Abstract
Background
Assessment of events by adjudication committees (ACs) is recommended in multicentre randomised controlled trials (RCTs). However, its usefulness has been questioned.
Objectives
The aim of this systematic review was to compare 1) treatment effect estimates of subjective clinical events assessed by onsite assessors versus by AC, and 2) treatment effect estimates according to the blinding status of the onsite assessor as well as the process used to select events to adjudicate.
Search methods
We searched Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, EMBASE, PsycINFO, CINAHL and Google Scholar (25 August 2015 as the last updated search date), using a combination of terms to retrieve RCTs with commonly used terms to describe ACs.
Selection criteria
We included all reports of RCTs and the published RCTs included in reviews and meta‐analyses that reported the same subjective outcome event assessed by both an onsite assessor and an AC.
Data collection and analysis
We extracted the odds ratio (OR) from onsite assessment and the corresponding OR from AC assessment and calculated the ratio of the odds ratios (ROR). A ratio of odds ratios < 1 indicated that onsite assessors generated larger effect estimates in favour of the experimental treatment than ACs.
Main results
Data from 47 RCTs (275,078 patients) were used in the meta‐analysis. We excluded 11 RCTs because of incomplete outcome data to calculate the OR for onsite and AC assessments. On average, there was no difference in treatment effect estimates from onsite assessors and AC (combined ROR: 1.00, 95% confidence interval (CI) 0.97 to 1.04; I2 = 0%, 47 RCTs). The combined ROR was 1.00 (95% CI 0.96 to 1.04; I2 = 0%, 35 RCTs) when onsite assessors were blinded; 0.76 (95% CI 0.48 to 1.12, I2 = 0%, two RCTs) when AC assessed events identified independently from unblinded onsite assessors; and 1.11 (95% CI 0.96 to 1.27, I2 = 0%, 10 RCTs) when AC assessed events identified by unblinded onsite assessors. However, there was a statistically significant interaction between these subgroups (P = 0.03)
Authors' conclusions
On average, treatment effect estimates for subjective outcome events assessed by onsite assessors did not differ from those assessed by ACs. Results of subgroup analysis showed an interaction according to the blinded status of onsite assessors and the process used to submit data to AC. These results suggest that the use of ACs might be most important when onsite assessors are not blinded and the risk of misclassification is high. Furthermore, research is needed to explore the impact of the different procedures used to select events to adjudicate.
Plain language summary
Comparison of central adjudication of outcomes and onsite outcome assessment on treatment effect estimates
It is widely recommended that multicentre randomised controlled trials (RCTs) should have a central process for assessing whether or not a patient has had an event, rather than relying solely on the outcomes reported by assessors at the relevant site where the decision might be subjective. These Adjudication Committees (ACs) are commonly used, especially in large trials. For example, the US Food and Drug Administration (FDA) and the European Medicine Agency (EMA) recommend assessment of events by such committees to harmonise and standardise outcome assessment across a trial. However, there is a need for evidence to justify the use of ACs and to decide on how central adjudication of clinical events should be conducted. This is the first large meta‐analysis across medical areas to evaluate the impact of central adjudication on the estimates for treatment effect produced by RCTs. We investigated whether using the event data from ACs produced different treatment effect estimates than the data from onsite for subjective outcomes in RCTs.
We defined an AC as a committee of clinical experts in a specific medical area that seeks to harmonise and standardise the outcome assessment; whereas onsite assessors would be investigators, research nurses, data collectors, or patients themselves doing an onsite evaluation of the occurrence of the outcome during the RCT. Onsite assessors may, or may not, be blinded to the treatment assigned. We included all reports of RCTs and meta‐analyses of published RCTs that reported the same subjective binary clinical event outcome assessed by both an onsite assessor and an AC.
We combined the findings of 47 RCTs (275,078 patients) in our systematic review and meta‐analysis in order to see if there is a difference between the results from ACs and from onsite assessment. Our results showed that treatment effect estimates of subjective clinical events did not differ, on average, from those assessed by ACs. When we divided the data into whether or not the onsite assessors knew the patient's allocated treatment in the RCT and the various ways of submitting data to ACs, we found that there might be important differences between onsite assessment and ACs depending on which methods are used. Our findings, which are up to date as of August 2015, raise important uncertainty about whether ACs are being used appropriately across all RCTs.
Background
An adjudication committee (AC) consists of a group of clinical experts in a specific medical area who validate the assessment of outcomes in a randomised controlled trial (RCT). Central adjudication of clinical events is recommended and commonly used in large multicentre RCTs (Stuck 2014). For example, the US Food and Drug Administration (FDA) and the European Medicine Agency (EMA) recommend assessment of events by ACs to harmonise and standardise outcome assessment. ACs are usually blinded to the assigned treatment, regardless of whether the trial itself is conducted in a blinded manner. Such committees are considered valuable when outcomes are subjective and when the intervention is not delivered in a blinded fashion (Bellamy 1997), and ACs are used to reduce bias and to ensure more precise classification of events (Granger 2008).
Detection bias, which is also called ascertainment bias or observer bias, might be expected with onsite assessment, mainly due to the knowledge of the allocated interventions by the assessor (Higgins 2011). This implies, especially for subjective binary outcomes, that there is a high risk of a biased effect estimates in RCTs which might exaggerate the effect estimates (Hróbjartsson 2012). Therefore, an AC might be a useful way to address such bias.
Description of the problem or issue
The importance of ACs has been advocated in some studies and challenged in others. Some studies have shown that the classification of events could change after outcome assessment by an AC (Naslund 1999; Mahaffey 2001b; O'Connor 2005). In contrast, other studies have shown that adjudicated data usually match well with onsite outcome assessment (Kirwan 2007; Granger 2008; Pogue 2009; Hata 2013).
Description of the methods being investigated
We investigated the impact of the use of an AC on treatment effect estimates in RCTs. An AC is defined as a committee of clinical experts in a specific medical area that harmonises and standardises the outcome assessment. The onsite assessors represent investigators, research nurses, data collectors, or patients themselves doing an onsite evaluation of the occurrence of the outcome during the RCT. Onsite assessors may, or may not, be blinded to the treatment assigned.
How these methods might work
The AC aims to increase the reliability of assessing outcomes by a more accurate assessment of events, discarding events that are potentially not valid and minimising bias (Boutron 2006; Boutron 2007; Dechartres 2009; Vannabouathong 2012). Hence, an AC would provide a systematic, unbiased, and independent assessment of outcomes by using a set of predefined criteria developed before the initiation of the RCT. Adjudication of outcomes should theoretically minimise bias through a blinded outcome assessment (without knowledge of the patient's allocated treatment) and reduce the variance that would exist between different site investigators through a standardised assessment with clearly defined endpoint definitions (Vannabouathong 2011). The central assessment carried out by the AC may be more accurate because it is performed by an independent group of trained clinicians with substantial expertise in the field, who are not otherwise involved in the RCT and who are blinded to the treatment allocation as well as to other factors (such as who is responsible for the care of the patient). In contrast, endpoint assessment performed by onsite assessors may be affected by conscious or unconscious detection bias, especially in trials that do not use placebo controls and have subjective outcomes (Hróbjartsson 2012).
Researchers have outlined the importance of ACs, showing differences in classification of clinical events between onsite assessment and AC assessment (Mahaffey 2001a; Mahaffey 2002; O'Connor 2005; Mahaffey 2011; Eriksson 2012; Winston 2012). These differences may be the result of bias in the original classification but they might be due to other causes which might not lead to differences in the treatment effect estimates.
Why it is important to do this review
The use of ACs in RCTs is frequent, particularly in some medical areas such as cardiology. For example, in a sample of 969 trials of venous thromboembolism, 69% reported the use of an AC (Stuck 2014). In addition, in a sample of RCTs published in high‐impact journals, the use of AC was reported in 33% of the 314 trials, ranging from 9% of 34 RCTs in infectious diseases to 81% of 75 RCTs in cardiology (Dechartres 2009).
The adjudication process can be costly because it involves identifying the cases to be adjudicated, collecting all the data to be adjudicated (case report forms, biological tests, radiography and other complementary tests, etc), anonymising and masking the data, identifying and inviting the adjudication members, training adjudication members, adjudicating the data, organising regular consensus meetings, and so forth.
Adjudication outcome assessment is highly recommended for studies that include subjective outcomes when blinding is not possible, and there is clear evidence that unblinded outcome assessment of subjective outcomes will overestimate treatment effect estimates (Wood 2008; Hróbjartsson 2012; Savović 2012).
To our knowledge, no comprehensive systematic review across medical areas has been published on this topic.
Objectives
We sought to assess the impact of adjudication committee (ACs) versus onsite outcome assessment on treatment effect estimates for subjective outcomes in randomised controlled trials (RCTs).
Methods
Criteria for considering studies for this review
Types of studies
We included all reports of RCTs and any published RCTs included in reviews and meta‐analyses (using these as a source of data on the individual studies) that reported the same subjective binary clinical event outcome assessed by both an onsite assessor and an AC. So, to be eligible, a study had to provide data to calculate the odds ratio (OR) for onsite assessment and for AC separately.
We excluded RCTs comparing AC assessment with administrative data (e.g., death certificates) or with outcome assessment by a local outcome committee. We also excluded RCTs using the same treatment in the two allocated groups. Furthermore, reports describing a specific complementary examination such as phlebography (evaluated only by imaging) were excluded. We also excluded RCTs in which it is unclear which intervention is "experimental" and which is "control" because such RCTs did not allow us to determine the direction of any bias on the effect estimate.
We did not place any restrictions on the number of centres included in the RCTs selected for the review. Single‐ and multicentre trials were eligible for inclusion.
Types of data
Eligible studies reported a subjective binary clinical event outcome. An outcome was considered "subjective" if it was based on an observer exercising judgment while assessing an event or state and could consequently be influenced by the assessor's knowledge of the allocated treatment (Moustgaard 2014). Objective outcomes were those determined without exercising judgment. We selected trials with subjective clinical events because there is evidence that blinding of outcome assessors is particularly important for subjective outcomes, but not for objective outcomes (Wood 2008; Hróbjartsson 2012; Savović 2012).
Types of methods
All eligible RCTs directly compared central versus onsite assessment for the same outcome.
Types of outcome measures
Primary outcomes
Our primary outcome was the impact of the AC on the treatment effect estimate for a subjective binary clinical outcome in the relevant RCT, compared to onsite assessment.
Two review authors (LAND, AY) independently selected one outcome from the article reporting each included RCT. This outcome will have been assessed by both an onsite assessor and an AC, and the data needed to have been provided for each treatment group. The decision to label an outcome as "subjective" was made by two trained clinical epidemiologists (LAND, AY). Disagreements were resolved by discussion with a third review author (IB). We only referred to the adjudication of outcomes, not to the adjudication of other data in the trial (e.g., baseline characteristics). If the primary outcome was a composite of objective and subjective outcomes, we selected at least one subjective outcome component that had been assessed by both an onsite assessor and an AC.
If several outcomes in a RCT were assessed by an onsite assessor and an AC, we selected only one outcome. For this purpose, we first selected all efficacy outcomes when available. If several efficacy outcomes were available, we selected the efficacy outcome(s) reported as primary outcome(s) of the RCT (i.e., as clearly stated in the RCT article, described in the study objectives, or used for the sample size calculation). If none or several were reported as primary outcome(s), we selected the most clinically relevant outcomes, and among them the outcome with the most events. We used the same selection process if the outcomes assessed by both an onsite assessor and an AC were only safety outcomes. If several time points were reported for an outcome, we selected the first time point after the end of treatment.
Secondary outcomes
There were no secondary outcomes for this review because our aim was to evaluate the impact of AC versus onsite assessment on the treatment effect estimates.
Search methods for identification of studies
Electronic searches
We searched a variety of standard databases (Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, EMBASE, PsycINFO, CINAHL) up to 18 March 2014 and updated our search most recently in August 2015 using the search strategies listed in Appendix 1. We also searched the full‐text database (Google Scholar) with commonly used terms including adjudication committee, central adjudication, endpoint committee, clinical event committee, outcome committee, critical event committee.
Searching other resources
We checked the references of included studies to identify additional relevant reports (Horsley 2011).
Data collection and analysis
If any RCTs identified in our initial search only reported the results of clinical events assessed by an AC, we sought the authors’ email addresses and contacted them to ask whether they had collected onsite outcome data and whether they could provide these data.
Selection of studies
One review author (LAND) screened all titles, abstracts and text fragments retrieved from the databases. The information selected was reviewed by a second review author (CB) to confirm relevance to the review. The review authors obtained the full‐text study reports relating to every potentially eligible record. If the selected report was an ancillary analysis of the RCT comparing onsite assessor and AC assessments, the primary report of the RCT was also retrieved.
When the selected report was a report of a meta‐analysis pooling data of individual RCTs in which the treatment effect estimates from the AC and the onsite assessor was compared, the primary reports of the included RCTs were systematically searched for and evaluated. If primary reports of these RCTS were not available, we collected data from the meta‐analysis and reported the total number of events in each treatment group resulting from the onsite assessor and AC assessment for each RCT. However, we excluded the RCTs in the meta‐analysis report if the meta‐analysis reported only combined effect estimates, and did not provide the effect estimates for each RCT separately.
Data extraction and management
We used a pre‐tested data extraction form (Appendix 2) to collect general characteristics and outcome data of the eligible reports. Two review authors (LAND, CB) extracted the following data independently and disagreements were resolved by discussion.
General characteristics of the RCT: medical speciality, funding source, experimental intervention, comparator. We considered that onsite assessors were blinded if the study was reported as a double‐blind study, a similar placebo procedure was used, a double‐dummy procedure was used, or a specific method was reported to blind the onsite assessor when patients and care providers were not blinded.
Data on the functioning of the AC: blinding status of the AC in terms of allocated treatment and to the onsite assessment, training and independence of AC members, and information provided to the AC. We extracted the method for selecting cases to adjudicate (i.e., whether this used events identified by the onsite assessor, computer algorithms to identify suspected events, adjudication of all patients randomised and the assessment of all deaths in the context of determining a specific cause of death).
Outcome data: total number of events in each randomised group resulting from onsite‐assessor and AC assessments. When possible, we extracted paired patient‐level data for onsite‐assessor and AC assessment and constructed a 2×2 table (event/no event x onsite/AC) for the experimental group and a corresponding table for the control group. For RCTs with more than two groups, we combined the results for the experimental groups. We estimated treatment effects as odds ratios (ORs). Outcome events were recoded in all RCTs such that an OR < 1 indicates benefit from the experimental treatment.
Assessment of risk of bias in included studies
A possible risk of bias for the comparison of onsite assessment versus ACs is linked to the method used to select cases to adjudicate. This selection could be biased if it is not blinded to the allocated treatment, for example if the events to be adjudicated were identified by the unblinded onsite assessor. To investigate this, we collected the method for selecting cases to adjudicate. These methods were classified as "events identified independently of onsite‐assessor assessment" if 1) events to be adjudicated were identified with a computer algorithms, 2) all patients randomised were adjudicated, or 3) all deaths were adjudicated when the outcome of interest was a specific cause of death. Selection methods were considered as low risk of bias if the onsite assessor was blinded or if events were identified independently of onsite‐assessor assessment. If events were identified by unblinded onsite‐assessors, we considered this to have a high risk of bias. We used unclear risk of bias if the methods of selection and blinded status of onsite assessors were not clear.
Measures of the effect of the methods
We summarised the effects by comparing the effect estimates for the same clinical event outcome in each RCT that were calculated with the outcome data from the onsite assessor versus the same analysis using outcome data from the AC. For each RCT, we calculated the ratio of ORs (ROR) as the OR from onsite assessors relative to the corresponding OR from the AC (ROR = OROnsite/OR AC). An ROR < 1 indicated that onsite assessors generated larger effect estimates in favour of the experimental treatment than ACs.
Unit of analysis issues
Because the onsite assessor and the AC classified outcome events for the same study population, the two corresponding estimates were correlated. The standard error of the logROR was calculated as the square root of the sum of the variance of the logOR for AC and onsite assessment. It was our intention to use the delta method so that the standard error of the logROR took into account the correlation between onsite and AC assessment (Bagos 2012; Hróbjartsson 2012), but we were unable to do this because the data required to estimate the covariance of the two correlated logORs were not available.
Dealing with missing data
When data were incomplete (e.g., authors reported the total number of events resulting from the onsite‐assessor and AC assessment but did not provide the data separately for each randomised group), we wrote to the corresponding author to ask for the results by group.
Assessment of heterogeneity
Heterogeneity was assessed by the I2 statistic and the between‐trial variance τ². I2 was the proportion of total variation between the studies attributable to differences between RCTs rather than to sampling error (chance), with values < 30% representing low heterogeneity, ≤ 60% moderate heterogeneity, and > 60% high heterogeneity.
Assessment of reporting biases
To minimise reporting bias, for all RCTs identified in our initial search which were excluded because they only reported the results of clinical events assessed by an AC, we contacted the corresponding author to request the data for onsite assessors. We explored the impact of these data on the results in a sensitivity analysis.
We did not intend to perform any other specific assessment of reporting bias because the statistical tools commonly used to assess reporting bias and related small‐study effects in meta‐analysis (in particular the funnel plot) have not been transposed or extended to meta‐epidemiological studies.
Data synthesis
We pooled the individual RORs using a DerSimonian and Laird random‐effects meta‐analysis (DerSimonian 1986), and reported the results in a forest plot with 95% confidence intervals (CIs). The decision to pool the RORs was based on the assessment of statistical heterogeneity and methodological diversity. If data combination was deemed inappropriate, we presented the results of individual studies in a forest plot (without a meta‐analysis) and discussed them. We considered the point estimate of the ROR significant at P < 0.05 if the 95% CI did not include the value 1.
Subgroup analysis and investigation of heterogeneity
We tested the interaction between the ROR and the blinding status of onsite assessors and ACs as well as the method used to select cases to adjudicate (events identified by or independent of onsite‐assessor assessment).
Sensitivity analysis
We conducted a sensitivity analysis to explore the impact of unreported data on our results.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The screening process is described in Figure 1. We examined 1210 full‐text articles based on 7855 hits in standard databases and 1893 hits in the full‐text database. After reading the full‐text articles, we selected 25 reports of RCTs and four reports of meta‐analyses which had included 21 RCTs. Two reports of RCTs were identified from the personal collections of the authors. Of the 874 full‐text articles reporting only results for the AC, we obtained an e‐mail address for corresponding authors of 496 trials; 106 authors responded and we obtained the data for 10 RCTs. Finally, we included 47 RCTs (with a total of 275,078 patients) in our meta‐analysis.
1.
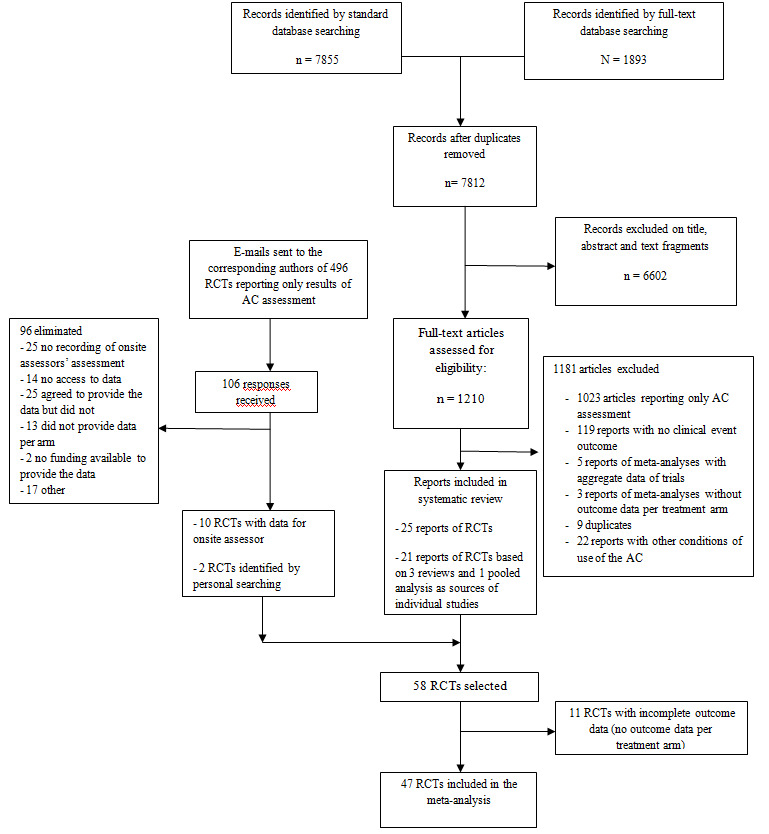
Included studies
Table 1 shows the baseline characteristics of the 47 RCTs included in the meta‐analysis. The median sample size was 3449 [interquartile range 1506 to 10,000], 83% (n = 39) of RCTs were in the field of cardiology, 89% (n = 42) were multicentre RCTs, and 94% were sponsored completely or partially by industry.
1. General characteristics.
| Characteristics | No. (%) |
| Type of journal | |
| Specialty journal | 16 (34.0) |
| General medical journal | 31 (66.0) |
| Medical specialty | |
| Cardiovascular system | 39 (83.0) |
| Neurology/psychiatry | 4 (8.5) |
| Orthopedics/ rheumatology | 2 (4.3) |
| Gastroenterology | 1 (2.1) |
| Oncology | 1 (2.1) |
| Study design | |
| Parallel groups | 47 (100.0) |
| Non‐inferiority/equivalence trial | 11 (23.4) |
| Multicentre studies | 42 (89.4) |
| Sample size (median [Q1‐Q3]) | 3,449 [1506 to 10,000] |
| Funding source | |
| Private | 30 (63.8) |
| Mixed | 14 (29.8) |
| Public | 2 (4.3) |
| Unclear | 1 (2.1) |
| Experimental treatment | |
| Drug | 43 (91.5) |
| Surgery and procedure | 3 (6.4) |
| Both | 1 (2.1) |
| Comparator | |
| Active treatment | 12 (25.5) |
| Placebo | 22 (46.8) |
| Usual care | 13 (27.7) |
| Risk of bias regarding treatment effect in selected RCTs | |
| Random allocation sequence adequately generated | 34 (72.3) |
| Random allocation sequence adequately concealed | 26 (55.3) |
| Patients blinded | 36 (76.6) |
| Care provider blinded | 34 (72.3) |
| Onsite assessor blinded | 35 (74.5) |
| Missing data < 10% of randomised population | 39 (83.0) |
| Outcome selected | |
| Efficacy | 41 (87.2) |
| Safety | 6 (12.8) |
| Primary outcome of the RCT | 39 (83.0) |
| Composite outcome | 32 (68.1) |
| Subjectivity of the outcome selected | |
| Subjective | 35 (74.5) |
| composite | 12 (25.5) |
Q1, Q3: quartile 1, quartile 3
The outcomes selected for assessment were mainly the RCT’s primary outcomes (n = 39, 83%). Many RCTs (n = 32; 68%) studied a composite outcome. Most of these composite outcomes included subjective outcomes only (n = 35; 75%), but 25% (n = 12) were a composite of subjective and objective outcomes. Details related to the AC are in Table 2. For 40 RCTs (85%), ACs were reported as blinded to the treatment allocated. The AC evaluated mainly suspected cases (failure events) identified by the onsite assessor (n = 37; 79%). For 35 RCTs (75%), the onsite assessor was blinded to the treatment allocated.
2. Functioning of the adjudication committee (AC) in 47 RCTs included in the meta‐analysis.
| Characteristics | No. (%) |
| Members of the AC independent | |
| Yes | 26 (55.3) |
| Not reported | 21 (44.7) |
| Training or education of AC members | |
| Yes | 23 (48.9) |
| Not reported | 24 (51.1) |
| AC blinded to treatment assignment | |
| Yes | 40 (85.1) |
| Not reported | 7 (14.9) |
| AC blinded to onsite outcome assessment | |
| Yes | 5 (10.6) |
| No | 2 (4.3) |
| Not reported | 40 (85.1) |
| Information provided to the AC | |
| Standard case report forms | 26 (55.3) |
| All medical files/some elements | 7 (14.9) |
| Not reported | 14 (29.8) |
| Methods used to select cases submitted to the AC for assessment | |
| Suspected events identified by onsite assessor | 37 (78.7) |
| Computer algorithm used to identify suspected events | 3 (6.4) |
| All patients adjudicated | 1 (2.1) |
| All deaths adjudicated | 3 (6.4) |
| Patient self‐reported events | 2 (4.3) |
| Not reported/unclear | 2 (4.3) |
Excluded studies
We excluded 11 studies because they did not provide the necessary data to calculate the OR for onsite assessment and for AC separately (Characteristics of excluded studies). We contacted the corresponding authors of these 11 studies but did not receive any responses after at least two reminders.
Risk of bias in included studies
A possible risk of bias is linked to the method for selecting cases to adjudicate, especially when the relevant events are identified by the unblinded onsite assessor.
Overall, 35 RCTs (75%) reported using blinded onsite assessors. Among the 12 RCTs with unblinded onsite assessors, events submitted to ACs were identified by the unblinded onsite assessors in 10 RCTs. In the two other unblinded RCTs, events submitted to ACs were identified independently of the onsite assessors.
Effect of methods
Treatment effect estimates from the onsite assessment and the ACs are shown in Figure 2 for the 47 included RCTs. We found no difference, on average, in treatment effect estimates between onsite assessment and ACs. The combined ROR was 1.00 (95% CI 0.97 to 1.04), with no heterogeneity (I2 = 0%, τ² = 0%) (n = 47 RCTs). Furthermore, we found no evidence of interaction by blinding status of onsite assessors ROR = 1.00, 95% CI 0.96 to 1.04 with blinded onsite assessors (n = 35 RCTs) and with unblinded onsite assessors (ROR 1.08, 95% CI 0.94 to 1.23, 12 RCTs); P = 0.07 (Table 3).
2.
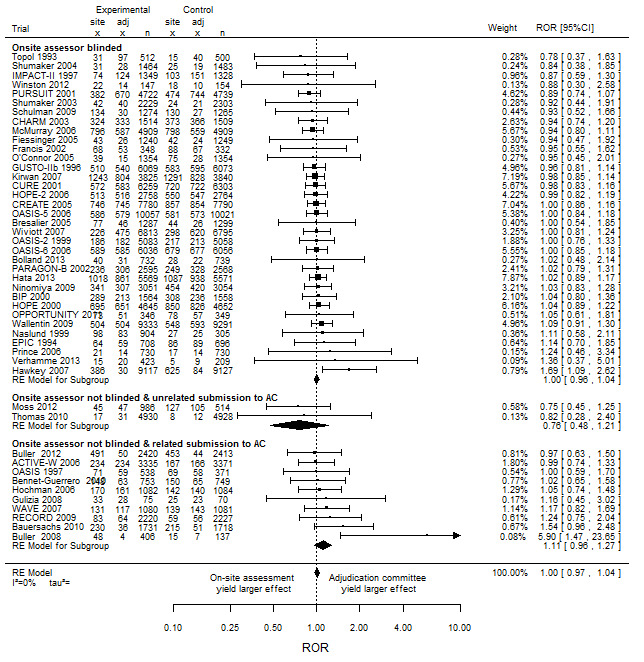
Impact of adjudication committee assessment on estimated intervention effects in randomised clinical trials measured as ratio of odds ratios (odds ratio based on onsite outcome assessment divided by odds ratio based on adjudication committee assessment)
3. Interaction between ratio of odds ratio (ROR) and blinding status of onsite assessors, ACs and method used to select cases submitted to ACs.
| Subgroup | No. of trials | ROR [95% CI] | I² (%) | P value for interaction |
| AC blinded AC with blinding status not reported |
40 7 |
1.00 [0.96 to 1.04] 1.04 [0.92 to 1.18] |
0 | 0.36 |
| Onsite assessor blinded Onsite assessor not blinded |
35 12 |
1.00 [0.96 to 1.04] 1.08 [0.94 to 1.23] |
0 4 |
0.07 |
| Onsite assessor blinded Onsite assessor not blinded and events submitted to ACs were identified independent of the onsite assessor assessment Onsite assessor not blinded and events submitted to AC were identified by onsite assessor |
35 2 10 |
1.00 [0.96 to 1.04] 0.76 [0.48 to 1.21] 1.11 [0.96 to 1.27] |
0 | 0.03 |
However, subgroup analysis showed a statistically significant interaction by blinding status of onsite assessor and the process for submitting data to the AC. The combined ROR was 1.00 (95% CI 0.96 to 1.04, I2 = 0%, 35 RCTs) with blinded onsite assessors; 0.76 (95% CI 0.48 to 1.21, I2 = 0%, two RCTs) with AC‐assessed events identified independent of unblinded onsite assessors; and 1.11 (95% CI 0.96 to 1.27, I2 = 0%, 10 RCTs) with AC‐assessed–only events identified by unblinded onsite assessors ; P = 0.03 (Figure 2, Table 3).
Discussion
Summary of main results
We performed a meta‐analysis of 47 randomised controlled trials (RCTs) (275,078 patients) to compare treatment effect estimates of subjective clinical events assessed by onsite assessors and by ACs. The combined ROR was 1.00 (95% CI 0.97 to 1.04). Results of subgroup analyses showed an interaction by blinding status of onsite assessors and the process used to submit data to ACs, with an increase in the effect estimate for the experimental treatment with onsite assessment compared to AC when the events were submitted by unblinded onsite assessors compared to when they were submitted by blinded onsite assessors or submitted independently of unblinded onsite assessors.
Overall completeness and applicability of evidence
The included RCTs are mainly large multicentre trials in cardiology. These results should be extrapolated to other medical areas with caution, but the included RCTs are representative of trials using ACs (Dechartres 2009; Stuck 2014).
Quality of the evidence
Our review has several strengths. First, our search strategy allowed for identifying trials with no restriction on medical area. Second, we identified a large sample of high‐quality RCTs with a large sample of patients in total. Third, our study featured low risk of confounding because of the direct comparison of onsite and AC assessment of the same outcome in the same study involving the same patients. Finally, the large number of included studies allowed us to perform a prespecified subgroup analysis related to the blinding status of onsite assessors and the submission of data to ACs.
Potential biases in the review process
Our review has some limitations. First, we cannot exclude a selective reporting bias, and investigators might be less prone to report the results of both AC and onsite assessors if the results differed. Furthermore, 11 RCTs (Characteristics of excluded studies) could not be included in the meta‐analysis because data were not available to compare the results for the randomised groups even though we contacted the corresponding authors of these RCTs to request the missing data. In contrast, we contacted authors reporting only outcomes assessed by ACs and obtained estimates for the onsite assessor for 10 RCTs. Second, the outcomes selected had variable levels of subjectivity, with 25% concerning composite outcomes including both subjective and objective outcomes (such as death). Third, we identified only two RCTs that had used unblinded onsite assessment, a blinded AC and an unbiased method for selecting cases to be adjudicated. Finally, we could not incorporate the correlation between effect estimates for ACs and onsite assessors because the data required to estimate the covariance were not available.
Agreements and disagreements with other studies or reviews
The largest previous study in this topic was published by Pogue and colleagues (Pogue 2009). They selected 10 RCTs conducted at the Population Health Research Institute in the field of cardiology. They did not detect any effect of event adjudication on the treatment effect estimates and raised the need to conduct more systematic analyses of the effect of the event adjudication in other trials to determine if this process is worthwhile. Other researchers have outlined the importance of ACs showing that misclassifications between onsite‐assessor and AC assessment can be frequent. However, these misclassifications may not be biased and might not lead to differences in treatment effect estimates (Hata 2013, Kirwan 2007). Because establishing and running an AC is time and resource consuming, some argue that ACs should be implemented only when the risk of misclassification is high (i.e., when onsite assessors are not blinded and the outcomes are subjective) (Granger 2008, Dechartres 2009). Indeed, we have previously shown that unblinded assessors of subjective clinical events generated substantially biased effect estimates in RCTs, exaggerating ORs by 36% (Hróbjartsson 2012). Prospective randomised, open, blinded end‐point (PROBE) studies are particularly recommended when blinding of patients and care providers is not feasible (Hansson 1992, Boutron 2006, Boutron 2007). Nevertheless, of the 47 trials we investigated, 35 (75%) had blinded onsite assessors, so the risk of biased misclassification was low. Similarly, previous work has shown that most ACs were implemented when onsite assessors are blinded or the outcome is objective (Dechartres 2009; Stuck 2014). This situation implies excessive cost and research waste (Ioannidis 2014, Al‐Shahi Salman 2014) .
In contrast, ACs would be important when onsite assessors are not blinded. We explored the impact of the blinding status of onsite assessors and did not detect an effect of AC assessment when onsite assessors were not blinded, with an estimated ROR of 1.08 (95% CI 0.94 to 1.23). These results could be related to the use of inadequate methods to capture suspected events, and biased submission of events by unblinded onsite assessors could result in a biased treatment effect estimated from ACs.
The question of the mode of data submission to the AC is important. In 79% of the RCTs included in our meta‐analysis, the method used to select cases to adjudicate was suspected events identified by the onsite assessor. Consequently, events that the onsite assessors had missed would not have been adjudicated. Therefore, when onsite assessors are not blinded, the estimated treatment effect by the AC could be biased because the AC will evaluate a biased sample of events identified by unblinded onsite assessors. The use of an AC could provide a false security because it does not control for the differential misclassification from onsite assessors. This issue was raised for the RECORD study and prompted the US FDA to modify the method for selecting cases to be adjudicated (Psaty 2010; Lopes 2013), although the “readjudication” of RECORD data also raised some concerns (Nissen 2013).
Authors' conclusions
Implication for methodological research.
Further research is needed to explore the impact of the different procedures used to select events to adjudicate.
Acknowledgements
We thank Laura Smales (BioMedEditing, Toronto, Canada) for editing this manuscript.
Appendices
Appendix 1. Search Strategy
Google scholar
(randomised OR random OR randomized) AND ("adjudication committee" OR "central adjudication" OR "endpoint committee" OR "clinical event committee" OR "outcome committee" OR "critical event committee")
EMBASE:
1.'randomized controlled trial'/exp
2.‘controlled clinical trial’/exp
3. randomized:ti,ab
4. placebo:ti,ab
5. randomly:ti,ab
6. trial:ti,ab
7. 1 OR 2 OR 3 OR 4 OR 5 OR 6
8. animal/exp NOT human/exp
9. 7 NOT 8
10.adjudicat*
11.’adjudication committee’
12. ‘central adjudication’
13.’clinical event committee’
14."endpoint committee"
15.’outcome committee’
16.’review committee’
17. ‘classification committee’
18.’critical event committee’
19.’central review’
20. 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19
21. 9 AND 20
CENTRAL:
adjudicat* OR "adjudication committee" OR "central adjudication" OR "endpoint committee" OR "clinical event committee" OR "outcome committee" OR "classification committee" OR "critical event committee" OR "central review" OR "consensus” OR "committee membership"
PubMed advanced with Cochrane filter:
1. “randomized controlled trial” [pt]
2. “controlled clinical trial” [pt]
3.”randomized” [tiab]
4. “placebo” [tiab]
5. ”drug therapy” [sh]
6. “randomly” [tiab]
7. “trial” [tiab]
8. “groups” [tiab]
9. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8
10.animals [mh] NOT humans [mh]
("Animals" [Mesh]) NOT "humans" [Mesh]
11. 9 NOT 10
12.adjudicat*
13."adjudication committee"
14. “central adjudication”
15."clinical event committee"
16."endpoint committee"
17."outcome committee"
18.“review committee"
19. "classification committee"
20."critical event committee"
21."central review"
22. 12 OR 13 OR 14 OR 15OR 16 OR 17 OR 18 OR 19 OR 20 OR 21
23. 11 AND 22
CINAHL:
( (MM "Research Methodology+") OR (MM "Clinical Trials+") ) TX
AND "adjudication committee" TX OR “central adjudication” TX OR "endpoint committee" TXOR "clinical event committee" TX OR "outcome committee" TX OR "critical event committee" TX OR "classification committee" TX OR "central review" TX
PsychINFO
{Clinical Trials} OR {Drug Therapy} OR {Evidence Based Practice} OR {Treatment Effectiveness Evaluation}
AND “adjudication committee" Any Field:
OR “central adjudication" Any Field OR "endpoint committee" Any Field OR “clinical event committee" Any Field OR "outcome committee" Any Field OR "critical event committee” Any Field OR "classification committee" Any Field OR "central review"Any Field
Appendix 2. Data extraction form
Adjudication committee versus Onsite investigator
● RCT included in a Meta‐analysis? Yes No
If yes, meta‐analysis number |__||__||__|
Review author………………………………................
RCT number |__||__||__| Date of publication (year) |__||__||__||__|
Title………………………………………………………………………
First author………………………………………………………………
Corresponding author (address)……………………………………
Journal………………………………………………………………
Funding sources: Public, Private, Both: public and private, Do not know
● Medical area of the patients
Critical care/Emergency medicine
Cardivascular system/peripheral vascular disease
Dermatology
Endocrinology and metabolism/Nutrition and dietetics
Geriatrics and Gerontology
Gastroenterology, hepatology, visceral surgery
Hematology/Oncology
Infectious diseases
Obstetric and gynecology
Internal medicine
Otorhinolaryngology/oral surgery and medicine/ophtalmology/dentistry
Pediatrics
Psychiatry/psychology
Musculo‐skeletal system (Orthopaedics/Rheumatology)
Nephrology/Urology
Neurology
Respiratory system
●Type of trial
What was the treatment being assessed? Pharmacological, Non pharmacological, Both
What was the comparator? Placebo, Active treatment, Usual care
Was it a multicenter study? Yes, No, Unclear
If yes, how many centers? |__||__||__| Do not know
Number of arms? |__| Sample size |__||__||__||__||__|
If more than 2 arms: Which were selected?
*Experimental arm………………………………*Control arm……………………………………
Number of patients randomized: Experimental group |__||__||__||__||__| Control group |__||__||__||__||__|
● Outcome selected 1……………………………………………………………………………..
Was it clearly reported as a primary outcome? Yes, No
Was the outcome:
1. Composite? Yes, No
2. Objective, Subjective, Mixed
3. Safety, Efficacy
● Were other outcomes compared? Yes, No, Number |__||__||__|
What were they?..................................................................................
● Blinding
Were the patients blinded? YES NO NOT REPORTED
Were the care providers blinded? YES NO NOT REPORTED
Were the site assessors blinded? YES NO NOT REPORTED
● Assessment of risk of bias
| Low risk | High risk | Unclear | |
| Random sequence generation Allocation concealment Performance bias Detection bias Incomplete outcome data |
●Description of the functioning of the adjudication committee (AC)
Was the AC independent? YES, NO, NOT, REPORTED
Was the AC blinded to the treatment assessed? YES, NO, NOT REPORTED
Was the AC blinded to onsite assessor’s assessment? YES, NO, NOT REPORTED
Were members of the AC trained? YES, NO, NOT REPORTED
What was the method used for selecting cases to adjudicate?
Suspected events identified by study investigators
Computer algorithms identifying all suspected events
Use of national registries to identify events
All patients adjudicated
Other; give a brief description…………………………………
Not reported
What was the information provided to the adjudication committee?
The entire medical file
Only some elements of medical file
A standardized case report form
Other; give a brief description……………………………………………………
Not reported
●Results of the selected outcome
Were those reported in the MA? Yes No
Were those reported in the RCT? Yes No
Table 1
ONSITE ASSESSOR
| Experimental arm | Control arm | Not available | |
| Number of randomized patients | |||
| Number of failures |
OR/RR IC95% …………………………………………………………………
ADJUDICATION COMMITTEE
| Experimental arm | Control arm | Not available | |
| Number of randomized patients | |||
| Number of failures |
OR/RR CI95% …………………………………………………………………
Was the agreement or disagreement per arm between the SI and AC available? Yes, No
If yes, complete the following table
Table 2
Experimental arm
Adjudication committee
| Success | Failure | Total | |
| Success | |||
| Failure | |||
| Total |
Onsite assessor
Control arm
Adjudication committee
| Success | Failure | Total | |
| Success | |||
| Failure | |||
| Total |
Onsite assessor
● Results for death
Were the number of deaths per arm reported? YES, NO, NOT AVAILABLE
Were these results compared between AC and site investigators? Yes, No
If yes, complete the table below
DEATHS REPORTED BY THE ONSITE ASSESSORS
| Experimental arm | Control arm | |
| Number of randomized patients | ||
| Number of deaths |
DEATHS REPORTED BY THE ADJUDICATION COMMITTEE
| Experimental arm | Control arm | |
| Number of randomized patients | ||
| Number of deaths |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ACTIVE‐W 2006.
| Methods | RCT comparing clopidogrel plus aspirin versus oral anticoagulation therapy for atrial fibrillation for prevention of vascular events. | |
| Data | 6706 patients were randomised (3371 in clopidogrel group/3335 in control group). | |
| Comparisons | Onsite assessment (local investigator non blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcomes selected were stroke or non‐central nervous systematic embolus or myocardial infarction or vascular death. | |
| Notes | The study was identified because it was included in a pooled analysis of 10 RCTs (Pogue 2009). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this meta‐analysis on the primary outcomes of the included studies. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | Yes | All suspect events adjudicated were identified by onsite assessor who was not blinded to allocated treatment. |
Bauersachs 2010.
| Methods | RCT comparing rivaroxaban versus enoxaparin in patients with acute and symptomatic deep‐vein thrombosis. | |
| Data | 3449 patients were randomised (1731/1718, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator non blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was recurrent venous thromboembolism. This was the study's primary outcome. | |
| Notes | Data related to the number of events in each treatment group resulting from onsite‐assessment were obtained directly from the study authors. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | Yes | All suspect events adjudicated were identified by onsite assessor who was not blinded to allocated treatment. |
Bennet‐Guerrero 2010.
| Methods | RCT evaluating effect of an Implantable Gentamicin‐Collagen Sponge versus no intervention (control) on sternal wound infections following cardiac surgery. | |
| Data | 1502 patients were randomised (753/749 respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator non blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The selected outcome was the incidence of sterna wound infection. This was the study's primary outcome. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | Yes | All suspect events adjudicated were identified by onsite assessor who was not blinded to allocated treatment. |
BIP 2000.
| Methods | RCT comparing bezafibrate versus placebo in patients with coronary artery disease. | |
| Data | 3122 patients were randomised (1542/1558, respectively in each treatment group), 32 patients were excluded from analysis because they never started the study medication. | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was fatal or non fatal myocardial infarction or sudden death. The study's primary outcome was time to one these events. | |
| Notes | Data related to the number of events in each treatment group resulting from onsite‐assessment were obtained directly from the study authors. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
Bolland 2013.
| Methods | RCT assessing calcium supplementation in healthy postmenopausal women | |
| Data | 1471 patients were randomised (732/739 respectively in each treatment group). | |
| Comparisons | Onsite assessment (patient self‐reported outcomes) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was stroke, which was not the study's primary outcome. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | Events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
Bresalier 2005.
| Methods | RCT comparing rofecoxib versus placebo for the prevention of colorectal adenoma. | |
| Data | A total of 3260 patients were screened for the study, of whom 2586 were deemed to be eligible (1287/1299 were randomised respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was the total number of thrombotic cardiovascular events. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
Büller 2008.
| Methods | RCT comparing rivaroxaban versus low molecular weight heparin in the treatment of patients with acute symptomatic deep vein thrombosis. | |
| Data | 543 patients were randomised (406/137, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator non blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was a composite outcome of symptomatic non fatal pulmonary embolism events or symptomatic recurrent deep venous thrombosis events. This was the study's primary outcome. | |
| Notes | Data related to the number of events in each treatment group resulting from onsite‐assessment were obtained directly from the study authors. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | Yes | All suspect events adjudicated were identified by onsite assessor who was not blinded to allocated treatment. |
Büller 2012.
| Methods | RCT comparing oral rivaroxaban versus enoxaparin for the treatment of symptomatic pulmonary embolism. | |
| Data | 4833 patients were randomised (2420/2413, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator non blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was recurrent venous thromboembolism. It was the study's primary outcome. | |
| Notes | Data related to the number of events in each treatment group resulting from onsite‐assessment were obtained directly from the study authors. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | Yes | All suspect events adjudicated were identified by onsite assessor who was not blinded to allocated treatment. |
CHARM 2003.
| Methods | RCT comparing candesartan versus placebo in patients with chronic heart failure and preserved left‐ventricular ejection fraction. | |
| Data | 3025 patients were randomised (1514/1509, respectively in each treatment group), including two patients who mistakenly received randomisation numbers but had no other data recorded and never received study medication. | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was admission to hospital for chronic heart disease or cardiovascular death. The study's primary outcome was cardiovascular death or admission to hospital for chronic heart failure. | |
| Notes | The study was identified because it was include in a review of six RCTs (Granger 2008). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this review . | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
CREATE 2005.
| Methods | RCT comparing reviparin versus placebo in patients with acute myocardial infarction presenting with ST‐segment elevation. | |
| Data | 15,570 patients were randomised (7780/7790, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was myocardial infarction or stroke at 7 days or death. | |
| Notes | The study was identified because it was included in a pooled analysis of 10 RCTs (Pogue 2009). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this meta‐analysis on the primary outcomes of the included studies. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
CURE 2001.
| Methods | RCT comparing clopidogrel plus aspirin versus placebo in patients with acute coronary syndromes without ST‐segment elevation. | |
| Data | 12,562 patients were randomised (6259/6303, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was myocardial infarction, stroke or cardiovascular death. | |
| Notes | The study was identified because it was included in a pooled analysis of 10 RCTs (Pogue 2009). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this meta‐analysis on the primary outcomes of the included studies. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
EPIC 1994.
| Methods | RCT comparing c7E3 Fab bolus and infusion versus placebo in high‐risk patients ongoing coronary angioplasty. | |
| Data | 2099 patients were randomised (1403/696, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was non fatal myocardial infarction or death from any cause. This was a prespecified composite of any of the following events in the first 30 days after randomisation. | |
| Notes | The study was identified because it was include in a review of six RCTs (Granger 2008). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this review. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
Fiessinger 2005.
| Methods | RCT comparing ximelagatran versus low molecular weight heparin and warfarin for the treatment of deep vein thrombosis. | |
| Data | 2528 patients were randomised (1258/1270, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was recurrent venous thromboembolism. The primary composite outcome was recurrent venous thromboembolism, bleeding, and mortality. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
Francis 2002.
| Methods | RCT comparing ximelagatran versus warfarin for the prevention of thromboembolism after total knee arthroplasty. | |
| Data | 680 patients were randomised (348/332, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was total thromboembolism. The study's primary outcome was the incidence of deep venous thrombosis (proximal or distal) or pulmonary embolism. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
Gulizia 2008.
| Methods | RCT designed to test the non‐inferiority of class IC antiarrhythmic drugs to amiodarone, in patients paced for sinus node disease. | |
| Data | 176 patients were enrolled: 70 patients were discharged on amiodarone, 75 patients were discharged on class IC agents (38 on propafenone and 37 on flecainide), and 31 were discharged on sotalol. | |
| Comparisons | Onsite assessment (local investigator non blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was a composite outcome of death, permanent atrial tachyarrhythmias, cardiovascular hospitalisation, atrial cardioversion or antiarrhythmic drug change. This was the study's primary outcome. | |
| Notes | Data related to the number of events in each treatment group resulting from onsite‐assessment were obtained directly from the study authors. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | Yes | All suspect events adjudicated were identified by onsite assessor who was not blinded to allocated treatment. |
GUSTO‐IIb 1996.
| Methods | RCT comparing recombinant hirudin versus heparin for the treatment of acute coronary syndromes. | |
| Data | 12,142 patients randomised (6069/6073, respectively in each treatment group. Patients were stratified according to the presence of ST‐segment elevation on the base‐line electrocardiogram (4131 patients) or its absence (8011 patients), with the latter characteristic considered to indicate unstable angina or non–Q‐wave myocardial infarction. | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was a composite outcome of myocardial infarction or reinfarction at 30 days or death from any cause. This was the study's primary outcome. | |
| Notes | The study was identified because it was included in a review of six RCTs (Granger 2008). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this review. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
Hata 2013.
| Methods | RCT assessing the effects of the routine administration of an angiotensin‐ converting enzyme inhibitor‐diuretic combination on serious vascular events in patients with diabetes, irrespective of initial blood pressure levels or the use of other blood pressure lowering drugs. | |
| Data | 11,140 were randomised (5569/5571, respectively to perindopril‐indapamide and to placebo). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was a composite outcome of major macro (non fatal myocardial infarction, non fatal stroke and cardiovascular death) and microvascular events (new or worsening nephropathy and retinopathy). This was the study's primary outcome. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
Hawkey 2007.
| Methods | RCT comparing lumiracoxib versus nonsteroidal anti‐inflammatory drugs (naproxen or ibuprofen) in patients with osteoarthritis. | |
| Data | 18,325 patients were randomised and 18,244 received at least 1 dose of study medication: lumiracoxib (9117 patients), naproxen (4730 patients), oribuprofen (4397 patients) . | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was all definite or probable ulcer complications. This was the study's primary outcome. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
Hochman 2006.
| Methods | RCT comparing percutaneous coronary intervention plus thrombolytic therapy versus thrombolytic therapy alone in patients who had total occlusion of the infarct‐related artery 3 to 28 days after myocardial infarction. | |
| Data | 2166 patients were randomised (1082/1084, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator non blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The selected outcome was a composite outcome of reinfarction or heart failure or death from any cause.This was the study's primary outcome. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | Yes | All suspect events adjudicated were identified by onsite assessor who was not blinded to allocated treatment. |
HOPE 2000.
| Methods | RCT comparing ramipril versus placebo to prevent cardiovascular events in high‐risk patients. | |
| Data | 9297 patients randomised (4645/4652, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was myocardial infarction, stroke or cardiovascular death. | |
| Notes | The study was identified because it was included in a pooled analysis of 10 RCTs (Pogue 2009). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this meta‐analysis on the primary outcomes of the included studies. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
HOPE‐2 2006.
| Methods | RCT comparing folic acid plus vitamin B versus placebo to prevent cardiovascular events in high‐risk patients. | |
| Data | 5522 patients randomised (2758/2764,) respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was myocardial infarction, stroke or cardiovascular death. | |
| Notes | The study was identified because it was included in a pooled analysis of 10 RCTs (Pogue 2009). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this meta‐analysis on the primary outcomes of the included studies. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
IMPACT‐II 1997.
| Methods | RCT comparing eptifibatide versus placebo to prevent cardiovascular events in high‐risk patients. | |
| Data | 4010 patients randomised (2682/1328, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was a 30‐day composite of myocardial infarction, coronary stent implantation, percutaneous revascularisation or death. This was the study's primary outcome. | |
| Notes | The study was identified because it was include in a review of six RCTs (Granger 2008). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this review. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
Kirwan 2007.
| Methods | RCT investigating the effect of the calcium antagonist nifedipine versus placebo on long‐term outcome in patients with stable angina pectoris. | |
| Data | 7797 patients were randomly allocated study drug with 7665 patients included in intention‐to‐treat analyses (3825/3840, respectively in nifedipine and placebo group) | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was a composite outcome of acute myocardial infarction, refractory angina, stroke or death. This was the study's primary outcome. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
McMurray 2006.
| Methods | RCT comparing the effect of valsartan monotherapy versus valsartan plus captopril versus captopril monotherapy on atherosclerotic events in patients who had acute myocardial infarction . | |
| Data | 14,703 patients included in the intention‐to‐treat analysis of the study (4909/4885/4909, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was fatal myocardial infarction. The study's primary outcome was death from any cause. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | Specific cause of all deaths classified by onsite assessor were adjudicated. |
Moss 2012.
| Methods | RCT comparing high‐rate and delayed therapy versus conventional therapy (implantable cardioverter‐defibrillator) in arrhythmias (ischaemic or non ischaemic heart disease). | |
| Data | 1500 patients were randomised (986/514, respectively in each treatment group). | |
| Comparisons | Onsite assessment (unblinded local investigator) versus assessment by a device‐interrogation committee (blinding status not reported) reviewing suspected events by algorithm of implanted devices (i.e., all device interrogations with use of electronic media downloaded from device interrogations at the enrolling centres). | |
| Outcomes | The outcome selected was the first occurrence of inappropriate therapy.This was the study's prespecified primary outcome. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | Events identified as suspected by an algorithm of the implanted devices were reviewed independently to the onsite assessor. |
Ninomiya 2009.
| Methods | RCT comparing perindopril plus indapamide versus placebo in cerebrovascular disease. | |
| Data | 6105 patients were randomised (3051/3054, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was total stroke, which was the study's primary outcome. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | Specific cause of all deaths and suspected events identified by onsite assessor who was blinded to allocated treatment were adjudicated. |
Näslund 1999.
| Methods | RCT comparing inogatran versus heparin in unstable coronary disease. | |
| Data | 1209 patients were randomised (904/305, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC (blinding status not reported). | |
| Outcomes | The outcome selected was death, myocardial infarction (reinfarction), refractory angina or recurrent angina.The study's primary outcome was a composite of these events at 7 days. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
O' Connor 2005.
| Methods | RCT comparing bucindolol versus placebo in patients who had moderate to severe heart failure. | |
| Data | 2708 patients were analysed (1354/1354, respectively in each group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was non fatal myocardial infarction. The study's primary outcome was total mortality. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
OASIS‐1 1997.
| Methods | RCT comparing the effects of two doses of recombinant hirudin versus heparin in patients with acute myocardial ischaemia without ST elevation. | |
| Data | 909 patients randomised (538/371 respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC not blinded to allocated treatment. | |
| Outcomes | The outcome selected was myocardial infarction, angina at 7 days or cardiovascular death. | |
| Notes | The study was identified because it was included in a pooled analysis of 10 RCTs (Pogue 2009). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this meta‐analysis on the primary outcomes of the included studies. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
OASIS‐2 1999.
| Methods | RCT comparing the effects of recombinant hirudin (lepirudin) versus heparin on death, myocardial infarction, refractory angina, and revascularisation procedures in patients with acute myocardial ischaemia without ST elevation. | |
| Data | 10,141 patients randomised (5058/5083, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was myocardial infarction at 7 days or cardiovascular death. | |
| Notes | The study was identified because it was included in a pooled analysis of 10 RCTs (Pogue 2009). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this meta‐analysis on the primary outcomes of the included studies. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
OASIS‐5 2006.
| Methods | RCT comparing fondaparinux versus enoxaparin in acute coronary syndromes. | |
| Data | 20,078 patients randomised (10,057/10,021), respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was refractory ischaemia or myocardial infarction at 9 days or death. | |
| Notes | The study was identified because it was included in a pooled analysis of 10 RCTs (Pogue 2009). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this meta‐analysis on the primary outcomes of the included studies. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
OASIS‐6 2006.
| Methods | RCT analysing the effects of fondaparinux, a factor Xa inhibitor, versus usual care on mortality and reinfarction in patients with acute ST‐segment elevation myocardial infarction. | |
| Data | 12,092 patients randomised (6056/6036, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was reinfarction at 30 days or death. | |
| Notes | The study was identified because it was included in a pooled analysis of 10 RCTs (Pogue 2009). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this meta‐analysis on the primary outcomes of the included studies. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
OPPORTUNITY 2011.
| Methods | RCT comparing human growth hormone versus placebo in haemodialysis patients. | |
| Data | 712 patients were randomised and 695 patients who received at least one dose of trial medication (346/349, respectively in each treatment group) were considered in the full analysis set. | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was any cardiovascular event and death of any cause. The study's primary outcome was time to all‐cause death. | |
| Notes | Data related to the number of events in each treatment group resulting from onsite‐assessment were obtained directly from the study authors. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
PARAGON‐B 2002.
| Methods | RCT comparing lamifiban versus placebo in patients with acute coronary syndrome. | |
| Data | Of 5225 patients enrolled, 5163 were analysed (2568/2595, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The primary selected was a composite outcome myocardial infarction, ischaemia or death at 30 days. This was the study's primary outcome. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified through a computer algorithm. |
Prince 2006.
| Methods | RCT comparing calcium carbonate versus placebo to prevent osteoporotic fractures. | |
| Data | 1460 patients were randomised (730/730 respectively in each treatment group). | |
| Comparisons | Blinded patients self‐reported outcomes versus assessment by an AC (blinding not reported). | |
| Outcomes | The outcome selected was myocardial infarction. The study's primary outcome included clinical incident osteoporotic fractures, vertebral deformity, and adverse events ascertained in 5 years. | |
| Notes | The study was identified because it was included in a review of 2 RCTs (Lewis 2012). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this review. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | Unclear | The method used to select cases to adjudicate was not reported. |
PURSUIT 2001.
| Methods | RCT comparing eptifibatide versus placebo in patients with acute coronary syndromes. | |
| Data | 10,948 patients were randomised. Data were presented in detail for the primary comparison groups, those assigned to receive high‐dose eptifibatide or placebo (4722/4739, respectively in each treatment group); 1487 patients were allocated to the low‐dose eptifibatide group. | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was a composite of death or post‐enrolment myocardial infarction (or reinfarction if patients had a myocardial infarction at enrolment) by 30 days. This was the study's primary outcome. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified through a computer algorithm. |
RECORD 2009.
| Methods | RCT comparing rosiglitazone versus metformin plus sulphonylurea in patients with type 2 diabetes. | |
| Data | 4447 patients were randomised (2220/2227, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator non blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was myocardial infarction. The study's primary outcome was cardiovascular hospitalisation or cardiovascular death.. | |
| Notes | The study was identified because it was included in a review (Serebruany 2012). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this review. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | Yes | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
Schulman 2009.
| Methods | RCT comparing dabigatran versus warfarin in patients with acute venous thromboembolism. | |
| Data | 2564 patients were randomised (1274/1265, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The primary selected was a composite outcome of symptomatic venous thromboembolism or related death. This was the study's primary outcome. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
Shumaker 2003.
| Methods | RCT comparing oestrogen plus progestin versus placebo in post‐menopausal women. | |
| Data | 4532 patients were randomised (2229/2303, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was incidence of dementia.This was the study's primary outcome. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to treatment allocation. |
Shumaker 2004.
| Methods | RCT comparing oestrogen alone versus placebo in post‐menopausal women. | |
| Data | 2947 patients were randomised (1464/1483, respectively in each treatment group). | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was probable dementia.This was the study's primary outcome. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
Thomas 2010.
| Methods | RCT comparing sertindole versus risperidone in patients with schizophrenia. | |
| Data | 9858 patients were randomised (4930/4928, respectively in each treatment group). | |
| Comparisons | Onsite assessment (non blinded local investigator) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was fatal suicide. The study's primary outcome was all‐cause mortality. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All deaths were adjudicated for a specific cause of death. |
Topol 1993.
| Methods | RCT comparing atherectomy versus angiography in patients with coronary artery disease. | |
| Data | 1012 patients were randomised (512/500, respectively in each treatment group). | |
| Comparisons | Onsite assessment (blinded local investigator) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was myocardial infarction. The study's primary outcome was angiographic restenosis. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All patients randomised were adjudicated. |
Verhamme 2013.
| Methods | RCT comparing TB‐402 versus rivaroxaban for the prevention of venous thromboembolism after total hip replacement. | |
| Data | 632 patients were randomised (423/209, respectively in each treatment group). | |
| Comparisons | Onsite assessment (blinded local investigator) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was total venous thromboembolism. This was study's primary outcome. | |
| Notes | Data related to the number of events in each treatment group resulting from onsite‐assessment were obtained directly from the study authors. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
Wallantin 2009.
| Methods | RCT comparing ticagrelor versus clopidogrel in patients with acute coronary syndromes. | |
| Data | 18,624 patients were randomised (9333/9291, respectively in each treatment group). | |
| Comparisons | Onsite assessment (blinded local investigator) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was myocardial infarction. The study's primary outcome was the time to the first occurrence of composite of death from vascular causes, myocardial infarction, or stroke. | |
| Notes | The study was identified because it was included in a review (Serebruany 2012). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this review. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
WAVE 2007.
| Methods | RCT comparing aspirin plus clopidogrel versus clopidogrel alone in atherosclerotic peripheral arterial disease. | |
| Data | 2161 patients were randomised (1080/1081, respectively in each treatment group). | |
| Comparisons | Onsite assessment (non blinded local investigator) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The primary outcome selected was a composite outcome of myocardial infarction, stroke, or death from cardiovascular causes. | |
| Notes | The study was identified because it was included in a pooled analysis of 10 RCTs (Pogue 2009). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this meta‐analysis on the primary outcomes of the included studies. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | Yes | All suspect events adjudicated were identified by onsite assessor who was not blinded to allocated treatment. |
Winston 2012.
| Methods | RCT comparing the efficacy and safety of prophylactic oral maribavir versus oral ganciclovir for prevention of cytomegalovirus disease in cytomegalovirus‐seronegative liver transplant recipients with CMV‐seropositive donors. | |
| Data | 307 patients were randomised (147/156, respectively in each group). Four patients never received the study drug. | |
| Comparisons | Onsite assessment (local investigator blinded) versus assessment by an AC (blinding status not reported). | |
| Outcomes | The selected was the incidence of cytomegalovirus disease. This was the study's primary outcome. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | Unclear | The method used to select cases to adjudicate was not reported. |
Wiviott 2007.
| Methods | RCT comparing prasugrel versus clopidogrel in patients with acute coronary syndromes. | |
| Data | 13,608 patients were randomised (6813/6795, respectively in each treatment group). | |
| Comparisons | Onsite assessment (blinded local investigator) versus assessment by an AC blinded to allocated treatment. | |
| Outcomes | The outcome selected was myocardial infarction. The study's primary outcome was death from cardiovascular causes, non fatal myocardial infarction, or non fatal stroke. | |
| Notes | The study was identified because it was included in a review (Serebruany 2012). Data related to the number of events in each treatment group resulting from onsite‐assessor and AC assessments were extracted from this review. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Method for selecting cases to adjudicate? | No | All suspect events adjudicated were identified by onsite assessor who was blinded to allocated treatment. |
AC: adjudication committee RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arnold 2013 | The classification of events (bleed severity) by the onsite assessment and AC was not provided for each treatment group. |
| Epstein 1999 | The classification of cause of death (in ventricular fibrillation or sustained ventricular tachycardia) by the onsite assessment and AC was not provided for each treatment group. |
| Heagerty 2002 | The number of critical events (major cardiovascular outcomes) in each treatment group resulting from onsite assessment and AC classification was not provided. |
| Kestle 1999 | The number of clinical events (shunt failures) in each treatment group resulting from onsite assessment and AC classifications was not provided. Only the overall agreement between the AC and the onsite assessment was available. |
| Mahaffey 2011 | The classification of events (congestive heart failure and cardiogenic shock) by the onsite assessment and the AC was not provided for each treatment group. The study only reported the agreement rate between the onsite assessors and the AC. |
| McGarvey 2007 | The ascertainment of cause‐specific mortality (in obstructive pulmonary disease) by the onsite assessment and AC was not provided for each treatment group. |
| McGarvey 2012 | The classification of cause‐specific mortality (in chronic obstructive pulmonary disease) by the onsite assessment and AC was not provided for each treatment group. |
| O'Connor 2011 | The classification of cause‐specific mortality (in advanced heart failure) by the onsite assessment and AC was not provided for each treatment group. |
| Petersen 2006 | The classification of cause of death (in sinus node disfunction) by the onsite assessment and AC was not provided for each treatment group. |
| Slee 2010 | The number of events related to the classification of cause of death (cardiovascular or non cardiovascular) by the onsite assessment and AC was not provided for each treatment group. |
| Vejlstrup 2003 | The classification of events (reinfarction and strokes) by the onsite assessment and AC was not provided for each treatment group. |
AC: adjudication committee
Differences between protocol and review
None.
Contributions of authors
Drafting the protocol: LAND, IB, LT
Acquisition of data: LAND, CB, AY
Analysed and interpreted data: LAND, IB, LT, AH, PR
Drafted the article: LAND, IB, LT
Revising it critically for important intellectual content: LT, IB, AH, PR
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Isabelle Boutron and Asbjorn Hrobjartson are co‐convenors of the Cochrane Methods Bias Group. Philippe Ravaud is director of the French Cochrane Centre.
New
References
References to studies included in this review
ACTIVE‐W 2006 {published and unpublished data}
- ACTIVE Writing Group of the ACTIVE Investigators, Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006;367(9526):1903‐12. [PUBMED: 16765759] [DOI] [PubMed] [Google Scholar]
Bauersachs 2010 {published and unpublished data}
- EINSTEIN Investigators, Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, et al. Oral rivaroxaban for symptomatic venous thromboembolism. New England Journal of Medicine 2010;363(26):2499‐510. [DOI: 10.1056/NEJMoa1007903; NCT00439725; NCT00440193] [DOI] [PubMed] [Google Scholar]
Bennet‐Guerrero 2010 {published data only}
- Bennett‐Guerrero E, Ferguson TB Jr, Lin M, Garg J, Mark DB, Scavo VA Jr, et al. SWIPE‐1 Trial Group. Effect of an implantable gentamicin‐collagen sponge on sternal wound infections following cardiac surgery: a randomized trial. JAMA 2010;304(7):755‐62. [PUBMED: 20716738] [DOI] [PubMed] [Google Scholar]
BIP 2000 {published and unpublished data}
- Bezafibrate Infarction Prevention (BIP) study. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation 2000;102(1):21‐7. [PUBMED: 10880410] [DOI] [PubMed] [Google Scholar]
Bolland 2013 {published data only}
- Bolland MJ, Barber A, Doughty RN, Grey A, Gamble G, Reid IR. Differences between self‐reported and verified adverse cardiovascular events in a randomised clinical trial. BMJ Open 2013;3(3):pii: e002334. [DOI: 10.1136/bmjopen-2012-002334; PUBMED: 23512838] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bresalier 2005 {published data only}
- Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Adenomatous Polyp Prevention on Vioxx (APPROVe) Trial Investigators. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. New England Journal of Medicine 2005;352(11):1092‐102. [PUBMED: 15713943] [DOI] [PubMed] [Google Scholar]
Büller 2008 {published and unpublished data}
- Buller HR, Lensing AW, Prins MH, Agnelli G, Cohen A, Gallus AS, et al. Einstein‐DVT Dose‐Ranging Study investigators. A dose‐ranging study evaluating once‐daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein‐DVT Dose‐Ranging Study. Blood 2008;112(6):2242‐7. [PUBMED: 18621928] [DOI] [PubMed] [Google Scholar]
Büller 2012 {published and unpublished data}
- EINSTEIN–PE Investigators, Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. New England Journal of Medicine 2012;366(14):1287‐97. [PUBMED: 22449293] [DOI] [PubMed] [Google Scholar]
CHARM 2003 {published data only}
- Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved Trial. Lancet 2003;362(9386):777‐81. [PUBMED: 13678871] [DOI] [PubMed] [Google Scholar]
CREATE 2005 {published and unpublished data}
- Yusuf S, Mehta SR, Xie C, Ahmed RJ, Xavier D, Pais P, et al. CREATE Trial Group Investigators. Effects of reviparin, a low‐molecular‐weight heparin, on mortality, reinfarction, and strokes in patients with acute myocardial infarction presenting with ST‐segment elevation. JAMA 2005;293(4):427‐35. [PUBMED: 15671427] [DOI] [PubMed] [Google Scholar]
CURE 2001 {published data only}
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK, Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation. New England Journal of Medicine 2001;345(7):494‐502. [PUBMED: 11519503] [DOI] [PubMed] [Google Scholar]
EPIC 1994 {published and unpublished data}
- The EPIC Investigators. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high‐risk coronary angioplasty. The EPIC Investigation. New England Journal of Medicine 1994;330(14):956‐61. [PUBMED: 8121459 ] [DOI] [PubMed] [Google Scholar]
Fiessinger 2005 {published data only}
- Fiessinger JN, Huisman MV, Davidson BL, Bounameaux H, Francis CW, Eriksson H, et al. THRIVE Treatment Study Investigators. Ximelagatran vs low‐molecular‐weight heparin and warfarin for the treatment of deep vein thrombosis: a randomized trial. JAMA 2005;293(6):691‐9. [PUBMED: 15701909] [DOI] [PubMed] [Google Scholar]
Francis 2002 {published data only}
- Francis CW, Davidson BL, Berkowitz SD, Lotke PA, Ginsberg JS, Lieberman JR, et al. Ximelagatran versus warfarin for the prevention of venous thromboembolism after total knee arthroplasty. A randomized, double‐blind trial. Annals of Internal Medicine 2002;137(8):648‐55. [PUBMED: 12379064] [DOI] [PubMed] [Google Scholar]
Gulizia 2008 {published and unpublished data}
- Gulizia M, Mangiameli S, Orazi S, Chiarandà G, Piccione G, Giovanni N, et a, PITAGORA Study Investigators. A randomized comparison of amiodarone and class IC antiarrhythmic drugs to treat atrial fibrillation in patients paced for sinus node disease: the Prevention Investigation and Treatment: A Group for Observation and Research on Atrial arrhythmias (PITAGORA) trial. American Heart Journal 2008;155(1)(1):100‐7. [PUBMED: 18082498] [DOI] [PubMed] [Google Scholar]
GUSTO‐IIb 1996 {published and unpublished data}
- The global use of strategies to open occluded coronary arteries (GUSTO) IIb investigators. A comparison of recombinant hirudin with heparin for the treatment of acute coronary syndromes. The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) IIb investigators. New England Journal of Medicine 1996;335(11):775‐82. [PUBMED: 8778585] [DOI] [PubMed] [Google Scholar]
Hata 2013 {published data only}
- Hata J, Arima H, Zoungas S, Fulcher G, Pollock C, Adams M, et al. ADVANCE Collaborative Group. Effects of the endpoint adjudication process on the results of a randomised controlled trial: the ADVANCE trial. PLoS One 2013;8(2):e55807. [PUBMED: 23390553] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawkey 2007 {published data only}
- Hawkey CJ, Weinstein WM, Smalley W, Gitton X, Sallstig P, Stricker K, et al. Effect of risk factors on complicated and uncomplicated ulcers in the TARGET lumiracoxib outcomes study. Gastroenterology 2007;133(1):57‐64. [PUBMED: 17631131] [DOI] [PubMed] [Google Scholar]
Hochman 2006 {published data only}
- Hochman JS, Lamas GA, Buller CE, Dzavik V, Reynolds HR, Abramsky SJ, et al. Occluded Artery Trial Investigators. Coronary intervention for persistent occlusion after myocardial infarction. New England Journal of Medicine 2006;355(23):2395‐407. [PUBMED: 17105759] [DOI] [PMC free article] [PubMed] [Google Scholar]
HOPE 2000 {published and unpublished data}
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. New England Journal of Medicine 2000;342(3):145‐53. [PUBMED: 10639539] [DOI] [PubMed] [Google Scholar]
HOPE‐2 2006 {published and unpublished data}
- Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. New England Journal of Medicine 2006;354(15):1567‐77. [PUBMED: 16531613] [DOI] [PubMed] [Google Scholar]
IMPACT‐II 1997 {published and unpublished data}
- The IMPACT‐II Investigators. Randomised placebo‐controlled trial of effect of eptifibatide on complications of percutaneous coronary intervention: IMPACT‐II. Integrilin to Minimise Platelet Aggregation and Coronary Thrombosis‐II. Lancet 1997;349(9063):1422‐8. [PUBMED: 9164315] [PubMed] [Google Scholar]
Kirwan 2007 {published data only}
- Kirwan BA, Lubsen J, Brouwer S, Danchin N, Battler A, Bayes de Luna A, et al. ACTION (A Coronary disease Trial Investigating Outcome with Nifedipine GITS) investigators. Diagnostic criteria and adjudication process both determine published event‐rates: the ACTION trial experience. Contemporary Clinical Trials 2007;28(6):720‐9. [PUBMED: 17509947] [DOI] [PubMed] [Google Scholar]
McMurray 2006 {published data only}
- McMurray J, Solomon S, Pieper K, Reed S, Rouleau J, Velazquez E, et al. The effect of valsartan, captopril, or both on atherosclerotic events after acute myocardial infarction: an analysis of the Valsartan in Acute Myocardial Infarction Trial (VALIANT). Journal of the American College of Cardiology 2006;47(4):726‐33. [PUBMED: 16487836 ] [DOI] [PubMed] [Google Scholar]
Moss 2012 {published data only}
- Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, et al. MADIT‐RIT Trial Investigators. Reduction in inappropriate therapy and mortality through ICD programming. New England Journal of Medicine 2012;367(24):2275‐83. [PUBMED: 23131066] [DOI] [PubMed] [Google Scholar]
Näslund 1999 {published data only}
- Näslund U, Grip L, Fischer‐Hansen J, Gundersen T, Lehto S, Wallentin L. The impact of an end‐point committee in a large multicentre, randomized, placebo‐controlled clinical trial: results with and without the end‐point committee's final decision on end‐points. European Heart Journal 1999;20(10):771‐7. [PUBMED: 10329069] [DOI] [PubMed] [Google Scholar]
Ninomiya 2009 {published data only}
- Ninomiya T, Donnan G, Anderson N, Bladin C, Chambers B, Gordon G, et al. PROGRESS Collaborative Group. Effects of the end point adjudication process on the results of the Perindopril Protection Against Recurrent Stroke Study (PROGRESS). Stroke 2009;40(6):2111‐5. [PUBMED: 19359647] [DOI] [PubMed] [Google Scholar]
O' Connor 2005 {published data only}
- O'Connor CM, Gottlieb S, Bourque JM, Krause‐Steinrauf H, Anand I, Anderson JL, et al. BEST Investigators. Impact of nonfatal myocardial infarction on outcomes in patients with advanced heart failure and the effect of bucindolol therapy. American Journal of Cardiology 2005;95(5):558‐64. [PUBMED: 15721091] [DOI] [PubMed] [Google Scholar]
OASIS‐1 1997 {published and unpublished data}
- Organization to Assess Strategies for Ischemic Syndromes (OASIS) Investigators. Comparison of the effects of two doses of recombinant hirudin compared with heparin in patients with acute myocardial ischemia without ST elevation: a pilot study. Circulation 1997;96(3):769‐77. [PUBMED: 9264481] [DOI] [PubMed] [Google Scholar]
OASIS‐2 1999 {published and unpublished data}
- Organisation to Assess Strategies for Ischemic Syndromes (OASIS‐2) Investigators. Effects of recombinant hirudin (lepirudin) compared with heparin on death, myocardial infarction, refractory angina, and revascularisation procedures in patients with acute myocardial ischaemia without ST elevation: a randomised trial. Lancet 1999;353(9151):429‐38. [PUBMED: 9989712] [PubMed] [Google Scholar]
OASIS‐5 2006 {published and unpublished data}
- Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators1, Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. New England Journal of Medicine 2006;354(14):1464‐76. [PUBMED: 16537663] [DOI] [PubMed] [Google Scholar]
OASIS‐6 2006 {published and unpublished data}
- Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, et al. OASIS‐6 Trial Group. Effects of fondaparinux on mortality and reinfarction in patients with acute ST‐segment elevation myocardial infarction: the OASIS‐6 randomized trial. JAMA 2006;295(13):1519‐30. [PUBMED: 16537725] [DOI] [PubMed] [Google Scholar]
OPPORTUNITY 2011 {published and unpublished data}
- Kopple JD, Cheung AK, Christiansen JS, Djurhuus CB, Nahas M, Feldt‐Rasmussen B, et al. OPPORTUNITY: a large‐scale randomized clinical trial of growth hormone in hemodialysis patients. Nephrology Dialysis Transplantation 2011;26(12):4095‐103. [PUBMED: 21750157] [DOI] [PubMed] [Google Scholar]
PARAGON‐B 2002 {published data only}
- Mahaffey KW, Roe MT, Dyke CK, Newby LK, Kleiman NS, Connolly P, et al. Misreporting of myocardial infarction end points: results of adjudication by a central clinical events committee in the PARAGON‐B trial. Second Platelet IIb/IIIa Antagonist for the Reduction of Acute Coronary Syndrome Events in a Global Organization Network Trial. American Heart Journal 2002;143(2):242‐8. [PUBMED: 11835026] [DOI] [PubMed] [Google Scholar]
Prince 2006 {published data only}
- Prince RL, Devine A, Dhaliwal SS, Dick IM. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5‐year, double‐blind, placebo‐controlled trial in elderly women. Archives of Internal Medicine 2006;166(8):869‐75. [PUBMED: 16636212] [DOI] [PubMed] [Google Scholar]
PURSUIT 2001 {published data only}
- Mahaffey KW, Harrington RA, Akkerhuis M, Kleiman NS, Berdan LG, Crenshaw BS, et al. For the PURSUIT Investigators. Disagreements between central clinical events committee and site investigator assessments of myocardial infarction endpoints in an international clinical trial: review of the PURSUIT study. Current Controlled Trials in Cardiovascular Medicine 2001;2(4):187‐94. [PUBMED: 11806794] [DOI] [PMC free article] [PubMed] [Google Scholar]
RECORD 2009 {published and unpublished data}
- Home PD, Pocock SJ, Beck‐Nielsen H, Curtis PS, Gomis R, Hanefeld M, et al. RECORD Study Team. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open‐label trial. Lancet 2009;373(9681):2125‐35. [PUBMED: 19501900] [DOI] [PubMed] [Google Scholar]
Schulman 2009 {published data only}
- Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. RE‐COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. New England Journal of Medicine 2009;361(24):2342‐52. [PUBMED: 19966341] [DOI] [PubMed] [Google Scholar]
Shumaker 2003 {published data only}
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289(20):2651‐62. [PUBMED: 12771112 ] [DOI] [PubMed] [Google Scholar]
Shumaker 2004 {published data only}
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, et al. Women's Health Initiative Memory Study. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA 2004;291(24):2947‐58. [PUBMED: 15213206] [DOI] [PubMed] [Google Scholar]
Thomas 2010 {published data only}
- Thomas SH, Drici MD, Hall GC, Crocq MA, Everitt B, Lader MH, et al. Safety of sertindole versus risperidone in schizophrenia: principal results of the sertindole cohort prospective study (SCoP). Acta Psychiatrica Scandinavica 2010;122(5):345‐55. [PUBMED: 20384598] [DOI] [PubMed] [Google Scholar]
Topol 1993 {published data only}
- Topol EJ, Leya F, Pinkerton CA, Whitlow PL, Hofling B, Simonton CA, et al. A comparison of directional atherectomy with coronary angioplasty in patients with coronary artery disease. The CAVEAT Study Group. New England Journal of Medicine 1993;329(4):221‐7. [PUBMED: 8316266] [DOI] [PubMed] [Google Scholar]
Verhamme 2013 {published and unpublished data}
- Verhamme P, Gunn S, Sonesson E, Peerlinck K, Vanassche T, Vandenbriele C, et al. Single‐dose TB‐402 or rivaroxaban for the prevention of venous thromboembolism after total hip replacement. A randomised, controlled trial. Thrombosis and Haemostasis 2013;109(6):1091‐8. [PUBMED: 23615791] [DOI] [PubMed] [Google Scholar]
Wallantin 2009 {published and unpublished data}
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. PLATO Investigators, Freij A, Thorsén M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. New England Journal of Medicine 2009;361(11):1045‐57. [DOI: 10.1056/NEJMoa0904327; PUBMED: 19717846 ] [DOI] [PubMed] [Google Scholar]
WAVE 2007 {published and unpublished data}
- Warfarin Antiplatelet Vascular Evaluation Trial Investigators, Anand S, Yusuf S, Xie C, Pogue J, Eikelboom J, Budaj A, et al. Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. New England Journal of Medicine 2007;357(3):217‐27. [PUBMED: 17634457] [DOI] [PubMed] [Google Scholar]
Winston 2012 {published data only}
- Winston DJ, Saliba F, Blumberg E, Abouljoud M, Garcia‐Diaz JB, Goss JA, et al. 1263‐301 Clinical Study Group. Efficacy and safety of maribavir dosed at 100 mg orally twice daily for the prevention of cytomegalovirus disease in liver transplant recipients: a randomized, double‐blind, multicenter controlled trial. American Journal of Transplantation 2012;12(11):3021‐30. [PUBMED: 22947426] [DOI] [PubMed] [Google Scholar]
Wiviott 2007 {published and unpublished data}
- Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. TRITON‐TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. New England Journal of Medicine 2007;357(20):2001‐15. [PUBMED: 17982182] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arnold 2013 {published and unpublished data}
- Arnold DM, Lauzier F, Rabbat C, Zytaruk N, Barlow Cash B, Clarke F, et al. PROTECT Investigators, on behalf of the Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Adjudication of bleeding outcomes in an international thromboprophylaxis trial in critical illness. Thrombosis Research 2013;131(3):204‐9. [PUBMED: 23317632 ] [DOI] [PubMed] [Google Scholar]
Epstein 1999 {published data only}
- Epstein AE, Powell J, Yao Q, Ocampo C, Lancaster S, Rosenberg Y, et al. In‐hospital versus out‐of‐hospital presentation of life‐threatening ventricular arrhythmias predicts survival: results from the AVID Registry. Antiarrhythmics Versus Implantable Defibrillators. Journal of the American College of Cardiology 1999;34(4):1111‐6. [PUBMED: 10520799] [DOI] [PubMed] [Google Scholar]
Heagerty 2002 {published and unpublished data}
- Heagerty A, Deverly A, Palmer C, Kaplinsky E, Salvetti A, Wahlgren NG, et al. The role of the critical event committee in a major cardiovascular outcome study. Blood Pressure 2002;11(6):339‐44. [PUBMED: 12523676] [DOI] [PubMed] [Google Scholar]
Kestle 1999 {published and unpublished data}
- Kestle J, Milner R, Drake D. An assessment of observer bias in the shunt design trial. Pediatric Neurosurgery 1999;30(2):57‐61. [PUBMED: 10325560] [DOI] [PubMed] [Google Scholar]
Mahaffey 2011 {published data only}
- Mahaffey KW, Wampole JL, Stebbins A, Berdan LG, McAfee D, Rorick TL. Strategic lessons from the clinical event classification process for the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX‐AMI) trial. Contemporary Clinical Trials 2011;32(2):178‐87. [PUBMED: 21220052] [DOI] [PubMed] [Google Scholar]
McGarvey 2007 {published and unpublished data}
- McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA, TORCH Clinical Endpoint Committee. Ascertainment of cause‐specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax 2007;62(5):411‐5. [PUBMED: 17311843 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGarvey 2012 {published and unpublished data}
- McGarvey LP, Magder S, Burkhart D, Kesten S, Liu D, Manuel RC, et al. Cause‐specific mortality adjudication in the UPLIFT® COPD trial: findings and recommendations. Respiratory Medicine 2012;106(4):515‐21. [DOI: 10.1016/j.rmed.2011.10.009; PUBMED: 22100536] [DOI] [PubMed] [Google Scholar]
O'Connor 2011 {published and unpublished data}
- O'Connor CM, Fiuzat M, Lindenfeld J, Miller A, Lombardi C, Carson P, et al. Mode of death and hospitalization from the Second Follow‐up Serial Infusions of Nesiritide (FUSION II) trial and comparison of clinical events committee adjudicated versus investigator reported outcomes. American Journal of Cardiology 2011;108(10):1449‐57. [DOI: 10.1016/j.amjcard.2011.06.065; PUBMED: 21890092] [DOI] [PubMed] [Google Scholar]
Petersen 2006 {published and unpublished data}
- Petersen JL, Haque G, Hellkamp AS, Flaker GC, Mark Estes NA 3rd, Marchlinski FE, et al. MOST Clinical Events Committee. Comparing classifications of death in the Mode Selection Trial: agreement and disagreement among site investigators and a clinical events committee. Contemporary Clinical Trials 2006;27(3):260‐8. [PUBMED: 16574497 ] [DOI] [PubMed] [Google Scholar]
Slee 2010 {published and unpublished data}
- Slee A, Nasco E, Hart R. Effect of adjudication on cause of death in the affirm trial: evidence for bias by unblinded local investigators. Clinical Trials. 2010; Vol. 7:418–504.
Vejlstrup 2003 {published data only}
- Vejlstrup N, Clemmensen P, Steinmetz E, Krusell LR, Hansen KN, Christiansen I, et al. Blinded end point adjudication in the 'Danish multicenter randomized study on fibrinolytic therapy versus acute coronary angioplasty in acute myocardial infarction' (DANAMI‐2 trial). Heart Drug 2003;3(3):127‐33. [DOI: 10.1159/000073837] [DOI] [Google Scholar]
Additional references
Al‐Shahi Salman 2014
- Al‐Shahi Salman R, Beller E, Kagan J, Hemminki E, Phillips RS, Savulescu J, et al. Increasing value and reducing waste in biomedical research regulation and management. Lancet 2014;383(9912):176‐85. [PUBMED: 24411646] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bagos 2012
- Bagos PG. On the covariance of two correlated log‐odds ratios. Statistics in Medicine 2012;31(14):1418‐31. [PUBMED: 22302419] [DOI] [PubMed] [Google Scholar]
Bellamy 1997
- Bellamy N, Kirwan J, Boers M, Brooks P, Strand V, Tugwell P, et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis. Consensus development at OMERACT III. Journal of Rheumatology 1997;24(4):799‐802. [PUBMED: 9101522] [PubMed] [Google Scholar]
Boutron 2006
- Boutron I, Estellat C, Guittet L, Dechartres A, Sackett DL, Hróbjartsson A, et al. Methods of blinding in reports of randomized controlled trials assessing pharmacologic treatments: a systematic review. PLoS Medicine 2006;3(10):e425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutron 2007
- Boutron I, Guittet L, Estellat C, Moher D, Hrobjartsson A, Ravaud P. Reporting methods of blinding in randomized trials assessing nonpharmacological treatments. PLoS Medicine 2007;4(2):e61. [PUBMED: 17311468] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dechartres 2009
- Dechartres A, Boutron I, Roy C, Ravaud P. Inadequate planning and reporting of adjudication committees in clinical trials: recommendation proposal. Journal of Clinical Epidemiology 2009;62(7):695‐702. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [PUBMED: 3802833] [DOI] [PubMed] [Google Scholar]
Eriksson 2012
- Eriksson BI, Smith JJ, Caprini J, Hantel S, Clemens A, Feuring M, et al. Evaluation of the acute coronary syndrome safety profile of dabigatran etexilate in patients undergoing major orthopedic surgery: findings from four Phase 3 trials. Thrombosis Research 2012;130(3):396‐402. [DOI] [PubMed] [Google Scholar]
Granger 2008
- Granger CB, Vogel V, Cummings SR, Held P, Fiedorek F, Lawrence M, et al. Do we need to adjudicate major clinical events?. Clinical Trials 2008;5(1):56‐60. [PUBMED: 18283081] [DOI] [PubMed] [Google Scholar]
Hansson 1992
- Hansson L, Hedner T, Dahlof B. Prospective randomized open blinded end‐point (PROBE) study. A novel design for intervention trials. Prospective Randomized Open Blinded End‐Point. Blood Pressure 1992;1(2):113‐9. [PUBMED: 1366259] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [PUBMED: 22008217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Horsley 2011
- Horsley T, Dingwall O, Sampson M. Checking reference lists to find additional studies for systematic reviews. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.MR000026.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hróbjartsson 2012
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non‐blinded outcome assessors. BMJ 2012;344:e1119. [DOI] [PubMed] [Google Scholar]
Ioannidis 2014
- Ioannidis JP, Greenland S, Hlatky MA, Khoury MJ, Macleod MR, Moher D, et al. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 2014;383(9912):166‐75. [PUBMED: 24411645 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2012
- Lewis JR, Zhu K, Prince RL. Adverse events from calcium supplementation: relationship to errors in myocardial infarction self‐reporting in randomized controlled trials of calcium supplementation. Journal of Bone and Mineral Research 2012;27(3):719–22. [DOI: 10.1002/jbmr.1484] [DOI] [PubMed] [Google Scholar]
Lopes 2013
- Lopes RD, Dickerson S, Hafley G, Burns S, Tourt‐Uhlig S, White J, et al. Methodology of a reevaluation of cardiovascular outcomes in the RECORD trial: study design and conduct. Amercan Heart Journal 2013;166(2):208‐16 e28. [PUBMED: 23895802] [DOI] [PubMed] [Google Scholar]
Mahaffey 2001a
- Mahaffey KW, Harrington RA, Akkerhuis M, Kleiman NS, Berdan LG, Crenshaw BS, et al. Systematic adjudication of myocardial infarction end‐points in an international clinical trial. Current Controlled Trials in Cardiovascular Medicine 2001;2(4):180‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahaffey 2001b
- Mahaffey KW, Harrington RA, Akkerhuis M, Kleiman NS, Berdan LG, Crenshaw BS, et al. Disagreements between central clinical events committee and site investigator assessments of myocardial infarction endpoints in an international clinical trial: review of the PURSUIT study. Current Controlled Trials in Cardiovascular Medicine 2001;2(4):187‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahaffey 2002
- Mahaffey KW, Roe MT, Dyke CK, Newby LK, Kleiman NS, Connolly P, et al. Misreporting of myocardial infarction end points: results of adjudication by a central clinical events committee in the PARAGON‐B trial. Second Platelet IIb/IIIa Antagonist for the Reduction of Acute Coronary Syndrome Events in a Global Organization Network Trial. American Heart Journal 2002;143(2):242‐8. [DOI] [PubMed] [Google Scholar]
Moustgaard 2014
- Moustgaard H, Bello S, Miller FG, Hróbjartsson A. Subjective and objective outcomes in randomized clinical trials: definitions differed in methods publications and were often absent from trial reports. Journal of Clinical Epidemiology 2014;67(12):1327‐34. [DOI] [PubMed] [Google Scholar]
Naslund 1999
- Naslund U, Grip L, Fischer‐Hansen J, Gundersen T, Lehto S, Wallentin L. The impact of an end‐point committee in a large multicentre, randomized, placebo‐controlled clinical trial: results with and without the end‐point committee's final decision on end‐points. European Heart Journal 1999;20(10):771‐7. [PUBMED: 10329069] [DOI] [PubMed] [Google Scholar]
Nissen 2013
- Nissen SE. Rosiglitazone: a case of regulatory hubris. BMJ 2013;347:f7428. [ 24335808] [DOI] [PubMed] [Google Scholar]
O'Connor 2005
- O'Connor CM, Gottlieb S, Bourque JM, Krause‐Steinrauf H, Anand I, Anderson JL, et al. Impact of nonfatal myocardial infarction on outcomes in patients with advanced heart failure and the effect of bucindolol therapy. American Journal of Cardiology 2005;95(5):558‐64. [DOI] [PubMed] [Google Scholar]
Pogue 2009
- Pogue J, Walter SD, Yusuf S. Evaluating the benefit of event adjudication of cardiovascular outcomes in large simple RCTs. Clinical Trials 2009;6(3):239‐51. [PUBMED: 19528133] [DOI] [PubMed] [Google Scholar]
Psaty 2010
- Psaty BM, Prentice RL. Minimizing bias in randomized trials: the importance of blinding. JAMA 2010;304(7):793‐4. [PUBMED: 20716744] [DOI] [PubMed] [Google Scholar]
Savović 2012
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Serebruany 2012
- Serebruany VL, Atar D. Viewpoint: Central adjudication of myocardial infarction in outcome‐driven clinical trials‐‐common patterns in TRITON, RECORD, and PLATO?. Thrombosis and Haemostasis 2012;108(3):412‐4. [PUBMED: 22836596] [DOI] [PubMed] [Google Scholar]
Stuck 2014
- Stuck AK, Fuhrer E, Limacher A, Mean M, Aujesky D. Adjudication‐related processes are underreported and lack standardization in clinical trials of venous thromboembolism: a systematic review. Journal of Clinical Epidemiology 2014;67(3):278‐84. [PUBMED: 24290147] [DOI] [PubMed] [Google Scholar]
Vannabouathong 2011
- Vannabouathong C, Sprague S, Bhandari M. Guidelines for fracture healing assessments in clinical trials. Part I: definitions and endpoint committees. Injury 2011;42(3):314‐6. [DOI] [PubMed] [Google Scholar]
Vannabouathong 2012
- Vannabouathong C, Saccone M, Sprague S, Schemitsch EH, Bhandari M. Adjudicating outcomes: fundamentals. Journal of Bone and Joint Surgery. American Volume 2012;94(Suppl 1):70‐4. [PUBMED: 22810452] [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Ndounga Diakou 2015
- Ndounga Diakou LA, Trinquart L, Hróbjartsson A, Barnes C, Yavchitz A, Ravaud P, et al. Comparison of central adjudication of outcomes and onsite outcome assessment on treatment effect estimates. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.MR000043] [DOI] [PMC free article] [PubMed] [Google Scholar]


