Abstract
Importance:
Genetic testing results can provide guidance in developing personalized treatment plans for patients with vascular anomalies.
Objective:
To explore the efficacy of tramitinib in the treatment of an extracranial arteriovenous malformation with a somatic MAP2K1 mutation.
Design:
Case report of a child with an arteriovenousu malformation that was successfully treated with trametinib after identification of a specific somatic mutation within the malformation.
Setting:
Outpatient Vascular Anomalies Clinic, Stanford University, Stanford, CA.
Participant:
An 11-year-old girl with an arteriovenous malformation on the back who had failed treatment with systemic sirolimus.
Intervention:
Paired exome sequencing using tumor and saliva DNA was performed and identified a somatic mutation within the MAP2K1 gene. The patient was then transitioned to trametinib at a starting dose of 0.5 mg once daily then increased to 0.5 mg twice daily after one month.
Main Outcome:
Tramitinib was effective in reducing the size of and blood flow to the arteriovenous malformation.
Results:
After one month on tramitinib, the patient and parents noticed the malformation reduced in size and became lighter in color. After six months of treatment, quantitative analyses were performed using magnetic resonance imaging and showed significant interval decrease in the volume of the malformation and the caliber of the vasculature compared to prior examinations. To date, she has tolerated the treatment well with development of a mild acneiform eruption responding well to over the counter adapalene and benzoyl peroxide.
Conclusion and Relevance:
Management of arteriovenous malformations is very challenging due to almost inevitable disease progression and high recurrence rates after surgical resection. The discovery of a somatic mutation associated with this arteriovenous malformation provided guidance for targeted therapy. Trametinib may be a promising targeted therapeutic option for sporadic extracranial arteriovenous malformations harboring MAP2K1 mutations.
Introduction
Arteriovenous malformations (AVMs) are rare, congenital vascular malformations composed of abnormal connections between arteries and veins and missing the normal capillary beds that typically lie in between. Comprising up to 4.7% of all vascular anomalies1, AVMs are the most difficult type of vascular malformations to manage. Currently, there are no FDA-approved treatments for AVMs. Here, we report a child with an AVM that successfully responded to trametinib using genotyping as guidance.
Case report
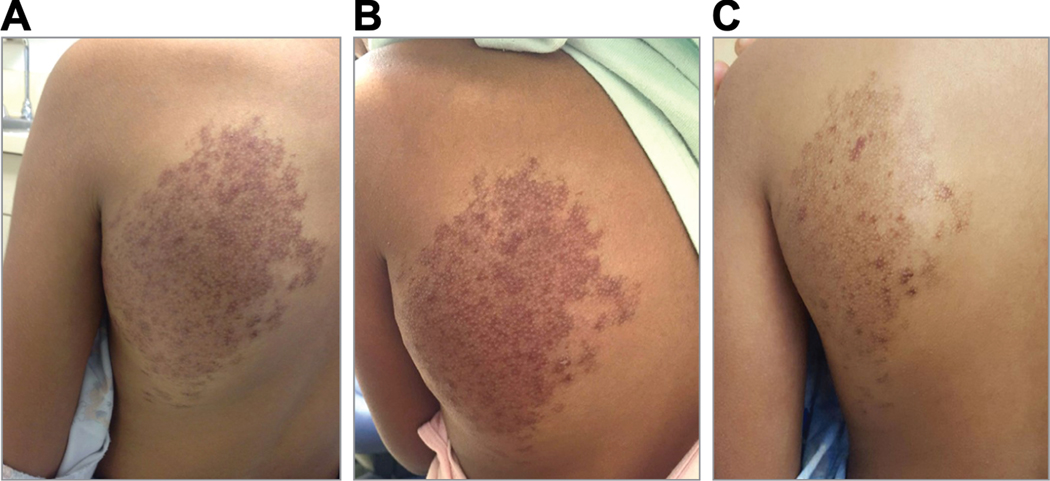
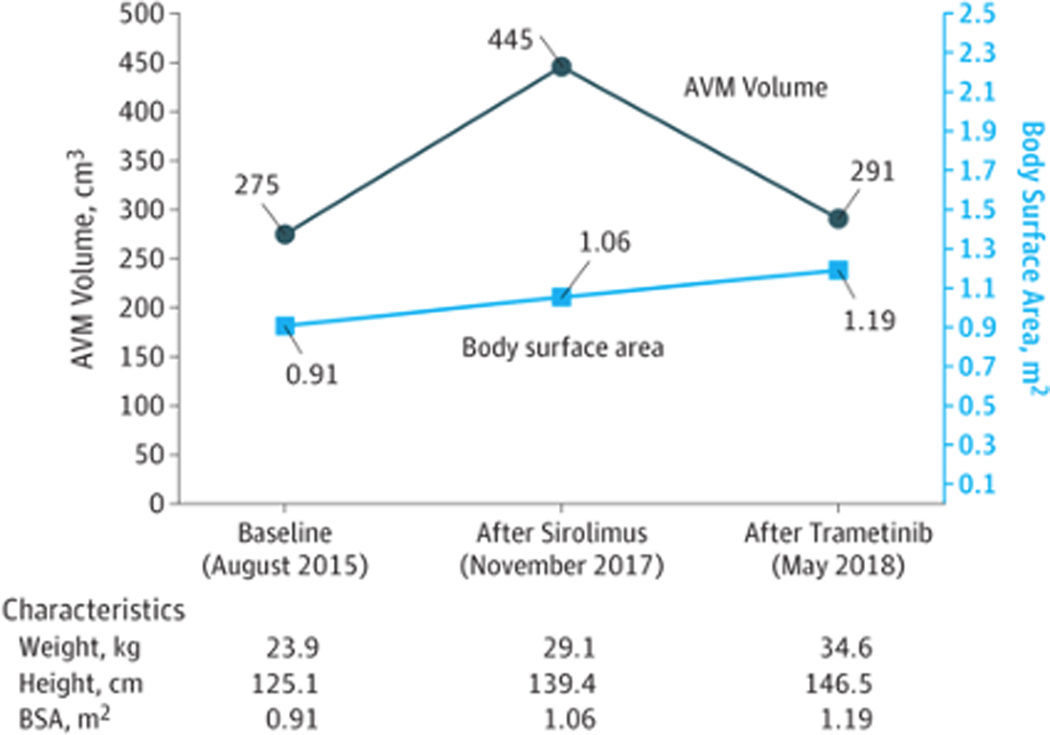
An 11-year-old girl presented with an enlarging congenital vascular mass on her back, which had grown notably in size and darkened in color since 3 years of age. She denied restriction of shoulder movement. Physical examination revealed a 17 × 12 cm, compressible, warm, vascular mass overlying the left scapular region and extending to the mid upper back (Fig. 1A). Radioimaging and clinical evaluation were most consistent with an AVM (Fig. 1D). Due to rapid disease progression, the patient was started on systemic sirolimus with target trough levels of 10–15 ng/dl. She remained at a therapeutic dose and tolerated the treatment for nearly 8 months, with the exception of intermittent oral ulcers. There was no improvement noted in the size of the AVM either on clinical exam or with MRI (Fig. 1b and 1e). Genomic DNA was extracted from a saliva sample and tumor biopsy, and paired exome sequencing was performed, identifying a somatic MAP2K1 c.169_183del (p.Lys57_Gly61del) in-frame deletion. Deletions in this region were previously reported to activate MAPK signaling via increasing levels of pERK.2 In light of this finding, in addition to the lack of clinical improvement on her current regimen, the risk of cardiac comorbidity from disease progression, and concerns regarding sirolimus side effects, the decision was made to stop sirolimus treatment. The patient was then transitioned to trametinib, a MEK inhibitor, at a starting dose of 0.5 mg once daily which was then increased to 0.5 mg twice daily after one month. At the one month follow up, the patient and her parents noted the lesion had reduced in size, lightened in color, and had decreased warmth to touch. MRI evaluation and volumetric analysis were performed following 6 months of treatment, which confirmed significant interval decrease in the extent of the malformation and the caliber of the vasculature compared to prior examinations (Fig. 1c, 1f and Fig. 2). To date, she has tolerated treatment well with only mild acne, which has responded well to over the counter benzoyl peroxide wash and adapalene 0.1% gel.
Figure 1:
Clinical presentation and imaging studies of an arteriovenous malformation on the left scapular region of an 11 year-old girl. Clinical photos at (A) baseline; (B) after 8 months of sirolimus treatment; and (C) after 6 months trametinib treatment are shown. In addition, corelating magnetic resonance imaging (D-F).
Figure 2:
Volumetric analysis of arteriovenous malformation response to sirolimus and trametinib treatment.
Discussion
All AVMs progress throughout life, with up to 80 percent of lesions beginning this progression during childhood and adolescence.3 Without treatment, arteriovenous shunting can cause tissue ischemia leading to tissue destruction, bleeding, functional deficits, deformity and rarely cardiac overload. Embolization and surgical debulking have transient benefits due to often rapid recurrence.3 With limited therapeutic options, there is a significant unmet medical need.
Genetic sequencing has recently been employed in diagnosing many vascular malformations and malformation syndromes. This diagnostic tool has enabled identification of pathogenic mutations associated with specific lesions which likely drive their growth. Prior studies have found somatic mutations in patients with sporadic extracranial AVMs, the most common of which are in MAP2K1.4 Additional mutations within KRAS and BRAF have also been reported.5 MAP2K1 encodes mitogen-activated protein kinase 1 (MEK1), which plays a key role in RAS/RAF/MAPK signaling and angiogenesis. MAP2K1 mutant endothelial cells demonstrate disrupted vascular channel formation, leading to abnormal capillary networks between developing arteries and veins.4,5 In-frame deletions at c.159_173del, c.170_184del, and c.172_186del, which encompass the deletion in our patient, have been shown to activate ERK phosphorylation in vitro for downstream MAPK signaling.2 Trametinib is a MEK inhibitor used for the treatment of many oncologic disorders, as well as neurofibromatosis in children.6 Given its relatively high safety profile, we initiated this treatment based on her genetic testing results and to specifically target the over-activated MAPK pathway. The patient clearly demonstrated superior clinical response to trametinib in comparison to sirolimus, which is a known anti-angiogenic agent targeting the mTOR pathway. Mild acne was the only noted side effect; repeated electrocardiogram, ocular exam and laboratory studies were all within normal. Our findings suggest that MEK inhibitors may be an effective treatment for extra-cranial AVMs, especially for patients carrying somatic MAP2K1 mutations. Further well-controlled, large-scale studies are needed to investigate the optimal dosing, duration, long-term safety and efficacy of this treatment.
Conclusion
Trametinib may be a promising new, targeted therapeutic option for sporadic extracranial AVMs harboring MAP2K1 mutations. This case report highlights the value of employing genetic testing in developing personalized treatments for patients with these challenging lesions.
Key Points.
Question:
Can tramitinib be an effective treatment for extracranial arteriovenous malformation?
Findings:
Tramitinib can be an effective and well-tolerated treatment for a child with an extracranial arteriovenous malformation due to a MAP2K1 mutation.
Meaning:
MEK inhibitor may offer a new, targeted therapeutic option for sporadic extracranial arteriovenous malformations that carry MAP2K1 mutations.
Acknowledgments
Funding/Support: No
| Funding/Sponsor was involved? | ||
| Design and conduct of the study | Yes |  |
| Collection, management, analysis and interpretation of data | Yes |  |
| Preparation, review, or approval of the manuscript | Yes |  |
| Decision to submit the manuscript for publication | Yes |  |
Footnotes
Financial disclosure: None reported
References
- 1.Visser A, FitzJohn T, Tan ST. Surgical management of arteriovenous malformation. J Plast Reconstr Aesthet Surg 2011;64(3):283–91. [DOI] [PubMed] [Google Scholar]
- 2.Chakraborty R, Hampton OA, Shen X, et al. Mutually exclusive recurrent somatic mutations in MAP2K1 and BRAF support a central role for ERK activation in LCH pathogenesis. Blood 2014;124(19):3007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu AS, Mulliken JB, Zurakowski D, et al. Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg 2010;125(4):1185–94. [DOI] [PubMed] [Google Scholar]
- 4.Couto JA, Huang AY, Konczyk DJ, et al. Somatic MAP2K1 Mutations Are Associated with Extracranial Arteriovenous Malformation. Am J Hum Genet 2017;100(3):546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Olabi L, Polubothu S, Dowsett K, et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J Clin Invest 2018;128(4):1496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trametinib Zeiser R..Recent Results Cancer Res 2014;201:241–8. [DOI] [PubMed] [Google Scholar]