ABSTRACT
Objective
EXTrauterine Environment for Neonatal Development (EXTEND) is a system to support ongoing fetal growth and organ development in an extrauterine environment, utilizing a pumpless low‐resistance oxygenator circuit. The aim of this study was to evaluate hemodynamics and cardiac function in fetal sheep sustained on the EXTEND system.
Methods
This was a prospective study of fetal sheep supported for a minimum of 3 weeks on EXTEND. Hemodynamic parameters were assessed weekly and included heart rate, mean arterial pressure (MAP), Doppler‐echocardiography‐derived cardiac output (CO), pulsatility indices (PIs) of the fetal middle cerebral artery (MCA), umbilical artery (UA) and ductus venosus and cardiac function, as assessed by speckle‐tracking‐derived global longitudinal strain and strain rate in the right (RV) and left (LV) ventricles. Parameters were compared at 0 days and 1, 2 and 3 weeks following placement on EXTEND.
Results
Of 10 fetal sheep enrolled, seven survived for 3 weeks and were included in the analysis. Median gestational age at cannulation was 107 (range, 95–109) days. Heart rate decreased and MAP increased significantly, but within acceptable ranges, during the study period. The quantities and relative ratios of right and left CO remained stable within the anticipated physiological range throughout the study period. Vascular tracings and PIs appeared to be similar to those seen normally in the natural in‐utero state, with MCA‐PI being higher than UA‐PI. UA tracings demonstrated maintained abundant diastolic flow despite the absence of placental circulation. In both the RV and LV, strain decreased significantly at 1 and 2 weeks relative to baseline but returned to baseline values by week 3.
Conclusions
The EXTEND mechanical support system replicates natural physiology and creates a stable and sustainable cardiovascular construct that supports growth over a 3‐week period. However, there is a period of depressed contractility within the first week with subsequent improvement by week 3. This may reflect a period of physiological accommodation that warrants further investigation. This study lays the foundation for further exploration as the EXTEND system moves towards human application. © 2019 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of the International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: EXTrauterine Environment for Neonatal Development, fetal cardiac function, fetal echocardiography, speckle tracking, strain, strain rate
CONTRIBUTION —
What are the novel findings of this work?
Fetal sheep can be sustained on a mechanical support system that replaces the placenta, maintains a stable cardiovascular state and replicates natural physiology to support growth over a period of 3 weeks. There is an initial phase of depressed myocardial contractility within the first week that improves by week 3.
What are the clinical implications of this work?
The EXTrauterine Environment for Neonatal Development (EXTEND) system offers the promise of supporting a premature infant through maintenance of fetal physiology and bridging to improved maturity. As we move towards application in humans, exploration of the impact of this mechanical support on the cardiovascular system and other organs will be of importance.
INTRODUCTION
Creation of a physiological extrauterine environment to support ongoing fetal growth and organ development, without connection to a biological placenta, would have the potential to substantially improve fetal survival and reduce the morbidity of prematurity. We have reported previously the development of such a system (EXTEND; EXTrauterine Environment Neonatal Development) which utilizes a pumpless low‐resistance oxygenator circuit in fetal sheep and allows survival for up to 4 weeks with good somatic growth and apparent normal organ development1. As EXTEND sustains growth and development in an unnatural state, understanding the effects on cardiovascular physiology is of importance. Theoretically, the circuit may alter loading conditions and cardiac function as an artificial arteriovenous shunt, with the potential for high‐output cardiac failure2. Alternatively, it may create afterload mismatch if the circuit imparts supraphysiological resistance. Such manifestations may impact fetal wellbeing negatively if present in a sustained manner while on EXTEND.
Numerous echocardiographic parameters characterizing the clinical status of the fetal cardiovascular system currently exist. Doppler ultrasound techniques are used routinely to assess placental and fetal cerebrovascular flow and vascular resistance through calculation of the pulsatility index (PI) in the umbilical artery (UA) and fetal middle cerebral artery (MCA), respectively. Cardiac function can be assessed using two‐dimensional (2D) strain and strain rate (SR), obtained using speckle‐tracking analysis for evaluation of myocardial deformation, and is relatively independent of ultrasound beam angulation3, 4. Exploration of these parameters in fetuses on EXTEND would be of significant value as we begin to assess the impact of the EXTEND system on organ functionality and natural development.
The fetal cardiovascular impact of sustained exposure to support provided via the EXTEND system is unknown. The purpose of this study was to evaluate hemodynamics and cardiac function in fetal sheep while on sustained support using the EXTEND system. Specifically, we aimed to explore the circulatory parameters of fetal MCA‐PI and UA‐PI as a reflection of vascular impedance, and investigate cardiac function as characterized by myocardial speckle‐tracking‐derived strain and SR in the right (RV) and left (LV) ventricles.
METHODS
This was a prospective study of fetal sheep in experiments performed between September 2017 and May 2018. Animals were housed, cared for and maintained as per guidelines and with approval of the Institutional Animal Care and Use Committee at The Children's Hospital of Philadelphia, PA, USA (IBC 15‐0000270). The inclusion criterion was survival for a minimum of 3 weeks on the EXTEND system. Fetal sheep undergoing induced hypoxia, growth restriction and chorioamnionitis/sepsis as part of other experimental protocols were excluded.
EXTrauterine Environment for Neonatal Development (EXTEND) system
The EXTEND system is a pumpless, arteriovenous oxygenator circuit connected via an umbilical vascular interface within a closed sterile fluid environment, with blood flow driven exclusively by the fetal heart, as described previously (Figure 1)1. The fetal lamb is exposed by Cesarean section. A double UA and single umbilical vein cannula connection is made between the fetus and a very low‐resistance oxygenator5. The fetal sheep is placed in a biobag closed system that continuously exchanges amniotic fluid and can be customized to replicate closely the size and shape of the uterus. The biobag consists of a polyethylene film that is translucent, sonolucent and flexible to permit monitoring, scanning and manipulation of the fetus as necessary. An open sealable side facilitates insertion of the fetus at the time of cannulation and various water‐tight ports are designed to accommodate cannulae, temperature probes and sterile tubing. After cannulation and fetal insertion, the biobag is sealed and transferred to a mobile support platform that incorporates temperature and pressure regulation, padding, fluid reservoirs and fluid exchange circuitry. Normal fetal oxygen delivery is maintained at between 18 and 25 mL/kg/min6 for physiological metabolic support, as well as substrate delivery of parenteral nutrition and dextrose administration for growth and development1.
Figure 1.
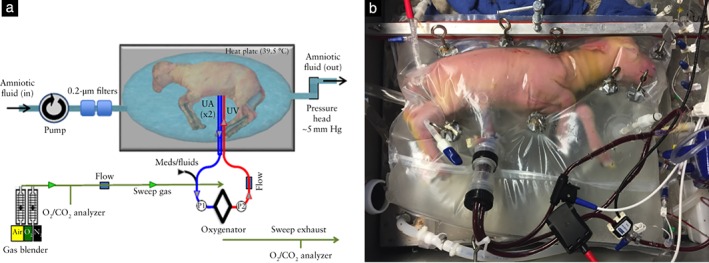
(a) EXTEND biobag system design. Circuit and system components include pumpless low‐resistance oxygenator, closed fluid environment with fluid exchange and umbilical vascular interface. (b) Lamb on EXTEND at 111 days' gestation. UA, umbilical artery; UV, umbilical vein.
Study parameters
Fetal sheep echocardiography was performed daily using an established protocol. All ultrasound parameters and images were acquired using a dedicated iE33 ultrasound system (Philips Healthcare, Bothell, WA, USA) with a S3–9‐MHz phased‐array transducer. Parameters collected included: heart rate; PI (calculated as peak systolic velocity − end‐diastolic velocity/mean velocity) of the fetal MCA, UA and ductus venosus (DV); cardiac output (CO) of each individual ventricle (calculated as: (diameter of the semilunar valve (cm) × 1/2)2 × 3.14 × velocity time integral (VTI; cm) × heart rate, indexed to body weight in kg); combined cardiac output (CCO; calculated as the sum of CO in both ventricles); and global longitudinal strain and SR of the LV and RV using speckle‐tracking analysis. For MCA sampling, the Doppler interrogation sampling gate was placed midway between the origin from the circle of Willis and the periphery of the skull; for the UA, as there was no placental attachment, the sampling gate was placed just distal to the origin of the UA from the internal iliac artery, within the fetal body prior to vessel exit into the biobag sac and cannulation site. DV flow was sampled at the site of vessel narrowing as the umbilical vein enters the body of the liver proximal to the pathway into the right atrium. Mean arterial pressure (MAP) measured proximal to the oxygenator and circuit flow volume through the oxygenator were collected and reported as a daily average.
Body weight of the fetal sheep in the biobag was estimated initially by ultrasound and then calculated assuming growth of 20 g/kg/day, as demonstrated previously1. Study parameters were reported and analyzed starting from when the fetus was placed into the biobag on the first day of EXTEND support (day 0) and then on the 7th day (± 1 day), 14th day (± 1 day) and 21st day (± 1 day). The partial pressure of oxygen (pO2), partial pressure of carbon dioxide (pCO2), pH, hemoglobin concentration and lactate concentrations were collected and reported as an average of all days while on support.
Speckle‐tracking analysis
Global longitudinal strain and SR were obtained using a 2D videoclip of a modified apical four‐chamber view of the heart that was stored for offline analysis. It was not possible to obtain a four‐chamber view with the apex vertically up or down due to the longitudinal position of the fetus within the biobag. However, we obtained readily images of a modified apical four‐chamber view, with the angle between the apex of the heart and ultrasound beam of approximately 30–45°. Care was taken to make certain that the same position and view of the heart was obtained in each animal at each study interval. Special caution was taken to obtain a clear image of the ventricular walls and a minimum frame rate of > 60 frames/s was aimed for. At least three cine loops of a modified four‐chamber view were acquired while the fetal sheep was not moving. DICOM datasets were transferred to a workstation for analysis using TomTec analysis software (Image Arena 2D version 4.6, TomTec Imaging Systems GmbH, Unterschleissheim, Germany). LV and RV endocardial borders were defined manually at end diastole and end systole and tracking curves of endocardial borders were created automatically by the software in subsequent frames. Automated segmentation provided segmental strain and SR for six segments as well as global longitudinal measures. Adequacy of tracking was assessed visually for each segment. As a high frame rate is required for high heart rate7, 8, strain and SR were obtained at 10‐ms intervals using cubic spline interpolation for all measurements9, 10. The average of the maximum global longitudinal strain and SR for three cardiac cycles was collected twice in both ventricles, using the same technique by a single investigator (K.O.), and intraobserver variability was calculated. The mean of the two measurements was used for analyzing strain and SR.
Statistical analysis
Hemodynamic parameters and strain and SR measures of cardiac function, obtained on the first day (day 0) and at 7, 14 and 21 days on EXTEND following placement into the biobag, were analyzed using repeated‐measures ANOVA and post‐hoc pairwise comparisons. Analysis was performed using Stata 15.1 software (Stata Corp., College Station, TX, USA). A P‐value < 0.05 was considered statistically significant.
RESULTS
Ten fetal sheep were enrolled for investigation, of which seven survived for 3 weeks and were included in the study analysis. Median gestational age at cannulation was 107 (range, 95–109) days. Median time on EXTEND was 22 (range, 21–27) days. Metabolic parameters were stable in the seven experimental animals throughout the study period, with samples obtained proximal to the oxygenator demonstrating pH of 7.40 ± 0.03 (mean ± SD), pO2 of 25.1 ± 6.7 mmHg, pCO2 of 39.1 ± 2.4 mmHg and hemoglobin of 11.7 ± 1.8 g/dL. Median serum lactate was 0.80 (range, 0.51–1.08) mmol/L. Weight gain was achieved in all seven animals over the 3‐week study period (P < 0.01) (Figure 2).
Figure 2.
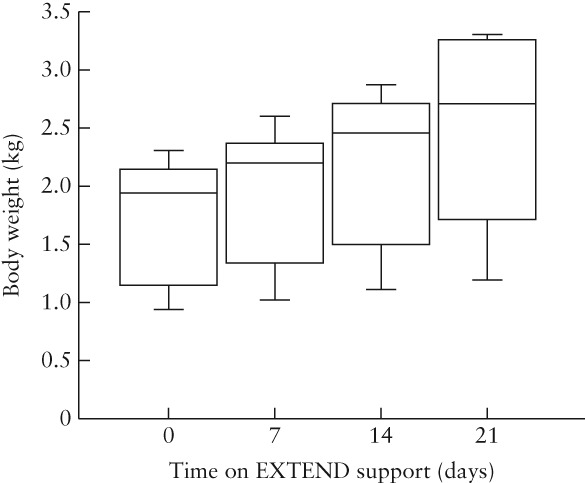
Box‐and‐whiskers plot of body weight estimated by ultrasound in seven fetal sheep at varying time intervals following placement on EXTEND support. Boxes are median and interquartile range and whiskers are range.
All imaging was performed during periods of sustained hemodynamic stability. Hemodynamic parameters on the first postoperative day (day 0) and at 1, 2 and 3 weeks are shown in Table 1. Heart rate decreased significantly and blood pressure increased over the study period. LV‐ and RV‐CO and CCO remained stable throughout the 3‐week study period. The ratio of RV‐ to LV‐CO remained stable at 1.41 to 1.44. With the decrease in heart rate, but stable CO, stroke volume increased. The quantity of circuit flow (equivalent to placental blood flow) as well as the ratio of circuit flow to CCO did not change.
Table 1.
Hemodynamic and physiological parameters in seven fetal sheep at different time intervals following placement on EXTEND support
| Parameter | Time since placement on EXTEND support | F | P | |||
|---|---|---|---|---|---|---|
| Day 0 | Week 1 | Week 2 | Week 3 | |||
| HR (bpm) | 184 ± 20 | 189 ± 7 | 168 ± 22 | 152 ± 15 | 6.52 | 0.002* |
| MAP (mmHg) | 28.0 ± 8.0 | 32.4 ± 4.5 | 32.7 ± 2.9 | 37.4 ± 5.7 | 3.28 | 0.04† |
| LV‐CO (mL/min/kg) | 215 ± 42 | 218 ± 35 | 213 ± 36 | 212 ± 50 | 0.04 | 0.99 |
| RV‐CO (mL/min/kg) | 309 ± 81 | 310 ± 57 | 301 ± 56 | 299 ± 73 | 0.04 | 0.98 |
| CCO (mL/min/kg) | 524 ± 122 | 528 ± 92 | 514 ± 92 | 511 ± 123 | 0.04 | 0.99 |
| Circuit flow (mL/min/kg) | 208 ± 37 | 247 ± 101 | 213 ± 98 | 193 ± 103 | 0.45 | 0.72 |
| Circuit flow/CCO | 0.41 ± 0.09 | 0.45 ± 0.10 | 0.40 ± 0.11 | 0.36 ± 0.10 | 0.98 | 0.42 |
| UA‐PI | 0.46 ± 0.14 | 0.46 ± 0.09 | 0.41 ± 0.09 | 0.55 ± 0.09 | 2.35 | 0.1 |
| MCA‐PI | 0.83 ± 0.20 | 0.81 ± 0.27 | 0.69 ± 0.19 | 0.76 ± 0.12 | 0.63 | 0.61 |
| DV‐PI | 0.43 ± 0.11 | 0.46 ± 0.19 | 0.41 ± 0.05 | 0.45 ± 0.21 | 0.14 | 0.93 |
Data are given as mean ± SD.
Variables compared between time intervals using repeated‐measures ANOVA with post‐hoc pairwise comparisons performed in case of significant difference.
Post‐hoc comparison showed significant difference compared with 3 weeks at:
0 days (P = 0.01) and 1 week (P = 0.004); and
0 days (P = 0.03).
CCO, combined cardiac output divided by body weight; CO, cardiac output divided by body weight; DV, ductus venosus; HR, heart rate; LV, left ventricle; MAP, mean arterial pressure proximal to oxygenator; MCA, middle cerebral artery; PI, pulsatility index; RV, right ventricle; UA, umbilical artery.
MCA‐PI, UA‐PI and DV‐PI values remained stable over the 3‐week study period. MCA and UA Doppler flow‐pattern waveforms appeared similar to those normally seen in the natural in‐utero state, with respect to MCA‐PI being higher than UA‐PI, thus quantifiably reflecting increased diastolic flow in the UA in comparison to lower diastolic flow in the MCA. Of note, despite the abundance of diastolic flow in the UA, an early diastolic notch was uniformly present. DV flow patterns appeared phasic, responding to cardiac cycle events and similar to normal in‐utero patterns (Figure 3).
Figure 3.
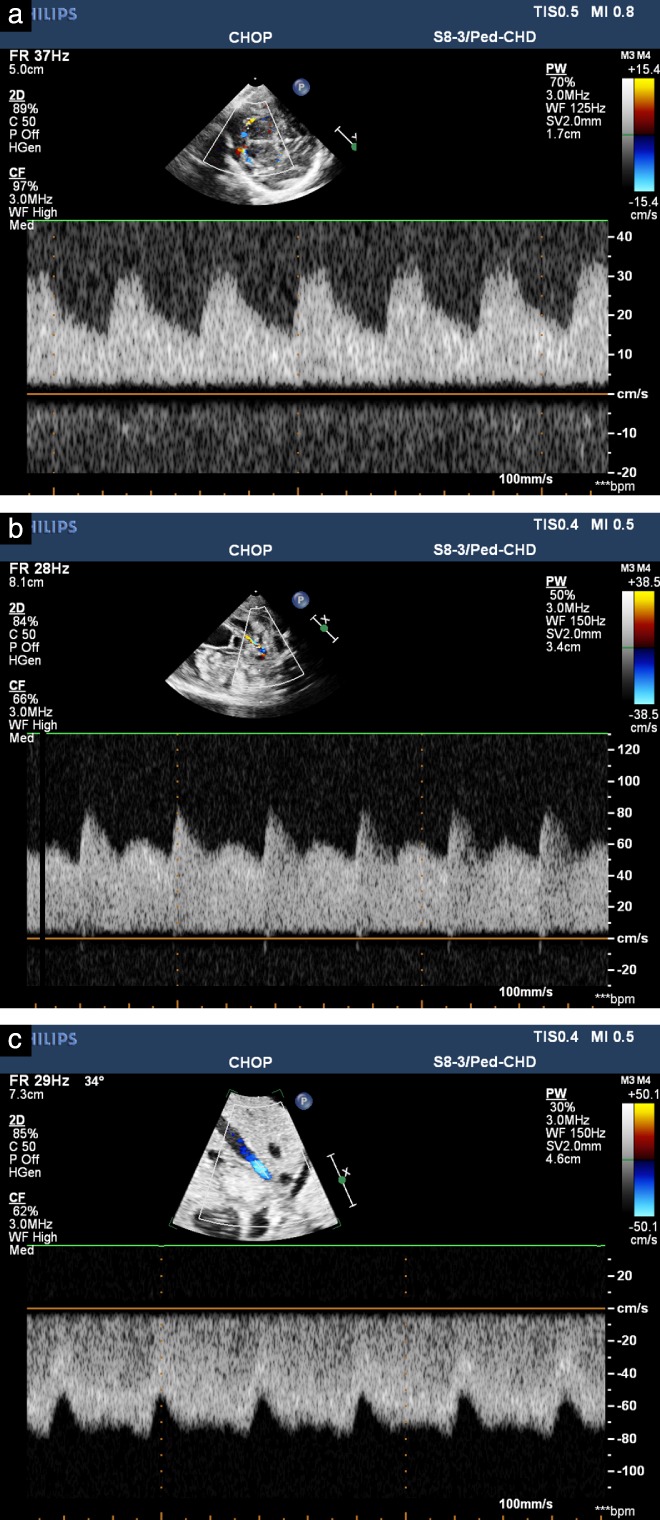
Doppler‐echocardiography‐derived spectral tracings in middle cerebral artery (MCA) (a), umbilical artery (UA) (b) and ductus venosus (c) obtained from fetal lamb on EXTEND support. Patterns appear similar to those acquired in utero in normal fetal lamb. Note abundance of diastolic flow seen in UA as compared to MCA, thus resulting in higher pulsatility in MCA than in UA, as in normal in‐utero state.
No obvious depression in systolic RV or LV contractility was noted on subjective qualitative 2D fetal echocardiographic assessment and there was no evidence of significant tricuspid or mitral regurgitation on color Doppler imaging at any timepoint. There was no evidence for ductal constriction and velocity in the ductus arteriosus was < 100 cm/s on all assessments. Myocardial strain and SR on the first day and at 1, 2 and 3 weeks on EXTEND are shown in Figure 4. The median frame rate for measurements was 61 (range, 60–131) frames/s. The average (± SD) difference between the maximum values of LV and RV strain and LV‐ and RV‐SR obtained using cubic spline interpolation and those obtained just from the software for analysis were 0.13 ± 0.10, 0.18 ± 0.14, 0.03 ± 0.02 and 0.02 ± 0.01, respectively. In both the RV and LV, strain decreased significantly (diminished negative value) at 1 and 2 weeks relative to baseline (day 0). Strain returned to baseline values by 3 weeks in both the RV and LV. No difference in LV strain was noted between 1 and 2 weeks and between 2 and 3 weeks, while RV strain demonstrated an increase (increased negative value) between 1 and 3 weeks. LV‐SR did not change during the study period, while RV‐SR at 1 week was significantly lower than at baseline (day 0), but was increased at 3 weeks compared to 1 week, similar to the pattern seen for RV strain. Interclass correlation coefficients for intraobserver variability of measurements of LV and RV strain and LV‐ and RV‐SR were 0.973 (95% CI, 0.947–0.986), 0.978 (95% CI, 0.957–0.989), 0.936 (95% CI, 0.879–0.967) and 0.976 (95% CI, 0.954–0.977), respectively.
Figure 4.
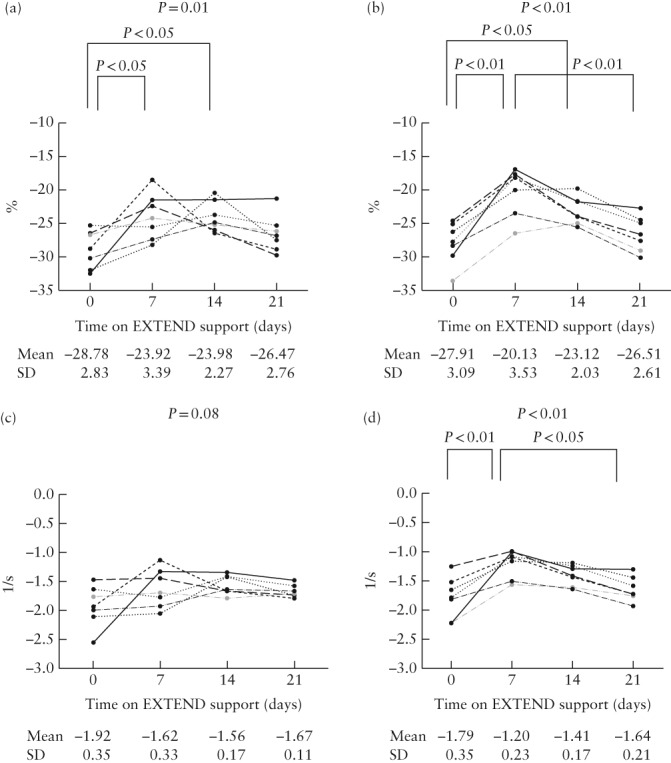
Individual global longitudinal strain (a,b) and strain rate (c,d) values in left (a,c) and right (b,d) ventricles in seven fetal sheep at varying time intervals following placement on EXTEND support. More negative strain/strain rate value indicates greater strain/strain rate, and vice versa. Overall P‐value reflects repeated‐measures ANOVA and P‐values over brackets reflect significant post‐hoc pairwise comparisons.
Three of the 10 enrolled fetal sheep (gestational ages 95, 108 and 109 days) were excluded from the above analysis due to death between weeks 1 and 2 of the study. The main reason for death was low mechanical circuit flow due to umbilical vascular spasm. We were not able to obtain ultrasound data immediately before the unanticipated death; however, data at baseline and 1 week were available. Compared with survivors, no differences in parameters at baseline were noted. However, at 1 week, ultrasound showed a trend towards a lower ratio of circuit flow to CCO (0.346 ± 0.09; P = 0.09), higher UA‐PI (0.67 ± 0.18; P = 0.03) and a higher DV‐PI (0.73 ± 0.29; P = 0.07) in the three non‐survivors compared to survivors. No other differences in hemodynamic parameters were noted and strain and SR values were not different between non‐survivors and survivors.
DISCUSSION
This study has demonstrated that EXTEND approximates the natural in‐utero state11 and creates a stable, sustainable hemodynamic construct for 3 weeks; however, there are notable effects on fetal cardiovascular physiology. Ventricular output ratios reflected RV dominance, as is the case in the normal in‐utero state; however, we found a RV‐ to LV‐CO ratio of approximately 1.4 to 1, which is lower than that found by Rudolph and Heymann12 using microsphere techniques, or by Acharya et al.13 using Doppler techniques. This may be inherent to EXTEND, explained by the slightly increased vascular resistance of the mechanical oxygenator compared to a natural placenta, with resistance imposed more selectively upon the RV. DV flow patterns and PI values (range, 0.41–0.46) were maintained throughout the study period, identical to those in the natural ovine fetus14. Little information exists on MCA flow in the fetal sheep. However, carotid flow PI is normally higher than UA‐PI15. In fetuses on EXTEND, we similarly found cerebrovascular resistance, as measured by MCA‐PI, to be consistently higher than UA‐PI, indicating preservation of the natural ratio of vascular tone and blood flow distribution between the brain and umbilical circulations. Umbilical cord midloop interrogation of a natural UA was not possible in our model. Nevertheless, we were eager to characterize UA flow and the vascular response to a circuit oxygenator, and thus measured the PI in the UA at the point of origin from the internal iliac artery, proximal to its exit from the body. PI at this site is similar to that found in the ovine UA in a natural state15. An important determinant of diastolic UA flow is the downstream low‐resistance sink of the placental vasculature. The finding of an abundance of diastolic flow in our UA sampling site, despite the absence of a placenta, suggests that the mechanical oxygenator circuit resistance is indeed relatively low. However, uniform appearance of an early diastolic notch may reflect a lack of biological compliance, as is normally exhibited in healthy placental vasculature16, and may be due to downstream physical properties of the cannulae or non‐compliant passages within the oxygenator17. Additional factors may include downstream impedance in the DV or upstream elastic properties that influence diastolic recoil of the iliac arteries at the origin of the UAs. Using EXTEND to manipulate these variables experimentally, to model the circulation and study the magnitude of their impact on UA Doppler flow, provides a research tool with which to explore growth restriction, placental insufficiency and other cardiovascular conditions.
Global longitudinal strain and SR assessed by 2D speckle‐tracking analysis is a sophisticated tool for assessing cardiac function in normal fetuses and fetuses with a variety of conditions18, 19, 20, 21, 22, 23, 24, 25, 26. Global longitudinal strain is also reported for assessing the effect of hypoxemia on cardiac function in fetal sheep27. We found that RV and LV strain have worsened at 1 week on EXTEND, compared to baseline, but then gradually improve by week 3. SR in the LV did not change, but in the RV it changed in a parallel manner to strain. As expected, the RV is apparently more affected than is the LV in fetuses on EXTEND. Generally, the fetal RV is influenced by the ductal arch and lower body vasculature, while the LV ejects more selectively to the upper body vasculature and cerebral circulation. Circuit flow, which is the equivalent of placental blood flow, was measured to be 36–45% of overall CO. We speculate that, at placement on EXTEND, RV contractility is impacted to a greater degree than is LV contractility, due to acute removal of the low‐resistance placental vasculature, with the burden of circuit flow imposed predominantly upon the RV. The LV is less perturbed as it perfuses selectively the upper body and cerebral circulations.
Placement on EXTEND may perturb the natural state to a greater degree in our younger animals. Overall, MAP on day 0 was lower than that in the subsequent weeks. Two of the youngest animals (95 and 104 days) exhibited MAP < 20 mmHg, while the others exhibited MAP > 30 mmHg. The trend over 3 weeks demonstrates a decrease in heart rate and increase in blood pressure with steady maintenance of CCO, indicating an increase in stroke volume. Sustained support on EXTEND does not impart any significant deleterious effects on the fetal cardiovascular system. However, there appears to be a period of depressed contractility within the first week, with subsequent improvement by week 3. This important finding focuses our attention on a potential period of physiological accommodation and myocardial adaptation that warrants further exploration.
There are limitations to this study. We did not perform fetal echocardiography in the natural state, prior to placement on EXTEND; thus, we cannot evaluate the acute physiological impact. Absolute values for blood flow in our model may be notably different from those in earlier published series of in‐utero hemodynamic research in whole animals. This may be due to differences in experimental methodologies, conditions and techniques of determining blood flow or possibly due to fundamental differences between mechanical placental support and natural connection to a biological placenta. Observations made in this study are in the group of survivors. Within this temporal cohort, three fetal sheep died due to technical failure (umbilical vascular spasm) at less than 3 weeks on EXTEND and were thus not included, creating possible selection bias towards more robust fetal sheep. Fetal death was not predictable based on initial baseline data but, by week 1, some differences reflecting vascular instability emerged. Fetal sheep were placed on EXTEND at a median gestational age of 107 days, which, from the perspective of lung development, is at the mid‐to‐late canalicular phase, the equivalent of a 22–24‐week human fetus28. Thus, our data inform on the responsiveness of the fetal cardiovascular system within a specific period of gestation. Due to differences in developmental maturity, the response to EXTEND may vary by gestational age.
Fetal sheep on EXTEND for 3 weeks have circulatory resistance patterns similar to normal and sustain fetal CO for growth. Myocardial contractility, mostly of the RV, is diminished early on but recovers. Further investigation of the impact of EXTEND on the cardiovascular system and other organs is underway29, as we move toward the aspiration of applying this novel technology in the human fetus.
ACKNOWLEDGMENTS
We wish to acknowledge the fetal sheep imaging contributions of Drs S. Zhao and Z. Gou. Dr Rychik's efforts are supported by the Robert and Dolores Harrington Endowed Chair in Pediatric Cardiology at The Children's Hospital of Philadelphia.
Disclosure
M.G.D. and A.W.F. hold patents on technologies included in this report.
REFERENCES
- 1. Partridge EA, Davey MG, Hornick MA, McGovern PE, Mejaddam AY, Vrecenak JD, Mesas‐Burgos C, Olive A, Caskey RC, Weiland TR, Han J, Schupper AJ, Connelly JT, Dysart KC, Rychik J, Hedrick HL, Peranteau WH, Flake AW. An extra‐uterine system to physiologically support the extreme premature lamb. Nat Commun 2017; 8: 15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Byrne FA, Lee H, Kipps AK, Brook MM, Moon‐Grady AJ. Echocardiographic risk stratification of fetuses with sacrococcygeal teratoma and twin‐reversed arterial perfusion. Fetal Diagn Ther 2011; 30: 280–288. [DOI] [PubMed] [Google Scholar]
- 3. Forsha D, Risum N, Rajagopal S, Dolgner S, Hornik C, Barnhart H, Kisslo J, Barker P. The influence of angle of insonation and target depth on speckle‐tracking strain. J Am Soc Echocardiogr 2015; 28: 580–586. [DOI] [PubMed] [Google Scholar]
- 4. Langeland S, D'Hooge J, Wouters PF, Leather HA, Claus P, Bijnens B, Sutherland GR. Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation 2005; 112: 2157–2162. [DOI] [PubMed] [Google Scholar]
- 5. Hornick MA, Davey MG, Partridge EA, Mejaddam AY, McGovern PE, Olive AM, Hwang G, Kim J, Castillo O, Young K, Han J, Zhao S, Connelly JT, Dysart KC, Rychik J, Peranteau WH, Flake AW. Umbilical cannulation optimizes circuit flows in premature lambs supported by the EXTra‐uterine Environment for Neonatal Development (EXTEND). J Physiol 2018; 596: 1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Itskovitz J, LaGamma EF, Rudolph AM. The effect of reducing umbilical blood flow on fetal oxygenation. Am J Obstet Gynecol 1983; 145: 813–818. [DOI] [PubMed] [Google Scholar]
- 7. DeVore GR, Polanco B, Satou G, Sklansky M. Two‐Dimensional Speckle Tracking of the Fetal Heart: A Practical Step‐by‐Step Approach for the Fetal Sonologist. J Ultrasound Med 2016; 35: 1765–1781. [DOI] [PubMed] [Google Scholar]
- 8. Enzensberger C, Achterberg F, Graupner O, Wolter A, Herrmann J, Axt‐Fliedner R. Wall‐motion tracking in fetal echocardiography‐Influence of frame rate on longitudinal strain analysis assessed by two‐dimensional speckle tracking. Echocardiography 2017; 34: 898–905. [DOI] [PubMed] [Google Scholar]
- 9. Ledesma‐Carbayo MJ, Kybic J, Desco M, Santos A, Suhling M, Hunziker P, Unser M. Spatio‐temporal nonrigid registration for ultrasound cardiac motion estimation. IEEE Trans Med Imaging 2005; 24: 1113–1126. [DOI] [PubMed] [Google Scholar]
- 10. Takeuchi M, Nakai H, Kokumai M, Nishikage T, Otani S, Lang RM. Age‐related changes in left ventricular twist assessed by two‐dimensional speckle‐tracking imaging. J Am Soc Echocardiogr 2006; 19: 1077–1084. [DOI] [PubMed] [Google Scholar]
- 11. Partridge EA, Davey MG, Hornick MA, Flake AW. An EXTrauterine environment for neonatal development: EXTENDING fetal physiology beyond the womb. Semin Fetal Neonatal Med 2017; 22: 404–409. [DOI] [PubMed] [Google Scholar]
- 12. Rudolph AM, Heymann MA. The circulation of the fetus in utero. Methods for studying distribution of blood flow, cardiac output and organ blood flow. Circ Res 1967; 21: 163–184. [DOI] [PubMed] [Google Scholar]
- 13. Acharya G, Erkinaro T, Makikallio K, Lappalainen T, Rasanen J. Relationships among Doppler‐derived umbilical artery absolute velocities, cardiac function, and placental volume blood flow and resistance in fetal sheep. Am J Physiol Heart Circ Physiol 2004; 286: H1266–1272. [DOI] [PubMed] [Google Scholar]
- 14. Schmidt KG, Silverman NH, Rudolph AM. Assessment of flow events at the ductus venosus‐inferior vena cava junction and at the foramen ovale in fetal sheep by use of multimodal ultrasound. Circulation 1996; 93: 826–833. [DOI] [PubMed] [Google Scholar]
- 15. Sonesson SE, Fouron JC, Teyssier G, Bonnin P. Effects of increased resistance to umbilical blood flow on fetal hemodynamic changes induced by maternal oxygen administration: a Doppler velocimetric study on the sheep. Pediatr Res 1993; 34: 796–800. [DOI] [PubMed] [Google Scholar]
- 16. Abuhamad A, Sclater AJ, Carlson EJ, Moriarity RP, Aguiar MA. Umbilical artery Doppler waveform notching: is it a marker for cord and placental abnormalities? J Ultrasound Med 2002; 21: 857–860. [DOI] [PubMed] [Google Scholar]
- 17. Tejada‐Martinez AE, Borberg CJ, Venugopal R, Carballo C, Moreno WA, Quintero RA. Computational fluid dynamic analysis of flow velocity waveform notching in umbilical arteries. Am J Physiol Regul Integr Comp Physiol 2011; 300: R76–84. [DOI] [PubMed] [Google Scholar]
- 18. Rychik J, Zeng S, Bebbington M, Szwast A, Quartermain M, Natarajan S, Johnson M, Tian Z. Speckle tracking‐derived myocardial tissue deformation imaging in twin‐twin transfusion syndrome: differences in strain and strain rate between donor and recipient twins. Fetal Diagn Ther 2012; 32: 131–137. [DOI] [PubMed] [Google Scholar]
- 19. Van Mieghem T, Giusca S, DeKoninck P, Gucciardo L, Done E, Hindryckx A, D'Hooge J, Deprest J. Prospective assessment of fetal cardiac function with speckle tracking in healthy fetuses and recipient fetuses of twin‐to‐twin transfusion syndrome. J Am Soc Echocardiogr 2010; 23: 301–308. [DOI] [PubMed] [Google Scholar]
- 20. Germanakis I, Matsui H, Gardiner HM. Myocardial strain abnormalities in fetal congenital heart disease assessed by speckle tracking echocardiography. Fetal Diagn Ther 2012; 32: 123–130. [DOI] [PubMed] [Google Scholar]
- 21. Ishii T, McElhinney DB, Harrild DM, Marcus EN, Sahn DJ, Truong U, Tworetzky W. Circumferential and longitudinal ventricular strain in the normal human fetus. J Am Soc Echocardiogr 2012; 25: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kapusta L, Mainzer G, Weiner Z, Deutsch L, Khoury A, Haddad S, Lorber A. Second trimester ultrasound: reference values for two‐dimensional speckle tracking‐derived longitudinal strain, strain rate and time to peak deformation of the fetal heart. J Am Soc Echocardiogr 2012; 25: 1333–1341. [DOI] [PubMed] [Google Scholar]
- 23. Ishii T, McElhinney DB, Harrild DM, Marcus EN, Sahn DJ, Truong U, Tworetzky W. Ventricular strain in fetuses with aortic stenosis and evolving hypoplastic left heart syndrome before and after prenatal aortic valvuloplasty. Fetal Diagn Ther 2014; 35: 18–26. [DOI] [PubMed] [Google Scholar]
- 24. Miranda JO, Cerqueira RJ, Ramalho C, Areias JC, Henriques‐Coelho T. Fetal Cardiac Function in Maternal Diabetes: A Conventional and Speckle‐Tracking Echocardiographic Study. J Am Soc Echocardiogr 2018; 31: 333–341. [DOI] [PubMed] [Google Scholar]
- 25. Truong UT, Sun HY, Tacy TA. Myocardial deformation in the fetal single ventricle. J Am Soc Echocardiogr 2013; 26: 57–63. [DOI] [PubMed] [Google Scholar]
- 26. Brooks PA, Khoo NS, Hornberger LK. Systolic and diastolic function of the fetal single left ventricle. J Am Soc Echocardiogr 2014; 27: 972–977. [DOI] [PubMed] [Google Scholar]
- 27. Bhide A, Rasanen J, Huhta H, Junno J, Erkinaro T, Ohtonen P, Haapsamo M, Acharya G. Effect of Hypoxemia on Fetal Ventricular Deformation in a Chronically Instrumented Sheep Model. Ultrasound Med Biol 2017; 43: 967–973. [DOI] [PubMed] [Google Scholar]
- 28. Joshi S, Kotecha S. Lung growth and development. Early Hum Dev 2007; 83: 789–794. [DOI] [PubMed] [Google Scholar]
- 29. Lawrence KM, Hennessy‐Strahs S, McGovern PE, Mejaddam AY, Rossidis AC, Baumgarten HD, Bansal E, Villeda M, Han J, Gou Z, Zhao S, Rychik J, Peranteau WH, Davey MG, Flake AW, Gaynor JW, Bartoli CR. Fetal hypoxemia causes abnormal myocardial development in a preterm ex‐utero fetal ovine model. JCI Insight 2018; 3: e124338. [DOI] [PMC free article] [PubMed] [Google Scholar]


