Abstract
There is an ongoing discussion regarding the impact of adjuvant chemotherapy in Stage II colon cancer. We therefore estimated adjuvant treatment effect in Stage II colon cancer using pooled disease‐free survival (DFS) data from randomized clinical trials (RCT approach) and compared this to real‐world data (RWD approach) estimates. First, we estimated the treatment effect in RCTs by (i) searching relevant trials reporting DFS data, (ii) generating patient‐level data from reported DFS data and (iii) estimating treatment effect in the patient‐level data. Second, the treatment effect was estimated in an observational cohort of 1,947 patients provided by the Netherlands Cancer Registry using three propensity score methods; matching, weighting and stratification. In the RCT approach, patient‐level data of 4,489 patients (events: 853) were generated from seven trials which compared two of the following treatment arms: control, 5FU/LV or FOLFOX. A Cox model was used to estimate a hazard ratio (HR) of 0.77 (0.43;1.10) for 5FU/LV vs. control and 0.93 (0.72;1.15) for FOLFOX vs. 5FU/LV. In the RWD approach, HRs for any adjuvant treatment vs. control were 0.95 (0.50;1.80), 0.88 (0.24;3.21) and 1.05 (0.04;2.06) using matching, weighting and stratification, respectively. There was no significant difference with the estimates from the RCT approach (interaction test, p > 0.10). The RCT data suggest a clinically relevant benefit of adjuvant chemotherapy in terms of DFS, but the estimate did not reach statistical significance. Stratified analyses are required to evaluate whether treatment effect differs in specific subgroups.
Keywords: colon cancer, treatment effect, randomized clinical trial, real‐world data, adjuvant chemotherapy
Short abstract
What's new?
There is an ongoing discussion regarding the impact of adjuvant chemotherapy in stage II colon cancer. This study presents the most recent pooled estimate based on available RCT data since 1999, resulting in a pooled hazard ratio of 0.77 (95% CI 0.43;1.10) for fluoropyrimidine compared to no treatment. Even though no significant treatment effect was found, neither in the RCT approach nor in the real‐world data approach, the RCT data suggest a clinically‐relevant benefit of adjuvant chemotherapy. To improve guidance in treatment decisions, larger sample sizes, pooling of true patient‐level data with covariate information, and subgroup specific analyses are required.
Abbreviations
- DFS
disease‐free survival
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- HR
hazard ratio
- NCR
Netherlands Cancer Registry
- OS
overall survival
- RCT
randomized clinical trial
- RWD
real‐world data
Introduction
There is an ongoing clinical dilemma of whether or not to provide adjuvant treatment to Stage II colon cancer patients after surgery. High‐risk patients who are eligible for adjuvant treatment are identified according to the European Society for Medical Oncology guidelines based on clinical and pathological factors. The most commonly used high‐risk factors include pT4 stage, less than 10–12 lymph nodes evaluated, the presence of perforation and/or obstruction, extramural vascular invasion, perineural invasion, poorly or undifferentiated tumor and mismatch repair status.1
Such guideline recommendations are developed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. GRADE assigns most value to randomized clinical trials (RCTs) and meta‐analyses, because of the methodologically strong character.2 An essential advantage of RCTs is the unbiased estimation of the treatment effect due to randomized allocation which can balance both observed and unobserved confounders. This randomized allocation is often combined with strict inclusion and exclusion criteria to minimize bias in the evaluation of treatment response.3, 4 Because of this strict patient selection in RCTs, the patient population may differ in daily clinical practice. Thus, treatment effect in clinical practice may be different from an RCT‐based treatment effect.3, 5, 6
Observational studies without patient selection, although lower on the GRADE scale, are closer to clinical practice. In particular, after the introduction of electronic medical records, more and more scientists and decision makers are arguing for the use of observational studies in addition to RCTs.4, 7 Nationwide registries are an example of such observational studies and often contain many patients. However, comparing treatment groups in observational studies is challenging because selection bias arises as a result of the nonrandom treatment allocation.4, 8 For that reason, appropriate statistical methodology to correct for confounding by indication should be applied.9 Even then, cautious interpretation of the results is necessary. Despite these limitations, observational studies could give important insights into real‐world effectiveness of treatment regimens.4, 10, 11
In the field of colon cancer, the literature is contradictory regarding treatment effects based on RCTs compared to those based on real‐world oncology registry data. For example, Iwashyna et al.12 concluded that a comparable adjuvant treatment effect is found in real‐world data (RWD) and RCTs in Stage III colon cancer patients. On the other hand, Meyerhardt et al.13 concluded that in metastatic colorectal cancer patients, treatment effect based on RCTs seems much stronger than the effect estimated using registry data. The authors speculate that the main explanation for this difference in effect is uncorrectable heterogeneity between the populations at baseline.
In patients diagnosed with Stage II colon cancer, results from RCTs were not supportive of prescribing adjuvant chemotherapy to all patients.14, 15, 16, 17, 18, 19 For example, the IMPACT meta‐analysis showed in a pooled analysis a HR of 0.83 (90% CI 0.72;1.07) for disease‐free survival (DFS) and a HR of 0.86 (90% CI 0.68;1.07) for overall survival (OS) for fluoropyrimidine monotherapy compared to no treatment.19 Combination regimens in which oxaliplatin is given in addition to fluoropyrimidine were not included in IMPACT, although these combination regimens are nowadays recommended in Dutch and international guidelines.1, 20 Furthermore, the trials included in IMPACT were not optimally designed to determine the treatment effect in Stage II colon cancer as patients with rectal cancer or Stage III disease were included as well, leading to relatively few Stage II colon cancer patients.21
Therefore, the aim of our study was to estimate adjuvant treatment effects in Stage II colon cancer patients using pooled DFS data from RCTs. Given the dilemma regarding applicability of outcomes from RCTs to the real‐world population, our secondary aim was to compare the RCT estimates to estimates based on a national oncology registry.
Methods
To estimate treatment effect in Stage II colon cancer patients, two approaches were used which we refer to as the “RCT approach” and “RWD approach.” In the RCT approach, we estimated the treatment effect in RCT data using the following three steps: (i) systematically searching relevant trials for which aggregated data on DFS was reported for Stage II colon cancer patients, (ii) generating patient‐level data from reported aggregated data and (iii) estimating a hazard ratio (HR) for treatment effect in the obtained patient‐level data. In the RWD approach, treatment effects for DFS were estimated in an observational cohort of 1,947 patients provided by the Netherlands Cancer Registry (NCR). Three methods were used to estimate the treatment effects: (i) propensity score matching (matching), (ii) inverse propensity score weighting (weighting) and (iii) propensity score stratification (stratification). All treatment effects were estimated using both parametric and semiparametric survival analyses. The semiparametric estimates were considered as main analysis. Supporting Information Table S1 describes the rationale, methods and results for the parametric analyses.
RCT approach
Systematical search for relevant trials
Studies that used a RCT design were included when they compared an adjuvant treatment arm to another adjuvant treatment arm or to a control group, and when Stage II colon cancer patients were at least a subgroup of the included patients. In line with the Dutch and international guidelines applicable during the literature search, the included adjuvant treatment regimens had to have a duration of at least 6 months.1, 20 Only studies published after 1987 in Western countries were taken into account. Finally, a Kaplan–Meier curve stratified for Stage II colon cancer patients with DFS as outcome had to be reported, as well as the associated numbers at risk.
For the identification of studies, we conducted a systematic search in MEDLINE, EMBASE and the Cochrane library similar to the search described in the Cochrane review by Figueredo et al.22 Reference lists of relevant studies were also searched and there were no language restrictions. A more detailed description of our search strategy is provided in Supporting Information. Inclusion criteria were applied by one researcher (GJ) to titles and abstracts. Full texts were obtained for hits that were considered relevant by one researcher (GJ). When in doubt, the eligibility was established by discussion (GJ, MG and VC). All authors agreed on the included studies before data extraction. Reasons for exclusion were documented and are presented in the PRISMA flow diagram (Fig. 1).23, 24 The main reasons for excluding studies were a population that did not include Stage II colon cancer patients or not reporting a stratified Kaplan–Meier curve for Stage II patients. Of each included trial, the following characteristics were extracted: time period, country, Stage II sample size, treatment regimens and 5‐year DFS (Tables 1 and 2). DFS was defined as the length of time after surgery during which no recurrence was detected.
Figure 1.
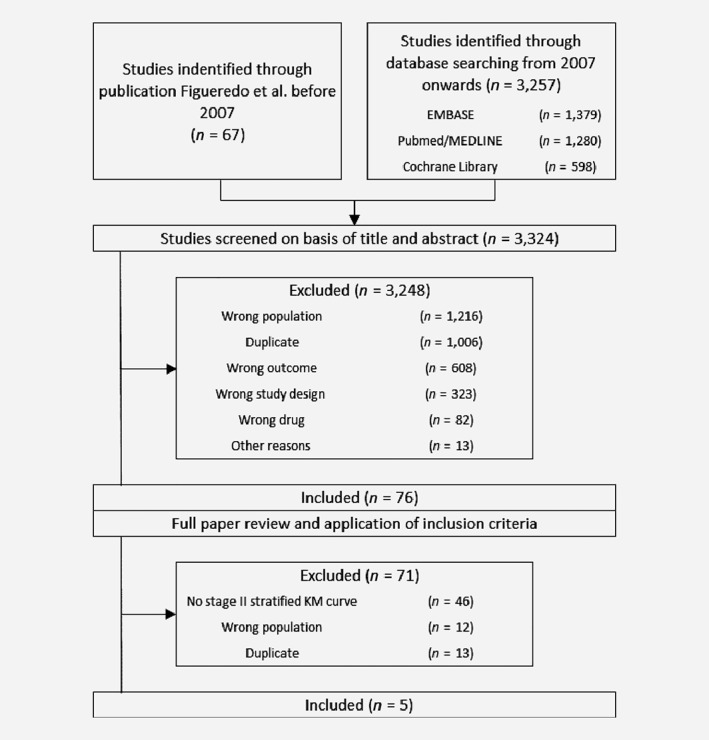
Flowchart of study selection.
Table 1.
Trial and patient characteristics
| Recruitment | Eligibility criteria | Treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Date of first randomization | Total randomized | Stage II randomized | Tumor site | Age limit (years) | Tumor stage | Start therapy after surgery (days) | Comparator | Treatment | Number of cycles | Duration (months) | Median follow‐up (years) | |
| IMPACT (1999) | ||||||||||||
| GIVIO | January 1989 | 888 | 449 | Colon | None | II and III1 | 35 | Control | FU + LV | 6 | 6 | 5.3 |
| NCIC‐CTG | May 1987 | 370 | 221 | Colon | None | II and III1 | 56 | Control | FU + LV | 6 | 6 | 5.9 |
| FFCD | October 1982 | 268 | 168 | Colon | <75 | II and III1 | 35 | Control | FU + LV | 6 | 6 | 5.2 |
| NNCTG | February 1988 | 317 | 57 | Colon | None | II and III1 | 35 | Control | FU + LV | 6 | 6 | 6.4 |
| Sienna | January 1985 | 239 | 121 | Colon | None | II and III1 | 21 | Control | FU + LV | 12 | 12 | 8.5 |
| QUASAR (2007) | May 1994 | 3,239 | 2,963 | Colon/Rectum | None | I, II and III | 42 | Control | FU + LV | 6 | 6 | 5.5 |
| Schippinger et al. (2007) | November 1993 | 500 | 500 | Colon | <80 | II | 42 | Control | 5‐FU + LV | 7 | 13 | 8.0 |
| MOSAIC (2009) | October 1998 | 2,246 | 899 | Colon | <75 | II, III | 42 | LV5FU2 | FOLFOX4 | 12 | 6 | 6.8 |
| NSABP C07 (2011) | February 2000 | 2,492 | 695 | Colon | None | II,III | NR | FULV | FLOX | 3 | 6 | 8.0 |
Dukes classification was used in these studies. Stage II: Dukes B2, tumor has grown through the gut, but not yet in lymph nodes. Stage III: Dukes C, tumor has grown into the regional lymph nodes.
Abbreviations: FOLFOX, regimen that includes the drugs leucovorin, fluoropyrimidine and oxaliplatin; FU, fluoropyrimidine; LV, leucovorin; NA, not applicable; NR, not reported.
Table 2.
Baseline characteristics of the patients included in the RCTs
| IMPACT | QUASAR | Schippinger et al. | MOSAIC | NSABP C07 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FU + LV | Control | FU + LV | Control | 5‐FU/LV | Control | FOLFOX4 | LV5FU2 | FOLFOX | FU + LV | |
| 5‐year DFS Stage II | 0.77 | 0.74 | 0.80 | 0.77 | 0.85 | 0.80 | 0.83 | 0.80 | 0.83 | 0.80 |
| Stage | ||||||||||
| I | 0 (0) | 0 (0) | 8 (1) | 8 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| II | 507 (100) | 509 (100) | 1,483 (91) | 1,480 (91) | 252 (100) | 248 (100) | 451 (40) | 448 (40) | 360 (29) | 359 (29) |
| III | 0 (0) | 0 (0) | 131 (8) | 129 (8) | 0 (0) | 0 (0) | 672 (60) | 675 (60) | 884 (71) | 878 (71) |
| Site | ||||||||||
| Colon | 507 (100) | 509 (100) | 1,148 (71) | 1,143 (71) | 252 (100) | 248 (100) | NR | NR | 1,247 (100) | 1,245 (100) |
| Left | 256 (50) | 280 (55) | NR | NR | 122 (48) | 124 (50) | NR | NR | 247 (20) | 263 (21) |
| Right | 236 (47) | 220 (43) | NR | NR | 130 (52) | 124 (50) | NR | NR | 576 (46) | 507 (41) |
| Recto sigmoid | NR | NR | NR | NR | NR | NR | NR | NR | 412 (33) | 459 (37) |
| Multiple | 6 (1) | 3 (1) | NR | NR | NR | NR | NR | NR | 10 (1) | 15 (1) |
| Unknown | 9 (2) | 6 (1) | NR | NR | NR | NR | NR | NR | 0 (0) | 0 (0) |
| Rectum | 0 (0) | 0 (0) | 474 (29) | 474 (29) | 0 (0) | 0 (0) | NR | NR | 0 (0) | 0 (0) |
| Gender | ||||||||||
| Male | 272 (54) | 287(56) | 1,006 (62) | 973 (60) | 137 (54.4) | 134 (54.0) | 630 (56) | 588 (52) | 690 (55.3) | (58) |
| Age | 61 (22–79) | 62 (26–86) | 63 (23–84) | 63 (23–86) | 65 (29–79) | 65 (30–80) | 61 (NR) | 60 (NR) | 59 (NR) | 59 (NR) |
| pT stage | ||||||||||
| T2 | 0 (0) | 0 (0) | NR | NR | 0 (0) | 0 (0) | 51 (4) | 54 (5) | NR | NR |
| T3 | 429 (85) | 437 (86) | NR | NR | 217 (86) | 214 (86) | 853 (76) | 852 (76) | NR | NR |
| T4 | 9 (1) | 6 (1) | NR | NR | 35 (14) | 34 (14) | 213 (20) | 208 (19) | NR | NR |
| Unknown | 69 (14) | 6 (13) | NR | NR | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NR | NR |
| Tumor differentiation | ||||||||||
| Moderate/well | 432 (85) | 421 (83) | NR | NR | NR | NR | 934 (83) | 914 (81) | NR | NR |
| Poor | 51 (10) | 63 (12) | NR | NR | NR | NR | 141 (13) | 148 (13) | NR | NR |
| Other | 8 (2) | 10 (2) | NR | NR | NR | NR | 0 (0) | 0 (0) | NR | NR |
| Unknown | 16 (3) | 15 (3) | NR | NR | NR | NR | 47 (4) | 61 (5) | NR | NR |
| Perforation present | NR | NR | NR | NR | NR | NR | 77 (7) | 77 (7) | NR | NR |
| Bowel obstruction | NR | NR | NR | NR | NR | NR | 201 (18) | 217 (19) | NR | NR |
Baseline characteristics of Stage II patients were not reported separately in most of the included trials. For these studies, the table shows the baseline characteristics of all patients included in the trial. Data are presented as numbers (%) except for 5‐year DFS and age. For DFS, data were presented as proportion disease‐free. For age, data were presented as mean (range).
Abbreviations: DFS, disease‐free survival; FOLFOX, regimen that includes the drugs leucovorin, fluoropyrimidine and oxaliplatin; FU, fluoropyrimidine; LV, leucovorin; m, months; NR, not reported.
Generating patient‐level data
DFS for Stage II patients was extracted from the included publications. First, all data points from the Kaplan–Meier curves for DFS that were required to reproduce the figure were read using GetData Graph Digitizer 2.26.25 Then, a curve fitting approach developed by Hoyle and Henley26 was used to generate the patient‐level data including data on treatment (yes/no), recurrence (yes/no) and time to recurrence. To maintain the randomization of the original trials, the extracted patient‐level data for all included studies were pooled in two separate analyses: (i) an analysis of the trials that compared fluoropyrimidine monotherapy to a control arm and (ii) an analysis of the trials that compared fluoropyrimidine in combination with oxaliplatin to fluoropyrimidine monotherapy. The first analysis was considered as the main outcome. To assess whether the study populations in the pooled RCTs were homogeneous, we compared 5‐year DFS using an interaction test.27
Estimating a HR for treatment effect
Then, HRs for DFS were estimated by adding treatment as a covariate in a Cox model. To account for the potential heterogeneity between trials a multilevel Cox regression was conducted. Literature suggests that a HR of 0.80 or less may be considered clinically meaningful.28 Therefore, this threshold value was used to judge clinical relevance. All survival models were estimated using the coxme package in Rstudio version 3.4.2.29
RWD approach
Real‐world observational cohort
The treatment effect was also estimated in a real‐world observational cohort from the NCR. The dataset consisted of 1,947 patients diagnosed with Stage II colon cancer between 2002 and 2008.30 The majority of patients had a pT3 stage (90.0%), less than 10 evaluated lymph nodes (53.9%) and a well/moderate tumor differentiation (83.3%). About 114 patients received adjuvant treatment (5.9%) and 1,833 did not (94.1%). Treatment regimens were fluoropyrimidine monotherapy (33.3%) or fluoropyrimidine with oxaliplatin (66.7%). Data were available on patient and tumor characteristics, time to recurrence and death. Follow‐up duration of the patients was at least 36 months, with a maximum of 179 months. The median follow‐up duration was 53 months for DFS. Recurrences could either be diagnosed due to symptoms or during regular follow‐up visits. This follow‐up consists of consultations every half‐year during the first 2–3 years after surgery and yearly thereafter until 5 years after surgery. In these consultations, either an ultrasound scan of the liver or CT scan of the abdomen is made. Also, the CEA values are determined at each visit.20 The baseline characteristics of the cohort are shown in columns 2–4 of Table 3.
Table 3.
Patient characteristics of the observational NCR cohort
| Variable | Whole population (n = 1,947) | Untreated (n = 1,833) | Treated (n = 114) | t‐test p‐value treated/untreated | Untreated match 11 (n = 76) | Treated match 11 (n = 76) | Untreated match 22 (n = 113) | Treated match 22 (n = 113) |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 70.9 (11.0) | 71.5 (10.8) | 61.7 (10.6) | <0.01 | 64.0 (8.2) | 64.0 (8.2) | 59.5 (11.6) | 61.9 (10.0) |
| pT stage | ||||||||
| T3 | 1,753 (90.0) | 1,678 (91.5) | 75 (65.8) | <0.01 | 68 (89.5) | 68 (89.5) | 85 (75.2) | 75 (66.4) |
| T4 | 194 (10.0) | 155 (8.5) | 39 (34.2) | 8 (10.5) | 8 (10.5) | 28 (24.8) | 38 (33.6) | |
| Number of evaluated lymph nodes | ||||||||
| <10 | 1,050 (53.9) | 975 (53.2) | 75 (65.8) | 0.01 | 57 (75.0) | 57 (75.0) | 82 (72.6) | 75 (66.4) |
| ≥10 | 897 (46.1) | 858 (46.8) | 39 (34.2) | 19 (25.0) | 19 (25.0) | 31 (27.4) | 38 (33.6) | |
| Tumor site | ||||||||
| Right | 1,173 (60.2) | 1,106 (60.3) | 67 (58.8) | 0.82 | 43 (56.6) | 43 (56.6) | 65 (57.5) | 67 (59.3) |
| Left | 774 (39.8) | 727 (39.7) | 47 (41.2) | 33 (43.4) | 33 (43.4) | 48 (42.5) | 46 (40.7) | |
| Tumor differentiation | ||||||||
| Well/moderate | 1,623 (83.3) | 1,543 (84.2) | 80 (70.2) | <0.01 | 58 (76.3) | 58 (76.3) | 89 (78.8) | 79 (69.9) |
| Poor/not | 324 (16.7) | 290 (15.8) | 34 (29.8) | 18 (23.7) | 18 (23.7) | 24 (21.2) | 34 (30.1) |
Data are presented as means (±SD) or numbers (%).
Match 1 is the matching sample with caliper score of 0.
Match 2 is the matching sample with caliper score of 0.2 multiplying by standard deviation of the logit propensity score.
Propensity score risk adjustments
Due to the nonrandomized nature of the cohort, comparisons between patients who received adjuvant chemotherapy with nonrecipients are potentially biased due to differences between both groups at baseline, that is, confounding by indication. The use of specialized methods is necessary to correct for confounding by indication. Although there are several methods available, there is no consensus on a gold standard.8 Therefore, three methods were used to estimate the treatment effects: (i) propensity score matching (matching), (ii) inverse propensity score weighting (weighting) and (iii) propensity score stratification (stratification). The choice for these methods is in line with Austin et al. (2013) and Gayat et al. (2012) who showed that these methods have a good performance for time‐to‐event data.31, 32 Propensity score estimation using observed confounders that are determined prior to treatment administration allows for unbiased estimation of treatment effects under the assumption of no unobserved confounding.33 This assumption cannot be formally tested. However, the most relevant clinical and pathological correlates of treatment assignment and survival, as reported in the literature, were available in our dataset (i.e., gender, age, pT stage, differentiation grade, lymph nodes evaluated and tumor site).34
The propensity score represents the probability that a patient would receive adjuvant treatment. Propensity scores were determined on the basis of a logistic regression model in which the dependent variable was administration of adjuvant treatment and the independent variables were all available factors potentially associated with administration of adjuvant treatment (gender, age, pT stage, number of evaluated lymph nodes, grade of differentiation and tumor site). Interactions between treatment and any of these factors were included as well. A backward variable selection based on Akaike Information Criterium (AIC) was done to select the most relevant covariates in the propensity score model.
Survival models
First, the naïve treatment effect, that is, without correcting for confounding by indication, was estimated in the observational cohort. Second, HRs for DFS were estimated including a correction for confounding by indication by matching, weighting and stratification. In the matching method, patients who did receive adjuvant treatment were 1:1 matched based on the propensity score to patients who did not receive adjuvant treatment. A caliper score was used to determine the maximum deviation in propensity score for matched pairs. In the inverse propensity score weighting method treatment effects were estimated by weighting the individuals based on the propensity score. In the stratification method, the sample was stratified into five mutually exclusive subclasses based on the propensity score. A detailed description of the confounding by indication methods is provided in Supporting Information.
Model selection
For each Cox model, a forward covariate selection was performed. All multivariate survival models included the covariates that were significant in one or more survival models to ensure comparability of the results: age, pT stage, evaluated lymph nodes, tumor site and differentiation grade.
Comparison RCT approach and RWD approach
An interaction test was used for significance testing of the differences between the estimates based on the RCT and RWD approach.27 A significance threshold of p < 0.10 was used to avoid type II error rate.
Data availability
The generated patient‐level data used for the RCT approach is available as Supporting Information. The registry data that support the findings of the RWD approach in this study are upon request available from the NCR.
Results
RCT approach
Eligible studies
We identified 3,324 potentially eligible studies that provided survival data on DFS for Stage II colon cancer patients. Of these, five publications met the inclusion criteria. The five publications reported nine trials which were included in the current study.16, 17, 19, 35, 36 Four of the five included publications were RCTs and one was a meta‐analysis of five RCTs.
Study characteristics
Characteristics of the nine included studies are presented in Table 1. The studies were published between 1999 and 2011. Five studies originated from European centers, two from North America and two included patients from multiple Western countries. Comparison of baseline characteristics was hampered, because three of the five publications did not report baseline characteristics for Stage II colon cancer separately. The total sample size of Stage II patients in the nine included studies was 6,076 patients.
Pooled treatment groups
Included trials either compared fluoropyrimidine monotherapy to a control arm, that is, IMPACT, QUASAR and Schippinger et al. or fluoropyrimidine in combination with oxaliplatin to fluoropyrimidine monotherapy, that is, NSABP C07 and MOSAIC. To maintain the randomization of the trials, HRs were estimated in two separate pooled analyses. In the first analysis, we pooled trials that compared fluoropyrimidine monotherapy to a control arm whereas, in the second analysis, we pooled trials that compared fluoropyrimidine in combination with oxaliplatin to fluoropyrimidine monotherapy. The 5‐year DFS of the pooled studies were in the same range (Table 2). Based on the interaction test, no significant differences were found in 5‐year DFS between the pooled study arms (Supporting Information Table S2).
Survival analyses
The Kaplan–Meier curves for DFS are shown by trial arm in Supporting Information Figure S1 a for the fluoropyrimidine monotherapy group which was compared to the control group, in Supporting Information Figure S1 b for the control group, in Supporting Information Figure S1 c for the combination therapy group and in Supporting Information Figure S1 d for the fluoropyrimidine monotherapy group which was compared to the combination therapy group. In the population for the first pooled analysis, there were 454 recurrences among the 2,244 patients in the control group after 5 years of follow‐up. For the treatment group with fluoropyrimidine monotherapy, the number of recurrences was 399 in a population of 2,245 patients. A pooled Kaplan–Meier curve is shown in Figure 2 a. In the population for the second pooled analysis, there were 175 recurrences among 788 patients in the fluoropyrimidine monotherapy group. In the group which received fluoropyrimidine in combination with oxaliplatin the number of recurrences was 166 in 799 patients. A pooled Kaplan–Meier curve is shown in Figure 2 b. The HR for treatment effect in the first pooled analysis, fluoropyrimidine compared to no treatment, was 0.77 (95% CI 0.43;1.10) for DFS. In the second pooled analysis, in which fluoropyrimidine combined with oxaliplatin was compared to fluoropyrimidine monotherapy, a HR of 0.93 (95% CI 0.72;1.15) was found for DFS. Both treatment effects were estimated with a multilevel Cox model. HRs for the multilevel and non‐multilevel Cox survival models are shown in Table 4.
Figure 2.
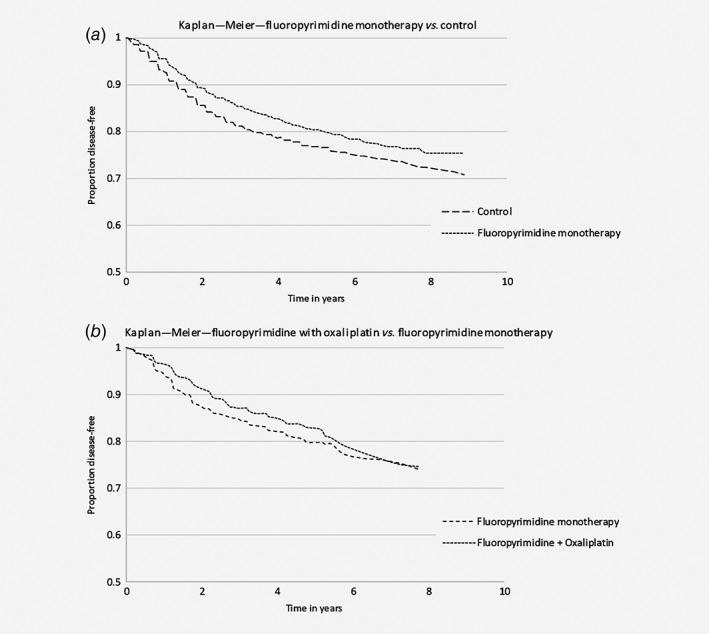
Kaplan–Meier curves for the treatment and control groups for pooled population 1 (a) and the curves for fluoropyrimidine combined with oxaliplatin compared to fluoropyrimidine monotherapy in pooled population 2 (b).
Table 4.
Estimated Treatment effects
| Cox proportional hazard | |||
|---|---|---|---|
| Hazard ratio (95% CI) | p‐value | p‐value of comparison RWD to RCT | |
| RCT approach—trial data | |||
| Survival model 1 | 0.78 (0.68;0.89) | <0.01 | NA |
| Multilevel survival model 1 | 0.77 (0.43;1.10) | 0.13 | Comparator |
| Survival model 2 | 0.93 (0.76;1.16) | 0.55 | NA |
| Multilevel survival model 2 | 0.93 (0.72;1.15) | 0.55 | NA |
| RWD approach—observational data unadjusted | |||
| Naive survival model | 1.65 (1.13;2.42) | 0.01 | 0.12 |
| RWD approach—observational data adjusted based on propensity scores | |||
| PS matching—caliper 0 | |||
| Multivariate survival model | 0.95 (0.50;1.80) | 0.88 | 0.49 |
| PS matching—caliper 0.2*SD logit propensity | |||
| Multivariate survival model | 1.00 (0.58;1.70) | 0.98 | 0.41 |
| PS inverse weighting | |||
| Multivariate survival model | 0.88 (0.24;3.21) | 0.99 | 0.71 |
| PS stratification | |||
| Multivariate survival model | 1.05 (0.04;2.06) | 0.99 | 0.47 |
Survival model 1 refers to the analysis in which a treatment effect was estimated for a fluoropyrimidine regimen compared to control. IMPACT, QUASAR and Schippinger et al. were included in this analysis. Survival model 2 refers to the analysis in which a treatment effect was estimated for fluoropyrimidine in combination with oxaliplatin compared to fluoropyrimidine monotherapy. MOSAIC and NSABP C07 were included in this analysis. Bold results are considered as main results.
Abbreviations: NA, not applicable; PS, propensity score; RCT, randomized clinical trial; RWD, real‐world data; SD, standard deviation.
RWD approach
Table 4 shows the treatment effect estimates based on the NCR cohort. The unadjusted naive Cox model estimated an HR of 1.65 (95% CI 1.13;2.42, p = 0.01) for DFS.
Propensity score risk adjustments
Adjuvant treatment propensity scores ranged from <0.01 to 0.84 in the control group and from 0.01 to 0.89 in the treated group. Thus, the treatment group showed sufficient overlap with the control group. Means of the distribution of the confounders age, pT stage, evaluated lymph nodes, tumor site and differentiation grade were equal in the matched sample, after weighting, and in all propensity score strata.
For matching, weighting and stratification, both univariate and multivariate survival models were fitted. Below, only HRs for DFS of the multivariate survival models are described as these are considered as most reliable. Results of the univariate models are shown in Supporting information Table S3. For the matching method, two samples were defined: (i) a sample of 76 matched pairs based on a caliper score of 0 and (ii) a sample of 112 matched pairs based on a caliper score of 0.2 of the standard deviation of the logit propensity score (Table 3). Estimated multivariate HRs were 0.95 (95% CI 0.50;1.80) and 1.00 (95% CI 0.58;1.70), respectively. For the weighting method, a HR of 0.88 (95% CI 0.24;3.21) was estimated in a multivariate survival model. A pooled HR of 1.05 (95% CI 0.04;2.06) was found for the stratification method (Table 4). It should be noted that all confidence intervals are wide and do not reach clinical relevance or statistical significance.
Comparison RCT and RDW approach
In Table 4, the p values of the interaction tests are shown. No significant differences were found between the estimates based on the RCT and RDW approach, which is potentially due to the small sample size of treated patients in the RWD approach. Furthermore, the estimate derived from the RCT approach suggests a clinically relevant treatment effect, while the treatment effect found in the RWD approach was not clinically relevant.
Discussion
The primary aim of this study was to estimate adjuvant treatment effects for DFS in Stage II colon cancer patients using pooled summary survival data from RCTs (RCT approach). Given the dilemma regarding the applicability of outcomes from RCTs to the real‐world population, our secondary aim was to compare the RCT estimate to estimates based on a national oncology registry (RWD approach). The RCT approach resulted in a HR of 0.77 (95% CI 0.43;1.10) for fluoropyrimidine monotherapy compared to no treatment. In addition, a HR of 0.93 (95% CI 0.72;1.15) was found for fluoropyrimidine in combination with oxaliplatin compared to fluoropyrimidine monotherapy. Although point estimates from the separate studies as well as our pooled estimate suggests a clinically relevant benefit (HR < 0.80) in terms of DFS from adjuvant chemotherapy in Stage II colon cancer, no statistical significance was reached. For the RWD approach, in which we compared patients treated with adjuvant chemotherapy (33.3% fluoropyrimidine monotherapy and 66.6% fluoropyrimidine therapy with oxaliplatin) to patients who did not receive treatment, none of the applied propensity score methods resulted in a clinically relevant or significant treatment effect. Finally, no significant differences were found between estimates based on the RCT and RWD approach. It should be noted that the sample size of the cohort used for the RWD estimates was small, resulting in large confidence intervals. This is also the likely explanation for insignificance of the interaction test on the difference between estimates based on the RCT and RWD approach. Overall, no significant treatment effect was found, neither in the RCT approach nor in the RWD approach. Nevertheless, the point estimate in the RCT approach suggests a clinically relevant benefit of adjuvant chemotherapy. To improve guidance in adjuvant treatment decisions in Stage II colon cancer, larger sample sizes, the pooling of true patient‐level data with covariate information and/or subgroup‐specific analyses are required.
The nonsignificant result for the comparison of fluoropyrimidine to control in the RCT approach is probably related to the following two aspects. First, we used a multilevel Cox proportional hazard model to estimate treatment effect. The variance in a multilevel model consists of two components; the variance around the treatment effect and the variance around the added random effect.37 As a result, the variance is increased compared to a non‐multilevel approach. This is underlined by our results in which the multilevel estimate of 0.77 has a much wider confidence interval (95% CI 0.43;1.10, p = 0.13) than the non‐multilevel estimate of 0.78 (0.68;0.89, p < 0.01). Second, we did not have the original patient‐level data to our disposition. Therefore, we opted for using a curve fitting approach developed by Hoyle and Henley. This method precludes the inclusion of relevant covariates in the survival models, which may narrow down confidence intervals around the estimate for treatment effect. Moreover, it should be noted that statistical inference is increasingly questioned in the literature.38, 39 That is, a p‐value does not measure the size of an effect nor the importance of a result. Results should always be interpreted within their context; taking into account the sample size, the methods used to estimate the effect as precisely as possible, and relevance of a result in daily clinical practice.28 Taking these arguments into account, we would consider the treatment effect found in the RCT approach as clinically relevant.
In the current study, DFS was used as outcome measure to estimate treatment effect. Although OS is acknowledged as the gold standard outcome in cancer trials,40 DFS can be considered as a surrogate endpoint for OS. This is underlined by Sargent et al.41 who showed that DFS and OS are highly correlated in colon cancer trials evaluating fluorouracil‐based regimens in Stage III colon cancer patients. Also, the majority of trials included in the current study reported similar results for DFS and OS in terms of clinical relevance and statistical significance. Only in Schippinger et al.,35 the results for DFS and OS were not consistent in terms of clinical relevance, which may be explained by the small sample size.
Previously, Sargent et al.42 reported a relationship between treatment effect and microsatellite instability (MSI). In addition to improved prognosis, it was shown that patients with an MSI tumor have a certain resistance to fluoropyrimidine‐based chemotherapy. It is possible that, in addition to MSI, more molecular characteristics can be identified that may influence the impact of treatment on DFS. Therefore, a stratified analysis would presumably have increased the ability to detect a stronger treatment effect in specific subgroups, such as patients with a MSS. Besides, data from other retrospective analyses strongly suggest pT4 as the strongest prognostic factor in Stage II colon cancer.43, 44 However, stratified analysis for both predictive and prognostic factors was hampered due to the absence of covariate information in the patient‐level data for our RCT‐based analysis. In the RWD approach, a stratified analysis was hampered due to the small sample size.
The presented treatment effect estimate for fluoropyrimidine monotherapy compared to no treatment, is based on updating the IMPACT analysis with the trials of QUASAR and Schippinger et al. It should be noted that in the Sienna trial (included in the IMPACT meta‐analysis) and the trial of Schippinger et al. a deviant treatment regimen of 12 or 13 months was prescribed instead of 6 months as in the other trials included in this study.19, 35 We believe that this did not influence our results as the treatment effect for the Sienna trial and the trial of Schippinger et al. were in the same range as the other trials included in the current study (range 0.69–0.83). Furthermore, we estimated a treatment effect for fluoropyrimidine combined with oxaliplatin compared to fluoropyrimidine monotherapy. No significant differences were found in DFS for the addition of oxaliplatin. This finding is in contrast with the effect of adding oxaliplatin to adjuvant treatment in Stage III colon cancer patients; both NSABP C07 and MOSAIC found a significant improvement in DFS for the addition of oxaliplatin in Stage III colon cancer.17, 36 Yothers et al. (2011) suggests that this difference found in effect between Stage II and Stage III patients can be explained by (i) the smaller sample size for Stage II patients compared to Stage III patients and (ii) the higher absolute survival probability for Stage II patients.
In the RWD approach, there was an imbalance between treated (n = 114) and untreated patients (1,833). The explanation for the imbalance is two‐sided; first, the majority of the population is not high‐risk and therefore not eligible for adjuvant chemotherapy according to guidelines. Second, only 5% of all high‐risk patients received chemotherapy in the dataset. From literature, we know that most guideline deviations are well‐substantiated, for example, due to the poor clinical condition of the patient or a patients’ preference. Other possible explanations mentioned in the literature are unfamiliarity with the guideline and differences in expert opinions.45 Furthermore, data for DFS is often not collected by default in registry data. Therefore, only a small subset (n = 1,947) out of approximately 10,000 Stage II cancer patients, was available to estimate the effect of treatment on recurrence. The small number of treated patients was a serious limitation in this analysis, causing large variance around the treatment effect estimates from the RWD approach compared to the RCT approach. These wide confidence intervals limit the power of the interaction test to detect a significant difference between the RCT and RWD approach, even though we used a lenient significance threshold of p < 0.10.
In observational data such as national registry data, there is the potential for bias due to confounding by indication. In this study, we used appropriate, though complex methods to correct for this bias. These methods assume that there are no unmeasured confounders. In the NCR dataset, the most important clinical and pathological factors that determine treatment allocation were included (i.e. gender, age, pT stage, differentiation grade, lymph nodes evaluated and tumor site). Nevertheless, the patient‐related factor performance status was not measured, while this variable is reported to affect treatment allocation.46 Furthermore, results of the RWD analysis were not entirely representative of the original sample. For example, in the matching analysis, many of the nontreated patients were excluded due to the differences in sample size between the treated and nontreated group. Moreover, the estimates of the three methods used in the RWD analysis were not consistent in effect direction, which complicates the interpretation. In summary, results of our RWD analysis should be interpreted carefully, taking the limitations of the study design and the statistical methods into account.
To summarize, the RCT data suggest a clinically relevant benefit of adjuvant chemotherapy in terms of DFS, although this benefit was not significant in our pooled analyses. To improve guidance in adjuvant treatment decisions in Stage II colon cancer, future studies should focus on the pooling of true patient‐level data with covariate information and/or subgroup‐specific analyses.
Supporting information
Appendix S1: Supplementary Information
Appendix S2: Supplementary Information
Acknowledgements
The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of the data for the Netherlands Cancer Registry.
Conflicts of interest: The authors declare no potential conflicts of interest.
References
- 1. Labianca R, Nordlinger B, Beretta GD, et al. Early colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2013;24(Suppl 6):vi64–72. [DOI] [PubMed] [Google Scholar]
- 2. Guyatt GH, Oxman AD, Kunz R, et al. What is "quality of evidence" and why is it important to clinicians? BMJ 2008;336:995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rothwell PM. External validity of randomised controlled trials:“to whom do the results of this trial apply?”. Lancet 2005;365:82–93. [DOI] [PubMed] [Google Scholar]
- 4. Booth C, Tannock I. Randomised controlled trials and population‐based observational research: partners in the evolution of medical evidence. Br J Cancer 2014;110:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer‐treatment trials. N Engl J Med 1999;341:2061–7. [DOI] [PubMed] [Google Scholar]
- 6. van Deudekom FJ, Postmus I, van der Ham DJ, et al. External validity of randomized controlled trials in older adults, a systematic review. PLoS One 2017;12:e0174053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garrison LP Jr, Neumann PJ, Erickson P, et al. Using real‐world data for coverage and payment decisions: the ISPOR real‐world data task force report. Value Health 2007;10:326–35. [DOI] [PubMed] [Google Scholar]
- 8. Schafer JL, Kang J. Average causal effects from nonrandomized studies: a practical guide and simulated example. Psychol Methods 2008;13:279–313. [DOI] [PubMed] [Google Scholar]
- 9. Rubin DB. Estimating causal effects of treatments in randomized and nonrandomized studies. J Educ Psychol 1974;66:688–701. [Google Scholar]
- 10. Barnish MS, Turner S. The value of pragmatic and observational studies in health care and public health. Pragmat Obs Res 2017;8:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Booth CM, Mackillop WJ. Translating new medical therapies into societal benefit: the role of population‐based outcome studies. JAMA 2008;300:2177–9. [DOI] [PubMed] [Google Scholar]
- 12. Iwashyna TJ, Lamont EB. Effectiveness of adjuvant fluorouracil in clinical practice: a population‐based cohort study of elderly patients with stage III colon cancer. J Clin Oncol 2002;20:3992–8. [DOI] [PubMed] [Google Scholar]
- 13. Meyerhardt JA, Li L, Sanoff HK, et al. Effectiveness of bevacizumab with first‐line combination chemotherapy for Medicare patients with stage IV colorectal cancer. J Clin Oncol 2012;30:608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil‐based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004;22:1797–806. [DOI] [PubMed] [Google Scholar]
- 15. Figueredo A, Charette ML, Maroun J, et al. Adjuvant therapy for stage II colon cancer: a systematic review from the Cancer Care Ontario program in evidence‐based care's gastrointestinal cancer disease site group. J Clin Oncol 2004;22:3395–407. [DOI] [PubMed] [Google Scholar]
- 16. Quasar Collaborative Group . Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020–9. [DOI] [PubMed] [Google Scholar]
- 17. André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16. [DOI] [PubMed] [Google Scholar]
- 18. Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C‐07. J Clin Oncol 2007;25:2198–204. [DOI] [PubMed] [Google Scholar]
- 19. IMPACT B2 Investigators . Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol 1999;17:1356–63. [PubMed] [Google Scholar]
- 20. National Working Group on Gastrointestinal Cancers . Guidelines colorectal cancer. Available at http://www.oncoline.nl.
- 21. Kannarkatt J, Joseph J, Kurniali PC, et al. Adjuvant chemotherapy for stage II colon cancer: a clinical dilemma. J Oncol Pract 2017;13:233–41. [DOI] [PubMed] [Google Scholar]
- 22. Figueredo A, Coombes ME, Mukherjee S. Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database Syst Rev 2008;3:CD005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions 5.1.0. London: The Cochrane Collaboration, 2011. 33–49. [Google Scholar]
- 24. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. GetData Graph Digitizer , Digitizing software. Digitize scanned graphs and get original (x,y) data. Available at http://getdata-graph-digitizer.com/, 2018.
- 26. Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol 2011;11:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ellis LM, Bernstein DS, Voest EE, et al. American Society of Clinical Oncology perspective: raising the bar for clinical trials by defining clinically meaningful outcomes. J Clin Oncol 2014;32:1277–80. [DOI] [PubMed] [Google Scholar]
- 29. Therneau TM, Therneau MTM. Package ‘coxme’. Mixed Effects Cox Models R package version, vol. 2 Vienna, Austria: R Foundation for Statistical Computing, 2018. [Google Scholar]
- 30. van Gestel YR, de Hingh IH, van Herk‐Sukel MP, et al. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol 2014;38:448–54. [DOI] [PubMed] [Google Scholar]
- 31. Gayat E, Resche‐Rigon M, Mary JY, et al. Propensity score applied to survival data analysis through proportional hazards models: a Monte Carlo study. Pharm Stat 2012;11:222–9. [DOI] [PubMed] [Google Scholar]
- 32. Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med 2013;32:2837–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. [Google Scholar]
- 34. O'Connor ES, Greenblatt DY, LoConte NK, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol 2011;29:3381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schippinger W, Samonigg H, Schaberl‐Moser R, et al. A prospective randomised phase III trial of adjuvant chemotherapy with 5‐fluorouracil and leucovorin in patients with stage II colon cancer. Br J Cancer 2007;97:1021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yothers G, O'Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C‐07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Diez‐Roux AV. Multilevel analysis in public health research. Annu Rev Public Health 2000;21:171–92. [DOI] [PubMed] [Google Scholar]
- 38. Wasserstein RL, Lazar NA. The ASA's statement on p‐values: context, process, and purpose. Am Stat 2016;70:129–33. [Google Scholar]
- 39. Wasserstein RL, Schirm AL, Lazar NA. Moving to a world beyond “p < 0.05”. Am Stat 2019;73:1–19. [Google Scholar]
- 40. Oba K, Paoletti X, Alberts S, et al. Disease‐free survival as a surrogate for overall survival in adjuvant trials of gastric cancer: a meta‐analysis. J Natl Cancer Inst 2013;105:1600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sargent DJ, Wieand HS, Haller DG, et al. Disease‐free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2005;23:8664–70. [DOI] [PubMed] [Google Scholar]
- 42. Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil‐based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Snaebjornsson P, Coupe V, Jonasson L, et al. pT4 stage II and III colon cancers carry the worst prognosis in a nationwide survival analysis. Shepherd's local peritoneal involvement revisited. Int J Cancer 2014;135:467–78. [DOI] [PubMed] [Google Scholar]
- 44. Kim JE, Hong YS, Kim HJ, et al. Defective mismatch repair status was not associated with DFS and OS in stage II colon cancer treated with adjuvant chemotherapy. Ann Surg Oncol 2015;22(Suppl 3):S630–7. [DOI] [PubMed] [Google Scholar]
- 45. Keikes L, van Oijen MGH, Lemmens V, et al. Evaluation of guideline adherence in colorectal cancer treatment in The Netherlands: a survey among medical oncologists by the Dutch colorectal cancer group. Clin Colorectal Cancer 2018;17:58–64. [DOI] [PubMed] [Google Scholar]
- 46. van Erning FN, Janssen‐Heijnen ML, Creemers GJ, et al. Deciding on adjuvant chemotherapy for elderly patients with stage III colon cancer: a qualitative insight into the perspectives of surgeons and medical oncologists. J Geriatr Oncol 2015;6:219–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information
Appendix S2: Supplementary Information
Data Availability Statement
The generated patient‐level data used for the RCT approach is available as Supporting Information. The registry data that support the findings of the RWD approach in this study are upon request available from the NCR.


