Abstract
A central theme of range‐limit theory (RLT) posits that abiotic factors form high‐latitude/altitude limits, whereas biotic interactions create lower limits. This hypothesis, often credited to Charles Darwin, is a pattern widely assumed to occur in nature. However, abiotic factors can impose constraints on both limits and there is scant evidence to support the latter prediction. Deviations from these predictions may arise from correlations between abiotic factors and biotic interactions, as a lack of data to evaluate the hypothesis, or be an artifact of scale. Combining two tenets of ecology—niche theory and predator–prey theory—provides an opportunity to understand how biotic interactions influence range limits and how this varies by trophic level.
We propose an expansion of RLT, interactive RLT (iRLT), to understand the causes of range limits and predict range shifts. Incorporating the main predictions of Darwin's hypothesis, iRLT hypothesizes that abiotic and biotic factors can interact to impact both limits of a species’ range. We summarize current thinking on range limits and perform an integrative review to evaluate support for iRLT and trophic differences along range margins, surveying the mammal community along the boreal‐temperate and forest‐tundra ecotones of North America.
Our review suggests that range‐limit dynamics are more nuanced and interactive than classically predicted by RLT. Many (57 of 70) studies indicate that biotic factors can ameliorate harsh climatic conditions along high‐latitude/altitude limits. Conversely, abiotic factors can also mediate biotic interactions along low‐latitude/altitude limits (44 of 68 studies). Both scenarios facilitate range expansion, contraction or stability depending on the strength and the direction of the abiotic or biotic factors. As predicted, biotic interactions most often occurred along lower limits, yet there were trophic differences. Carnivores were only limited by competitive interactions (n = 25), whereas herbivores were more influenced by predation and parasitism (77%; 55 of 71 studies). We highlight how these differences may create divergent range patterns along lower limits.
We conclude by (a) summarizing iRLT; (b) contrasting how our model system and others fit this hypothesis and (c) suggesting future directions for evaluating iRLT.
Keywords: abiotic stress, biotic interaction, climate change, condition‐specific competition, ecological niche, predator–prey theory, range limits, stress‐gradient hypothesis
Understanding why range limits form and shift is an important pursuit. The authors introduce a novel extension to a classic theory and provide evidence that range dynamics are the result of the interaction between abiotic and biotic factors. The approach is especially useful for predicting climate change impacts on species distributions.

1. INTRODUCTION
Understanding how and why geographical range limits form and change over time is a long‐standing inquiry of biogeographers and ecologists. An enduring hypothesis, dating back to Darwin (1859), posits that high‐latitude/altitude range limits are formed by stressful abiotic environments (e.g. cold climates), whereas lower limits are set by biotic interactions (e.g. competition, predation). This hypothesis is a major tenet of biogeography and has been subsumed in various definitions of the niche in ecology (Brown, Stevens, & Kaufmnan, 1996; Connell, 1961; Dobzhansky, 1950; MacArthur, 1984). It has undergone a recent resurgence given its potential to better understand the impacts of global change on species distributions (Anderegg & HilleRisLambers, 2019; Dvorský, Macek, Kopecký, Wild, & Doležal, 2017; Louthan, Doak, & Angert, 2015; Normand et al., 2009).
However, after more than a century of theoretical and empirical groundwork, there is little consensus on the extent to which abiotic and biotic factors (see Box 1 for definitions) determine range limits and how this varies by distributional edge position (Alexander, Diez, Usinowicz, & Hart, 2018; Godsoe, Jankowski, Holt, & Gravel, 2018; Louthan et al., 2015). Although many studies indicate that high‐latitude/altitude (hereafter upper) limits are formed by abiotic factors (see papers in Hargreaves, Samis, & Eckert, 2014), biotic factors can mediate abiotic stress along upper limits (Ettinger & HilleRisLambers, 2017; Pitt, Larivière, & Messier, 2008). Moreover, few studies have shown that low‐latitude/altitude (hereafter lower) limits are caused by biotic interactions (Cahill et al., 2014; Schemske, Mittelbach, Cornell, Sobel, & Roy, 2009). Potential explanations are that biotic interactions only have influence at local scales (Soberón, 2007; Wiens, 2011), or that the scant availability of biotic data at broad spatial scales (e.g. distribution of competitors) precludes meaningful inference (Wisz et al., 2013). Another possibility is that correlations between abiotic and biotic factors confound interpretations of the importance of either along range limits (Godsoe, Franklin, & Blanchet, 2017; Sexton, McIntyre, Angert, & Rice, 2009; Westoby, Kunstler, Leishman, & Morgan, 2017). Thus, it can appear that abiotic factors restrict populations along lower limits despite an underlying biotic constraint; the opposite process can also occur along upper limits. This correlation is rarely tested, yet it could provide important insight into the interactive nature of factors that form range limits and allow for predictions that will be valuable to conservation in the face of global change.
BOX 1. What is the difference between a biotic interaction and a biotic factor?
The terms ‘biotic interactions’ and ‘biotic factors’ are commonly used in range‐limit studies. However, they can have different meanings which can cause confusion. Biotic interactions are defined as direct intraspecific or interspecific interactions (e.g., competition, predation, mutualism) that have a negative, neutral, or positive effect on a focal species’ distribution or abundance and are typically limited to interactions within or between adjoining trophic levels (Anderson, 2017; Andrewartha & Birch, 1954; Krebs, 1972). Biotic factors, on the other hand, is a more general term that also includes unlinked biotic factors (density‐independent) that are not significantly consumed or contested and have influence at broad spatial and temporal scales (Anderson, 2017; Peterson et al., 2011; Soberón, 2007). These include ‘unlinked biotic predictors’ (e.g., habitat structure), ‘composite biotic predictors’ (e.g., habitat type), and ‘unlinked biotic interactors’ (e.g., distribution of a food resource) that can span multiple trophic levels and also have positive, negative, or neutral effects on a focal species (Anderson, 2017; Peterson et al., 2011). In some cases, positive or negative associations with unlinked biotic predictors/interactors (e.g., habitat type) have been used as proxies for biotic interactions, due to the paucity of interaction data at broad spatial scales (Morales‐Castilla, Matias, Gravel, & Araújo, 2015; Wisz et al., 2013); when this was evident, we included interpretations provided by studies. We refer to biotic interactions and unlinked biotic factors using the definitions described above and use biotic factors when studies combined these categories or were vague in their usage of them.
Recent scholarship (e.g. Godsoe, Jankowski, Holt, & Gravel, 2017) has emphasized the need to integrate ecological theory to better understand how and under what conditions biotic factors influence range limits, especially considering increasing threats from climate change, habitat conversion and species invasions (Guisan et al., 2013; Parmesan, 2006). To this end, we propose an expansion of current thinking on range limits—interactive range‐limit theory (iRLT)—which makes predictions for range limits and shifts. We begin by summarizing previous hypotheses and models on range limits to frame iRLT. We use a conceptual model to illustrate that range limits and shifts are the result of an interaction between abiotic and biotic factors, and provide evidence from an integrative review, primarily focused on North American mammals. We also investigate the evidence for how biotic interactions vary by trophic level and how this may create divergent range patterns for mammalian carnivores and herbivores. We end by outlining limitations and future directions of iRLT.
2. AN OVERVIEW OF RANGE‐LIMIT THEORY
Despite numerous theoretical and empirical investigations of range limits over the past centuries, there is not a clear definition of ‘range‐limit theory’. However, empirical models and hypotheses on ecological causes of range limits tend to group under three categories (Louthan et al., 2015; Sexton et al., 2009; Srinivasan, Elsen, Tingley, & Wilcove, 2018). The first category includes models that only consider abiotic factors to form range limits (Table 1). These include species distribution models which evaluate correlations between abiotic variables and empirical data or published physiological tolerances (Araujo & Peterson, 2012). These models assume that the geographical distributions are manifestations of a range of environmental conditions (i.e. the fundamental niche; Hutchinson, 1957). There are other abiotic‐only hypotheses of range limits that are not necessarily based on niche theory (climatic variability hypothesis). Some abiotic‐only hypotheses are edge‐specific such as that proposed by Darwin (1859) and derivatives thereof (Table 1) that posit abiotic stress forms upper limits (Louthan et al., 2015; Normand et al., 2009).
Table 1.
Summary of hypotheses and models on the causes of range limits
| Hypothesis/model | Category | Premise of hypothesis/model | Relevant taxa | Notable papers |
|---|---|---|---|---|
| Species distribution models/environmental niche models/climate envelope models | Abiotic | These models assume that animals and plants track a climate niche, i.e., their distributions are their fundamental niche. They are commonly used to evaluate abiotic constraints on species' distributions and to generate predictive maps. | Plants and animals | Pearson and Dawson (2003), Soberón (2007) |
| Climatic variability hypothesis | Abiotic | This hypothesis posits that species are more temperature limited in aseasonal environments and have narrow temperature niches than species living in seasonal and harsher climates, which explains narrower altitudinal distributions in tropical areas. | Plants and animals | Janzen (1967), Ghalambor, Huey, Martin, Tewksbury, & Wang (2006) |
| Abundant‐centre model/abundant‐centre hypothesis/Central margin hypothesis/Centre‐periphery hypothesis | Biotic | These hypotheses and models predict that abundance, fitness, or genetic diversity is highest at the centre of a species geographical range and declines towards each edge. | Plants and animals | Brown (1984), Gaston et al. (2000), Carter and Prince (1981), Pironon et al. (2017) |
| Asymmetric abiotic stress limitation hypothesis (AASL); species interactions‐abiotic stress hypothesis (SIASH); Stress‐trade‐off hypothesis (STH) | Abiotic or biotic | These contributions are centred around the classic hypothesis described by Darwin (1859), Connell (1961), Dobzhansky (1950) and MacArthur (1984), which posits that abiotic factors form high‐latitude/altitude limits and biotic interactions form lower limits. | Plants and animals | Darwin (1859), Dobzhansky (1950), Connell (1961), MacArthur (1984), Normand et al. (2009), Louthan et al. (2015), Anderegg and HilleRisLambers (2019) |
| Stress‐gradient hypothesis (SGH) | Interactive | This hypothesis postulates that gradients of environmental stress determine the extent to which competition affects populations. Those living along lower edges, in less stressful environments, are more likely to experience competition, whereas those along upper edges, where abiotic stress is thought to be higher, are more likely to experience positive biotic interactions (e.g. facilitation). | Plants | Callaway et al. (2002), Ettinger and HilleRisLambers (2017) |
| Condition‐specific competition (CSC); resource availability hypothesis | Interactive | The main premise of this hypothesis is that interacting species will either gain or lose competitive advantage based on environmental conditions and this will, in turn, affect their distributions. | Animals | Connell (1961), Taniguchi & Nakano (2000), Malenke et al. (2011), Srinivasan et al. (2018) |
A second group considers only how biotic factors or interactions form range limits (Table 1). This includes the hypothesis that biotic interactions form lower limits originating from Darwin (1859) (Table 1). Another group of biotic models hypothesize that abundance, fitness and genetic diversity decrease outwards from the centre of a species geographical range (Table 1; abundant‐centre model and others) due to exogenous (e.g. patchy habitat) and/or endogenous (e.g. limited dispersal ability) factors (Brown, 1984; Carter & Prince, 1981). Like Darwin's hypothesis on range limits, the spatial patterns of these models are generally assumed to occur in nature, but evidence supporting their existence is equivocal (Pironon et al., 2017).
The third category for understanding causes of range limits explores interactions between abiotic and biotic factors. The stress‐gradient hypothesis (SGH) (Callaway et al., 2002) and condition‐specific competition (CSC) (Nagamitsu, Yamagishi, Kenta, Inari, & Kato, 2010) are two common approaches; the former has been applied primarily to plants and the latter to animals (Table 1). Both predict that environmental stress mediates biotic interactions across a gradient of conditions. They are commonly evaluated in altitudinal studies (Ettinger & HilleRisLambers, 2017; Twomey, Morales, & Summers, 2008) with some focus on geographical limits (Malenke, Newbold, & Clayton, 2011; Meier, Edwards, Kienast, Dobbertin, & Zimmermann, 2011). They are also consistent with Darwin's hypothesis on range limits, assuming abiotic and biotic factors have greater influence on either end of range limits, yet these assumptions are not explicit. One primary difference is the SGH predicts that positive biotic interactions are influential in stressful environments and negative biotic interactions in mild climates. CSC is similar to the SGH but, as the name implies, is limited to competitive interactions and does not predict positive biotic interactions in abiotically stressful environments.
In combination, these hypotheses comprise the commonly referenced (but previously undefined) ecological component of ‘range‐limit theory’ (Connallon & Sgrò, 2018; Hargreaves et al., 2014; Johansson, Frisk, Nemomissa, & Hylander, 2018; Louthan et al., 2015; Sexton et al., 2009). Hereafter, we refer to range‐limit theory as RLT, with an emphasis on the long‐standing hypothesis posited by Darwin (1859) and others since then (Table 1).
3. INTERACTIVE RANGE‐LIMIT THEORY
To formalize the interactive nature of abiotic and biotic factors along range limits that has been highlighted in previous research (Godsoe, Franklin, et al., 2017; Wisz et al., 2013), we propose the interactive range limit theory, iRLT, an expansion of RLT that incorporates interactions among abiotic and biotic factors. iRLT produces the primary predictions of RLT (Table 2; Figure 1a), with some major additions. In agreement with RLT, abiotic factors are more influential along upper limits of a species’ range. But iRLT hypothesizes that biotic factors can ameliorate abiotic conditions and moderate range‐limit dynamics (Table 2; Figure 1b). Similarly, biotic interactions are still predicted to be more important along lower limits, but iRLT hypothesizes that abiotic factors can mediate biotic interactions and thus range limits (Table 2; Figure 1b). We predict that the most pronounced shifts on either edge of a species’ distribution occur when abiotic and biotic factors oppose each other (i.e. marked expansion occurs along upper limits following a decrease in negative abiotic factors and a simultaneous increase in positive biotic factors, with the opposite pattern for lower limits).
Table 2.
Outline of predictions for range‐limit theory (RLT) and interactive range‐limit theory (iRLT)
| High‐latitude/altitude limit | Low‐latitude/altitude limit | |
|---|---|---|
| Predictions of factors causing range limits | ||
| RLT | Negative abiotic factors | Negative biotic interactions |
| iRLT | Negative abiotic factors AND Positive biotic factors | Negative biotic interactions AND Positive abiotic factors |
| Predictions for contraction along range limits | ||
| RLT | Negative abiotic factors increase | Negative biotic interactions increase |
| iRLT | Negative abiotic factors increase AND/OR Positive biotic factors decrease | Negative biotic interactions increase AND/OR Positive abiotic factors decrease |
| Predictions for expansion along range limits | ||
| RLT | Negative abiotic factors decrease | Negative biotic interactions decrease |
| iRLT | Negative abiotic factors reduce AND/OR Positive biotic factors increase | Negative biotic interactions reduce AND/OR Positive abiotic factors increase |
Figure 1.
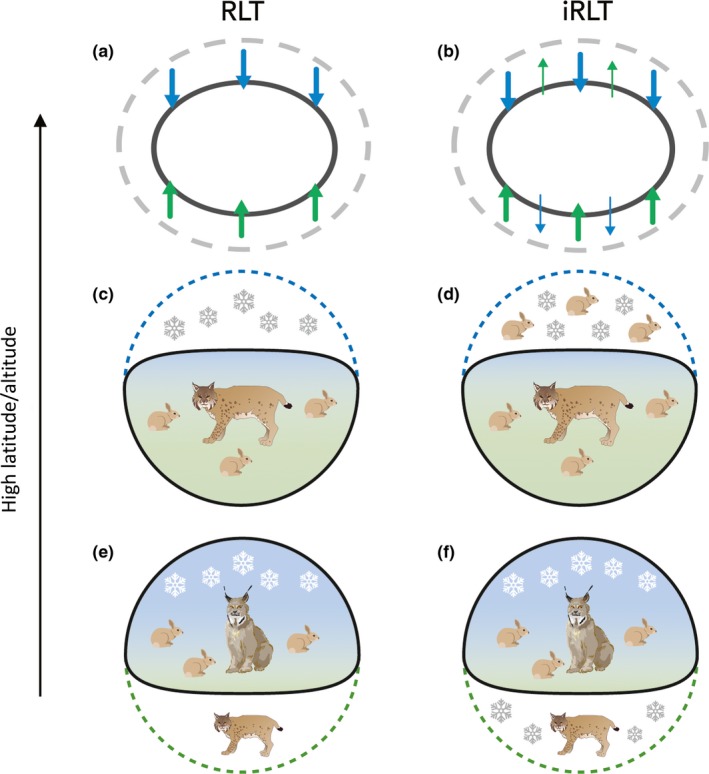
Range‐limit theory (RLT) (a) predicts that abiotic factors (blue) constrain the high‐latitude/altitude (upper) limit of the potential range (grey dashed lines) and biotic interactions (green) constrain the low‐latitude/altitude (lower) edge of the potential range, resulting in the black outlined observed range. Interactive range‐limit theory (iRLT) (b) extends RLT to predict that the interaction of abiotic and biotic factors forms limits at either edge of a range. Positive biotic factors can expand the range along upper limits despite negative abiotic factors, and expansion along lower edges can result if negative biotic interactions are ameliorated by stress from abiotic factors. RLT posits that (c) species like bobcat (Lynx rufus), (bottom) are limited by abiotic factors (e.g. climate) on the upper edge, and (e) those such as Canada lynx (Lynx canadensis) are limited by biotic interactions (e.g. competition for prey) along the lower limit. iRLT predicts that (d) positive biotic factors (more prey for bobcats) can ameliorate negative abiotic factors along high‐latitude/altitude limits and (f) positive abiotic factors (increase in snow for lynx) mediate negative biotic interactions along lower limits
Consider the following scenario of range dynamics along an upper limit. A population of Species A is limited by an abiotic factor. For example, exposure to cold reduces survival and lowers population growth rates, creating the upper limit of the species range, as predicted by RLT (Figure 1c). Accordingly, range expansion will follow periods of warming, whereas contraction will occur if temperatures decrease, indicating that climate ultimately forms range limits. Southern pine beetles in North America provide a contemporary example of expansion along upper limits due to anthropogenic warming (Lesk, Coffel, D'Amato, Dodds, & Horton, 2017). An extreme version of contraction occurred during glacial periods in North America, where ice forced populations to retreat downslope and southward (Lomolino, Riddle, & Whittaker, 2016).
iRLT, on the other hand, accounts for the complexity that spatial and temporal variation creates along range limits. A particularly beneficial biotic factor can ameliorate the negative influence of a harsh environment and allow for population persistence along high range limits (Figure 1d). For instance, populations of Species A may persist along upper range limits despite low winter temperatures because there is optimal habitat or abundant food resources that enable individuals to thermoregulate more easily and increase survival. However, if these positive biotic factors diminish, survival will decrease and result in contraction along upper limits (Figure 2a). Furthermore, if there is a coincident increase in cold temperature, contraction will be especially pronounced. Conversely, where negative abiotic factors lessen and positive biotic factors increase, range expansion is fuelled along upper limits for some species (Figure 2b) (e.g. Elmhagen et al., 2017). Range expansion along leading range edges in response to modern climate change is perhaps the most obvious example.
Figure 2.
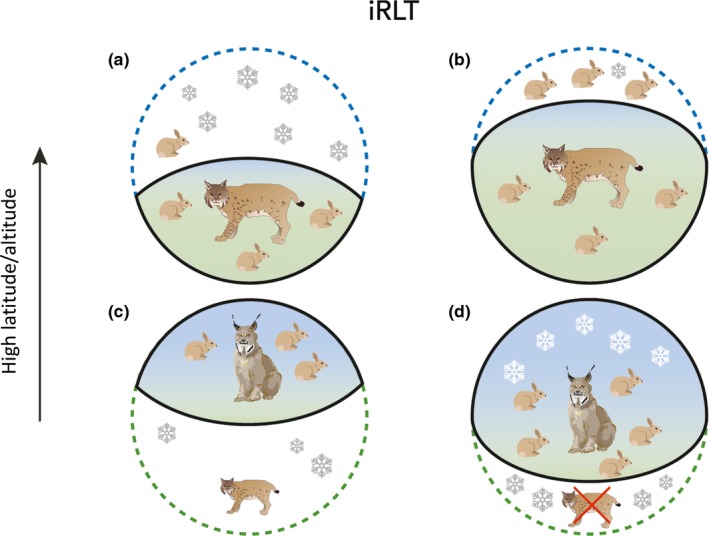
Interactive range‐limit theory (iRLT) provides predictions for expansion and contraction along each edge. For high‐latitude/altitude limits, (a) range contraction (e.g. of bobcat) occurs when abiotic stress is greater (increased snow) than the influence of positive biotic factors and (b) range expansion occurs when positive biotic factors (e.g. more prey) are greater than abiotic stress. For low‐latitude/altitude limits, (c) range contraction (e.g. of Canada lynx) occurs when negative biotic interactions (increased competition) are greater than the influence of abiotic factors (d) and expansion occurs when this dynamic is reversed. In summary, positive biotic factors can expand the range along upper limits despite the presence of stressful abiotic factors, and expansion along lower limits can result if negative biotic interactions are buffered by stress from abiotic factors; contraction occurs in the absence of these indirect and mediating factors along either edge
Now consider a population of Species A along its lower range margin, where, according to RLT, biotic interactions (e.g. competition) are considered the primary determinant of range limits (Table 2; Figure 1e). Although Species A is limited by competition, it has greater tolerance for a stressful abiotic factor (e.g. cold temperature) than its competitor along lower range margins. Thus, iRLT predicts that abiotic stress can act as a buffer by reducing the fitness of the competitor but not Species A (Figure 1f). However, when temperatures warm, the focal species becomes exposed to environments that are suitable for its competitor, resulting in range contraction of the focal species (Figure 2c). Conversely, range expansion will occur if the abiotic factor intensifies relative to the strength of the biotic interaction (Figure 2d).
Thus, the simplest case of iRLT, in the absence of interactive effects, produces the same predictions as RLT. However, the expectation is that interactive effects are common and affect many species on both edges of their ranges. We next set out to test this assumption.
4. REVIEW OF EVIDENCE FOR iRLT
4.1. Context
To provide evidence of the applicability of iRLT and to determine the extent to which biotic interactions varied by trophic level, we reviewed literature based on a specific set of criteria (see Text A1, Tables S1–S2; Supporting Information). First, we looked for evidence of RLT: that studies at upper limits would show negative impacts of abiotic factors, and that studies at lower limits would show negative impacts of biotic factors (Table 2). We further predicted, based on iRLT, studies of populations along upper limits would also document positive associations with biotic factors, whereas those along lower limits would detect positive associations with abiotic factors (Table 2). We used studies of mammalian carnivores and herbivores from North America with a focus on those occurring along the boreal‐temperate (Goldblum & Rigg, 2010) and forest‐tundra (Payette, Fortin, & Gamache, 2001) ecotones. We chose this region as many studies have been conducted along these ecotones over the past century (Eckert, Samis, & Lougheed, 2008), providing an opportunity to evaluate the extent to which abiotic and biotic factors influence range limits. Furthermore, ecotones, in general, are considered ideal regions to evaluate the influence of abiotic factors (e.g. climate) on species distributions as they often coincide with range limits (Kupfer & Cairns, 1996). Our review spanned five taxonomic orders and provided a total of 15 families, 31 genera and 52 species (Table S1).
4.2. Evidence for iRLT along high‐latitude/altitude limits
In concert with RLT, abiotic factors often imposed a negative influence on upper range limits of mammalian carnivores and herbivores from North America along the boreal‐temperate and forest‐tundra ecotones (n = 61 studies, Table 3), with deep snow or cold temperatures often considered the limiting factors. On the other hand, unlinked biotic factors (Box 1) such as habitat or prey availability had a positive influence along upper limits; this interaction of a biotic factor lessening the negative impact of an abiotic factor is evidence in support of iRLT (n = 57 studies, Table 3). This pattern was evident for carnivores and herbivores (Table S3) and for studies that only evaluated abiotic or biotic factors, instead of both (Table S4). However, our review process may have inflated the number of studies that reported positive associations with unlinked biotic factors along high range limits (see bias assessment, Text A1). Comparatively, there were fewer studies that found positive and negative associations with abiotic and biotic factors, respectively, along upper limits (Table 3). Limiting biotic factors were typically associated with food availability or habitat type (e.g. open tundra). Evidence for the impact of biotic interactions on upper limits was rare (n = 3 studies; Table 3); however, relatively few studies evaluated species interactions at broader spatial scales.
Table 3.
Number of studies that found positive, negative and neutral effects of abiotic and biotic factors on range limits of North American mammals
| Range limit | Factor | Positive | Negative | Neutral | Biotic interactiona | Totalb |
|---|---|---|---|---|---|---|
| High | Abiotic | 13 | 61 | 1 | 75 | |
| Biotic | 57 | 18 | 7 | 3 | 85 | |
| Low | Abiotic | 46 | 15 | 11 | 72 | |
| Biotic | 49 | 12 | 4 | 9 | 74 |
This table only includes studies that evaluated both abiotic and biotic factors along range limits (n = 138).
Few studies coincidentally evaluated biotic interactions (e.g. competition, predation) at broader spatial scales.
Note that some studies documented multiple abiotic or biotic factors, which occasionally had opposite signs. For example, if a study indicated that one abiotic variable had a positive effect and another had a strong negative effect, we tallied these as separate records, which increased the total number of studies.
Finally, a subset of the papers in our review evaluated range shifts along upper limits (Table S5). Most studies documented range expansion (n = 13), instead of contraction (n = 4) or stability (n = 1). The availability of habitat or prey often ameliorated the effect of negative abiotic factors. For instance, bobcats Lynx rufus, normally snow limited, can persist for years in deep snow locales along high‐latitude limits if there are large or abundant prey (Litvaitis & Harrison, 1989; Major & Sherburne, 1987; Newbury & Hodges, 2018). A similar pattern has been inferred for other purportedly snow‐limited carnivores, including fisher Pekania pennanti (Jensen & Humphries, 2019; McLellan, Vashon, Johnson, Crowley, & Vashon, 2018) and coyotes Canis latrans (Litvaitis & Harrison, 1989; Patterson, Benjamin, & Messier, 1998). These findings indicate, in support of iRLT, that populations may persist along upper limits if a positive biotic factor can overcome the negative abiotic impacts.
Range contraction along upper limits was often associated with a decline in positive unlinked biotic factors, such as prey and habitat availability. This dynamic occurred for felids (Litvaitis, Tash, & Stevens, 2006), ungulates (D'Eon & Serrouya, 2005) and small mammals (Wolff, 1996). A notable example is the southward contraction of white‐tailed deer Odocoileus virginianus range in New England due to the loss of mature conifer forest—a habitat that provides refuge during deep snow winters (Simons‐Legaard, Harrison, & Legaard, 2018). Another study documented a 240‐km range contraction of southern flying squirrels Glaucomys volans along its northern limit during a shortage of tree seeds that coincided with a severe winter (Bowman, Holloway, Malcolm, Middel, & Wilson, 2005). However, the authors found that these populations persisted during harsh winters when seeds were especially abundant. A similar food‐related shift in abundance was also observed for two mouse species (Peromyscus spp.) along an altitudinal gradient in the Appalachian Mountains (Wolff, 1996).
Range expansion along upper limits was especially evident when a negative abiotic factor decreased along with a corresponding increase in positive unlinked biotic factors (Dawe & Boutin, 2016; Lavoie et al., 2009). Some of the best examples include the northward expansion of opossum Didelphis virginiana and raccoons Procyon lotor in response to increasing food availability in conjunction with warming climate (Kanda, Fuller, Sievert, & Kellogg, 2009; Larivière, 2004; Pitt et al., 2008). Additionally, experimental work at local scales indicates abundant forage can buffer the negative effects of harsh climate for mule deer Odocoileus hemionus (Baker & Hobbs, 1985) and New England cottontails Sylvilagus transitionalis (Weidman & Litvaitis, 2011). These studies support the iRLT prediction of range expansion along upper limits, where the strength of biotic factors ameliorate harsh abiotic conditions (Figure 2b).
There are some studies where biotic interactions were considered the direct limiting factor, or complex interactions between abiotic and biotic factors formed upper limits. For instance, predation rates were higher in open tundra for snowshoe hares Lepus americanus along its northern range limit in Canada (Barta, Keith, & Fitzgerald, 1989). Consequently, this species has benefitted from the northward expansion of shrubs in the arctic tundra (Tape, Christie, Carroll, & O’Donnell, 2016). Conversely, abiotic factors such as snow have been shown to increase the susceptibility of swamp rabbits Sylvilagus aquaticus and eastern cottontails to predation (Boland & Litvaitis, 2008; Hillard et al., 2018), yet anthropogenic refuges can buffer losses for the latter species (Keith & Bloomer, 1993). Many of these studies, however, were not able to differentiate between direct and indirect causal effects.
4.3. Evidence for iRLT along low‐latitude/altitude limits
Supporting iRLT, many species had positive associations with winter climate along lower limits (n = 46 studies, Table 3). Specifically, abiotic factors, such as snow or cold temperatures, were positively correlated with the distribution of carnivores (n = 29 studies; Table S3). A similar, but less pronounced, pattern prevailed for herbivores (n = 17 studies; Table S3). Interestingly, unlinked biotic factors, such as prey or habitat availability, also had a strong and positive effect on range limits for both trophic levels (n = 49 studies, Table 3). This pattern was present for studies that only evaluated abiotic or biotic factors, instead of both (Table S4). Comparatively, there were fewer studies that found negative associations with abiotic or unlinked biotic factors along lower limits (Table 3). In general, negative relationships with the latter were associated with anthropogenic habitat (e.g. roads) and considered a proxy of negative biotic interactions (e.g. predation; Beguin et al., 2013). Although fewer studies reported biotic interactions at the distributional scale, there were a higher number along lower limits, which is predicted by RLT (Table 3).
The few papers we found that evaluated shifts along lower edges primarily documented contraction (n = 14), yet some found expansion (n = 5) or stability (n = 2) (Table S5). Similar to studies along high limits, range stability is likely more common than reported in the literature due to the bias against reporting negative results (Fanelli, 2012). Range contraction along lower limits was especially evident when positive abiotic factors reduced in strength relative to that of negative biotic factors. This occurred for carnivores when buffering from abiotic factors diminished and provided a competitive advantage for sympatric species (Elmhagen et al., 2017; Krohn, 2012). For example, a 175‐km contraction along the southeastern edge of the snow‐adapted Canada lynx Lynx canadensis range was associated with several decades of mild winters that enabled competitors to expand (Koen, Bowman, Murray, & Wilson, 2014; Peers, Thornton, & Murray, 2013). These patterns can occur locally at seasonal scales (Scully, Fisher, Miller, & Thornton, 2018) or geographically over longer time periods (Hoving, Joseph, & Krohn, 2003; Krohn, 2012). Range contraction was also observed for herbivores when the strength of an abiotic factor reduced, exposing populations to predation, disease or parasitism. For example, recent studies indicate snowshoe hares experience higher predation rates and population declines when their white winter coats contrast with snowless environments (Wilson, Shipley, Zuckerberg, Peery, & Pauli, 2018; Zimova, Mills, & Nowak, 2016). Consequently, reduced snow duration over the past several decades is associated with range contraction along the snowshoe hare's southern limit (Burt, Roloff, & Etter, 2017; Sultaire et al., 2016) with future declines expected due to climate change (Zimova et al., 2016). These studies are in accordance with the iRLT prediction of range contraction along lower limits when the positive effect from an abiotic factor diminishes (Figure 2c).
Range expansion was evident for carnivore populations along lower limits when abiotic factors were exceptionally strong; again, this dynamic occurred over short and long time scales (Hornocker & Hash, 1981; Krohn, 2012). This is well illustrated by the historical ranges of extant species such as American marten Martes americana whose southern limit extended farther south in the northeastern United States during the Little Ice Age (Krohn, 2012). Expansion was also associated with the emergence of positive unlinked biotic factors (e.g. habitat availability); however, this occurred within the range of environmental conditions that suited the focal species but not its competitors (Hoving et al., 2003; Kelly, Fuller, & Kanter, 2009; Simons‐Legaard, Harrison, & Legaard, 2016). Most of the latter examples occurred over longer time scales and were attributed to habitat availability. This dynamic indicates that a number of conditions may be required for range expansion along lower limits (Anderson et al., 2009; Hoving, Harrison, Krohn, Joseph, & O’Brien, 2005; McCann & Moen, 2011). Indeed, our review indicates that the ratio of positive abiotic to positive biotic factors along lower edges was relatively equal (46:49) compared to upper limits (Table 3). A common theme of these studies was that a strong abiotic factor was required for range expansion along lower limits.
Several authors indicate that a suite of complex interactions form lower limits. For instance, Belding's ground squirrels Urocitellus beldingi moved upslope in response to climate change during the past century, yet anthropogenic refugia, artificially supplementing food and water resources, facilitated population persistence along its low‐altitude limit (Morelli et al., 2012). Another study found that porcupines Erethizon dorsatum had lower survival in the presence of a recolonizing carnivore (fisher), and this was exacerbated during severe winter weather (Pokallus & Pauli, 2015). Additionally, the recolonization or reintroduction of martens to historical locales indicates that a combination of factors, including climate, competition with sympatric carnivores and prey availability forms their lower limit (Carlson et al., 2014; Manlick, Woodford, Zuckerberg, & Pauli, 2017; Zielinski, Tucker, & Rennie, 2017). One of the most interesting examples includes wolverines Gulo gulo in North America whose lower limit is positively associated with deep snowpack that is hypothesized to help preserve cached food and provide protection from competitors (Inman, Magoun, Persson, & Mattisson, 2012).
Other studies provide evidence that biotic factors alone can form lower limits. For example, shrub habitats were considered population sinks for Arctic ground squirrels Urocitellus parryii due to high predation rates (Donker & Krebs, 2012). This dynamic was also confirmed for arctic hares Lepus arcticus through a series of experiments (Barta et al., 1989; Small & Keith, 1992). There are also notable examples that indicate abiotic factors alone are the ultimate limits for low‐latitude populations (Lenarz, Nelson, Schrage, & Edwards, 2009; Wattles, Zeller, & DeStefano, 2018). Similar to the examples provided previously, many of these studies could not identify the direct and indirect causal mechanisms that formed range limits.
5. EXAMPLES FROM OTHER TAXA AND REGIONS
There are numerous examples of taxa or mammals from other regions that support iRLT. We did not conduct a comprehensive review of these but present some to serve as starting points for future studies. There was support for iRLT along upper limits for European mammals (Acevedo, Jiménez‐Valverde, Melo‐Ferreira, Real, & Alves, 2012; Levänen, Kunnasranta, & Pohjoismäki, 2018; Taulman & Robbins, 1996), birds (Plummer, Siriwardena, Conway, Risely, & Toms, 2015), plants (Hargreaves et al., 2014) and even bacteria (Simon et al., 2014). One study found that older‐aged trees can facilitate survival and growth for seedling trees along high‐altitude limits by providing shelter from harsh climate (Ettinger & HilleRisLambers, 2017). Other examples include the expansion of rats (Rattus spp.) (Varudkar & Ramakrishnan, 2015) and ticks (Leighton, Koffi, Pelcat, Lindsay, & Ogden, 2012) to high‐latitude/altitude regions via indirect (rats) or direct (ticks) facilitation by humans.
We also found support for iRLT along lower limits for birds (Waite & Strickland, 2006), European mammals (Atmeh, Andruszkiewicz, & Zub, 2018; Levänen et al., 2018; Pedersen, Odden, & Pedersen, 2017), amphibians (Cunningham, Rissler, & Apodaca, 2009) and especially plants (Callaway et al., 2002; Hargreaves et al., 2014; Johansson et al., 2018; Loehle, 1998). For example, Canada jays Perisoreus canadensis rely on snow and cold weather to cache food for early breeding, yet warmer winters have exposed caches to rot and resulted in subsequent declines in reproduction (Derbyshire, Strickland, & Norris, 2015); ultimately, this abiotic constraint could determine the low‐latitude range limit for the species.
Overall, we found overwhelming support for abiotic and biotic factors impacting both limits of the range for the North American mammal studies that we reviewed. Although there was evidence for the classic predictions of RLT, much more evidence was found for the interactive effects predicted by our extension, iRLT.
6. BIOTIC INTERACTIONS VARY BY TROPHIC LEVEL
Our review provided insight on the biotic interactions that limit mammalian carnivore and herbivore populations along range edges. In accordance with RLT, biotic interactions were approximately three times as prevalent along lower limits (Table 4). We also found clear differences between carnivores and herbivores, providing support for trophic theory (Hairston & Hairston, 1993); competition was the only biotic interaction associated with carnivores (25 studies), whereas predation or parasitism was considered the limiting factor for 77% (55 of the 71 studies) of herbivore studies along range limits (Table 4). It is important to note, though, that many studies assume competition (Barrio, Hik, Bueno, & Cahill, 2013) when other biotic interactions might be structuring populations and communities. Also, there is known publication bias towards negative biotic interactions (Barrio et al., 2013), especially along lower edges (Cahill et al., 2014). The latter bias may have occurred for studies in our review. Even those that were not following RLT were likely predisposed to evaluate biotic interactions along lower limits given the prevalent assumption of this hypothesis in biogeography and ecology (Cahill et al., 2014).
Table 4.
Number of biotic interactions by trophic level and range‐limit position reported by 92 of 290 studies (32%) included in the integrative review
| Trophic level | Range limit | Competition | Predation/parasitism |
|---|---|---|---|
| Carnivore | High | 6 | 0 |
| Low | 19 | 0 | |
| Herbivore | High | 6 | 18 |
| Low | 10 | 37 |
Our findings highlight the different types of spatial patterns that biotic interactions can impart along range limits (Bull, 1991; Holt & Barfield, 2009) and provide insight into the underlying processes. Competition can create a variety of range‐limit patterns (abrupt–diffuse) depending on phylogenetic and ecological similarity (Bull, 1991; Godsoe, Holland, et al., 2017; Wisz et al., 2013). For example, competition between highly similar carnivore species pairs (e.g. lynx‐bobcats, red fox‐arctic fox) is thought to create parapatric distributions (Hersteinsson & Macdonald, 1992; Peers et al., 2013). Species pairs that are still within the same taxonomic family but have contrasting body sizes (e.g. marten‐fisher, red fox‐coyotes) often have greater geographical and regional overlap (Jensen & Humphries, 2019; Krohn, Elowe, & Boone, 1995; Murray & Larivière, 2002). Contrastingly, near sympatry can occur for phylogenetically dissimilar species pairs with similar ecological associations (e.g. lynx‐coyote; Guillaumet, Bowman, Thornton, & Murray, 2015). There are notable exceptions (e.g. mesopredator release; Crooks & Soulé, 1999; Ritchie & Johnson, 2009), though, indicating that competition between similar species is not always the dominant biotic interaction that forms range limits for carnivores (see e.g. Davis et al., 2018).
Comparatively, the patterns that predation and parasitism create along range limits are less well understood (Godsoe, Holland, et al., 2017). These biotic interactions can confer patterns similar to competition (e.g. parapatry) as shown by theoretical and empirical studies (e.g. apparent competition; Holt & Barfield, 2009; Poley et al., 2014). However, the mechanisms underlying predation and parasitism may also lead to divergent range patterns. In particular, the functional response of predators and parasites varies based on their degree of specialization and the density of prey and host populations (Holling, 1959). For example, snowshoe hares at lower latitudes, beyond the range of their specialist predator (lynx), often persist at low densities (Hodges, Mills, & Murphy, 2009; Linden, Campa, Roloff, Beyer, & Millenbah, 2011). In regions where lynx are absent, generalist carnivores may exhibit a Type III functional response (density‐dependent predation) (Chan et al., 2017; Todd, Keith, & Fischer, 1981) that potentially affords hares a low‐density refuge from predation (Holt & Barfield, 2009; Oaten & Murdoch, 1975). Similarly, a low‐density refuge may allow moose Alces alces to escape high parasite loads and explain their persistence in some regions along their low‐latitude limit in North America (Samuel, 2007).
Low‐density refuges occur in some aquatic ecosystems (Griffen & Williamson, 2008; Seitz, Lipcius, Hines, & Eggleston, 2001) and are akin to Janzen‐Connell effects where plant seeds occurring at low density escape predation by seed predators (see review in Comita et al., 2014). We propose that Janzen‐Connell effects, which describe predation patterns at local scales, may be extended to other trophic levels and at broader spatial scales. We suggest that a low‐density refuge from predation provides a plausible explanation of why the ranges of some prey/host species extend further towards the equator than their carnivore/parasite counterparts. The extent at which this occurs, though, might be predicated on the quality and availability of unlinked biotic factors (e.g. habitat), which vary in space and time (e.g. Sinclair et al., 1998). The spatial pattern associated with our hypothesis might produce diffuse range margins compared to abrupt limits which are often associated with competition. As mentioned previously, there are other outcomes associated with predation or parasitism (e.g. apparent competition) that can lead to variable patterns along range limits (Bull, 1991; Holt & Barfield, 2009). However, few of these hypotheses have been tested experimentally or using empirical data.
7. CONCLUSIONS, LIMITATIONS AND FUTURE DIRECTIONS
Our review indicates that the long‐standing theory on range limits, proposed by Darwin (1859) and others since then, deserves to be broadened to include the interactive nature of abiotic and biotic factors along range margins. For populations along upper limits, abiotic factors will likely have more importance and directly influence range dynamics, whereas positive biotic factors have the potential to ameliorate harsh abiotic conditions. Conversely, biotic interactions will have greater importance along lower limits, but abiotic factors can mediate negative biotic interactions. For both scenarios of iRLT, the strength and direction of abiotic and biotic factors can be used to predict range expansion, contraction or stability.
iRLT has properties comparable to the stress‐gradient hypothesis (SGH) and condition‐specific competition (CSC) (Table 1). It is most similar to the SGH, yet this hypothesis has only recently been advocated for understanding animal distributions (Barrio et al., 2013) with only a few tests (e.g. Peoples, Blanc, & Frimpong, 2015). However, SGH focuses solely on biotic interactions which may be an incomplete model for mobile animals that are influenced by unlinked biotic factors (e.g. habitat or prey availability) and biotic interactions (Jensen & Humphries, 2019). Similar to the SGH, iRLT provides a conceptual framework to evaluate positive biotic factors which is important as the inclusion of these are lacking in range‐limit studies, especially for animals (Barrio et al., 2013). However, SGH does not predict interactions between abiotic and biotic factors along lower limits. As shown below, CSC is more similar to iRLT in this regard.
Like iRLT, CSC provides a framework to evaluate asymmetric competition through the lens of environmental gradients. The premise of CSC is that interacting species will either gain or lose competitive advantage based on the environmental conditions. For example, Taniguchi & Nakano (2000) found that salmonids adapted to colder conditions performed better than closely related species but performed poorly when temperatures were higher. Although CSC is focused on animals (Connell, 1961; Nagamitsu et al., 2010), it has not been applied to mammals. Our review indicates that CSC is applicable to mammals. For example, abiotic factors associated with winter (e.g. snow, cold temperature) were often positively correlated with the distribution of boreal carnivores along lower limits; in these cases, harsh climate was thought to mediate competitive interactions with more temperate species (Dekker, 1989; Jensen & Humphries, 2019; Krohn et al., 1995; Peers et al., 2013). However, unlike CSC, iRLT includes other biotic interactions such as predation, parasitism and facilitation, which can lead to a variety of range‐limit patterns. Indeed, snow or cold temperatures can have a strong positive effect on boreal herbivores, buffering predation (Bastille‐Rousseau et al., 2018; Zimova et al., 2016) and parasitism (Bowman et al., 2005; Murray et al., 2006).
Interactive models of range limits such as ours, CSC and the SGH may require a different inferential framework to understand causes of range limits. Most studies in our review were correlative and did not evaluate fitness (e.g. growth rates) along range limits. Modelling frameworks that allow for the inclusion of correlated direct and indirect predictors, like structural equation modelling (Joseph, Preston, & Johnson, 2016), provide a promising avenue (see for example Duclos, DeLuca, & King, 2019). Future research could prioritize large‐scale observational studies that collect data on direct and indirect effects at the same spatial and temporal scale, as well as extend beyond the range of the focal species to identify limiting factors (Louthan et al., 2015; Westoby et al., 2017). This type of experimental design is well suited for evaluating direct and indirect effects using a causal modelling framework (Joseph et al., 2016). Ideally, though, large‐scale studies should be integrated with laboratory experiments (e.g. Malenke et al., 2011) to determine how gradients of abiotic stress and biotic factors influence population growth rates and thus range limits (Godsoe, Jankowski, et al., 2017; Louthan et al., 2015).
We consider iRLT to be applicable to different taxa and regions and encourage researchers to think critically of the biotic interactions and factors that limit each trophic level. Competition appears to be a limiting factor for plants and carnivores along lower margins (Hargreaves et al., 2014; Peers et al., 2013), whereas predation/parasitism likely regulates herbivores (Anderson et al., 2009; Murray et al., 2006). Regardless of these differences, the predictions of iRLT remain similar. However, the types of biotic interactions, which vary by trophic level, may create different patterns along range limits and result in differences in range contraction, expansion and stability. Our examples using snowshoe hares and moose provide a starting point to explore the interactive nature across trophic levels. Specifically, the lower limits of herbivores, which (like carnivores) appear to be influenced by climate‐mediated biotic interactions, may contract at different rates and lag those of their carnivore counterparts. This may be a particularly interesting avenue of research to explore considering climate change predictions.
Our review does not incorporate many intraspecific factors or evolutionary considerations (e.g. dispersal ability, Allee effects) which could greatly influence range limits (Parmesan, 2006; Sexton et al., 2009). However, there are numerous examples that indicate iRLT is relevant for understanding the influence of these factors on range limits. For instance, phenotypic and/or genotypic variability may rescue populations along lower limits, either of which can occur naturally or from facilitation by humans (Atmeh et al., 2018; Jones et al., 2018; Mills et al., 2018). Furthermore, population size can ameliorate the influence of harsh climate along range limits and influence the rate of expansion (Grayson & Johnson, 2018). There are other eco‐evolutionary dynamics such as within‐species trait differences associated with dispersal along upper range limits (Hughes, Dytham, & Hill, 2007; Simmons & Thomas, 2004).
Identifying abiotic and biotic mechanisms that limit ranges is critical for predicting future distributions and developing appropriate conservation and management strategies. This is especially important considering current and anticipated threats from climate change, habitat loss and species invasions (Mantyka‐Pringle, Martin, & Rhodes, 2012). iRLT can improve predictions of species responses to global change and thus lead to better decision‐making. We encourage future research to explore the interactive nature of abiotic and biotic factors to better understand why range limits form and change over time.
AUTHORS' CONTRIBUTIONS
A.P.K.S. conceived the thesis and developed the review; T.L.M. assisted with the concepts and the development of the review; A.P.K.S. led the writing of the manuscript. Both authors contributed critically to the drafts and gave final approval for publication.
Supporting information
ACKNOWLEDGEMENTS
This research was funded by the Department of the Interior Northeast Climate Adaptation Science Center, which is managed by the USGS National Climate Adaptation Science Center. We thank M. Zimova and Morelli laboratory members for comments on earlier drafts. We also thank E. Johnson for help with figure illustrations.
Sirén APK, Morelli TL. Interactive range‐limit theory (iRLT): An extension for predicting range shifts. J Anim Ecol. 2020;89:940–954. 10.1111/1365-2656.13150
DATA AVAILABILITY STATEMENT
This article does not use data.
REFERENCES
- Acevedo, P. , Jiménez‐Valverde, A. , Melo‐Ferreira, J. , Real, R. , & Alves, P. C. (2012). Parapatric species and the implications for climate change studies: A case study on hares in Europe. Global Change Biology, 18(5), 1509–1519. 10.1111/j.1365-2486.2012.02655.x [DOI] [Google Scholar]
- Alexander, J. M. , Diez, J. M. , Usinowicz, J. , & Hart, S. P. (2018). Species’ distributions as a coexistence problem: A response to Godsoe et al. Trends in Ecology & Evolution, 33(3), 144–145. 10.1016/j.tree.2018.01.001 [DOI] [PubMed] [Google Scholar]
- Anderegg, L. D. L. , & HilleRisLambers, J. (2019). Local range boundaries vs. large‐scale trade‐offs: Climatic and competitive constraints on tree growth. Ecology Letters, 22(5), 787–796. 10.1111/ele.13236 [DOI] [PubMed] [Google Scholar]
- Anderson, R. P. (2017). When and how should biotic interactions be considered in models of species niches and distributions? Journal of Biogeography, 44, 8–17. 10.1111/jbi.12825 [DOI] [Google Scholar]
- Anderson, B. J. , Akcakaya, H. R. , Araujo, M. B. , Fordham, D. A. , Martinez‐Meyer, E. , Thuiller, W. , & Brook, B. W. (2009). Dynamics of range margins for metapopulations under climate change. Proceedings of the Royal Society B: Biological Sciences, 276(1661), 1415–1420. 10.1098/rspb.2008.1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrewartha, H. G. , & Birch, L. C. (1954). The distribution and abundance of animals (p. 782). Chicago, IL: Chicago University Press. [Google Scholar]
- Araujo, M. B. , & Peterson, A. T. (2012). Uses and misuses of bioclimatic envelope modeling. Ecology, 93(7), 1527–1539. 10.1002/ecy.1674 [DOI] [PubMed] [Google Scholar]
- Atmeh, K. , Andruszkiewicz, A. , & Zub, K. (2018). Climate change is affecting mortality of weasels due to camouflage mismatch. Scientific Reports, 8(1), 1–7. 10.1038/s41598-018-26057-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, D. L. , & Hobbs, N. T. (1985). Emergency feeding of mule deer during winter: Tests of a supplemental ration. Journal of Wildlife Management, 49(4), 934–942. 10.2307/3801374 [DOI] [Google Scholar]
- Barrio, I. C. , Hik, D. S. , Bueno, C. G. , & Cahill, J. F. (2013). Extending the stress‐gradient hypothesis – Is competition among animals less common in harsh environments? Oikos, 122(4), 516–523. 10.1111/j.1600-0706.2012.00355.x [DOI] [Google Scholar]
- Barta, R. M. , Keith, L. B. , & Fitzgerald, S. M. (1989). Demography of sympatric arctic and snowshoe hare populations: And experimental assessment of interspecific competition. Canadian Journal of Zoology, 67, 2762–2775. 10.1139/z89-392 [DOI] [Google Scholar]
- Bastille‐Rousseau, G. , Schaefer, J. A. , Peers, M. J. L. , Ellington, E. H. , Mumma, M. A. , Rayl, N. D. , … Murray, D. L. (2018). Climate change can alter predator–prey dynamics and population viability of prey. Oecologia, 186(1), 141–150. 10.1007/s00442-017-4017-y [DOI] [PubMed] [Google Scholar]
- Beguin, J. , McIntire, E. J. B. , Fortin, D. , Cumming, S. G. , Raulier, F. , Racine, P. , & Dussault, C. (2013). Explaining geographic gradients in winter selection of landscapes by boreal caribou with implications under global changes in eastern Canada. PLoS ONE, 8(10), 1–10. 10.1371/journal.pone.0078510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland, K. M. , & Litvaitis, J. A. (2008). Role of predation and hunting on eastern cottontail mortality at Cape Cod National Seashore, Massachusetts. Canadian Journal of Zoology, 86, 918–927. 10.1139/Z08-064 [DOI] [Google Scholar]
- Bowman, J. , Holloway, G. L. , Malcolm, J. R. , Middel, K. R. , & Wilson, P. J. (2005). Northern range boundary dynamics of southern flying squirrels: Evidence of an energetic bottleneck. Canadian Journal of Zoology, 83(11), 1486–1494. 10.1139/z05-144 [DOI] [Google Scholar]
- Brown, J. H. (1984). On the relationship between abundance and distribution of species. The American Naturalist, 124(2), 255–279. 10.1086/284267 [DOI] [Google Scholar]
- Brown, J. H. , Stevens, G. C. , & Kaufmnan, D. M. (1996). The geographic range: Size, shape, boundaries, and internal structure. Annual Review of Ecology and Systematics, 27(1996), 597–623. 10.1146/annurev.ecolsys.27.1.597 [DOI] [Google Scholar]
- Bull, C. M. (1991). Ecology of parapatric distributions. Annual Review of Ecology, Evolution, and Systematics, 22, 19–36. 10.1146/annurev.es.22.110191.000315 [DOI] [Google Scholar]
- Burt, D. M. , Roloff, G. J. , & Etter, D. R. (2017). Climate factors related to localized changes in snowshoe hare (Lepus americanus) occupancy. Canadian Journal of Zoology, 95, 15–22. https://doi.org/10.0.4.115/cjz-2016-0180 [Google Scholar]
- Cahill, A. E. , Aiello‐Lammens, M. E. , Caitlin Fisher‐Reid, M. , Hua, X. , Karanewsky, C. J. , Ryu, H. Y. , … Wiens, J. J. (2014). Causes of warm‐edge range limits: Systematic review, proximate factors and implications for climate change. Journal of Biogeography, 41(3), 429–442. 10.1111/jbi.12231 [DOI] [Google Scholar]
- Callaway, R. M. , Brooker, R. W. , Choler, P. , Kikvidze, Z. , Lortie, C. J. , Michalet, R. , … Cook, B. J. (2002). Positive interactions among alpine plants increase with stress. Nature, 417, 844–848. 10.1038/nature00805.1 [DOI] [PubMed] [Google Scholar]
- Carlson, J. E. , Gilbert, J. H. , Pokallus, J. W. , Manlick, P. J. , Moss, W. E. , & Pauli, J. N. (2014). Potential role of prey in the recovery of American martens to Wisconsin. Journal of Wildlife Management, 78(8), 1499–1504. 10.1002/jwmg.785 [DOI] [Google Scholar]
- Carter, R. N. , & Prince, S. D. (1981). Epidemic models used to explain biogeographical distribution limits. Nature, 293, 644–645. 10.1038/293644a0 [DOI] [Google Scholar]
- Chan, K. , Boutin, S. , Hossie, T. J. , Krebs, C. J. , O’Donoghue, M. , & Murray, D. L. (2017). Improving the assessment of predator functional responses by considering alternate prey and predator interactions. Ecology, 98(7), 1787–1796. 10.1002/ecy.1828 [DOI] [PubMed] [Google Scholar]
- Comita, L. S. , Queenborough, S. A. , Murphy, S. J. , Eck, J. L. , Xu, K. , Krishnadas, M. , … Zhu, Y. (2014). Testing predictions of the Janzen‐Connell hypothesis: A meta‐analysis of experimental evidence for distance‐ and density‐dependent seed and seedling survival. Journal of Ecology, 102(4), 845–856. 10.1111/1365-2745.12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connallon, T. , & Sgrò, C. M. (2018). In search of a general theory of species’ range evolution. PLoS Biology, 16(6), 1–6. 10.1371/journal.pbio.2006735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell, J. H. (1961). The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus . Ecology, 42(4), 710–723. 10.2307/1933500 [DOI] [Google Scholar]
- Crooks, K. , & Soulé, M. (1999). Mesopredator release and avifaunal extinctions in a fragmented system. Nature, 400, 563–566. 10.1038/23028 [DOI] [Google Scholar]
- Cunningham, H. R. , Rissler, L. J. , & Apodaca, J. J. (2009). Competition at the range boundary in the slimy salamander: Using reciprocal transplants for studies on the role of biotic interactions in spatial distributions. Journal of Animal Ecology, 78, 52–62. 10.1111/j.1365-2656.2008.01468.x [DOI] [PubMed] [Google Scholar]
- D’Eon, R. , & Serrouya, R. (2005). Mule deer seasonal movements and multiscale resource selection using global positioning system radiotelemetry. Journal of Mammalogy, 86(4), 736–744. 10.1644/1545-1542(2005)086[0736:mdsmam]2.0.co;2 [DOI] [Google Scholar]
- Darwin, C. (1859). On the origin of species. London, UK: Murray; 10.5117/9781904633785 [DOI] [Google Scholar]
- Davis, C. L. , Rich, L. N. , Farris, Z. J. , Kelly, M. J. , Di Bitetti, M. S. , Blanco, Y. D. , … Miller, D. A. W. (2018). Ecological correlates of the spatial co‐occurrence of sympatric mammalian carnivores worldwide. Ecology Letters, 21, 1401–1412. 10.1111/ele.13124 [DOI] [PubMed] [Google Scholar]
- Dawe, K. L. , & Boutin, S. (2016). Climate change is the primary driver of white‐tailed deer (Odocoileus virginianus) range expansion at the northern extent of its range; land use is secondary. Ecology and Evolution, 6(18), 6435–6451. 10.1002/ece3.2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker, D. (1989). Population fluctuations and spatial relationships among wolves, Canis lupus, coyotes, Canis latrans, and red foxes, Vulpes vulpes, in Jasper National Park, Alberta. The Canadian Field‐Naturalist, 103(2), 261–264. [Google Scholar]
- Derbyshire, R. , Strickland, D. , & Norris, D. R. (2015). Experimental evidence and over forty years of monitoring data show that food limits reproductive success in a boreal food‐caching passerine. Ecology, 96(11), 3005–3015. 10.1890/15-0191.1 [DOI] [PubMed] [Google Scholar]
- Dobzhansky, T. (1950). Evolution in the tropics. American Scientist, 38(2), 208–221. 10.2307/2989767 [DOI] [Google Scholar]
- Donker, S. A. , & Krebs, C. J. (2012). Evidence for source – Sink dynamics in a regional population of arctic ground squirrels (Urocitellus parryii plesius). Wildlife Research, 39(2), 163–170. 10.1071/WR11167 [DOI] [Google Scholar]
- Duclos, T. R. , DeLuca, W. V. , & King, D. I. (2019). Direct and indirect effects of climate on bird abundance along elevation gradients in the Northern Appalachian mountains. Diversity and Distributions, 25(11), 1670–1683. 10.1111/ddi.12968 [DOI] [Google Scholar]
- Dvorský, M. , Macek, M. , Kopecký, M. , Wild, J. , & Doležal, J. (2017). Niche asymmetry of vascular plants increases with elevation. Journal of Biogeography, 44(6), 1418–1425. 10.1111/jbi.13001 [DOI] [Google Scholar]
- Eckert, C. G. , Samis, K. E. , & Lougheed, S. C. (2008). Genetic variation across species’ geographical ranges: The central‐marginal hypothesis and beyond. Molecular Ecology, 17(5), 1170–1188. 10.1111/j.1365-294X.2007.03659.x [DOI] [PubMed] [Google Scholar]
- Elmhagen, B. , Berteaux, D. , Burgess, R. M. , Ehrich, D. , Gallant, D. , Henttonen, H. , … Angerbjörn, A. (2017). Homage to Hersteinsson and Macdonald: Climate warming and resource subsidies cause red fox range expansion and Arctic fox decline. Polar Research, 36(3), 3 10.1080/17518369.2017.1319109 [DOI] [Google Scholar]
- Ettinger, A. , & HilleRisLambers, J. (2017). Competition and facilitation may lead to asymmetric range shift dynamics with climate change. Global Change Biology, 23(9), 3921–3933. 10.1111/gcb.13649 [DOI] [PubMed] [Google Scholar]
- Fanelli, D. (2012). Negative results are disappearing from most disciplines and countries. Scientometrics, 90(3), 891–904. 10.1007/s11192-011-0494-7 [DOI] [Google Scholar]
- Gaston, K. J. , Blackburn, T. M. , Greenwood, J. D. , Gregory, R. D. , Quinn, R. M. , & Lawton, J. H. (2000). Abundance-occupancy relationships. Journal of Applied Ecology, 37, 39–59. [Google Scholar]
- Ghalambor, C. K. , Huey, R. B. , Martin, P. R. , Tewksbury, J. J. , & Wang, G. (2006). Are mountain passes higher in the tropics? Janzen’s hypothesis revisited. Integrative and Comparative Biology, 46(1), 5–17. 10.1093/icb/icj003 [DOI] [PubMed] [Google Scholar]
- Godsoe, W. , Franklin, J. , & Blanchet, F. G. (2017). Effects of biotic interactions on modeled species’ distribution can be masked by environmental gradients. Ecology and Evolution, 7(2), 654–664. 10.1002/ece3.2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsoe, W. , Holland, N. J. , Cosner, C. , Kendall, B. E. , Brett, A. , Jankowski, J. , & Holt, R. D. (2017). Interspecific interactions and range limits: Contrasts among interaction types. Theoretical Ecology, 10(2), 167–179. 10.1007/s12080-016-0319-7 [DOI] [Google Scholar]
- Godsoe, W. , Jankowski, J. , Holt, R. D. , & Gravel, D. (2017). Integrating biogeography with contemporary niche theory. Trends in Ecology & Evolution, 32(7), 488–499. 10.1016/j.tree.2017.03.008 [DOI] [PubMed] [Google Scholar]
- Godsoe, W. , Jankowski, J. , Holt, R. D. , & Gravel, D. (2018). Which coexistence mechanisms should biogeographers quantify? A reply to Alexander et al. Trends in Ecology & Evolution, 33(3), 145–147. 10.1016/j.tree.2018.01.003 [DOI] [PubMed] [Google Scholar]
- Goldblum, D. , & Rigg, L. S. (2010). The deciduous forest – Boreal forest ecotone. Geography Compass, 7, 701–717. 10.1111/j.1749-8198.2010.00342.x [DOI] [Google Scholar]
- Grayson, K. L. , & Johnson, D. M. (2018). Novel insights on population and range edge dynamics using an unparalleled spatiotemporal record of species invasion. Journal of Animal Ecology, 87, 581–593. 10.1111/1365-2656.12755 [DOI] [PubMed] [Google Scholar]
- Griffen, B. D. , & Williamson, T. (2008). Influence of predator density on nonindependent effects of multiple predator species. Oecologia, 155, 151–159. 10.1007/s00442-007-0889-6 [DOI] [PubMed] [Google Scholar]
- Guillaumet, A. , Bowman, J. , Thornton, D. , & Murray, D. L. (2015). The influence of coyote on Canada lynx populations assessed at two different spatial scales. Community Ecology, 16(2), 135–146. 10.1556/168.2015.16.2.1 [DOI] [Google Scholar]
- Guisan, A. , Tingley, R. , Baumgartner, J. B. , Naujokaitis‐Lewis, I. , Sutcliffe, P. R. , Tulloch, A. I. T. , … Buckley, Y. M. (2013). Predicting species distributions for conservation decisions. Ecology Letters, 16(12), 1424–1435. 10.1111/ele.12189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hairston, N. G. , & Hairston, N. G. (1993). Cause‐effect relationships in energy flow, trophic structure, and interspecific interactions. The American Naturalist, 142(3), 379–411. 10.1086/285546 [DOI] [Google Scholar]
- Hargreaves, A. L. , Samis, K. E. , & Eckert, C. G. (2014). Are species’ range limits simply niche limits writ large? A review of transplant experiments beyond the range. The American Naturalist, 183(2), 157–173. 10.1086/674525 [DOI] [PubMed] [Google Scholar]
- Hersteinsson, P. , & Macdonald, D. W. (1992). Interspecific competition and the geographical distribution of red and arctic foxes Vulpes vulpes and Alopex lagopus . Oikos, 64(3), 505–515. 10.2307/3545168 [DOI] [Google Scholar]
- Hillard, E. M. , Edmund, A. C. , Crawford, J. C. , Nielsen, C. K. , Schauber, E. M. , & Groninger, J. W. (2018). Winter snow cover increases swamp rabbit (Sylvilagus aquaticus) mortality at the northern extent of their range. Mammalian Biology, 93, 93–96. 10.1016/j.mambio.2018.09.001 [DOI] [Google Scholar]
- Hodges, K. E. , Mills, L. S. , & Murphy, K. M. (2009). Distribution and abundance of snowshoe hares in Yellowstone National Park. Journal of Mammalogy, 90(4), 870–878. 10.1644/08-MAMM-A-303.1 [DOI] [Google Scholar]
- Holling, C. S. (1959). The components of predation as revealed by a study of small‐mammal predation of the European pine sawfly. The Canadian Entomologist, 91(5), 293–320. 10.4039/Ent91293-5 [DOI] [Google Scholar]
- Holt, R. D. , & Barfield, M. (2009). Trophic interactions and range limits: The diverse roles of predation. Proceedings of the Royal Society B: Biological Sciences, 276(1661), 1435–1442. 10.1098/rspb.2008.1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornocker, M. G. , & Hash, H. S. (1981). Ecology of the wolverine in northwestern Montana. Canadian Journal of Zoology, 59, 1286–1301. 10.1139/z81-181 [DOI] [Google Scholar]
- Hoving, C. L. , Harrison, D. J. , Krohn, W. B. , Joseph, R. A. , & O’Brien, M. (2005). Broad‐scale predictors of Canada lynx occurrence in eastern North America. Journal of Wildlife Management, 69(2), 739–751. 10.2193/0022-541X(2005)069[0739:BPOCLO]2.0.CO;2 [DOI] [Google Scholar]
- Hoving, C. L. , Joseph, R. A. , & Krohn, W. B. (2003). Recent and historical distributions of Canada Lynx in Maine and the Northeast. Northeastern Naturalist, 10(4), 363–382. 10.1656/1092-6194(2003)010[0363:RAHDOC]2.0.CO;2 [DOI] [Google Scholar]
- Hughes, C. L. , Dytham, C. , & Hill, J. K. (2007). Modelling and analysing evolution of dispersal in populations at expanding range boundaries. Ecological Entomology, 32(5), 437–445. 10.1111/j.1365-2311.2007.00890.x [DOI] [Google Scholar]
- Hutchinson, G. E. (1957). Concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology, 22, 415–427. 10.1101/SQB.1957.022.01.039 [DOI] [Google Scholar]
- Inman, R. M. , Magoun, A. J. , Persson, J. , & Mattisson, J. (2012). The wolverine’s niche: Linking reproductive chronology, caching, competition, and climate. Journal of Mammalogy, 93(3), 634–644. 10.1644/11-MAMM-A-319.1 [DOI] [Google Scholar]
- Janzen, D. H. (1967). Why mountain passes are higher in the tropics. The American Naturalist, 101(919), 233–249. [Google Scholar]
- Jensen, P. G. , & Humphries, M. M. (2019). Abiotic conditions mediate intraguild interactions between mammalian carnivores. Journal of Animal Ecology, 88, 1305–1318. 10.1111/1365-2656.13024 [DOI] [PubMed] [Google Scholar]
- Johansson, M. U. , Frisk, C. A. , Nemomissa, S. , & Hylander, K. (2018). Disturbance from traditional fire management in subalpine heathlands increases Afro‐alpine plant resilience to climate change. Global Change Biology, 24(7), 2952–2964. 10.1111/gcb.14121 [DOI] [PubMed] [Google Scholar]
- Jones, M. R. , Mills, L. S. , Alves, P. C. , Callahan, C. M. , Alves, J. M. , Lafferty, D. J. R. , … Good, J. M. (2018). Adaptive introgression underlies polymorphic seasonal camouflage in snowshoe hares. Science, 360, 1355–1358. 10.1126/science.aar5273 [DOI] [PubMed] [Google Scholar]
- Joseph, M. B. , Preston, D. L. , & Johnson, P. T. J. (2016). Integrating occupancy models and structural equation models to understand species occurrence. Ecology, 97(3), 765–775. 10.1890/15-0833.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda, L. , Fuller, T. K. , Sievert, P. R. , & Kellogg, R. R. (2009). Seasonal source‐sink dynamics at the edge of a species’ range. Ecology, 90(6), 1574–1585. 10.1890/08-1263.1 [DOI] [PubMed] [Google Scholar]
- Keith, L. B. , & Bloomer, S. E. M. (1993). Differential mortality of sympatric snowshoe hares and cottontail rabbits in central Wisconsin. Canadian Journal of Zoology, 71(8), 1694–1697. 10.1139/z93-238 [DOI] [Google Scholar]
- Kelly, J. R. , Fuller, T. K. , & Kanter, J. J. (2009). Records of recovering American Marten, Martes americana in New Hampshire. Canadian Field‐Naturalist, 123(1), 1–6. 10.22621/cfn.v123i1.668 [DOI] [Google Scholar]
- Koen, E. L. , Bowman, J. , Murray, D. L. , & Wilson, P. J. (2014). Climate change reduces genetic diversity of Canada lynx at the trailing range edge. Ecography, 37(8), 754–762. 10.1111/j.1600-0587.2013.00629.x [DOI] [Google Scholar]
- Krebs, C. J. (1972). Ecology; the experimental analysis of distribution and abundance (p. 694). San Francisco, CA: Harper & Row. [Google Scholar]
- Krohn, W. B. (2012). Distribution changes of American martens and fishers in eastern North America, 1699–2001 In Aubry K. B., Zielinski W. J., Raphael M. G., Proulx G., & Buskirk S. W. (Eds.), Biology and conservation of martens, sables, and fishers – A new synthesis (pp. 58–73). Ithaca, NY: Cornell University Press; 10.7591/9780801466076-007 [DOI] [Google Scholar]
- Krohn, W. B. , Elowe, K. D. , & Boone, R. B. (1995). Relations among fishers, snow, and martens: Development and evaluation of two hypotheses. Forestry Chronicle, 71(1), 97–105. 10.5558/tfc71097-1 [DOI] [Google Scholar]
- Kupfer, J. A. , & Cairns, D. M. (1996). The suitability of montane ecotones as indicators of global climatic change. Progress in Physical Geography, 20(3), 253–272. 10.1177/030913339602000301 [DOI] [Google Scholar]
- Larivière, S. (2004). Canadian expansion of raccoons in the Canadian prairies: Review of hypotheses. Wildlife Society Bulletin, 32, 955–963. 10.2193/0091-7648(2004)032[0955:REORIT]2.0.CO;2 [DOI] [Google Scholar]
- Lavoie, M. , Collin, P.‐Y. , Lemieux, F. , Jolicoeur, H. , Canac‐Marquis, P. , & Larivire, S. (2009). Understanding fluctuations in bobcat harvest at the northern limit of their range. Journal of Wildlife Management, 73(6), 870–875. 10.2193/2008-275 [DOI] [Google Scholar]
- Leighton, P. A. , Koffi, J. K. , Pelcat, Y. , Lindsay, L. R. , & Ogden, N. H. (2012). Predicting the speed of tick invasion: An empirical model of range expansion for the Lyme disease vector Ixodes scapularis in Canada. Journal of Applied Ecology, 49, 457–464. 10.1111/j.1365-2664.2012.02112.x [DOI] [Google Scholar]
- Lenarz, M. S. , Nelson, M. E. , Schrage, M. W. , & Edwards, A. J. (2009). Temperature mediated moose survival in northeastern Minnesota. Journal of Wildlife Management, 73(4), 503–510. 10.2193/2008-265 [DOI] [Google Scholar]
- Lesk, C. , Coffel, E. , D’Amato, A. W. , Dodds, K. , & Horton, R. (2017). Threats to North American forests from southern pine beetle with warming winters. Nature Climate Change, 7, 713–717. 10.1038/nclimate3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levänen, R. , Kunnasranta, M. , & Pohjoismäki, J. (2018). Mitochondrial DNA introgression at the northern edge of the brown hare (Lepus europaeus) range. Annales Zoologici Fennici, 55(1–3), 15–24. 10.5735/086.055.0103 [DOI] [Google Scholar]
- Linden, D. W. , Campa, H. , Roloff, G. J. , Beyer, D. E. , & Millenbah, K. F. (2011). Modeling habitat potential for Canada lynx in Michigan. Wildlife Society Bulletin, 35(1), 20–26. 10.1002/wsb.3 [DOI] [Google Scholar]
- Litvaitis, J. A. , & Harrison, D. J. (1989). Bobcat–coyote niche relationships during a period of coyote population increase. Canadian Journal of Zoology, 67(5), 1180–1188. 10.1139/z89-170 [DOI] [Google Scholar]
- Litvaitis, J. A. , Tash, J. P. , & Stevens, C. L. (2006). The rise and fall of bobcat populations in New Hampshire: Relevance of historical harvests to understanding current patterns of abundance and distribution. Biological Conservation, 128(4), 517–528. 10.1016/j.biocon.2005.10.019 [DOI] [Google Scholar]
- Loehle, C. (1998). Height growth rate tradeoffs determine northern and southern range limits for trees. Journal of Biogeography, 25(4), 735–742. 10.1046/j.1365-2699.1998.2540735.x [DOI] [Google Scholar]
- Lomolino, M. V. , Riddle, B. R. , & Whittaker, R. J. (2016). Glaciation and biogeographic dynamics of the pleistocene In Biogeography: Biological diversity across space and time (5th Ed., pp. 271–310). Sunderland, MA: Sinauer Associates; 10.1016/B978-1-85617-679-8.00009-6 [DOI] [Google Scholar]
- Louthan, A. M. , Doak, D. F. , & Angert, A. L. (2015). Where and when do species interactions set range limits? Trends in Ecology and Evolution, 30(12), 780–792. 10.1016/j.tree.2015.09.011 [DOI] [PubMed] [Google Scholar]
- MacArthur, R. (1984). Geographical ecology; patterns in the distribution of species (2nd Ed.). Princeton, NJ: Princeton University Press. [Google Scholar]
- Major, J. T. , & Sherburne, J. A. (1987). Interspecific relationships of coyotes, bobcats, and red foxes in western Maine. Journal of Wildlife Management, 51(3), 606–616. 10.2307/3801278 [DOI] [Google Scholar]
- Malenke, J. R. , Newbold, N. , & Clayton, D. H. (2011). Condition‐specific competition governs the geographic distribution and diversity of ectoparasites. The American Naturalist, 177(4), 522–534. 10.1086/658176 [DOI] [PubMed] [Google Scholar]
- Manlick, P. J. , Woodford, J. E. , Zuckerberg, B. , & Pauli, J. N. (2017). Niche compression intensifies competition between reintroduced martens (Martes americana) and fishers (Pekania pennanti). Journal of Mammalogy, 3, 690–702. 10.1093/jmammal/gyx030 [DOI] [Google Scholar]
- Mantyka‐Pringle, C. S. , Martin, T. G. , & Rhodes, J. R. (2012). Interactions between climate and habitat loss effects on biodiversity: A systematic review and meta‐analysis. Global Change Biology, 18(4), 1239–1252. 10.1111/j.1365-2486.2011.02593.x [DOI] [Google Scholar]
- McCann, N. P. , & Moen, R. A. (2011). Mapping potential core areas for lynx (Lynx canadensis) using pellet counts from showshoe hares (Lepus americanus) and satellite imagery. Canadian Journal of Zoology, 89, 509–516. 10.1139/Z11-016 [DOI] [Google Scholar]
- McLellan, S. R. , Vashon, J. H. , Johnson, E. L. , Crowley, S. M. , & Vashon, A. D. (2018). Fisher predation on Canada lynx in the northeastern United States. Journal of Wildlife Management, 82, 1775–1783. 10.1002/jwmg.21538 [DOI] [Google Scholar]
- Meier, E. S. , Edwards, T. C. , Kienast, F. , Dobbertin, M. , & Zimmermann, N. E. (2011). Co‐occurrence patterns of trees along macro‐climatic gradients and their potential influence on the present and future distribution of Fagus sylvatica L. Journal of Biogeography, 38, 371–382. 10.1111/j.1365-2699.2010.02405.x [DOI] [Google Scholar]
- Mills, L. S. , Bragina, E. V. , Kumar, A. V. , Zimova, M. , Lafferty, D. J. R. , Feltner, J. , … Fay, K. (2018). Winter color polymorphisms identify global hot spots for evolutionary rescue from climate change. Science, 1036(March), eaan8097 10.1126/SCIENCE.AAN8097 [DOI] [PubMed] [Google Scholar]
- Morales-Castilla, I. , Matias, M. G. , Gravel, D. , & Araújo, M. B. (2015). Inferring biotic interactions from proxies. Trends in Ecology and Evolution, 30, 347–356. 10.1016/j.tree.2015.03.014 [DOI] [PubMed] [Google Scholar]
- Morelli, T. L. , Smith, A. B. , Kastely, C. R. , Mastroserio, I. , Moritz, C. , & Beissinger, S. R. (2012). Anthropogenic refugia ameliorate the severe climate‐related decline of a montane mammal along its trailing edge. Proceedings of the Royal Society B: Biological Sciences, 279(1745), 4279–4286. 10.1098/rspb.2012.1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, D. L. , Cox, E. W. , Ballard, W. B. , Whitlaw, H. A. , Lenarz, M. S. , Custer, T. W. , … Fuller, T. K. (2006). Pathogens, nutritional deficiency, and climate influences on a declining moose population. Wildlife Monographs, 166, 1–30. 10.2193/0084-0173(2006)166[1:PNDACI]2.0.CO;2 [DOI] [Google Scholar]
- Murray, D. L. , & Larivière, S. (2002). The relationship between foot size of wild canids and regional snow conditions: Evidence for selection against a high footload? Journal of Zoology, 256, 289–299. 10.1017/S095283690200033X [DOI] [Google Scholar]
- Nagamitsu, T. , Yamagishi, H. , Kenta, T. , Inari, N. , & Kato, E. (2010). Competitive effects of the exotic Bombus terrestris on native bumble bees revealed by a field removal experiment. Population Ecology, 52(1), 123–136. 10.1007/s10144-009-0151-7 [DOI] [Google Scholar]
- Newbury, R. K. , & Hodges, K. E. (2018). Regional differences in winter diets of bobcats in their northern range. Ecology and Evolution, 8(22), 11100–11110. 10.1002/ece3.4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normand, S. , Treier, U. A. , Randin, C. , Vittoz, P. , Guisan, A. , & Svenning, J. C. (2009). Importance of abiotic stress as a range‐limit determinant for European plants: Insights from species responses to climatic gradients. Global Ecology and Biogeography, 18(4), 437–449. 10.1111/j.1466-8238.2009.00451.x [DOI] [Google Scholar]
- Oaten, A. , & Murdoch, W. W. (1975). Switching, functional response, and stability in predator‐prey systems. The American Naturalist, 109(967), 299–318. 10.1086/282999 [DOI] [Google Scholar]
- Parmesan, C. (2006). Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics, 37(1), 637–669. 10.1146/annurev.ecolsys.37.091305.110100 [DOI] [Google Scholar]
- Patterson, B. R. , Benjamin, L. K. , & Messier, F. (1998). Prey switching and feeding habits of eastern coyotes in relation to snowshoe hare and white‐tailed deer densities. Canadian Journal of Zoology, 76, 1885–1897. 10.1139/z98-135 [DOI] [Google Scholar]
- Payette, S. , Fortin, M.‐J. , & Gamache, I. (2001). The subarctic forest–tundra: The structure of a biome in a changing climate. BioScience, 51(9), 709 10.1641/0006-3568(2001)051[0709:TSFTTS]2.0.CO;2 [DOI] [Google Scholar]
- Pearson, R. G. , & Dawson, T. P. (2003). Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecology and Biogeography, 12, 361–371. 10.1109/CLOUD.2010.16 [DOI] [Google Scholar]
- Pedersen, S. , Odden, M. , & Pedersen, H. C. (2017). Climate change induced molting mismatch? Mountain hare abundance reduced by duration of snow cover and predator abundance. Ecosphere, 8(3), 1–8. 10.1002/ecs2.1722 29552374 [DOI] [Google Scholar]
- Peers, M. J. L. , Thornton, D. H. , & Murray, D. L. (2013). Evidence for large‐scale effects of competition: Niche displacement in Canada lynx and bobcat. Proceedings of the Royal Society, 280(1773), 20132495 10.1098/rspb.2013.2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples, B. K. , Blanc, L. A. , & Frimpong, E. A. (2015). Lotic cyprinid communities can be structured as nest webs and predicted by the stress‐gradient hypothesis. Journal of Animal Ecology, 84(6), 1666–1677. 10.1111/1365-2656.12428 [DOI] [PubMed] [Google Scholar]
- Peterson, T. , Soberón, J. , Pearson, R. G. , Anderson, R. P. , Martínez-Meyer, E. , Nakamura, M. , & Araújo, M. B. (2011). Ecological niches and geographic distributions. Princeton, NJ: Princeton University Press. [Google Scholar]
- Pironon, S. , Papuga, G. , Villellas, J. , Angert, A. L. , García, M. B. , & Thompson, J. D. (2017). Geographic variation in genetic and demographic performance: New insights from an old biogeographical paradigm. Biological Reviews, 92(4), 1877–1909. 10.1111/brv.12313 [DOI] [PubMed] [Google Scholar]
- Pitt, J. A. , Larivière, S. , & Messier, F. (2008). Survival and body condition of raccoons at the edge of the range. Journal of Wildlife Management, 72(2), 389–395. 10.2193/2005-761 [DOI] [Google Scholar]
- Plummer, K. E. , Siriwardena, G. M. , Conway, G. J. , Risely, K. , & Toms, M. P. (2015). Is supplementary feeding in gardens a driver of evolutionary change in a migratory bird species? Global Change Biology, 21(12), 4353–4363. 10.1111/gcb.13070 [DOI] [PubMed] [Google Scholar]
- Pokallus, J. W. , & Pauli, J. N. (2015). Population dynamics of a northern‐adapted mammal: Disentangling the influence of predation and climate change. Ecological Applications, 25(6), 1546–1556. 10.1111/oik.01559 [DOI] [PubMed] [Google Scholar]
- Poley, L. G. , Pond, B. A. , Schaefer, J. A. , Brown, G. S. , Ray, J. C. , & Johnson, D. S. (2014). Occupancy patterns of large mammals in the Far North of Ontario under imperfect detection and spatial autocorrelation. Journal of Biogeography, 41(1), 122–132. 10.1111/jbi.12200 [DOI] [Google Scholar]
- Ritchie, E. G. , & Johnson, C. N. (2009). Predator interactions, mesopredator release and biodiversity conservation. Ecology Letters, 12(9), 982–998. 10.1111/j.1461-0248.2009.01347.x [DOI] [PubMed] [Google Scholar]
- Samuel, W. M. (2007). Factors affecting epizootics of winter ticks and mortality of moose. Alces, 43, 39–48. [Google Scholar]
- Schemske, D. W. , Mittelbach, G. G. , Cornell, H. V. , Sobel, J. M. , & Roy, K. (2009). Is there a latitudinal gradient in the importance of biotic interactions? Annual Review of Ecology Evolution and Systematics, 40, 245–269. 10.1146/annurev.ecolsys.39.110707.173430 [DOI] [Google Scholar]
- Scully, A. E. , Fisher, S. , Miller, D. A. W. , & Thornton, D. H. (2018). Influence of biotic interactions on the distribution of Canada lynx (Lynx canadensis) at the southern edge of their range. Journal of Mammalogy, 99(4), 760–772. 10.1093/jmammal/gyy053 [DOI] [Google Scholar]
- Seitz, R. D. , Lipcius, R. N. , Hines, A. H. , & Eggleston, D. B. (2001). Density‐dependent predation, habitat variation, and the persistence of marine bivalve prey. Ecology, 82(9), 2435–2451. 10.1890/0012-9658(2001)082[2435:DDPHVA]2.0.CO;2 [DOI] [Google Scholar]
- Sexton, J. P. , McIntyre, P. J. , Angert, A. L. , & Rice, K. J. (2009). Evolution and ecology of species range limits. Annual Review of Ecology, Evolution, and Systematics, 40(1), 415–436. 10.1146/annurev.ecolsys.110308.120317 [DOI] [Google Scholar]
- Simmons, A. D. , & Thomas, C. D. (2004). Changes in dispersal during species’ range expansions. The American Naturalist, 164(3), 378–395. 10.1086/423430 [DOI] [PubMed] [Google Scholar]
- Simon, J. A. , Marrotte, R. R. , Desrosiers, N. , Fiset, J. , Gaitan, J. , Gonzalez, A. , … Millien, V. (2014). Climate change and habitat fragmentation drive the occurrence of Borrelia burgdorferi, the agent of Lyme disease, at the northeastern limit of its distribution. Evolutionary Applications, 7(7), 750–764. 10.1111/eva.12165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons‐Legaard, E. M. , Harrison, D. J. , & Legaard, K. R. (2016). Habitat monitoring and projections for Canada lynx: Linking the Landsat archive with carnivore occurrence and prey density. Journal of Applied Ecology, 53, 1260–1269. 10.1111/1365-2664.12611 [DOI] [Google Scholar]
- Simons‐Legaard, E. M. , Harrison, D. J. , & Legaard, K. R. (2018). Ineffectiveness of local zoning to reduce regional loss and fragmentation of wintering habitat for white‐tailed deer. Forest Ecology and Management, 427(May), 78–85. 10.1016/j.foreco.2018.05.027 [DOI] [Google Scholar]
- Sinclair, A. R. E. , Pech, R. P. , Dickman, C. R. , Hik, D. , Mahon, P. , & Newsome, A. E. (1998). Predicting effects of predation on conservation of endangered prey. Conservation Biology, 12(3), 564–575. 10.1046/j.1523-1739.1998.97030.x [DOI] [Google Scholar]
- Small, R. J. , & Keith, L. B. (1992). An experimental study of red fox predation on arctic and snowshoe hares. Canadian Journal of Zoology, 70(8), 1614–1621. 10.1139/z92-222 [DOI] [Google Scholar]
- Soberón, J. (2007). Grinnellian and Eltonian niches and geographic distributions of species. Ecology Letters, 10(12), 1115–1123. 10.1111/j.1461-0248.2007.01107.x [DOI] [PubMed] [Google Scholar]
- Srinivasan, U. , Elsen, P. R. , Tingley, M. W. , & Wilcove, D. S. (2018). Temperature and competition interact to structure himalayan bird communities. Proceedings of the Royal Society B: Biological Sciences, 285(1874), 20172593 10.1098/rspb.2017.2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultaire, S. M. , Pauli, J. N. , Martin, K. J. , Meyer, M. W. , Notaro, M. , & Zuckerberg, B. (2016). Climate change surpasses land‐use change in the contracting range boundary of a winter‐adapted mammal. Proceedings of the Royal Society B, 283, 20153104 10.1098/rspb.2015.3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi, Y. , & Nakano, S. (2000). Condition-specific competition: Implications for the altitudinal distribution of stream fishes. Ecology, 81(7), 2027–2039. [Google Scholar]
- Tape, K. D. , Christie, K. , Carroll, G. , & O’Donnell, J. A. (2016). Novel wildlife in the Arctic: The influence of changing riparian ecosystems and shrub habitat expansion on snowshoe hares. Global Change Biology, 22(1), 208–219. 10.1111/gcb.13058 [DOI] [PubMed] [Google Scholar]
- Taulman, J. F. , & Robbins, L. W. (1996). Recent range expansion and distributional limits of the nine‐banded armadillo (Dasypus novemcinctus) in the United States. Journal of Biogeography, 23(5), 635–648. 10.1111/j.1365-2699.1996.tb00024.x [DOI] [Google Scholar]
- Todd, A. W. , Keith, L. B. , & Fischer, C. A. (1981). Population ecology of coyotes during a fluctuation of snowshoe hares. Journal of Wildlife Management, 45(3), 629–640. 10.2307/3808696 [DOI] [Google Scholar]
- Twomey, E. , Morales, V. , & Summers, K. (2008). Evaluating condition‐specific and asymmetric competition in a species‐distribution context. Oikos, 117(8), 1175–1184. 10.1111/j.0030-1299.2008.16676.x [DOI] [Google Scholar]
- Varudkar, A. , & Ramakrishnan, U. (2015). Commensalism facilitates gene flow in mountains: A comparison between two Rattus species. Heredity, 115(3), 253–261. 10.1038/hdy.2015.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite, T. A. , & Strickland, D. (2006). Climate change and the demographic demise of a hoarding bird living on the edge. Proceedings of the Royal Society B: Biological Sciences, 273(1603), 2809–2813. 10.1098/rspb.2006.3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattles, D. W. , Zeller, K. A. , & DeStefano, S. (2018). Range expansion in unfavorable environments through behavioral responses to microclimatic conditions: Moose (Alces americanus) as the model. Mammalian Biology, 93, 189–197. 10.1016/j.mambio.2018.05.009 [DOI] [Google Scholar]
- Weidman, T. , & Litvaitis, J. A. (2011). Can supplemental food increase winter survival of a threatened cottontail rabbit? Biological Conservation, 144(7), 2054–2058. 10.1016/j.biocon.2011.04.027 [DOI] [Google Scholar]
- Westoby, M. , Kunstler, G. , Leishman, M. R. , & Morgan, J. (2017). How species boundaries are determined: A response to Alexander et al. Trends in Ecology & Evolution, 32(1), 7–8. 10.1016/j.tree.2016.10.014 [DOI] [PubMed] [Google Scholar]
- Wiens, J. J. (2011). The niche, biogeography and species interactions. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1576), 2336–2350. 10.1098/rstb.2011.0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, E. C. , Shipley, A. A. , Zuckerberg, B. , Peery, M. Z. , & Pauli, J. N. (2018). An experimental translocation identifies habitat features that buffer camouflage mismatch in snowshoe hares. Conservation Letters, 12, e12614 10.1111/conl.12614 [DOI] [Google Scholar]
- Wisz, M. S. , Pottier, J. , Kissling, W. D. , Pellissier, L. , Lenoir, J. , Damgaard, C. F. , … Svenning, J.‐C. (2013). The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biological Reviews, 88(1), 15–30. 10.1111/j.1469-185X.2012.00235.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff, J. O. (1996). Coexistence of white‐footed mice and deer mice may be mediated by fluctuating environmental conditions. Oecologia, 108(3), 529–533. 10.1007/BF00333730 [DOI] [PubMed] [Google Scholar]
- Zielinski, W. J. , Tucker, J. M. , & Rennie, K. M. (2017). Niche overlap of competing carnivores across climatic gradients and the conservation implications of climate change at geographic range margins. Biological Conservation, 209(March), 533–545. 10.1016/j.biocon.2017.03.016 [DOI] [Google Scholar]
- Zimova, M. , Mills, L. S. , & Nowak, J. J. (2016). High fitness costs of climate change induced camouflage mismatch in a seasonally colour moulting mammal. Ecology Letters, 19, 299–307. 10.1111/ele.12568 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article does not use data.


