Abstract
BACKGROUND:
Clinical monitoring of patients with Parkinson’s disease (PD) for cognitive decline is an important element of care. The Montreal Cognitive Assessment (MoCA) has been proposed to be a sensitive tool for assessing cognitive impairment in PD. The aim of our study was to compare the responsiveness of the MoCA to decline in cognition to the responsiveness of the Mini Mental State Examination (MMSE) and the Scales for Outcomes of Parkinson’s disease-cognition (SCOPA-Cog).
METHODS:
PD patients without dementia were enrolled at 6 North American movement disorders centers between 2008 and 2011. Participants received annual evaluations including the MoCA, MMSE, and SCOPA-Cog followed by formal neuropsychological testing. The gold standard for change in cognition was defined as the change on the neuropsychological test scores over the annual assessments. The Reliable Change Method was used to provide an estimate of the probability that a given difference score would be obtained by chance. The sensitivity of the MoCA, MMSE, and SCOPA-Cog to change was quantified using receiver operating characteristics (ROC) curves.
RESULTS:
One hundred seventeen patients were included in the analysis. Participants were followed at mean intervals of 11 ± 2 months for a median of 2 (maximum 5) visits. According to the reliable change index, 56 intervals of cognitive testing showed a decline in global cognition. ROC analysis of change in MoCA, MMSE, and SCOPA-Cog global scores compared to gold standard testing found an area under the curve (AUC) of 0.55 (95% CI 0.48–0.62), 0.56 (0.48–0.63), and 0.63 (0.55–0.70) respectively. There were no significant differences in the AUCs across the tests. The sensitivity of the MoCA, MMSE, and SCOPA-Cog to change at various thresholds for decline in scores reached a maximum of 71% for a cut-off of 1 point change on the SCOPA-Cog.
CONCLUSION:
Using neuropsychological testing as a gold standard comparator, the performance of the MoCA, MMSE, and SCOPA-Cog for detecting decline in non-demented PD patients over a 1-year interval is poor. This has implications for clinical practice; stable scores may not be taken as reassurance of the absence of cognitive decline.
Introduction
Cognitive impairment in Parkinson’s Disease (PD) is associated with impaired functioning and caregiver distress [1–4]. Clinical monitoring of patients with PD for cognitive decline is an important element of PD care. For this purpose, cognitive scales covering multiple cognitive domains are used. Two such instruments are routinely used: the Montreal Cognitive Assessment (MoCA) and the Mini Mental State Examination (MMSE).
The MoCA has been proposed to be a practical and sensitive tool for assessing cognitive impairment in PD [5, 6]. A study assessing cognitive decline and quality of life in patients with PD determined that a decline in MoCA scores over a 3-year period can predict a decline in quality of life [7]. The MoCA can detect mild impairment with less ceiling effect than the MMSE and includes executive function, complex visuospatial and language tasks [8, 9]. In addition, its use as a sensitive tool to monitor cognitive changes over time has been evaluated in different neurological conditions such as mild cognitive impairment, Alzheimer’s disease and cerebrovascular disease [10–12] and recently also in people with PD [13, 14]. The studies in PD are conflicting with respect to the comparative ability of the MoCA and MMSE to detect change in cognition over time [13, 14]. A third such instrument, the Scales for Outcomes of Parkinson’s disease-cognition (SCOPA-Cog), was designed for research purposes. It covers the cognitive areas most affected by PD [15] and has been found to be sensitive to detect cognitive deficits in PD [16]. However, a recent review on cognitive tests in PD reported that there is not enough evidence regarding its responsiveness to recommend its use for clinical trials [17].
Evidence for the responsiveness of the MoCA, MMSE, and SCOPA-Cog to change is scarce and conflicting, and no studies to date have examined this using a gold standard of change. In order to identify a preferred instrument for clinical monitoring, comparative evidence for responsiveness to change over time is needed. The aim of our study was to compare the responsiveness of MoCA, MMSE, and SCOPA-Cog to changes in cognition over time. Given their component items, we hypothesized that the SCOPA-Cog would be most responsive to change in global cognition and executive test performance followed by the MoCA and that the MMSE would be least responsive.
Methods
Participants
PD patients without dementia were enrolled at 6 North American movement disorders centers from 2008 through 2011. Inclusion criteria were:
Diagnosis of PD according to the UK PD Society Brain Bank criteria [18]
No planned medication changes over the following 3 weeks
A close contact available for collateral history (contact at least 2 times per week)
No clinically significant depressive disorder (15-item Geriatric Depression Scale score less than 5) [19]
No currently unstable psychiatric disorder
No significant functional impairment due to cognitive problems as assessed by the Disability Assessment for Dementia [20]. The Disability Assessment for Dementia assesses impairment on specific Activities of daily living (ADLs) and Instrumental Activities of Daily Living (IADLs), for example, managing finances, grocery shopping.
English as a first language
Each participating institution received local IRB approval before study enrolment. Written informed consent was obtained from all study participants and close contacts.
Assessments
Participants received annual clinical evaluations including the MoCA (version 7.1 at each annual visit), MMSE and SCOPA-Cog followed 1–3 weeks later by formal neuropsychological testing. The neuropsychological test battery was given and the administrator was blinded to the scores on the MoCA, MMSE, and SCOPA-Cog. Two neuropsychological tests for each of the 5 following cognitive domains (a total of ten tests) were administered:
For Attention/working memory domain Wechsler Memory Scale-III letter-number sequencing test [21] and Delis Kaplan Executive Function System Color Word Interference Color Naming test [22] were used. For the language 30-item Boston Naming Test [23] and Delis Kaplan Executive Function System Verbal Fluency Category Fluency test [22] were administered. Visuospatial function was assessed by Benton Judgment of Line Orientation [24] test and Copy Trial of the Rey Complex Figure Test and Recognition Trial [25]. Memory tests included Rey Complex Figure Test and Recognition Trial Delayed Recall [25] California Verbal Learning Test-II Long Delay Free Recall test [26]. Finally, Executive function was assessed using the Visual Verbal Test abbreviated 10-item version [27] and the Trail Making Test B minus A [28].
To be eligible for inclusion in the current analysis, at least 2 evaluations (baseline and 1 year follow-up) with MoCA, MMSE, SCOPA-Cog and neuropsychological test battery were required. Thus, the analysis comprised of all subjects who were followed at multiple points.
Statistical Analysis
The gold standard for the definition of change in cognition is the change seen on neuropsychological test scores over annual assessments. At each follow-up visit, the results of the neuropsychological test battery were compared to the previous visit and the participant was classified as either unchanged or changed during that interval. As there is no established definition for clinically meaningful change in studies using a neuropsychological test battery, the Reliable Change Method [29, 30] was used to provide an estimate of the probability that a given difference score would be obtained by chance. The Reliable Change Index (RCI) is calculated as the difference between the change in score for the individual and the change in score for the entire cohort divided by the standard deviation of the change in score for the entire cohort:
([X2 – X1] – [M2 – M1])/SED where:
X1 = individual baseline score,
X2 = the individual follow-up score,
M1 = normative mean baseline score,
M2 = normative mean follow-up score,
SED = Standard Error of the Difference, SED = (2*SEM2)1/2,
SEM = Standard Error of Measurement SEM = SD ([1-r]1/2),
SD = pre-test SD
r = reliability coefficient [30].
An RCI less than –1.96 or greater than +1.96 was used to define reliable change and indicates that the observed change falls outside the 95% CI for change. Reliable change was determined as present or absent for each interval (baseline-year 1, year 1 – year 2, etc.) on each of the 10 core tests (2 tests × 5 domains) for each subject. For the Visual Verbal Test, Rey Complex Figure Test, Boston Naming Test and Trail Making Test the relevant test-retest reliability data are not available and a change of more than 1.65 SDs was considered a significant change.
The gold standard for global change (yes/no) in cognition was defined as RCI greater than +1.96 or less than –1.96 on 2 or more tests. We also determined domain-specific cognitive change. There are 2 cognitive tests in each domain; the RCI for the 2 tests was averaged and this domain-specific average RCI was used to determine change in that domain. The executive domain was of primary interest due to a high prevalence of impairment in this domain in PD, as well as because it is variably represented across the tests being compared; all other domain-specific comparisons were considered to be exploratory.
The change in score on the MoCA, MMSE, or SCOPA-Cog for each interval and the RCI classification for the same interval formed pairs of observations for the analysis. Intervals were the unit of observation and most commonly represented 1-year period. The sensitivity of the MoCA, MMSE, and SCOPA-Cog to decline at various thresholds of differences in scores (1 point decline, 2 point decline, etc.) was calculated. The results were quantified using receiver operating characteristics (ROC) curves, with a larger area under the curve (AUC) reflecting better responsiveness to change. AUC were compared according to the method of DeLong et al. [31]. For the ROC analysis, improvement was classified as unchanged.
Standardized effect size (SES, mean observed change/standard deviation of baseline scores) and the standardized response mean (SRM, mean observed change/SD of the change in scores) for each test (MoCA, MMSE, SCOPA-Cog) were also calculated as indices of responsiveness.
Results
One hundred thirty-nine participants were enrolled in the study. The longitudinal study is ongoing; at the time of this analysis, 117 participants had a minimum of 2 and a maximum of 5 evaluations and were thus included in this analysis. The mean interval between evaluations was 46.45 ± 9.85 weeks. Out of the 117 participants who were followed in the first year, 104 participants continued follow-up for 2 years, 38 participants for 3 years and 24 for more than 3 years, resulting in 282 intervals for analysis. Reasons for dropout were: insertion of deep brain stimulation (n = 2), death (n = 2), other medical conditions (n = 4). Other participants provided no specific reason for discontinuation.
Baseline characteristics of the 117 participants are shown in Table 1. At baseline 44% of the subjects were classified as PD-MCI according to the MDS task force level II criteria [32]. The remainder were classified as having normal cognition (individuals with dementia were excluded from the study).
Table 1.
Baseline characteristics for patients with repeat visits
| Total participants, n | 0.117 |
| Age, years, mean (SD) | 071.0 (5.4) |
| Men, % | 068.0 |
| Years of education, mean (SD) | 015.9 (2.5) |
| Premorbid IQ, mean (SD) | 113.4 (9.0) |
| MoCA score, mean (SD) | 025.5 (2.9) |
| Scopa-Cog score, mean (SD) | 027.9 (4.9) |
| MMSE score, mean (SD) | 028.4 (1.8) |
| Percentage with normal cognition, % | 066.1 |
| Disease duration, years, mean (SD) | 004.7 (4.0) |
| MDS-UPDRS III score, mean (SD) | 026.9 (11.0) |
Frequency of Change
According to the RCI (Table 2), 56/282 intervals across the years showed a decline in global cognition and 14/282 intervals showed improvement. In each period, most of the patients remained unchanged. Decline in the visuospatial domain (55/282 intervals) was most frequent, whereas decline in the attention/working memory domain was least frequent (18/282 intervals). V-S = Visuospatial.
Table 2.
Number of participants declining, improving, or remaining unchanged in each cognitive domain on neuropsychological testing
| Executive | Attention/ working memory | Language | V-S | Learning/ memory | Global | |
|---|---|---|---|---|---|---|
| Baseline – Year 1 | ||||||
| Declined | 22 (19)* | 010 (9) | 25 (21) | 22 (19) | 28 (24) | 27 (23) |
| Unchanged | 82 (70) | 104 (89) | 88 (75) | 84 (72) | 80 (68) | 87 (74) |
| Improved | 13 (11) | 003 (3) | 04 (3) | 11 (9) | 09 (8) | 03 (3) |
| Year 1 – Year 2 | ||||||
| Declined | 16 (15) | 004 (4) | 11 (11) | 15 (14) | 16 (15) | 15 (14) |
| Unchanged | 69 (66) | 096 (92) | 76 (73) | 78 (75) | 82 (79) | 80 (77) |
| Improved | 19 (18) | 004 (4) | 17 (16) | 11 (11) | 06 (6) | 09 (9) |
| Year 2 – Year 3 | ||||||
| Declined | 09 (24) | 003 (8) | 13 (35) | 13 (35) | 05 (14) | 10 (27) |
| Unchanged | 24 (65) | 034 (92) | 23 (62) | 19 (51) | 31 (83) | 25 (68) |
| Improved | 04 (11) | 000 (0) | 01 (3) | 05 (14) | 01 (3) | 02 (5) |
| Year 3+ | ||||||
| Declined | 03 (13) | 001 (4) | 02 (8) | 05 (21) | 04 (17) | 04 (17) |
| Unchanged | 21 (88) | 023 (96) | 21 (88) | 18 (75) | 20 (83) | 20 (83) |
| Improved | 00 (0) | 000 (0) | 01 (4) | 01 (4) | 00 (0) | 00 (0) |
Values expressed in parentheses are percentages.
Because of rounding up of numbers, some sums of percentages do not add to 100%.
Global Cognitive Change
The AUCs did not differ meaningfully when evaluated separately for each year of study (baseline-year 1, year 1 – year 2, etc.); thus, the observations were curated into a single database of 1-year intervals. ROC analysis of decline in MoCA, MMSE, and SCOPA-Cog global scores compared to gold standard testing found an AUC of 0.55 (95% CI 0.48–0.62), 0.56 (0.48–0.63) and 0.63 (0.55–0.70) respectively (Fig. 1). There were no significant differences in the AUC of the MoCA compared to that of the MMSE (p = 0.82) or SCOPA-Cog (p = 0.14), nor for the SCOPA-Cog compared to the MMSE (p = 0.21). The sensitivity of the MoCA, MMSE, and SCOPA-Cog to change at various thresholds of decline in scores was low, reaching a maximum of 71% for a cut-off of 1 point change on the SCOPA-Cog and was lower for the other tests (Table 3). Specificity greater than 80% was achieved for a decline of 3 or more on the MoCA (87%), 2 or more on the MMSE (83%), and 4 or more on the SCOPA-Cog (83%; online suppl. Table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000496454).
Fig. 1.
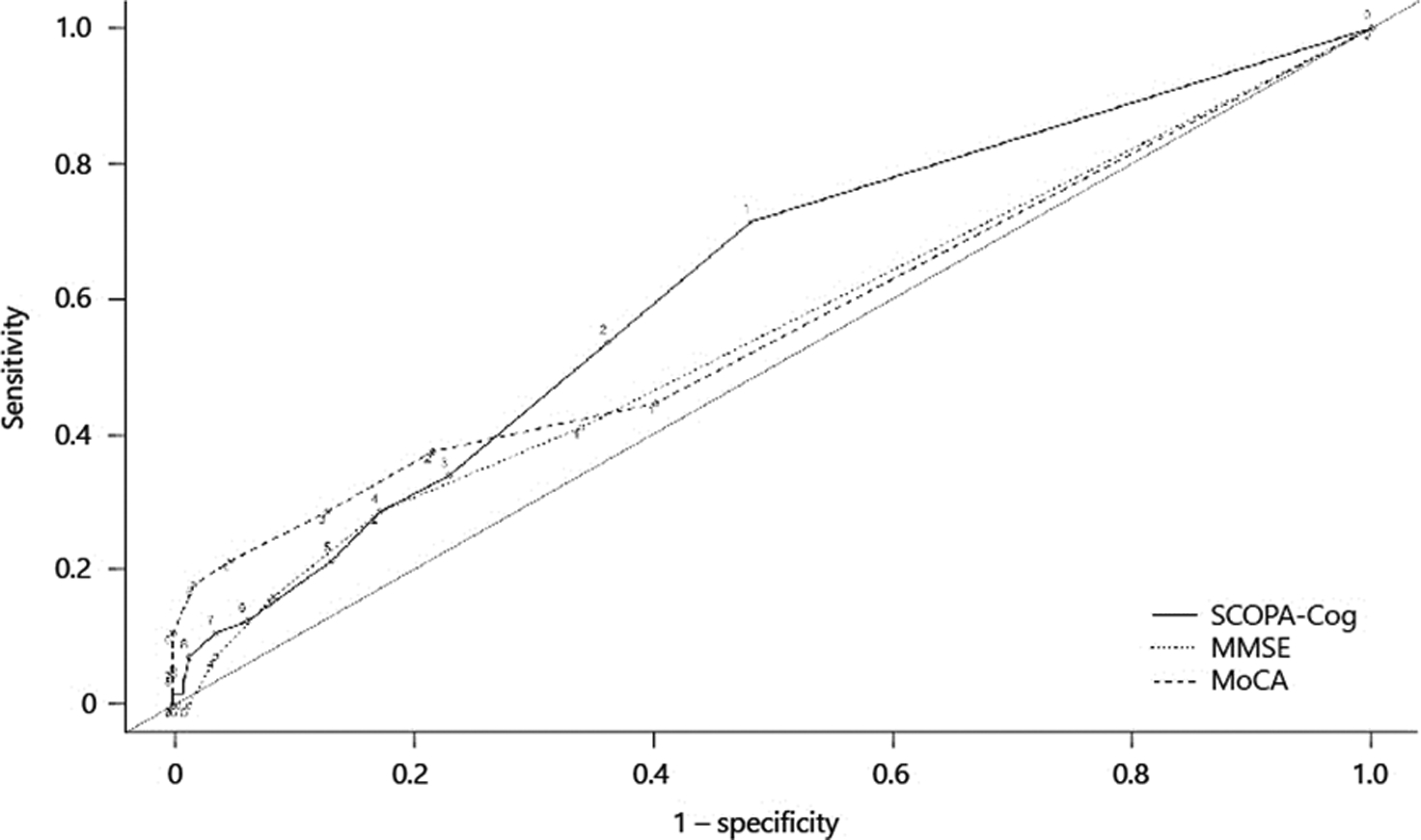
ROC curves for change in Montreal Cognitive Assessment (MoCA), Mini Mental State Examination (MMSE), and SCOPA-Cog scores using global cognitive decline in neuropsychological testing as the gold standard.
Table 3.
Sensitivity of MMSE, MoCA, and SCOPA-Cog to global cognitive decline at different thresholds of change in scores
| Threshold for decline | Sensitivity (95% CI) | ||
|---|---|---|---|
| MoCA | MMSE | SCOPA-Cog | |
| –1 | 45 (31–59) | 41 (28–55) | 71 (56–82) |
| –2 | 38 (24–50) | 29 (16–41) | 54 (38–69) |
| –3 | 29 (16–41) | 16 (7–28) | 34 (21–48) |
Domain-Specific Cognitive Change
Given our primary interest in the executive domain, since it is more highly represented in the MoCA than in the MMSE, we performed an ROC analysis of MoCA, MMSE, and SCOPA-Cog executive domain change scores compared to gold standard testing (Fig. 2). The AUCs were 0.59, 0.52, and 0.59 respectively (Table 4). The best sensitivity to decline was found for a 1-point change of the Scopa-Cog: 62% (online suppl. Table 1). There were no statistically significant pairwise differences in AUC across the 3 tests.
Fig. 2.
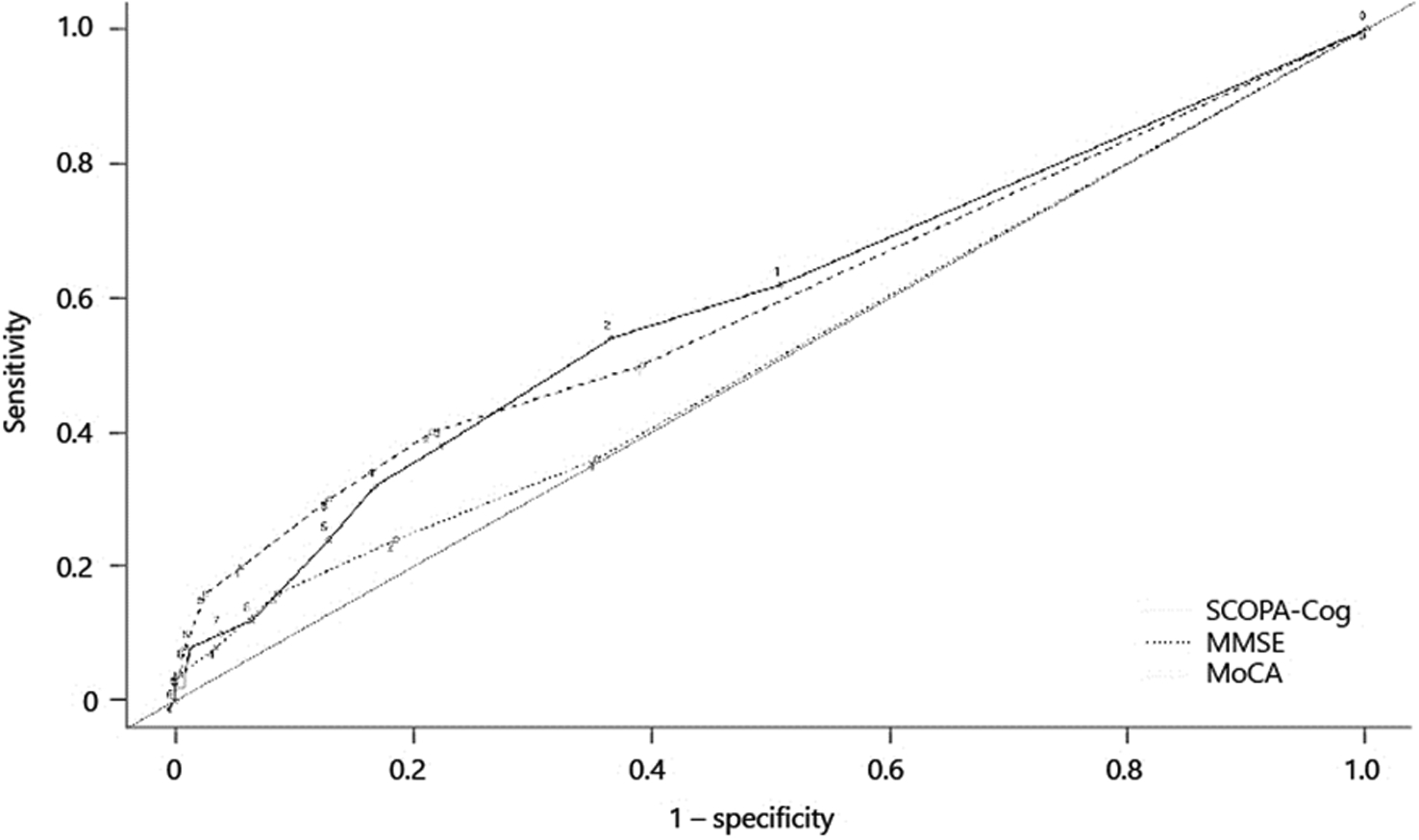
ROC curves for change in Montreal Cognitive Assessment (MoCA), Mini Mental State Examination (MMSE), and SCOPA-Cog scores using executive domain change as gold standard.
Table 4.
Areas under the ROC curves for domain-specific change
| MoCA | MMSE | SCOPA-Cog | |
|---|---|---|---|
| Executive change | 0.59 | 0.52 | 0.59 |
| Visuospatial change | 0.60 | 0.53 | 0.58 |
| Learning/memory change | 0.49 | 0.45 | 0.56 |
| Attention change | 0.64 | 0.58 | 0.59 |
| Language change | 0.52 | 0.59 | 0.60 |
Areas under the ROC curves for the different cognitive domains are shown in Table 4. The highest AUC was for the attention domain when comparing the MoCA to the gold standard of neuropsychological testing. However, even this value was relatively low (0.64).
Other Indices of Responsiveness
Measures of internal responsiveness, the SES and the SRM were similar for all 3 tests: MoCA (SES –0.344, SRM –0.615), MMSE (SES –0.386, SRM –0.602), SCOPA-Cog (SES –0.352, SRM –0.733).
Discussion
It is common in clinical practice to administer screening tests to determine the cognitive abilities of patients with PD. The MoCA has been suggested by some to be a sensitive and specific test for PD-associated mild cognitive impairment [6, 33, 34]. As a complement to single episodes of testing, it is common to apply the tests serially to provide insight into the trajectory of cognitive change. Given the widespread clinical use of the MoCA for measuring cognition over time, understanding the performance of this test for detecting decline is important. Using neuropsychological testing as a gold standard comparator, we found that the performance of the MoCA, MMSE, and SCOPA-Cog for detecting statistically significant decline in non-demented PD patients was poor. This has implications for clinical practice; stable scores may not be taken as reassurance for the absence of cognitive decline, as the sensitivity of all tests was low. High specificity can be achieved with larger values of change on all 3 tests but not accompanied by reasonable sensitivity.
Only a few studies have evaluated sensitivity to decline in cognition of these cognitive tests, with conflicting results. A previous 3-year longitudinal study of PD patients with different cognitive status found that MMSE scores but not MoCA scores declined significantly in a linear mixed effects model over time, suggesting that the MMSE was more sensitive to cognitive decline [13]. Their results are in contrast with those of an 18-month prospective longitudinal study looking at predictors for cognitive impairment in patients with early PD [35]. This latter study concluded that the MoCA was more sensitive than MMSE in detecting cognitive decline, based on a statistically significant decline in MoCA (but not MMSE) scores over the period of observation. Another longitudinal study compared the responsiveness to change of the MoCA and MMSE over 1 year in PD and in patients with Dementia with Lewy Bodies [14]. Using Cohen’s D for change over time, they found a similar and small effect size for both the MMSE and the MoCA. The 1-year mean change in scores for the PD population was –0.33 (1.14) for MMSE and –0.30 (1.32) for the MoCA. In a Chinese 30-month prospective study of cognitive changes in PD patients, the mean MMSE score decreased by 2.05 points, and the MoCA score decreased by 2.56 points. They concluded that the MoCA score registered a more obvious decline [36] (see Table 5 for a comparison of previous studies to the present study).
Table 5.
Summary of previous studies evaluating sensitivity to decline using different cognitive tests
| Authors | Year | Subjects number for longitudinal analysis | Follow-up duration, years | Baseline characteristics, mean ± SD | |||||
|---|---|---|---|---|---|---|---|---|---|
| age, years | education, years | disease duration, years | UPDRS III | MMSE | MoCA | ||||
| Lessig et al. [13] | 2012 | 98 PD patients | Up to 3 | 068.8±1.02 | 015.3±2.6 | 06.7±5.4 | 026.4±11.9 | 027.5±2.3 | 00.24±3.6 |
| Biundo et al. [14] | 2016 | 139 PD, 14 DLBD patients |
1 | 65.53±8.95 (PD group) | 12.04±3.66 | 8.90±5.37 | 43.06±14.54 | 027.5±2.34 | 24.53±3.9 |
| Hu et al. [35] | 2014 | 155 PD patients | 1.5 | 67.8±9.4 | 013.6±3.5 | 01.5±1 | 026.8±11 | 027.4±2.4 | 024.9±3.4 |
| Chen et al. [36] | 2016 | 102 PD patients | 2.5 | 64.32±9 | 09.71±4.06 | 6.43±4.55 | 18.76±10.29 | 28.16±2.29 | 024.6±4.23 |
To our knowledge, ours is the first study using a gold standard of cognitive change to assess the responsiveness to change of commonly used measures of global cognition in PD. The evaluation of responsiveness depends upon the choice of gold standard. We chose a statistical criterion to define cognitive change, and it must be kept in mind that this is not based on an assessment of clinically meaningful change. There are no widely accepted methods for integrating the results on serial application of a neuropsychological test battery into a single determination of changed or unchanged. We chose an RCI threshold of more than 1.96 SD to define change, which is large compared to the threshold used by others who have defined change as more than 1.65 SD [37]. However, smaller changes should be more difficult to detect, and therefore allowing smaller changes to be defined as true change would be expected to worsen the sensitivity performance.
Limitations of our study include the selected nature of our cohort, recruited from an academic center with a high level of education. This might be the explanation for the fact that only a relatively small proportion of our cohort met criteria for reliable change.
High level of education has been found to be associated with cognitive reserve and a slower rate of cognitive decline [38–40]. Previous studies that described changes in cognition of non-demented PD patients re-assessed patients at longer intervals [6, 41], although some were not able to detect meaningful changes even after durations as long as 17 months between evaluations [36]. Despite the low proportion of individuals experiencing change, our 95% CIs on measures of test performance rule out satisfactory sensitivity of the MoCA, MMSE, and SCOPA-Cog to cognitive decline demonstrated by neuropsychological testing over 1 year in the non-demented PD population. The implication of this is that, the absence of decline on these screening tests may not mean that cognition is stable. Longer interval studies could show better responsiveness to change in these screening tests. Some of our participants’ performance on these tests improved with time, which has several possible explanations. Practice effects are possible, particularly since alternative versions of the MoCA were not employed. However, assessments were performed at least 1 year apart, so the impact of practice effects is likely to be limited. This is another factor for reducing the proportion of individuals declining. It is also recognized that cognitive impairment in PD may represent a heterogeneous syndrome, not always reflecting a neurodegenerative process (e.g., vascular component). Similar improvements over time were demonstrated in other longitudinal studies using different patient populations. For example, a study of older adults with MCI or who were cognitively normal (n = 106) used the MoCA to detect cognitive change over 3.5 years [10]. Using an RCI cutoff of ±1.73; 42% of MCI participants significantly declined, 49% remained stable, and 9% demonstrated increased scores. Another longitudinal study of the MoCA in healthy older adults demonstrated significant improvements in MoCA scores over 1 and 4 years from baseline [42]. Improvement over time in older adults has also been found in the MMSE literature [30]. Although a proportion of the cohort improved, we did not examine the performance of the MoCA, MMSE, or SCOPA-Cog to detect cognitive improvement. Our results do not reflect the ability of these tests to detect clinically meaningful improvement in cognition in the context of clinical trials. Indeed, several randomized controlled trials of cognitive interventions in PD have incorporated the MoCA as an outcome measure and have shown improvement post intervention at a group level [43, 44].
In summary, in this prospective longitudinal study comparing the responsiveness of MoCA, MMSE, and SCOPA-Cog to decline in cognition over time, we detected poor responsiveness when using a neuropsychological test battery as a gold standard. It is important not to assume that patients with unchanging scores on these tests over 1-year intervals have stable cognitive abilities.
Supplementary Material
Footnotes
Disclosure Statement
The authors declare that they have no conflicts of interest to disclose.
References
- 1.Aarsland D, Larsen JP, Karlsen K, Lim NG, Tandberg E. Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. Int J Geriatr Psychiatry. 1999. October;14(10):866–74. [PubMed] [Google Scholar]
- 2.Post B, Merkus MP, de Haan RJ, Speelman JD; CARPA Study Group. Prognostic factors for the progression of Parkinson’s disease: a systematic review. Mov Disord. 2007. October;22(13):1839–51. [DOI] [PubMed] [Google Scholar]
- 3.Marras C, Rochon P, Lang AE. Predicting motor decline and disability in Parkinson disease: a systematic review. Arch Neurol. 2002. November;59(11):1724–8. [DOI] [PubMed] [Google Scholar]
- 4.Reginold W, Duff-Canning S, Meaney C, Armstrong MJ, Fox S, Rothberg B, et al. Impact of mild cognitive impairment on health-related quality of life in Parkinson’s disease. Dement Geriatr Cogn Disord. 2013;36(1–2):67–75. [DOI] [PubMed] [Google Scholar]
- 5.Holden SK, Jones WE, Baker KA, Boersma IM, Kluger BM. Outcome measures for Parkinson’s disease dementia: a systematic review. Mov Disord Clin Pract (Hoboken). 2016. Jan-Feb;3(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kandiah N, Zhang A, Cenina AR, Au WL, Nadkarni N, Tan LC. Montreal Cognitive Assessment for the screening and prediction of cognitive decline in early Parkinson’s disease. Parkinsonism Relat Disord. 2014. November;20(11):1145–8. [DOI] [PubMed] [Google Scholar]
- 7.Lawson RA, Yarnall AJ, Duncan GW, Breen DP, Khoo TK, Williams-Gray CH, et al. ; ICICLE-PD study group. Cognitive decline and quality of life in incident Parkinson’s disease: the role of attention. Parkinsonism Relat Disord. 2016. June;27:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trzepacz PT, Hochstetler H, Wang S, Walker B, Saykin AJ; Alzheimer’s Disease Neuroimaging Initiative. Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatr. 2015. September;15(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005. April;53(4):695–9. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan K, Rossetti H, Hynan LS, Carter K, Falkowski J, Lacritz L, et al. Changes in Montreal Cognitive Assessment Scores Over Time. Assessment. 2017. September;24(6):772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa AS, Reich A, Fimm B, Ketteler ST, Schulz JB, Reetz K. Evidence of the sensitivity of the MoCA alternate forms in monitoring cognitive change in early Alzheimer’s disease. Dement Geriatr Cogn Disord. 2014;37(1–2):95–103. [DOI] [PubMed] [Google Scholar]
- 12.Popović IM, Serić V, Demarin V. Mild cognitive impairment in symptomatic and asymptomatic cerebrovascular disease. J Neurol Sci. 2007. June;257(1–2):185–93. [DOI] [PubMed] [Google Scholar]
- 13.Lessig S, Nie D, Xu R, Corey-Bloom J. Changes on brief cognitive instruments over time in Parkinson’s disease. Mov Disord. 2012. August;27(9):1125–8. [DOI] [PubMed] [Google Scholar]
- 14.Biundo R, Weis L, Bostantjopoulou S, Stefanova E, Falup-Pecurariu C, Kramberger MG, et al. MMSE and MoCA in Parkinson’s disease and dementia with Lewy bodies: a multicenter 1-year follow-up study. J Neural Transm (Vienna). 2016. April;123(4):431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isella V, Mapelli C, Morielli N, Siri C, De Gaspari D, Pezzoli G, et al. Diagnosis of possible mild cognitive impairment in Parkinson’s disease: validity of the SCOPA-Cog. Parkinsonism Relat Disord. 2013. December;19(12):1160–3. [DOI] [PubMed] [Google Scholar]
- 16.Marinus J, Visser M, Verwey NA, Verhey FR, Middelkoop HA, Stiggelbout AM, et al. Assessment of cognition in Parkinson’s disease. Neurology. 2003. November;61(9):1222–8. [DOI] [PubMed] [Google Scholar]
- 17.Skorvanek M, Goldman JG, Jahanshahi M, Marras C, Rektorova I, Schmand B, et al. ; members of the MDS Rating Scales Review Committee. Global scales for cognitive screening in Parkinson’s disease: critique and recommendations. Mov Disord. 2018. February;33(2):208–18. [DOI] [PubMed] [Google Scholar]
- 18.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992. March;55(3):181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gélinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. Am J Occup Ther. 1999. Sep-Oct;53(5):471–81. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D Wechsler memory scale (WMS-III). Psychological corporation; San Antonio, TX; 1997. [Google Scholar]
- 21.McDonald WM, Holtzheimer PE, Haber M, Vitek JL, McWhorter K, Delong M. Validity of the 30-item geriatric depression scale in patients with Parkinson’s disease. Mov Disord. 2006. October;21(10):1618–22. [DOI] [PubMed] [Google Scholar]
- 22.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system: Technical manual. Psychological Corporation; 2001. [Google Scholar]
- 23.Williams BW, Mack W, Henderson VW. Boston Naming Test in Alzheimer’s disease. Neuropsychologia. 1989;27(8):1073–9. [DOI] [PubMed] [Google Scholar]
- 24.Benton AL. Contributions to Neuropsychological Assessment: A Clinical Manual. Oxford University Press; 1994. [Google Scholar]
- 25.Meyers JE, Meyers KR. Rey complex figure test and recognition trial professional manual. Odessa, Flor.: Psychological Assessment Resources; 1995. [Google Scholar]
- 26.Delis DC, Kramer JH, Kaplan E, Ober BA. CVLT-II: California verbal learning test: adult version. Psychological Corporation; 2000. [Google Scholar]
- 27.Wicklund AH, Johnson N, Weintraub S. Preservation of reasoning in primary progressive aphasia: further differentiation from Alzheimer’s disease and the behavioral presentation of frontotemporal dementia. J Clin Exp Neuropsychol. 2004. May;26(3):347–55. [DOI] [PubMed] [Google Scholar]
- 28.Reitan RM. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Percept Mot Skills. 1958;8(3):271–6. [Google Scholar]
- 29.Chelune GJ, Naugle RI, Lüders H, Sedlak J, Awad IA. Individual change after epilepsy surgery: practice effects and base-rate information. Neuropsychology. 1993;7(1):41–52. [Google Scholar]
- 30.Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini-Mental State Examination. J Neurol Neurosurg Psychiatry. 2007. December;78(12):1298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988. September;44(3):837–45. [PubMed] [Google Scholar]
- 32.Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012. March;27(3):349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalrymple-Alford JC, MacAskill MR, Nakas CT, Livingston L, Graham C, Crucian GP, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010. November;75(19):1717–25. [DOI] [PubMed] [Google Scholar]
- 34.Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009. November;73(21):1738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu MT, Szewczyk-Królikowski K, Tomlinson P, Nithi K, Rolinski M, Murray C, et al. Predictors of cognitive impairment in an early stage Parkinson’s disease cohort. Mov Disord. 2014. March;29(3):351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L, Yu C, Zhang N, Liu J, Liu W. Cognitive impairment in patients with Parkinson’s disease: A 30-month follow-up study. Clin Neurol Neurosurg. 2016. December;151:65–9. [DOI] [PubMed] [Google Scholar]
- 37.Tröster AI, Woods SP, Morgan EE. Assessing cognitive change in Parkinson’s disease: development of practice effect-corrected reliable change indices. Arch Clin Neuropsychol. 2007. August;22(6):711–8. [DOI] [PubMed] [Google Scholar]
- 38.Hindle JV, Martyr A, Clare L. Cognitive reserve in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2014. January;20(1):1–7. [DOI] [PubMed] [Google Scholar]
- 39.Hindle JV, Hurt CS, Burn DJ, Brown RG, Samuel M, Wilson KC, et al. The effects of cognitive reserve and lifestyle on cognition and dementia in Parkinson’s disease—a longitudinal cohort study. Int J Geriatr Psychiatry. 2016. January;31(1):13–23. [DOI] [PubMed] [Google Scholar]
- 40.Perneczky R, Drzezga A, Boecker H, Ceballos-Baumann AO, Granert O, Förstl H, et al. Activities of daily living, cerebral glucose metabolism, and cognitive reserve in Lewy body and Parkinson’s disease. Dement Geriatr Cogn Disord. 2008;26(5):475–81. [DOI] [PubMed] [Google Scholar]
- 41.Caspell-Garcia C, Simuni T, Tosun-Turgut D, Wu IW, Zhang Y, Nalls M, et al. ; Parkinson’s Progression Markers Initiative (PPMI). Multiple modality biomarker prediction of cognitive impairment in prospectively followed de novo Parkinson disease. PLoS One. 2017. May;12(5):e0175674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooley SA, Heaps JM, Bolzenius JD, Salminen LE, Baker LM, Scott SE, et al. Longitudinal change in performance on the Montreal Cognitive Assessment in older adults. Clin Neuropsychol. 2015;29(6):824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gélinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. Am J Occup Ther. 1999. Sep-Oct;53(5):471–81. [DOI] [PubMed] [Google Scholar]
- 44.Rios Romenets S, Anang J, Fereshtehnejad SM, Pelletier A, Postuma R. Tango for treatment of motor and non-motor manifestations in Parkinson’s disease: a randomized control study. Complement Ther Med. 2015. April;23(2):175–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


