Abstract
Herein, we present the synthesis of the bench‐stable sodium bicyclo[1.1.1]pentanesulfinate (BCP‐SO2Na) and its application in the synthesis of bicyclo[1.1.1]pentyl (BCP) sulfones and sulfonamides. The salt can be obtained in a four‐step procedure from commercially available precursors in multigram scale without the need for column chromatography or crystallization. Sulfinates are known to be useful precursors in radical and nucleophilic reactions and are widely used in medicinal chemistry. This building block enables access to BCP sulfones and sulfonamides avoiding the volatile [1.1.1]propellane which is favorable for the extension of SAR studies. Further, BCP‐SO2Na enables the synthesis of products that were not available with previous methods. A chlorination of BCP‐SO2Na and subsequent reaction with a Grignard reagent provides a new route to BCP sulfoxides. Several products were analyzed by single‐crystal X‐ray diffraction.
Keywords: bicyclo[1.1.1]pentane, bioisosteres, propellanes, sulfonamides, sulfur
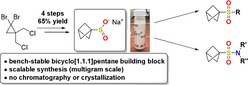
Scaling the chemical heights! We present a scalable synthesis of the bench‐stable sodium bicyclo[1.1.1]pentane sulfinate (BCP‐SO2Na) in four steps without the need of chromatography or crystallization. Further, its application in the synthesis of BCP sulfones and sulfonamides is described (see scheme).
Sulfones and sulfonamides are, among other sulfur‐containing groups, common moieties in drug compounds,1 with eletriptan (1), a serotonin receptor agonist, and bosentan (2), an endothelin receptor antagonist, just two of many examples (Figure 1 a).2 ‘Escaping the flatland’ is a common trend in recent years, in which the bioisosteric replacement of planar aromatic moieties by saturated hydrocarbons can improve pharmacological properties of drug candidates.3 The rigid bicyclo[1.1.1]pentanes (BCPs) have become famous target structures in these approaches.4 There have been studies that have used BCPs successfully as a replacement of benzene (Figure 1 b),5 alkyne,6 and tert‐butyl7 groups.
Figure 1.
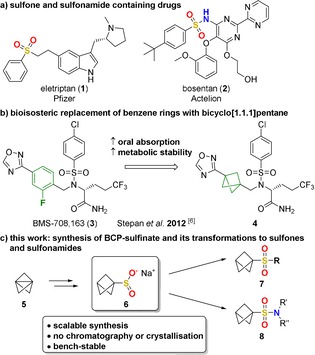
a) Examples for sulfone‐ and sulfonamide‐containing drugs. b) The replacement of a para‐substituted fluorobenzene with BCP in the γ‐secretase inhibitor 3 led to improved pharmacological properties. c) Content of this work.
Most BCPs are accessed by radical or anionic reactions with the strained tricyclic compound [1.1.1]propellane (5).8 The latter can react with Grignard reagents,6, 9 or alkyl iodides9a, 10 to provide aryl‐ and alkyl‐substituted BCPs. BCP amines can be obtained by the reaction of turbo‐amides with 5.11 Sulfur‐based functional groups allow the radical opening of 5 as well, as shown for thiols,12 disulfides,13 and xanthates.14
However, all of these reactions require the handling of the volatile precursor 5 and the necessity of Schlenk techniques in the preparation. Bench‐stable precursors facilitate the use of this interesting group and a variety of BCP amines, acids and esters are already commercially available. Recently, Kanazawa, Uchiyama et al. developed a gram‐scale synthesis of a silaborated BCP.15 The availability of sulfur‐based BCP building blocks is still limited and therefore the broad application of this bioisostere is prevented.16
Sulfinates seem to be ideal candidates for this purpose as they are highly versatile reagents. They can be employed in nucleophilic reactions, transition‐metal catalysis or serve as radical precursors.17
We, herein, report the synthesis of sodium bicyclo[1.1.1]pentanesulfinate (BCP‐SO2Na, 6) and the utilization of this building block in different reactions to obtain BCP sulfones 7 and sulfonamides 8 (Figure 1 c).
The bench‐stable salt could be obtained in good yield and purity without the need of purification by column chromatography or crystallization (Scheme 1). In the first step, [1.1.1]propellane (5) was prepared from the commercially available precursor 9 with phenyllithium as previously described and distilled together with diethyl ether (see the Supporting Information for details).11a The obtained solution was used directly to perform a thiol addition with 10.12 After one washing step with NaOH‐solution and removal of the solvent, pure 11 was obtained in 79 % yield from 9. The sulfide 11 was oxidized with 3‐chloroperoxybenzoic acid (mCPBA), which led to formation of 12 in 82 % yield. The purity of 12 could be successfully increased by changing the oxidant to oxone, yielding 72 % after extraction with dichloromethane. The sulfone 12 was converted to the respective sulfinate in a retro‐Michael reaction initiated by sodium methoxide.18 Without further purification, product 6 was obtained in quantitative yield. The synthesis was performed on a multigram‐scale (9.4 g, 61 mmol) in an overall yield of 65 % (with mCPBA) or 57 % (with oxone) over four steps.
Scheme 1.
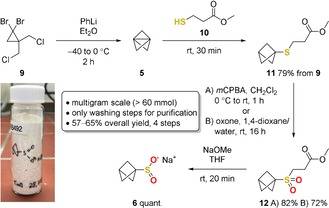
Synthesis of sodium BCP‐SO2Na (6) from commercially available cyclopropane 9. The synthesis was performed on a multigram‐scale (9.4 g, 61 mmol for method A). Oxone: 2 KHSO5 ⋅KHSO4 ⋅K2SO4.
With the novel building block 6 in hand, we performed several reactions that prove its versatility. Nucleophilic aromatic substitutions (SNAr) resulted in good to excellent yields with electron‐deficient aryl fluorides (7 a–c), while poor to fair yields were observed with heteroaryl chlorides (7 d,e) (Scheme 2 a). It should be noted that attempts to obtain heteroaryl‐substituted BCP sulfides through aromatic thiol addition to 5 were not successful. This method is the first to provide access to heteroaryl‐containing structures, for example, 7 d and 7 e, to our current knowledge.
Scheme 2.
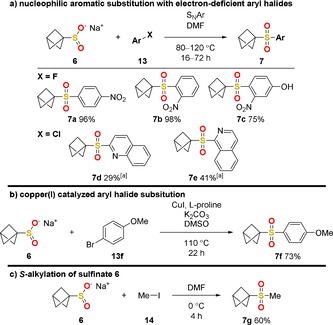
Syntheses of sulfones 7 through (a) SNAr, (b) copper(I) catalysis, and (c) alkylation. [a] Addition of 1.50 equiv of K2CO3.
To access BCP sulfones with aryl substituents with higher electron density, such as 7 f, a copper(I)‐catalyzed reaction was deployed successfully (Scheme 2 b). Simple alkylation with methyl iodide (14) could be performed without the addition of a base at low temperature (0 °C) (Scheme 2 c).
Numerous procedures can be found to convert sulfinates, isolated or in situ, into sulfonamides. For a summary of recent methods we refer the reader to review articles by Messaoudi, Alami and Hamze et al.17a and Maulide et al.17c For the conversion of 6 into N‐alkyl sulfonamides 8 a,b we chose the simple conditions by Yuan et al. (Scheme 3 a).19 This reaction enables access to BCP sulfonamides for the first time. Previous attempts with protected BCP thiols (Bn‐ and TIPS‐protected) were unsuccessful as the BCP thiol seemed to rearrange during the deprotection step and no desired product could be detected.
Scheme 3.
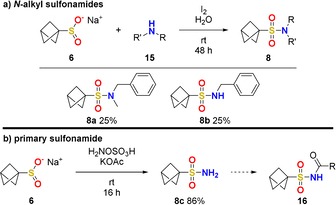
Syntheses of N‐alkyl sulfonamides 8 a,b (a) and primary sulfonamide 8 c (b).
The primary BCP sulfonamide 8 c could be obtained in very good yield from 6 with hydroxylamine‐O‐sulfonic acid (Scheme 3 b). This compound provides easy access to N‐acyl sulfonamides 16, another medicinally relevant class of compounds.20
To further extend the possible modifications of 6, a chlorination was performed and the sulfinyl chloride 17 was reacted with phenylmagnesium bromide in situ to obtain sulfoxide 18 (Scheme 4).
Scheme 4.
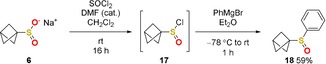
Chlorination of 6 and subsequent reaction with phenylmagnesium bromide to sulfoxide 18.
For several products, we were able to obtain single crystals and determine the structure by X‐ray diffraction (Supporting Information). Three of those structures (7 a, 7 g, 8 b) are shown in Figure 2.
Figure 2.
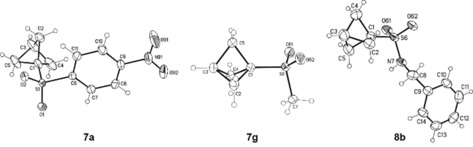
Molecular structures of 7 a, 7 g, and 8 b determined by X‐ray diffraction. The displacement parameters are drawn at 50 % probability level.
In conclusion, we have developed a four‐step synthesis of BCP‐SO2Na (6) from commercially available precursors. The synthesis is scalable and requires no chromatography or crystallization to purify the product. We have shown the application of this building block in the syntheses of several sulfones and sulfonamides. The synthesis of sulfoxides was shown for one example as a proof‐of‐concept.
This building block will be a useful tool in novel structure–activity‐relationship studies and will expand the application of BCPs in medicinal chemistry.
Experimental Section
Full experimental details and analytical data (1H NMR, 13C NMR, X‐ray analysis) are provided in the Supporting Information.
CCDC https://www.ccdc.cam.ac.uk/services/strctures?id=doi:10.1002/chem.2000097 contain the supplementary crystallographic data for this paper. These data are provided free of charge by http://www.ccdc.cam.ac.uk/.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
R.M.B. acknowledges the SFB 1176 funded by the German Research Foundation (DFG) in the context of projects A4 & B3 for funding.
R. M. Bär, P. J. Gross, M. Nieger, S. Bräse, Chem. Eur. J. 2020, 26, 4242.
References
- 1. Feng M., Tang B., Liang S. H., Jiang X., Curr. Top. Med. Chem. 2016, 16, 1200–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scott K. A., Njardarson J. T., Top. Curr. Chem. 2018, 376, 1–34. [DOI] [PubMed] [Google Scholar]
- 3. Lovering F., Bikker J., Humblet C., J. Med. Chem. 2009, 52, 6752–6756. [DOI] [PubMed] [Google Scholar]
- 4. Locke G. M., Bernhard S. S. R., Senge M. O., Chem. Eur. J. 2019, 25, 4590–4647. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. Pellicciari R., Filosa R., Fulco M. C., Marinozzi M., Macchiarulo A., Novak C., Natalini B., Hermit M. B., Nielsen S., Sager T. N., Stensbøl T. B., Thomsen C., ChemMedChem 2006, 1, 358–365; [DOI] [PubMed] [Google Scholar]
- 5b. Stepan A. F., Subramanyam C., Efremov I. V., Dutra J. K., O'Sullivan T. J., DiRico K. J., McDonald W. S., Won A., Dorff P. H., Nolan C. E., Becker S. L., Pustilnik L. R., Riddell D. R., Kauffman G. W., Kormos B. L., Zhang L., Lu Y., Capetta S. H., Green M. E., Karki K., Sibley E., Atchison K. P., Hallgren A. J., Oborski C. E., Robshaw A. E., Sneed B., O'Donnell C. J., J. Med. Chem. 2012, 55, 3414–3424; [DOI] [PubMed] [Google Scholar]
- 5c. Nicolaou K. C., Vourloumis D., Totokotsopoulos S., Papakyriakou A., Karsunky H., Fernando H., Gavrilyuk J., Webb D., Stepan A. F., ChemMedChem 2016, 11, 31–37; [DOI] [PubMed] [Google Scholar]
- 5d. Auberson Y. P., Brocklehurst C., Furegati M., Fessard T. C., Koch G., Decker A., La Vecchia L., Briard E., ChemMedChem 2017, 12, 590–598; [DOI] [PubMed] [Google Scholar]
- 5e. Goh Y. L., Tam E. K., Bernardo P. H., Cheong C. B., Johannes C. W., William A. D., Adsool V. A., Org. Lett. 2014, 16, 1884–1887; [DOI] [PubMed] [Google Scholar]
- 5f. Measom N. D., Down K. D., Hirst D. J., Jamieson C., Manas E. S., Patel V. K., Somers D. O., ACS Med. Chem. Lett. 2017, 8, 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Makarov I. S., Brocklehurst C. E., Karaghiosoff K., Koch G., Knochel P., Angew. Chem. Int. Ed. 2017, 56, 12774–12777; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 12949–12953. [Google Scholar]
- 7.
- 7a. Barbachyn M. R., Hutchinson D. K., Toops D. S., Reid R. J., Zurenko G. E., Yagi B. H., Schaadt R. D., Allison J. W., Bioorg. Med. Chem. Lett. 1993, 3, 671–676; [Google Scholar]
- 7b. Westphal M. V., Wolfstädter B. T., Plancher J.-M., Gatfield J., Carreira E. M., ChemMedChem 2015, 10, 461–469. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. Dilmaç A. M., Spuling E., de Meijere A., Bräse S., Angew. Chem. Int. Ed. 2017, 56, 5684–5718; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 5778–5813; [Google Scholar]
- 8b. Kanazawa J., Uchiyama M., Synlett 2019, 30, 1–11. [Google Scholar]
- 9.
- 9a. Messner M., Kozhushkov S. I., de Meijere A., Eur. J. Org. Chem. 2000, 1137–1155; [Google Scholar]
- 9b. Daniel Rehm J. D., Ziemer B., Szeimies G., Eur. J. Org. Chem. 1999, 1999, 2079–2085. [Google Scholar]
- 10.
- 10a. Nugent J., Arroniz C., Shire B. R., Sterling A. J., Pickford H. D., Wong M. L. J., Mansfield S. J., Caputo D. F. J., Owen B., Mousseau J. J., Duarte F., Anderson E. A., ACS Catal. 2019, 9, 9568–9574; [Google Scholar]
- 10b. Caputo D. F. J., Arroniz C., Dürr A. B., Mousseau J. J., Stepan A. F., Mansfield S. J., Anderson E. A., Chem. Sci. 2018, 9, 5295–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.
- 11a. Gianatassio R., Lopchuk J. M., Wang J., Pan C.-M., Malins L. R., Prieto L., Brandt T. A., Collins M. R., Gallego G. M., Sach N. W., Spangler J. E., Zhu H., Zhu J., Baran P. S., Science 2016, 351, 241–246; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11b. Hughes J. M. E., Scarlata D. A., Chen A. C. Y., Burch J. D., Gleason J. L., Org. Lett. 2019, 21, 6800–6804. [DOI] [PubMed] [Google Scholar]
- 12. Bär R. M., Kirschner S., Nieger M., Bräse S., Chem. Eur. J. 2018, 24, 1373–1382. [DOI] [PubMed] [Google Scholar]
- 13. Bär R. M., Heinrich G., Nieger M., Fuhr O., Bräse S., Beilstein J. Org. Chem. 2019, 15, 1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rout S. K., Marghem G., Lan J., Leyssens T., Riant O., Chem. Commun. 2019, 55, 14976–14979. [DOI] [PubMed] [Google Scholar]
- 15. Kondo M., Kanazawa J., Ichikawa T., Shimokawa T., Nagashima Y., Miyamoto K., Uchiyama M., Angew. Chem. Int. Ed. 2020, 59, 1970–1974; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 1986–1990 [Google Scholar]
- 16.To the best of our knowledge there are only few sulfur-containing BCPs available from Enamine Ltd. BCP thiol costs ≈2000 $ g−1.
- 17.
- 17a. Aziz J., Messaoudi S., Alami M., Hamze A., Org. Biomol. Chem. 2014, 12, 9743–9759; [DOI] [PubMed] [Google Scholar]
- 17b. Smith J. M., Dixon J. A., deGruyter J. N., Baran P. S., J. Med. Chem. 2019, 62, 2256–2264; [DOI] [PubMed] [Google Scholar]
- 17c. Kaiser D., Klose I., Oost R., Neuhaus J., Maulide N., Chem. Rev. 2019, 119, 8701–8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baskin J. M., Wang Z., Tetrahedron Lett. 2002, 43, 8479–8483. [Google Scholar]
- 19. Pan X., Gao J., Liu J., Lai J., Jiang H., Yuan G., Green Chem. 2015, 17, 1400–1403. [Google Scholar]
- 20. Ammazzalorso A., De Filippis B., Giampietro L., Amoroso R., Chem. Biol. Drug Des. 2017, 90, 1094–1105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


