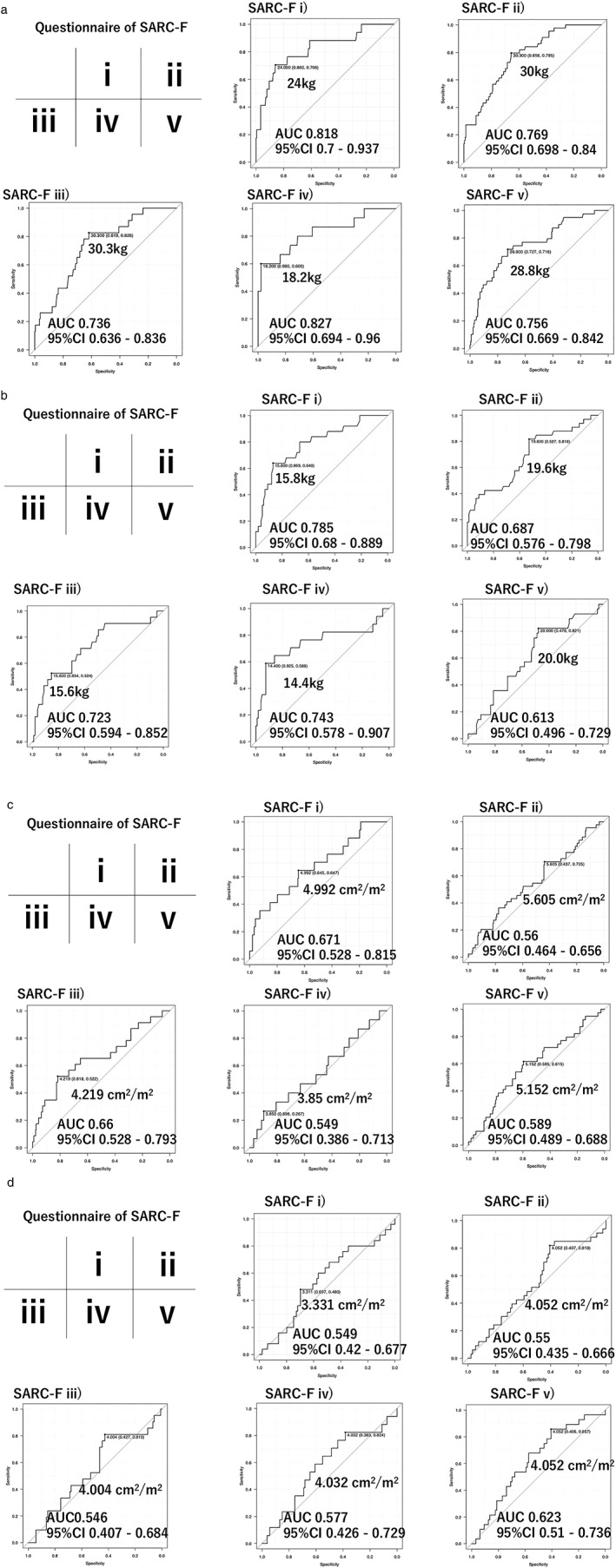
Relationship between muscle strength decline and each SARC‐F item. (a) Muscle strength decline (MSD; handgrip strength) in men. (b) MSD in women. (c) Psoas index (PI) in men. (d) PI in women. AUC, area under the curve.
Discussion
Muscle abnormalities, including MSD, MVL, and sarcopenia, are known to occur in CLD patients,5, 6 with MVL reported to be an important prognostic factor for death in patients with HCC,16, 17, 18, 19, 20 as well as those with liver cirrhosis.7 A previous meta‐analysis concluded that MVL is an independent prognostic factor for poor prognosis in HCC patients treated with not only curative, but also palliative, methods.21 In addition, muscle function decline has been found to have a close relationship with cognitive status. In our previous reports, a decline in peak expiratory flow rate was shown to be a risk factor other than age for postoperative delirium in HCC patients treated with surgical resection,20 whereas CLD patients with handgrip strength decline had significantly more frequent falls within a 1‐month period as compared with those without handgrip strength decline.22 Using Mini‐Mental State Examination results, Alfaro‐Acha et al. reported a close relationship between handgrip strength decline and cognitive function in elderly people in Mexico over a 7‐year period.23 That report also noted that individuals with reduced handgrip strength at the baseline showed a statistically significant decline in cognitive function, whereas those in the highest handgrip strength quartile maintained a higher level of cognitive function. In another study, Tinetti et al. reported that falling was associated with cognitive function in elderly patients.24 Also, as noted above, MSD and MVL, factors related to sarcopenia, each have important roles in QOL and survival of patients with CLD.
Recently, the JSH proposed criteria for sarcopenia in cases of CLD, in order to increase awareness of that age‐related disease among hepatologists in Japan. However, there are clinical issues that must be considered. Although MSD can be evaluated by handgrip strength by use of a hand dynamometer, more specialized equipment, such as CT, dual‐energy X‐ray absorptiometry, and BIA, for evaluation of MVL are far too expensive for use by general clinical physicians, and establishment of an easy method for assessment of muscle volume is required. CC testing was reported to be easy to perform and useful for muscle volume abnormality (cut‐off values: men 34 cm; women 33 cm).14 Furthermore, the finger‐circle (yubi‐wakka) test, in which the fingers of the patient are used for assessment, has been proposed,8 after which its usefulness for screening of pre‐MVL in CLD patients was shown (sensitivity/specificity/AUC: 0.619/0.667/0.654 in men, 0.740/0.583/0.698 in women).9 The EWGSOP recently upgraded their surveillance strategy for sarcopenia, termed EWGSOP‐2,10 in which SARC‐F was added as a first‐step questionnaire.11
Among the present patients with a high SARC‐F score and positive for MSD, 56.0% showed a CC abnormality and 40.0% had finger circle test results showing pre‐MVL, whereas those values were 14.5% and 10.6%, respectively, in patients with a low SARC‐F score. Additionally, 36.0% of the patients with a high SARC‐F score were positive for both MSD and pre‐MVL shown by PI, whereas those findings were found in 13.6% of the patients with a low SARC‐F score. Similar results were obtained with each evaluation. The present results show that use of SARC‐F for assessment of CLD patients has a high level of specificity, but low sensitivity, for high risk of sarcopenia in CLD patients. SARC‐F might play a different role for muscle assessment in CLD outpatients compared with that in general elderly people. A prior study found that the use of a questionnaire for determining QOL in CLD patients is important, because those patients do not generally complain, even if they have subjective symptoms, until asked by a physician and are often unaware that QOL has declined.25 In addition to assessments for muscle dysfunction (handgrip strength, CC, or finger‐circle test), SARC‐F testing as a questionnaire for QOL should be performed to make it easier for CLD patients to actively engage in the prevention of progression of sarcopenia.
In the present study, the cut‐off values for handgrip strength for each SARC‐F item in men ranged from 18.8 to 30.3 kg with good AUC values, whereas those for women ranged from 15.6 to 20.0 kg, also with good AUC values. In contrast, cut‐off values for PI for each item of SARC‐F had a wide range for both sexes, as well as poor AUC values. Our SARC‐F questionnaire results showed that decline in muscle function has a large impact on QOL decline in CLD patients. Recently, Ida et al. reported the usefulness of EWGSOP‐2 in 140 patients with cut‐off values for handgrip strength and muscle volume based on the JSH sarcopenia criteria.26 That report noted sensitivity and specificity for sarcopenia in men of 16.3% and 95.3%, respectively (PPV/NPV: 81.8%/47.1%), and 45.0% and 90.8%, respectively, in women (PPV/NPV: 81.8%/64.5%). The present results obtained with SARC‐F, handgrip strength, and CC or finger‐circle test results were favorable, and the methods can easily be used by general practitioners, although additional analysis with a greater number of patients and cut‐off values for muscle volume based on the JSH criteria is required, because the assessment method used for muscle volume was PI rather than those criteria. Furthermore, no report has provided suitable CC cut‐off values for muscle volume decline in CLD patients. In the near future, in addition to cut‐off values for CC, those for the finger‐circle tests according to the JSH criteria should be examined for prediction of muscle volume decline, as well as CT or BIA values, in CLD patients.
The present study had some limitations. First, we did not use JSH criteria, because assessment modalities for evaluation of total muscle area at L3 level and BIA are not available at our institution. Additional analysis should be performed to elucidate the relationship of sarcopenia or muscle volume abnormality with the JSH criteria. Second, this was a single‐center study, and additional analysis in a multicenter study format should be performed. Third, our cohort was limited, as the population comprised of outpatients who underwent CT for HCC screening. Fourth, the items examined were subjective, not objective. A study with a greater number of patients in each CLD stage is required in order to obtain more concrete results. For more detailed surveillance of muscle volume abnormalities, an additional examination to establish the exact cut‐off values for CC and finger‐circle tests, along with assessment modalities based on the JSH criteria, is necessary.
In conclusion, SARC‐F has limitations as a first step surveillance tool for CLD patients with a high risk of sarcopenia because of its low sensitivity and PPV. However, the present findings obtained using a combination of handgrip strength and CC or finger‐circle test results (assessment for muscle function and volume), along with complementary use of SARC‐F (assessment for QOL) show this method for surveillance of patients at high risk for sarcopenia and QOL decline to be easily performed in CLD outpatients.
Conflict of interest
Dr Atsushi Hiraoka gave lectures at Otsuka, Eisai and Bayer (2018–2019). The other authors declare no conflict of interest.
Acknowledgments
We are thankful for the cooperation of Ms Miho Oonishi and Ms Satsuki Koyama with CC measurements, the finger‐circle tests, and handgrip strength measurements.
Hiraoka, A. , Nagamatsu, K. , Izumoto, H. , Yoshino, T. , Adachi, T. , Tsuruta, M. , Aibiki, T. , Okudaira, T. , Yamago, H. , Suga, Y. , Iwasaki, R. , Mori, K. , Miyata, H. , Tsubouchi, E. , Ninomiya, T. , Hirooka, M. , Abe, M. , Matsuura, B. , Hiasa, Y. , and Michitaka, K. (2020) SARC‐F combined with a simple tool for assessment of muscle abnormalities in outpatients with chronic liver disease. Hepatol Res, 50: 502–511. 10.1111/hepr.13469.
References
- 1. Rosenberg I. Summary comments and methodological problems in determining nutritional status of older persons. Am J Clin Nutr 1989; 50: 1231–1233. [PubMed] [Google Scholar]
- 2. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM et al Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen LK, Liu LK, Woo J et al Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014; 15: 95–101. [DOI] [PubMed] [Google Scholar]
- 4. Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 2016; 46: 951–963. [DOI] [PubMed] [Google Scholar]
- 5. Hanai T, Shiraki M, Ohnishi S et al Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol Res 2016; 46: 743–751. [DOI] [PubMed] [Google Scholar]
- 6. Hiraoka A, Michitaka K, Ueki H et al Sarcopenia and two types of presarcopenia in Japanese patients with chronic liver disease. Eur J Gastroenterol Hepatol 2016; 28: 940–947. [DOI] [PubMed] [Google Scholar]
- 7. Hiraoka A, Kitahata S, Izumoto H et al Muscle volume loss a prognostic factor for death in liver cirrhosis patients and special relationship to portal hypertension. Hepatol Res 2018; 48: E354–e359. [DOI] [PubMed] [Google Scholar]
- 8. Tanaka T, Takahashi K, Akishita M, Tsuji T, Iijima K. "Yubi‐wakka" (finger‐ring) test: A practical self‐screening method for sarcopenia, and a predictor of disability and mortality among Japanese community‐dwelling older adults. Geriatr Gerontol Int 2018; 18: 224–232. [DOI] [PubMed] [Google Scholar]
- 9. Hiraoka A, Izumoto H, Ueki H et al Easy surveillance of muscle volume decline in chronic liver disease patients using finger‐circle (yubi‐wakka) test. J Cachexia Sarcopenia Muscle 2019; 10: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cruz‐Jentoft AJ, Bahat G, Bauer J et al Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC‐F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016; 7: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The Liver Cancer Study Group of Japan . The general rules for the clinical and pathological study of primary liver cancer, 6th edn. : Tokyo: Kanehara, 2015; 26. [Google Scholar]
- 13. Hiraoka A, Aibiki T, Okudaira T et al Muscle atrophy as pre‐sarcopenia in Japanese patients with chronic liver disease: computed tomography is useful for evaluation. J Gastroenterol 2015; 50: 1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawakami R, Murakami H, Sanada K et al Calf circumference as a surrogate marker of muscle mass for diagnosing sarcopenia in Japanese men and women. Geriatr Gerontol Int 2015; 15: 969–976. [DOI] [PubMed] [Google Scholar]
- 15. Kanda Y. Investigation of the freely available easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplant 2013; 48: 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Masuda T, Shirabe K, Ikegami T et al Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl 2014; 20: 401–407. [DOI] [PubMed] [Google Scholar]
- 17. Iritani S, Imai K, Takai K et al Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastroenterol 2015; 50: 323–332. [DOI] [PubMed] [Google Scholar]
- 18. Harimoto N, Yoshizumi T, Shimokawa M et al Sarcopenia is a poor prognostic factor following hepatic resection in patients aged 70 years and older with hepatocellular carcinoma. Hepatol Res 2016; 46: 1247–1255. [DOI] [PubMed] [Google Scholar]
- 19. Watanabe T, Tokumoto Y, Joko K et al Effects of long‐term entecavir treatment on the incidence of hepatocellular carcinoma in chronic hepatitis B patients. Hepatol Int 2016; 10: 320–327. [DOI] [PubMed] [Google Scholar]
- 20. Hiraoka A, Otsuka Y, Kawasaki H et al Impact of muscle volume and muscle function decline in patients undergoing surgical resection for hepatocellular carcinoma. J Gastroenterol Hepatol 2018; 33: 1271–1276. [DOI] [PubMed] [Google Scholar]
- 21. Chang KV, Chen JD, Wu WT, Huang KC, Hsu CT, Han DS. Association between Loss of Skeletal Muscle Mass and Mortality and Tumor Recurrence in Hepatocellular Carcinoma: A Systematic Review and Meta‐Analysis. Liver Cancer 2018; 7: 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hiraoka A, Tamura R, Oka M et al Prediction of risk of falls based on handgrip strength in chronic liver disease patients living independently. Hepatol Res 2019; 49: 823–829. [DOI] [PubMed] [Google Scholar]
- 23. Alfaro‐Acha A, Al Snih S, Raji MA, Kuo YF, Markides KS, Ottenbacher KJ. Handgrip strength and cognitive decline in older Mexican Americans. J Gerontol A Biol Sci Med Sci 2006; 61: 859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988; 319: 1701–1707. [DOI] [PubMed] [Google Scholar]
- 25. Hiraoka A, Yoshiji H, Iwasa M et al Clinical features of liver cirrhosis patients with muscle cramping: a multicenter study. Eur J Gastroenterol Hepatol 2019; 31: 1557–1562. [DOI] [PubMed] [Google Scholar]
- 26. Ida S, Kojima Y, Hamaoka S et al Validity of Japanese version of SARC‐F questionnaire in patients with chronic liver disease. J Gastroenterol Hepatol 2019; 34: 947–953. [DOI] [PubMed] [Google Scholar]


