Abstract
The class of rapid‐acting insulin analogues were introduced more than 20 years ago to control postprandial plasma glucose (PPG) excursions better than unmodified regular human insulin. Insulins, lispro, aspart and glulisine all achieved an earlier onset of action, greater peak effect and shorter duration of action resulting in lower PPG levels and a reduced risk of late postprandial hypoglycaemia. However, the subcutaneous absorption rate of these analogues still fails to match the physiological profile of insulin in the systemic circulation following a meal. Recent reformulations of aspart and lispro have generated a second generation of more rapid‐acting insulin analogue candidates, including fast‐acting aspart (faster aspart), ultra‐rapid lispro and BioChaperone Lispro. These modifications have the potential to mimic physiological prandial insulin secretion better with an even earlier onset of action with improved PPG control, shorter duration of effect and reduced risk of hypoglycaemia. Recent phase 3 trials in type 1 and type 2 diabetes show that faster aspart and ultra‐rapid lispro compared with conventional aspart and lispro, achieved fewer PPG excursions with a small increase in post‐meal hypoglycaemia but similar or marginally superior glycated haemoglobin levels, and suggest the need for parallel optimization of basal insulin replacement. Phase 1 trials for BioChaperone Lispro are equally encouraging with phase 3 trials yet to be initiated. Comparative analysis of the clinical and pharmacological evidence for these new prandial insulin candidates in the treatment of type 1 and type 2 diabetes is the main focus of this review.
Keywords: clinical trials, hypoglycaemia, insulin therapy, pharmacodynamics, pharmacokinetics, postprandial glucose, type 1 diabetes, type 2 diabetes
1. INTRODUCTION
An essential component in diabetes management is to achieve and maintain good glycaemic control in an attempt to reduce the risk of micro‐ and macrovascular complications.1, 2, 3 Both fasting plasma glucose and postprandial plasma glucose (PPG) contribute to overall glycaemic control, as assessed clinically by the monitoring of glycated haemoglobin (HbA1c) levels. The greater recognition of the contribution of postprandial hyperglycaemia to elevated HbA1c and consequent risk for diabetes complications highlights the importance of reducing PPG excursions,4 which remains a challenging aspect in clinical practice.5
The primary aim of subcutaneously administered mealtime‐related insulin formulations is to replicate the dynamics of endogenous prandial insulin secretion, thereby controlling PPG excursions.6 In contrast to unmodified regular human insulin (RHI), the current rapid‐acting insulin analogues (insulins lispro, aspart and glulisine), developed more than 20 years ago, through faster subcutaneous absorption, have an earlier onset and shorter duration of action.7, 8, 9 These “first‐generation” rapid‐acting insulin analogues, when compared with RHI in type 1 diabetes (T1DM) in a multiple daily injection regimen, improve HbA1c by 0.15%10 and by 0.20%11 when delivered via subcutaneous insulin pumps. However, when rapid‐acting analogues and basal insulin preparations are both optimized, HbA1c decreases by more than 0.3% versus RHI,12, 13 while also decreasing the rate of hypoglycaemia.13
The enhanced absorption meant patient convenience was improved, as administration of these analogues closer to, or at mealtimes became possible.12, 13 In current practice, to optimize PPG control the analogues are recommended to be administered up to 20 min before meals or within 20 min after starting a meal.5, 14, 15, 16 Despite their enhanced subcutaneous absorption, the physiological mealtime insulin response is not fully replicated, as the tissue barrier still delays insulin entry in blood with consequent elevated PPG.9, 17 Attempts to overcome PPG by increasing the dose resulted in an increased risk of delayed inter‐prandial hypoglycaemia. Further improvements are required to simulate endogenous mealtime insulin secretion better18 and alleviate the inconvenience of anticipating meal timing, content and/or dosing requirements.5, 14, 15, 16
Further adjustments have been made to the pharmaceutical formulations of insulin to match normal prandial insulin secretion better, to control meal‐related glucose excursions without increasing the risk of delayed hypoglycaemia.17, 19, 20 Initial attempts at a “second generation” of rapid‐acting insulin analogues involved the co‐administration of recombinant hyaluronidase enzyme (rHuPH20) to insulin lispro, aspart or glulisine thereby disrupting the interstitial matrix of the subcutaneous tissue and facilitating absorption.21, 22 These analogues did not progress into phase 3 clinical trials and the programme was discontinued. Subsequent approaches included altering the excipients with several compounds developed by Biodel (BIOD‐100/123/238/250, Albireo Pharma Inc, Boston, Massachusetts); the first (BIO‐100) involved adding ethylenediaminetetraacetic acid and citrate to RHI,23 followed by BIOD‐12324 and BIOD‐25025 with magnesium sulphate added to lispro, which also reduced discomfort at the local injection site. These excipients acted to destabilize the insulin hexamer enhancing its dissociation into dimers and monomers.23 This programme has also been discontinued.26 However, the remaining members of the second generation of rapid‐acting insulin analogues that have or are due to enter phase 3 trials, namely fast‐acting aspart (hereafter called faster aspart), ultra‐rapid lispro and BioChaperone Lispro, are the main focus of this review, describing their individual structure, mechanism of action and pharmacology, along with evidence from their respective phase 1 and 3 clinical studies.
2. SECOND GENERATION RAPID‐ACTING INSULIN ANALOGUES
2.1. Structure and mechanism of action
The three candidates differ in terms of primary structure and mechanism of faster absorption (Table 1). Faster aspart contains two additional excipients, niacinamide and l‐arginine. The niacinamide acts to increase subcutaneous blood flow to enhance absorption while the amino acid l‐arginine serves as a stabilizing agent.27 Ultra‐rapid lispro (LY900014) contains the excipients citrate and treprostinil. The citrate enhances vascular permeability at the injection site, while treprostinil accelerates lispro absorption by increasing local vasodilation with no measurable systemic exposure.28, 29 BioChaperone Lispro contains the excipients citrate and BioChaperone BC222.30 Citrate increases vascular permeability at the injection site, while BioChaperone BC222 forms a physical complex with insulin protecting it from enzymatic degradation while enhancing both its stability and solubility increasing the rate of hexamer dissociation and monomer absorption from the subcutaneous tissue.
Table 1.
Current second‐generation rapid‐acting insulin analogues in development
| Drug | Company | Core insulin structure | Added excipients | Mechanism of action |
|---|---|---|---|---|
| Faster aspart27 | Novo Nordisk, Bagsværd, Denmark | Insulin aspart | Niacinamide (vitamin B3), l‐arginine | Increased subcutaneous blood flow |
| Ultra‐rapid lispro28, 29 | Eli Lilly, Indianapolis, IN | Insulin lispro | Treprostinil, citrate | Enhanced vascular permeability and increased local vasodilation |
| BioChaperone Lispro30 | Adocia, Lyon, France | Insulin lispro | BioChaperone BC222,a citrate | Enhanced diffusion |
An oligosaccharide modified with natural molecules.
2.2. Pharmacokinetic/pharmacodynamic studies
The pharmacological properties for each of the second‐generation rapid‐acting analogues have been characterized in a number of phase 1 trials.31, 32, 33 Most of these trials were single‐dose studies comparing each treatment with conventional aspart or lispro in subjects with T1DM, administered by either subcutaneous injection or via continuous subcutaneous insulin infusion (CSII). The majority of the faster aspart trials have been published,34 while results of the trials for ultra‐rapid lispro are currently only in abstract form.29, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 Two of the BioChaperone Lispro trials have been published30, 45 with several trials reported as abstracts.46, 47, 48, 49, 50, 51 Key findings from the completed trials are summarized below.
2.2.1. Features following subcutaneous injection
The pharmacokinetics of faster aspart (0.2 U/kg dose) were characterized in a pooled analysis of six studies in 218 adults with T1DM showing that faster aspart was detected in the blood 5 min earlier than aspart with onset of exposure (time to reach 50% of the maximum concentration) 9.5 min earlier and an offset of exposure (time to late half‐maximum exposure) 12.2 min earlier (Table 2, Figure 1A).52 Initial insulin exposure during the first 30 min after administration was twofold higher with faster aspart. Three 12‐h euglycaemic clamp trials characterized the pharmacodynamics of faster aspart.52 Similar to the pharmacokinetic profile, early glucose‐lowering, as measured by the glucose infusion rate (GIR) during the first 30 min was 74% greater for faster aspart (Figure 2A). In meal tests in persons with T1DM, faster aspart (0.2 U/kg) improved PPG when given immediately before a liquid meal (Figure 3A); mean reductions versus aspart during the first 2 h were in the range of 10–26 mg/dL (0.6–1.4 mmol/L) in adults and 4–27 mg/dL (0.2–1.5 mmol/L) in children/adolescents.53, 54
Table 2.
Key pharmacokinetic and pharmacodynamic results of second‐generation rapid‐acting insulins versus comparators in subjects with T1DM
| Comparator | Administration | Faster aspart | Ultra‐rapid lispro | BioChaperone Lispro | ||||
|---|---|---|---|---|---|---|---|---|
| Aspart | Lispro | Aspart | Faster aspart | Lispro | Aspart | Faster aspart | ||
| Pharmacokinetics | ||||||||
| Onset (t Early50%Cmax) | sc injection | −9.5 min 52 | −8.8 36 to − 12.5 43 min | −13.9 min 43 | −5.9 min 43 | −10.7 min a48 | NR | NR |
| CSII | −11.8 min 55 | −8.6 min 40 | NR | NR | NR | −11.7 min 30 | −0.7 min30 | |
| Offset (t Late50%Cmax) | sc injection | −12.2 min 52 | −7.0 36 to − 13.8 43 min | −21.1 min 43 | −9.5 min 43 | −28.2 min a48 | NR | NR |
| CSII | −35.4 min 55 | −12.2 min40 | NR | NR | NR | −38.2 min 30 | −19.6 min 30 | |
| Early exposure (AUC30 min) | sc injection | ↑~2‐fold 52 | ↑~2.2 36 ‐ to 2.9 43 ‐fold | ↑2.4‐fold 43 | ↑1.2‐fold 43 | ↑2.5 a43 ‐ to 2.7 49 ‐fold | NR | NR |
| CSII | ↑~3‐fold 55 | ↑1.5‐fold 40 | NR | NR | NR | NR | NR | |
| Pharmacodynamic | ||||||||
| Onset (t Early50%GIRmax) | sc injection | −9.5 min 52 | −12.2 min b35 | NR | NR | −10.0 min 47 | ||
| CSII | −11.1 min 55 | NR | NR | NR | NR | −13.0 min 30 | +1.3 min30 | |
| Offset (t Late50%GIRmax) | sc injection | −14.3 min 52 | 0 minb35 | NR | NR | NR | NR | NR |
| CSII | −24.0 min 55 | NR | NR | NR | NR | −38.2 min 30 | −19.6 min 30 | |
| Early effect (AUCGIR,30 min) | sc injection | ↑~1.7‐fold 52 | NR | NR | NR | ↑>3‐fold 47 | NR | NR |
| CSII | ↑~2‐fold 55 | NR | NR | NR | NR | NR | NR | |
Table shows mean time difference versus comparators. Items in bold are statistically significant. Table adapted from oral presentation by Tim Heise at American Diabetes Association Symposium on June 25, 2018.
Abbreviations: AUC, area under the insulin concentration curve; CSII, continuous subcutaneous insulin infusion; GIR, glucose infusion rate; NR, not reported; sc, subcutaneous; t Early50%Cmax, time to 50% of maximum insulin concentration in the early part of the pharmacokinetic profile; t Late50%Cmax, time to late half‐maximum insulin exposure; T1DM, type 1 diabetes.
Mean data from two studies in patients with T1DM.48
In healthy subjects.
Figure 1.
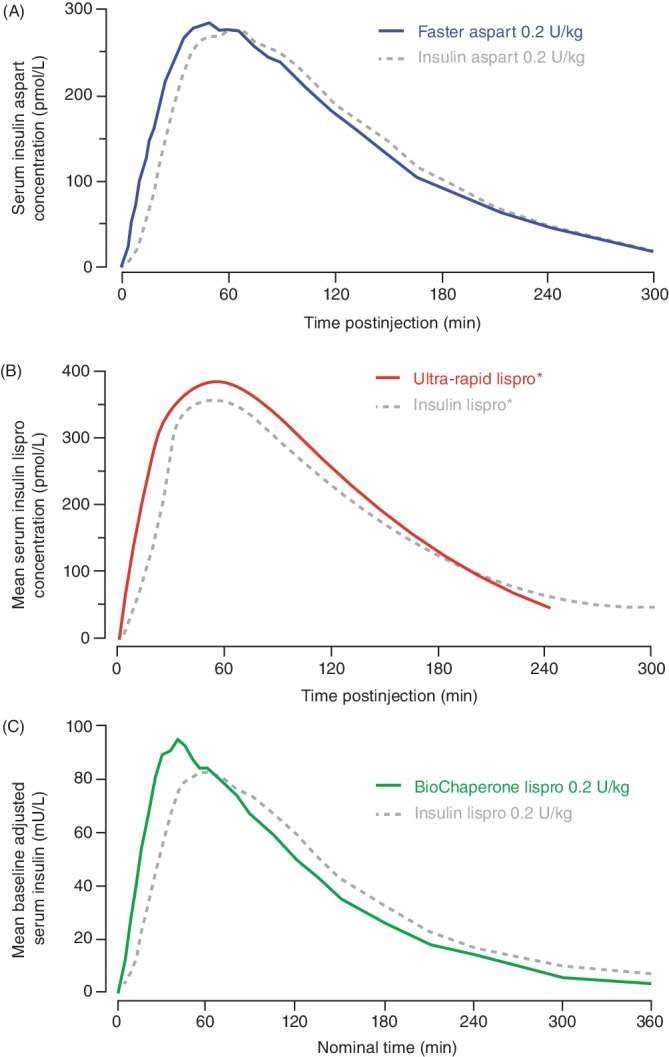
Pharmacokinetic action profiles (serum insulin levels) after subcutaneous injection of (A) faster aspart [adapted from Heise et al52 under Creative Commons Attribution‐NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/)], (B) ultra‐rapid lispro (adapted from Kazda et al36) and (C) BioChaperone Lispro (adapted from Andersen et al47), all versus insulin aspart or lispro in people with type 1 diabetes. *Dose based on individual insulin‐carbohydrate ratios
Figure 2.
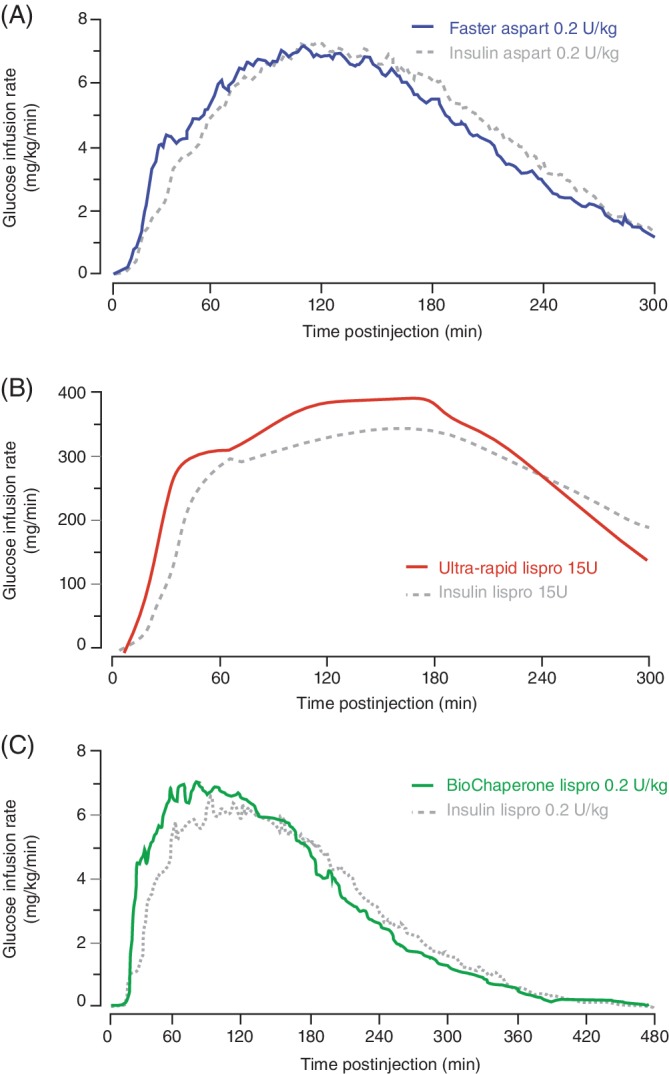
Pharmacodynamic action profiles (glucose infusion rate) in clamp studies (fasting) after subcutaneous injection of (A) faster aspart [adapted from Heise et al52 under Creative Commons Attribution‐NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/)], (B) ultra‐rapid lispro (adapted from Leohr et al35) and (C) BioChaperone Lispro (adapted from Andersen et al47), all versus insulin aspart or lispro in healthy volunteers or people with type 1 diabetes
Figure 3.
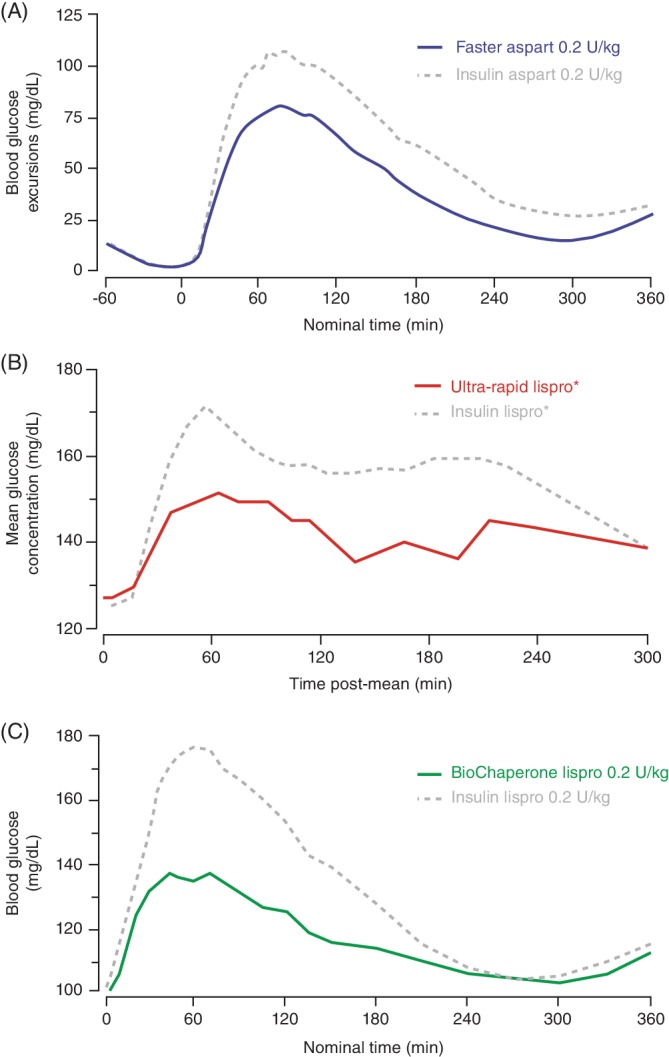
Postprandial blood glucose profiles after subcutaneous injection of (A) faster aspart following a standardized liquid meal (67% carbohydrate, 600 kcal) (adapted from Heise et al53), (B) ultra‐rapid lispro following a mixed meal (adapted from Kazda et al36) and (C) BioChaperone Lispro following a standardized liquid meal (adapted from Andersen et al49), all versus insulin aspart or lispro in people with type 1 diabetes. *Dose based on individual insulin‐carbohydrate ratios
The pharmacological features of ultra‐rapid lispro were characterized in a single‐dose study in 36 people with T1DM showing that ultra‐rapid lispro was detected in the blood 8.8 min earlier (15.5 vs. 24.3 min) than lispro (Table 2) with a twofold higher insulin exposure over the first 30 min (Figure 1B).36 Ultra‐rapid lispro also showed a greater early glucose‐lowering effect, as measured by GIR, when compared with lispro (Figure 2B). In meal tests, ultra‐rapid lispro improved PPG control when given immediately before a mixed meal (composition not reported) in persons with either T1DM36, 37, 40 or type 2 diabetes (T2DM).39 Glucose excursions with ultra‐rapid lispro were lower by 39%–47% during the first 2 h after the start of this (presumably liquid) meal (Figure 3B).37, 39 In a further multiple comparison study in 68 people with T1DM, time to onset of ultra‐rapid lispro exposure occurred earlier than with faster aspart, lispro and aspart (Table 2).43 Initial insulin exposure during the first 30 min after administration of ultra‐rapid lispro was also higher than faster aspart (1.2‐fold), lispro (2.9‐fold) and aspart (2.4‐fold). Offset of exposure occurred earlier than with the other analogues. PPG excursions following a liquid meal test (100 g of carbohydrate) were numerically lower with ultra‐rapid lispro than faster aspart and significantly lower versus lispro and aspart.
The pharmacokinetics of BioChaperone Lispro (0.2 U/kg) were characterized in a pooled analysis of two studies in 76 adults with T1DM showing that BioChaperone Lispro was detected in the blood 11 min earlier than lispro (Figure 1C) with offset of exposure occurring 28.2 min earlier (Table 2).48 Initial insulin exposure during the first 30 min after administration was more than twofold higher with BioChaperone.48 BioChaperone Lispro showed a greater glucose‐lowering effect, as measured by GIR, within the first hour compared with lispro (Figure 2C).47 In standardized liquid (80 g carbohydrate) or individualized solid (50% carbohydrate) meal tests in persons with T1DM45, 49 or T2DM,50 BioChaperone Lispro reduced glucose excursions by 22%–61% during the first 2 h compared with lispro (Figure 3C).
2.2.2. Features when used in continuous subcutaneous insulin infusion (pumps)
When faster aspart was given via CSII as a bolus of 0.15 U/kg on top of a basal rate of 0.02 U/kg/h, plasma insulin levels were threefold higher than aspart during the first 30 min.55 Onset of exposure occurred 11.8 min earlier (20.7 vs. 32.5 min) with faster aspart and the glucose‐lowering effect during the first 30 min was increased by more than 100%.
When ultra‐rapid lispro was given via CSII (bolus dose 15 U, basal dose not reported), insulin exposure [area under the insulin concentration curve (AUC)] was more than 50% higher than lispro during the first 15 min and the onset of exposure occurred 8.6 min earlier (15.6 vs. 24.1 min) (Table 2).40
When BioChaperone Lispro was given via CSII (0.15 U/kg bolus on top of basal rate 0.01 U/kg/h), early insulin exposure (AUC) during the first hour was more than 60% higher than aspart, with offset of exposure occurring 38.2 min earlier (Table 2).30 In the same study, early insulin exposure during the first hour was 14% higher than faster aspart with offset of exposure occurring 19.6 min earlier.
In summary, despite differences in the structure and mechanism of action, the pharmacokinetic profiles of the three candidates are quite similar (Table 2), demonstrating an earlier and higher peak concentration with a shorter duration of action relative to their predecessors (Figure 1). The differences in onset of action range from 9 to 14 min with an offset of effect in the range of 7–28 min with respect to their half‐maximal metabolic effects (Table 2).56 This degree of difference is qualitatively similar to that seen previously between the current conventional rapid‐acting insulin analogues and RHI. However, none has been compared against insulin glulisine, which has been shown to have a slightly faster onset of action than both aspart and lispro.57, 58, 59, 60
2.3. Phase 3 clinical trials
Eight phase 3 trials have reported the efficacy and safety of faster aspart (Table 3A).61, 62, 63, 64, 65, 66, 67, 68, 69, 70 Seven phase 3 trials are evaluating the efficacy and safety of ultra‐rapid lispro versus lispro,71, 72, 73, 74, 75, 76, 77 with preliminary results for three of these trials reported as abstracts.71, 74, 76, 78 The phase 3 trial programme for BioChaperone Lispro is yet to be announced.
Table 3A.
Key features and results of phase 3 trials comparing mealtime faster aspart with different comparators
| Parameter | T1DM basal‐bolus | T2DM basal‐bolus | T1DM pump | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Onset 161, 62 | Onset 863, 64 | Onset 765 | Onset 266 | Onset 970 | Onset 367 | Onset 468 | Onset 569 | ||
| Comparator | Aspart | Aspart | Aspart | Aspart | Aspart | Basal insulin only | CSII aspart | CSII aspart | |
| Basal insulin used | IDet | IDeg | IDeg | Gla‐100 | IDeg | IDet/Gla‐100/NPH | – | – | |
| Participants (n) | 381 vs. 380 | 381 vs. 380 | 342 vs. 342 | 260 vs. 258 | 345 vs. 344 | 546 vs. 545 | 116 vs. 120 | 25 vs. 12 | 236 vs. 236 |
| Duration (weeks) | 26 | 52 | 26 | 26 | 26 | 16 | 18 | 6 | 16 |
| Glycaemic control | |||||||||
| ΔHbA1c, % | −0.15 | −0.10 | −0.02 | −0.17 | −0.02 | −0.04 | −0.94 | −0.14 | +0.09 a |
| HbA1c <7.0%, OR | 1.47 | 0.97 | 0.88 | 1.33b | 1.01 | NR | 9.31 | NR | 0.76 |
| Meal test | |||||||||
| Composition | Liquidc | Liquidc | Liquid, 78 g CHO | Liquidd, 1.5 g CHO/kg | Liquid, ~80 g CHO | Liquidc | NA | NA | Liquidc |
| Consumption time | ≤12 min | ≤12 min | ≤12 min | NR | ≤12 min | NR | NA | NA | ≤12 min |
| ΔPPG1‐h, mmol/L | −1.18 | −0.91 | −0.90 | NSd | −0.59 | −0.40 | NA | NA | −0.91 |
| ΔPPG2‐h, mmol/L | −0.67 | −0.42 | −0.35 | NSd | −0.36 | −0.30 | NA | NA | −0.90 |
| SMPG | |||||||||
| ΔPPG1‐h, mmol/L | NR | NR | −0.48 e | −0.93 f | NR | −0.25 f | −1.14 e | NR | −0.46 e , f |
| ΔPPG2‐h, mmol/L | −0.21e | −0.25e , f | NR | NR | NR | NR | −2.48 e | −0.77f , g | NR |
| PPG2‐h ≤ 7.8 mmol/L, OR | 1.33 | 1.57 | 1.54 h | NR | 1.18 | NR | 41.9 | NR | NR |
| PPG2‐h ≤ 7.8 mmol/L without SH, OR | NR | 1.47 | NR | NR | 1.23 | NR | 44.2 | NR | NR |
| Hypoglycaemia | |||||||||
| Overalli, RR | 1.01 | 1.01 | 0.84 | 1.11 | 1.09 | 0.81 | 8.24 | 0.98j | 1.00 |
| PM within 1 h, RR | 1.48 | 1.37 | 1.09 | NS | 1.29 | 1.16 | NR | NR | 1.78 |
| PM within 2 h, RR | NR (NS)k | NR | 0.75l | NS | 1.60 | 0.97 | NR | NR | NR (NS) |
Table shows mean difference versus comparators. Confidence intervals around point estimates are not shown for simplicity. Items in bold are statistically significant.
Abbreviations: ‐, no basal insulin; Δ, change; aspart, insulin aspart; CHO, carbohydrate; ΔHbA1c, ETD in mean ΔHbA1c from baseline (%); ΔPPG1‐h, ETD in 1‐h PPG increment; IDeg, insulin degludec; IDet, insulin detemir; ETD, estimated treatment difference; Gla‐100, insulin glargine 100 U/mL; NA, not applicable as no meal test was performed in the study; NR, not reported; NS, no statistically significant difference; OR, estimated odds ratio; PM, post‐meal; PPG, postprandial glucose; RR, rate ratio; SH, severe hypoglycaemia; SMPG, self‐monitored plasma glucose; T1DM, type 1 diabetes; T2DM, type 2 diabetes.
P < 0.02 in favour of aspart, P < 0.001 for non‐inferiority of faster aspart versus aspart.
Odds of achieving HbA1c <7.5%.
78–80 g CHO (Ensure, Abbott Nutrition, Columbus, Ohio).
Meal test performed in a subgroup of subjects from selected sites aged ≥8 years at screening.
Estimated change from baseline in mean PPG increments (SMPG profiles).
Based on PPG increment across all meals.
Converted from mg/dL to mmol/L using the conversion factor 0.0555.
OR of 1‐h PPG ≤7.8 mmol/L.
Hypoglycaemia defined as severe or blood glucose confirmed (<3.1 mmol/L [56 mg/dL]) events.
After adjusting for imbalance in severe or blood glucose‐confirmed hypoglycaemia in the run‐in period.
Meal‐related hypoglycaemia within 2 h after meal.
During period 1–2 h after meal.
2.3.1. Trials in adults with type 1 diabetes
Two similarly designed studies compared mealtime faster aspart (administered 0–2 min before a meal) with mealtime aspart in a basal‐bolus regimen in a 52‐week trial with insulin detemir (Onset 1) and in a 26‐week trial with insulin degludec (Onset 8).61, 62, 63, 64 In Onset 1, HbA1c was reduced with faster aspart versus aspart by 0.15% after week 2661 and by 0.10% at week 52.62 No significant difference in HbA1c was observed in Onset 8 [estimated treatment difference (ETD) of −0.02% in favour of faster aspart].63 The rate of severe or blood glucose (BG)‐confirmed hypoglycaemia was comparable in both trials. PPG increment at 1‐h post‐meal in a liquid meal test was reduced with faster aspart versus aspart in both trials at week 26,61, 63 and at week 52 in Onset 1 (see Table 3A for details).62 When faster aspart was administered within 20 min after meal initiation PPG increment at 1‐h post‐meal in a meal test was higher versus mealtime aspart in both trials.61, 63 Mean PPG levels based on the results of self‐monitored plasma glucose (SMPG) profiles with consumption of solid mixed meals, were reduced with faster aspart across all meals in both trials.62, 63 In the 26‐week Onset 8 trial, the self‐measured PPG increment at 1 h was also reduced for faster aspart but there was a trend for higher levels at bedtime.63 Hypoglycaemia within the first hour from meal initiation was increased with faster aspart in Onset 1 at 26 and 52 weeks,61, 62 while in Onset 8, a reduced rate of hypoglycaemia 3–4 h after meal initiation was seen with faster aspart.63 The cause of the discrepancy between these two studies is not apparent, although the use of different basal insulins may play a role.
A single trial (PRONTO‐T1D) compared mealtime ultra‐rapid lispro (administered 0–2 min before a meal) with lispro in a basal‐bolus treatment regimen (Table 3B).71, 78 The change from baseline in HbA1c after 26 weeks was similar with both treatments (ETD −0.08%, P = 0.06). Administration of ultra‐rapid lispro 20 min after the start of a meal was less effective (but still non‐inferior) than pre‐meal lispro (HbA1c ETD +0.13%, P = 0.003). PPG increment at 1 and 2 h post‐meal in a liquid meal test at week 26 (see Table 3B for details) was reduced with ultra‐rapid lispro. Similarly, PPG increment at 1 h based on SMPG was reduced with ultra‐rapid lispro at breakfast, with no difference at midday or following the evening meal. Severe, overall and early (≤4 h) postprandial BG‐documented hypoglycaemia was similar between treatments with fewer episodes observed with mealtime ultra‐rapid lispro in the late (>4 h) postprandial period.
Table 3B.
Key features and results of reported phase 3 trials comparing mealtime ultra‐rapid lispro with mealtime lispro
| Parameter | T1DM basal‐bolus | T2DM basal‐bolus |
|---|---|---|
| PRONTO‐T1D71, 78 | PRONTO‐T2D76, 79 | |
| Comparator | Mealtime lispro | Mealtime lispro |
| Basal insulin used | Gla‐100/IDeg | Gla‐100/IDeg |
| Participants (n) | 451 vs. 442 | 336 vs. 337 |
| Duration (weeks) | 26 (52) | 26 |
| Glycaemic control | ||
| Δ HbA1c (%) | −0.08 | +0.06 |
| Meal testa | ||
| Composition | Liquida, ~100 g CHO (~57% kcal) | Liquida |
| Consumption time | NR | NR |
| ΔPPG1‐h, mmol/L | −1.55 | −0.66 |
| ΔPPG2‐h, mmol/L | −1.73 | −0.96 |
| ΔPPG3‐h, mmol/L | NR (P < 0.001) | NR (P < 0.001) |
| ΔPPG4‐h, mmol/L | NR (P < 0.05) | NR (P < 0.05) |
| Hypoglycaemia rateb | ||
| Documented, RR | 0.92 | 1.02 |
| Post‐meal ≤1 h, RR | 1.16 | 1.14 |
| Post‐meal ≤2 h, RR | 1.11 | 1.33 |
| Post‐meal >1 to ≤2 h, RR | 1.07 | 2.31 |
| Post‐meal >2 to ≤4 h, RR | 1.01 | 1.44 |
| Post‐meal ≤4 h, RR | 1.06 | NR |
| Post‐meal >4 h, RR | 0.62 (P < 0.001) | 0.95 |
Table shows mean difference versus mealtime lispro. Confidence intervals around point estimates are not shown for simplicity. Items in bold are statistically significant.
Abbreviations: Δ, change; lispro, insulin lispro; ΔHbA1c, ETD in mean HbA1c Δ from baseline (%); ΔPPG1‐h, ETD in 1‐h PPG increment; IDeg, insulin degludec; ETD, estimated treatment difference; Gla‐100, insulin glargine 100 U/mL; NR, not reported; PPG, postprandial glucose; RR, relative rate; SH, severe hypoglycaemia; SMPG, self‐monitored plasma glucose; T1DM, type 1 diabetes; T2DM, type 2 diabetes.
Standardized liquid mixed meal tolerance test (MMTT).
Hypoglycaemia defined as blood glucose documented [<3.0 mmol/L (54 mg/dL)] events with or without symptoms.
2.3.2. Trials in children and young persons with type 1 diabetes
A single trial (Onset 7) in children and young persons (1–17 years) compared faster aspart with aspart in a basal‐bolus regimen.65 HbA1c was reduced with faster aspart versus aspart by 0.17% at week 26 with a comparable rate of severe or BG‐confirmed hypoglycaemia. PPG increment at 1 h based on the SMPG profile was reduced with faster aspart at breakfast, dinner and across all meals (0.93 mmol/L improvement). Administration of post‐meal faster aspart was less effective (but still non‐inferior) than aspart with the 1‐h PPG increment from SMPG across all meals also slightly worse than aspart (+0.43 mmol/L). No studies are available with ultra‐rapid lispro or BioChaperone Lispro.
2.3.3. Trials in type 2 diabetes
Two studies compared mealtime faster aspart with aspart in a basal‐bolus regimen in a 26‐week trial with insulin glargine 100 U/mL (glargine‐100) (Onset 2)66 and in a 16‐week trial with insulin degludec (Onset 9).70 No significant difference in HbA1c at the end of treatment was observed in either trial (ETD −0.02% in Onset 2, −0.04% in Onset 9; both in favour of faster aspart). The rate of severe or BG‐confirmed hypoglycaemia was comparable in the 26‐week trial, but significantly lower for faster aspart in the 16‐week trial. PPG increment at 1 h post‐meal in liquid meal tests at the end of each trial was reduced with faster aspart. However, PPG across all daily meals based on the SMPG was comparable in the 26‐week trial but significantly lower in the 16‐week trial (along with a reduced 1‐h PPG increment after lunch and the evening meal) for faster aspart. The improved PPG control in the 16‐week trial was counter‐balanced by an increase in early (0–2 h) post‐meal hypoglycaemia.
A further 18‐week trial evaluated the addition of faster aspart versus placebo to basal insulin and metformin (Onset 3).67 As expected, a significant improvement in HbA1c (ETD −0.94%) and self‐monitored 2‐h PPG across all meals was seen with the basal‐bolus using faster aspart versus basal insulin only.
A single trial (PRONTO‐T2D) has compared mealtime ultra‐rapid lispro with lispro in a basal‐bolus regimen (Table 3B).76 HbA1c was reduced with both treatments by a similar amount after 26 weeks (ETD +0.06%) with a comparable rate of severe or BG‐confirmed hypoglycaemia. PPG increment at 1 and 2 h post‐meal in a liquid meal test at week 26 was reduced with ultra‐rapid lispro, with the benefits extending out to 4 h. PPG increment at 1 and 2 h and daily mean PPG levels/excursions based on the SMPG profile were reduced with ultra‐rapid lispro.79
2.3.4. Trials in pumps and closed‐loop systems
Two trials (Onset 4 and 5) evaluated the use of faster aspart versus aspart when delivered via CSII pumps. Pump compatibility of faster aspart over 6 weeks in terms of the risk of catheter occlusions was investigated in 37 persons with T1DM.68 Although there was a trend towards better glycaemic control with faster aspart and a similar risk of hypoglycaemia, unexplained hyperglycaemia and premature infusion set changes (<72 h) were more common. There were 21 premature infusion‐set changes in 11 (44%) of the faster aspart users compared with four in two (17%) of the aspart users.80 Only one‐third of those in the faster aspart group could be attributed to purely technical issues (e.g. empty reservoir, kinked or dislodged infusion‐set tubing) compared with three‐quarters explainable in aspart users. No confirmed cases of microscopic occlusions of the infusion sets (Quick‐Set® or Silhouette® [Medtronic, Northridge, CA]) were seen when faster aspart (25 persons) or aspart (12 persons) were used in connection with a MiniMed Paradigm® (Medtronic, Northridge, California) pump.
In the larger Onset 5 trial, the change from baseline in HbA1c after 16 weeks was slightly in favour of aspart (ETD 0.09%, P < 0.02) with a comparable rate of severe or overall BG‐confirmed hypoglycaemia.69 PPG increment at 1 and 2 h post‐meal in a liquid meal test at 16 weeks was reduced with faster aspart. Changes from baseline in 1‐ and 2‐h interstitial glucose (IG) increments assessed by continuous glucose monitoring were also significantly lower with faster aspart across all meals. However, the proportion of IG values in the target range of 4–10 mmol/L (71–180 mg/dL) was the same in each treatment group. Nocturnal, pre‐meal and 4 h post‐meal (particularly the evening meal) IG levels were higher with faster aspart. It was postulated that the elevated nocturnal IG levels with faster aspart might have been because of suboptimal pump settings for the evening meal bolus, lack of basal insulin compensation because of the shorter bolus insulin action, or suboptimal basal insulin rates at night.81 Hypoglycaemia within the first hour from meal initiation was increased with faster aspart. Similar to Onset 4, a higher number of participants on faster aspart experienced events related to infusion‐site reactions versus aspart {5.5% of participants [0.29 events/patient‐years of exposure (PYE)] vs. 3.8% of participants (0.18 events/PYE), respectively}. In addition, a greater number of participants required non‐regular change of the infusion set [45.8% of participants (2.76 events/PYE) vs. 31.8% of participants (2.87 events/PYE), respectively].
Two small trials have reported on the use of faster aspart in closed‐loop delivery systems. The first trial in 20 participants with T1DM showed a similar proportion of time in target range (primary endpoint) versus aspart.82 A further study in 15 adults with T2DM showed that faster aspart achieved comparable glucose control (time in target range) vs aspart although a higher dose of faster aspart was required to achieve this outcome.83
Two trials (PRONTO‐Pump and PRONTO‐Pump 2) are evaluating ultra‐rapid lispro use via CSII pumps.74, 75 Pump compatibility of ultra‐rapid lispro versus lispro over 6 weeks in terms of the rate of infusion set failures (premature infusion set changes because of a pump occlusion alarm or unexplained hyperglycaemia) was investigated in 49 persons with T1DM (PRONTO‐Pump).74 Although there was no difference in the rate of infusion set failures between the two treatments, premature infusion set changes (≤72 h) were more common with the use of ultra‐rapid lispro. There were 77 premature infusion‐set changes in ultra‐rapid lispro users versus 52 in lispro users (aggregate rate: 1.13 vs. 0.78 events per 30 days, P = 0.028). A trend towards better glycaemic control with ultra‐rapid lispro during the 6 weeks was observed with no difference in the risk of hypoglycaemia. The results of the PRONTO‐Pump 2 study are not yet available.75
2.4. Regulatory status
Faster aspart was approved in 2017 in Europe and the USA for use in adults with diabetes,84, 85 and subsequently in both regions for use in children or adolescents aged ≥1 year. Prescribing guidelines recommend faster aspart should be given immediately before a meal, although it may be given up to 20 min after starting a meal if necessary.85, 86 Faster aspart is also approved in Europe and the USA for CSII by external delivery systems.85, 86 Regulatory applications for ultra‐rapid lispro have been submitted in Europe and Japan, and were intended in the USA in late 2019.87
3. WHAT IS THE POTENTIAL ROLE FOR THE SECOND‐GENERATION OF RAPID‐ACTING INSULINS?
Faster aspart is the first and most extensively investigated of the three newer rapid‐acting insulins and the only one currently approved for clinical use. Trials in patients with T1DM show a significant improvement in PPG increment at 1 h (mean decrease −0.90 to −1.18 mmol/L) compared with aspart.61, 63, 69 The limitation of the liquid meal tests in these trials should be noted as in real‐life, solid mixed meals are more commonly consumed with a slower and more prolonged absorption period. The observed improvement in the 1 to 2‐h PPG increment is sometimes offset by a slightly higher 4‐h PPG (Onset 5), suggesting a need for optimizing the basal insulin and/or adjusting the prandial insulin during an extended inter‐prandial period. This is conceptually similar to what was observed more than 20 years ago with the introduction of rapid‐insulin analogues.13, 88 Trials in people with T2DM show a similar but lesser, yet statistically significant, reduction in the 1‐h PPG increment (mean decrease −0.40 to −0.59 mmol/L) compared with aspart.66, 70 Despite reducing PPG levels in the trials reported, the overall glucose‐lowering ability of faster aspart compared with aspart (based on HbA1c levels) was comparable in T2DM66, 70 or slightly better in T1DM.61, 62, 63, 65 SMPG profile data in several trials indicate that faster aspart has a minimal effect on reducing PPG after mixed meals. It could be that the duration of increased bioavailability in the early phase of their absorption is too short with mixed meals thereby exposing patients to late postprandial hyperglycaemia 3–4 h after meals. Several studies observed a slight increase in hypoglycaemia within the first 1–2 h following pre‐meal faster aspart,61, 62, 66, 69 although other studies showed no difference in the risk. In this regard, the possible risk of early hypoglycaemia in individual patients should be considered in relation to meal composition, thereby placing the patient at the centre of controlling postprandial glycaemic excursions with glucose monitoring.16
Preliminary data for ultra‐rapid lispro from two phase 3 trials have recently been presented. In people with T1DM, ultra‐rapid lispro improved PPG increment at 1 h (mean decrease −1.55 mmol/L) in a liquid meal test compared with lispro. Similarly, in persons with T2DM, a lesser, although significant, improvement in the 1‐h PPG increment (−0.66 mmol/L) was observed. In both trials, PPG excursions with ultra‐rapid lispro were significantly lower within 30 min and persisted for up to 4 h after the test meal. Despite reducing PPG levels, HbA1c levels were comparable with lispro in both populations. Safety data reported to date do not indicate an increase in postprandial hypoglycaemia risk compared with lispro. The limited data currently presented preclude comparison with the data for faster aspart.
Each of the phase 3 clinical studies comparing faster aspart or ultra‐rapid lispro with aspart/lispro have generally given each drug just before the meal (0–2 min), although some studies had a third group with the faster‐acting analogues given post‐meal. No study has compared the current rapid‐acting insulins (aspart/lispro/glulisine) given 15–20 min before the meal with the new faster‐acting analogue given at the time of the meal (0–2 min). In this situation, the faster‐acting analogue might not demonstrate superior 1 and 2‐h PPG control. Similarly, it is not known whether the risk of hypoglycaemia with aspart/lispro/glulisine given 15–20 min before the meal is similar or lower than the faster‐acting analogues given with a meal.
CSII is an increasingly popular and effective method of subcutaneous insulin administration.56 Insulins with a faster onset and offset of glucose‐lowering effect are particularly attractive in a CSII setting to improve PPG control further while limiting the risk of late post‐meal hypoglycaemia.89 However, the results of the two clinical trials of faster aspart appear to suggest that this analogue is less stable and has a higher occlusion rate of CSII catheters than aspart. The cause of this lower physico‐chemical stability is currently being explored and in view of the uncertainty about a more frequent need to replace the infusion sets and a lack of practical guidance on the optimal use of faster aspart,81 the current rapid‐acting insulin therapies could probably remain the preferred analogues for use in CSII. Limited data have so far been presented for ultra‐rapid lispro.74 Neither insulin has been evaluated in children or young persons with diabetes. The potential that these new rapid‐acting insulins could improve the performance of closed‐loop automated insulin delivery systems (e.g. the artificial pancreas) remains of great interest,90 with ongoing trials evaluating their use in this environment.
4. CONCLUSIONS
The new second‐generation of rapid‐acting insulin analogues described in this review have applied different modifications in pharmaceutical formulation to enhance further the rate of subcutaneous absorption of the insulin, which is an attempt to achieve better control of PPG when compared with their predecessors. This has been successfully achieved. However, there is only a minimal improvement in overall glycaemic control (HbA1c), and no lesser risk of inter‐prandial hypoglycaemia as observed in a limited number of long‐term studies in persons with T1DM and T2DM. To achieve optimal glycaemic control, individualized adjustments of basal insulin (subcutaneous bolus or CSII) and/or additional small boluses of the faster‐acting insulin analogue may also be required to accommodate prolonged inter‐ or postprandial periods. Because the majority of trials evaluating PPG excursions have used liquid meals, additional information is required for solid meals with different compositions mimicking real‐life conditions.
Future studies are needed to answer the question as to which patients would benefit most from these new insulin analogues, as compared with those who would not. At present, it is tempting to speculate that the newer rapid‐acting insulin analogues would be of particular value for individuals with marked post‐breakfast hyperglycaemia, because of the dawn phenomenon91 and/or insulin resistance, individuals on corticosteroids, fertile women in the second half of the menstrual cycle and those individuals who regularly or intermittently consume meals with a high content of refined carbohydrates. On the other hand, there are limited data available on the benefit of these new insulins in people experiencing delayed gastric emptying who may be at greater risk of hypoglycaemia soon after their meals. In addition, it is also important to recognize the possible need to optimize basal insulin supplementation, when introducing these newer prandial insulins. Therefore, future trials should clarify their role in various clinical scenarios typically encountered in daily clinical practice. Their application in CSII devices also remains at an early phase with compatibility issues to be clarified.
CONFLICTS OF INTEREST
D.R.O. has received lecture fees/honoraria from Sanofi, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Mendor and Roche Diagnostics. G.B.B. is a consultant to Menarini and Sanofi; has provided research support to Sanofi; and is a member of the speaker's bureau for Menarini and Sanofi. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
AUTHOR CONTRIBUTIONS
Both authors contributed equally to the literature search, interpretation and writing of this review. Both authors approved the final submitted version.
ACKNOWLEDGMENTS
The authors wish to acknowledge the support of Oberon Ltd for figure preparation, article formatting and editing, funded by Sanofi.
Owens DR, Bolli GB. The continuing quest for better subcutaneously administered prandial insulins: a review of recent developments and potential clinical implications. Diabetes Obes Metab. 2020;22:743–754. 10.1111/dom.13963
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13963.
Funding information Funding was provided by Sanofi for figure preparation, article formatting and editing
REFERENCES
- 1. The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329:977‐986. [DOI] [PubMed] [Google Scholar]
- 2. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577‐1589. [DOI] [PubMed] [Google Scholar]
- 3. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group , Lachin JM, White NH, et al. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow‐up in the DCCT/EDIC. Diabetes. 2015;64:631‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woerle HJ, Neumann C, Zschau S, et al. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract. 2007;77:280‐285. [DOI] [PubMed] [Google Scholar]
- 5. Luijf YM, van Bon AC, Hoekstra JB, DeVries JH. Premeal injection of rapid‐acting insulin reduces postprandial glycemic excursions in type 1 diabetes. Diabetes Care. 2010;33:2152‐2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shiramoto M, Nishida T, Hansen AK, Haahr H. Fast‐acting insulin aspart in Japanese patients with type 1 diabetes: Faster onset, higher early exposure and greater early glucose‐lowering effect relative to insulin aspart. J Diabetes Investig. 2018;9:303‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bolli GB, Di Marchi RD, Park GD, Pramming S, Koivisto VA. Insulin analogues and their potential in the management of diabetes mellitus. Diabetologia. 1999;42:1151‐1167. [DOI] [PubMed] [Google Scholar]
- 8. Home PD. The pharmacokinetics and pharmacodynamics of rapid‐acting insulin analogues and their clinical consequences. Diabetes Obes Metab. 2012;14:780‐788. [DOI] [PubMed] [Google Scholar]
- 9. Hermansen K, Bohl M, Schioldan AG. Insulin aspart in the management of diabetes mellitus: 15 years of clinical experience. Drugs. 2016;76:41‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fullerton B, Siebenhofer A, Jeitler K, et al. Short‐acting insulin analogues versus regular human insulin for adults with type 1 diabetes mellitus. Cochrane Database Syst Rev. 2016;6:CD012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siebenhofer A, Plank J, Berghold A, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev. 2006;2:CD003287. [DOI] [PubMed] [Google Scholar]
- 12. Del Sindaco P, Ciofetta M, Lalli C, et al. Use of the short‐acting insulin analogue lispro in intensive treatment of type 1 diabetes mellitus: importance of appropriate replacement of basal insulin and time‐interval injection‐meal. Diabet Med. 1998;15:592‐600. [DOI] [PubMed] [Google Scholar]
- 13. Lalli C, Ciofetta M, Del Sindaco P, et al. Long‐term intensive treatment of type 1 diabetes with the short‐acting insulin analog lispro in variable combination with NPH insulin at mealtime. Diabetes Care. 1999;22:468‐477. [DOI] [PubMed] [Google Scholar]
- 14. Cobry E, McFann K, Messer L, et al. Timing of meal insulin boluses to achieve optimal postprandial glycemic control in patients with type 1 diabetes. Diabetes Technol Ther. 2010;12:173‐177. [DOI] [PubMed] [Google Scholar]
- 15. Muchmore DB. The need for faster insulin. J Diabetes Sci Technol. 2017;11:157‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slattery D, Amiel SA, Choudhary P. Optimal prandial timing of bolus insulin in diabetes management: a review. Diabet Med. 2018;35:306‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heinemann L, Muchmore DB. Ultrafast‐acting insulins: state of the art. J Diabetes Sci Technol. 2012;6:728‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Home PD. Plasma insulin profiles after subcutaneous injection: how close can we get to physiology in people with diabetes? Diabetes Obes Metab. 2015;17:1011‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zaykov AN, Mayer JP, DiMarchi RD. Pursuit of a perfect insulin. Nat Rev Drug Discov. 2016;15:425‐439. [DOI] [PubMed] [Google Scholar]
- 20. Mathieu C, Gillard P, Benhalima K. Insulin analogues in type 1 diabetes mellitus: getting better all the time. Nat Rev Endocrinol. 2017;13:385‐399. [DOI] [PubMed] [Google Scholar]
- 21. Muchmore DB, Vaughn DE. Review of the mechanism of action and clinical efficacy of recombinant human hyaluronidase coadministration with current prandial insulin formulations. J Diabetes Sci Technol. 2010;4:419‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morrow L, Muchmore DB, Hompesch M, Ludington EA, Vaughn DE. Comparative pharmacokinetics and insulin action for three rapid‐acting insulin analogs injected subcutaneously with and without hyaluronidase. Diabetes Care. 2013;36:273‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krasner A, Pohl R, Simms P, Pichotta P, Hauser R, De Souza E. A review of a family of ultra‐rapid‐acting insulins: formulation development. J Diabetes Sci Technol. 2012;6:786‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krasner A, Brazg RL, Blevins TC, et al. Safety and efficacy of ultra‐rapid‐acting human insulin formulation BIOD‐123 in patients with type 1 diabetes. Diabetes. 2014;63(Suppl 1):A34. [Google Scholar]
- 25. Krasner A, Canney L, Pichotta P, et al. Lispro formulations BIOD‐238 and BIOD‐250 associated with faster absorption and declines from peak concentrations compared to Humalog [abstract 44‐LB]. Diabetes. 2013;62(Suppl 1A):LB12. [Google Scholar]
- 26. Hedrington MS, Davis SN. Discontinued drug therapies to treat diabetes in 2015. Expert Opin Investig Drugs. 2017;26:219‐225. [DOI] [PubMed] [Google Scholar]
- 27. Kildegaard J, Buckley ST, Nielsen RH, et al. Elucidating the mechanism of absorption of fast‐acting insulin aspart: The role of niacinamide. Pharm Res. 2019;36:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Michael MD, Zhang C, Siesky AM, et al. Exploration of the mechanism of accelerated absorption for a novel insulin lispro formulation [abstract 968‐P]. Diabetes. 2017;66(Suppl 1):A250. [Google Scholar]
- 29. Pratt E, Leohr J, Heilmann C, Johnson J, Landschulz W. Treprostinil causes local vasodilation, is well tolerated, and results in faster absorption of insulin lispro [abstract 975‐P]. Diabetes. 2017;66(Suppl 1):A253. [Google Scholar]
- 30. Heise T, Meiffren G, Alluis B, et al. BioChaperone Lispro versus faster aspart and insulin aspart in patients with type 1 diabetes using continuous subcutaneous insulin infusion: a randomized euglycemic clamp study. Diabetes Obes Metab. 2019;21:1066‐1070. [DOI] [PubMed] [Google Scholar]
- 31. http://clinicaltrials.gov. Faster‐acting insulin aspart Phase 1 studies. https://clinicaltrials.gov/ct2/results?term=NN1218. Accessed November 21, 2019.
- 32. http://clinicaltrials.gov. Ultra rapid lispro (LY900014) Phase 1 studies. https://clinicaltrials.gov/ct2/results?term=LY900014. Accessed November 21, 2019.
- 33. http://clinicaltrials.gov. BioChaperone Lispro Phase 1/2 studies. https://clinicaltrials.gov/ct2/results?term=BioChaperoneLispro Accessed November 21, 2019.
- 34. Haahr H, Heise T. Fast‐acting insulin aspart: a review of its pharmacokinetic and pharmacodynamic properties and the clinical consequences. Clin Pharmacokinet. 2019; https://doi:10.1007/s40262-019-00834-5. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leohr J, Pratt EJ, Heilmann C, Johnson J, Kelly RP, Lanschulz W. A novel insulin lispro formulation containing citrate and treprostinil demonstrates faster absorption and onset of insulin action in healthy subjects [abstract 976‐P]. Diabetes. 2017;66(Suppl 1):A253. [Google Scholar]
- 36. Kazda C, Leohr J, Liu R, et al. A novel formulation of insulin lispro containing citrate and treprostinil shows faster absorption and improved postprandial glucose excursions vs. Humalog in patients with T1DM [abstract 959‐P]. Diabetes. 2017;66(Suppl 1):A248‐A249. [Google Scholar]
- 37. Plum‐Möerschel L, Leohr J, Liu R, et al. Ultra‐rapid Lispro (URLi) Reduces postprandial glucose excursions vs. Humalog in patients with T1D at multiple meal‐to‐dose timing intervals [abstract 1010‐P]. Diabetes. 2018;67(Suppl 1):A264‐A265. [Google Scholar]
- 38. Kapitza C, Leohr J, Liu R, et al. A novel formulation of insulin lispro containing citrate and treprostinil shows significantly faster absorption and an improvement in postprandial glucose excursions vs. humalog in patients with T2DM [abstract 978‐P]. Diabetes. 2017;66(Suppl 1):A253‐A254. [Google Scholar]
- 39. Kapitza C, Leohr J, Liu R, et al. Ultra‐rapid lispro (URLi) reduces postprandial glucose excursions vs. Humalog® in patients with T2D at multiple meal‐to‐dose timing intervals [abstract 1009‐P]. Diabetes. 2018;67(Suppl 1):A264. [Google Scholar]
- 40. Kazda CM, Leohr J, Liu R, et al. Ultra‐rapid Lispro (URLi) shows faster absorption of insulin lispro vs. Humalog® during insulin pump (CSII) use in patients with T1D [abstract 1006‐P]. Diabetes. 2018;67(Suppl 1):A264. [Google Scholar]
- 41. Linnebjerg H, Zhang Q, Labell ES, et al. Ultra rapid lispro (URLi) accelerates insulin lispro absorption and insulin action vs. Humalog (lispro) in patients with T1D [abstract]. Diabetes. 2019;68(Suppl 1):1107‐P. [Google Scholar]
- 42. Leohr J, Dellva MA, Coutant DE, et al. Ultra rapid lispro (URLi) accelerates insulin lispro absorption and insulin action vs. Humalog (Lispro) in patients with T2D [abstract]. Diabetes. 2019;68(Suppl 1):1100‐P. [Google Scholar]
- 43. Heise T, Linnebjerg H, Cao D, et al. Ultra rapid lispro (URLi) lowers postprandial glucose (PPG) and more closely matches normal physiological glucose response compared with other rapid insulin analogs [abstract]. Diabetes. 2019;68(Suppl 1):1112‐P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Plum‐Möerschel L, Linnebjerg H, Labell ES, et al. Greater reduction in postprandial glucose excursions with ultra rapid lispro (URLi) vs lispro in patients with type 2 diabetes at different meal‐to‐dose timings [abstract 787]. Diabetologia. 2019;62(Suppl 1):S382. [Google Scholar]
- 45. Andersen G, Meiffren G, Lamers D, et al. Ultra‐rapid BioChaperone Lispro improves postprandial blood glucose excursions vs insulin lispro in a 14‐day crossover treatment study in people with type 1 diabetes. Diabetes Obes Metab. 2018;20:2627‐2632. [DOI] [PubMed] [Google Scholar]
- 46. Andersen G, Alluis B, Meiffren G, et al. The ultra‐rapid biochaperone insulin lispro shows a faster onset of action and stronger early metabolic effect than insulin lispro alone [abstract 934]. Diabetologia. 2014;57(Suppl 1):S381. [Google Scholar]
- 47. Andersen G, Alluis B, Meiffren G, et al. Ultra‐rapid Biochaperone insulin Lispro (BC LIS): Linear dose–response and faster absorption than insulin lispro (LIS) [abstract 979‐P]. Diabetes. 2015;64(Suppl 1):A248. [Google Scholar]
- 48. Heise T, Ranson A, Gaudier M, et al. Pooled analysis of clinical trials investigating the pharmacokinetics (PK) of ultra‐rapid insulin BioChaperone Lispro (BCLIS) vs. Lispro (LIS) in subjects with Type 1 (T1D) and Type 2 (T2D) Diabetes [abstract 998‐P]. Diabetes. 2018;67(Suppl 1):A261. [Google Scholar]
- 49. Andersen G, Meiffren G, Alluis B, et al. Ultra‐rapid Biochaperone Lispro ameliorates postprandial blood glucose (PPG) control compared with humalog in subjects with type 1 diabetes mellitus [abstract 294‐OR]. Diabetes. 2016;65(Suppl 1):A77. [Google Scholar]
- 50. Heise T, Meiffren G, Lamers D, et al. Ultra‐rapid Biochaperone Lispro (BCLIS) improves postprandial blood glucose (PPG) control vs. insulin lispro (LIS) in a 14‐day treatment study in subjects with type 2 diabetes (T2DM) [abstract 994‐P]. Diabetes. 2017;66(Suppl 1):A259. [Google Scholar]
- 51. Meiffren G, Andersen G, Klein O, et al. Proportional dose‐exposure relationship of ultra‐rapid BioChaperone Lispro (BCLIS) in healthy Japanese subjects [abstract 996‐P]. Diabetes. 2017;66(Suppl 1):A260. [Google Scholar]
- 52. Heise T, Pieber TR, Danne T, Erichsen L, Haahr H. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast‐acting insulin aspart in adults with type 1 diabetes. Clin Pharmacokinet. 2017;56:551‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Heise T, Haahr HL, Jensen L, Erichsen L, Hompesch M. Faster‐acting insulin aspart improves postprandial glycemia vs. insulin aspart in patients with type 1 diabetes mellitus (T1DM) [abstract 129‐OR]. Diabetes. 2014;63(Suppl 1):A34. [Google Scholar]
- 54. Fath M, Danne T, Biester T, Erichsen L, Kordonouri O, Haahr H. Faster‐acting insulin aspart provides faster onset and greater early exposure vs insulin aspart in children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2017;18:903‐910. [DOI] [PubMed] [Google Scholar]
- 55. Heise T, Zijlstra E, Nosek L, Rikte T, Haahr H. Pharmacological properties of faster‐acting insulin aspart vs insulin aspart in patients with type 1 diabetes receiving continuous subcutaneous insulin infusion: a randomized, double‐blind, crossover trial. Diabetes Obes Metab. 2017;19:208‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Heinemann L, Muchmore D. Coverage of prandial insulin requirements: an elusive goal. Diabetes Technol Ther. 2017;19:7‐8. [DOI] [PubMed] [Google Scholar]
- 57. Becker RH, Frick AD, Burger F, Potgieter JH, Scholtz H. Insulin glulisine, a new rapid‐acting insulin analogue, displays a rapid time‐action profile in obese non‐diabetic subjects. Exp Clin Endocrinol Diabetes. 2005;113:435‐443. [DOI] [PubMed] [Google Scholar]
- 58. Heise T, Nosek L, Spitzer H, et al. Insulin glulisine: a faster onset of action compared with insulin lispro. Diabetes Obes Metab. 2007;9:746‐753. [DOI] [PubMed] [Google Scholar]
- 59. Luzio S, Peter R, Dunseath GJ, Mustafa L, Owens DR. A comparison of preprandial insulin glulisine versus insulin lispro in people with Type 2 diabetes over a 12‐h period. Diabetes Res Clin Pract. 2008;79:269‐275. [DOI] [PubMed] [Google Scholar]
- 60. Arnolds S, Rave K, Hövelmann U, Fischer A, Sert‐Langeron C, Heise T. Insulin glulisine has a faster onset of action compared with insulin aspart in healthy volunteers. Exp Clin Endocrinol Diabetes. 2010;118:662‐664. [DOI] [PubMed] [Google Scholar]
- 61. Russell‐Jones D, Bode BW, De Block C, et al. Fast‐acting insulin aspart improves glycemic control in basal‐bolus treatment for type 1 diabetes: results of a 26‐week multicenter, active‐controlled, treat‐to‐target, randomized, parallel‐group trial (onset 1). Diabetes Care. 2017;40:943‐950. [DOI] [PubMed] [Google Scholar]
- 62. Mathieu C, Bode BW, Franek E, et al. Efficacy and safety of fast‐acting insulin aspart in comparison with insulin aspart in type 1 diabetes (onset 1): a 52‐week, randomized, treat‐to‐target, phase III trial. Diabetes Obes Metab. 2018;20:1148‐1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Buse JB, Carlson AL, Komatsu M, et al. Fast‐acting insulin aspart versus insulin aspart in the setting of insulin degludec‐treated type 1 diabetes: efficacy and safety from a randomized double‐blind trial. Diabetes Obes Metab. 2018;20:2885‐2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Buse JB, Carlson A, Komatsu M, et al. Efficacy and safety of faster aspart compared with insulin aspart both with insulin degludec in adults with T1D [abstract 1000‐P]. Diabetes. 2018;67(Suppl 1):A261‐A262. [Google Scholar]
- 65. Bode BW, Iotova V, Kovarenko M, et al. Efficacy and safety of fast‐acting insulin aspart compared with insulin aspart, both in combination with insulin degludec, in children and adolescents with type 1 diabetes: the onset 7 trial. Diabetes Care. 2019;42:1255‐1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bowering K, Case C, Harvey J, et al. Faster aspart versus insulin aspart as part of a basal‐bolus regimen in inadequately controlled type 2 diabetes: the onset 2 trial. Diabetes Care. 2017;40:951‐957. [DOI] [PubMed] [Google Scholar]
- 67. Rodbard HW, Tripathy D, Vidrio Velázquez M, Demissie M, Tamer SC, Piletic M. Adding fast‐acting insulin aspart to basal insulin significantly improved glycaemic control in patients with type 2 diabetes: A randomized, 18‐week, open‐label, phase 3 trial (onset 3). Diabetes Obes Metab. 2017;19:1389‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zijlstra E, Demissie M, Graungaard T, Heise T, Nosek L, Bode B. Investigation of pump compatibility of fast‐acting insulin aspart in subjects with type 1 diabetes. J Diabetes Sci Technol. 2018;12:145‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Klonoff D, Evans M, Lane W, et al. A randomized, multicentre trial evaluating the efficacy and safety of fast‐acting insulin aspart in continuous subcutaneous insulin infusion in adults with type 1 diabetes (onset 5). Diabetes Obes Metab. 2019;21:961‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lane W, Bozkurt K, Favaro E, Jang HC, Kjærsgaard MIS. Efficacy and safety of fast‐acting insulin aspart compared with insulin aspart, both with insulin degludec with or without metformin, in adults with type 2 diabetes [abstract 17]. Diabetologia. 2019;62(Suppl 1):S10‐S11. [Google Scholar]
- 71. Klaff LJ, Cao D, Dellva MA, et al. Ultra rapid lispro (URLi) improves postprandial glucose (PPG) control vs. Humalog (lispro) in T1D: PRONTO‐T1D study [abstract]. Diabetes. 2019;68(Suppl 1):144‐OR. [Google Scholar]
- 72.Eli Lilly and Company. A study comparing LY900014 to insulin lispro (Humalog) in children and adolescents with type 1 diabetes (PRONTO‐Peds). In: http://clinicaltrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US); 2000. https://clinicaltrials.gov/ct2/show/NCT03740919 NLM Identifier: NCT03740919. Accessed June 14, 2019.
- 73.Eli Lilly and Company. A study of LY900014 compared to insulin lispro (Humalog) in adults with type 1 diabetes. In: http://ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US); 2000. https://clinicaltrials.gov/ct2/show/NCT03952130 NLM Identifier: NCT03952130. Accessed June 14, 2019.
- 74. Bode BW, Liu R, Hardy TA, Ignaut DA. Compatibility and safety of Ultra Rapid Lispro (URLi) with continuous subcutaneous insulin infusion (CSII) in patients with type 1 diabetes: PRONTO‐pump study [abstract 790]. Diabetologia. 2019;62(Suppl 1):S383. [DOI] [PubMed] [Google Scholar]
- 75.Eli Lilly and Company. A study comparing LY900014 to insulin lispro (Humalog) in adults with type 1 diabetes using insulin pump therapy (PRONTO‐Pump‐2). In: http://ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US); 2000. https://clinicaltrials.gov/ct2/show/NCT03830281 NLM Identifier: NCT03830281. Accessed June 14, 2019.
- 76. Blevins T, Zhang Q, Frias JP, Jinnouchi H, Chang AM. Ultra rapid lispro (URLi) improves postprandial glucose (PPG) control vs. Humalog (lispro) in patients with type 2 diabetes (T2D): PRONTO‐T2D [abstract]. Diabetes. 2019;68(Suppl 1):145‐OR. [Google Scholar]
- 77.Eli Lilly and Company. A study of LY900014 compared to insulin lispro (Humalog) in adults with type 2 diabetes. In: http://ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US); 2000. https://clinicaltrials.gov/ct2/show/NCT03952143 NLM Identifier: NCT03952143. Accessed June 14, 2019.
- 78. Dahl D, Cao D, Dellva M, et al. Ultra rapid lispro (URLi) improves postprandial glucose (PPG) control vs lispro in type 1 diabetes: PRONTO‐T1D study [abstract 181]. Diabetologia. 2019;62(Suppl 1):S93. [Google Scholar]
- 79. Frias JP, Zhang Q, Blevins T, Jinnouchi H, Chang AM. Ultra rapid lispro (URLi) improves postprandial glucose (PPG) control vs lispro in patients with type 2 diabetes: PRONTO‐T2D study [abstract 791]. Diabetologia. 2019;62(Suppl 1):S384. [Google Scholar]
- 80. Muchmore DB. Pump users clamor for faster insulin: is fast‐acting insulin aspart ready for them? J Diabetes Sci Technol. 2018;12:152‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Evans M, Ceriello A, Danne T, et al. Use of fast‐acting insulin aspart in insulin pump therapy in clinical practice. Diabetes Obes Metab. 2019;21:2039‐2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dovc K, Piona C, Yeşiltepe Mutlu G, et al. Faster compared with standard insulin aspart during day‐and‐night fully closed‐loop insulin therapy in type 1 diabetes: a double‐blind randomized crossover trial. Diabetes Care. 2020;43:29‐36. [DOI] [PubMed] [Google Scholar]
- 83. Bally L, Herzig D, Ruan Y, et al. Short‐term fully closed‐loop insulin delivery using faster insulin aspart compared with standard insulin aspart in type 2 diabetes. Diabetes Obes Metab. 2019;21:2718‐2722. [DOI] [PubMed] [Google Scholar]
- 84.European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) Assessment Report for Fiasp (insulin aspart). 10 November 2016. https://www.ema.europa.eu/en/documents/assessment-report/fiasp-epar-public-assessment-report_en.pdf. Accessed August 6, 2019.
- 85. Nordisk Novo. Fiasp® (insulin aspart injection), for subcutaneous or intravenous use [package insert]. Novo Nordisk A/S, Bagsvaerd, Denmark; December 2019. https://www.novo-pi.com/fiasp.pdf. Accessed January 7, 2020. [Google Scholar]
- 86.European Medicines Agency. Fiasp. Summary of product characteristics, October 30, 2019. https://www.ema.europa.eu/documents/product-information/fiasp-epar-product-information_en.pdf. Accessed November 21, 2019.
- 87.Eli Lilly and Company. Lilly's ultra rapid lispro provided similar A1C reductions compared to Humalog® (insulin lispro), with superior post‐meal blood glucose reductions. Press release, June 9, 2019. https://investor.lilly.com/node/41421/pdf. Accessed June 14, 2019.
- 88. Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care. 2001;24:1722‐1727. [DOI] [PubMed] [Google Scholar]
- 89. Kruszynska YT, Home PD, Hanning I, Alberti KG. Basal and 24‐h C‐peptide and insulin secretion rate in normal man. Diabetologia. 1987;30:16‐21. [DOI] [PubMed] [Google Scholar]
- 90. Gingras V, Taleb N, Roy‐Fleming A, Legault L, Rabasa‐Lhoret R. The challenges of achieving postprandial glucose control using closed‐loop systems in patients with type 1 diabetes. Diabetes Obes Metab. 2018;20:245‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Porcellati F, Lucidi P, Bolli GB, Fanelli CG. Thirty years of research on the dawn phenomenon: lessons to optimize blood glucose control in diabetes. Diabetes Care. 2013;36:3860‐3862. [DOI] [PMC free article] [PubMed] [Google Scholar]


