Abstract
Locally administered steroids have a long history in ophthalmology for the treatment of inflammatory conditions. Anterior segment conditions tend to be treated with topical steroids whilst posterior segment conditions generally require periocular, intravitreal or systemic administration for penetration. Over recent decades, the clinical applications of periocular steroid delivery have expanded to a wide range of conditions including macular oedema from retino‐vascular conditions. Formulations have been developed with the aim to provide practical, targeted, longer‐term and more efficacious therapy whilst minimizing side effects. Herein, we provide a comprehensive overview of the types of periocular steroid delivery, their clinical applications in ophthalmology and their side effects.
Keywords: corticosteroid, dexamethasone, Fluocinolone acetonide, prednisolone acetate, triamcinolone acetonide
1. INTRODUCTION
The first use of corticosteroids in ophthalmology by Gordon and McLean1 in the 1950s was a landmark event that revolutionized the management of inflammatory eye disease. The following decades led to further research into the mechanisms and immunological pathways within the eye, as well as the development of various forms of steroid that are locally administered in clinical practice today. Variations in ocular steroid delivery sites, dosages and preparations have all improved efficacy and durability whilst minimizing side effects. Despite development of systemic immunomodulatory (steroid‐sparing) agents and intravitreal monoclonal antibodies, locally administered steroids continue to retain a fundamental role in the management of many ophthalmic diseases. This paper reviews the mechanism of action, preparations, indications and side effects of locally administered steroids.
2. STEROID SUBTYPES AND MECHANISM OF ACTION
Steroids are organic compounds with 17 core carbon atoms bonded in three fused cyclohexane and one fused cyclopentane ring. The main two groups are corticosteroids (glucocorticoids and mineralocorticoids) and sex steroids (progestogens, androgens and estrogens; Figure 1).2
Figure 1.
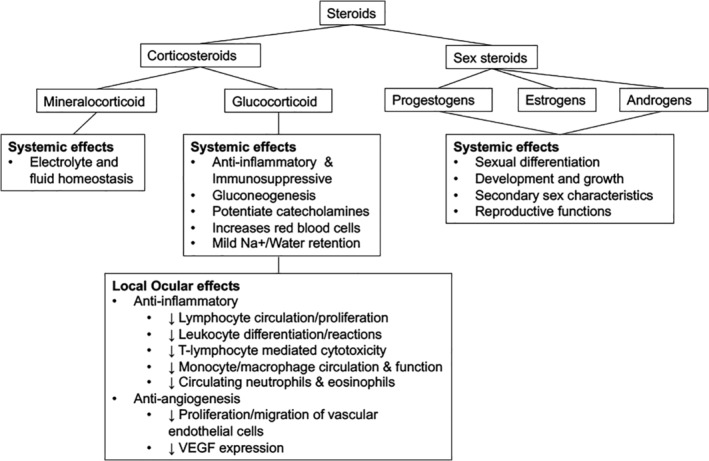
Classification of steroids and actions
Most steroids used in ophthalmology are glucocorticoids, which have anti‐inflammatory and immunosuppressive activity. The synthetic steroid prednisolone has both glucocorticoid and mineralocorticoid receptor activity, whilst the other three main ocular steroids (triamcinolone acetonide [TA], dexamethasone acetonide [DA] and fluocinolone acetonide [FA]) are predominantly active against glucocorticoid receptors (Table 1).2
Table 1.
Potency in receptor activation determined in engineered human HeLa cells3
| Glucocorticoid receptor activation potency HeLa cells | Mineralocorticoid receptor activation potency HeLa cells | |||
|---|---|---|---|---|
| Absolute (nM) | Relative to Cortisol | Absolute (nM) | Relative to cortisol | |
| Short acting | ||||
|
72 | 100% | 0.04 | 100% |
| Intermediate‐acting | ||||
|
8 | 900% | 0.015 | 267% |
|
1 | 7200% | >100 | <0.04% |
| Long‐acting | ||||
|
3 | 2400% | 0.3 | 13% |
|
0.4 | 18 000% | >100 | <0.04% |
The therapeutic effect of glucocorticoids are mediated via the glucocorticoid receptor in the cytosol which upon activation, undergoes conformational changes and translocate toward the cell nucleus. This activated glucocorticoid receptor signals the transactivation or trans‐repression of gene transcription factors which cause both therapeutic and side effects. There are over 40 distinct isoforms of the glucocorticoid receptor which have varying distribution within the tissues of the eye, each with different downstream signalling effects allowing for diverse cell‐specific actions.4
The anti‐inflammatory effect of steroids is caused by inhibiting the transcription of inflammatory and immune genes. These actions block the release of arachidonic acid and its subsequent eicosanoids (prostaglandins, thromboxanes, prostacyclins and leukotrienes).5 This affects the blood‐retinal barrier with a reduction in fibroblast proliferation, collagen and scar formation, retinal oedema, fibrin deposition, capillary leakage, intraretinal migration of inflammatory cells and levels of vascular endothelial growth factor (VEGF).
3. STEROID PREPARATIONS AND METHODS OF LOCAL ADMINISTRATION TO TREAT OPHTHALMIC DISEASE
Glucocorticoids may be locally administered in the following ways: topical, sub‐conjunctival, periocular (sub‐Tenon, orbital floor, peribulbar) and intravitreal (Figure 2). Regional administration allows for high levels of ocular delivery (intravitreal steroid bypasses the blood‐retinal barrier) whilst minimizing systemic side effects. An overview of steroid preparations and their local delivery methods are presented in Table 2.
Figure 2.
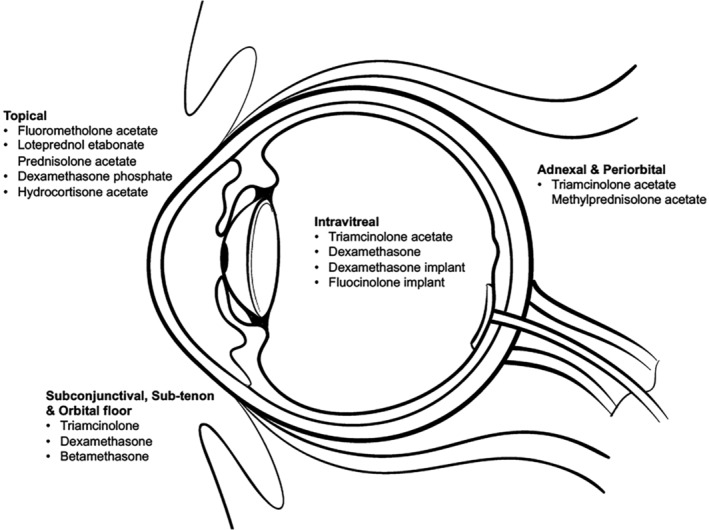
Common locally administered ophthalmic steroids
Table 2.
Ocular steroid preparations and their delivery sites, generic name (trade name)
| 1. Topical |
Dexamethasone sodium phosphate 0.1% (MAXIDEX, Decadron) Dexamethasone sodium phosphate ointment 0.05% (Dexadron) Prednisolone acetate 1% (Pred Forte, Econopred Plus, AK‐Tate) Prednisolone acetate 0.12% (Pred Mild, Econopred) Prednisolone sodium phosphate 1% (Inflamase Forte, AK‐Pred) Prednisolone sodium phosphate 0.5% (Prednisolone Minims, Metreton) Prednisolone phosphate 0.5%, 0.25% ointment (Hydeltrasol) Fluorometholone alcohol 0.1% or 0.25% suspension (FML Forte, FML, FML liquifilm) Fluorometholone ointment 0.1% (FML SOP) Fluorometholone acetate 0.1% (FLAREX) Hydrocortisone acetate 1% ointment (Siguent Hycor) Medrysone 1% suspension (HMS) Rimexolone 1% (Vexol) Medroxyprogesterone acetate 1% (Provera) Loteprednol etabonate 0.5% (Lotemax, Alrex) Difluprednate 0.05% emulsion (Durezol) |
| 2. Sub‐conjunctival |
Hydrocortisone 100 to 1000 mg powder (hydrocortisone sodium succinate) Methylprednisolone sodium succinate 40 mg/mL, 125 mg/mL, 2 g/40 mL solution (Solu‐Medrol) Methylprednisolone acetate 40 mg/mL (Depo‐Medrol) Triamcinolone diacetate 25 to 40 mg/mL suspension (Aristocort) Triamcinolone acetonide 10 to 40 mg/mL suspension (Kenalog, Kenacort‐A 10, Kenacort‐A 40) Triamcinolone acetonide 40 mg/mL (Triescence) Dexamethasone acetate 6 to 16 mg/mL (Decadron‐LA) Betamethasone acetate and sodium phosphate 3 mg/mL suspension (Celestone Soluspan) |
| 3. Periocular (intra‐lesional [eyelids], Juxtascleral, sub‐Tenon, orbital floor, peribulbar) |
Hydrocortisone 100 to 1000 mg powder (hydrocortisone sodium succinate) Methylprednisolone sodium succinate 40 mg/mL, 125 mg/mL, 2 g/40 mL solution (Solu‐Medrol) Methylprednisolone acetate 20 to 80 mg/mL (Depo‐Medrol) Triamcinolone diacetate 25 to 40 mg/mL suspension (Aristocort) Triamcinolone acetonide 10 to 40 mg/mL suspension (Kenalog, Kenacort‐A 10, Kenacort‐A 40) Triamcinolone acetonide 40 mg/mL (Triescence) Dexamethasone 0.4 mg implant (Dextenza), inserted into lacrimal puncta Dexamethasone acetate 6 to 16 mg/mL (Decadron‐LA) Dexamethasone sodium phosphate 4, 10, 24 mg/mL solution (Decadron Phosphate) Betamethasone acetate and sodium phosphate 3 mg/mL suspension (Celestone Soluspan) |
| 4. Intravitreal |
Triamcinolone acetonide 10 to 40 mg/mL suspension (Kenalog, Kenacort‐A 10, Kenacort‐A 40) Triamcinolone acetonide 40 mg/mL (Triescence) Dexamethasone solution 9% (DEXYCU) Dexamethasone 0.7 mg implant (OZURDEX) Fluocinolone acetonide 0.19 mg implant (Iluvien) Fluocinolone acetonide 0.18 mg implant (Yutiq) Fluocinolone acetonide 0.59 mg implant (Retisert) |
3.1. Topical
Topical steroids are used to treat inflammation of the conjunctiva, cornea and the anterior segment. In certain circumstances they can also be useful in treating uveitic or postoperative macular oedema. Penetration into the aqueous humour occurs by diffusion across the cornea.6
Dexamethasone is approximately 25 to 30 times intrinsically more potent than hydrocortisone.7 However, the efficacy of each preparation depends not only on the drug's intrinsic potency, but its penetration and durability. Acetate preparations are more lipophilic than those with phosphate preparations, and hence have greater corneal penetration.8 Although prednisolone acetate is six times less potent on a molar basis than dexamethasone or betamethasone, due to the acetate preparation, topical prednisolone acetate 1% provides greater anti‐inflammatory effect than either dexamethasone or betamethasone phosphate 0.1%.9 Solutions with preservatives also have greater penetrance than those without, as the preservative disrupts tight junctions between corneal epithelial cells. The frequency of application also increases the anti‐inflammatory effect. A study on corneal inflammation demonstrated greater anti‐inflammatory effects when topical prednisolone acetate is applied every 15 minutes (or five doses at 1 minute intervals each hour) versus hourly.10 It is important that suspensions are shaken immediately prior to use, otherwise the administered dosage will vary.
3.2. Sub‐conjunctival
Sub‐conjunctival steroids are frequently administered at the conclusion of intraocular surgery. The most common preparation used is dexamethasone, although methylprednisolone may also be given.11 Dexamethasone has been shown to achieve good ocular penetration following sub‐conjunctival injection, with higher levels of concentration in the aqueous and vitreous than when it is administered as a peribulbar injection or orally.12 Sub‐conjunctival TA has been shown to be efficacious and safe for anterior uveitis and non‐necrotising, non‐infectious anterior scleritis.13, 14
3.3. Periocular
Sub‐Tenon, orbital floor and peribulbar steroids are frequently used to treat ocular inflammatory conditions, particularly when there is associated macular oedema and in whom systemic side effects are less desirable. After 30 days following a single sub‐Tenon injection of 40 mg of TA, corticosteroid levels can be found in all ocular tissues, with highest levels within the choroid and retinal pigment epithelium, whilst systemic levels remain low.15 The drug of choice is usually TA, formulations of which include Kenacort and Triesence.
Posterior juxtascleral depot injection of anecortave acetate was previously used to treat choroidal neovascularisation. Anecortave acetate (Retaane) is a synthetic angiostatic steroid that was formulated to be devoid of glucocorticoid receptor‐mediated activity. It was delivered as a posterior juxtascleral depot every 6 months. Although its efficacy in neovascular age‐related macular degeneration (AMD) was shown against placebo,16 it did not strongly demonstrate significant benefit against photodynamic therapy with verteporforin.17 The role of anecortave acetate was soon superceded by the emergence of intravitreal anti‐VEGF.
3.4. Intravitreal
Steroids are most potent against retinal disease when delivered intravitreally. Intravitreal steroids are used for macular oedema, uveitis and to stain the vitreous during intraocular surgery for improved visualization. As the procedure involves globe penetration, it must be done under aseptic conditions. It may be given as an intravitreal injection (Kenacort, Triesence), or as a slow‐release intravitreal implant (OZURDEX, Iluvien, Retisert).
3.4.1. Triamcinolone acetonide
TA is a minimally water‐soluble suspension. After intravitreal injection, triamcinolone crystals slowly dissolve into the vitreous. This creates a diffusional gradient from the vitreous to the macula with minimal systemic exposure. While a portion of the drug targets the macula, another portion either clears through the retina or diffuses to the anterior segment where it can cause cataract or elevation of intraocular pressures (IOPs).
Kenacort was formulated for intra‐articular and intramuscular injection and thus its application in ophthalmology is off‐label. In contrast, Triesence is a preservative‐free preparation of TA. The pharmacokinetics and pharmacodynamics of different TA preparations have been shown to differ in animal studies.18, 19 Since only dissolved free triamcinolone has a therapeutic effect, durability depends on multiple factors such as pH, particle size (smaller and more uniform for Triesence compared with Kenacort), crystallinity, solubility and dissolution kinetics in the vitreous.19 The duration of effect of intravitreal TA (IVTA) lasts between 320 and 621 months in non‐vitrectomised eyes, but is up to six times shorter in vitrectomised eyes.20
Kenacort comes in two dosages: Kenacort‐A 10 (10 mg/ml) and Kenacort‐A 40 (40 mg/ml). As its use in ophthalmology is off‐label, no specific dosage is recommended however most studies for diabetic macular oedema (DMO) have injected 4 mg in 0.1 mL. The SCORE studies for macular oedema secondary to retinal vein occlusion showed no significant differences between the 1 mg/0.1 mL and 4 mg/0.1 mL preservative‐free IVTA (Trivaris Allergan, Inc., Irvine, California) arms.22, 23 The manufacturer of Triesence (Alcon) recommends an initial dosage of 4 mg/0.1 ml for therapeutic purposes, and 1 to 4 mg for visualization during vitrectomy.24
3.4.2. Dexamethasone intravitreal implant
OZURDEX is a biodegradable intravitreal implant that contains 0.7 mg dexamethasone in a NOVADUR solid rod‐shaped polymer drug delivery system. It is designed to release drug over 3 to 6 months in a biphasic fashion with higher doses in the initial 6 weeks followed by lower doses for up to 6 months. It is injected using a single‐use intravitreal applicator with a stepped technique. It is used to treat DMO, macular oedema due to branch or central retinal vein occlusion (BRVO, CRVO) and non‐infectious posterior uveitis.
OZURDEX is contraindicated if there is active ocular infection, hypersensitivity to the drug, advanced glaucoma or posterior lens capsule rupture.25 In vitrectomised eyes, there may be an advantage in using the dexamethasone intravitreal implant (DII) over other bolus intravitreal therapies which have a reduced half‐life.20 Rabbit‐studies have shown no difference in clearance rates of DII in vitrectomised and non‐vitrectomised eyes.26 The efficacy of DII has also been shown to be similar in vitrectomised and non‐vitrectomised eyes when used to treat macular oedema secondary to CRVO.27
3.4.3. FA implant
Fluocinolone acetonide implants are synthetic corticosteroid with low solubility in aqueous allowing extended drug release.
Iluvien is an injectable intravitreal 0.19 mg implant within a rod‐shaped (3.5 × 0.37 mm) non‐biodegradable reservoir that has a duration of action of 18 to 36 months. The FAMOUS study demonstrated a sustained release by measuring aqueous concentrations, with levels of slightly more than 2 ng/mL for the first 3 months followed by maintained concentrations of 0.5 to 1.0 ng/mL from 6 to 36 months.28
Retisert is a non‐biodegradable disc‐shaped intravitreal implant containing 0.59 mg of FA within a silicone elastomer. It is surgically inserted through a pars plana incision and removed by a second surgical procedure. It has a duration of 18 to 30 months with an initial active drug release of 0.6 μg/day to an eventual steady‐state release of 0.3 to 0.4 μg/day for 30 months.29
Yutiq is a recently developed intravitreal implant containing 0.18 mg of FA which is designed to deliver sustained release for up to 36 months. Fluocinolone is contained in the core of a polyamide polymeric cylinder (3.5 × 0.37 mm) with a permeable polyvinyl alcohol membrane. It is injected by a pre‐loaded sterile applicator with a 25‐gauge needle. The drug delivers an approximate initial rate of 0.2 μg daily followed by 0.1 μg daily over 36 months.30
4. USES OF LOCALLY ADMINISTERED STEROIDS
An outline of studies on the local delivery of corticosteroids in clinical ophthalmology is presented in Table 3.
Table 3.
Outline of studies on local delivery of corticosteroids in clinical ophthalmology
| Conditions | Authors, (Year) | Design | No. of eyes/(patients) | Treatment steroid | Study aims and outcome measures | Conclusions |
|---|---|---|---|---|---|---|
| Adnexal | ||||||
| TED |
Bordaberry et al, (2009)31 |
RCT | 21 |
|
To assess the efficacy of peribulbar TA to treat inflammatory signs of moderate to severe Graves' orbitopathy and associated optic neuropathy
|
Peribulbar TA reduced inflammatory signs of moderate Graves' orbitopathy as measured by the clinical activity score |
|
Ebner et al, (2004)32 |
Multi‐centre RCT | 41 |
|
To assess the efficacy of peribulbar TA vs control to treat TED
|
Peribulbar TA is effective in reducing diplopia and extraocular muscle size in TED | |
|
Alkawas et al, (2010)226 |
RCT | 12 |
|
To assess the efficacy of peribulbar TA vs oral prednisolone to treat TED
|
No statistical difference found in study sample between peribulbar TA and oral prednisolone in treating TED | |
| Lee et al, (2013)35 | Single‐blinded RCT | (106) |
|
To assess the efficacy of sub‐conjunctival TA in treatment of TED related lid retraction
|
Sub‐conjunctival TA was effective in treating TED related lid retraction and persisted through to 24 weeks of follow‐up | |
| Nasolacrimal disease | McNeill et al, (2005)44 | RCT | 11 |
|
To assess the efficacy of nasal corticosteroids in treating functional epiphoria in patients with rhinitis
|
Epiphoria secondary to rhinitis can be treated successfully with intranasal beclomethasone |
| Chalazia | Goawalla and Lee, (2007)46 | RCT | 136 |
|
To compare intra‐lesional TA, incision and curettage and hot compresses in the treatment of chalazia
|
Resolution rates between intra‐lesional TA and incision and curettage were similar and both were significantly greater than conservative group. There was less pain and patient inconvenience with intra‐lesional TA compared to incision and curettage |
| Ben Simon et al, (2011)47 | RCT | 94 |
|
To compare intra‐lesional TA against incision and curettage for the treatment of chalazia
|
Intra‐lesional TA was as effective as incision and curettage in primary chalazia | |
| Anterior segment | ||||||
| Bacterial keratitis | Srinivasan et al, (2012)60 | Multi‐centre placebo‐controlled double‐blinded RCT |
500 (3 mo) 399 (12 mo) |
|
To compare the benefit in clinical outcomes of adjunctive topical corticosteroids in the treatment of bacterial corneal ulcers
|
No significant differences in clinical outcomes with topical prednisolone sodium phosphate 1% compared to placebo in non‐Nocardia species. Ulcers caused by Nocardia may fare worse with topical steroids |
| HSK | Wilhelmus et al, (1994)62 | Multi‐centre placebo‐controlled double‐blinded RCT | 106 |
|
To compare the benefit in clinical outcomes of adjunctive topical corticosteroids in the treatment of HSV keratitis
|
Topical prednisolone phosphate was significantly better than placebo in reducing persistence or progression of stromal inflammation (by 68%) |
| Allergic eye disease | Singh et al, (2001)69 | Double‐blinded RCT | 90 (45) |
|
To compare three types of supratarsal steroid injections for the treatment of refractory VKC
|
All three drugs were equally effective with no statistically significant difference in the time of resolution. Recurrence was seen within six in all cases irrespective of the steroid used |
| Saini et al, (1999)70 | Double‐blinded RCT | 38 (19) |
|
To compare supratarsal TM vs supratarsal DM for the treatment of refractory VKC
|
Both were equally effective in controlling symptoms and signs however supratarsal TM had a lower recurrence rate | |
| KCS | Pflugfelder et al, (2004)81 | Multi‐centre double‐blinded RCT | 128 (64) |
|
To assess the efficacy of loteprednol etabonate 0.5% vs placebo for KCS
|
Topical loteprednol etabonate may be beneficial in KSC with moderate clinical inflammation |
| Sheppard et al, (2014)84 | Multi‐centre double‐blinded RCT | (119) |
|
To assess the efficacy of loteprednol etabonate 0.5% with topical cyclosporin 0.05% in dry eye disease
|
Loteprednol showed greater efficacy in dry eye signs and symptoms than topical cyclosporin or artificial tears alone. It also provided rapid relief of dry eye disease | |
| Lin and Gong, (2015)86 | Multi‐centre double‐blinded RCT | (41) |
|
To compare topical FML vs cyclosporin A for the treatment of dry eye disease in Sjogren syndrome
|
Both medications gave similar improvement from baseline, however topical FML provided faster improvement in symptoms of ocular dryness | |
| Pinto‐Fraga et al, (2016)87 | Double‐blinded RCT | (42) |
|
To assess the efficacy of topical FML in dry eye disease when exposed to adverse environments
|
Topical FML was effective in alleviating dry eye disease but also especially in preventing exacerbation caused by exposure to a desiccating stress | |
| GVHD | Yin et al, (2018)89 | Double‐blinded RCT | 42 |
|
To assess the efficacy of topical loteprednol in dry eye disease associated with GVHD
|
Topical loteprednol had a less favourable response in treating dry eye disease in GVHD compared to those without GVHD |
| Chemical Injury | Brodovsky et al, (2000)93 | Retrospective series | 177 (121) |
|
To compare treatment outcomes of a standard protocol of intensive treatment vs conservative management in alkali‐burned corneas
|
Patients with intensive treatment had a trend for rapid healing and better final visual outcomes in grade 3 chemical burns but no difference in grade 4 burns |
| Anterior scleritis | Sohn et al, (2011)13 | Retrospective multi‐centre cohort | 68 (53) |
|
To assess the efficacy of sub‐conjunctival TA for non‐necrotising anterior scleritis
|
After one injection sub‐conjunctival TA gave improvement of symptoms and signs in 97% and eyes remained recurrence‐free in 67.6% at 24 mo. Sub‐conjunctival TA is a useful adjuvant therapy that may reduce the burden of systemic medication |
| Glaucoma surgery | ||||||
| Glaucoma filtration surgery | Araujo et al, (1995)100 | RCT | 46 (35) |
|
To compare no adjunctive steroids vs topical prednisolone vs topical prednisolone and oral steroids in glaucoma filtration surgery after 10 y
|
Patients treated with steroids (groups 2 and 3) had significantly improved outcomes compared with patients without steroids (group 1). Group 1 had more additional procedures, higher IOPs, more additional glaucoma drops and lower rate of stabilized glaucoma |
| Yuki et al, (2009)102 | RCT | 53 |
|
To assess the efficacy of intraoperative sub‐Tenon TA on the success rate of trabeculectomy in secondary glaucoma
|
Intraoperative sub‐Tenon TA neither increased intermediate‐term success nor decreased postoperative complications | |
| Breusegem et al, (2010)227 | RCT | 54 |
|
To compare preoperative treatment of topical ketorolac or FML vs placebo on trabeculectomy outcomes
|
Use of topical ketorolac or fluorometholone 1 mo prior to trabeculectomy was associated with less likelihood of postoperative needling and less need for IOP‐lowering medication | |
| Yazdani et al, (2017)110 | Triple‐blinded RCT | 90 |
|
To compare intraoperative sub‐Tenon TA vs without in Ahmed glaucoma valve implantation
|
Sub‐Tenon IOP resulted in a lower mean IOP at the first mo and was 1.5 mmHg lower throughout the study period. Peak postoperative IOP was also lower. The rates of success, occurrence of hypertensive phase and complications were similar between the two groups | |
| Posterior segment | ||||||
| DMO | Gillies et al, (2006)115 | Double‐blinded RCT | 69 (43) |
|
To assess the efficacy of outcomes of IVTA in the treatment of refractory DMO
|
IVTA had significantly greater proportion of patients (56%) achieving ≥15 letters of improvement in BCVA than placebo (26%). IVTA was also found to reduce central macular thickening however adverse events included cataract and glaucoma |
|
Bressler et al, (2010)117 DRCR Protocol I |
Multi‐centre double‐blinded RCT | 828 |
|
To compare intravitreal ranibizumab plus prompt or deferred laser vs prompt laser or IVTA plus prompt laser in DMO
|
Eyes receiving initial ranibizumab for centre‐involving DMO had better long‐term vision and reduced central subfield thickness | |
|
Boyer et al, (2014)118 MEAD study |
Two identical, parallel multi‐centre double‐blinded RCT | (1048) |
|
To assess safety and efficacy of DII in the treatment of DMO
|
DII had significantly greater proportion of patients achieving ≥15‐letters of improvement in BCVA (22.2% for 0.7 mg, 18.4% for 0.35 mg and 12.0% for sham) | |
|
Fraser‐Bell et al, (2016)120 BEVORDEX study |
Multi‐centre single‐blinded RCT | 88 (61) |
|
To compare DII vs intravitreal bevacizumab for the treatment of DMO
|
DII achieved similar rates of BCVA improvement with bevacizumab and superior anatomic outcomes with fewer injections at 12 mo. At 24‐mo, there was no significant difference of improvement in BCVA but less burden of injections | |
| Callanan et al, (2013)189 | Double‐blinded multi‐centre RCT | 253 |
|
To compare DII combined with laser photocoagulation compared with laser alone for treatment of diffuse DMO
|
DII combined with laser resulted in significantly greater mean improvement in BCVA at all time points through month 9. Combination treatment also reduced areas of diffuse vascular leakage on angiography. At 12 mo, there was no significant difference between the two groups | |
|
Campochiaro et al, (2011)121 FAME A and B |
Two identical parallel, multi‐centre double‐blinded RCT | 392 |
|
To compare efficacy and safety of IVFA implants for treatment of DMO
|
Low‐dose and high‐dose IVFA implant groups had greater percentage of patients with ≥15 letters of improvement in BCVA at 24 mo (28.7% and 28.6%) compared with sham (16.2%). There was also more improvement in foveal thickness compared to sham. A significant percentage (7.6%) of the high‐dose group required incisional glaucoma surgery | |
| CMO in RVO |
Ip et al, (2009)22 SCORE‐CRVO |
Multi‐centre RCT | 271 |
|
To assess the efficacy and safety of IVTA for treatment of macular oedema secondary to central retinal vein occlusion
|
IVTA had significantly greater proportion of patients with ≥15 letter improvement in BCVA (27% for 1 mg, 26% for 4 mg and 7% for sham). Superior safety profile of 1 mg dose compared with 4 mg dose IVTA with respect to glaucoma and cataract |
| Scott et al, (2009)23 SCORE‐BRVO | Multi‐centre RCT | 411 |
|
To assess the efficacy and safety of IVTA for treatment of macular oedema secondary to branch retinal vein occlusion
|
Treatment with IVTA with 1 mg or 4 mg or standard of care did not demonstrate a significant difference in visual acuity outcomes in macular oedema secondary to branch retinal vein occlusion | |
|
Haller et al, (2010)123 GENEVA |
Two identical, parallel multi‐centre double‐blinded RCT | 1267 |
|
To assess the efficacy for DII for treatment of macular oedema secondary to CRVO or BRVO
|
DII had significantly greater proportion of patients with ≥15 letter improvement in BCVA, mean BCVA and less proportion of patients losing ≥15 letters in BCVA | |
| Posterior non‐infectious uveitis |
Sen et al, (2014)140 SITE |
Retrospective review of multi‐centre cohort | 1192 (914) | Periocular corticosteroid (including sub‐Tenon and orbital floor) | To assess the efficacy and safety of periocular corticosteroid injections in uveitis
|
Over 50% of eyes demonstrated improved VA at some point within 6 mo of receiving periocular steroid. Periocular corticosteroids were also effective in treating acute inflammation or macular oedema |
|
Kempen et al, (2015)141 MUST study |
Multi‐centre RCT | 479 (255) |
|
To compare IV FA implant with systemic immunosuppression in the treatment of posterior non‐infectious uveitis
|
No significant difference in BCVA at 2 and 5 y. Systemic immunosuppression had better BCVA outcome at 7 y | |
| Jaffe et al, (2019)30 | Multi‐centre, double‐blinded sham‐controlled RCT | 129 |
|
To assess efficacy and safety of IVFA implant on recurrence rates in chronic posterior non‐infectious uveitis
|
IVFA provided a greater proportion of patients with ≥15 letter improvement as well as effective management of intraocular inflammation and lower recurrence rates during the first 12 mo | |
|
Lowder et al, (2011)146 HURON study |
Parallel‐group, multi‐centre, blinded RCT | 229 |
|
Efficacy of DII on treating inflammation and CMO in non‐infectious posterior uveitis or panuveitis
|
Both doses of DII showed a significant reduction of posterior inflammation and CMO compared to sham which persisted through week 25. The proportion of patients with ≥15 letter improvement of BCVA was also significantly higher in the DII groups compared to sham | |
|
Thorne et al, (2019)228 POINT trial |
Multi‐centre, parallel‐treatment comparative RCT | 235 |
|
To compare the efficacy of periocular TA, IVTA and OZURDEX for treatment of uveitic macular oedema
|
Improvements of CMT were seen in all three groups, periocular TA (23%), IVTA (39%), OZURDEX (46%). Greater improvements in BCVA were also seen with IVTA and OZURDEX. No significant differences between IVTA and OZURDEX in central subfield thickness or BCVA | |
| Das et al, (1999)151 | RCT | 63 |
|
To compare adjunctive IVDM vs intravitreal antibiotics‐only during vitrectomy for suspected postoperative or post‐traumatic bacterial endophthalmitis
|
A reduction of inflammation was observed in the IV DM group at 1 week and 1 mo (although topical corticosteroids were not given in the intravitreal antibiotic‐only group). Final visual outcomes at 3 mo were not significantly different | |
| Gan et al, (2005)152 | RCT | 29 |
|
To compare adjunctive IVDM vs intravitreal antibiotics alone in postoperative endophthalmitis
|
No statistically significant difference on visual acuity at 3 and 12 mo between the two groups. Trial terminated prematurely due to the study drug (dexamethasone sodium diphosphate was no longer available) | |
| Albrecht et al, (2011)153 | Double‐masked RCT | 62 |
|
To compare adjunctive IV DM vs intravitreal antibiotics alone in presumed bacterial endophthalmitis
|
No statistically significant difference in visual outcomes in short‐term (2 weeks) or intermediate‐term (2–4 mo post‐treatment) between the two groups | |
| Manning et al, (2018)154 | Multi‐centre RCT | 167 |
|
To compare adjunctive IVDM vs intravitreal antibiotics alone in patients with suspected bacterial endophthalmitis post‐cataract surgery
|
No statistically significant difference in final visual outcomes between IVDM and placebo group | |
| Postoperative CMO | Konstantopoulos et al, (2008)158 | Retrospective case series | 21 (20) |
|
To assess efficacy and safety of IVTA in postoperative CMO
|
All patients had significantly improved BCVA from baseline which was maintained at 6 mo |
| Thach et al, (1997)229 | Retrospective review | 49 (48) |
|
To compare the retrobulbar TA vs posterior sub‐Tenon's TA for pseudophakic CMO refractory to topical medications
|
There was significant improvement in BCVA compared to baseline for both groups but no statistically significant difference was found between the two groups | |
Abbreviations: CMO, cystoid macular oedema; DM, dexamethasone; DMO, diabetic macular oedema; FML, fluoromethalone; HC, hydrocortisone; HSK, herpes simples keratitis; IV, intravitreal; JXH, Juvenile xanthogranuloma; KCS, keratoconjunctivits sicca; LCH, Langerhan's cell histiocytosis; MP, methylprednisolone; PA, prednisolone acetate; RCT, Randomized Controlled Clinical Trial; RVO, retinal vein occlusion; TA, triamcinolone; TED, thyroid eye disease; VKC, vernal keratoconjunctivitis.
4.1. Ocular adnexae‐eyelids, lacrimal gland and orbit
Oculoplastic uses of topical or intra‐lesional steroids is limited to a few conditions.
4.1.1. Thyroid eye disease
Peribulbar and intra‐orbital steroids have been used in management of active thyroid eye disease (TED). Two randomized studies, with small patient numbers, showed reduction in clinical activity score and extraocular muscle size.31, 32 Both studies required multiple peribulbar injections of TA 20 mg. There were few reported side effects‐only two cases developed raised IOP. Although they can have an adjunctive role in active TED, they are not first‐line therapy.
Peribulbar and sub‐conjunctival steroids have also been used for upper eyelid retraction in TED.33, 34, 35, 36 Studies show that repeat injections of 20 mg of TA are required (one to four injections at monthly intervals) and the response rate is better in patients with recent onset of upper lid retraction or active disease. Xu et al37 noted an improvement in 83.3% of patients with symptom duration less than 6 months, compared to 36.4% who responded if symptoms were greater than 6 months. Joos et al36 showed that a superior orbital peri‐levator injection technique improved lid retraction and demonstrated reduction in size of the levator/superior rectus complex on MRI imaging after repeat injections.
4.1.2. Histiocytic orbital lesions
Histiocytic lesions are divided into Langerhans cell histiocytosis (LCH) and non‐LCH lesions, of which Juvenile Xanthogranuloma (JXG) is the most common. Langerhans cell histiocytosis can be monofocal (often seen in the frontal bone of the orbit), or systemic. It often presents in children, but can occur in all age groups. It results in bone destruction and secondary soft tissue expansion.38 The treatment of choice for orbital LCH is incisional biopsy with curettage of the lesion and intra‐lesional steroids (triamcinolone 40 mg/mL or methylprednisone).39, 40 JXG often presents with cutaneous involvement. Eyelid and orbital lesions are rare and can be managed with a combination of intra‐lesional steroids with or without surgical debulking.41
4.1.3. Periorbital capillary haemangiomas
The treatment of choice for periorbital infantile haemangiomas has traditionally been intra‐lesional and systemic steroids. In 2008 Léaute‐Labrèze et al42 published a series of 11 cases of infantile haemangioma cases managed with propranolol which has revolutionized treatment. Two systematic reviews show both intra‐lesional steroids and propranolol are effective, though less side effects occur with propranolol.37, 43 Propranolol is now the mainstay of treatment for periorbital infantile haemangioma and intra‐lesional steroids are used as an adjunct in resistant cases.
4.1.4. Nasolacrimal disease
Topical steroid drops and steroid nasal spray have been used in management of nasolacrimal duct obstruction (NLDO), with little evidence for their use. In functional NLDO with associated symptoms of rhinitis, topical steroid nasal spray (eg, mometasone and budesonide) may improve epiphora.44 The anecdotal improvement of epiphora and mucus discharge in complete NLDO with topical steroid drops is likely secondary to the anti‐inflammatory effect and reduced mucus in the lacrimal sac. Mansur et al45 recently assessed the lacrimal complications associated with systemic chemotherapy agents and suggested minor canalicular blockages may be effectively treated with probing and topical steroid drops. If there is a more significant blockage or likely long‐term chemotherapy, then lacrimal surgery is advised.45
4.1.5. Chalazia
Traditionally non‐resolving or large chalazia of the eyelids are treated by surgical incision. However, studies show equally effective results with 0.2 to 0.4 mL of 10 mg/mL intra‐lesional triamcinolone.46, 47 A meta‐analysis of randomized studies showed incision and curettage was more effective than steroid injection as a single procedure, but with repeat procedures similar outcomes were shown.48 Intra‐lesional steroid for chalazia is an acceptable treatment for primary and recurrent chalazia.
4.1.6. Periocular scarring
Hypertrophic scarring following surgery or trauma has traditionally been managed with a mixture of topical or intra‐lesional steroids or surgery. Recently, combination therapy of intra‐lesional triamcinolone and 5‐FU has shown great promise in improving periocular scarring post‐surgery.49
4.2. Anterior segment
Steroids are used frequently in anterior segment diseases,4 however there is a considerable lack of randomized control trials (RCTs) to guide treatment.
4.2.1. Corneal transplants
Corticosteroids are the principle medication in the management of corneal transplantation. They are readily absorbed through the cornea and achieve a high concentration in the anterior chamber through topical application. Prednisolone and dexamethasone are the most commonly used forms.50 Multiple treatment regimes exist, but as a guide prednisolone 1% or dexamethasone 0.1% drops are used every 2 hours initially, tapered over a period of 6 to 12 months, and a mild steroid used daily for maintenance treatment in endothelial keratoplasties.
Some have advocated for the use of steroids prior to high‐risk transplantation50, 51, 52, 53 but this has been variably adopted. A survey of the Bowman's Club (The UK Society of corneal surgeons) found that topical dexamethasone was used in 33%, oral prednisolone by 22% and single dose IV methylprednisolone (IVMP) by 14%.54
During corneal allograft rejection, topical, sub‐conjunctival, periocular and/or systemic corticosteroid use is the treatment of choice, with the majority of corneal specialists favouring topical formulations (with a preference for prednisolone acetate 1%).55, 56 In severe cases of allograft rejection oral or IVMP is often added, and one prospective study suggested that a single dose of 500 mg of IVMP is more effective and better tolerated than daily oral prednisolone.57 Unfortunately there are no RCTs to add weight to this study.
4.2.2. Bacterial keratitis
The greatest evidence for the use of steroids in bacterial keratitis come from the Steroids for Corneal Ulcers Trials (SCUT).58, 59, 60 A cochrane review of steroid use in bacterial keratitis61 found four RCTs that met inclusion criteria, but only the SCUT trial was of sufficient power to determine the effect of steroids in bacterial keratitis.
This SCUT trial examined the outcomes of 500 cases of culture‐positive bacterial keratitis where fungal, acanthamoeba, HSV, impending perforation and previous corneal transplant patients were all excluded. All cases received moxifloxacin q1h for 48 hours prior to randomization; at randomization, half the patients received prednisolone 1% for a total of 3 weeks only (QID for 1 week, BD for 1 week and daily for 1 week) compared to placebo. In both 3 and 12 months reports, there were no difference between groups in any parameters measured (best‐subjective corrected visual acuity [BSCVA], scar size, rate of re‐epithelialization, rate of perforation). This report added to the weight of evidence that steroids do not cause corneal perforation in bacterial keratitis. The IOP was lower in the steroid group at 3 months as inflammation was better controlled (p = .04).
However, subsequent subgroup analysis demonstrated a benefit for the use of steroids. In the 3‐month report, those with baseline BSCVA of CF or worse and those with an infiltrate covering the central 4 mm of the cornea performed better with early introduction of steroids compared to placebo (a two‐line difference in vision, p < .05) indicating that there is a benefit of steroids in severe, central infections in the early stage of recovery. At 12 months, when Nocardia infections were removed from the cohort, those who had steroids after 48 hours of antibiotic treatment had a one‐line improvement in BSCVA compared to those who did not have steroids.
4.2.3. Herpes simplex keratitis
Steroid use in herpes simples keratitis (HSK) is mainly for stromal and endothelial keratitis. Much of the evidence for the use of steroids in HSK comes from the double blind, placebo‐controlled RCT known as the Herpetic Eye Disease Study (HEDS).62 The HEDS demonstrated a clear benefit of the use of topical prednisolone in the treatment of stromal keratitis.62 Those on trifluridine plus prednisolone had a treatment failure rate of 26% compared to 73% on trifluridine plus placebo (p < .001). The study also demonstrated that a 10‐week tapering course of steroids was too brief as 50% developed a recurrence within 6 weeks. Thus, for non‐necrotising stromal keratitis without an epithelial defect, antiviral treatment in conjunction with topical steroids for at least 10 weeks is recommended.
Endothelial disease typically presents independently of other forms of HSV keratitis and only few studies are available to guide treatment.63, 64, 65 These compare topical betamethasone with topical acyclovir against topical acyclovir alone (all five times a day) and found that the addition of steroid resulted in a faster response and fewer treatment failures than antiviral alone. Thus, the recommendation for HSV endothelial disease is the combination of antiviral treatments with topical steroids, tapered according to patient signs and symptoms.
4.2.4. Allergic eye diseases
Allergic eye diseases cover a spectrum from seasonal allergic disease through to vernal keratoconjunctivitis (VKC) and atopic keratoconjunctivitis. Corticosteroids play an important role in controlling acute exacerbations; however, they should not be used as long‐term maintenance due to their side effects.66 In children with severe VKC, intraocular pressure rises have been reported in up to 28.3% of patients, with 5.5% progressing to glaucoma.67 Various regimes of topical steroids can be employed depending on severity of disease with early introduction of a steroid‐sparing agent when the patient is expected to require long‐term disease control.
Supratarsal injection of steroid is effective in refractory, severe and challenging cases of allergic eye diseases.68 Two prospective, randomized, double‐masked, case‐control trials showed no difference between dexamethasone sodium (2 mg) phosphate, TA (10‐20 mg) and hydrocortisone sodium succinate (50 mg) in improving severe refractory VKC with resolution of many symptoms by 3 weeks.69, 70 However, symptoms recurred about 12 weeks post‐treatment without anti‐allergy medication.69
4.2.5. Corneal neovascularization
Topical steroids are the mainstay of treatment for the suppression of early proliferating corneal vessels.71, 72, 73, 74, 75 They act primarily due to suppression of inflammation associated with new vessels and are not necessarily angio‐regressive.76 As such, steroids are most effective when applied before, or immediately after corneal injury.71
4.2.6. Keratoconjunctivitis sicca
Topical steroids have a role for treating keratoconjunctivitis sicca (KCS), as outlined in the Tear Film and Ocular Surface Society Dry Eye Workshop II (TFOS DEWS II) report.77 This report summarizes the currently available evidence on managing dry eye disease, including results from several RCTs,78, 79, 80, 81, 82, 83, 84, 85, 86, 87 and concluded that short courses of corticosteroid are effective in improving symptoms of KCS. However, this is not an effective long‐term strategy due to potential side effects. Typically, low strength steroids such as FML were used QID.
4.2.7. Graft vs host disease
Ocular involvement of Graft Versus Host Disease (GVHD) may cause an acute or chronic immunologically mediated inflammatory disease of the ocular surface. Whilst systemic corticosteroids are the mainstay of controlling the acute exacerbations of chronic GVHD, adjunctive topical steroids is often used to allow tapering and cessation of systemic immunosuppression. Small series have retrospectively demonstrated efficacy of topical steroid treatment in controlling acute conjunctival inflammation and reducing scarring, however signs of KCS remained.88
Long‐term topical steroids are not recommended after the acute inflammatory phase, when other anti‐inflammatory agents, such as cyclosporin A and tacrolimus may be employed. This is supported by a recent RCT of 42 patients that assessed dry eye disease in chronic GVHD. Topical loteprednol etabonate 0.5% was found to have a minimal effect in ocular surface disease index (OSDI) and corneal fluorescein staining compared to topical lubricants.89
4.2.8. Cicatrising conjunctival disorders
Chemical and thermal injury
The goal of therapy following chemical and thermal anterior segment injuries is to restore the ocular surface and maintain long‐term corneal clarity by preventing cicatrisation and limbal stem‐cell deficiency. Along with other important aspects of treatment, topical steroids may be used to limit the profound associated inflammation and promote healing. However, there has been controversy regarding the use and timing of topical corticosteroids.
Corticosteroids may have beneficial effects on inflammatory cell suppression and collagenase inhibition, however they may also suppress keratocyte migration and collagen production and thus cause corneal thinning.90 Generally, their anti‐inflammatory effect is maximal in the first week after which they should be tapered due to the risk of corneal thinning. Their use alone has been cautioned as it has been shown to cause further corneoscleral melt.91 Monitoring for infection or prophylactically adding topical antibiotics prior to epithelialisation is also recommended. One study found a risk of corneoscleral melting if topical steroids were used after 10 days of the chemical injury. The timing coincides where suppression of keratocyte collagen production by corticosteroids may outweigh the advantages of inflammatory cells suppression and collagenase inhibition to promote corneal ulceration.90 Davis et al and Brodovsky et al, found in their retrospective series that the prolonged use of topical prednisolone 0.5% used concurrently with topical ascorbate 10% was not associated with corneoscleral melt.92, 93
Ocular cicatricial pemphigoid/mucous membrane pemphigoid
Mucous membrane pemphigoid is a systemic disease primarily affecting mucous membranes. When localized to the conjunctiva, the condition is known as ocular cicatricial pemphigoid. It manifests as a chronically progressive conjunctival inflammation causing bilateral blindness. Systemic immunosuppression is required to halt the progressive inflammation and achieve adequate long‐term remission.94 Topical and sub‐conjunctival corticosteroids are used adjunctively with systemic therapy. They may offer short‐term relief of symptoms but are not effective in halting progression of the systemic autoimmune disease.95 Due to the infrequency and nature of the condition, there have been no studies assessing their role. Other topical agents shown to give variable results include: calcineurin inhibitors, cyclosporine A, tacrolimus and mitomycin C.94
4.2.9. Anterior uveitis
Topical corticosteroids are the mainstay of treating uncomplicated anterior uveitis as it has fewer local and systemic side effects compared to periocular or systemic administration. The interval of drop instillation is tailored to each patient; however, it is generally initiated on frequent intervals then slowly tapered according to the clinical response to prevent rebound inflammation. Where anterior uveitis has not adequately responded to topical corticosteroids, periocular steroids such as sub‐conjunctival dexamethasone may provide greater therapeutic effect with a short duration of action of 1 to 2 days.96 Similarly, sub‐conjunctival triamcinolone or betamethasone has also been shown to be safe and effective in severe cases of anterior uveitis.14
4.2.10. Non‐necrotising, non‐infectious anterior scleritis
Sub‐conjunctival TA may be given for the treatment of non‐necrotising, non‐infectious anterior scleritis.13, 97
4.3. Glaucoma surgery
The use of corticosteroids in modulating conjunctival wound healing is essential in glaucoma surgery. Topical corticosteroids are routinely used postoperatively and their frequency is often titrated according to the desired effect on wound healing. Sub‐conjunctival corticosteroids are also often injected at the end of surgery, though not usually at the surgical filtration site.
4.3.1. Glaucoma filtration surgery
Glaucoma filtration surgery is aimed at creating a filtering bleb which allows aqueous drainage and thus lowers the IOP. The long‐term success of surgery is dependent on modulating wound healing at the site of filtration, namely the scleral flap and overlying conjunctiva and Tenon's capsule.
Topical postoperative corticosteroids after trabeculectomy have been widely used since the apparent effect on filtering blebs was first described in 1965.98 The beneficial effects of steroids after trabeculectomy were first demonstrated in a prospective study in 1985, before the revolutionary widespread use of adjunctive local antimetabolites.99 Forty‐six eyes of 35 patients with a diagnosis of primary open‐angle glaucoma or primary angle‐closure glaucoma underwent trabeculectomy were randomized into three groups. Group 1 received no additional steroids, group 2 received topical 1% prednisolone acetate initially every 3 hours then tapered over 20 days, group 3 received the same treatment as in group 2 with additional of oral prednisone (80 mg daily) with a progressive taper over 16 days. The results were followed after 1.5 years, and long‐term data were later published at 5 and 10 years on 58 and 46 eyes, respectively. At 10 years, patients in group 1 (who did not have steroids) had a significantly higher rate (66.7%) of additional glaucoma procedures compared to those in group 2 (11.1%) and group 3 (38.5%). Furthermore, patients in group 1 had higher IOPs, were treated with more glaucoma drops and had lower rate of stabilized glaucoma (based on optic disc photography and visual fields).100
Perioperative injection of sub‐conjunctival corticosteroids at the filtering site have been demonstrated to give favourable bleb formation and IOP control in a small pilot series,101 In contrast, the only prospective randomized study comparing postoperative topical steroids to postoperative topical steroids in addition to steroid depot injection of TA found no significant differences in outcomes.102 Nevertheless, since the introduction of locally administered antimetabolites such as 5‐fluorouracil and mitomycin C were found to be more potent in impairing wound healing and thus increase long‐term success rates of trabeculectomy, the potential role of perioperative sub‐conjunctival steroid administration had become less significant.103
Currently, locally administered antimetabolite therapy is routinely used in conjunction with topical postoperative corticosteroids to modulate conjunctival wound healing in glaucoma filtration surgery. Corticosteroids predominantly modulate wound healing by reducing the release of acute inflammatory mediators and fibroblast recruitment. They also have a lesser effect in the proliferative phase of wound healing by limiting fibroblast activity. In contrast, antimetabolites modulate wound healing by inhibiting proliferation of fibroblasts and their profibrotic mechanisms.104, 105 Modulating both the inflammatory and proliferative phases of the wound healing response with these agents increases the likelihood of long‐term filtration and lower postoperative IOPs.106
4.3.2. Aqueous shunt surgery
Modulation of wound healing is important in the process of bleb encapsulation in aqueous shunt surgery. The use of corticosteroids to control postoperative inflammation is thought to influence the hypertensive phase after glaucoma drainage implantation. The hypertensive phase is characterized by a rise in IOP due to bleb encapsulation or capsular fibrosis that occurs at approximately 1 to 3 months postoperatively.107 It is particularly observed after implantation of non‐valved glaucoma drainage devices such as the Ahmed glaucoma device where it may occur in 56% to 82%.108 It has been reported that in 72% of these cases, the elevated IOP does not resolve indicating early surgical failure.109
Turalba and Pasquale110 retrospectively compared patients who received intraoperative sub‐Tenon during Ahmed device implantation with those who did not. The hypertensive phase was found to be 26% in those who received triamcinolone compared to those without (52%). There was no difference in final IOP outcomes and the authors warned of a higher rate of early complications including tube erosion and endophthalmitis. Yadnazi et al111 demonstrated in a prospective randomized trial of 90 eyes that adjunctive sub‐Tenon TA during Ahmed valve implantation had significantly lower IOPs at 1 month and lower peak IOPs, however had no difference in the rates of success or incidence of a hypertensive phase.
4.4. Posterior segment
Ocular steroids are used to treat macular oedema of varied aetiology (diabetic, retinal vein occlusion, postoperative and inflammatory), intraocular inflammation including uveitis and scleritis and to assist visualization of the vitreous during vitrectomy.
4.4.1. Diabetic macular oedema
Diabetic macular oedema is the most common cause of vision loss in people with diabetes.112 The pathogenesis of DMO is multifactorial, with not only VEGF but other pro‐inflammatory factors involved in breaking down the blood‐retinal barrier and increasing vascular permeability.113 Although intravitreal anti‐VEGF therapy remains first‐line treatment for centre‐involving DMO in phakic patients, ocular steroids may be considered for pseudophakic patients, those with planned cataract surgery, or in patients with suboptimal response or contraindication to intravitreal anti‐VEGF therapy.114 A suboptimal response to intravitreal anti‐VEGF therapy is not uncommon. In the RISE/RIDE registration trials, BCVA was worse than 6/12 in 42.8% of patients and central foveal thickness greater than 250 μm in 24.8% of patients despite 2 years of monthly intravitreal ranibizumab (0.3 mg) injections.25
Intravitreal TA for diabetic macular oedema
Intravitreal TA (Kenacort‐A 40, Bristol‐Myers Squibb Pharmaceuticals, Noble Park, Australia) was first shown to be superior to sham treatment for BCVA in patients with centre‐involving DMO in a prospective, double‐masked, placebo‐controlled randomized controlled trial.115 Compared with those receiving placebo, eyes receiving TA had a 5.7 Logarithm of Minimum Angle of Resolution (LogMAR) letter better improvement at 2 years.
The Diabetic Retinopathy Clinical Research Network (DRCR.Net) Protocol I study was a multi‐centre RCT comparing sham injection + prompt laser, 0.5 mg ranibizumab + prompt laser, 0.5 mg ranibizumab + deferred laser and 4 mg TA + prompt laser for centre‐involving DMO.116 The main outcome measure was improvement in BCVA at 1 year, and 854 study eyes of 691 patients were enrolled. The ranibizumab arms showed greater improvement in BCVA compared with laser alone. Although overall the TA + prompt laser arm did not do as well as the ranibizumab arms, TA was shown to be as effective as ranibizumab when only pseudophakic patients were analysed. However, at 5‐year follow‐up the TA arm was inferior to ranibizumab arms, even when only analysing pseudophakic patients and allowing for the addition of “very deferred ranibizumab” after 74 weeks from baseline.117
DII for diabetic macular oedema
The MEAD study included two 3‐year, multi‐centre, masked, randomized controlled phase III clinical trials that compared a minimum of 6‐monthly dosing with DII 0.7 mg, 0.35 mg and sham procedure. Patients had centre‐involving DMO, visual acuities between 6/15 and 6/60 and central retinal thickness ≥300 μm on optical coherence tomography scans.118 The main outcome measures were proportion of patients achieving ≥15 LogMAR letters of improvement in BCVA and safety profile. At baseline there were 1048 patients enrolled, and 57.9% completed the 3‐year study. Both DII doses had a significantly greater proportion of patients achieving ≥15‐letters of improvement in BCVA (22.2% for 0.7 mg, 18.4% for 0.35 mg and 12.0% for sham).
The BEVORDEX study was a prospective, multi‐centre, randomized single‐masked clinical trial comparing 4‐weekly bevacizumab and DII 0.7 mg (OZURDEX) that could be given more frequently (4‐monthly) in 88 eyes of 61 patients with centre‐involving DMO.119 The main outcome measure was the proportion of patients achieving an improvement in vision of 10 LogMAR letters. Each arm had similar proportion of patients reaching the main outcome measure at 12 and 24120 months (40% with bevacizumab and 41% with DII at 12 months). The group receiving DII had fewer mean injections (2.7) compared to the bevacizumab arm (8.6) over the first 12 months with a greater reduction in central macular thickness at 12 but not 24 months. However, more patients in the DII arm lost vision, mainly because of cataract progression.
FA intravitreal implant for diabetic macular oedema
Iluvien is a sustained delivery FA injectable implant that has been shown to treat patients with DMO. The FAME A and B studies were identically designed parallel‐group, phase 3 double‐masked, randomized controlled phase III clinical trials that compared two doses (0.2 and 0.5 μg/day) of FA over a 3‐year period. The primary end point was a gain of ≥15 letters at 24 months with follow‐up to 36 months.
A pre‐planned subgroup analysis examined visual outcomes as a function of duration of DMO at randomization revealed that the treatment effect resided primarily in patients with chronic DMO (duration ≥3 years). At month 36, a significantly higher proportion of FA treated patients from both studies showed an improvement of ≥15 letters from baseline compared to the sham group (FAME A: 31.8% for 0.2 μg/day, 13.5% for sham; FAME B: 36.4% for 0.2 μg/day, 13.2% for sham). In patients with non‐chronic DMO (duration <3 years), the proportion of patients gaining ≥15 letters were similar between the FA and sham groups in both studies.121 In Europe and in the United Kingdom, it has been approved for the treatment of persistent DMO that has not sufficiently responded to available therapies. In the USA, it is approved for the treatment of DMO in patients who have been previously treated with corticosteroids without a clinically significant rise in IOP.
4.4.2. Macular oedema secondary to retinal vein occlusion
Macular oedema is the most common cause of visual loss in RVO.22, 122, 123 Like DMO, anti‐VEGF therapy remains first‐line treatment in phakic patients. This is based on multiple RCTs demonstrating visual benefit of anti‐VEGF for macular oedema secondary to CRVO (ranibizumab: CRUISE124, 125; aflibercept: COPERNICUS,126 GALILEO,127 SCORE2128; bevacizumab: SCORE2,128 Epstein et al129) and BRVO (ranibizumab: BRAVO130, 131; aflibercept: VIBRANT132, 133; bevacizumab134).
Intravitreal TA for retinal vein occlusion
Ocular steroids may be considered in pseudophakic eyes in which anti‐VEGF is contraindicated or failing to provide an adequate result.135 Ocular steroids not only inhibit VEGF, but their anti‐inflammatory and neuroprotective effects may also benefit eyes with RVO.22, 123 The Standard Care versus Corticosteroid for Retinal Vein Occlusion (SCORE) studies were multi‐centre randomized clinical trials evaluating the benefit of IVTA for the treatment of macular oedema secondary to retinal vein occlusion.22, 23 In the SCORE‐CRVO Study (Report 5), 271 patients were randomized to observation (the standard of care at that time), 1 or 4 mg preservative‐free IVTA (Trivaris).22 The main outcome measure was the proportion of patients with ≥15 letter improvement from baseline to month 12. This was achieved in significantly more patients on 1 or 4 mg triamcinolone (27 and 26%, respectively) than those who were observed (7%). For the SCORE‐BRVO Study (Report 6) the observation arm was replaced with grid photocoagulation because grid laser was the standard of care at that time for treating macular oedema secondary to BRVO according to the Branch Vein Occlusion Study.136, 137 Unlike SCORE‐CRVO, there was no significant difference between the arms in the proportion of patients achieving a ≥15 letter improvement from baseline to month 12.
Intravitreal dexamethasone implant for retinal vein occlusion
The Global Evaluation of Implantable Dexamethasone in Retinal Vein Occlusion with Macular Edema (GENEVA) study included two identical multi‐centre, masked, 6‐month, sham‐controlled RCTs assessing the efficacy of DII implant for vision loss due to macular oedema from both CRVO and BRVO.123 A total of 1267 patients were randomized to receive a single treatment of DII 0.7, 0.35 mg, or sham procedure. Both DII groups performed significantly better than the sham arm in the time to reach a ≥15 letter improvement in BCVA, proportion of patients achieving a ≥15 letter improvement in BCVA, mean BCVA and proportion of patients losing ≥15 letters.
Intravitreal fluocinolone implant for retinal vein occlusion
There is a lack of studies on intravitreal FA implants for the treatment of CMO from retinal vein occlusions. The Fluocinolone Acetonide Intravitreal Inserts for Vein Occlusion in Retina (FAVOUR) study started recruiting patients however the study was terminated early. Currently, Iluvien has not been approved for macular oedema from retinal vein occlusions in the USA, UK or Europe and any use for this indication is off‐label.
Retisert has been used for CMO in retinal vein occlusions within a small pilot series by Ramachandran et al138 which demonstrated 69% of eyes showing visual acuity improvement, 15% were stable and 15% lost two lines from baseline at 12 months. Cataract formation occurred in almost all patients and 39% eyes required glaucoma filtration surgery by 12 months. A follow‐up study recruited 10 further patients and indicated sustained benefit up to 30 months.139
4.4.3. Posterior non‐infectious uveitis
For patients with posterior segment inflammation and macular oedema, topical steroid therapy is often inadequate. These patients have the option of periocular (sub‐Tenon, orbital floor, peribulbar), intravitreal (IVTA, OZURDEX) and systemic steroids. In an attempt to minimize the systemic side effects (such as Cushingoid state, osteoporosis and elevated blood glucose), local steroid is often considered, especially for unilateral inflammation. In the retrospective cohort of the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) study, over half of 1192 eyes in 914 patients with uveitis demonstrated improved visual acuity at some point within 6 months of receiving periocular steroid.140
Intravitreal steroids are particularly useful in two groups of patients: those with severe vitritis or cystoid macular oedema (CMO) that is unlikely to respond rapidly to periocular corticosteroids, and those with inflammation that are refractory to other treatment. In patients with persistent disease, these options may also be combined effectively with systemic steroids and steroid‐sparing agents (eg, methotrexate, mycophenolate and cyclosporin) for acute unilateral relapses or persistent disease activity to reduce the dosages and side effects of the systemic treatment.
It should also be noted that although ongoing repeated depot steroid injections could be considered as a treatment option for chronic persistent intermediate, posterior or panuveitis, such a management approach must be considered with caution given the recent 7‐year follow‐up findings from the NIH sponsored MUST (Multi‐centre Uveitis Steroid Treatment) trial. This study was also a prospective RCT that compared the FA containing Retisert implant with standard systemic immunosuppression in 479 eyes. Although the implant group initially had a faster gain in BCVA, the systemic treatment group had a more gradual gain in BCVA such that there was no significant difference at 2 and 5 years.141 However, at 7 years, the systemic group overtook the implant group in terms of BCVA outcomes,142 with the implication being that the uveitis relapses occurring once the depot steroid “wears off” are more severe and more likely to result in more (irreversible) damage than lower grade relapses seen with systemic treatment when oral prednisolone/immunosuppression is being gradually weaned.
A prospective 3‐year randomized, sham‐controlled study is comparing Yutiq with placebo. Yutiq is designed to release FA for up to 36 months and the 12‐month data has shown it was effective in lowering the rate of recurrence of posterior uveitis. At 36‐months, the effect of reducing recurrence rate was still significantly lower with Yutiq (56.3%) compared to sham‐treated eyes (92.9%).30
4.4.4. Uveitic macular oedema
Cystoid macular oedema is a common cause of vision loss in uveitis.24 Intravitreal TA has been shown to effectively reduce uveitic CMO.143 Visual acuity improvements are more significant if the CMO is present for 12 months or less and for patients aged 60 years or younger. It is useful in improving visual acuity in patients with CMO, even when their non‐infectious uveitis has been quiescent.144 As a single IVTA injection lasts approximately 3 to 6 months, repeated injections may be required.145
Alternatives to IVTA for treating uveitis include DII or FA implants. The HURON trial demonstrated efficacy of a single OZURDEX injection in non‐infectious intermediate, posterior or panuveitis in comparison to placebo, with a reduction of inflammation and CMO in 47%, and ≥15 letter gain in up to 43%.146
Most recently, the National Institute of Health (NIH) funded POINT trial compared all three of the above depot steroid options (periocular TA, IVTA and OZURDEX) for the treatment of CMO secondary to uveitis in a prospective, multi‐centre RCT.147 In this trial, 235 eyes were randomized 1:1:1 to either periocular TA (40mg/1ml), unpreserved IVTA (4mg/0.1ml) or OZURDEX (0.7mg dexamethasone). The primary outcome was central subfield macular thickness (CMT) at 8 weeks, with secondary outcomes including visual acuity and rate of adverse events over 24 weeks of follow‐up. Overall, the CMT in all three groups improved, however IVTA and OZURDEX were found to be superior to periocular TA, with rates of improvement of 39% and 46%, respectively, vs 23% for periocular TA (p < .0001). However, no statistically significant difference was demonstrated between IVTA and OZURDEX. Similarly, BCVA also improved in all three groups, with greater gains (four to seven letters to more greater gains) seen with intravitreal treatments, with again no clinical or statistically significant differences seen between IVTA or OZURDEX. Interestingly, despite the findings in other studies, the duration of effect of OZURDEX on CMT was found to decrease after 8 weeks (rather than 12‐16 weeks) in this cohort of patients. This may indicate that patients with uveitic CMO may require intravitreal injections more frequently than for other indications. It should be noted that the POINT study design did allow the IVTA arm to have re‐treatments at 8 weeks, but only at 12 weeks for the OZURDEX arm.
4.4.5. Bacterial endophthalmitis
Intravitreal steroids have been described for the management of acute bacterial endophthalmitis in conjunction with intravitreal antibiotics, although their use remains controversial.148, 149 They may tamper the inflammatory response that causes damage to the retina, but, conversely, they may interfere with infection control, lower the concentration of intravitreal antibiotics and the additional volume may elevate the IOP.149 If they are used, intravitreal dexamethasone is preferred due to its rapid elimination from the eye.149 A dosage of 0.4 mg/0.1 mL is usually prescribed, as higher doses have been shown to cause Müller cell damage in animal studies.150 Evidence for intravitreal dexamethasone in acute endophthalmitis is limited to retrospective case series which gave mixed results, and four prospective RCTs151, 152, 153, 154 which failed to show statistically significant improvements in final visual outcomes.149 Although traditional teaching is to avoid intraocular steroids for fungal endophthalmitis, this fear may be exaggerated in patients treated with vitrectomy and intravitreal anti‐fungal therapy.155
4.4.6. Postoperative macular oedema
Macular oedema is a well‐known complication of cataract surgery. Topical non‐steroidal and steroidal therapy are usually first‐line treatment.156 Topical and oral carbonic anhydrase inhibitors and intravitreal anti‐VEGF agents have also been described, but strong evidence for these are lacking. In recalcitrant cases, local steroid injections may be considered. IVTA has been shown to reduce retinal thickness and improve vision in cases of persistent pseudophakic macular oedema157, 158, 159, 160 with an effect that may be sustained for more than 6 months.158
4.4.7. Other indications for intravitreal TA and dexamethasone implant
Intravitreal steroids have been used with variable results for a variety of other causes of macular oedema including: neovascular AMD,161 retinal angiomatous proliferation,162 macular telangiectasia,163, 164 Coat's disease,165 vasoproliferative tumour,166 radiation retinopathy,167, 168 retinitis pigmentosa,169, 170 proliferative vitreoretinopathy,171, 172, 173 following scleral buckling174 or vitrectomy surgery175 and from idiopathic CMO.160 , 176
Intravitreal TA can be used intraoperatively to visualize the vitreous. This is particularly useful for iatrogenic induction of a posterior vitreous detachment, peeling internal limiting membrane and when vitreous needs to be highlighted for clearance in complicated cataract surgery.177
5. COMPLICATIONS OF OCULAR AND PERIOCULAR STEROID DELIVERY
Complications arising from use of ocular steroids may be related to the procedure itself, or the pharmacological effects of the steroids.
5.1. Procedure related complications
5.1.1. Periocular injections
Periocular injections can be performed using different techniques: into the sub‐conjunctival space, into the sub‐Tenon space, into the orbital floor alongside the globe, (usually inferiorly, via a transcutaneous or transconjunctival injection), or into the peribulbar or retrobulbar space. Complications of these injections include: orbital swelling, chemosis, proptosis, sub‐conjunctival haemorrhage, retrobulbar haemorrhage, globe ischaemia, posterior ischaemic optic neuropathy, optic atrophy, globe perforation, orbital cellulitis, fat atrophy, fat herniation, damage to the rectus muscles resulting in diplopia, ptosis, dural puncture and an oculocardiac reflex.178, 179, 180, 181
The likelihood of complications differs depending on the site of the injection. Posterior injection reduces the chances of unsightly sub‐conjunctival plaques resulting from anterior seepage of depot, conjunctival or corneoscleral melting, depigmentation and granuloma related to the methylcellulose vehicle of the depot injection. Injection into the orbital floor is easily performed with a 25 mm 25‐gauge needle. It is well tolerated and carries only a very small risk of globe perforation if the needle is directed away from the globe at all times. It is frequently difficult to access the sub‐Tenon's space of patients who have had previous surgery (notably scleral buckling), these eyes may be more suited to an orbital floor injection. Peribulbar and retrobulbar injection are more likely to lead to globe perforation or inadvertent intravascular injection with vascular occlusion from embolization. Additional caution is required in myopic patients as they have a thinner sclera and larger globes which are at an increased risk of perforation.
5.1.2. Intravitreal injections
Intravitreal injections may be associated with endophthalmitis, ocular inflammation, vitreous haemorrhage, retinal tears, rhegmatogenous retinal detachment, IOP elevation, cataract and lens subluxation.182 Although rare (with a reported incidence of 0.09%‐0.87%),183 acute bacterial endophthalmitis is the most serious of these complications and requires immediate treatment with intravitreal antibiotics. Rates of vitreous haemorrhage,118 wound leak hypotony and retinal tears and detachment123 may be higher with DII, as the needle is larger (23‐gauge) and the force of injection greater than for standard 30‐gauge needle intravitreal injections. DII is contraindicated in aphakic and pseudophakic patients with posterior capsular rupture, as the implant may migrate into the anterior chamber.184 If this occurs, early removal is recommended to avoid chronic corneal oedema.184
5.2. Pharmacologic related complications
The two most important pharmacologic related complications of ocular steroids are raised IOP and development of cataract, which are more frequent with intravitreal injections compared to periocular injections.
5.2.1. Raised IOP
Raised IOP with subsequent development of glaucomatous optic neuropathy is one of the most significant complications of locally administered corticosteroids. If other therapeutic options are available, ocular steroids are best avoided in patients with pre‐existing glaucoma. The pathogenesis is not well understood but may involve downregulation of trabecular meshwork matrix metalloproteinase activity, increased myocilin production and/or decreased trabecular meshwork phagocytic activity that increases aqueous outflow resisance.185 The susceptibility to pressure response may be due to genetic differences and variations in corticosteroid receptors whilst the degree of effect on IOP appears to be dose‐dependent.
Raised IOP with TA
In the SCORE Study Report 15, the proportion of patients being treated for BRVO or CRVO with a cumulative incidence of IOP elevation ≥10 mm Hg from baseline to 36 months was 2% (no IVTA), 9% (1 mg IVTA) and 45% (4 mg IVTA). Consideration of a lower dose (1 or 2 mg) of IVTA to treat RVO may be appropriate in patients at risk of an IOP‐related event, particularly as little difference has been reported in efficacy between the 1 and 4 mg doses.22, 23, 185 Other risk factors for an IOP‐related event include higher baseline IOP and younger age. A cumulative incidence of 32% of patients in the SCORE study reached an IOP ≥25mm Hg at 12 months. The incidence of IOP elevation in other reports is comparable.186, 187, 188 Although most cases of IOP elevation occur in the first 1 to 2 months of initiating therapy, in some cases it may take several months to develop (in SCORE Study Report 15 this ranged up to 598 days), so long‐term vigilance is required even if no IOP rise is seen after the first few injections.185
Raised IOP with DII
IOP elevation can also occur with DII, although possibly at lower rates than for IVTA. In the MEAD study for DMO, the incidence of IOP elevation ≥10 mm Hg from baseline to 36 months at any visit for those receiving DII 0.7 mg was 27.7%.118 The cumulative incidence of at least one visit with IOP ≥25 mm Hg or ≥35 mm Hg was 32% and 6.6%. The large majority of these patients with IOP elevation could be managed with medical therapy. Only one patient required incisional glaucoma surgery, and no patients required removal of the implant. Mean IOP returned to baseline by month 6 after each injection, and there did not appear to be a cumulative effect on IOP elevation with repeated injections. Similar findings were found in a 12‐month trial by the OZURDEX PLACID Study Group189 and a recent large retrospective analysis of 2736 eyes of 1441 patients treated with a total of 6015 DII in which 26.5% of eyes had an IOP rise >25 mm Hg but only 0.91% required glaucoma filtration surgery.190
Raised IOP with FA intravitreal implant
In the FAME trials, an IOP of ≥30 mm Hg developed in 16.3% of FA injectable implant treatment groups at month 23 and 18.4% by month 36. Elevated IOP that required incisional surgery by 36 months was 4.8% in the low‐dose group, 8.1% in the high‐dose group and 0.5% in the sham group.191
Raised IOP with periocular steroid
IOP elevation can also occur with periocular steroid. In a study by Sen et al140 of patients being treated with periocularly administered corticosteroid (predominantly TA 40mg) for uveitis, the cumulative incidence of at least 1 visit with IOP ≥24 mm Hg or ≥30 mm Hg at 12 months was 34% and 15%.
Raised IOP in uveitic patients
Corticosteroid‐induced raised IOP is much more common in the uveitic population than for other indications, and even higher in paediatric patients.192, 193, 194 In the POINT trial 20%, 30% and 41% recorded an IOP of ≥24 mm Hg by 24 weeks in the periocular, IVTA and OZURDEX groups, respectively, with 9%, 18% and 39%, respectively, developing an IOP rise of ≥10 mm Hg from baseline.147 Interestingly, only 4% to 6% overall developed an IOP of ≥30 mm Hg. Although both intravitreal treatments had a significantly higher rate of raised IOP when compared with periocular steroid, there was no significant difference seen between IVTA and OZURDEX in a direct comparison.
Raised IOP in children
Children are more likely than adults to have an IOP response to steroids.195 Compared to adult patients, the IOP rise can be more severe and resulting glaucoma may progress more rapidly and has even been reported within hours of starting treatment.196 The effect can result from topical, periocular, intravitreal, oral and intravitreal dosing regimes.
Dexamethasone is more likely to cause a steroid response than FML in children. A study by Kwok et al195 included 19 Chinese children undergoing bilateral strabismus surgery, with one eye randomized to receive topical dexamethasone 0.1% and the other to receive FML 0.1% six times per day for 4 weeks postoperatively. The mean increase in IOP in eyes receiving dexamethasone (15.48 ± 8.71 mm Hg) was almost double that of eyes receiving FML (5.83 ± 4.96 mm Hg; P = .001).195
FML also causes ocular hypertension, in a dose‐dependent manner in children. In a study by Fan et al,197 31 children undergoing bilateral strabismus surgery had one eye randomized to receive topical FML six times per day and the other eye to receive topical FML three times per day, each for 4 weeks postoperatively. The IOP increased significantly from baseline in both groups, but the peak IOP was higher (19.0 ± 5.06 mm Hg vs 17.13 ± 3.32 mm Hg, P < .001) and the net increase was also greater (4.37 ± 4.79 vs 2.57 ± 3.32 mm Hg, P = .005) in the group with more frequent dosing. More frequent dosing was correlated with reaching peak IOP sooner (6 days vs 13 days; P = .033), but there was no difference in postoperative inflammation between the groups.197
5.2.2. Cataract
Cataract is the other common complication of ocular and periocular delivered steroids. There are few studies that directly compare rates of cataract progression with different types of intravitreal steroids. However, the rates from various studies may suggest higher rates of cataract progression with IVTA and FA than DII. A head to head comparison between intravitreal FA and DII in uveitis patients showed a significantly higher incidence of cataract (and raised IOP) with FA.198
In the SCORE Study Report 5 (CRVO), the estimate through month‐12 of phakic patients developing new‐onset lens opacity or progression of existing opacity was 18%, 26% and 33%, respectively, in the observation, 1 mg IVTA and 4 mg IVTA groups. The percentage of patients requiring cataract surgery by 24 months in these respective groups were 0%, 0% and 33%.22 In the SCORE Study Report 6 (BRVO) this was 13%, 25% and 35%, respectively, in the laser, 1 mg IVTA and 4 mg IVTA groups.23 In the MEAD study for DMO, DII 0.7 mg was associated with cataract‐related adverse events in 67.9% of patients over the course of the 3‐year study, with 59.2% requiring cataract surgery during the study.118 A recent large retrospective analysis of over 6000 DII injections found a statistically significant increase in cataract progression in eyes receiving injections (P = .004) although with a small co‐relation co‐efficient (r = .057).190
In the FAME trials, the development of cataract and the proportion of patients requiring cataract surgery were significantly higher in both the low‐ and high‐dose FA treatment arms than in the sham‐control group. At 36 months, the percentage of patients developing cataracts were 81.7%, 88.7% and 50.7% in the low‐dose, high‐dose and control group, respectively.199
Uveitis patients may develop cataract from both intraocular inflammation and corticosteroid treatment. In the SITE cohort, 1192 eyes of 914 patients received periocular injections (sub‐Tenon's or orbital floor) for uveitis. Cataract development attributing to an incident reduction in visual acuity of worse than 6/12 occurred in 20.2%, whilst cataract surgery was performed within 12 months in 13.8% of initially phakic eyes.140
Periocular injection of corticosteroid used in the management of paediatric uveitis is associated with a high rate of cataract formation, [4 of 19 (21%) eyes]200 and IVTA used to manage uveitic macular oedema in children has been found to induce cataract in 6 of 11 eyes (55%).145
5.2.3. Non‐infectious endophthalmitis and pseudoendophthalmitis
Although acute bacterial endophthalmitis is the most serious complication of intraocularly administered steroids, acute non‐infectious endophthalmitis following IVTA injection is more common, with most studies reporting an incidence of 0.5% to 2.0%.201, 202 Non‐infectious endophthalmitis refers to a transient, inflammatory reaction, typically with hypopyon that presents within a day or two post injection of IVTA.201 There is usually no or minimal inflammation of the sclera and conjunctiva, minimal anterior chamber fibrin and pain is rare, as might be expected since steroids are anti‐inflammatory.201 The condition is usually self‐limiting and resolves in 1 to 2 weeks but may cause persistent vitreous opacification in a minority of cases.
Some authors have attributed non‐infectious endophthalmitis following IVTA injection to the preservative vehicle 0.99% benzyl alcohol found in Kenacort.202 In one study, the incidence of severe sterile endophthalmitis fell from 13.0% to 4.3% after switching from preserved to preservative‐free TA.203 However another study found no difference in the incidence of non‐infectious endophthalmitis after removing benzyl alcohol204 and cases of non‐infectious endophthalmitis have been reported with benzyl‐alcohol free Triesence.205, 206 In fact, one study found a higher rate of non‐infectious endophthalmitis following administration of Triescence compared with Kenalog‐40 and preservative‐free TA.207 The authors attributed this result to the smaller particle size and higher particle load of Triesence. The pathogenesis for this phenomenon is likely to be multifactorial.
A non‐infectious, non‐inflammatory pseudoendopthalmitis can also occur when TA particles migrate and settle in the anterior chamber, resembling a hypopyon.201 Whenever there is uncertainty regarding whether an endophthalmitis is infectious or non‐infectious, it should be managed with the standard treatment for infectious endophthalmitis.
5.2.4. Activation of ocular or periocular infection
Corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections. This can prolong the course and/or exacerbate the severity of viral (eg, herpes simplex epithelial keratitis), bacterial and fungal infections of the eye. In acute purulent infections of the eye, steroids may mask infection or exacerbate existing infection. Since corticosteroids are known to reduce resistance to infections, simultaneous bilateral intraocular injections of steroids should, if possible, be avoided to limit the potential for bilateral postoperative infection.208
Infectious scleritis is a rare complication of sub‐Tenon TA with a reported incidence of 0.04%.209 A recent review found nine reported cases in which four were caused by fungal organisms.210
Use of intraocular or periocular administered corticosteroids may reactivate HSK and concurrent antiviral prophylaxis for those with a history of HSK is advised.211, 212
Viral retinitis has been reported following local administration of corticosteroids. A recent review found 30 cases with causative viruses identified as cytomegalovirus in 76.7%, HSV in 16.7%, with one case of varicella zoster virus and another unspecified. Steroids were administered by IVTA (33.3%), intravitreal FA acetonide implant Retisert (33.3%), posterior sub‐Tenon TA (6.6%) and anterior sub‐Tenon TA (3.3%).208
Unmasking or activation of dormant ocular syphilitic infection following IVTA have been reported in five cases. All cases manifested with small round yellow retinal lesions, retinovasculitis and optic disc involvement.213, 214, 215 Three of these cases presented with acute syphilitic posterior placoid chorioretinitis and all had devastating visual outcomes.213, 214
In contrast, a small series of seven patients demonstrated safety of DII in treating CMO in patients with infectious uveitis where other treatments for CMO had failed. All patients were concurrently treated on the appropriate antimicrobial agent and had resolution of CMO without reactivation of the infectious ocular disease.216
5.2.5. Steroid induced central serous chorioretinopathy
It is well established that any exogenous use of corticosteroids may precipitate or exacerbate central serous chorioretinopathy (CSC).217 Despite topical, periocular and intravitreal steroids being common ophthalmic treatments, CSR is rarely reported in association with administration of ophthalmic steroids.218
A recent literature review found only one case of IVTA causing new‐onset CSC,219 and another case of worsening pre‐existing CSC.220 Interestingly, a reported case of intravitreal Ozurdex implantation for refractory Irvine‐Gass syndrome was found to precipitate CSC in the fellow eye.221 One case presenting with bilateral multifocal serous retinal detachments initially diagnosed as Vogt‐Koyanagi‐Harada syndrome received high‐dose oral steroids and IVTA. The treatment ultimately worsened the condition later diagnosed as CSC.222
In contrast, IVTA experimentally used to treat chronic CSC and CMO secondary to CSC, did not improve or worsen the CSC.223, 224 Surprisingly, a recent literature review found only one report of CSC associated with periocular steroid use,225 and no cases of CSC related to topical steroid eye drops.218
6. CONCLUSION
For over 70 years, ocular steroids have proven to be a major weapon in the treatment of ocular inflammation. An increasing variety of preparations and delivery sites have maximized their efficacy whilst attempting to minimize their major side effects‐ raised IOP and cataract. Multiple RCTs have improved our understanding of the utility and limitations of this versatile medication.
FINANCIAL DISCLOSURE
Adrian Fung, Honoraria and travel support: Allergan, Bayer, Novartis. Lyndell Lim, Research grant: Abbvie, Bayer. Advisory Board work and consultant fees: Bayer, Abbvie, Allergan. Chameen Samarawickrama, Research funding: Westmead Charitable Trust Early Career Research Fellowship. Jennifer Arnold, Advisory Board work and honoraria: Allergan, Novartis, Bayer, Alcon. Mark Gillies, Research funding, honoraria and travel support: Novartis, Bayer, Allergan. Andrew Symons, Research grant: Topaz Study.
Fung AT, Tran T, Lim LL, et al. Local delivery of corticosteroids in clinical ophthalmology: A review. Clin Experiment Ophthalmol. 2020;48:366–401. 10.1111/ceo.13702
REFERENCES
- 1. Gordon DM, McLean JM. Effects of pituitary adrenocorticotrophic hormone (ACTH) therapy in ophthalmologic conditions. JAMA. 1950;142:1271‐1276. [Google Scholar]
- 2. Edelman JL. Differentiating intraocular glucocorticoids. Ophthalmologica. 2010;224(Suppl 1):25‐30. 10.1159/000315158. [DOI] [PubMed] [Google Scholar]
- 3. Whitcup SM, Cidlowski JA, Csaky KG, et al. Pharmacology of corticosteroids for diabetic macular edema. Invest Ophthalmol Vis Sci. 2018;59:1‐12. 10.1167/iovs.17-22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sulaiman RS, Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in the eye. Steroids. 2018;133:60‐66. 10.1016/j.steroids.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711‐1723. [DOI] [PubMed] [Google Scholar]
- 6. Doane MG, Jensen AD, Dohlman CH. Penetration routes of topically applied eye medications. Am J Ophthalmol. 1978;85:383‐386. [DOI] [PubMed] [Google Scholar]
- 7. van Rensburg E, Meyer D. Astute and safe use of topical ocular corticosteroids in general practice: practical guidelines. Contin Med Educ. 2013;31:127‐129. [Google Scholar]
- 8. Jones R 3rd, Rhee DJ. Corticosteroid‐induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163‐167. [DOI] [PubMed] [Google Scholar]
- 9. Cunningham ET, Wender JD. Practical approach to the use of corticosteroids in patients with uveitis. Can J Ophthalmol. 2010;45:352‐358. [DOI] [PubMed] [Google Scholar]
- 10. Leibowitz HM, Kupferman A. Optimal frequency of topical prednisolone administration. Arch Ophthalmol. 1979;97:2154‐2156. [DOI] [PubMed] [Google Scholar]
- 11. Merkoudis N, Wikberg Matsson A, Granstam E. Comparison of peroperative subconjunctival injection of methylprednisolone and standard postoperative steroid drops after uneventful cataract surgery. Acta Ophthalmol (Oxf). 2014;92:623‐628. 10.1111/aos.12358. [DOI] [PubMed] [Google Scholar]
- 12. Weijtens O, Feron EJ, Schoemaker RC, et al. High concentration of dexamethasone in aqueous and vitreous after subconjunctival injection. Am J Ophthalmol. 1999;128:192‐197. [DOI] [PubMed] [Google Scholar]
- 13. Sohn E, Wang R, Read R, et al. Long‐term, multicenter evaluation of subconjunctival injection of triamcinolone for non‐necrotizing, noninfectious anterior scleritis. Ophthalmology. 2011;118:1932‐1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Athanasiadis Y, Tsatsos M, Sharma A, et al. Subconjunctival triamcinolone acetonide in the management of ocular inflammatory disease. J Ocul Pharmacol Ther. 2013;29:516‐522. 10.1089/jop.2012.0208. [DOI] [PubMed] [Google Scholar]
- 15. Nan K, Sun S, Li Y, et al. Characterisation of systemic and ocular drug level of triamcinolone acetonide following a single sub‐Tenon injection. Br J Ophthalmol. 2010;94:654‐658. 10.1136/bjo.2009.172106. [DOI] [PubMed] [Google Scholar]
- 16. D'Amico DJ, Goldberg MF, Hudson H, et al. Anecortave acetate as monotherapy for treatment of subfoveal neovascularization in age‐related macular degeneration: twelve‐month clinical outcomes. Ophthalmology. 2003;110:2372‐2383. 10.1016/j.ophtha.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 17. Slakter JS, Bochow TW, D'Amico DJ, et al. Anecortave acetate (15 milligrams) versus photodynamic therapy for treatment of subfoveal neovascularization in age‐related macular degeneration. Ophthalmology. 2006;113:3‐13. 10.1016/j.ophtha.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 18. Zacharias LC, Lin T, Migon R, et al. Assessment of the differences in pharmacokinetics and pharmacodynamics between four distinct formulations of triamcinolone acetonide. Retina. 2013;33:522‐531. 10.1097/IAE.0b013e3182647f69. [DOI] [PubMed] [Google Scholar]
- 19. Chen H, Sun S, Li J, et al. Different intravitreal properties of three triamcinolone formulations and their possible impact on retina practice. Invest Ophthalmol Vis Sci. 2013;54:2178‐2185. 10.1167/iovs.12-11460. [DOI] [PubMed] [Google Scholar]
- 20. Beer PM, Bakri SJ, Singh RJ, et al. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology. 2003;110:681‐686. [DOI] [PubMed] [Google Scholar]
- 21. Young S, Larkin G, Branley M, Lightman S. Safety and efficacy of intravitreal triamcinolone for cystoid macular oedema in uveitis. Clin Experiment Ophthalmol. 2001;29:2‐6. [DOI] [PubMed] [Google Scholar]
- 22. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the standard care vs corticosteroid for retinal vein occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101‐1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scott IU, Ip MS, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the standard care vs corticosteroid for retinal vein occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127:1115‐1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rothova A, Suttorp‐van Schulten MS, Frits Treffers W, et al. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80:332‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789‐801. 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 26. Chang‐Lin JE, Burke JA, Peng Q, et al. Pharmacokinetics of a sustained‐release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Invest Ophthalmol Vis Sci. 2011;52:4605‐4609. 10.1167/iovs.10-6387. [DOI] [PubMed] [Google Scholar]
- 27. Shaikh AH, Petersen MR, Sisk RA, et al. Comparative effectiveness of the dexamethasone intravitreal implant in vitrectomized and non‐vitrectomized eyes with macular edema secondary to central retinal vein occlusion. Ophthalmic Surg Lasers Imaging Retina. 2013;44:28‐33. 10.3928/23258160-20121221-09. [DOI] [PubMed] [Google Scholar]
- 28. Campochiaro PA, Nguyen QD, Hafiz G, et al. Aqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implants. Ophthalmology. 2013;120:583‐587. 10.1016/j.ophtha.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 29. Kiernan DF, Mieler WF. The use of intraocular corticosteroids. Expert Opin Pharmacother. 2009;10:2511‐2525. 10.1517/14656560903160671. [DOI] [PubMed] [Google Scholar]
- 30. Jaffe GJ, Foster CS, Pavesio CE, et al. Effect of an injectable fluocinolone acetonide insert on recurrence rates in chronic noninfectious uveitis affecting the posterior segment: twelve‐month results. Ophthalmology. 2019;126:601‐610. 10.1016/j.ophtha.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 31. Bordaberry M, Marques DL, Pereira‐Lima JC, et al. Repeated peribulbar injections of triamcinolone acetonide: a successful and safe treatment for moderate to severe graves' ophthalmopathy. Acta Ophthalmol. 2009;87:58‐64. 10.1111/j.1755-3768.2008.01171.x. [DOI] [PubMed] [Google Scholar]
- 32. Ebner R, Devoto MH, Weil D, et al. Treatment of thyroid associated ophthalmopathy with periocular injections of triamcinolone. Br J Ophthalmol. 2004;88:1380‐1386. 10.1136/bjo.2004.046193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chee E, Chee SP. Subconjunctival injection of triamcinolone in the treatment of lid retraction of patients with thyroid eye disease: a case series. Eye (Lond). 2008;22:311‐315. 10.1038/sj.eye.6702933. [DOI] [PubMed] [Google Scholar]
- 34. Xu D, Liu Y, Xu H, et al. Repeated triamcinolone acetonide injection in the treatment of upper‐lid retraction in patients with thyroid‐associated ophthalmopathy. Can J Ophthalmol. 2012;47:34‐41. 10.1016/j.jcjo.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 35. Lee SJ, Rim TH, Jang SY, et al. Treatment of upper eyelid retraction related to thyroid‐associated ophthalmopathy using subconjunctival triamcinolone injections. Graefes Arch Clin Exp Ophthalmol. 2013;251:261‐270. 10.1007/s00417-012-2153-y. [DOI] [PubMed] [Google Scholar]
- 36. Joos ZP, Sullivan TJ. Peri‐levator palpebrae superioris triamcinolone injection for the treatment of thyroid eye disease‐associated upper eyelid retraction. Clin Exp Ophthalmol. 2017;45:651‐652. 10.1111/ceo.12939. [DOI] [PubMed] [Google Scholar]
- 37. Xu S, Jia R, Ge S, et al. Treatment of periorbital infantile haemangiomas: a systematic literature review on propranolol or steroids. J Paediatr Child Health. 2014;50:271‐279. 10.1111/jpc.12464. [DOI] [PubMed] [Google Scholar]
- 38. Herwig MC, Wojno T, Zhang Q, et al. Langerhans cell histiocytosis of the orbit: five clinicopathologic cases and review of the literature. Surv Ophthalmol. 2013;58:330‐340. 10.1016/j.survophthal.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Woo KI, Harris GJ. Eosinophilic granuloma of the orbit: understanding the paradox of aggressive destruction responsive to minimal intervention. Ophthalmic Plast Reconstr Surg. 2003;19:429‐439. 10.1097/01.IOP.0000092800.86282.27. [DOI] [PubMed] [Google Scholar]
- 40. Singh S, Kaliki S, Reddy Palkonda VA, et al. Langerhans cell histiocytosis of the orbit: a study of eight cases. Oman J Ophthalmol. 2018;11:134‐139. 10.4103/ojo.OJO_226_2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Samara WA, Khoo CT, Say EA, et al. Juvenile Xanthogranuloma involving the eye and ocular adnexa: tumor control, visual outcomes, and globe salvage in 30 patients. Ophthalmology. 2015;122:2130‐2138. 10.1016/j.ophtha.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 42. Léauté‐Labrèze C, De La Roque ED, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649‐2651. [DOI] [PubMed] [Google Scholar]
- 43. Hoornweg MJ, Saeed P, Tanck MW, et al. Comparison of intralesional corticosteroid and propranolol treatment of periorbital infantile hemangiomas: an outcome study of 61 cases. Eur J Ophthalmol. 2014;24:940‐947. 10.5301/ejo.5000467. [DOI] [PubMed] [Google Scholar]
- 44. McNeill EJ, Kubba H, Bearn MA, et al. The management of rhinitis in patients with functional epiphora: a randomized controlled crossover trial. Am J Rhinol. 2005;19:588‐590. [PubMed] [Google Scholar]
- 45. Mansur C, Pfeiffer ML, Esmaeli B. Evaluation and management of chemotherapy‐induced epiphora, punctal and canalicular stenosis, and nasolacrimal duct obstruction. Ophthalmic Plast Reconstr Surg. 2017;33:9‐12. 10.1097/IOP.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 46. Goawalla A, Lee V. A prospective randomized treatment study comparing three treatment options for chalazia: triamcinolone acetonide injections, incision and curettage and treatment with hot compresses. Clin Exp Ophthalmol. 2007;35:706‐712. 10.1111/j.1442-9071.2007.01617.x. [DOI] [PubMed] [Google Scholar]
- 47. Ben Simon GJ, Rosen N, Rosner M, et al. Intralesional triamcinolone acetonide injection versus incision and curettage for primary chalazia: a prospective, randomized study. Am J Ophthalmol. 2011;151:714‐718. 10.1016/j.ajo.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 48. Aycinena AR, Achiron A, Paul M, et al. Incision and curettage versus steroid injection for the treatment of chalazia: a meta‐analysis. Ophthalmic Plast Reconstr Surg. 2016;32:220‐224. 10.1097/IOP.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 49. Yoo DB, Azizzadeh B, Massry GG. Injectable 5‐FU with or without added steroid in periorbital skin grafting: initial observations. Ophthalmic Plast Reconstr Surg. 2015;31:122‐126. 10.1097/IOP.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 50. Randleman JB, Stulting RD. Prevention and treatment of corneal graft rejection: current practice patterns (2004). Cornea. 2006;25:286‐290. 10.1097/01.ico.0000178731.42187.46. [DOI] [PubMed] [Google Scholar]
- 51. Rapuano CJ, Cohen EJ, Brady SE, et al. Indications for and outcomes of repeat penetrating keratoplasty. Am J Ophthalmol. 1990;109:689‐695. [DOI] [PubMed] [Google Scholar]
- 52. Kharod‐Dholakia B, Randleman JB, Bromley JG, et al. Prevention and treatment of corneal graft rejection: current practice patterns of the cornea society (2011). Cornea. 2015;34:609‐614. 10.1097/ICO.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 53. Kim HK, Choi JA, Uehara H, et al. Presurgical corticosteroid treatment improves corneal transplant survival in mice. Cornea. 2013;32:1591‐1598. 10.1097/ICO.0b013e31829ebb0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Koay PY, Lee WH, Figueiredo FC. Opinions on risk factors and management of corneal graft rejection in the United Kingdom. Cornea. 2005;24:292‐296. [DOI] [PubMed] [Google Scholar]
- 55. Rinne JR, Stulting RD. Current practices in the prevention and treatment of corneal graft rejection. Cornea. 1992;11:326‐328. [DOI] [PubMed] [Google Scholar]
- 56. The Collaborative Corneal Transplantation Studies (CCTS) . Effectiveness of histocompatibility matching in high‐risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch Ophthalmol. 1992;110:1392‐1403. [PubMed] [Google Scholar]
- 57. Hill JC, Maske R, Watson P. Corticosteroids in corneal graft rejection. Oral versus single pulse therapy. Ophthalmology. 1991;98:329‐333. [DOI] [PubMed] [Google Scholar]
- 58. Srinivasan M, Mascarenhas J, Rajaraman R, et al. Corticosteroids for bacterial keratitis: the Steroids for Corneal Ulcers Trial (SCUT). Arch Ophthalmol. 2012;130:143‐150. 10.1001/archophthalmol.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Srinivasan M, Mascarenhas J, Rajaraman R, et al. The steroids for corneal ulcers trial (SCUT): secondary 12‐month clinical outcomes of a randomized controlled trial. Am J Ophthalmol. 2014;157:327‐333. 10.1016/j.ajo.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ray KJ, Srinivasan M, Mascarenhas J, et al. Early addition of topical corticosteroids in the treatment of bacterial keratitis. JAMA Ophthalmol. 2014;132:737‐741. 10.1001/jamaophthalmol.2014.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Herretes S, Wang X, Reyes JM. Topical corticosteroids as adjunctive therapy for bacterial keratitis. Cochrane Database Syst Rev. 2014;10:CD005430 10.1002/14651858.CD005430.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wilhelmus KR, Gee L, Hauck WW, et al. Herpetic Eye Disease Study. A controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmology. 1994;101:1883‐1895. [DOI] [PubMed] [Google Scholar]
- 63. Collum LM, Logan P, Ravenscroft T. Acyclovir (Zovirax) in herpetic disciform keratitis. Br J Ophthalmol. 1983;67:115‐118. 10.1136/bjo.67.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Collum LM, Power WJ, Collum A. The current management of herpetic eye disease. Doc Ophthalmol. 1992;80:201‐205. [DOI] [PubMed] [Google Scholar]
- 65. Power WJ, Hillery MP, Benedict‐Smith A, et al. Acyclovir ointment plus topical betamethasone or placebo in first episode disciform keratitis. Br J Ophthalmol. 1992;76:711‐713. 10.1136/bjo.76.12.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Singhal D, Sahay P, Maharana PK, et al. Vernal keratoconjunctivitis. Surv Ophthalmol. 2019;64:289‐311. 10.1016/j.survophthal.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 67. Ang M, Ti SE, Loh R, et al. Steroid‐induced ocular hypertension in Asian children with severe vernal keratoconjunctivitis. Clin Ophthalmol. 2012;6:1253‐1258. 10.2147/OPTH.S32936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Costa AXD, Gomes JAP, Marculino LGC, et al. Supratarsal injection of triamcinolone for severe vernal keratoconjunctivitis in children. Arq Bras Oftalmol. 2017;80:186‐188. 10.5935/0004-2749.20170045. [DOI] [PubMed] [Google Scholar]
- 69. Singh S, Pal V, Dhull CS. Supratarsal injection of corticosteroids in the treatment of refractory vernal keratoconjunctivitis. Indian J Ophthalmol. 2001;49:241‐245. [PubMed] [Google Scholar]
- 70. Saini JS, Gupta A, Pandey SK, et al. Efficacy of supratarsal dexamethasone versus triamcinolone injection in recalcitrant vernal keratoconjunctivitis. Acta Ophthalmol Scand. 1999;77:515‐518. [DOI] [PubMed] [Google Scholar]
- 71. Gupta D, Illingworth C. Treatments for corneal neovascularization: a review. Cornea. 2011;30:927‐938. 10.1097/ICO.0b013e318201405a. [DOI] [PubMed] [Google Scholar]
- 72. Qazi Y, Wong G, Monson B, et al. Corneal transparency: genesis, maintenance and dysfunction. Brain Res Bull. 2010;81:198‐210. 10.1016/j.brainresbull.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chang JH, Gabison EE, Kato T, et al. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242‐249. [DOI] [PubMed] [Google Scholar]
- 74. Boneham GC, Collin HB. Steroid inhibition of limbal blood and lymphatic vascular cell growth. Curr Eye Res. 1995;14:1‐10. [DOI] [PubMed] [Google Scholar]
- 75. Kim TI, Kim SW, Kim S, et al. Inhibition of experimental corneal neovascularization by using subconjunctival injection of bevacizumab (Avastin). Cornea. 2008;27:349‐352. 10.1097/ICO.0b013e31815cf67d. [DOI] [PubMed] [Google Scholar]
- 76. Nirankari VS, Baer JC. Corneal argon laser photocoagulation for neovascularization in penetrating keratoplasty. Ophthalmology. 1986;93:1304‐1309. [DOI] [PubMed] [Google Scholar]
- 77. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15:575‐628. 10.1016/j.jtos.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 78. Marsh P, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjogren syndrome. Ophthalmology. 1999;106:811‐816. 10.1016/S0161-6420(99)90171-9. [DOI] [PubMed] [Google Scholar]
- 79. Sainz De La Maza Serra M, Simon Castellvi C, Kabbani O. Nonpreserved topical steroids and lacrimal punctal occlusion for severe keratoconjunctivitis sicca. Arch Soc Esp Oftalmol. 2000;75:751‐756. [PubMed] [Google Scholar]
- 80. Avunduk AM, Avunduk MC, Varnell ED, et al. The comparison of efficacies of topical corticosteroids and nonsteroidal anti‐inflammatory drops on dry eye patients: a clinical and immunocytochemical study. Am J Ophthalmol. 2003;136:593‐602. [DOI] [PubMed] [Google Scholar]
- 81. Pflugfelder SC, Maskin SL, Anderson B, et al. A randomized, double‐masked, placebo‐controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138:444‐457. 10.1016/j.ajo.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 82. Lee HK, Ryu IH, Seo KY, et al. Topical 0.1% prednisolone lowers nerve growth factor expression in keratoconjunctivitis sicca patients. Ophthalmology. 2006;113:198‐205. 10.1016/j.ophtha.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 83. Jonisch J, Steiner A, Udell IJ. Preservative‐free low‐dose dexamethasone for the treatment of chronic ocular surface disease refractory to standard therapy. Cornea. 2010;29:723‐726. 10.1097/ICO.0b013e3181b765a6. [DOI] [PubMed] [Google Scholar]
- 84. Sheppard JD, Donnenfeld ED, Holland EJ, et al. Effect of loteprednol etabonate 0.5% on initiation of dry eye treatment with topical cyclosporine 0.05%. Eye Contact Lens. 2014;40:289‐296. 10.1097/ICL.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 85. Kheirkhah A, Dohlman TH, Amparo F, et al. Effects of corneal nerve density on the response to treatment in dry eye disease. Ophthalmology. 2015;122:662‐668. 10.1016/j.ophtha.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lin T, Gong L. Topical fluorometholone treatment for ocular dryness in patients with Sjogren syndrome: a randomized clinical trial in China. Medicine (Baltimore). 2015;94:e551 10.1097/MD.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pinto‐Fraga J, Lopez‐Miguel A, Gonzalez‐Garcia MJ, et al. Topical fluorometholone protects the ocular surface of dry eye patients from desiccating stress: a randomized controlled clinical trial. Ophthalmology. 2016;123:141‐153. 10.1016/j.ophtha.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 88. Robinson MR, Lee SS, Rubin BI, et al. Topical corticosteroid therapy for cicatricial conjunctivitis associated with chronic graft‐versus‐host disease. Bone Marrow Transplant. 2004;33:1031‐1035. 10.1038/sj.bmt.1704453. [DOI] [PubMed] [Google Scholar]
- 89. Yin J, Kheirkhah A, Dohlman T, et al. Reduced efficacy of low‐dose topical steroids in dry eye disease associated with graft‐versus‐host disease. Am J Ophthalmol. 2018;190:17‐23. 10.1016/j.ajo.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wagoner MD. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol. 1997;41:275‐313. 10.1016/s0039-6257(96)00007-0. [DOI] [PubMed] [Google Scholar]
- 91. Donshik PC, Berman MB, Dohlman CH, et al. Effect of topical corticosteroids on ulceration in alkali‐burned corneas. Arch Ophthalmol. 1978;96:2117‐2120. 10.1001/archopht.1978.03910060497024. [DOI] [PubMed] [Google Scholar]
- 92. Davis AR, Ali QK, Aclimandos WA, et al. Topical steroid use in the treatment of ocular alkali burns. Br J Ophthalmol. 1997;81:732‐734. 10.1136/bjo.81.9.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Brodovsky SC, McCarty CA, Snibson G, et al. Management of alkali burns: an 11‐year retrospective review. Ophthalmology. 2000;107:1829‐1835. 10.1016/s0161-6420(00)00289-x. [DOI] [PubMed] [Google Scholar]
- 94. Queisi MM, Zein M, Lamba N, et al. Update on ocular cicatricial pemphigoid and emerging treatments. Surv Ophthalmol. 2016;61:314‐317. 10.1016/j.survophthal.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 95. Foster CS. Cicatricial pemphigoid. Trans Am Ophthalmol Soc. 1986;84:527‐663. [PMC free article] [PubMed] [Google Scholar]
- 96. Foster CS, Kothari S, Anesi SD, et al. The ocular immunology and uveitis foundation preferred practice patterns of uveitis management. Surv Ophthalmol. 2016;61:1‐17. 10.1016/j.survophthal.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 97. Roufas A, Jalaludin B, Gaskin C, et al. Subconjunctival triamcinolone treatment for non‐necrotising anterior scleritis. Br J Ophthalmol. 2010;94:743‐747. 10.1136/bjo.2009.164962. [DOI] [PubMed] [Google Scholar]
- 98. Sugar HS. Clinical effect of corticosteroids on Conjunctival filtering blebs; a case report. Am J Ophthalmol. 1965;59:854‐860. 10.1016/0002-9394(65)93017-5. [DOI] [PubMed] [Google Scholar]
- 99. Starita RJ, Fellman RL, Spaeth GL, et al. Short‐ and long‐term effects of postoperative corticosteroids on trabeculectomy. Ophthalmology. 1985;92:938‐946. 10.1016/s0161-6420(85)33931-3. [DOI] [PubMed] [Google Scholar]
- 100. Araujo SV, Spaeth GL, Roth SM, Starita RJ. Ten‐year follow‐up on a prospective, randomized trial of postoperative corticosteroids after trabeculectomy. Ophthalmology. 1995;102:1753‐1759. 10.1016/s0161-6420(95)30797-x. [DOI] [PubMed] [Google Scholar]
- 101. Giangiacomo J, Dueker DK, Adelstein E. The effect of preoperative subconjunctival triamcinolone administration on glaucoma filtration. I. Trabeculectomy following subconjunctival triamcinolone. Arch Ophthalmol. 1986;104:838‐841. 10.1001/archopht.1986.01050180072032. [DOI] [PubMed] [Google Scholar]
- 102. Yuki K, Shiba D, Kimura I, et al. Trabeculectomy with or without intraoperative sub‐tenon injection of triamcinolone acetonide in treating secondary glaucoma. Am J Ophthalmol. 2009;147:1055‐1060. 10.1016/j.ajo.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 103. Koike KJ, Chang PT. Trabeculectomy: a brief history and review of current trends. Int Ophthalmol Clin. 2018;58:117‐133. 10.1097/IIO.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 104. Zada M, Pattamatta U, White A. Modulation of fibroblasts in conjunctival wound healing. Ophthalmology. 2018;125:179‐192. 10.1016/j.ophtha.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 105. Seibold LK, Sherwood MB, Kahook MY. Wound modulation after filtration surgery. Surv Ophthalmol. 2012;57:530‐550. 10.1016/j.survophthal.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 106. Costa VP, Spaeth GL, Eiferman RA, et al. Wound healing modulation in glaucoma filtration surgery. Ophthalmic Surg. 1993;24:152‐170. [PubMed] [Google Scholar]
- 107. Fargione RA, Tansuebchueasai N, Lee R, et al. Etiology and management of the hypertensive phase in glaucoma drainage‐device surgery. Surv Ophthalmol. 2019;64:217‐224. 10.1016/j.survophthal.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 108. Ayyala RS, Zurakowski D, Smith JA, et al. A clinical study of the Ahmed glaucoma valve implant in advanced glaucoma. Ophthalmology. 1998;105:1968‐1976. 10.1016/S0161-6420(98)91049-1. [DOI] [PubMed] [Google Scholar]
- 109. Nouri‐Mahdavi K, Caprioli J. Evaluation of the hypertensive phase after insertion of the Ahmed glaucoma valve. Am J Ophthalmol. 2003;136:1001‐1008. 10.1016/s0002-9394(03)00630-5. [DOI] [PubMed] [Google Scholar]
- 110. Turalba AV, Pasquale LR. Hypertensive phase and early complications after Ahmed glaucoma valve implantation with intraoperative subtenon triamcinolone acetonide. Clin Ophthalmol. 2014;11:1311‐1316. 10.2147/OPTH.S64257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yazdani S, Doozandeh A, Pakravan M, et al. Adjunctive triamcinolone acetonide for Ahmed glaucoma valve implantation: a randomized clinical trial. Eur J Ophthalmol. 2017;27:411‐416. 10.5301/ejo.5000892. [DOI] [PubMed] [Google Scholar]
- 112. Lee R, Wong T, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015;2:17 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Romero‐Aroca P, Baget‐Bernaldiz M, Pareja‐Rios A, et al. Diabetic macular edema pathophysiology: vasogenic versus inflammatory. J Diabetes Res. 2016;2016:2156273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Regillo CD, Callanan DG, Do DV, et al. Use of corticosteorids in the treatment of patients with diabetic macular edema who have a suboptimal repsponse to anti‐VEGF: recommendations of an expert panel. OSLI. 2017;48:291‐301. [DOI] [PubMed] [Google Scholar]
- 115. Gillies MC, Sutter FK, Simpson JM, et al. Intravitreal triamcinolone for refractory diabetic macular edema: two‐year results of a double‐masked, placebo‐controlled, randomized clinical trial. Ophthalmology. 2006;113:1533‐1538. [DOI] [PubMed] [Google Scholar]
- 116. Diabetic Retinopathy Clinical Research N , Elman MJ, Aiello LP, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064‐1077.e1035. 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bressler SB, Glassman AR, Almukhtar T, et al. Five‐year outcomes of ranibizumab with prompt or deferred laser versus laser or triamcinolone plus deferred ranibizumab for diabetic macular edema. Am J Ophthalmol. 2016;164:57‐68. 10.1016/j.ajo.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Boyer DS, Yoon YH, Belfort R Jr, et al. Three‐year, randomized, sham‐controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904‐1914. 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 119. Gillies MC, Lim LL, Campain A, et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology. 2014;121:2473‐2481. 10.1016/j.ophtha.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 120. Fraser‐Bell S, Lim LL, Campain A, et al. Bevacizumab or dexamethasone implants for DME: 2‐year results (The BEVORDEX Study). Ophthalmology. 2016;123:1399‐1401. 10.1016/j.ophtha.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 121. Campochiaro PA, Brown DM, Pearson A, et al. Long‐term benefit of sustained‐delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626‐635.e622. 10.1016/j.ophtha.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 122. Ehlers JP, Kim SJ, Yeh S, et al. Therapies for macular edema associated with branch retinal vein occlusion: a report by the American Academy of Ophthalmology. Ophthalmology. 2017;124:1412‐1423. 10.1016/j.ophtha.2017.03.060. [DOI] [PubMed] [Google Scholar]
- 123. Haller JA, Bandello F, Belfort R Jr, et al. Randomized, sham‐controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134‐1146.e1133. 10.1016/j.ophtha.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 124. Brown DM, Campochiaro PA, Singh RJ, et al. Ranibizumab for macular edema following central retinal vein occlusion six‐month primary end point results of a phase III study. Ophthalmology. 2010;117:1124‐1133. [DOI] [PubMed] [Google Scholar]
- 125. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve‐month outcomes of a phase III study. Ophthalmology. 2011;118:2041‐2049. [DOI] [PubMed] [Google Scholar]
- 126. Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1‐year results from the phase 3 COPERNICUS Study. Am J Ophthalmol. 2013;155:429‐437. [DOI] [PubMed] [Google Scholar]
- 127. Holz FG, Roider J, Ogura Y, et al. VEGF trap‐eye for macular oedema secondary to central retinal vein occlusion: 6‐month results of the phase III GALILEO study. Br J Ophthalmol. 2015;99:1746‐1746. [DOI] [PubMed] [Google Scholar]
- 128. Scott IU, van Veldhuisen PC, Ip MS, et al. Effect of Bevacizumab vs Aflibercept on visual acuity among patients with macular Edema due to central retinal vein occlusion: the SCORE2 randomized clinical trial. JAMA. 2017;317:2072‐2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Epstein D, Algvere P, von Wendt G, et al. Long‐term benefit from bevacizumab for macular edema in central retinal vein occlusion: 12‐month results of a prospective study: Mo‐Sw1‐1. Acta Ophthalmologica. 2012;90:48. [DOI] [PubMed] [Google Scholar]
- 130. Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six‐month primary end point results of a phase III study. Ophthalmology. 2010;117:1102‐1112.e1101. 10.1016/j.ophtha.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 131. Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12‐month outcomes of a phase III study. Ophthalmology. 2011;118:1594‐1602. 10.1016/j.ophtha.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 132. Campochiaro PA, Clark WL, Boyer DS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24‐week results of the VIBRANT study. Ophthalmology. 2015;122:538‐544. 10.1016/j.ophtha.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 133. Clark WL, Boyer DS, Heier JS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52‐week results of the VIBRANT Study. Ophthalmology. 2016;123:330‐336. 10.1016/j.ophtha.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 134. Russo V, Barone A, Conte E, et al. Bevacizumab compared with macular laser grid photocoagulation for cystoid macular edema in branch retinal vein occlusion. Retina. 2009;29:511‐515. 10.1097/IAE.0b013e318195ca65. [DOI] [PubMed] [Google Scholar]
- 135. Alshareef RA, Garg SJ, Hsu J, et al. Intravitreal triamcinolone acetonide injection for macular edema due to central retinal vein occlusion persisting despite multiple intravitreal bevacizumab injections. JOcul Pharmacol Ther. 2014;30:512‐516. [DOI] [PubMed] [Google Scholar]
- 136. Anonymous . Argon laser photocoagulation for macular edema in branch vein occlusion. The Branch Vein Occlusion Study Group. Am J Ophthalmol. 1984;98:271‐282. [DOI] [PubMed] [Google Scholar]
- 137. Anonymous . Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion. A randomized clinical trial. Branch Vein Occlusion Study Group. Arch Ophthalmol. 1986;104:34‐41. [DOI] [PubMed] [Google Scholar]
- 138. Ramchandran RS, Fekrat S, Stinnett SS, et al. Fluocinolone acetonide sustained drug delivery device for chronic central retinal vein occlusion: 12‐month results. Am J Ophthalmol. 2008;146:285‐291. 10.1016/j.ajo.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 139. Jain N, Stinnett SS, Jaffe GJ. Prospective study of a fluocinolone acetonide implant for chronic macular edema from central retinal vein occlusion: thirty‐six‐month results. Ophthalmology. 2012;119:132‐137. 10.1016/j.ophtha.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 140. Sen HN, Vitale S, Gangaputra SS, et al. Periocular corticosteroid injections in uveitis: effects and complications. Ophthalmology. 2014;121:2275‐2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Multicenter Uveitis Steroid Treatment Trial Research Group , Kempen JH, Altaweel MM, et al. Benefits of systemic anti‐inflammatory therapy versus fluocinolone acetonide intraocular implant for intermediate uveitis, posterior uveitis, and panuveitis: fifty‐four‐month results of the multicenter uveitis steroid treatment (MUST) trial and follow‐up study. Ophthalmology. 2015;122:1967‐1975. 10.1016/j.ophtha.2015.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Writing Committee for the Multicenter Uveitis Steroid Treatment Trial , Follow‐up Study Research Group , Kempen JH, et al. Association between long‐lasting intravitreous fluocinolone acetonide implant vs systemic anti‐inflammatory therapy and visual acuity at 7 years among patients with intermediate, posterior, or panuveitis. JAMA. 2017;317:1993‐2005. 10.1001/jama.2017.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kok H, Lau C, Maycock N, et al. Outcome of intravitreal triamcinolone in uveitis. Ophthalmology. 1916;2005(112):e1911‐e1917. [DOI] [PubMed] [Google Scholar]
- 144. Maca SM, Abela‐Formanek C, Kiss CG, et al. Intravitreal triamcinolone for persistent cystoid macular oedema in eyes with quiescent uveitis. Clin Experiment Ophthalmol. 2009;37:389‐396. 10.1111/j.1442-9071.2009.02033.x. [DOI] [PubMed] [Google Scholar]
- 145. Sallam A, Taylor SR, Habot‐Wilner Z, et al. Repeat intravitreal triamcinolone acetonide injections in uveitic macular oedema. Acta Ophthalmol (Oxf). 2012;90:e323‐e325. 10.1111/j.1755-3768.2011.02247.x. [DOI] [PubMed] [Google Scholar]
- 146. Lowder C, Belfort R Jr, Lightman S, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129:545‐553. 10.1001/archophthalmol.2010.339. [DOI] [PubMed] [Google Scholar]
- 147. Thorne JE, Sugar EA, Holbrook JT, et al. Periocular triamcinolone vs. intravitreal triamcinolone vs. intravitreal dexamethasone implant for the treatment of uveitic macular edema: the PeriOcular vs. INTravitreal corticosteroids for uveitic macular edema (POINT) trial. Ophthalmology. 2019;126(2):283‐295. 10.1016/j.ophtha.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Moisseiev E, Abbassi S, Park SS. Intravitreal dexamethasone in the management of acute endophthalmitis: a comparative retrospective study. Eur J Ophthalmol. 2017;27:67‐73. 10.5301/ejo.5000866. [DOI] [PubMed] [Google Scholar]
- 149. Bui DK, Carvounis PE. Evidence for and against intravitreous corticosteroids in addition to intravitreous antibiotics for acute endophthalmitis. Int Ophthalmol Clin. 2014;54:215‐224. 10.1097/IIO.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kwak HW, D'Amico DJ. Evaluation of the retinal toxicity and pharmacokinetics of dexamethasone after intravitreal injection. Arch Ophthalmol. 1992;110:259‐266. [DOI] [PubMed] [Google Scholar]
- 151. Das T, Jalali S, Gothwal VK, et al. Intravitreal dexamethasone in exogenous bacterial endophthalmitis: results of a prospective randomised study. Br J Ophthalmol. 1999;83:1050‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Gan IM, Ugahary LC, van Dissel JT, et al. Intravitreal dexamethasone as adjuvant in the treatment of postoperative endophthalmitis: a prospective randomized trial. Graefes Arch Clin Exp Ophthalmol. 2005;243:1200‐1205. [DOI] [PubMed] [Google Scholar]
- 153. Albrecht E, Richards JC, Pollock T, et al. Adjunctive use of intravitreal dexamethasone in presumed bacterial endophthalmitis: a randomised trial. BrJ Ophthalmol. 2011;95:1385‐1388. 10.1136/bjo.2010.187963. [DOI] [PubMed] [Google Scholar]
- 154. Manning S, Ugahary LC, Lindstedt EW, et al. A prospective multicentre randomized placebo‐controlled superiority trial in patients with suspected bacterial endophthalmitis after cataract surgery on the adjuvant use of intravitreal dexamethasone to intravitreal antibiotics. Acta Ophthalmol (Oxf). 2018;96:348‐355. 10.1111/aos.13610. [DOI] [PubMed] [Google Scholar]
- 155. Majji AB, Jalali S, Das T, et al. Role of intravitreal dexamethasone in exogenous fungal endophthalmitis. Eye. 1999;13:660‐665. [DOI] [PubMed] [Google Scholar]
- 156. Shelsta HN, Jampol LM. Pharmacologic therapy of pseudophakic cystoid macular edema: 2010 update. Retina. 2010;31:4‐12. [DOI] [PubMed] [Google Scholar]
- 157. Karacorlu M, Ozdemir H, Karacorlu S. Intravitreal triamcinolone acetonide for the treatment of chronic pseudophakic cystoid macular oedema. Acta Ophthalmol Scand. 2003;81:648‐652. [DOI] [PubMed] [Google Scholar]
- 158. Konstantopoulos A, Williams CP, Luff AJ. Outcome of intravitreal triamcinolone acetonide in postoperative cystoid macular oedema. Eye. 2008;22:219‐222. [DOI] [PubMed] [Google Scholar]
- 159. Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide for pseudophakic cystoid macular edema. Am J Ophthalmol. 2003;136:384‐386. [DOI] [PubMed] [Google Scholar]
- 160. Bonfiglio V, Reibaldi M, Fallico M, et al. Widening use of dexamethasone implant for the treatment of macular edema. Drug Design Dev Therapy. 2017;11:2359‐2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ranchod TM, Ray SK, Daniels SA, et al. LuceDex: a prospective study comparing ranibizumab plus dexamethasone combination therapy versus ranibizumab monotherapy for neovascular age‐related macular degeneration. Retina. 2013;33:1600‐1604. 10.1097/IAE.0b013e318285cb71. [DOI] [PubMed] [Google Scholar]
- 162. Rouvas AA, Chatziralli IP, Theodossiadis PG, et al. Long‐term results of intravitreal ranibizumab, intravitreal ranibizumab with photodynamic therapy, and intravitreal triamcinolone with photodynamic therapy for the treatment of retinal angiomatous proliferation. Retina. 2012;32:1181‐1189. 10.1097/IAE.0b013e318235d8ce. [DOI] [PubMed] [Google Scholar]
- 163. Wu LM, Evans TM, Arevalo JFMF, et al. Long‐term effect of intravitreal triamcinolone in the nonproliferative state of type II idiopathic parafoveal telangiectasia. Retina. 2008;28:314‐319. [DOI] [PubMed] [Google Scholar]
- 164. Sandali O, Akesbi J, Rodallec T, Laroche L, Nordmann JP. Dexamethasone implant for the treatment of edema related to idiopathic macular telangiectasia. Can J Ophthalmol. 2013;48:e78‐e80. [DOI] [PubMed] [Google Scholar]
- 165. Ghazi NG, Al Shamsi H, Larsson J, et al. Intravitreal triamcinolone in coats' disease. Ophthalmology. 2012;119:648‐649. [DOI] [PubMed] [Google Scholar]
- 166. Cebeci Z, Oray M, Tuncer S, et al. Intravitreal dexamethasone implant (Ozurdex) and photodynamic therapy for vasoproliferative retinal tumours. Can J Ophthalmol. 2014;49:e83‐e84. 10.1016/j.jcjo.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 167. Sutter FK, Gillies MC. Intravitreal triamcinolone for radiation‐induced macular edema. Arch Ophthalmol. 2003;121:1491‐1493. [DOI] [PubMed] [Google Scholar]
- 168. Caminal JM, Flores‐Moreno I, Arias L, et al. Intravitreal dexamethasone implant for radiation maculopathy secondary to plaque brachytherapy in choroidal melanoma. Retina. 2015;35:1890‐1897. 10.1097/IAE.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 169. Scorolli L, Morara M, Meduri A, et al. Treatment of cystoid macular edema in retinitis pigmentosa with intravitreal triamcinolone. Arch Ophthalmol. 2007;125:759‐764. [DOI] [PubMed] [Google Scholar]
- 170. Ahn SJ, Kim KE, Woo SJ, et al. The effect of an intravitreal dexamethasone implant for cystoid macular edema in retinitis pigmentosa: a case report and literature review. Ophthalmic Surg Lasers Imaging Retina. 2014;45:160‐164. 10.3928/23258160-20140131-03. [DOI] [PubMed] [Google Scholar]
- 171. Banerjee PJ, Quartilho A, Bunce C, et al. Slow‐release dexamethasone in proliferative vitreoretinopathy: a prospective, randomized controlled clinical trial. Ophthalmology. 2017;124:757‐767. 10.1016/j.ophtha.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 172. Chen W, Chen H, Hou P, et al. Midterm results of low‐dose intravitreal triamcinolone as adjunctive treatment for proliferative vitreoretinopathy. Retina. 2011;31:1137‐1142. 10.1097/IAE.0b013e3181fe5427. [DOI] [PubMed] [Google Scholar]
- 173. Ahmadieh H, Feghhi M, Tabatabaei H, et al. Triamcinolone acetonide in silicone‐filled eyes as adjunctive treatment for proliferative vitreoretinopathy: a randomized clinical trial. Ophthalmology. 2008;115:1938‐1943. 10.1016/j.ophtha.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 174. Bonfiglio V, Fallico MR, Russo A, et al. Intravitreal dexamethasone implant for cystoid macular edema and inflammation after scleral buckling. Eur J Ophthalmol. 2015;25:e98‐e100. 10.5301/ejo.5000599. [DOI] [PubMed] [Google Scholar]
- 175. Taney LS, Baumal CR, Duker JS. Sustained‐release dexamethasone intravitreal implant for persistent macular edema after vitrectomy for epiretinal membrane. Ophthalmic Surg Lasers Imaging Retina. 2015;46:224‐228. 10.3928/23258160-20150213-01. [DOI] [PubMed] [Google Scholar]
- 176. Scott IU, Flynn HW Jr, Rosenfeld PJ. Intravitreal triamcinolone acetonide for idiopathic cystoid macular edema. Am J Ophthalmol. 2003;136:737‐739. [DOI] [PubMed] [Google Scholar]
- 177. Couch SM, Bakri SJ. Use of triamcinolone during vitrectomy surgery to visualize membranes and vitreous. Clin Ophthalmol. 2008;2:891‐896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Kumar CM, Eid H, Dodds C. Sub‐Tenon's anaestheia: complications and their prevention. Eye. 2011;25:694‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Kumar CM. Orbital regional anesthesia: complications and their prevention. Indian J Ophthalmol. 2006;54:77‐84. [DOI] [PubMed] [Google Scholar]
- 180. Gupta OP, Boynton JR, Sabini P, et al. Proptosis after retrobulbar corticosteroid injections. Ophthalmology. 2003;110:443‐447. [DOI] [PubMed] [Google Scholar]
- 181. Smith JR, George RK, Rosenbaum JT. Lower eyelid herniation of orbital fat may complicate periocular corticosteroid injection. Am J Ophthalmol. 2002;133:845‐847. [DOI] [PubMed] [Google Scholar]
- 182. Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti‐VEGF agents: a review of literature. Eye. 2013;27:787‐794. 10.1038/eye.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Westfall AC, Osborn A, Kuhl D, et al. Acute endophthalmitis incidence: intravitreal triamcinolone. Arch Ophthalmol. 2005;123:1075‐1077. [DOI] [PubMed] [Google Scholar]
- 184. Khurana RN, Appa SN, McCannel CA, et al. Dexamethasone implant anterior chamber migration: risk factors, complications, and management strategies. Ophthalmology. 2014;121:67‐71. 10.1016/j.ophtha.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 185. Aref AA, Scott IU, Oden NL, et al. Timing of elevated intraocular pressure after intravitreal triamcinolone acetonide injection for macular edema secondary to retinal vein occlusion: SCORE study report 15. JAMA Ophthalmol. 2015;133:1022‐1029. 10.1001/jamaophthalmol.2015.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Vasconcelos‐Santos DV, Nehemy PG, Schachat AP, et al. Secondary ocular hypertension after intravitreal injection of 4 mg of triamcinolone acetonide: incidence and risk factors. Retina. 2008;28:573‐580. 10.1097/IAE.0b013e31816079e8. [DOI] [PubMed] [Google Scholar]
- 187. Smithen LM, Ober MD, Maranan L, et al. Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004;138:740‐743. [DOI] [PubMed] [Google Scholar]
- 188. Roth DB, Verma V, Realini T, et al. Long‐term incidence and timing of intraocular hypertension after intravitreal triamcinolone acetonide injection. Ophthalmology. 2009;116:455‐460. 10.1016/j.ophtha.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 189. Callanan DG, Gupta S, Boyer DS, et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology. 2013;120:1843‐1851. 10.1016/j.ophtha.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 190. Rajesh B, Zarranz‐Ventura J, Fung AT, et al. Safety of 6000 intravitreal dexamethasone implants. Br J Ophthalmol. 2019;04:30 10.1136/bjophthalmol-2019-313991. [DOI] [PubMed] [Google Scholar]
- 191. Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119:2125‐2132. 10.1016/j.ophtha.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 192. Totan Y, Guler E, Guragac FB, et al. Cystoid macular edema associated with juvenile idiopathic arthritis resolved by a dexamethasone intravitreal implant. J Pediatr Ophthalmol Strabismus. 2014;51 :e25‐e28. 10.3928/01913913-20140429-01. [DOI] [PubMed] [Google Scholar]
- 193. Winterhalter S, Behrens UD, Salchow D, et al. Dexamethasone implants in paediatric patients with noninfectious intermediate or posterior uveitis: first prospective exploratory case series. BMC Ophthalmol. 2017;17:252 10.1186/s12886-017-0648-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Sella R, Oray M, Friling R, et al. Dexamethasone intravitral implant (Ozurdex) for pediatric uveitis. Graefes Arch Clin Exp Ophthalmol. 2015;253:1777‐1782. [DOI] [PubMed] [Google Scholar]
- 195. Kwok AK, Lam DS, Ng JS, et al. Ocular‐hypertensive response to topical steroids in children. Ophthalmology. 1997;104:2112‐2116. [DOI] [PubMed] [Google Scholar]
- 196. Nuyen B, Weinreb RN, Robbins SL. Steroid‐induced glaucoma in the pediatric population. J Aapos. 2017;21:1‐6. 10.1016/j.jaapos.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 197. Fan DS, Ng JS, Lam DS. A prospective study on ocular hypertensive and antiinflammatory response to different dosages of fluorometholone in children. Ophthalmology. 2001;108:1973‐1977. [DOI] [PubMed] [Google Scholar]
- 198. Arcinue CA, Ceron OM, Foster CS. A comparison between the fluocinolone acetonide (Retisert) and dexamethasone (Ozurdex) intravitreal implants in uveitis. J Ocul Pharmacol Ther. 2013;29:501‐507. 10.1089/jop.2012.0180. [DOI] [PubMed] [Google Scholar]
- 199. Yang Y, Bailey C, Holz FG, et al. Long‐term outcomes of phakic patients with diabetic macular oedema treated with intravitreal fluocinolone acetonide (FAc) implants. Eye (Lond). 2015;29:1240 10.1038/eye.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Habot‐Wilner Z, Sallam A, Roufas A, et al. Periocular corticosteroid injection in the management of uveitis in children. Acta Ophthalmol (Oxf). 2010;88:e299‐e304. 10.1111/j.1755-3768.2010.02025.x. [DOI] [PubMed] [Google Scholar]
- 201. Roth DB, Flynn HW Jr. Distinguishing between infectious and noninfectious endophthalmitis after intravitreal triamcinolone injection. Am J Ophthalmol. 2008;146:346‐347. 10.1016/j.ajo.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 202. Chang AA, Li H, Broadhead GK, et al. Safety and efficacy of intravitreal preservative‐free triamcinolone acetonide (Triesence) for macular edema. J Ocul Pharmacol Ther. 2015;31:563‐569. 10.1089/jop.2015.0021. [DOI] [PubMed] [Google Scholar]
- 203. Otsuka H, Kawano H, Sonoda S, et al. Particle‐induced endophthalmitis: possible mechanisms of sterile endophthalmitis after intravitreal triamcinolone. Invest Ophthalmol Vis Sci. 2013;54:1758‐1766. 10.1167/iovs.12-11247. [DOI] [PubMed] [Google Scholar]
- 204. Carrerro JL, Barcia MG, Flores IP. Sterile endophthalmitis after benzyl alcohol–filtered triamcinolone acetonide injection. Arch Ophthalmol. 2008;126:142‐143. [DOI] [PubMed] [Google Scholar]
- 205. Krah JL, Krimmel D, Dodwell D. Sterile endophthalmitis secondary to intravitreal triesence (triamcinolone acetonide). Invest Ophthalmol Vis Sci. 2009;50:3565. [Google Scholar]
- 206. Roth DB, Prenner JL, Krajnky O. Incidence of non‐infectious endophthalmitis after intravitreal injection of preservative‐free triamcinolone acetonide. Retin Cases Brief Rep. 2008;2:247‐249. [DOI] [PubMed] [Google Scholar]
- 207. Dodwell DG, Krimmell DA, de Fiebre CM. Sterile endophthalmitis rates and particle size analyses of different formulations of triamcinolone acetonide. Clin Ophthalmol. 2015;9:1033‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Takakura A, Tessler HH, Goldstein DA, et al. Viral retinitis following intraocular or periocular corticosteroid administration: a case series and comprehensive review of the literature. Ocul Immunol Inflamm. 2014;22:175‐182. 10.3109/09273948.2013.866256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Bakri SJ, Kaiser PK. Posterior sub‐Tenon triamcinolone acetonide for refractory diabetic macular edema. Am J Ophthalmol. 2005;139:290‐294. 10.1016/j.ajo.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 210. Todokoro D, Hoshino J, Yo A, et al. Scedosporium apiospermum infectious scleritis following posterior sub‐Tenon triamcinolone acetonide injection: a case report and literature review. BMC Ophthalmol. 2018;18:40 10.1186/s12886-018-0707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Jusufbegovic D, Schaal S. Quiescent herpes simplex keratitis reactivation after intravitreal injection of dexamethasone implant. Retinal Cases Brief Reports. 2017;11:296‐297. 10.1097/ICB.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 212. Gulkilik G, Demirci G, Ozdamar AM, et al. A case of herpetic keratitis after intravitreal triamcinolone injection. Cornea. 2007;26:1000‐1001. 10.1097/ICO.0b013e3180cab659. [DOI] [PubMed] [Google Scholar]
- 213. Song JH, Hong YT, Kwon OW. Acute syphilitic posterior placoid chorioretinitis following intravitreal triamcinolone acetonide injection. Graefes Arch Clin Exp Ophthalmol. 2008;246:1775‐1778. 10.1007/s00417-008-0928-y. [DOI] [PubMed] [Google Scholar]
- 214. Mushtaq B, Gupta R, Elsherbiny S, et al. Ocular syphilis unmasked following intravitreal triamcinolone injection. Ocul Immunol Inflamm. 2009;17:213‐215. 10.1080/09273940902745411. [DOI] [PubMed] [Google Scholar]
- 215. Erol N, Topbas S. Acute syphilitic posterior placoid chorioretinitis after an intravitreal triamcinolone acetonide injection. Acta Ophthalmol Scand. 2006;84:435 10.1111/j.1600-0420.2005.00641.x. [DOI] [PubMed] [Google Scholar]
- 216. Fonollosa A, Llorenc V, Artaraz J, et al. Safety and efficacy of intravitreal dexamethasone implants in the management of macular edema secondary to infectious uveitis. Retina. 2016;36:1778‐1785. 10.1097/IAE.0000000000001001. [DOI] [PubMed] [Google Scholar]
- 217. Carvalho‐Recchia CA, Yannuzzi LA, Negrao S, et al. Corticosteroids and central serous chorioretinopathy. Ophthalmology. 2002;109:1834‐1837. 10.1016/s0161-6420(02)01117-x. [DOI] [PubMed] [Google Scholar]
- 218. Nicholson BP, Atchison E, Idris AA, et al. Central serous chorioretinopathy and glucocorticoids: an update on evidence for association. Surv Ophthalmol. 2018;63:1‐8. 10.1016/j.survophthal.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 219. Imasawa M, Ohshiro T, Gotoh T, et al. Central serous chorioretinopathy following vitrectomy with intravitreal triamcinolone acetonide for diabetic macular oedema. Acta Ophthalmol Scand. 2005;83:132‐133. 10.1111/j.1600-0420.2005.00379.x. [DOI] [PubMed] [Google Scholar]
- 220. Kocabora MS, Durmaz S, Kandemir N. Exacerbation of central serous chorioretinopathy following intravitreal triamcinolone injection. Graefes Arch Clin Exp Ophthalmol. 2008;246:1783‐1786. 10.1007/s00417-008-0932-2. [DOI] [PubMed] [Google Scholar]
- 221. Georgalas I, Petrou P, Pagoulatos D, et al. Central serous chorioretinopathy in the fellow eye as a complication of intravitreal dexamethasone implant for the treatment of Irvine‐Gass syndrome. Clin Exp Optom. 2016;99:601‐603. 10.1111/cxo.12420. [DOI] [PubMed] [Google Scholar]
- 222. Bowie EM, Folk JC, Barnes CH. Corticosteroids, central serous chorioretinopathy, and neurocysticercosis. Arch Ophthalmol. 2004;122:281‐283. 10.1001/archopht.122.2.281. [DOI] [PubMed] [Google Scholar]
- 223. Jonas JB, Kamppeter BA. Intravitreal triamcinolone acetonide and central serous chorioretinopathy. Br J Ophthalmol. 2005;89:386‐387. 10.1136/bjo.2004.054247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Patron ME, Vuong LN, Duker JS. Intravitreal triamcinolone acetonide for cystoid macular edema secondary to central serous chorioretinopathy. Retinal Cases Brief Rep. 2009;3:319‐322. 10.1097/ICB.0b013e31819b19ee. [DOI] [PubMed] [Google Scholar]
- 225. Baumal CR, Martidis A, Truong SN. Central serous chorioretinopathy associated with periocular corticosteroid injection treatment for HLA‐B27‐associated iritis. Arch Ophthalmol. 2004;122:926‐928. 10.1001/archopht.122.6.926. [DOI] [PubMed] [Google Scholar]
- 226. Alkawas AA, Hussein AM, Shahien EA. Orbital steroid injection versus oral steroid therapy in management of thyroid‐related ophthalmopathy. Clin Exp Ophthalmol. 2010;38:692‐697. 10.1111/j.1442-9071.2010.02332.x. [DOI] [PubMed] [Google Scholar]
- 227. Breusegem C, Spielberg L, Van Ginderdeuren R, et al. Preoperative nonsteroidal anti‐inflammatory drug or steroid and outcomes after trabeculectomy: a randomized controlled trial. Ophthalmology. 2010;117:1324‐1330. 10.1016/j.ophtha.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 228. Thorne JE, Sugar EA, Holbrook JT, et al. Periocular triamcinolone vs. intravitreal triamcinolone vs. intravitreal dexamethasone implant for the treatment of uveitic macular edema: the PeriOcular vs. INTravitreal corticosteroids for uveitic macular edema (POINT) trial. Ophthalmology. 2019;126:283‐295. 10.1016/j.ophtha.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Thach AB, Dugel PU, Flindall RJ, Sipperley JO, Sneed SR. A comparison of retrobulbar versus sub‐Tenon's corticosteroid therapy for cystoid macular edema refractory to topical medications. Ophthalmology. 1997;104:2003‐2008. 10.1016/s0161-6420(97)30065-7. [DOI] [PubMed] [Google Scholar]


