Abstract
Aim
Treat‐to‐target, randomized controlled trials have confirmed lower rates of hypoglycaemia at equivalent glycaemic control with insulin degludec (degludec) versus insulin glargine 100 units/mL (glargine U100) in patients with type 1 (T1D) or type 2 diabetes (T2D). Treat‐to‐target trials are designed to enable comparisons of safety and tolerability at a similar HbA1c level. In this post hoc analysis of the SWITCH 1 and 2 trials, we utilised a patient‐level modelling approach to compare how glycaemic control might differ between basal insulins at a similar rate of hypoglycaemia.
Materials and Methods
Data for HbA1c and symptomatic hypoglycaemia from the SWITCH 1 and SWITCH 2 trials were analyzed separately for patients with type 1 diabetes and type 2 diabetes, respectively. The association between the individual patient‐level risk of hypoglycaemia and HbA1c was investigated using a Poisson regression model and used to estimate potential differences in glycaemic control with degludec versus glargine U100, at the same rate of hypoglycaemia.
Results
Improvements in glycaemic control increased the incidence of hypoglycaemia with both basal insulins across diabetes types. Our analysis suggests that patients could achieve a mean HbA1c reduction of 0.70 [0.05; 2.20]95% CI (for type 1 diabetes) or 0.96 [0.39; 1.99]95% CI (for type 2 diabetes) percentage points (8 [1; 24]95% CI or 10 [4; 22]95% CI mmol/mol, respectively) further with degludec than with glargine U100 before incurring an equivalent risk of hypoglycaemia.
Conclusion
Our findings suggest that patients in clinical practice may be able to achieve lower glycaemia targets with degludec versus glargine U100, before incurring an equivalent risk of hypoglycaemia.
Keywords: basal insulin analogues, glycaemic control, hypoglycaemia, insulin therapy, type 1 diabetes, type 2 diabetes
1. INTRODUCTION
Tight glycaemic control is important to reduce the risk of diabetes‐related complications and the associated morbidity, mortality and healthcare costs.1, 2 Glycaemic control requires a delicate balancing act between keeping blood glucose levels close to normal, and minimizing the risk of hypoglycaemia.3 Consequently, hypoglycaemia is the main limiting factor to achieving optimal glycaemic control in clinical practice, with implications for diabetes management and clinical outcomes.4 Insulin degludec (degludec) has the longest half‐life of currently marketed insulin analogues.5 The stable and consistent duration of the blood glucose‐lowering action of insulin degludec (degludec) has the potential to minimize fluctuations in a patient's blood glucose levels.6 An assessment of two pharmacodynamic studies confirmed consistently lower relative within‐day (estimated treatment ratio [degludec/glargine U100]: 0.61 [0.54; 0.69]95% CI) and between‐day variability (variance ratio [glargine U100/degludec]: 12.50 [7.14; 21.74]95% CI) in glucose‐lowering effect with degludec versus insulin glargine 100 units/mL (glargine U100), while accounting for potential experimental confounders.7 Randomized controlled trials (RCTs) suggest that these pharmacological properties translate into less frequent hypoglycaemia with degludec versus glargine U100 at equivalent glycaemic control, across a broad spectrum of patients with diabetes.8, 9, 10, 11
RCTs have established that intensive glycaemic control, targeting a lower HbA1c level through more aggressive insulin dose titration and/or the use of additional antihyperglycaemic agents, increases the risk of hypoglycaemia.12, 13, 14 However, the relationship between treatment regimen intensity and hypoglycaemia fundamentally differs from the relationship between glycaemic control and hypoglycaemia. The association between glycaemic control and hypoglycaemia rates in insulin‐treated patients with diabetes has been investigated by a small number of RCTs that have shown an inverse relationship between end‐of‐trial HbA1c level and hypoglycaemia rates.15, 16 Treat‐to‐target RCTs are designed to enable the comparison of safety endpoints, such as hypoglycaemia rates, at glycaemic parity, but this in turn confounds our understanding of the relative risks of hypoglycaemia for comparators at different HbA1c levels. Less is known about this relationship outside of the well‐controlled, clinical trial setting under conditions of routine clinical care. Observational studies show an inconsistent relationship between glycaemic control and hypoglycaemia rates in patients with diabetes.17, 18, 19 These inconsistencies most probably reflect inter‐individual differences in hypoglycaemia propensity,20 in combination with varying patient tolerance and awareness thresholds of hypoglycaemic events and a tendency to titrate insulin to this individual threshold of hypoglycaemia (rather than to a glycaemic target).
According to the US Food and Drug Administration guidance on study design for the investigation of new treatments for diabetes, the aim should be to achieve similar levels of glycaemic control in the new and comparator treatments to enable a better comparison of safety endpoints, such as hypoglycaemia, and thus establish comparable risk–benefit profiles.21 Such treat‐to‐target study designs have limited utility for comprehensive evaluations of treatment efficacy, as dosing and/or treatment intensification are manipulated in order to achieve the same glucose target with each treatment. However, a converse approach of comparing glycaemic control at similar incidence rates of defined safety endpoints would be unethical, as in order to obtain a robust comparison potentially damaging adverse events would need to be induced rather than avoided. Furthermore, high levels of inter‐individual variability in hypoglycaemia propensity,20, 22 the influence of lifestyle and/or stochastic factors on individual hypoglycaemia rates,20 and relatively low rates of hypoglycaemia would complicate the titration of insulin to target rates of hypoglycaemia.
In the current post hoc analysis, we utilized a patient‐level modelling approach and data from the SWITCH trials to compare how glycaemic control might differ between basal insulins at a similar rate of hypoglycaemia.
2. MATERIALS AND METHODS
2.1. SWITCH trial designs
The trial design and primary results of the SWITCH trials have been previously published.10, 11 In brief, SWITCH 1 (NCT02034513) and SWITCH 2 (NCT02030600) were both randomized, double‐blind, multicentre, two‐period crossover, treat‐to‐target clinical trials in patients with diabetes (please refer to Appendix 1 in the supporting information for the trial designs). In SWITCH 1, patients with type 1 diabetes (T1D) (N = 501) received a basal–bolus regimen with mealtime insulin aspart (two to four times daily); in SWITCH 2, patients with type 2 diabetes (T2D) (N = 721) received basal‐only insulin therapy with or without oral antidiabetic drugs (OADs); all pretrial OADs were continued at their pretrial dose. Both trials enrolled participants with a high risk of hypoglycaemia (refer to Table 1), reflecting the patients seen in clinical practice. Baseline characteristics of patients in the SWITCH trials are summarized in Table 1.
Table 1.
Baseline characteristics of patients in SWITCH 1 and SWITCH 2
| Characteristic | SWITCH 110 | SWITCH 211 |
|---|---|---|
| Full analysis set, N | 501 | 720 |
| Male, n (%) | 269 (53.7) | 382 (53.1) |
| Race, n (%) | ||
| White | 462 (92.2) | 578 (80.3) |
| Black | 32 (6.4) | 106 (14.7) |
| Asian | 2 (0.4) | 22 (3.1) |
| Other | 5 (1.0) | 14 (1.9) |
| Ethnicity: Hispanic or Latino, n (%) | 51 (10.2) | 262 (36.4) |
| Age, years | 45.9 (14.2) | 61.4 (10.5) |
| BMI, kg/m2 | 27.5 (4.8) | 32.2 (5.6) |
| Duration of diabetes, years | 23.4 (13.4) | 14.1 (8.1) |
| HbA1c, % [mmol/mol] |
7.6 (1.0) [59 (11)] |
7.6 (1.1) [59 (12)] |
| eGFR, mL/min/1.73m2 | 90.0 (21.1) | 78.3 (21.3) |
| Pretrial insulin, n (%) | ||
| NPH insulin | 97 (19.4) | 59 (8.2) |
| Insulin detemir | 305 (60.9) | 159 (22.2) |
| Glargine U100 | 1 (0.2)a | 502 (69.7) |
| Rapid‐acting insulin (CSII) | 97 (19.4) | 0 (0) |
| Pretrial treatment regimen, n (%) | ||
| Basal OD | 0 (0) | 606 (84.2) |
| Basal BID | 0 (0) | 114 (15.8) |
| Basal OD + 2–4 bolus injections | 224 (44.7) | 0 (0) |
| Basal BID +2–4 bolus injections | 179 (35.7) | 0 (0) |
| CSII | 97 (19.4) | 0 (0) |
| OADs at screening, n (%) | ||
| 0 agents | 100 (0) | 150 (20.8) |
| 1 agent | 0 | 448 (62.2) |
| ≥2 agents | 0 | 122 (16.9) |
| Hypoglycaemia risk, n (%) | ||
| ≥1 severe hypoglycaemic episodea in the last year | 125 (25.0) | 118 (16.4) |
| Moderate chronic renal failure | 42 (8.4) | 159 (22.1) |
| Hypoglycaemia unawareness | 104 (20.8) | 129 (17.6) |
| Exposure to insulin for ≥5 years | N/A | 356 (49.4) |
| Diabetes for ≥15 years | 332 (66.3) | N/A |
| Hypoglycaemic episodeb in the last 12 weeks | 459 (91.6) | 478 (66.4) |
Abbreviations: BID, twice daily; BMI, body mass index; CSII, continuous subcutaneous insulin infusion; eGFR, estimated glomerular filtration rate; glargine U100, glargine 100 units/mL; N, number of patients; n, number in sample; N/A, not applicable; NPH, neutral protamine Hagedorn; OAD, oral antidiabetic drug; OD, once daily; %, percentage of patients.
Data are mean (standard deviation) unless otherwise stated.
One patient was randomized in error (treatment with glargine U100 within the last 26 weeks was an exclusion criteria in SWITCH 1).
Defined as requiring third‐party assistance.23
Defined as symptoms of hypoglycaemia, blood glucose level ≤70 mg/dL, or both.
In both trials, patients were randomized 1:1 to receive either once‐daily degludec (100 units/mL) for 32 weeks followed by glargine U100 for a further 32 weeks, or vice versa. Patients attended up to 14 scheduled clinic visits where blood samples were taken for a central laboratory measurement of HbA1c (refer to Appendix 1 for the visit schedule). The primary endpoint was the rate of symptomatic hypoglycaemia during the maintenance periods (weeks 16–32 and weeks 48–64). The titration periods (weeks 1–16 and weeks 32–48) were included to ensure complete washout of previous therapies, minimize the effect of treatment switch on hypoglycaemia rates, and facilitate an accurate comparison of safety endpoints at stable insulin dose and glycaemic control.
Symptomatic hypoglycaemia was defined in both studies as severe (according to the American Diabetes Association definition as requiring third‐party assistance)23 or blood glucose‐confirmed (<3.1 mmol/L [56 mg/dL]) symptomatic hypoglycaemia. Suspected events of severe hypoglycaemia were confirmed by an external, blinded, event adjudication committee. The design and primary findings of SWITCH 1 and 2 are summarized in Table 2.
Table 2.
Design and primary findings of SWITCH 1 and SWITCH 2
| SWITCH 110 | SWITCH 211 | |
|---|---|---|
| Design | Multicentre (USA: 84 sites; Poland: 6 sites), randomized, double‐blind, two‐period crossover | Multicentre (USA: 152 sites), randomized, double‐blind, two‐period crossover |
| Participants | N = 501 adults | N = 721 adults |
| Inclusion criteria | T1D ≥52 weeks, BB regimen or CSII ≥26 weeks, HbA1c ≤10%, BMI ≤45 kg/m2, ≥1 hypoglycaemia risk factor | T2D ≥26 weeks, basal insulin ± OADs ≥26 weeks, HbA1c ≤9.5%, BMI ≤45 kg/m2, ≥1 hypoglycaemia risk factor |
| Treatment | Degludec or glargine U100 OD + mealtime IAsp (2–4 times daily) | Degludec or glargine U100 OD ± OAD(s) |
| Randomization | 1:1 to treatment sequence (degludec followed by glargine U100 or glargine U100 followed by degludec); 1:1 to morning or evening dosing | 1:1 to treatment sequence (degludec followed by glargine U100 or glargine U100 followed by degludec); 1:1 to morning or evening dosing |
| Duration | Two x 32‐week treatment periods (titration: weeks 1–16 and 32–48; maintenance: weeks 16–32 and 48–64) | Two x 32‐week treatment periods (titration: weeks 1–16 and 32–48; maintenance: weeks 16–32 and 48–64) |
| Titration BG target | Basal insulin: 4.0–5.0 mmol/L (71–90 mg/dL); IAsp: 4.0–6.0 mmol/L (71–108 mg/dL) | Basal insulin: 4.0–5.0 mmol/L (71–90 mg/dL) |
| Rate of symptomatic hypoglycaemiaa in the maintenance periodb |
Significantly lower with degludec versus glargine U100 HR: 0.89 [0.85; 0.94]95% CI, P < 0.001 |
Significantly lower with degludec versus glargine U100 HR: 0.70 [0.61; 0.80]95% CI, P < 0.001 |
| Change in HbA1c from baseline after 32 weeks of treatmentc | Non‐inferiority of degludec versus glargine U100 confirmed for both treatment periods | Non‐inferiority of degludec versus glargine U100 confirmed for both treatment periods |
Abbreviations: BB, basal–bolus; BG, blood glucose; BMI, body mass index; CI, confidence interval; CSII, continuous subcutaneous insulin infusion; glargine U100, glargine 100 units/mL; HR, hazard ratio; IAsp, insulin aspart; OAD, oral antidiabetic drug; OD, once daily; T1D, type 1 diabetes; T2D, type 2 diabetes.
Symptomatic hypoglycaemia was defined as severe (requiring third‐party assistance)23 as confirmed by an event adjudication committee or BG‐confirmed (<3.1 mmol/L [56 mg/dL]) accompanied by symptoms.
Primary endpoint; analysed using a Poisson model with patient as random effect; treatment, period, sequence, and time of dosing as fixed effects; and logarithm of the observation time (100 years) as offset.
Analysed separately for each treatment period with a mixed model for repeated measurements including treatment, visit, sex, region, pre‐trial insulin regimen, and time of dosing as fixed effects, and age and baseline HbA1c as covariates; all fixed factors and covariates are nested within visit.
Both trials were conducted in accordance with the Declaration of Helsinki and International Conference of Harmonisation Good Clinical Practice.24, 25 Before trial initiation, protocols, consent forms and patient information sheets were reviewed and approved by the appropriate health authorities and an independent ethics committee or institutional review board at each site. Written informed consent was obtained from all participating patients.
2.2. Post hoc analysis of the SWITCH trials
Data were analyzed separately for patients with T1D and for those with T2D. The study population included all patients with at least one postbaseline HbA1c measurement until treatment discontinuation or end of trial. Appendix 2 (see the supporting information) presents a schematic illustration of the data, utilized in these post hoc analyses, for hypoglycaemia and HbA1c at three fictional clinic visits.
2.2.1. Patient‐level association between glycaemic control and hypoglycaemia
Frequency of symptomatic hypoglycaemia between scheduled clinic visits was analyzed for each patient group using a Poisson regression model with treatment, treatment period, sequence and time of dosing as fixed effects. The interaction between treatment and HbA1c at the end of each interval (between scheduled clinic visits) was included as covariate, patient as random effect, and interval duration as offset.
2.2.2. Estimated difference in glycaemic control at the same rate of hypoglycaemia
Differences in glycaemic control (change in HbA1c) between degludec and glargine U100 at the same rate of hypoglycaemia were determined from the estimated rate ratios (ERRs) of symptomatic hypoglycaemia in the maintenance periods of the SWITCH trials and this model (described above). ERRs were applied to the modelled patient‐level associations between hypoglycaemia and HbA1c reduction to estimate the increase in hypoglycaemia for a standard (1.0 percentage point [11 mmol/mol]) decrease in HbA1c for each treatment in the SWITCH trials using the following calculation: 1/(−log[a] * log[b]), where a is the slope of the patient‐level association between glycaemic control and hypoglycaemia with degludec in the corresponding SWITCH trial, and b is the ERR (degludec/glargine U100) for symptomatic hypoglycaemia in the maintenance periods of the corresponding SWITCH trial. These estimates utilized the assumption that hypoglycaemia is the limiting factor to glycaemic control in clinical practice. It is worth noting that the patient‐level models did not rely on this assumption.
2.2.3. Population‐level association between glycaemic control and hypoglycaemia
In addition to the patient‐level modelling approach, a population‐level approach was also undertaken to enable comparison and to facilitate discussion regarding the two approaches. Data for symptomatic hypoglycaemic events and HbA1c in the maintenance period were retrieved from the databases of the SWITCH trials. The number of symptomatic hypoglycaemic events during the maintenance period was analyzed using a Poisson model with patient as random effect; treatment, period, sequence and time of dosing as fixed effects; mean HbA1c in the maintenance period and mean HbA1c in the maintenance period squared as covariates; and exposure time to trial drug in each hypoglycaemia‐counting period as an offset term. The population‐level associations between mean HbA1c level and symptomatic hypoglycaemia rate were estimated from these models.
3. RESULTS
At baseline, the range of HbA1c was 4.1%–9.8% (21–84 mmol/mol) in SWITCH 1 and 4.7%–10.8% (28–95 mmol/mol) in SWITCH 2 with a median HbA1c of 7.6% (60 mmol/mol) in both trials (Figure 1). For each randomized treatment, data from treatment periods 1 (weeks 1–32) and 2 (weeks 33–64; after treatment switch) were combined. The most frequent HbA1c reduction was in the range of 0–1 percentage points (0–11 mmol/mol).
Figure 1.
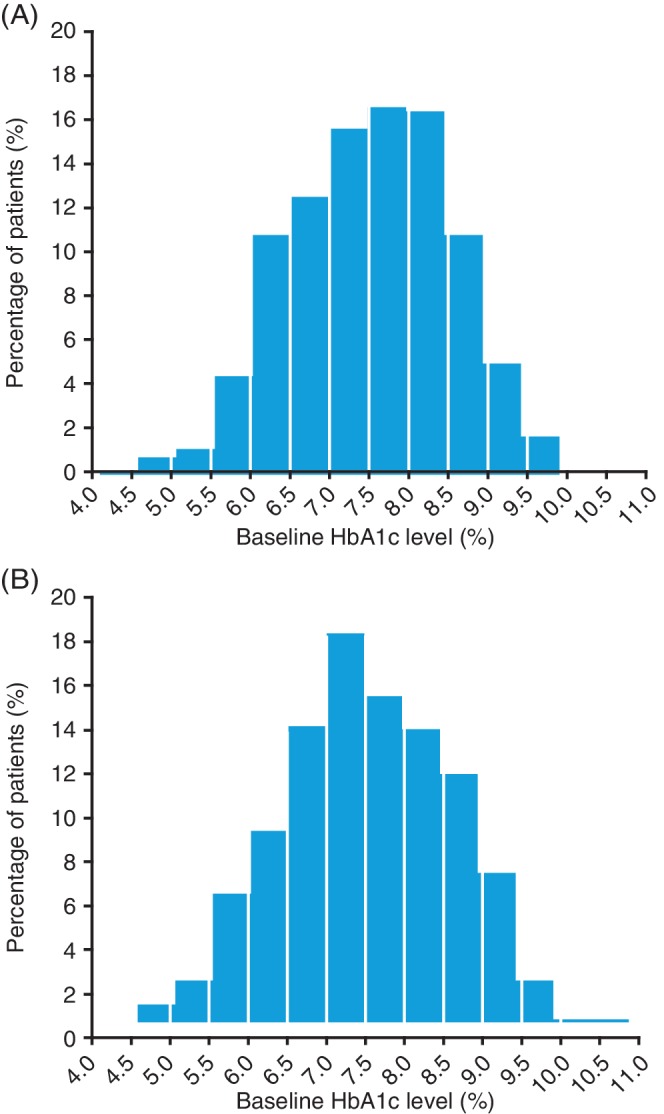
Frequency distribution of HbA1c at baseline in (A) SWITCH 1 and (B) SWITCH 2. Full analysis set
3.1. Patient‐level association between glycaemic control and hypoglycaemia
Reduction in HbA1c increased the incidence of hypoglycaemic events with both basal insulins across both diabetes types in the SWITCH trials (Figure 2). For the same decrease in HbA1c, there was a smaller relative increase in hypoglycaemic events with degludec compared with glargine U100 for patients with T1D or T2D. For a 1.0 percentage point (11 mmol/mol) decrease in HbA1c, hypoglycaemia rates increased relatively by: 18% [12; 25]95% CI with degludec versus 34% [27; 42]95% CI with glargine U100 in patients with T1D, and 45% [31; 60]95% CI versus 67% [51; 85]95% CI, respectively, in patients with T2D.
Figure 2.
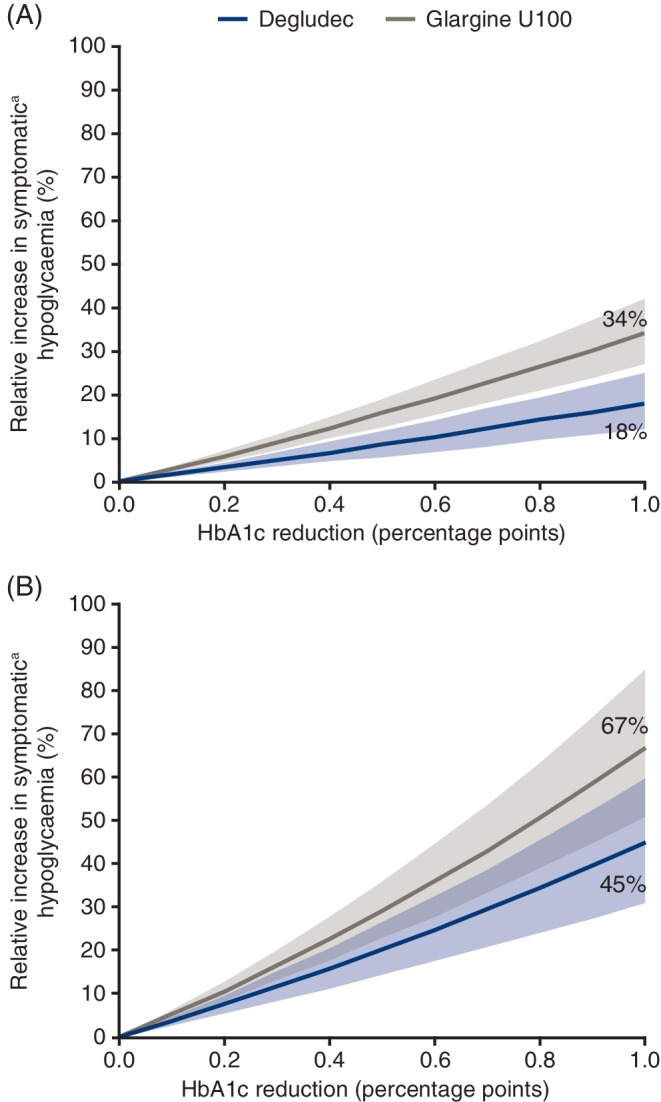
Individual patient‐level association between HbA1c reduction and the incidence of hypoglycaemia in patients with (A) type 1 diabetes or (B) type 2 diabetes, based on data from the maintenance period of the SWITCH trials.
Glargine U100, insulin glargine 100 units/mL.
Based on the full analysis set. Frequency of overall symptomatic hypoglycaemia was analyzed using Poisson regression with treatment, treatment period, sequence and time of dosing as fixed effects. The interaction between treatment and HbA1c at the end of each period was included as covariate, patient as random effect and duration of the period as offset. Data plotted are the estimated relative change in incidence of symptomatic hypoglycaemia by HbA1c reduction and 95% confidence interval.
a Symptomatic hypoglycaemia was defined as severe (requiring third‐party assistance)23 as confirmed by an event adjudication committee or blood glucose‐confirmed (<3.1 mmol/L [56 mg/dL]) accompanied by symptoms
3.2. Estimated difference in glycaemic control at the same rate of hypoglycaemia
Our analysis suggests that patients could achieve a mean HbA1c reduction of 0.70 [0.05; 2.20]95% CI (for T1D) or 0.96 [0.39; 1.99]95% CI (for T2D) percentage points (8 [1; 24]95% CI or 10 [4; 22]95% CI mmol/mol, respectively) further with degludec than with glargine U100 before incurring an equivalent risk of hypoglycaemia.
3.3. Population‐level association between glycaemic control and hypoglycaemia
For T1D, there are broadly similar curvilinear relationships between mean glycaemic control and the rate of hypoglycaemic events with degludec and glargine U100, although the peak is more pronounced and at a lower mean HbA1c level with glargine U100 compared with degludec (Figure 3A). For T2D, the association between mean glycaemic control and hypoglycaemic events varied substantially between basal insulins, with a similar (as described above for T1D), although less pronounced convex relationship with degludec, but a concave relationship with glargine U100 (Figure 3B). This resulted in a large divergence between basal insulins in the modelled number of hypoglycaemic events in patients with near‐normal glycaemic control (HbA1c <6.0% [42 mmol/mol]), with a lower rate of these events for degludec compared with glargine U100.
Figure 3.
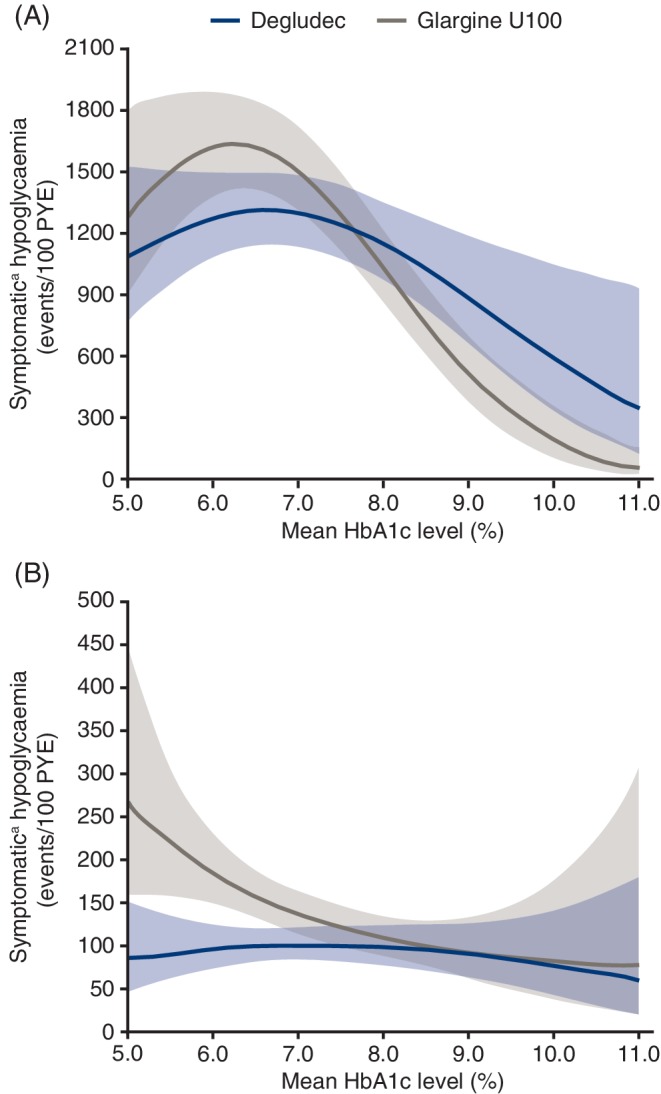
Population‐level association between mean HbA1c level and hypoglycaemia rate in patients with (A) type 1 diabetes and (B) type 2 diabetes based on data from the maintenance period of the SWITCH trials.
Glargine U100, insulin glargine 100 units/mL; PYE, patient‐year of exposure.
Symptomatic hypoglycaemiaa in the maintenance period was analyzed using a Poisson model with patient as random effect; treatment, period, sequence and time of dosing as fixed effects; mean HbA1c in the maintenance period and mean HbA1c in the maintenance period squared as covariates; and exposure time to trial drug in each hypoglycaemia counting period as an offset term. The population‐level associations between mean HbA1c level and symptomatic hypoglycaemia rate were estimated from these models. Data shown are the estimated population mean and the 95% confidence interval.
aSymptomatic hypoglycaemia was defined as severe (requiring third‐party assistance)23 as confirmed by an event adjudication committee or blood glucose‐confirmed (<3.1 mmol/L [56 mg/dL]) accompanied by symptoms
4. DISCUSSION
In this post hoc analysis of SWITCH 1 and SWITCH 2, improvements in glycaemic control increased the patient‐level risk of hypoglycaemia irrespective of the basal insulin treatment. In both T1D and T2D, however, a given level of reduction in HbA1c was associated with a smaller relative increase in hypoglycaemic events in patients receiving degludec compared with glargine U100. Based on the lower rates of symptomatic hypoglycaemia with degludec versus glargine U100 reported in the SWITCH primary manuscripts, and the modelled associations between glycaemic control and hypoglycaemia reported here, our findings suggest that patients with diabetes could target a lower individualized HbA1c goal with degludec than with glargine U100. When interpreting the results of the present patient‐level analyses, it should be noted that these are for relative change in hypoglycaemia frequency. Therefore, despite comparatively larger relative increases in the frequency of hypoglycaemia for a given HbA1c reduction in patients with T1D compared with T2D, in absolute terms, hypoglycaemic events are expected to occur more frequently in patients with T1D than in those with T2D.26
In clinical practice, a large percentage of patients with diabetes do not achieve glycaemic targets. Results from the 2016/17 National Diabetes Audit indicate that, in England and Wales, only 30% of patients with T1D and 67% of patients with T2D are achieving HbA1c <7.5% (58 mmol/mol)27 while in the United States just 50% of adult patients with diabetes are attaining HbA1c <7.0% (53 mmol/mol).28 Our findings suggest that treatment with degludec might help to alleviate a limitation to glycaemic control—hypoglycaemia—and thus might facilitate improved glycaemic control in patients with diabetes when compared with glargine U100 treatment. Our findings are supported by real‐world evidence of degludec providing better glycaemic control and a reduced incidence of hypoglycaemia after switching from other basal insulins, including glargine U100, under conditions of routine clinical practice.29, 30, 31, 32
There are numerous clinical risk factors for hypoglycaemia that influence patient propensity towards hypoglycaemia, including age, diabetes duration, co‐morbidities, therapeutic regimen, impaired hypoglycaemia awareness and experience of previous hypoglycaemic events.20, 33 Additionally, glycaemic variability may contribute to overall glycaemic control,34 with retrospective study data indicating that high glycaemic variability is associated with poor glycaemic control and also with the risk of severe hypoglycaemia.35 Furthermore, in a post hoc analysis of clinical trial data, both intra‐ and inter‐day glycaemic variability were associated with hypoglycaemia risk in insulin‐treated patients with T2D.22 The crossover SWITCH trial design and the patient‐level modelling approach utilized in the current analysis will have minimized the influence of inter‐individual variability in hypoglycaemia propensity on these findings.
Unlike the patient‐level results, the population‐level associations cannot assist in an estimation of the change in an individual's hypoglycaemia rate with improved glycaemic control, but simply provide an overall picture of these variables and their associations from a population level. From a clinical viewpoint, our patient‐level findings may be of greater interest and/or value, as they can assist with clinical decision‐making and discussions between patients and physicians regarding hypoglycaemia risk, regimen intensification and glycaemic targets.
Results from the population‐level analysis are more difficult to interpret as they suggest that the relationship between glycaemic control and hypoglycaemia rate is different in patients with T2D treated with glargine U100 compared with the other patient groups in the SWITCH trials. Those with either very poor or near‐normal glycaemic control experienced fewer hypoglycaemic events than those with intermediate control. However, patients with T2D treated with glargine U100 who had near‐normal glycaemic control experienced the highest incidence of hypoglycaemia, with fewer events in patients with intermediate or poor control. Our observation that patients with poor glycaemic control had fewer hypoglycaemia events seems intuitive. Meanwhile, the paradox of lower rates in those with near‐normal control with degludec (T1D and T2D) and glargine U100 (T1D only) may reflect high levels of compliance or excellent diabetes self‐management skills in certain patients or, perhaps, a subset of patients with some preservation of the endogenous insulin response. Alternatively, this paradox may signal a reduced awareness of hypoglycaemia in a subset of patients with near‐normal control, relaxing the limitation of symptomatic hypoglycaemia to optimal glycaemic control, presumably at the expense of a greater frequency of asymptomatic events. These findings exemplify the difficulty in interpreting population‐level associations from a clinical standpoint because of bidirectional associations between glycaemic control and hypoglycaemia rates, in combination with high levels of variability between patients in their propensity towards hypoglycaemia at a given HbA1c level.
One of the main strengths of our post hoc analysis is that data were extracted from two large rigorously designed, double‐blind clinical trials. Clinical trials are a powerful tool for developing evidence on the safety and efficacy of a therapeutic intervention, but their limitations can make it difficult to generalize their findings to more inclusive populations of patients and diverse settings that reflect clinical practice.36 By contrast, real‐world studies often lack the scientific rigour of RCTs and may suffer from selection biases or confounding factors.36 These limitations have been overcome in the SWITCH trials to some extent, where generalizability was enhanced through the inclusion of patients who better represent those encountered in clinical practice.10, 11
Our analysis is subject to several limitations. Like all models, the results largely depend on the underlying assumptions. While the present analysis was conducted at the individual patient level and, therefore, may have accounted for a substantial proportion of consistent inter‐individual variation in hypoglycaemia risk, other factors, including increased activity without additional carbohydrate, decreased carbohydrate or caloric intake without compensation in insulin dose, unplanned or strenuous exercise, alcohol consumption and stress, which could have influenced hypoglycaemia rates, were not captured.20 The SWITCH trials had a two‐period, crossover design, whereby patients were randomized to a treatment sequence (degludec followed by glargine U100 or glargine U100 followed by degludec) and, therefore, results in the second period may have been influenced by events/treatment in the first period. However, we adjusted our analyses for both period and sequence effects and, consequently, we would not expect either to have exerted a large influence on our results. In addition, as this was a post hoc analysis, our findings need to be confirmed in further clinical or real‐world studies. However, as discussed previously, the challenging design and ethical considerations prevent the comparison of glycaemic control between treatments at comparable rates of hypoglycaemia in a clinical trial setting. Our analyses captured symptomatic hypoglycaemic events, as per the SWITCH trial endpoints, but not all events are symptomatic and some patients have a reduced awareness of the symptoms of hypoglycaemia. The authors look forward to the availability of flash or continuous glucose monitoring data from future clinical trials in this patient population to assist with our understanding of hypoglycaemia risk through the collection of data at high temporal resolution and detection sensitivity.
In conclusion, the findings of this post hoc analysis suggest that patients in clinical practice may be able to achieve lower glycaemic targets with degludec compared with glargine U100, before incurring an equivalent risk of hypoglycaemia. In patients who are suitable for insulin intensification, this may give both the patient and clinician greater confidence in titrating to a lower glycaemic target with degludec versus glargine U100.
CONFLICT OF INTEREST
A.P.T. has participated on advisory boards for AstraZeneca, Dexcom, Eli Lilly and Co., Merck, Novo Nordisk and Sanofi; and received research support from Dexcom, Mylan, Novo Nordisk and Sanofi. W.L. has participated on advisory boards for Novo Nordisk; participated in speakers’ bureaux for Novo Nordisk, Dexcom and Insulet; and received research support from Novo Nordisk, Sanofi and Dexcom. U.P.B. has participated on advisory boards for AstraZeneca, Novo Nordisk, Sanofi and Zealand Pharma; received consultancy fees from MSD and Novo Nordisk; and received research support from Novo Nordisk. C.W. has participated on advisory boards for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lily and Co., Janssen and Sanofi; participated on speakers’ bureaux for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Co., Janssen, Insulet, Novo Nordisk and Sanofi; and reports consultancy fees from AstraZeneca, Janssen, Novo Nordisk and Sanofi. L.B. and S.Ha are both employees of Novo Nordisk and L.B. also holds shares in Novo Nordisk. S.He reports consultancy fees for Eli Lilly and Co., Novo Nordisk, Takeda and Boehringer Ingelheim, Zealand Pharma and UNEEG; has participated in speakers’ bureaux for Novo Nordisk; Eli Lilly and Co, Merck Sharp & Dohme, Takeda and AstraZeneca; and has participated on advisory panels for Novo Nordisk and Eli Lilly and Co.
AUTHOR CONTRIBUTIONS
A.P.T. is the guarantor of this work and, as such, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
All authors confirm that they meet the International Committee of Medical Journal Editors (ICJME) uniform requirements for authorship and that they have contributed to: critical analysis and interpretation of the data, drafting and/or critically revising the article and sharing in the final responsibility for the content of the manuscript and the decision to submit it for publication.
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
FUNDING INFORMATION
Sponsorship for the SWITCH 1 and 2 clinical trials, this study and article processing charges were funded by Novo Nordisk A/S.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
We thank the trial investigators, staff and patients for their participation in the SWITCH 1 and SWITCH 2 trials. The authors also thank Charlotte Thim Hansen and Jens Gundgaard (Novo Nordisk) for their review and input to the manuscript; and acknowledge Lars Lynne Hansen's contribution to the data analysis. Medical writing and submission support were provided by Anna Campbell and Beverly La Ferla of Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc, funded by Novo Nordisk.
Parts of this analysis were presented as an oral presentation at the American Diabetes Association (ADA), 78th Scientific Sessions, 22‐26 June 2018, Orlando, Florida.
Parts of this analysis were presented as a poster at both the European Association for the Study of Diabetes (EASD), 54th Annual Meeting, 1‐5 October 2018, Berlin, Germany, and the Australasian Diabetes Congress 2018 (ADS/ADEA) Annual Scientific Meeting, 22‐24 August 2018, Adelaide, SA, Australia.
Sponsorship for the SWITCH 1 and 2 clinical trials, this study and article processing charges were funded by Novo Nordisk A/S.
Philis‐Tsimikas A, Lane W, Pedersen‐Bjergaard U, et al. The relationship between HbA1c and hypoglycaemia in patients with diabetes treated with insulin degludec versus insulin glargine 100 units/mL. Diabetes Obes Metab. 2020;22:779–787. 10.1111/dom.13954
Funding information Novo Nordisk A/S
DATA ACCESSIBILITY
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Tancredi M, Rosengren A, Svensson AM, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373:1720‐1732. [DOI] [PubMed] [Google Scholar]
- 2. Aronson D. Hyperglycemia and the pathobiology of diabetic complications. Adv Cardiol. 2008;45:1‐16. [DOI] [PubMed] [Google Scholar]
- 3. Cryer PE. Glycemic goals in diabetes: Trade‐off between glycemic control and iatrogenic hypoglycemia. Diabetes. 2014;63:2188‐2195. [DOI] [PubMed] [Google Scholar]
- 4. Cryer PE. Hypoglycaemia: The limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia. 2002;45:937‐948. [DOI] [PubMed] [Google Scholar]
- 5. Anderson SL, Trujillo JM, Anderson JE, Tanenberg RJ. Switching basal insulins in type 2 diabetes: Practical recommendations for health care providers. Postgrad Med. 2018;130:229‐238. [DOI] [PubMed] [Google Scholar]
- 6. Heise T, Nosek L, Bottcher SG, Hastrup H, Haahr H. Ultra‐long‐acting insulin degludec has a flat and stable glucose‐lowering effect in type 2 diabetes. Diabetes Obes Metab. 2012;14:944‐950. [DOI] [PubMed] [Google Scholar]
- 7. Heise T, Kaplan K, Haahr HL. Day‐to‐day and within‐day variability in glucose‐lowering effect between insulin degludec and insulin glargine (100 U/mL and 300 U/mL): A comparison across studies. J Diabetes Sci Technol. 2018;12:356‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ratner RE, Gough SC, Mathieu C, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre‐planned meta‐analysis of phase 3 trials. Diabetes Obes Metab. 2013;15:175‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marso SP, McGuire DK, Zinman B, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017;377:723‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lane W, Bailey TS, Gerety G, et al. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 1 diabetes: The SWITCH 1 randomized clinical trial. JAMA. 2017;318:33‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wysham C, Bhargava A, Chaykin L, et al. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 2 diabetes: The SWITCH 2 randomized clinical trial. JAMA. 2017;318:45‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545‐2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329:977‐986. [DOI] [PubMed] [Google Scholar]
- 14. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837‐853. [PubMed] [Google Scholar]
- 15. Rosenstock J, Fonseca V, Schinzel S, Dain MP, Mullins P, Riddle M. Reduced risk of hypoglycemia with once‐daily glargine versus twice‐daily NPH and number needed to harm with NPH to demonstrate the risk of one additional hypoglycemic event in type 2 diabetes: Evidence from a long‐term controlled trial. J Diabetes Complications. 2014;28:742‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hermansen K, Davies M, Derezinski T, Martinez RG, Clauson P, Home P. A 26‐week, randomized, parallel, treat‐to‐target trial comparing insulin detemir with NPH insulin as add‐on therapy to oral glucose‐lowering drugs in insulin‐naive people with type 2 diabetes. Diabetes Care. 2006;29:1269‐1274. [DOI] [PubMed] [Google Scholar]
- 17. Lipska KJ, Warton EM, Huang ES, et al. HbA1c and risk of severe hypoglycemia in type 2 diabetes: The Diabetes and Aging Study. Diabetes Care. 2013;36:3535‐3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weinstock RS, Xing D, Maahs DM, et al. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: Results from the T1D Exchange clinic registry. J Clin Endocrinol Metab. 2013;98:3411‐3419. [DOI] [PubMed] [Google Scholar]
- 19. Miller CD, Phillips LS, Ziemer DC, Gallina DL, Cook CB, El‐Kebbi IM. Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med. 2001;161:1653‐1659. [DOI] [PubMed] [Google Scholar]
- 20. Kalra S, Mukherjee JJ, Venkataraman S, et al. Hypoglycemia: The neglected complication. Indian J Endocrinol Metab. 2013;17:819‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Food and Drug Administration . Center for Drug Evaluation and Research (CDER). Guidance for industry. Diabetes mellitus: Developing drugs and therapeutic biologics for treatment and prevention. Draft guidance. In: U.S. Department of Health and Human Services. 2008.
- 22. Qu Y, Jacober SJ, Zhang Q, Wolka LL, DeVries JH. Rate of hypoglycemia in insulin‐treated patients with type 2 diabetes can be predicted from glycemic variability data. Diabetes Technol Ther. 2012;14:1008‐1012. [DOI] [PubMed] [Google Scholar]
- 23. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: A report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Medical Association . World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191‐2194. [DOI] [PubMed] [Google Scholar]
- 25. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use . Integrated addendum to ICH E6(R1): Guideline for good clinical practice E6(R2). 2016. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4_2016_1109.pdf. Accessed June 2019.
- 26. Khunti K, Alsifri S, Aronson R, et al. Rates and predictors of hypoglycaemia in 27 585 people from 24 countries with insulin‐treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metab. 2016;18:907‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NHS digital. National Diabetes Audit, 2016–17: Care Processes and Treatment Targets. 2018. https://files.digital.nhs.uk/pdf/s/k/national_diabetes_audit_2016-17_report_1__care_processes_and_treatment_targets.pdf. Accessed July 2018.
- 28. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med. 2013;368:1613‐1624. [DOI] [PubMed] [Google Scholar]
- 29. Siegmund T, Tentolouris N, Knudsen ST, et al. A European, multicentre, retrospective, non‐interventional study (EU‐TREAT) of the effectiveness of insulin degludec after switching basal insulin in a population with type 1 or type 2 diabetes. Diabetes Obes Metab. 2018;20:689‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ponzani P, Berra C, Di Lelio A, et al. Impact of insulin degludec in type 2 diabetes: Real‐world data on effectiveness and safety. Diabetes Ther. 2018;9:2209‐2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tibaldi J, Hadley‐Brown M, Liebl A, et al. A comparative effectiveness study of degludec and insulin glargine 300 U/mL in insulin‐naive patients with type 2 diabetes. Diabetes Obes Metab. 2019;21:1001‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fadini GP, Feher M, Hansen TK, et al. Switching to degludec from other basal insulins is associated with reduced hypoglycemia rates: A prospective study. J Clin Endocrinol Metab. 2019;104:5977‐5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: The diabetes and aging study. Diabetes Care. 2011;34:1329‐1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Inchiostro S, Candido R, Cavalot F. How can we monitor glycaemic variability in the clinical setting? Diabetes Obes Metab. 2013;15(Suppl 2):13‐16. [DOI] [PubMed] [Google Scholar]
- 35. Williams ME, Garg R, Wang W, Lacson R, Maddux F, Lacson E Jr. High hemoglobin A1c levels and glycemic variability increase risk of severe hypoglycemia in diabetic hemodialysis patients. Hemodial Int. 2014;18:423‐432. [DOI] [PubMed] [Google Scholar]
- 36. Sherman RE, Anderson SA, Dal Pan GJ, et al. Real‐world evidence ‐ what is it and what can it tell us? N Engl J Med. 2016;375:2293‐2297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


