Abstract
Background & Aims
Human immunodeficiency virus (HIV)/hepatitis C virus (HCV) coinfection is common in people who inject drugs (PWIDs). Recently, ‘high‐risk’ behaviour among men who have sex with men (MSM) has emerged as another main route of HCV transmission. We analysed temporal trends in HCV epidemiology in a cohort of Viennese HIV+ patients.
Methods
Hepatitis C virus parameters were recorded at HIV diagnosis (baseline [BL]) and last visit (follow‐up [FU]) for all HIV+ patients attending our HIV clinic between January 2014 and December 2016. Proportions of HIV+ patients with anti‐HCV(+) and HCV viraemia (HCV‐RNA(+)) at BL/FU were assessed and stratified by route of transmission.
Results
In all, 1806/1874 (96.4%) HIV+ patients were tested for HCV at BL. Anti‐HCV(+) was detected in 93.2% (276/296) of PWIDs and in 3.7% (31/839) of MSM. After a median FU of 6.9 years, 1644 (91.0%) patients underwent FU HCV‐testing: 167 (90.3%) of PWIDs and 49 (6.7%) of MSM showed anti‐HCV(+). Among 208 viraemic HCV‐RNA(+) patients at BL, 30 (14.4%) had spontaneously cleared HCV, 76 (36.5%) achieved treatment‐induced eradication and 89 (42.8%) remained HCV‐RNA(+) at last FU. Among 1433 initially HCV‐naive patients, 45 (3.5%) acquired de‐novo HCV infection (11.1% PWIDs/80.0% MSM; incidence rate (IR) 0.004%; 95% confidence interval [CI] 0.0%‐0.022%) and 14 had HCV reinfections (85.7% PWIDs/14.3% other; IR 0.001%; 95% CI 0.0%‐0.018%) during a median FU of 6.7 years (interquartile range 7.4).
Conclusion
Hepatitis C virus testing was successfully implemented in the Viennese HIV(+) patients. Anti‐HCV(+) prevalence remained stable in HIV+ PWIDs but almost doubled in HIV+ MSM. De‐novo HCV infection occurred mostly in MSM, while HCV reinfections were mainly observed in PWIDs. HCV treatment uptake was suboptimal with 42.8% remaining HCV‐RNA(+) at FU.
Keywords: epidemiology, Hepatitis C virus, human immunodeficiency virus, MSM, PWIDs
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- ALT
alanine aminotransferase
- BL
baseline
- CI
confidence interval
- DAA
direct acting antiviral
- FIB4‐Score
fibrosis‐4 Score
- FU
follow‐up
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IDU
intravenous drug use
- IFN
interferon
- IQR
interquartile range
- IR
incidence rate
- MSM
men who have sex with men
- PCR
polymerase chain reaction
- PEGIFN
pegylated interferon
- PWIDs
people who inject drugs
- RBV
ribavirin
- RNA
ribonucleic acid
- SVR
sustained virological response
- TE
transient elastography
- WHO
World Health Organization
Key points.
Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) coinfection used to mostly affect people with injection drug use.
Our analysis of temporal trends of HCV coinfection among Viennese HIV(+) patients showed a high overall HCV test rate.
Interestingly, increasing numbers of new HCV/HIV coinfection in men who have sex with men, likely due to high‐risk sexual behaviour were documented.
HCV treatment uptake needs to be improved in Viennese HIV+ patients in order to reach the World Health Organization goal of HCV elimination by 2030.
1. INTRODUCTION
Hepatitis C virus (HCV) infection remains a leading cause for cirrhosis, hepatocellular carcinoma and liver‐related death worldwide.1 While human immunodeficiency virus (HIV) incidence and acquired immunodeficiency syndrome (AIDS)‐related deaths show a global decrease, hepatitis C‐induced liver disease – mainly due to the shared transmission routes – remains a major contributor to morbidity and mortality in the HIV+ patient population.2, 3
Human immunodeficiency virus infection accelerates the progression of liver disease towards end‐stage liver disease and portal hypertension, being most pronounced in immunocompromised patients.4, 5 Therefore, early diagnosis and treatment of HCV as well as follow‐up (FU) after achieving sustained virological response (SVR) are essential.6
While people who inject drugs (PWIDs) are known to have an increased risk for the acquisition of HIV and HCV, recent trends in risk behaviour, especially among HIV+ men who have sex with men (MSM) and transgender women, have been reported as another main HCV transmission route.7, 8, 9, 10 The use of drugs to enhance sexual experience (‘chemsex’) has become a major contributing factor in the current HCV epidemic among MSM as it stimulates high‐risk sexual behaviour including promiscuity, traumatic anal intercourse and extensive sex duration as well as condomless sex, which in turn promotes the transmission of HIV, HCV and other sexually transmitted infections.9, 10, 11, 12, 13 While in PWIDs, especially in case of ongoing intravenous drug use (IDU) or opioid substitution, rates of HCV diagnosis and treatment uptake still remain low, MSM tend to show high treatment uptake and SVR rates.13, 14 French data collected between 2012 and 2016 showed a considerable fluctuation in the incidence of HCV reinfections after treatment‐induced SVR in HIV+ MSM that exceeded the incidence of first infections, suggesting that a subgroup of MSM continues to engage in high‐risk practices after SVR.13
The advent of highly effective direct acting antiviral (DAA) has revolutionized HCV treatment. Modern DAA‐based HCV therapy results in SVR rates >95% across all HCV genotypes and HIV coinfected patients.1, 2, 15 While the historic HCV treatment with pegylated interferon (PEGIFN) and ribavirin (RBV) showed only modest SVR rates and was limited by various patient characteristics,16, 17, 18, 19 the introduction of DAAs now enables curative treatment at a favourable tolerability in HIV/HCV coinfected individuals including HIV+ patients with acute hepatitis C as well as patients with cirrhosis and prior HCV treatment failure.20, 21, 22, 23, 24, 25, 26, 27, 28
Yet, the HCV‐associated burden of disease remains high in HIV patients and efforts are needed to expand screening and treatment as well as provide education on risk factors for HCV transmission and effective treatment options.1, 11, 29, 30, 31, 32, 33, 34 The unrestricted access to novel DAA‐regimens in Vienna since September 2017 marked an important step towards the World Health Organization (WHO)‐goal of HCV elimination by 2030.35 With this study, we aimed to collect and report recent epidemiology data in order to inform and support national HCV elimination plans.
2. MATERIAL AND METHODS
This retrospective data analysis was carried out at the Medical University of Vienna – a tertiary care centre hosting large HIV and HCV clinics. The HIV cohort studied here included consecutive adult HIV+ patients attending the HIV clinic between 01 January 2014 and 31 December 2016. Electronic medical records were searched for the first available HCV serology and HCV‐RNA test after HIV diagnosis (defined as the baseline [BL] date). In an additional systematic data collection, we recorded the last available (FU date) HCV serology and HCV‐RNA polymerase chain reaction (PCR). Additional laboratory parameters, including aspartate aminotransferase, alanine aminotransferase (ALT), gamma‐glutamyl transferase, alkaline phosphatase, serum bilirubin, albumin, prothrombin time, international normalized ratio and platelet count were recorded at BL and FU. The fibrosis‐4 Score (FIB4‐Score) was calculated based on BL and FU parameters.36 For patients that had undergone a liver fibrosis evaluation by transient elastography (TE), results recorded.
2.1. HCV testing and virological case definitions
At BL, the rate of HCV testing by anti‐HCV or HCV‐RNA PCR was assessed. During the study period, our hospital used the VERSANT® HCV Genotype 2.0 Assay Line Probe Assay (LiPA) (Siemens Healthcare Diagnostics) and the Abbott RealTime HCV assay (Abbott Molecular) for HCV GT determination and HCV RNA quantification respectively. The HCV‐RNA PCR assay is capable of quantifying HCV‐RNA to a lower limit of 12 IU/mL. According to anti‐HCV and HCV‐RNA status at BL, patients were divided into:
BL–HCV‐naïve: anti‐HCV(−) and/or HCV‐RNA(−)
BL–HCV‐exposed: anti‐HCV(+) and HCV‐RNA(−)
BL–HCV‐viraemic: HCV‐RNA(+)
In patients with virological clearance, defined as anti‐HCV(+) and HCV‐RNA(−) at FU, antiviral therapies against HCV were recorded if prescribed/administered:
FU–HCV spontaneous clearance: anti‐HCV (+) and HCV‐RNA(−) at FU without antiviral therapy
FU–HCV SVR: anti‐HCV(+) and HCV‐RNA(−) at FU due to antiviral therapy
In addition, we recorded the following scenarios during FU:
FU–HCV de‐novo infection: anti‐HCV(−) and/or HCV‐RNA(−) at BL but HCV‐RNA(+) at FU
FU–HCV reinfection: any sequence of anti‐HCV(+) followed by HCV‐RNA(−) and subsequently by HCV‐RNA(+) but excluding HCV treatment failures.
Essentially, the percentage of HIV+ patients tested for HCV was assessed and the prevalences and incidences of HCV infection as well as virological clearance were calculated. In patients with virological clearance, the percentage of spontaneous vs treatment‐induced clearance was investigated. Furthermore, we assessed HCV coinfection status according to the suspected route of HIV/HCV transmission. In the era of PEGIFN/RBV, SVR was evaluated 6 months (i.e. 24 weeks) after cessation of antiviral therapy, while SVR was evaluated at 3 months (i.e. 12 weeks) after cessation of therapy in case of DAA therapy.
2.2. Suspected routes of transmission
The main routes of HIV and/or HCV transmission were determined by the medical records. In MSM with a history of IDU, the route of HIV infection could not clearly be identified given the two potential modes of transmission. Since IDU has become part of frequently performed high‐risk sex practices in MSM and IDU can therefore hardly be ruled out as a risk factor in this cohort, these patients were included in the subgroup of MSM for further analyses.
2.3. Statistics
Statistical analyses were performed using IBM SPSS Statistics 25 (IBM). Figures were generated using Graph Pad Prism 8 (GraphPad Software). Descriptive statistics including the calculation of median and interquartile range (IQR) were performed. Continuous variables were reported as median (IQR). Categorical variables were reported as number of patients with/without (proportion of patients with) the characteristic of interest. Case numbers, frequencies and percentages were calculated, and prevalence and incidence were concluded.
2.4. Ethics statement
The study was approved by the ethics committee of the Medical University of Vienna on 7 July 2017 (EC Number: 1527/2017).
3. RESULTS
3.1. Study cohort (Figure 1, Table 1)
Figure 1.
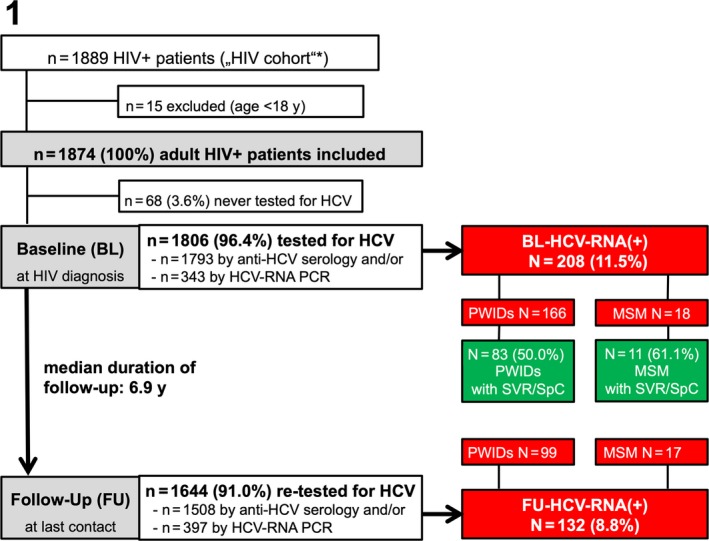
Patient flowchart. BL, baseline; FU, follow‐up; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MSM, men who have sex with men; PCR, polymerase chain reaction; PWIDs, people who inject drugs; RNA, ribonucleic acid; SpC, spontaneous clearance; SVR, sustained virological response
Table 1.
Baseline characteristics
| Variable | Overall | PWIDs | MSM | Other |
|---|---|---|---|---|
| Overall [n (%)] | 1874 (100) | 307 (16.4) | 892 (47.6) | 675 (36.0) |
| Sex [n (%)] | ||||
| Male | 1439 (76.8) | 196 (63.8) | 892 (100) | 351 (52.0) |
| Female | 435 (23.2) | 111 (36.2) | 0 (0.0) | 324 (48.0) |
| Age (years) at BL [median (IQR)] | 36.9 (15.2) | 31.7 (12.2) | 37.0 (13.4) | 37.7 (16.2) |
| Advanced fibrosis at BL [n (%)] according to | ||||
| Transient elastography >9.5 kPa | 19 (1.0) | 10 (3.3) | 6 (0.7) | 3 (0.4) |
| Missing values | 1694 (90.4) | 192 (62.5) | 846 (94.8) | 656 (97.2) |
| FIB4‐Score >3.25a | 88 (4.7) | 36 (11.7) | 26 (2.9) | 26 (3.9) |
| No fibrosis evaluation | 267 (14.2) | 40 (13.0) | 139 (15.6) | 88 (13.0) |
| HCV‐RNA at BL | ||||
| >12 IU/mL [n (%)] | 208 (11.1) | 166 (54.1) | 18 (2.0) | 24 (3.6) |
| Viral load (IU/mL) [median (IQR)] | 673 500 (3 671 930) | 113 500 (1 343 370) | 183 500 (2 302 490) | 530 500 (3 505 400) |
| HCV genotype [n (%b)] | ||||
| 1 | 122 (58.7) | 100 (60.2) | 12 (66.7) | 10 (16.7) |
| 2 | 5 (2.4) | 4 (2.4) | 1 (5.6) | 0 (0.0) |
| 3 | 39 (18.8) | 32 (19.3) | 1 (5.6) | 6 (12.5) |
| 4 | 18 (8.7) | 11 (6.6) | 4 (22.2) | 3 (4.2) |
| Unknown | 24 (11.5) | 19 (11.4) | 0 (0.0) | 5 (66.7) |
| ART [n (%)] | ||||
| No ART at BL, but later | 1802 (96.2) | 297 (96.7) | 850 (95.3) | 655 (97.0) |
| No ART at BL, never ART during FU | 60 (3.2) | 8 (2.6) | 33 (3.7) | 19 (2.8) |
| ART at baseline | 12 (0.6) | 2 (0.7) | 9 (1.0) | 1 (0.1) |
| HIV‐RNA at BL | ||||
| Viral load (log10/mL) [median (IQR)] | 4.8 (1.1) | 4.8 (1.3) | 4.9 (1.0) | 4.8 (1.1) |
| HIV‐RNA suppression (<1.7 log10/mL) [n (%)] | 290 (15.6) | 37 (12.1) | 145 (16.3) | 108 (16.0) |
| Missing values [n (%)] | 49 (2.6) | 4 (1.3) | 34 (3.8) | 11 (1.6) |
| CD4 count at BL (cells/µL) [median (IQR)] | 347 (369.0) | 400 (416.5) | 373 (356.0) | 327 (323.5) |
| Unknown [n (%)] | 40 (2.1) | 2 (0.7) | 27 (3.0) | 11 (1.6) |
| CDC stage at BL [n (%)] | ||||
| A1‐A3 | 1481 (79.0) | 236 (76.9) | 712 (80.0) | 533 (79.0) |
| B1‐B3 | 93 (5.0) | 15 (4.9) | 44 (5.0) | 34 (5.0) |
| C1‐C3 | 139 (7.4) | 26 (8.5) | 57 (6.4) | 56 (8.3) |
| AIDS | 433 (23.1) | 82 (26.7) | 179 (20.1) | 172 (25.5) |
| Unknown | 161 (8.6) | 30 (9.8) | 79 (8.9) | 52 (7.7) |
Abbreviations: AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; BL, baseline; CD4, cluster of differentiation 4; CDC, centers for disease control and prevention; FIB4‐Score, fibrosis‐4 score; FU, follow‐up; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; MSM, men who have sex with men; PWIDs, people with injection drug use; RNA, ribonucleic acid.
Applied upper limit of normal (ULN) for AST and ALT: 35 IU/L (females) and 50 IU/L (males).
Of HCV‐RNA at BL >12 IU/mL.
In all, 1874/1889 adult HIV+ patients were included in the study. The median age was 36.9 years (IQR 15.2) and 1439 (76.8%) patients were male. Among the main suspected routes of HIV transmission, we recorded 307 (16.4%) PWIDs, 892 MSM (47.6%) and 675 (36.0%) with other or unknown routes of HIV infection. The subgroup of ‘other’ routes of HIV transmission included 569 with heterosexual transmission, 16 recipients of blood transfusions, 10 patients with haemophilia or other coagulopathies, 6 patients with nosocomial HIV infection, 2 patients with vertical HIV transmission and 72 cases in which the route of HIV transmission ultimately could not be determined (Figure 1, Table 1).
3.2. HCV testing (Figure 1)
In all, 1806/1874 (96.4%) were tested at least once for HCV infection, that is, by anti‐HCV serology and/or by HCV‐RNA PCR. While 99.3% (1793/1806) of the patients were tested by anti‐HCV serology, only 19% (343/1806) underwent HCV‐RNA PCR testing. In the majority (298/343; 86.9%) of cases HCV‐RNA PCR tests were performed after the detection of anti‐HCV(+).
3.3. Seroprevalence of anti‐HCV and HCV‐RNA viraemia at BL (Figure 2A‐C)
Figure 2.
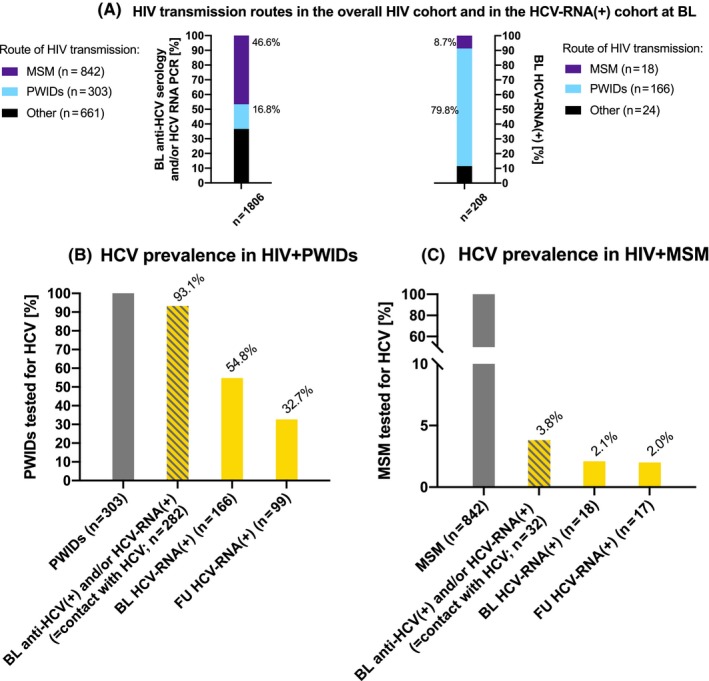
HCV testing and prevalence in the HIV cohort by HIV transmission route. A, HIV transmission routes in the overall HIV cohort and in the HCV‐RNA(+) cohort; B, HCV prevalence in HIV+ PWIDs; C, HCV prevalence in HIV+ MSM. BL, baseline; FU, follow‐up; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MSM, men who have sex with men; PWIDs, people who inject drugs; RNA, ribonucleic acid
Among 1806 patients tested at BL, the anti‐HCV seroprevalence was 19.9% (359/1806) and the HCV‐RNA viraemia rate was 11.5% (208/1806). The HCV‐RNA viraemia rate among the patients undergoing HCV‐PCR testing was 60.6% (208/343) suggesting a strict pre‐selection for PCR testing.
Among these 208 HCV‐RNA viraemic patients at BL, there were 166 PWIDs, 18 MSM and 24 with other routes of HIV transmission (Figure 2A). The prevalence of HCV‐RNA viraemia at BL was 54.8% (166/303) in PWIDs (Figure 2B) and 2.1% (18/842) in MSM (Figure 2C). One PWID showed HCV‐RNA viraemia and a negative anti‐HCV serology, suggesting acute HCV infection at BL.
3.4. HCV and HIV characteristics (Table 1)
While many patients (1228; 65.5%) showed HIV viral loads >4 log10 copies/mL upon HIV diagnosis, most patients still presented at an early clinical stage of the disease (Centers for Disease Control and Prevention stage A in 1481; 79.0%). Yet, 23.1% (433/1874) had AIDS‐defining cluster of differentiation 4 T‐lymphocyte counts below 200 cells/µL. The vast majority of patients consecutively started combined antiretroviral therapy (1814; 96.8%).
Among the 184/208 (88.5%) HCV viraemic patients that were evaluated for their HCV genotype, HCV genotype 1 (n = 122; 58.7%) and 3 (n = 39; 18.8%) were documented most frequently. According to non‐invasive fibrosis assessment by TE and FIB‐4 score, the majority (1530/1607; 95.2%) of the patients did not show significant liver fibrosis.
3.5. Seroprevalence and HCV viraemia at FU (Figure 3A,B)
Figure 3.
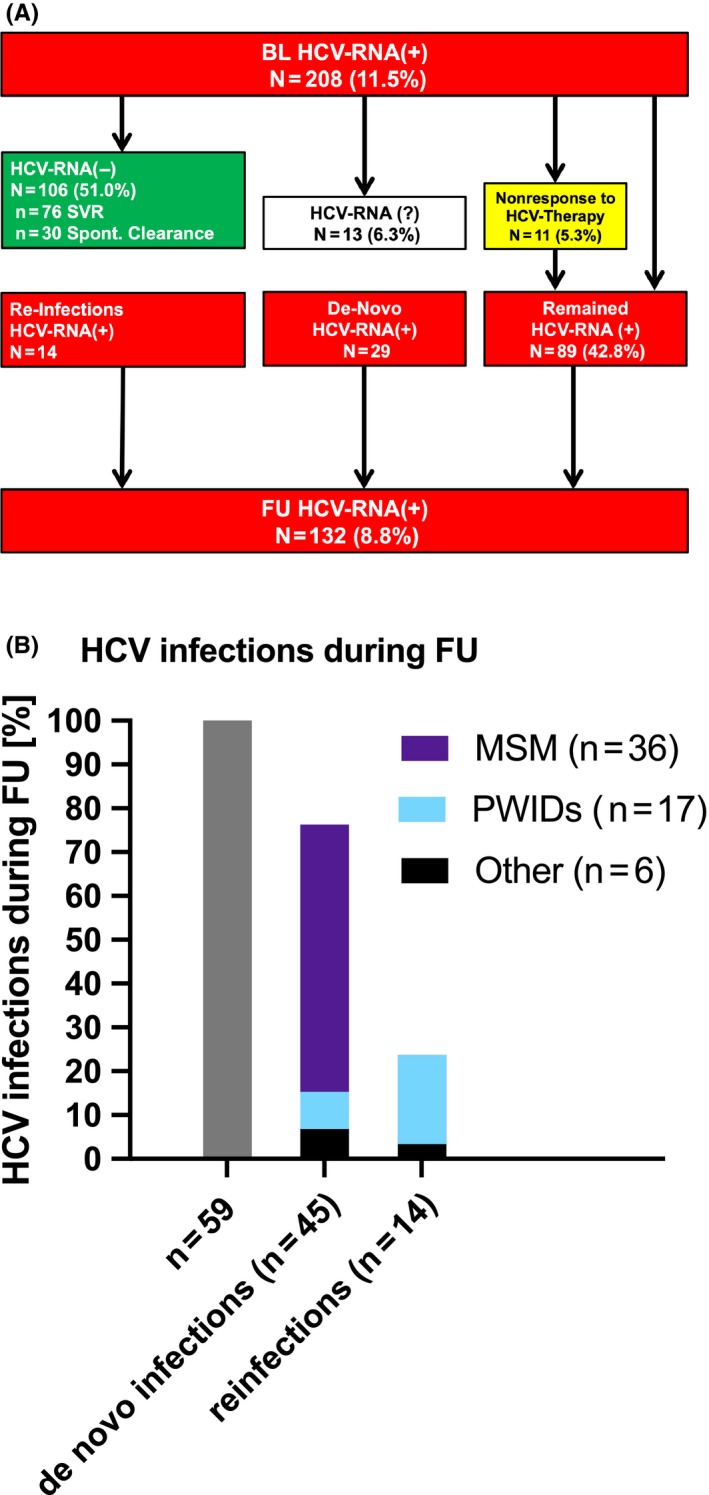
Follow‐up of the HIV cohort. A, FU of patients with HCV viraemia at BL; B, HCV infections during FU. BL, baseline; FU, follow‐up; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MSM, men who have sex with men; PWIDs, people who inject drugs; RNA, ribonucleic acid; SVR, sustained virological response
The median documented FU period of our HIV+ cohort that underwent at least one FU HCV test was of 6.9 years (Figure 3).
In all, 195/208 (93.8%) of patients with HCV viraemia at BL had a FU HCV‐RNA PCR available. Among 13 initially HCV viraemic patients without a FU HCV‐RNA PCR, one patient died within the observation period. Persisting HCV viraemia was detected in 89/195 (45.6%) patients. The patient who initially presented with acute HCV infection at BL showed a positive anti‐HCV serology and persisting HCV viraemia at FU, suggesting HCV chronification. Overall, 106 (54.4%) patients with HCV‐RNA viraemia at BL, were negative HCV‐RNA(−) at FU including 76 (71.7%) with treatment‐induced clearance and 30 (28.3%) with spontaneous clearance.
Treatment uptake (n = 9/18; 50.0%) and virological clearance (n = 11/18; 61.1%) were highest in MSM compared to the other subgroups while PWIDs showed the highest percentage of persisting HCV viraemia (n = 74/166; 44.6%). Among 76 patients who achieved SVR, 30 (39.5%) received interferon (IFN)‐based regimens and 46 (60.5%) received DAAs. 10/76 (13.2%) patients were re‐treated, among which two patients received a second course of PEGIFN/RBV and eight patients were treated with IFN‐free DAA regimens. One patient received a third HCV treatment cycle with DAAs during the observation period.
Among the 1433 HIV+ patients who were HCV‐naïve at BL there were 19 PWIDs and 808 MSM. Overall, a FU anti‐HCV serology was performed in 1281 patients, including 16/19 HCV‐naïve PWIDs and 708/808 HCV‐naïve MSM. Importantly, 5 PWIDs and 34 MSM showed anti‐HCV(+) seroconversion during FU, corresponding to a seroconversion rate of 26.3% and 4.2% respectively. Ultimately, 2 PWIDs (10.5% of HCV‐naïve PWIDs at BL) and 11 MSM (1.4% of HCV‐naïve MSM at BL) showed newly documented HCV‐RNA viraemia at FU.
3.6. New cases of HCV infection and HCV reinfections (Figure 3A,B)
Of the 135/343 patients (39.4%; 76 PWIDs; 33 MSM; 26 with other routes of HIV transmission) with a negative HCV‐RNA PCR at BL, 98 (72.6%) patients showed anti‐HCV(+) at BL. In 99/135 (73.3%) a FU HCV‐RNA PCR was performed. All of the 14/99 (14.1%) patients (12 PWIDs; 2 with other routes of HIV transmission) who presented with new HCV viraemia upon last FU, anti‐HCV(+) had already been detected at first examination (i.e. reinfections).
Overall, 43 of the 1281 anti‐HCV(−) at BL converted to anti‐HCV(+) at last FU, among whom 13/43 presented with additional HCV viraemia. Moreover one patient showed new HCV viraemia and was not retested for anti‐HCV while another patient was HCV viraemic with a negative HCV serology, suggesting acute HCV infection.
Therefore, 59 new HCV‐infections (including 17 PWIDs and 36 MSM) were recorded during a median FU of 6.7 years, resulting in an incidence rate (IR) of 0.004% (95% confidence interval [CI] 0.0%‐0.022%) for de‐novo HCV infections and 0.001% (95% CI 0.0%‐0.018%) for HCV reinfections. While 18.1% of the initially anti‐HCV(−) and/or HCV‐RNA(−) PWIDs acquired a new HCV infection during FU, MSM made up for 61.0% of all incident HCV infections (i.e. de‐novo infections or reinfections) throughout the observation period.
3.7. Severity of HCV‐related liver disease at BL and at last FU (Figure 4A,B)
Figure 4.
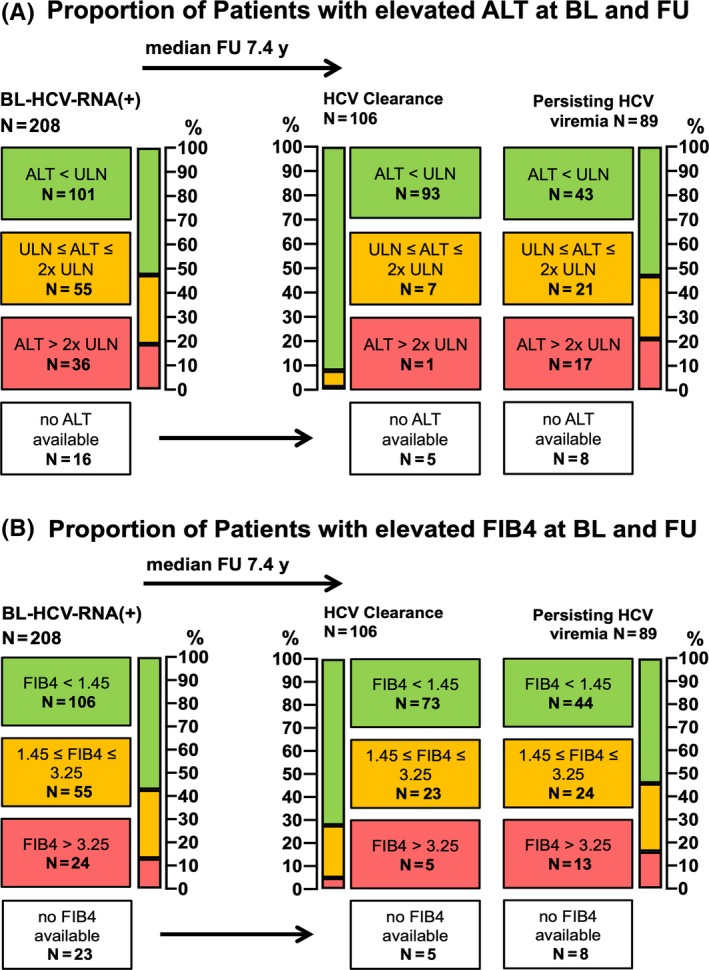
Evaluation of biochemical liver damage and advanced fibrosis by ALT‐levels and FIB4‐Score in HIV+ patients with HCV viraemia at baseline. A, Proportion of Patients with elevated ALT at BL and FU; B, Proportion of Patients with elevated FIB4 at BL and FU. ALT, alanine aminotransferase (ULN <35 U/L (f); <50 U/L (m)); BL, baseline; FIB4, fibrosis‐4 Score; FU, follow‐up; HCV, hepatitis C virus; HIV, human immunodeficiency virus; RNA, ribonucleic acid; ULN, upper limit of normal
Among the 208 patients that presented with HCV viraemia at BL, ALT was available in 192 (92.3%) (Figure 4A). 36/192 (17.3%) showed an ALT level >2x sex‐adjusted upper limit of normal as a marker of biochemical liver damage, while 101 (48.6%) had an ALT level within the normal range. After a FU of 7.4 years, ALT was evaluated in 182 patients, the majority (n = 136; 70.0%) of which showed ALT levels within the normal range.
In all, 24/185 (11.5%) patients with an available FIB4‐Score showed a FIB4 >3.25, indicating advanced liver fibrosis at BL, while in 106 (51%), significant liver fibrosis was ruled out based on a FIB4‐Score <1.45 (Figure 4B). At FU, 18/182 (9.9%) had FIB4‐Score values indicating advanced liver fibrosis, while in 117 (64.3%), advanced liver fibrosis was ruled out.
Among the 101 patients with HCV clearance during FU, 92.1% finally showed an ALT level within the normal range and 72.3% showed a FIB4‐Score <1.45, while only 43/81 (53.1%) and 44/81 (54.3%) of the patients with persisting viraemia showed normal ALT and FIB4, respectively.
4. DISCUSSION
The aim of this study was to assess temporal trends of HCV epidemiology in a Viennese cohort of HIV infected patients in order to inform future HCV elimination strategies and targeted HCV microelimination projects. Our study was performed at the largest Austrian HIV treatment institution and thus, most likely provides representative results for the Austrian population living with HIV. Patients were analysed according to the main suspected route of HIV acquisition as documented by in the standardized medical HIV records.
Overall, we observed a successful implementation of routine screening for HCV coinfection in our HIV+ cohort (n = 1806/1874; 96.4%). While HIV+ PWIDs remain an important reservoir for HCV (anti‐HCV seroprevalence 93.2% at BL and 90.3% at FU), we observed a relevant increase in anti‐HCV seroprevalence among MSM from 3.7% at BL to 6.5% at last FU. Importantly, 61.0% of all incident HCV diagnoses were recorded in MSM. Considering the fact that historically, HIV/HCV coinfection used to almost exclusively affect PWIDs,7, 37, 38, 39 these findings mark an important change in HIV/HCV epidemiology in Austria and support previously published data, reporting an increasing HCV incidence in MSM that is likely driven by high‐risk sex practices including chemsex.8, 9, 10, 11, 12, 13 However, the potential use of intravenously injected drugs within our cohort of MSM remains unclear as the available data are solely based on self‐reported behaviour. The HCV transmission risk in MSM specifically related to IDU versus high‐risk sex practices should be assessed in future studies in order to provide more detailed data on these combined risk factors.
We observed an excellent compliance concerning the performance of FU examinations after the diagnosis of HCV viraemia in MSM with a FU HCV‐RNA PCR being available in 94.4% of all initially HCV‐RNA(+) MSM. These observations highlight the potential to successfully link HIV+ MSM to HCV‐specific care and treatment in order to achieve the WHO goal of eliminating hepatitis C in the subgroup of MSM.9
People who inject drugs with HCV‐RNA(+) PCR at BL often showed persisting HCV viraemia at last FU. While 91.1% of PWIDs tested for HCV showed anti‐HCV(+) at first examination, only 79.9% received a confirmatory HCV‐RNA PCR test. Among our study population, one PWID presented with acute HCV infection at BL and showed persisting viraemia at FU. Although the overall test rate in our population was high, this goes in line with recently published findings from the EuroSIDA cohort, where in almost 20% of anti‐HCV(+) individuals no subsequent HCV‐RNA PCR was performed and only a low proportion of HIV/HCV coinfected patients achieved SVR.40
While response to DAA treatment in HIV/HCV coinfected patients tended to be lower in the early DAA era,41 modern HCV DAA regimens yield SVR rates in HIV/HCV coinfected patients of >95%, and thus, HIV/HCV coinfected patients no longer represent a ‘difficult‐to‐cure’ patient population.23, 24, 26 We observed a response rate of 64.7% (22/34) to PEGIFN‐based treatment vs 87.5% (49/56) to DAA‐based regimens in our study cohort. However, addressing barriers to treatment and optimizing drug adherence remain essential in the population of HIV/HCV coinfected individuals.23, 26 Target‐oriented strategies such as directly‐observed DAA therapy in PWIDs on opioid substitution therapy proved to be effective and may help to overcome barriers and link this subgroup of patients to HCV treatment.14, 42, 43, 44, 45
Importantly, screening for HCV within at‐risk populations needs to be enforced. According to European guidelines, people at high risk for HCV infection should be screened by anti‐HCV serology at least once a year. These ‘key populations’ include HIV‐infected patients as well as HIV‐negative MSM, PWIDs, people in prisons, sex workers and transgender populations. Additionally, there are specific national recommendations in Austria including not only annual testing of key populations but also systematic recall strategies.46 In high‐risk populations, HCV‐RNA PCR is recommended as a primary screening test to detect acute infection. Yet, despite those efforts, incompliance and poor adherence remain the main reasons for lack of annual testing.
Among the 208 patients that initially presented with HCV viraemia, 13/208 patients remained without a FU HCV‐RNA PCR. While one patient died during the observation period, the other patients were lost to FU. Similarly, the rather low rate of HCV treatment‐uptake (76/208; 36.5%) and the high rate of persisting HCV viraemia (89/208; 42.8%) at last FU were partially attributed to loss to FU. Among the 89 patients with persisting HCV viraemia at FU, 10 received treatment during the observation period: 5/10 were treated with PEGIFN and 5/10 received DAA. Due to the retrospective character of the study, we cannot completely exclude that some of these 10 treated patients might have been re‐infected as opposed to showing treatment failure.
One of the other 89 HCV viraemic patients was started on treatment with DAA but had not finished treatment at the end of the observation period. The remaining 78/89 patients had not received HCV therapy at our institution by the end of the observation period. The main reason why HCV viraemic patients were not started on treatment was incompliance and poor adherence.
Overall, in this study we observed a reduction in anti‐HCV(+) seroprevalence from 20.0% to 16.6% and a reduction in HCV viraemia from 60.6% to 33.2% between BL and FU respectively indicating an effect of HCV screening and treatment in the Viennese HIV+ population.
Yet, our data emphasizes that linkage to care needs to be improved among HIV+ patients: Overall, 132 patients showed HCV‐RNA(+) at FU, among which 23/132 (17.4%) deceased (according to medical history and the national death registry). The remaining 109 HIV+ patients may still require HCV therapy. Since the termination of this study, interventions to increase linkage to care for HIV/HCV coinfected patients have been intensified in Vienna47 and future data analyses will assess the epidemiological impact of these HCV microelimination strategies. Furthermore, similar to the EuroSIDA report,40 our observations are not limited to but also include time periods from the pre‐DAA era. Since then, the reimbursement limitations for HCV DAA therapy was eliminated and modern pangenotypic DAA regimens have been available, which will most likely impact HCV epidemiology in Austria.
While this study is limited by the lack of a standardized HCV testing protocol and some patients being lost to FU, our epidemiological results are essential for future HCV microelimination projects targeting the specific population of HIV+ individuals in Vienna and other parts of Austria. FU reports evaluating the successful implementation of pangenotypic DAA treatment for HCV will reveal their impact on the epidemiology of HCV coinfection in HIV+ individuals in Vienna.
CONFLICTS OF INTEREST
Schmidbauer C received travel support from Gilead and Abbvie. Chromy Dreceived payments for consulting from MSD, Abbvie and Gilead as well as travel support from Abbvie and Gilead. Bauer D received travel support from Gilead and Abbvie. Mandorfer M served as a speaker and/or consultant and/or advisory board member for AbbVie, Bristol‐Myers Squibb, Gilead, and W. L. Gore & Associates and received travel support from AbbVie, Bristol‐Myers Squibb, and Gilead. Simbrunner B received travel support from AbbVie and Gilead. Trauner M served as speaker for BMS, Falk Foundation, Gilead and MSD; advisory boards for Albireo, BiomX, Falk Pharma GmbH, Genfit, Gilead, Intercept, MSD, Novartis, Phenex and Regulus. He further received travel grants from Abbvie, Falk, Gilead and Intercept and research grants from Albireo, Cymabay, Falk, Gilead, Intercept, MSD and Takeda. He is also co‐inventor of patents on the medical use of norUDCA. Gschwantler M received grant support from Abbvie, Gilead and MSD; speaking honoraria from Abbvie, Gilead, Intercept, and MSD; consulting/advisory board fees from Abbvie, Gilead, Intercept and MSD; and travel support from Abbvie and Gilead. Reiberger T received grant support from Abbvie, Boehringer‐Ingelheim, Gilead, MSD, Philips Healthcare, Gore; speaking honoraria from Abbvie, Gilead, Gore, Intercept, Roche, MSD; consulting/advisory board fee from Abbvie, Bayer, Boehringer‐Ingelheim, Gilead, Intercept, MSD, Siemens; and travel support from Boehringer‐Ingelheim, Gilead and Roche. Schmidbauer V, Apata M, Nguyen D, Rieger A, Mayer F, Schmidt R and Holzmann H have nothing to disclose.
ACKNOWLEDGEMENTS
Nothing to disclose.
Schmidbauer C, Chromy D, Schmidbauer V, et al. Epidemiological trends in HCV transmission and prevalence in the Viennese HIV+ population. Liver Int. 2020;40:787–796. 10.1111/liv.14399
Handling Editor: Jürgen Rockstroh
REFERENCES
- 1. Polaris Observatory HCV Collaborators . Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161‐176. [DOI] [PubMed] [Google Scholar]
- 2. Mandorfer M, Schwabl P, Steiner S, Reiberger T, Peck‐Radosavljevic M. Advances in the management of HIV/HCV coinfection. Hepatol Int. 2016;10(3):424‐435. [DOI] [PubMed] [Google Scholar]
- 3. Tengan FM, Ibrahim KY, Dantas BP, Manchiero C, Magri MC, Bernardo WM. Seroprevalence of hepatitis C virus among people living with HIV/AIDS in Latin America and the Caribbean: a systematic review. BMC Infect Dis. 2016;16(1):663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mandorfer M, Payer BA, Schwabl P, et al. Revisiting liver disease progression in HIV/HCV‐coinfected patients: the influence of vitamin D, insulin resistance, immune status, IL28B and PNPLA3. Liver Int. 2015;35(3):876‐885. [DOI] [PubMed] [Google Scholar]
- 5. Reiberger T, Ferlitsch A, Sieghart W, et al. HIV‐HCV co‐infected patients with low CD4+ cell nadirs are at risk for faster fibrosis progression and portal hypertension. J Viral Hepat. 2010;17(6):400‐409. [DOI] [PubMed] [Google Scholar]
- 6. Mandorfer M, Schwabl P, Peck‐Radosavljevic M, Reiberger T, Vienna HIV & Liver Study Group and the Vienna Hepatic Hemodynamic Lab . Letter: sustained virological response and liver healing ‐ authors' reply. Aliment Pharmacol Ther. 2017;45(8):1173‐1174. [DOI] [PubMed] [Google Scholar]
- 7. Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Global Health. 2017;5(12):e1192‐e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co‐infection in people living with HIV: a global systematic review and meta‐analysis. Lancet Infect Dis. 2016;16(7):797‐808. [DOI] [PubMed] [Google Scholar]
- 9. Pufall EL, Kall M, Shahmanesh M, et al. Sexualized drug use (‘chemsex’) and high‐risk sexual behaviours in HIV‐positive men who have sex with men. HIV Med. 2018;19:261‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tieu H‐V, Laeyendecker O, Nandi V, et al. Prevalence and mapping of hepatitis C infections among men who have sex with men in New York City. PLoS ONE. 2018;13(7):e0200269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bourne A, Reid D, Hickson F, Torres‐Rueda S, Weatherburn P. Illicit drug use in sexual settings (‘chemsex’) and HIV/STI transmission risk behaviour among gay men in South London: findings from a qualitative study. Sex Transm Infect. 2015;91(8):564‐568. [DOI] [PubMed] [Google Scholar]
- 12. Hammoud MA, Vaccher S, Jin F, et al. The new MTV generation: Using methamphetamine, Truvada™, and Viagra™ to enhance sex and stay safe. Int J Drug Policy. 2018;55:197‐204. [DOI] [PubMed] [Google Scholar]
- 13. Pradat P, Huleux T, Raffi F, et al. Incidence of new hepatitis C virus infection is still increasing in French MSM living with HIV. AIDS. 2018;32(8):1077–1082. [DOI] [PubMed] [Google Scholar]
- 14. Falade‐Nwulia O, Irvin R, Merkow A, et al. Barriers and facilitators of hepatitis C treatment uptake among people who inject drugs enrolled in opioid treatment programs in Baltimore. J Subst Abuse Treat. 2019;100:45‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reiberger T. Chronic hepatitis C: treat everyone now or stratify by disease? Minerva Gastroenterol Dietol. 2015;61(1):11‐19. [PubMed] [Google Scholar]
- 16. Mandorfer M, Payer BA, Scheiner B, et al. Health‐related quality of life and severity of fatigue in HIV/HCV co‐infected patients before, during, and after antiviral therapy with pegylated interferon plus ribavirin. Liver Int Off J Int Assoc Study Liver. 2014;34(1):69‐77. [DOI] [PubMed] [Google Scholar]
- 17. Mandorfer M, Reiberger T, Payer BA, et al. Low vitamin D levels are associated with impaired virologic response to PEGIFN + RBV therapy in HIV‐hepatitis C virus coinfected patients. AIDS. 2013;27(2):227‐232. [DOI] [PubMed] [Google Scholar]
- 18. Mandorfer M, Reiberger T, Payer BA, et al. Revisiting predictors of virologic response to PEGIFN + RBV therapy in HIV‐/HCV‐coinfected patients: the role of metabolic factors and elevated GGT levels. J Viral Hepat. 2014;21(1):33‐41. [DOI] [PubMed] [Google Scholar]
- 19. Reiberger T, Rasoul‐Rockenschaub S, Rieger A, Ferenci P, Gangl A, Peck‐Radosavljevic M. Efficacy of interferon in immunocompromised HCV patients after liver transplantation or with HIV co‐infection. Eur J Clin Invest. 2008;38(6):421‐429. [DOI] [PubMed] [Google Scholar]
- 20. Berenguer J, Gil‐Martin Á, Jarrin I, et al. All‐oral direct‐acting antiviral therapy against hepatitis C virus (HCV) in human immunodeficiency virus/HCV‐coinfected subjects in real‐world practice: Madrid coinfection registry findings. Hepatol. 2018;68(1):32‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bischoff J, Mauss S, Cordes C, et al. Rates of sustained virological response 12 weeks after the scheduled end of direct‐acting antiviral (DAA)‐based hepatitis C virus (HCV) therapy from the National German HCV registry: does HIV coinfection impair the response to DAA combination therapy? HIV Med. 2018;19(4):299‐307. [DOI] [PubMed] [Google Scholar]
- 22. Gayam V, Hossain MR, Khalid M, et al. Real‐world clinical efficacy and tolerability of direct‐acting antivirals in hepatitis C monoinfection compared to hepatitis C/human immunodeficiency virus coinfection in a community care setting. Gut Liv. 2018;12(6):694‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim DY. Efficacy of direct‐acting antivirals in patients with hepatitis C virus/human immunodeficiency virus coinfection: a gap between clinical trial and real practice. Gut Liv. 2018;12(6):609‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roberson JL, Lagasca AM, Kan VL. Comparison of the hepatitis C continua of care between hepatitis C virus/HIV coinfected and hepatitis C virus mono‐infected patients in two treatment eras during 2008–2015. AIDS Res Hum Retroviruses. 2018;34(2):148‐155. [DOI] [PubMed] [Google Scholar]
- 25. Ryom L, Boesecke C, Bracchi M, et al. Highlights of the 2017 European AIDS Clinical Society (EACS) Guidelines for the treatment of adult HIV‐positive persons version 9.0. HIV Med. 2018;19:309‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sikavi C, Chen PH, Lee AD, Saab EG, Choi G, Saab S. Hepatitis C and human immunodeficiency virus coinfection in the era of direct‐acting antiviral agents: no longer a difficult‐to‐treat population. Hepatology. 2018;67(3):847‐857. [DOI] [PubMed] [Google Scholar]
- 27. Chromy D, Mandorfer M, Bucsics T, et al. Prevalence and predictors of hepatic steatosis in patients with HIV/HCV coinfection and the impact of HCV eradication. AIDS Patient Care STDs. 2019;33(5):197‐206. [DOI] [PubMed] [Google Scholar]
- 28. Chromy D, Mandorfer M, Bucsics T, et al. High efficacy of interferon‐free therapy for acute hepatitis C in HIV‐positive patients. United Eur Gastroenterol J. 2019;7(4):507‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baumert TF, Berg T, Lim JK, Nelson DR. Status of direct‐acting antiviral therapy for hepatitis C virus infection and remaining challenges. Gastroenterology. 2019;156(2):431‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. European Union HCV Collaborators . Hepatitis C virus prevalence and level of intervention required to achieve the WHO targets for elimination in the European Union by 2030: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(5):325‐336. [DOI] [PubMed] [Google Scholar]
- 31. Juanbeltz R, Pérez‐García A, Aguinaga A, et al. Progress in the elimination of hepatitis C virus infection: a population‐based cohort study in Spain. PLoS ONE. 2018;13(12):e0208554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Papatheodoridis GV, Hatzakis A, Cholongitas E, et al. Hepatitis C: the beginning of the end‐key elements for successful European and national strategies to eliminate HCV in Europe. J Viral Hepat. 2018;25(Suppl 1):6‐17. [DOI] [PubMed] [Google Scholar]
- 33. Pham T, Rathbun RC, Keast S, Nesser N, Farmer K, Skrepnek G. National estimates of case‐mix, mortality, and economic outcomes among inpatient HIV/AIDS mono‐infection and hepatitis C co‐infection cases in the US. J Eval Clin Pract. 2019;25:806‐821. [DOI] [PubMed] [Google Scholar]
- 34. Popping S, Bade D, Boucher C, et al. The global campaign to eliminate HBV and HCV infection: International Viral Hepatitis Elimination Meeting and core indicators for development towards the 2030 elimination goals. J Virus Erad. 2019;5(1):60‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marshall AD, Pawlotsky J‐M, Lazarus JV, Aghemo A, Dore GJ, Grebely J. The removal of DAA restrictions in Europe ‐ one step closer to eliminating HCV as a major public health threat. J Hepatol. 2018;69(5):1188‐1196. [DOI] [PubMed] [Google Scholar]
- 36. Chromy D, Schwabl P, Bucsics T, et al. Non‐invasive liver fibrosis assessment and HCV treatment initiation within a systematic screening program in HIV/HCV coinfected patients. Wien Klin Wochenschr. 2018;130(3‐4):105‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378(9791):571‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dolan K, Wirtz AL, Moazen B, et al. Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet. 2016;388(10049):1089‐1102. [DOI] [PubMed] [Google Scholar]
- 39. Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388(10049):1081‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Amele S, Peters L, Sluzhynska M, et al. Establishing a hepatitis C continuum of care among HIV/hepatitis C virus‐coinfected individuals in EuroSIDA. HIV Med. 2019;20:264‐273. [DOI] [PubMed] [Google Scholar]
- 41. Neukam K, Morano‐Amado LE, Rivero‐Juárez A, et al. HIV‐coinfected patients respond worse to direct‐acting antiviral‐based therapy against chronic hepatitis C in real life than HCV‐monoinfected individuals: a prospective cohort study. HIV Clin Trials. 2017;18(3):126‐134. [DOI] [PubMed] [Google Scholar]
- 42. Berg KM, Litwin A, Li X, Heo M, Arnsten JH. Directly observed antiretroviral therapy improves adherence and viral load in drug users attending methadone maintenance clinics: a randomized controlled trial. Drug Alcohol Depend. 2011;113(2‐3):192‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schütz A, Moser S, Schwanke C, et al. Directly observed therapy of chronic hepatitis C with ledipasvir/sofosbuvir in people who inject drugs at risk of nonadherence to direct‐acting antivirals. J Viral Hepat. 2018;25(7):870‐873. [DOI] [PubMed] [Google Scholar]
- 44. Schütz A, Moser S, Marchart K, Haltmayer H, Gschwantler M. Direct observed therapy of chronic hepatitis C with interferon‐free all‐oral regimens at a low‐threshold drug treatment facility‐a new concept for treatment of patients with borderline compliance receiving opioid substitution therapy. Am J Gastroenterol. 2016;111(6):903‐905. [DOI] [PubMed] [Google Scholar]
- 45. Kosloski MP, Zhao W, Asatryan A, Kort J, Geoffroy P, Liu W. No clinically relevant drug‐drug interactions between methadone or buprenorphine‐naloxone and antiviral combination glecaprevir and pibrentasvir. Antimicrob Agents Chemother. 2017;61(10): pii: e00958‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leierer G, Rappold M, Strickner S, Zangerle R. 35th Report of the Austrian HIV Cohort Study. 2018. https://www.pdffiller.com/457926326--35th-Report-ofthe-Austrian-HIV-Cohort-Study-AGES-Various-Fillable-Forms. Accessed March 1, 2020.
- 47. Steiner S, Bucsics T, Schwabl P, et al. Progress in eradication of HCV in HIV positive patients with significant liver fibrosis in Vienna. Wien Klin Wochenschr. 2017;129(15‐16):517‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]


