Abstract
Aim:
We evaluated the efficacy of a novel switch protocol for EGFR-TKIs for EGFR mutation-positive NSCLC.
Materials & methods:
Clinical records were collected from the patients who had received one of two sequential combination strategies of EGFR-TKIs: Salvage use of osimertinib for T790M-mediated acquired resistance to an prior EGFR-TKI or switch use of osimertinib where an EGFR-TKI was switched to osimertinib before disease progression.
Results:
Progression-free survival of osimertinib and time from the start of treatment until progression to osimertinib was comparable between the salvage use and switch use of osimertinib.
Conclusion:
Switch use of osimertinib seemed to produce improved efficacy for patients with activating EGFR mutations, because of the lack of patient selection via T790M.
Keywords: : EGFR-TKI, EGFR mutation, NSCLC, osimertinib, switch, time to failure of strategy
Practice points.
There are currently two popular clinical protocols for the use of single-agent osimertinib in the treatment of EGFR mutation-positive NSCLC; one is the front-line use and the other is salvage use for a preceding first- or second-generation EGFR-TKI.
Several types of sequential uses of different EGFR-TKIs have been studied extensively.
The salvage protocol of osimertinib has the fundamental disadvantage that osimertinib is applicable only for T790M-mediated disease progression.
In the present study, we report a novel protocol which we named switch osimertinib, where a first- or second-generation EGFR-TKI is changed to osimertinib prior to disease progression.
We focused on time to failure of strategy to evaluate the efficacy of protocols.
Time to failure of strategy of the treatment with EGFR-TKIs was similar between the patients in the salvage group and those in the switch group.
The switch protocol of osimertinib may be better than salvage protocol, given the poor detection rate of T790M mutation in acquired resistance to EGFR-TKIs.
Background
Molecular targeted therapy is a key strategy in the treatment of advanced NSCLC, with activated EGFR being one of the most frequent targets. EGFR-TKIs are critical drugs in the treatment of NSCLC harboring common EGFR mutations. Thus far, three generations of EGFR-TKIs have been clinically available. In the FLAURA study, front-line administration of osimertinib, a third-generation EGFR-TKI, produced longer progression-free survival (PFS) and overall survival (OS) with generally improved toxicity than seen with first-generation EGFR-TKIs [1,2]. Additionally, osimertinib is associated with the longest PFS among all EGFR-TKIs [1,3–5]. These results indicate that osimertinib is an important drug for the treatment of EGFR mutation-positive NSCLC. Osimertinib has an additional advantage of good penetration into the CNS.
There are currently two popular clinical protocols for the use of single-agent osimertinib in the treatment of EGFR mutation-positive NSCLC. One is the front-line use of osimertinib, while the other, known as salvage osimertinib, involves front-line treatment with a first- or second-generation EGFR-TKI, followed by osimertinib in the case of disease progression mediated by the EGFR T790M mutation. While the salvage protocol continues to be extensively studied and has been reviewed by Girard [6], the fundamental disadvantage of this strategy is that osimertinib is applicable only for T790M-mediated disease progression. This limits the number of patients receiving osimertinib when treatment is initiated with a first- or second-generation EGFR-TKI. In the FLAURA study, only 84 (39%) out of 213 patients who had discontinued standard EGFR-TKIs received subsequent anti-cancer treatment at data cut-off time. A real-world study in the USA reported that only 19% of patients were tested for the EGFR T790M mutation following first-line EGFR-TKI treatment [7]. As a result of these limitations, there is a growing trend to select front-line osimertinib as a treatment strategy, although there are several issues with this approach. For example, OS was not significantly different in comparison with first-generation EGFR-TKIs between second- and third-generation EGFR-TKIs in the first-line setting: osimertinib in the FLAURA study (hazard ratio; HR: 0.788) and dacomitinib in the ARCHER1050 study (HR: 0.760). In addition, a subset analysis of the FLAURA study revealed that the point estimate of the HR for OS was almost one in the Asian population and in patients who were EGFR L858R mutation positive. Finally, results from real-world studies have shown longer PFS or time to treatment failure when EGFR-TKIs are used in combination, especially when first-line afatinib is followed by osimertinib [6,8]. These results indicate that treatment outcomes may be further improved through the use of specific combinations of EGFR-TKIs.
In the present study, we focused on the protocol we named switch osimertinib, in which a first- or second-generation EGFR-TKI is changed to osimertinib prior to disease progression (PD). The advantage of this method is that detection of EGFR T790M is not required to use osimertinib because of the absence of PD. To our knowledge, there are no previous reports on the switch osimertinib protocol so far. In our hospital, this strategy was adopted in 20 patients, either due to toxicities of the preceding EGFR-TKIs or in response to the approval of front-line osimertinib in Japan. The purpose of the present study was to estimate the efficacy of the switch osimertinib protocol in comparison with the salvage use of osimertinib.
Materials & methods
This study was approved by the ethics committee of our hospital. Clinical records including age, sex, disease stage and clinical course were collected for patients who had an Eastern Cooperative Oncology Group performance status (PS) of 0–1 and had been diagnosed with NSCLC harboring common EGFR mutations (exon 19 deletion and L858R in exon21), who were treated with osimertinib in our hospital. To reduce variables, the analysis was limited to patients who had started first-line treatment using a first- or second-generation EGFR-TKI and received osimertinib as second therapy. In Japan, osimertinib was approved for EGFR T790M-mutation-positive NSCLC on 31 May 2016 and was extended for use as a front-line treatment in August 2018. Reflecting this, the patients were classified into three groups: salvage group 1 (SA1), salvage group 2 (SA2) and switch group (SW). In the patients in the salvage groups, a first- or second-generation EGFR-TKI was followed by osimertinib when T790M-mediated PD was proven. The patients in SA1 had started the first-line treatment prior to 31 May 2016, while those in SA2 started treatment after this date. As a result, patients who achieved lengthy responses to a first-line EGFR-TKI tended to accumulate in the SA1 group. In the patients in the SW, a first- or second-generation EGFR-TKI was switched directly to osimertinib prior to PD. The main reasons for the EGFR-TKI switch were adverse events and intentional replacements by attending physicians.
Since EGFR-TKIs are primarily beneficial to the survival of patients with EGFR mutation-positive NSCLC, the duration of the treatment strategy (i.e., treatment with EGFR-TKIs), rather than PFS of a particular EGFR-TKI, should be evaluated. Therefore, we considered that time to failure of strategy (TFS) was the best fit for an end point in this study [9,10]. Combined with the accessibility of the strategy of interest, TFS may be the best measure to properly determine the optimal combination of EGFR-TKIs. The TFS of EGFR-TKIs (TKI-TFS) can be defined as the time period from the start of an EGFR-TKI until death or PD of the last drug among EGFR-TKIs that had been used consecutively. Treatment with EGFR-TKIs after PD to osimertinib has not yet been established, therefore TKI-TFS was defined as the time period from the start of a first-line EGFR-TKI to patient death or PD to osimertinib in this study. Kaplan–Meier analysis was performed using SPSS Statistics version 23 (IBM, NY, USA), and the cut-off date for data collection was 2 December 2019.
Results
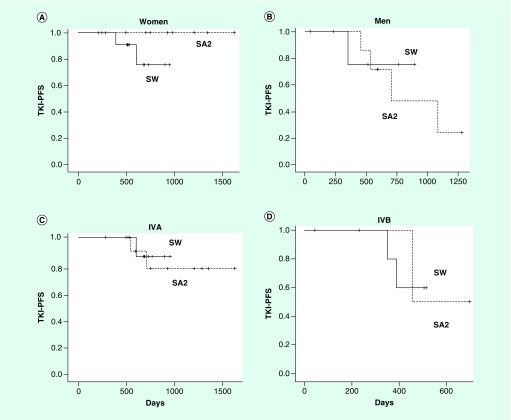
A total of 42 patients met the inclusion criteria. Patient characteristics are shown in Table 1. Variation between groups was observed with respect to age, sex, PS and disease stage. As shown in Figure 1, the PFS of osimertinib was longer in women and those with early-stage disease compared with their corresponding counterparts, but was similar between younger (<75) versus older (>75) patients, PS 0 versus PS 1 patients and between the types of EGFR mutations. Similar trends were observed for TKI-TFS.
Table 1. . Patients characteristics.
| Usage of osimertinib | SA1 | SA2 | SW |
|---|---|---|---|
| Number of patients | 7 | 15 | 20 |
| Men/women | 42.9/57.1 | 46.7/53.3 | 30.0/70.0 |
| Age, years (≥75/<75) | 57.1/42.9 | 20.0/80.0 | 35.0/65.0 |
| PS 0/1 | 28.6/71.4 | 26.7/73.3 | 60.0/40.0 |
| Stage (I–III/IV) | 28.6/71.4 | 13.3/86.7 | 17.6/82.4 |
| EGFR mutation (Ex19del/L858R) | 71.4/28.6 | 53.3/46.7 | 45.0/55.0 |
| EGFR-TKI (Gef/Erl/Afa) | 71.4/14.3/14.3 | 26.7/60.0/13.3 | 40.0/40.0/20.0 |
| Reason of EGFR-TKI switch | |||
| Adverse event | 25.0% | ||
| Intentional change | 75.0% | ||
Afa: Afatinib; Erl: Erlotinib; Gef: Gefitinib; PS: Ecog performance status; SA2: Salvage group 2; SW: Switch group; VSA1: Salvage group 1;.
Figure 1. . Progression-free survival of osimertinib by background factors.
(A) Age; (B) gender; (C) PS; (D) stage; (E) EGFR mutation type.
IVA: stage IVA; IVB: stage IVB; LD: disease confined to the thorax; PFS: progression-free survival, PS; ECOG performance status.
TKI-TFS & PFS of osimertinib in the three groups
No difference in the PFS of osimertinib was observed among the three groups (Figure 2). A slight improvement in the PFS in the SW patients compared with those in the SA2 group was observed. In contrast, the longest TKI-TFS was in the SA1 group, and TKI-TFS was similar between the SA2 and SW groups. This discrepancy is likely to be a reflection of the patient selection as described previously. Patients with a long PFS on a first-line EGFR-TKI had a higher probability of surviving until the time that osimertinib was approved, and therefore tended to accumulate in the SA1 group. The Kaplan–Meier curves of TKI-TFS did not reach 50% of events in either the SA2 or SW groups. However, the time points at which 40% of events occurred were approximately 32 months in both groups, which is much longer than the median PFS time of approximately 19 months in the FLAURA study. In the SW group, the median time period from the start of a first-line EGFR-TKI until the start of osimertinib was 182 days, ranging from 15 to 680 days.
Figure 2. . TKI-time to failure of strategy and progression-free survival of osimertinib in three groups.
Differences in TKI-TFS by EGFR-TKI, gender & stage
The combination of osimertinib with afatinib, a second-generation EGFR-TKI, appeared to be the most effective in prolonging TKI-TFS in both the SA2 and SW groups (Figure 3). No PD was observed with osimertinib in either SA2 or SW when afatinib had preceded osimertinib, although the number of patients receiving this combination was small. As shown in Table 1, the patients were not evenly distributed among the three groups, particularly with respect to gender and stage, and these two factors distinctly impact TKI-TFS. Therefore, we factored these variables into our analyses of TKI-TFS. As shown in Figure 4, inter-sex and inter-stage variations of TKI-TFS were insignificant or nonexistent between the SA1, SA2 and SW groups.
Figure 3. . Difference of tyrosine kinase inhibitor-time to failure of strategy by first-line EGFR-TKI in salvage group 2 and switch group.
(A) SA2; (B) SW.
SA2: Salvage group 2; SW: Switch group.
Figure 4. . Effect of treatment strategies in tyrosine kinase inhibitor-time to failure of strategy by patient subsets.
(A & B) Gender; (C & D) stage.
IVA: Stage IVA; IVB: Stage IVB; SA2: Salvage group 2; SW: Switch group.
Discussion
The present study showed that TKI-TFS is comparable between the salvage and switch osimertinib protocols. However, a disadvantage for the salvage use of osimertinib is that detection of the T790M mutation is required. The rate of T790M mutations in EGFR mutation-positive NSCLC with acquired resistance to a first- or second-generation EGFR-TKI is reported to be 40–70% in well-controlled studies [11,12]. It is also difficult to perform a re-biopsy to test for T790M in a real-world setting for various reasons, including poor PS of the patients or the anatomical location of recurrent lesions [13–15]. Therefore, the salvage use of osimertinib results in a substantial number of patients losing the opportunity to undergo treatment with osimertinib. In addition, salvage use of osimertinib is less effective than front-line osimertinib in PFS.
By definition, the patients in the SW group did not experience disease progression with the first EGFR-TKI prior to switching to osimertinib. The median duration of the first EGFR-TKI was 182 days. This could indicate that the patients whose NSCLC was responsive to an EGFR-TKI had been selected for in the SW group. However, the initial sections of the PFS curves of osimertinib in both SA1 and SA2 were flat (Figure 2), suggesting that all patients in SA1 and SA2 were responsive to EGFR-TKI, therefore the selection bias of SW may not have had a significant impact on the results.
Switch use has a clear advantage in that all patients, except those with early PD, can receive osimertinib, the most important drug for EGFR mutation-positive NSCLC, without waiting for the emergence of disease progression to other EGFR-TKIs. We could not determine how long the first EGFR-TKI should precede osimertinib to attain the longest TKI-TFS. In general, the PD rate during the first 6 months is estimated to be around 20% when patients with EGFR mutation-positive NSCLC were given a first- or second-generation EGFR-TKI as a front-line treatment [1,16–18]. Thus, approximately 20% of patients will fail to switch to osimertinib if the preceding first or second EGFR-TKI continues for 6 months. Liquid biopsy may be a good tool to solve this problem. Because the appearance of mutant EGFR circulating tumor DNA, including that carrying T790M, is reported to precede the radiographic progression by 2 to 3 months, sequential measurement of circulating tumor DNA may indicate appropriate timing of switching [19,20]. It should be noted that TKI-TFSs were nearly equal between the SA2 and SW groups. The results presented here suggest that both PFS of osimertinib and TKI-TFS can be optimized by the switch use of EGFR-TKIs.
At present, the mechanisms of this phenomenon are not precisely known, but several studies have presented clues to understanding it, as reviewed by Kohsaka et al. [21]. NSCLC harboring EGFR mutations are heterogeneous at the cellular level, as evidenced by the presence of cells with uncommon EGFR mutations or cells with compound EGFR mutations within a tumor [22,23]. These tumor cells are generally less sensitive to EGFR-TKIs, and each EGFR-TKI has a different potential to these tumors [24,25]. If de novo or acquired resistance to osimertinib is partly based on cancer cell clones with heterogeneous mutations [26], clonal convergence by a preceding EGFR-TKI different from osimertinib might have positive effects on the efficacy of subsequent use of osimertinib. Clinically, pretreatment with a first- or second-generation EGFR-TKI may potentiate the effect of osimertinib. In vitro, afatinib has been shown to have the strongest effect on tumor cells with heterogeneous mutations, except for the T790M mutation [24,27]. Afatinib is known to be active against NSCLC with uncommon EGFR mutations [28,29], and sequential administration of osimertinib following afatinib was reported to produce longer PFS in retrospective analysis [5,29,30]. Afatinib also produced the best results in our study, although the number of patients with afatinib was too small for statistical comparison.
Conclusion
Our analysis is limited by the small number of patients. In addition, the treatment outcomes in the three groups in our study were not comparable because of the retrospective nature of the study. However, the results of this study focusing on TFS provide a new perspective for evaluating the treatment of EGFR-mutation-positive NSCLC, and suggest that the switch use of EGFR-TKIs is a novel method to unleash their potential. TFS was a useful metric for the comparison of treatment strategies using EGFR-TKIs. The present study revealed the possibility that switch osimertinib might achieve TFS equivalent to that of salvage use, and that the switch use of osimertinib may be a better strategy, given the poor detection rate of T790M mutations in acquired resistance to EGFR-TKIs.
Future perspective
At present, at least three types of drugs are available for patients with EGFR mutation-positive NSCLC: EGFR-TKIs, cytotoxic drugs and immune checkpoint inhibitors. To further prolong patient OS, combination strategies of already-available drugs appear to be important, as well as the development of new drugs. The results of these novel combinations, such as the combination of an EGFR-TKI and cytotoxic chemotherapy, are now beginning to be published. Because EGFR-TKIs are most effective for EGFR mutation-positive NSCLC, the significance of combining different EGFR-TKIs should be studied. Recently, molecular-target therapies are expanding beyond the confines of the organs. Switch protocol, if effective, will also become an important strategy in molecular-target therapies in different areas in the future.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing. We also thank to the Osaka Foundation for the Prevention of Cancer and Lifestyle-related Diseases (Public Interest Incorporated Foundation) for their help.
Footnotes
Author contributions
Planning of research: F Imamura. Data analysis: A Kubota. Data collection: K Kunimasa, H Kuhara, M Tamiya, K Nishino, M Kimura, K Kuno, H Kawachi, T Kumagai.
Financial & competing interests disclosure
F Imamura, T Kumagai, K Nishino, and M Tamiya have received research funds and/or honoraria from AstraZeneca, Boehringer-Ingelheim, Chugai and Pfizer. The other authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Ethical conduct of research
The authors state that this study is approved by ethics committee of Osaka International Cancer Institute.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Soria JC, Ohe Y, Vansteenkiste J. et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 378(2), 113–125 (2018). [DOI] [PubMed] [Google Scholar]; •• Important manuscript for understanding osimertinib.
- 2.Ramalingam SS, Vansteenkiste J, Planchard D. et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 382(1), 41–50 (2020). [DOI] [PubMed] [Google Scholar]; • Important manuscript for understanding osimertinib.
- 3.Park K, Tan EH, O'Byrne K. et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a Phase IIB, open-label, randomised controlled trial. Lancet Oncol. 17(5), 577–589 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Wu YL, Cheng Y, Zhou X. et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, Phase III trial. Lancet Oncol. 18(11), 1454–1466 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Mok TS, Wu Y-L, Ahn M-J. et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N. Engl. J. Med. 376(7), 629–640 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girard N. Optimizing outcomes and treatment sequences in EGFR mutation-positive non-small-cell lung cancer: recent updates. Future Oncol. 15(25), 2983–2997 (2019). [DOI] [PubMed] [Google Scholar]; •• Describes importance of sequences of EGFR-tyrosine kinase inhibitors.
- 7.Nadler E, Pavilack M, Espirito JL. et al. Observational study of treatment patterns in patients with epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer after first-line EGFR-tyrosine kinase inhibitors. Adv. Ther. 37(2), 946–954 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Hochmair MJ, Morabito A, Hao D. et al. Sequential treatment with afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: an observational study. Future Oncol. 14(27), 2861–2874 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Allegra C, Blanke C, Buyse M. et al. End points in advanced colon cancer clinical trials: a review and proposal. J. Clin. Oncol. 25(24), 3572–3575 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Chibaudel B, Bonnetain F, Shi Q. et al. Alternative end points to evaluate a therapeutic strategy in advanced colorectal cancer: evaluation of progression-free survival, duration of disease control, and time to failure of strategy--an Aide et Recherche en Cancerologie Digestive Group Study. J. Clin. Oncol. 29(31), 4199–4204 (2011). [DOI] [PubMed] [Google Scholar]; • Describes time to failure of strategy to evaluate a therapeutic strategy.
- 11.Yang JC, Ahn MJ, Kim DW. et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study Phase II extension component. J. Clin. Oncol. 35(12), 1288–1296 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Wu SG, Liu YN, Tsai MF. et al. The mechanism of acquired resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib in lung adenocarcinoma patients. Oncotarget 7(11), 12404–12413 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chouaid C, Dujon C, Do P. et al. Feasibility and clinical impact of re-biopsy in advanced non small-cell lung cancer: a prospective multicenter study in a real-world setting (GFPC study 12-01). Lung Cancer 86(2), 170–173 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa T, Sawa T, Futamura Y. et al. Feasibility of rebiopsy in non-small cell lung cancer treated with epidermal growth factor receptor-tyrosine kinase inhibitors. Intern. Med. 54(16), 1977–1980 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Bosc C, Ferretti GR, Cadranel J. et al. Rebiopsy during disease progression in patients treated by TKI for oncogene-addicted NSCLC. Target. Oncol. 10(2), 247–253 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Sequist LV, Yang JC, Yamamoto N. et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 31(27), 3327–3334 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Wu YL, Zhou C, Hu CP. et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised Phase III trial. Lancet Oncol. 15(2), 213–222 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Kato T, Yoshioka H, Okamoto I. et al. Afatinib versus cisplatin plus pemetrexed in Japanese patients with advanced non-small cell lung cancer harboring activating EGFR mutations: subgroup analysis of LUX-Lung 3. Cancer Sci. 106(9), 1202–1211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imamura F, Uchida J, Kukita Y. et al. Monitoring of treatment responses and clonal evolution of tumor cells by circulating tumor DNA of heterogeneous mutant EGFR genes in lung cancer. Lung Cancer 94, 68–73 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Taus Á, Camacho L, Rocha P. et al. Dynamics of EGFR mutation load in plasma for prediction of treatment response and disease progression in patients with EGFR-mutant lung adenocarcinoma. Clin. Lung Cancer 19(5), 387–394 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Kohsaka S, Petronczki M, Solca F. et al. Tumor clonality and resistance mechanisms in EGFR mutation-positive non-small-cell lung cancer: implications for therapeutic sequencing. Future Oncol. 15(6), 637–665 (2019). [DOI] [PubMed] [Google Scholar]; • Important manuscript for understanding molecular events during therapies for EGFR mutation-positive NSCLC.
- 22.Hata AN, Niederst MJ, Archibald HL. et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat. Med. 22(3), 262–269 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamal-Hanjani M, Quezada SA, Larkin J. et al. Translational implications of tumor heterogeneity. Clin. Cancer Res. 21(6), 1258–1266 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohsaka S, Nagano M, Ueno T. et al. A method of high-throughput functional evaluation of EGFR gene variants of unknown significance in cancer. Sci. Transl. Med. 9(416), eaan6566 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Saxon JA, Sholl LM, Janne PA. EGFR L858M/L861Q cis mutations confer selective sensitivity to afatinib. J. Thorac. Oncol. 12(5), 884–889 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blakely CM, Watkins TBK, Wu W. et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR mutation-positive lung cancers. Nat. Genet. 49(12), 1693–1704 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banno E, Togashi Y, Nakamura Y. et al. Sensitivities to various epidermal growth factor receptor-tyrosine kinase inhibitors of uncommon epidermal growth factor receptor mutations L861Q and S768I: what is the optimal epidermal growth factor receptor-tyrosine kinase inhibitor? Cancer Sci. 107(8), 1134–1140 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim EY, Cho EN, Park HS. et al. Compound EGFR mutation is frequently detected with co-mutations of actionable genes and associated with poor clinical outcome in lung adenocarcinoma. Cancer Biol. Ther. 17(3), 237–245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang JC, Sequist LV, Geater SL. et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 16(7), 830–838 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Tamiya M, Tamiya A, Suzuki H. et al. Which is better EGFR-TKI followed by osimertinib between afatinib and gefitinib/erlotinib? Anticancer Res. 39(7), 3923–3929 (2019). [DOI] [PubMed] [Google Scholar]