Abstract
Objectives
To document the management of advanced prostate cancer including diagnosis, prognosis, treatment, and care, in real‐world practice in Asia using the United in Fight against prOstate cancer (UFO) registry.
Patients and Methods
We established a multi‐national, longitudinal, observational registry of patients with prostate cancer presenting to participating tertiary care hospitals in eight Asian countries. A total of 3636 eligible patients with existing or newly diagnosed high‐risk localised prostate cancer (HRL), non‐metastatic biochemically recurrent prostate cancer (M0), or metastatic prostate cancer (M1), were consecutively enrolled and are being followed‐up for 5 years. Patient history, demographic and disease characteristics, treatment and treatment decisions, were collected at first prostate cancer diagnosis and at enrolment. Patient‐reported quality of life was prospectively assessed using the European Quality of Life‐five Dimensions, five Levels (EQ‐5D‐5L) and Functional Assessment of Cancer Therapy for Prostate Cancer questionnaires. In the present study, we report the first interim analysis of 2063 patients enrolled from study start (15 September 2015) until 18 May 2017.
Results
Of the 2063 enrolled patients, 357 (17%), 378 (19%), and 1328 (64%) had HRL, M0 or M1 prostate cancer, respectively. The mean age at first diagnosis was similar in each group, 56% of all patients had extracapsular extension of their tumour, 28% had regional lymph node metastasis, and 53% had distant metastases. At enrolment, 62% of patients had at least one co‐morbidity (mainly cardiovascular disease or diabetes), 91.8% of M1 patients had an Eastern Cooperative Oncology Group performance score of <2 and the mean EQ‐5D‐5L visual analogue score was 74.6–79.6 across cohorts. Treatment of M1 patients was primarily with combined androgen blockade (58%) or androgen‐deprivation therapy (either orchidectomy or luteinising hormone‐releasing hormone analogues) (32%). Decisions to start therapy were mainly driven by treatment guidelines and disease progression. Decision to discontinue therapy was most often due to disease progression (hormonal drug therapy) or completion of therapy (chemotherapy).
Conclusion
In the UFO registry of advanced prostate cancer in Asia, regional differences exist in prostate cancer treatment patterns that will be explored more deeply during the follow‐up period; prospective follow‐up is ongoing. The UFO registry will provide valuable descriptive data on current disease characteristics and treatment landscape amongst patients with prostate cancer in Asia.
Keywords: prostate cancer, registry, quality of life, Asia, epidemiology, observational study, #ProstateCancer, #PCSM
Abbreviations
- ADT
androgen‐deprivation therapy
- CAB
combined androgen blockade
- EQ‐5D‐5L
European Quality of Life‐five Dimensions, five Levels
- HRL
high‐risk localised prostate cancer
- M0
non‐metastatic, biochemically recurrent prostate cancer
- M1
metastatic prostate cancer
- mCRPC
metastatic castration‐resistant prostate cancer
- mHSPC
metastatic hormone‐sensitive prostate cancer
- QoL
quality of life
- RP
radical prostatectomy
- RT
radiotherapy
Introduction
Prostate cancer is a common cancer in Asian men and is emerging as a health priority in the region as the incidence and mortality rates in Asia increase 1. Prostate cancer incidence rates vary more than 27‐fold across Asian countries; from 1.2 per 100 000 population in Nepal, to 33.1 per 100 000 population in Singapore (2012 data) 2. Differences in screening practices, access to treatments and healthcare services, and national cancer reporting capacity, are likely to contribute to this variability, although changes to Western‐style diets linked to increased incidence of prostate cancer and genetic differences between ethnicities may also play a role 2, 3, 4, 5, 6, 7.
Much about prostate cancer epidemiology, treatment, and outcome in Asia is not well understood. Not all countries have national cancer databases, and the types and quality of data where they exist are variable. The extent to which new treatment options for prostate cancer are used across the region and their impact in Asian patients is not known. The psychosocial consequences of prostate cancer and its treatment are not well described in Asian countries.
We aimed to document prostate cancer management and outcomes in real‐world practice in Asia by establishing a large, longitudinal, observational prostate cancer registry; the United in Fight against prOstate cancer (UFO) registry 8. The registry is compiling high‐quality, real‐world data to inform on country‐specific patterns of advanced prostate cancer in Asia, including data on prostate cancer screening, diagnosis, disease status, treatment patterns, the underlying clinical decisions that determine treatment choice, response to treatment, and patient‐reported quality of life (QoL). Ultimately, registry data will be used in exploratory analyses of potential associations between risk factors and disease progression, and evaluation of prostate cancer treatments.
The study commenced on 15 September 2015 and 2063 patients with advanced prostate cancer were enrolled from the eight participating countries: China, India, Japan, Malaysia, Singapore, South Korea, Taiwan, and Thailand, by 18 May 2017. In the present study, we report the first descriptive interim analysis of baseline characteristics, which includes all patients enrolled from the start of the study until 18 May 2017.
Patient and Methods
Registry Design
The registry design has been described in detail by Liu et al. 8 (http://www.clinicaltrials.gov NCT02546908, Registry Identifier: NOPRODPCR4001). In brief, ~3500 eligible patients with existing or newly diagnosed high‐risk localised prostate cancer (HRL), non‐metastatic biochemically recurrent prostate cancer (M0), or metastatic prostate cancer (M1), were to be enrolled and followed for up to 5 years. Patient history, demographic, and disease characteristics are collected either from the medical records of patients enrolled after their initial prostate cancer diagnosis, or prospectively for newly diagnosed and enrolled patients. Treatments, treatment decision‐making, and health‐related QoL outcomes are collected at the time of enrolment into the registry.
The decision of patients to participate in this study does not in any way impact upon the standard of care they are receiving or any benefits to which they are otherwise entitled. Patients may withdraw consent and discontinue participation in the study at any time, with no effect on their medical care or access to treatment. Clinical visits, medical interventions, treatments, laboratory tests, and procedures are neither mandated nor recommended as part of this study. The protocol was reviewed and approved by the Institutional Review Boards/Independent Ethics Committees in each of the participating countries.
Patient Population
Written informed consent was obtained from each patient or their legally acceptable representative to allow source data collection and verification in accordance with local laws. Eligible patients were aged ≥21 years with HRL, M0 (biochemical recurrence) or M1 prostate cancer. Enrolment of patients with HRL or M0 prostate cancer was capped at 600 per cohort to increase the proportion of M1 patients in the study. To overcome differences in prostate cancer staging used by individual institutions, disease stage definitions were standardised for registry enrolment (Fig. 1).
Figure 1.
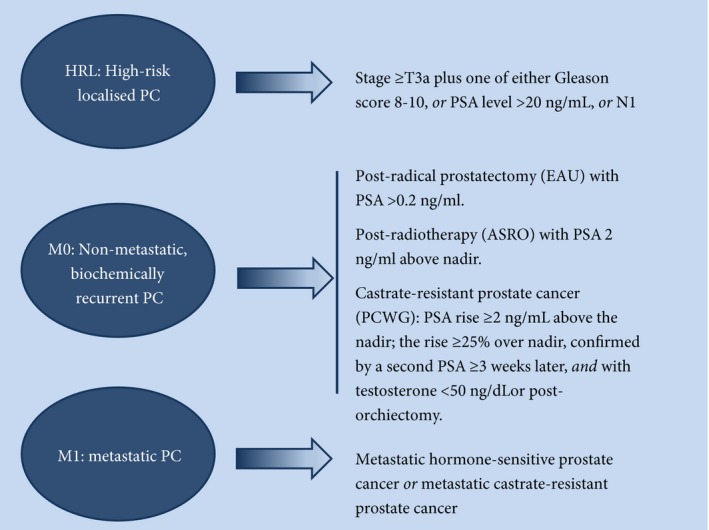
Disease eligibility criteria for registry enrolment. ASRO, American Society for Radiation Oncology guidelines; EUA, European Association of Urology guidelines; PC, prostate cancer; PCWG, Prostate Cancer Working Group.
Outcome Variables
Information recorded at study entry included: demographic and lifestyle characteristics; family medical history; detailed features of prostate cancer at diagnosis such as detection method, laboratory values, staging, Gleason score; treatment history; reasons underlying treatment decision‐making; and health‐related QoL. QoL was measured using the European Quality of Life‐five Dimensions, five Levels (EQ‐5D‐5L) and Functional Assessment of Cancer Therapy for Prostate Cancer (FACT‐P) questionnaires 9, 10.
Statistical Analysis
The analyses of interim demographic and medical characteristics, as well as treatment data were descriptive.
Results
Of 2063 eligible patients enrolled at the time of the interim analysis, 654 (31.7%) were from Japan, 292 (14.2%) from Thailand, 264 (12.8%) from Taiwan, 262 (12.7%) from South Korea, 200 (9.7%) from Malaysia, 193 (9.4%) from China, 134 (6.5%) from Singapore, and 64 (3.1%) from India.
There were 357 patients (17%) staged as HRL, 378 (19%) as M0, and 1328 as M1 (of whom 1038 (50%) had metastatic hormone‐sensitive prostate cancer [mHSPC] and 290 (14%) had metastatic castration‐resistant prostate cancer [mCRPC]).
Trigger for Diagnosis
The diagnosis of prostate cancer was driven by the investigation of symptoms in 68.6% of patients with mHSPC, 77.5% with mCRPC, and in 56.9% of patients with HRL (Fig. 2). For patients with M0, a similar percentage of patients were diagnosed by regular health screening (46.1%) and symptom investigation (42.1%).
Figure 2.

Triggers for diagnosis of prostate cancer by disease stage at diagnosis. All patients = all patients with available data.
Demographic Characteristics and Disease Features at Prostate Cancer Diagnosis
The mean age of patients at the time of their prostate cancer diagnosis was similar in each cohort, ranging from 65.7 years in patients with M0 to 69.1 years in patients with mHSPC (Table 1). The percentage of patients with extracapsular extension of their tumour (≥T3a) at diagnosis was 83.2% for patients with HRL, 34.4% for patients with M0, 58.1% for mHSPC, and 46.5% for mCRPC. Regional lymph node extension (N1) was most frequent in M1 patients (38.1% for mHSPC and 34.1% for mCRPC) and was least common amongst M0 patients (9.8%). A total of 84.5% of patients with mHSPC and 75.9% with mCRPC had de novo metastases. In 1937 patients with a Gleason score available at diagnosis, 29.0% of M0 patients had Gleason scores between 8 and 10, vs 74.4% of patients with HRL, 76.9% with mHSPC, and 71.6% with mCRPC.
Table 1.
Demographic characteristics and disease features at the time of diagnosis.
| Characteristic | HRL N = 357 | M0 N = 378 | mHSPC N = 1038 | mCRPC N = 290 |
|---|---|---|---|---|
| Age, years, mean (sd) | 68.3 (8.10) | 65.7 (6.83) | 69.1 (7.91) | 67.6 (8.38) |
| PSA level, ng/mL, median (IQR) | 29.1 (14.7–59.5) | 13.2 (7.7–25.1) | 105.0 (40.8–498) | 100.0 (3.5–9883) |
| TMN, n (%) | ||||
| <T3a | 58 (16.2) | 220 (58.2) | 256 (24.7) | 84 (29.1) |
| ≥T3a | 297 (83.2) | 130 (34.4) | 603 (58.1) | 135 (46.5) |
| Tx | 2 (0.6) | 28 (7.4) | 179 (17.2) | 71 (24.5) |
| N0 | 253 (70.9) | 319 (84.4) | 333 (32.1) | 83 (28.6) |
| N1 | 63 (17.6) | 19 (5.0) | 395 (38.1) | 99 (34.1) |
| Nx | 41 (11.5) | 18 (4.8) | 310 (29.9) | 108 (37.2) |
| M0 | 334 (93.6) | 350 (92.6) | 117 (11.2) | 55 (19.0) |
| M1 | 0 | 0 | 877 (84.5) | 220 (75.9) |
| Mx | 23 (6.4) | 28 (7.4) | 44 (4.2) | 15 (5.2) |
| Gleason score, N with data | 341 | 359 | 958 | 271 |
| 2–6*, n (%) | 19 (5.6) | 97 (27.0) | 39 (4.1) | 21 (7.7) |
| 7, n (%) | 69 (20.2) | 158 (44.0) | 182 (19.0) | 56 (20.7) |
| 8–10, n (%) | 253 (74.4) | 104 (29.0) | 737 (76.9) | 194 (71.6) |
SD, standard deviation; IQR, interquartile range; N, total number of patients; n (%), number/percentage of patients in each category; HRL, High‐risk localised prostate cancer; M0, non‐metastatic biochemically recurrent prostate cancer; M1, metastatic PC comprising mHSPC, metastatic hormone‐sensitive prostate cancer and mCRPC, metastatic castrate‐resistant prostate cancer.
Across all stages, 13 patients had a Gleason score of 3, nine had a score of 4, 17 had a score of 5, and 137 had a score of 6.
Demographic and disease features at diagnosis in individual countries generally reflected those of the overall analysis (Table S1). The age range at diagnosis was largest in China [mean age ranging from 58.0 years for M0 (three patients) to 69.8 years for mCRPC (20 patients)] and lowest in Malaysia [range from 67.5 years in HRL (22 patients) to 69.5 years in mCRPC (39 patients)]. The percentage of M1 patients with de novo metastases was: 73.2% in Singapore, 73.9% in Taiwan, 79.4% in Japan, 86.2% in Malaysia, 88.5% in Korea, 88.8% in Thailand, 91.7% in China, and 94.1% in India.
Disease Features at Registry Enrolment
The median duration between prostate cancer diagnosis and registry enrolment for each cohort ranged from 2.5 to 4.3 years for patients with HRL, mHSPC or mCRPC, and was 6.7 years for patients with M0 (Table 2). About two‐thirds of patients in each cohort had co‐morbidities. The most frequently reported co‐morbidities in all cohorts combined were: cardiovascular disease, including hypertension in 866 (42.0%) patients; diabetes in 341 (26.6%) patients; hypercholesterolaemia in 220 patients (10.6%); and other cardiovascular disease in 213 (10.3%) patients (data not shown). The percentage of patients with a known first‐degree family history of prostate cancer or breast cancer was similar in each cohort (range 2.7–4.3% for prostate cancer and 1.2–3.8% for breast cancer) (data not shown).
Table 2.
Demographic and disease features at the time of registry enrolment.
| Characteristic | HRL N = 357 | M0 N = 378 | mHSPC N = 1038 | mCRPC N = 290 |
|---|---|---|---|---|
| Years from diagnosis until enrolment, median (IQR) | 2.7 (0.5–4.0) | 6.7 (3.7–9.7) | 2.5 (0.2–3.5) | 4.3 (1.8–5.5) |
| N (%): | ||||
| Co‐morbidities * | 225 (63.0) | 224 (59.3) | 621 (59.8) | 205 (70.7) |
| Cardiovascular | 175 (49.0) | 171 (45.2) | 485 (46.7) | 165 (56.9) |
| Respiratory | 22 (6.2) | 9 (2.4) | 68 (6.6) | 16 (5.5) |
| Renal | 12 (3.4) | 20 (5.3) | 58 (5.6) | 33 (11.4) |
| Hepatic | 14 (3.9) | 20 (5.3) | 37(3.6) | 10 (3.4) |
| Neurological | 17 (4.8) | 20 (5.3) | 53 (5.1) | 18 (6.2) |
| Endocrine (DM) | 72 (20.2) | 58 (15.3) | 207 (19.9) | 67 (23.1) |
| Other | 39 (10.9) | 70 (18.5) | 137 (13.2) | 59 (20.3) |
| Skeletal‐related event | – | 2 (0.5)† | 55 (5.3) | 29 (10.0) |
| Prior bone‐targeted therapy | ||||
| Zoledronic acid | 4 (1.1) | 9 (2.4) | 145 (14.0) | 55 (19.0) |
| Denosumab | 4 (1.1) | 4 (1.1) | 115 (11.1) | 32 (11.0) |
| Radium 223 | 0 | (0) | 7 (0.7) | 1 (0.3) |
| Others | 1 (0.3) | (0) | 11 (1.1) | 4 (1.4) |
| Castration status at baseline | ||||
| Medically or surgically castrated | 275 (77.0) | 264 (69.8) | 887 (85.5) | 288 (99.3) |
| Castration resistant | 1 | 36 (9.5) | 0 | 290 (100) |
| Time from initial diagnosis to castration, years, median (IQR) | 0.1 (0–0.2) | 1 (0.2–3.9) | 0.1 (0–0.3) | 0.2 (0.1–0.6) |
DM, diabetes mellitus; SD, standard deviation; IQR, interquartile range; N, total number of patients; n (%), number/percentage of patients in each category; HRL, High‐risk localised prostate cancer; M0, non‐metastatic biochemically recurrent prostate cancer; M1, metastatic PC comprising mHSPC, metastatic hormone‐sensitive prostate cancer and mCRPC, metastatic castrate‐resistant prostate cancer.
Numbers are not additive, as patients may have had more than one co‐morbidity.
Patients with non‐metastatic CRPC.
Bone metastases were present in 79.8% of M1 patients at enrolment, of which the majority (~87%) were located axially (Table 3). In 798 of the M1 patients who underwent additional imaging, 49.9% had radiological evidence of non‐bone metastases. The most frequently implicated sites were lymph nodes (42.5% of all non‐bone metastases), lung and thoracic cavity (22.6%), abdominal cavity and pelvis (11.4%), and liver (4.6%). The site of the metastasis was not specified in 39.9% of patients with a non‐bone metastasis.
Table 3.
Characteristics of patients with metastatic (M1) prostate cancer.
| Characteristic, n (%) | M1 (N = 1328) |
|---|---|
| Radiological imaging | |
| Had undergone imaging at baseline* | 1328 (100) |
| Bone metastases in all M1 patients † | 1059 (79.8) |
| Axial | 919 (86.8) |
| Appendicular | 295 (27.9) |
| Unspecified | 22 (2.1) |
| Had undergone relevant imaging for non‐bone metastases | 798 |
| Any non‐bone metastasis | 393 (49.9) |
| Node – sub‐diaphragmatic | 114 (29.0) |
| Node – supra‐diaphragmatic | 40 (10.2) |
| Node – unspecified | 13 (3.3) |
| Liver | 18 (4.6) |
| Lung | 49 (12.5) |
| Abdominal cavity | 5 (1.3) |
| Pelvis | 40 (10.2) |
| Bladder | 2 (0.5) |
| Thoracic cavity | 40 (10.2) |
| Unspecified | 157 (39.9) |
M1, metastatic PC comprising mHSPC, metastatic hormone‐sensitive prostate cancer and mCRPC, metastatic castrate‐resistant prostate cancer.
Imaging includes CT, positron‐emission tomography‐CT, MRI, TRUS, X‐ray, other unspecified scans reporting non‐bone metastasis.
Numbers are not additive as patients may have had more than one metastasis.
By enrolment, 52.6% of all patients had undergone a bone scan, 30% had a CT scan, and 12.3% had MRI. The percentage of patients with prostate cancer who had undergone bone scan by enrolment was highest in Thailand (76%) and Malaysia (74%); and lowest in Singapore (24%) and India (36%). MRI was more common in India (25.5%) than in the other countries (range 7–17%). There were 93 patients (26.1%) with HRL and 65 (17.2%) with M0 who had undergone any radiological assessment prior to enrolment.
At the time of enrolment, skeletal‐related events had been experienced by 55 (5.3%) patients in the mHSPC cohort and in 32 (11.3%) patients with mCRPC (Table 2). The most frequently used bone‐targeted therapies were zoledronic acid and denosumab (15% and 11% of M1 patients, respectively).
Medical or surgical castration was undertaken in 77.0% of patients with HRL, 85.5% of patients with mHSPC, and almost all patients with mCRPC, within several months after the initial prostate cancer diagnosis (Table 2). In M0 patients, 69.8% underwent castration after a median period of 1 year after diagnosis. Few patients in the M0 cohort (9.5%) showed evidence of castration resistance.
Treatment Pattern at Registry Enrolment
Between 30% and 40% of patients with HRL or M0 received combined androgen blockade (CAB; an anti‐androgen drug and either orchidectomy or LHRH analogues) and 36–37% received androgen‐deprivation therapy (ADT; either orchidectomy or LHRH analogues; Fig. 3). CAB was used by 57.0% of patients with mHSPC and by 62.8% with mCRPC, and 31.1% and 34.5%, respectively, received ADT.
Figure 3.
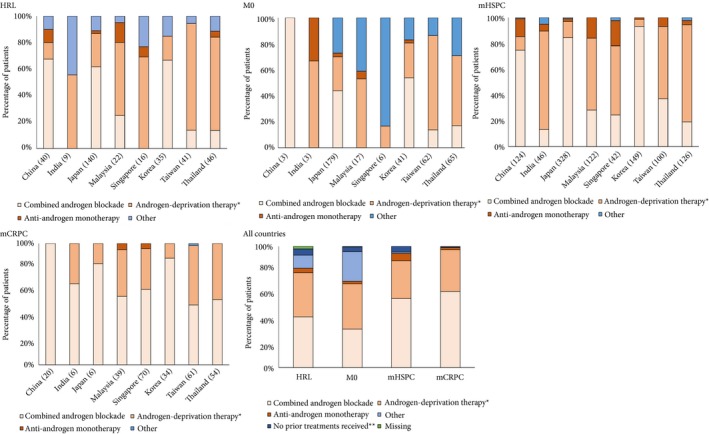
Drug treatment patterns amongst patients with prostate cancer in Asia by country and overall. *Either orchidectomy or LHRH analogues. ‘Other’ includes RP alone or with definitive RT, or chemotherapy alone. Results are tabulated in Table S2.
There were marked differences in treatment patterns between participating countries for each disease stage (Fig. 3, Table S2), although the small numbers of patients in some categories prevents drawing firm conclusions. CAB was the most common treatment choice for all disease stages in China, Japan, and Korea; and for treatment of mCRPC in all countries. ADT (either orchidectomy or LHRH analogues) was the most common treatment for HRL, M0, and mHSPC in India, Malaysia, Taiwan, and Thailand; and for HRL and mHSPC in Singapore. A small percentage of patients in each disease stage received anti‐androgen monotherapy.
Hormonal Therapy
Hormonal drug therapy (LHRH analogues, anti‐androgens or adrenal/androgen biosynthesis inhibitors) was preferred over surgical castration in all disease stages (Table 4). Prior to registry enrolment, hormonal drug therapy was administered to 55.1–58.2% of patients with HRL, M0 or mHSPC, and to 79.7% of patients with mCRPC; while 2.2–11.0% of patients with HRL, M0 or mHSPC, and 24.1% with mCRPC had undergone orchidectomy. In patients with M1 prostate cancer, orchidectomy was performed more frequently in India (56% of patients with M1) and in Thailand (58%) than in the other study countries (≤25%; data not shown).
Table 4.
Treatments received by patients with prostate cancer prior to enrolment.
| Treatment*, n (%) | HRL N = 357 | M0 N = 378 | mHSPC N = 1038 | mCRPC N = 290 |
|---|---|---|---|---|
| Prior treatment | ||||
| Radiotherapy | 132 (37.0) | 198 (52.4) | 122 (11.8) | 78 (26.9) |
| Hormonal drug therapy | 199 (55.7) | 220 (58.2) | 572 (55.1) | 231 (79.7) |
| Chemotherapy | 8 (2.2)† | 4 (1.1%)† | 90 (8.7) | 62 (21.4) |
| Orchidectomy | 8 (2.2) | 9 (2.4) | 114 (11.0) | 70 (24.1) |
| Radical prostatectomy | 132 (37.0) | 247 (65.3) | 81 (7.8) | 27 (9.3) |
| None | 0 | 0 | 2 (0.2) | 0 |
| Prior Radiotherapy | ||||
| Definitive only | 108 (30.3) | 158 (41.8) | 42 (4.0) | 29 (10.0) |
| Palliative only | 9 (2.5) | 16 (4.2) | 66 (3.4) | 41 (14.1) |
| Definitive and palliative | 13 (3.6) | 9 (2.4) | 8 (0.8) | 5 (1.7) |
| Type of radiotherapy | ||||
| Brachytherapy | 14 (3.9) | 24 (6.3) | 2 (0.2) | 3 (1.0) |
| EBRT | 58 (16.2) | 111 (29.4) | 86 (8.3) | 41(14.1) |
| Conformal | 65 (18.2) | 64 (16.9) | 27 (2.6) | 23 (7.9) |
| Cyberknife | 0.0 | 0.0 | 1 (0.1) | 0.0 |
| Others | 4 (1.1) | 6 (1.6) | 10 (1.0) | 13 (4.5) |
| Prior hormonal therapy | 202 | 222 | 573 | 231 |
| LHRH analogues | 170 (47.6) | 199 (52.6) | 380 (36.6) | 133 (45.9) |
| Buserelin | 0 | 0 | 1 (0.1) | 0 |
| Goserelin | 33 (9.2) | 69 (18.3) | 105 (10.1) | 48 (16.6) |
| Leuprorelin | 95 (26.6) | 142 (37.6) | 210 (20.2) | 88 (30.3) |
| Triptorelin | 24 (6.7) | 32 (8.5) | 37 (3.6) | 25 (8.6) |
| Degarelix | 36 (10.1) | 12 (3.2) | 109 (10.5) | 10 (3.4) |
| Anti‐androgens | 121 (33.9) | 144 (38.1) | 417 (40.2) | 200 (69.0) |
| Bicalutamide | 118 (33.1) | 139 (36.8) | 391 (37.7) | 188 (64.8) |
| Flutamide | 7 (2.0) | 10 (2.6) | 86 (8.3) | 34 (11.7) |
| Enzalutamide | 5 (1.4) | 7 (1.9) | 34 (3.3) | 11 (3.8) |
| Nilutamide | 0 | 0 | 0 | 0 |
| Cyproterone | 0 | 0 | 0 | 1 (0.3) |
| Adrenal/androgen biosynthesis inhibitors | 0 | |||
| Abiraterone acetate (Zytiga) + prednisone/ prednisolone | 0 | 3 (0.8) | 40 (3.9) | 20 (6.9) |
| Non‐branded abiraterone acetate | 0 | 0 | 4 (0.4) | 2 (0.7) |
| Others/unspecified | 0 | 0 | 1 (0.3) | 0 |
| Prior chemotherapy | ||||
| Cabazitaxel | – | – | 5 (0.5) | 0 |
| Docetaxel | – | – | 86 (8.3) | 59 (20.3) |
| Platinum | – | – | 1 (0.1) | 3 (1.0) |
| Other | – | – | 11 (1.1) | 5 (1.7) |
N, total number of patients; n (%), number/percentage of patients in each category; HRL, High‐risk localised prostate cancer; M0, non‐metastatic biochemically recurrent prostate cancer; M1, metastatic PC comprising mHSPC, metastatic hormone‐sensitive prostate cancer and mCRPC, metastatic castrate‐resistant prostate cancer; EBRT, external beam radiotherapy; LHRH, luteinizing hormone‐releasing hormone.
Numbers are not additive as patients could have undergone more than one treatment.
Some patients were enrolled in clinical trials; others were treated according to local guidelines, which allow use of chemotherapy in these stages.
LHRH agonists/antagonists were used by 47.6% of patients with HRL, 52.6% with M0, 36.6% with mHSPC, and 45.9% with mCRPC; of which the most commonly prescribed was leuprorelin (Table 5). The LHRH antagonist (degarelix) was used in up to 10.5% of patients. Only one patient (mHSPC) used buserelin.
Table 5.
Most frequent reasons for initiating and discontinuing treatment.*
| Variable, n (%) | HRL N = 357 | M0 N = 378 | mHSPC N = 1038 | mCRPC N = 290 |
|---|---|---|---|---|
| Initiated hormonal therapy | 217 | 203 | 885 | 249 |
| Following treatment guidelines† | 88 (40.6) | 47 (23.2) | 321 (36.3) | 139 (55.8) |
| Disease progression | 59 (27.2) | 118 (58.1) | 278 (31.4) | 123 (49.4) |
| PSA progression | 38 (17.5) | 106 (52) | 218 (24.6) | 89 (35.7) |
| Clinical progression | 23 (10.6) | 17 (8.3) | 101 (11.4) | 28 (11.2) |
| Radiological progression | 4 (1.8) | 1 (0.5) | 33 (3.7) | 33 (13.3) |
| Unspecified | 75 (34.6) | 58 (28.6) | 281 (31.8) | 55 (22.1) |
| Patient disease‐related characteristics | 16 (7.4) | 6 (3.0) | 120 (13.6) | 28 (11.2) |
| Safety profile | 4 (1.8) | 1 (0.5) | 25 (2.8) | 5 (2.0) |
| Patient preference | 1 (0.5) | 3 (1.5) | 30 (3.4) | 16 (6.4) |
| Discontinued hormonal therapy | 86 | 76 | 324 | 114 |
| Disease progression | 23 (26.7) | 30 (39.5) | 136 (42.0) | 71 (62.3) |
| Inconvenience | 2 (2.3) | 2 (2.6) | 10 (3.1) | 2 (1.8) |
| Treatment‐related side‐effects | 2 (2.3) | 2 (2.6) | 9 (2.8) | 16 (14.0) |
| Stable disease | 5 (5.8) | 7 (9.2) | 2 (0.6) | – |
| Unspecified | 20 (23.3) | 11 (14.4) | 102 (31.5) | 16 (14) |
| Patient decision | 1 (1.2) | 4 (5.3) | 19 (5.9) | 10 (8.8) |
| Affordability for the patient | 1 (1.2) | 1 (1.3) | 6 (1.9) | 2 (1.8) |
| No response to treatment | 1 (1.2) | – | 4 (1.2) | – |
| Lost to follow‐up | 1 (1.2) | – | 6 (1.9) | 1 (0.9) |
| New/deterioration of co‐morbidities | – | 2 (2.6) | 3 (0.9) | 6 (5.3) |
| Death | – | 2 (2.6) | 15 (4.6) | 7 (6.1) |
| Initiated chemotherapy | – | – | 168 | 60 |
| Disease progression | – | – | 72 (42.9) | 42 (70.0) |
| PSA progression | – | – | 51 (30.4) | 29 (48.3) |
| Clinical progression | – | – | 21 (12.5) | 9 (15.0) |
| Radiological progression | – | – | 14 (8.3) | 15 (25.0) |
| Following treatment guidelines† | – | – | 49 (29.2) | 9 (15.0) |
| Ongoing prior to enrolment | – | – | 15 (8.9) | 6 (10.0) |
| Patient disease‐related characteristics | – | – | 13 (7.7) | 1 (1.7) |
| Unspecified | – | – | 9 (5.4) | 2 (3.3) |
| Safety profile | – | – | 7 (4.2) | 1 (1.7) |
| Patient preference | – | – | 2 (1.2) | 1 (1.7) |
| Toxicity of previous treatment | – | – | 2 (1.2) | 1 (1.7) |
| Discontinued chemotherapy | 88 | 44 | ||
| Completed therapy | – | – | 48 (54.5) | 22 (50.0) |
| Disease progression | – | – | 18 (20.5) | 15 (34.1) |
| Treatment‐related side‐effects | – | – | 7 (8.0) | 4 (9.1) |
| Unspecified | – | – | 15 (17) | 1 (2.3) |
| Patient decision | – | – | 1 (1.1) | 1 (2.3) |
| Death | – | – | 3 (3.4) | – |
| Affordability for the patient | – | – | 1 (1.1) | – |
| New/worsening co‐morbidities | – | – | – | 2 (4.5) |
N, total number of patients; n (%), number/percentage of patients in each category; HRL, High‐risk localised prostate cancer; M0, non‐metastatic, biochemically recurrent prostate cancer; M1, metastatic PC comprising; mHSPC, metastatic hormone‐sensitive prostate cancer and mCRPC, metastatic castrate‐resistant prostate cancer.
Numbers are not additive as more than one reason could be reported.
International, national or site guidelines.
Anti‐androgens were used by 33.9% of patients with HRL, 38.1% with M0, 40.2% with mHSPC, and 69.0% with mCRPC; of which the most commonly prescribed was bicalutamide. Flutamide or enzalutamide were used by ≤11.7% of patients in each cohort, no patient received nilutamide, and one patient (mCRPC) received cyproterone.
Few patients received adrenal/androgen biosynthesis inhibitors, of which abiraterone acetate (plus prednisone/prednisolone) was administered most frequently (4.2% of patients with mHSPC and 7.6%with mCRPC (Table 4).
Chemotherapy was used by 9.9% of patients with mHSPC and by 23.1% with mCRPC. Docetaxel was the most frequently prescribed chemotherapeutic agent (8.3% of patients with mHSPC and 20.3% with mCRPC). Few patients received cabazitaxel or platinum‐based treatments.
Radical Prostatectomy
Rates of radical prostatectomy were highest in the M0 cohort (65.3%) and lowest in the mHSPC and mCRPC cohorts (7.8% and 9.3%, respectively; Table 4). The distribution of surgical procedure varied across the study countries (Fig. 4). Robot‐assisted radical prostatectomy dominated in Malaysia and Singapore, laparoscopic procedures were used more frequently than robot‐assisted or open procedures in China and India. The proportion of patients who received open radical prostatectomy was higher than those that received robot‐assisted or laparoscopic procedures in Japan and Taiwan.
Figure 4.
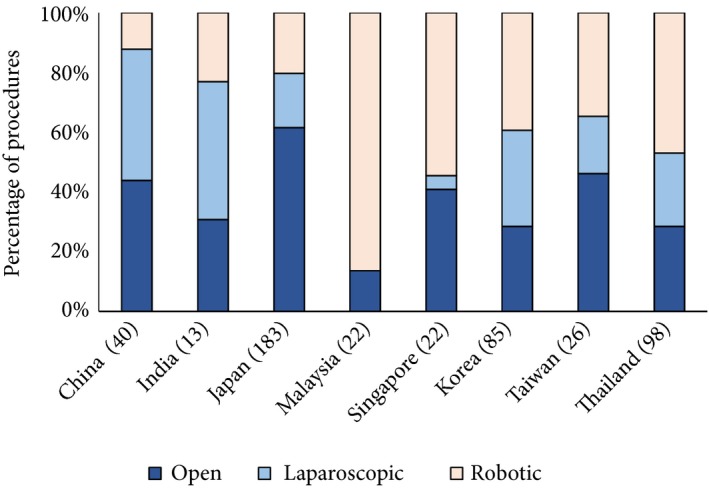
Surgical procedure in 489 patients who underwent RP by country. Brackets indicate number of patients.
Radiotherapy
Most radiotherapy was used with curative intent, with few patients in the HRL, M0 or mHSPC cohorts receiving palliative radiotherapy (Table 4). Palliative radiotherapy was administered to 15.8% of patients with mCRPC. External beam was undertaken most frequently in patients with M0 or M1 disease, whereas external beam and conformal RT was used similarly in patients with HRL.
Rationale for Treatment Decisions
Decisions to initiate hormonal drug therapy were mainly driven by PSA or clinical progression, and in compliance with international, national or site treatment guidelines (Table 5). There were 600 patients who discontinued hormone treatment, of whom 114 had mCRPC. The most common reason for discontinuation in all cohorts was disease progression. Few patients discontinued hormonal therapy due to costs (<2% in each cohort). In patients with mCRPC, 62.3% of patients who discontinued hormonal therapy did so due to progression despite treatment, 14.0% discontinued treatment due to treatment‐related side‐effects, 5.3% due to new/deterioration of co‐morbidities, and 6.1% of patients died. In 8.8% of cases the patient made the decision to stop treatment.
Among patients with mHSPC or mCRPC, disease progression (PSA or clinical progression), followed by compliance with treatment guidelines, were the most common reasons for initiating chemotherapy (Table 4). About one‐half of patients who underwent chemotherapy had completed treatment at enrolment. Others discontinued chemotherapy because of progression despite treatment and treatment‐related side‐effects.
Health Status and QoL
At enrolment, the percentage of patients in each cohort with an Eastern Cooperative Oncology Group (ECOG) performance score of 0 or 1 was 96.0% for HRL, 95.3% for M0, 92.7% for mHSPC, and 89.3%R for mCRPC (Table 6). In the QoL assessment using the EQ‐5D‐5L, there were significant differences between the cohorts in mean visual analogue scores. The highest visual analogue scores (indicating a perception of better health) were in the HRL [mean (sd) score 79.6 (15.66), out of a possible 100] and M0 [mean (sd) score of 79.1 (14.33)] cohorts (Table 6). The mean (sd) visual analogue scores in the mHSPC and mCRPC cohorts were 74.9 (17.62) and 73.6 (18.22), respectively. Furthermore, >80% of patients in each health dimension reported Level 1 to Level 2 scores (indicating no problem or slight problems, respectively). Not surprisingly, HRL and M0 had the highest proportion of patients who reported no to slight problems/limitations, while the mCRPC cohort has the lowest proportion; although overall the scores are still relatively better.
Table 6.
Quality of life at registry enrolment.
| HRL N = 357 | M0 N = 378 | mHSPC N = 1038 | mCRPC N=290 | P‐value between cohorts | |
|---|---|---|---|---|---|
| Baseline ECOG | 249 | 232 | 770 | 272 | |
| 0–1 n (%) | 239 (96.0) | 221 (95.3) | 714 (92.7) | 243 (89.3) | |
| ≥2 n (%) | 10 (4.0) | 11 (4.7) | 56 (7.3) | 29 (10.7) | |
| EQ5D questionnaire completed | 353 | 375 | 1034 | 287 | |
| Baseline EQ5D VAS, mean (sd) | 79.6 (15.66) | 79.1 (14.33) | 74.9 (17.62) | 73.6 (18.22) | <0.001 |
| Baseline utility score, mean (sd) | |||||
| Mobility issues | 1.4 (0.80) | 1.4 (0.78) | 1.6 (0.93) | 1.6 (0.97) | |
| Self‐care issues | 1.2 (0.65) | 1.1 (0.50) | 1.2 (0.66) | 1.3 (0.82) | |
| Issues with performing usual activities | 1.3 (0.78) | 1.3 (0.75) | 1.5 (0.86) | 1.5 (0.92) | |
| Anxiety/depression | 1.3 (0.68) | 1.3 (0.60) | 1.4 (0.72) | 1.4 (0.69) | |
| Pain and discomfort | 1.5 (0.78) | 1.5 (0.76) | 1.8 (0.87) | 1.8 (0.88) | |
| FACT‐P questionnaire completed | 353 | 375 | 1033 | 187 | |
| Baseline FACT‐P score, mean (sd) | |||||
| Total score | 118.5 (19.46) | 117.1 (19.17) | 113.7 (20.98) | 113.3 (23.14) | <0.001 |
| Physical well‐being | 24.7 (4.04) | 25.3 (3.33) | 23.4 (4.85) | 22.6 (5.76) | <0.001 |
| Social and family well‐being | 18.8 (6.62) | 17.2 (7.24) | 19.3 (6.44) | 20.4 (5.60) | <0.001 |
| Emotional well‐being | 19.9 (4.06) | 19.7 (3.66) | 19.1 (4.24) | 18.8 (4.71) | <0.001 |
| Functional‐well bring | 20.8 (6.38) | 20.8 (6.42) | 19.4 (6.83) | 19.1 (6.44) | <0.001 |
| Prostate cancer subscale | 34.4 (6.75) | 34.1 (6.51) | 32.6 (7.72) | 32.3 (8.30) | <0.001 |
HRL, High‐risk localised prostate cancer; M0, non‐metastatic, biochemically recurrent prostate cancer; M1, metastatic PC comprising; mCRPC, metastatic castrate‐resistant prostate cancer; mHSPC, metastatic hormone‐sensitive prostate cancer; n (%), number/percentage of patients in each category; N, total number of patients; SD, standard deviation; VAS, visual analogue score.
The FACT‐P total scores and prostate cancer subscale scores showed similar trends, reflecting higher scores (indicating good health) in HRL and M0, and slightly lower mean scores in the mHSPC and mCRPC cohorts. Of 397 patients who reported use of pain medications, strong opioids were used for symptom management by 4% (one of 24) of patients in the HRL cohort, 11% (three of 27) in the M0 cohort, 15% (36/242) in the mHSPC cohort, and 18% (19/104) in the mCRPC cohort.
Discussion
This first interim analysis of data from the UFO registry describes the patterns of diagnosis, disease characteristics, and treatment patterns in a large cohort of patients with prostate cancer in Asia. Most of the prostate cancer diagnoses in the study were symptom‐driven, reflecting the absence of routine screening programmes in all of the participating countries 11. De novo metastases were present in 82.6% of all patients with M1 disease at enrolment, ranging from 73.2% to 94.1% across the eight participating countries. The highest percentages of patients with de novo metastases were enrolled in China and India, possibly due to late detection, as most patients consult only following onset of symptoms. It is also worthy to note that the Registry specifically enrolled patients with metastatic prostate cancer, which means that the percentage of patients presenting with M1 disease is likely to be higher than that observed in the general prostate cancer population. For example, in South Korea, de novo metastases are encountered only in up to 9% of patients at initial prostate cancer diagnosis 12. A large study in the USA, where routine PSA screening is performed, reported de novo metastases in 5.20–6.85% of patients with prostate cancer (depending on ethnicity) 13. On the other hand, although several professional societies recommend routine imaging of patients with high‐risk prostate cancer 14, only 17.2% of M0 patients and 26.1% of HRL patients had undergone any radiological imaging at enrolment.
Significantly increased mortality in patients presenting with M1 disease compared with those that present without de novo metastases suggests that earlier detection in these patients could contribute to improved outcomes for patients in Asia 13. However, low incidence rates, uncertainty about the validity of PSA screening cut‐offs for Asian populations, and other healthcare priorities within the available budget, have to date worked against implementation of routine PSA screening in Asia 11.
Most patients were already on treatment at baseline, which may have positively impacted QoL. Despite the advanced disease of the Registry cohort, most patients reported no limitations or only slight limitations in their health‐related QoL. QoL measures were poorest amongst patients with M1 disease, of whom 16% required strong‐acting opioids for pain control. Only 25.2% of patients with M1 disease received targeted‐bone therapy, suggesting that most patients with bone metastases from prostate cancer were undertreated for the prevention of skeletal complications at the time of enrolment. This could reflect the high cost of therapy, and/or limited access to drugs in some study centres.
Inter‐country differences were evident in treatment practices for prostate cancer. The use of CAB was relatively more common in Japan, China, and Korea for all patient groups; whereas, ADT was more common in Taiwan, India, Thailand, Malaysia, and Singapore. Use of CAB and/or ADT was widespread in all countries for patients with mHSPC and mCRPC, whereas patients with HRL or M0 disease also received anti‐androgen monotherapy and/or other treatments (usually radical prosatectomy , chemotherapy or radiotherapy). These differences may reflect local practices and guidelines or drug/procedure availability and national reimbursement schemes. The heterogeneity between different countries in Asia is also highlighted by the Asian Prostate Cancer study, which is collecting information on patients with all stages of prostate cancer in Asia 15, 16.
Few patients received chemotherapy, and docetaxel use in particular was recorded only in ~8% of patients with mHSPC and 20% with mCRPC. Although utilisation was low, completion of chemotherapy was high amongst those who received it, suggesting strong compliance. The low use of chemotherapy could reflect preferences and experience of the treating physician (mainly urologists) and/or patient preference to avoid possible chemotherapy‐related adverse events. In addition, fewer patients received the novel hormonal agents, abiraterone and enzalutamide, by the time of study enrolment. This could possibly be due to unavailability of drugs commercially at the time of patient diagnosis, or lack of access thereof due to early issues in including the drugs under healthcare reimbursement. The limited availability and scant use of novel agents contrasts with some Western countries. In the USA, where abiraterone has been available since 2011, 34% of patients with mCRPC received first‐line treatment with abiraterone acetate (plus prednisone/prednisolone) and 26.0% received enzalutamide 17. We also found regional variations in the distribution of surgical procedures for radical prostatectomy; although this may reflect practice preferences in the background of the surgeon’s type of procedural qualification (whether robot‐assisted or laparoscopic qualification vs extensive experience in performing open radical prostatectomy), as well as availability of specialised facilities to perform the procedure at the individual study sites, rather than national or regional trends. This is consistent with regional reports that have noted a decrease in the use of radical prostatectomy in Asia between 2006 and 2013, and increasing use of robot‐assisted surgery in some countries, e.g., in Japan, where robot‐assisted surgery has been approved under the Japanese health insurance system 11, 15.
Almost 39% of patients who initiated hormonal drug treatment later discontinued or changed therapy, including 46% of patients with mCRPC, most frequently due to disease progression or treatment‐related side‐effects.
Strengths of the present study include enrolment of a large population of men from countries across Asia, which differ significantly in their healthcare infrastructure. To our knowledge, this is the only registry to differentiate between patients with mHSPC and mCRPC, and thus will provide an important picture on how these diseases are managed in Asia. By ensuring that a high percentage of enrolled patients had advanced disease, meaningful information on disease treatment and progression will be possible over the 5‐year study period. Finally, unlike the clinical trial environment, this study is using real‐world data, as well as patient‐reported outcomes, to understand the treatment and outcomes of patients with prostate cancer in Asia.
Potential limitations of the study include the risk of selection bias and case ascertainment bias, which we have minimised by consecutive patient enrolment using standardised disease stage definitions. Additionally, some information based on recall (such as reasons for treatment decision‐making) cannot be validated and could be subject to recall bias. The proportion and number of patients from different countries may be influenced by the timing of site activation and does not reflect the percentage of patients with prostate cancer in each country. In that regard, countries that started enrolling earlier may have a relatively higher proportion of patients with M0/HRL due to the cap enrolment rule for these stages.
The data presented here combine results from eight economically diverse countries, and deeper investigation of each country‐specific setting is needed to understand differences between countries and the reasons that drive those differences. Follow‐up in the UFO registry will continue to 5 years, and interim analyses of data will be reported regularly during this time.
Conclusion
In the UFO registry of advanced prostate cancer in Asia, regional differences exist in prostate cancer characteristics and treatment patterns, with more use of CAB in some countries compared with other regions. Overall, the use of chemotherapy and novel agents is low. Prospective follow‐up is ongoing. The UFO registry will provide valuable descriptive data on current disease characteristics and the treatment landscape amongst patients with prostate cancer in Asia.
Conflict of Interests
Hirotsugu Uemura reports grants from: Janssen, Astellas, Takeda, AstraZeneca; personal fees from: AstraZeneca, Astellas, Sanofi; other from: Sanofi, outside the submitted work.
Edmund Chiong reports receiving institutional grants from Janssen during the conduct of the study, and honoraria and conference support from Janssen outside the submitted work.
Ravindran Kanesvaran reports grants and personal fees from Johnson & Johnson Pte Ltd outside the submitted work.
Yanfang Liu is an employee of the Department of Global Epidemiology, Johnson & Johnson Pte Ltd.
Weiping Liu is an employee of Johnson & Johnson (China) Investment Ltd.
Marxengel Asinas‐Tan is an employee of Johnson & Johnson Pte Ltd at the time of the study.
Maximillian Van Kooten Losio was an employee of Johnson & Johnson Pte Ltd at the time of the study.
Yanfang Liu, and Maximillian Van Kooten Losio hold stock in Johnson & Johnson Pte Ltd.
Dingwei Ye, Sudhir Rawal, Azad Hassan Addual Razack, Yeong‐Shiau Pu, Hao Zeng, Byung Ha Chung, Yuh‐Shyan Tsai, Bannakij Lojanapiwat, Choung Soo Kim, Sunai Leewansangtong, Chikara Ohyama, and Noor Ashani Md Yusoff report that they have nothing to disclose.
Funding
All costs associated with development of this manuscript were funded by Janssen. The corresponding author had final responsibility for the decision to submit for publication.
Authors’ contributions
Hirotsugu Uemura, Dingwei Ye, Ravindran Kanesvaran, Hao Zeng, Yeong‐Shiau Pu, Edmund Chiong, Bannakij Lojanapiwat, Sudhir Kumar Rawal, Byung Ha Chung, Azad Hassan Addual Razack, Choung Soo Kim, Noor Ashani Md Yusoff, Chikara Ohyama, Sunai Leewansangtong, Yuh‐Shyan Tsai: Study design, data collection, interpretation of data. Yanfang Liu and Maximillian Van Kooten Losio: Study design and protocol development, statistical analysis, interpretation of data. Marxengel Asinas‐Tan: Statistical analysis and interpretation of data. Weiping Liu: Statistical analysis. All authors critically reviewed the manuscript. Each author gave final approval of the version to be published.
Supporting information
Table S1 . Demographic characteristics and disease features by country.
Table S2 . Hormone treatment patterns in patients with prostate cancer in Asia by country and overall.
Acknowledgements
Operations support for data collection and data cleaning was provided by Angelia Sim (Global Trial Manager at Johnson & Johnson, Pte. Ltd.). Investigators and site coordinators from the following sites have graciously given their time and effort in entering and reviewing data for the benefit of this registry: Gao Xin, the 3rd Affiliated Hospital, Sun Yat‐sen University, Guangzhou, Guangdong, China; Hu Zhiquan, Tongi Hospital, Tongi Medical College, Huazhong University, Wuhan, Hubei, China; Radheshyam Devanna Naik, Healthcare Global (HCG) Hospital, Bengaluru, Karnataka, India; Amlesh Seth, All India Institute of Medical Sciences, New Delhi, Delhi, India; Amit Joshi, Tata Memorial Hospital, Mumbai, Maharashtra, India; Gaku Arai, Dokkyo Medical University Koshigaya Hospital, Koshigaya, Saitama, Japan; Motohide Uemura, Osaka University Hospital, Suita, Osaka, Japan. Junya Furukawa, Kobe University Hospital, Kobe, Hyogo, Japan; Katsuyoshi Hashine NHO Shikoku Cancer Center, Matsuyama, Ehime, Japan; Ji Youl Lee, St. Mary’s Hospital, The Catholic University of Korea, Seoul, South Korea; Cheol Kwak, Seoul National University Hospital, Seoul, South Korea; Hyun Moo Lee, Samsung Medical Center, Seoul, South Korea; Yew Lam Chong, Tan Tock Seng Hospital, Singapore, Singapore; Apirak Santingamkun, King Chulalongkorn Memorial Hospital, Bangkok, Thailand; Choosak Pripatananont, Songkhla Hospital, Songkhla, Thailand; Tong‐Lin Wu, Kaohsiung Veterans General Hospital, Kaohsiung City, Taiwan. Writing assistance was provided by Joanne Wolter (independent writer on behalf of Janssen).
Trial registration: http://www.clinicaltrials.gov NCT02546908. Registry Identifier: NOPRODPCR4001.
References
- 1. Zhu Y, Wang HK, Qu YY, Ye DW. Prostate cancer in East Asia: evolving trend over the last decade. Asian J Androl 2015; 17: 48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kimura T, Egawa S. Epidemiology of prostate cancer in Asian countries. Int J Urol 2018; 25: 524–31 [DOI] [PubMed] [Google Scholar]
- 3. Baade PD, Youlden DR, Cramb SM, Dunn J, Gardiner RA. Epidemiology of prostate cancer in the Asia‐Pacific region. Prostate Int 2013; 1: 47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hsing AW, Devesa SS, Jin F, Gao YT. Rising incidence of prostate cancer in Shanghai, China. Cancer Epidemiol Biomarkers Prev 1998; 7: 83–4 [PubMed] [Google Scholar]
- 5. Katanoda K, Matsuda T, Matsuda A et al. An updated report of the trends in cancer incidence and mortality in Japan. Jpn J Clin Oncol 2013; 43: 492–507 [DOI] [PubMed] [Google Scholar]
- 6. Lim GH, Chow KY, Lee HP. Singapore cancer trends in the last decade. Singapore Med J 2012; 53: 3–9 [PubMed] [Google Scholar]
- 7. Ito K. Prostate cancer in Asian men. Nat Rev Urol 2014; 11: 197–212 [DOI] [PubMed] [Google Scholar]
- 8. Liu Y, Uemura H, Ye D et al. Prostate cancer in Asia: design of a patient registry to inform real‐world treatments, outcomes, and quality of life. Prostate Int 2019; 7: 108–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. EuroQol G. EuroQol – a new facility for the measurement of health‐related quality of life. Health Policy 1990; 16: 199–208 [DOI] [PubMed] [Google Scholar]
- 10. Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy‐prostate instrument. Urology 1997; 50: 920–8 [DOI] [PubMed] [Google Scholar]
- 11. Chen R, Ren S, Chinese Prostate Cancer Consortium et al. Prostate cancer in Asia: a collaborative report. Asian J Urol 2014; 1: 15–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tejedor JC, Omeñaca F, García‐Sicilia J et al. Immunogenicity and reactogenicity of a three‐dose primary vaccination course with a combined diphtheria‐tetanus‐acellular pertussis‐hepatitis B‐inactivated polio‐haemophilus influenzae type b vaccine coadministered with a meningococcal C conjugate vaccine. Pediatr Infect Dis J 2004; 23: 1109–15 [PubMed] [Google Scholar]
- 13. Akinyemiju T, Sakhuja S, Waterbor J, Pisu M, Altekruse SF. Racial/ethnic disparities in de novo metastases sites and survival outcomes for patients with primary breast, colorectal, and prostate cancer. Cancer Med 2018; 7: 1183–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wollin DA, Makarov DV. Guideline of guidelines: imaging of localized prostate cancer. BJU Int 2015; 116: 526–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lojanapiwat B, Lee JY, Gang Z et al. Report of the third Asian Prostate Cancer study meeting. Prostate Int 2019; 7: 60–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Youl Lee J, Taniguchi T, Zhang K et al. Report of the forth Asian Prostate Cancer (A‐CaP) study meeting. Jpn J Clin Oncol 2019; 49: 581–6 [DOI] [PubMed] [Google Scholar]
- 17. Caram ME, Estes JP, Griggs JJ, Lin P, Mukherjee B. Temporal and geographic variation in the systemic treatment of advanced prostate cancer. BMC Cancer 2018; 18: 258 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 . Demographic characteristics and disease features by country.
Table S2 . Hormone treatment patterns in patients with prostate cancer in Asia by country and overall.


