Abstract
Achieving target glycaemic control is essential in people with diabetes to minimize the risk of long‐term complications, and many people with type 2 diabetes will ultimately require basal insulin (BI) therapy to achieve their individualized glycaemic targets. Usually, the first 12 weeks following initiation of BI therapy represents the period when the greatest dose increases and glycaemic reductions occur. Effective glycaemic control combined with minimizing the risk of hypoglycaemia is important to enable the achievement of glycaemic control in the longer term. However, substantial therapeutic inertia exists in clinical practice, both in initiation and up‐titration of BI, owing to patient‐, physician‐ and healthcare system‐related barriers, including fear of hypoglycaemia and the perception of a burdensome regimen. The more prolonged duration of action, reduced glycaemic variability and lower risk of hypoglycaemia seen with second‐generation versus first‐generation BI analogues may help alleviate patients’ and physicians’ concerns and facilitate titration. In turn, optimal BI titration and subsequent metabolic benefits may help improve therapy adherence and self‐management. This review details the clinical implications of prompt titration of BI to achieve early glycaemic control, and the importance of minimizing hypoglycaemia risk within the initial titration period. Facilitation of patients’ self‐management of BI is also addressed.
Keywords: basal insulin, glycaemic control, hypoglycaemia, insulin analogues, insulin therapy
1. INTRODUCTION
Tight glycaemic control is essential in people with diabetes to promote better clinical outcomes, particularly to reduce the incidence and progression of microvascular complications such as retinopathy and nephropathy.1, 2, 3, 4, 5 The benefits of tight glycaemic control are particularly pronounced if control is achieved soon after the initiation of antihyperglycaemic therapy, especially during the first year. A 10‐year post‐trial follow‐up of the UK Prospective Diabetes Study showed that early intensive glycaemic treatment (and initial greater reductions in HbA1c) during the first year resulted in significant improvements in long‐term clinical outcomes, including microvascular disease, myocardial infarction and mortality.6 Additionally, a retrospective analysis on data from the UK Clinical Practice Research Datalink on patient records from people with type 2 diabetes (T2D) showed that a 1‐year delay in antihyperglycaemic therapy intensification (either adding further oral antihyperglycaemic drugs [OADs] or insulin) was associated with significantly increased risk of adverse cardiovascular outcomes (eg, myocardial infarction, heart failure and stroke).7
Despite this, real‐world evidence has consistently shown that many people with T2D still have poor glycaemic control for prolonged periods of time.8 Clinical practice guidelines recommend therapy intensification by the addition of basal insulin (BI) therapy in people with T2D inadequately controlled with OADs and glucagon‐like peptide‐1 receptor agonists.9, 10 Despite these recommendations, therapeutic inertia with insulin therapy is widespread. The Study of Once Daily Levemir (SOLVE) trial showed that the majority of patients with T2D are poorly controlled at the time of BI therapy initiation; regional differences were observed, but the proportion of patients with HbA1c ≥9.0% when insulin therapy was initiated ranged from 23% to 64%.11 Even after BI initiation, attainment of the general HbA1c target is often inadequate in clinical practice, with one large observational study of European and US people with T2D showing that 79.1% and 72.2% of people initiating insulin failed to achieve an HbA1c of ≤7.0% after 3 and 24 months, respectively.12 This agrees with another European study in both insulin‐naïve people initiating BI, or those previously treated who switched to a new BI, which showed poor achievement of HbA1c <7.0% (53.6%) after 12 months.13 Data from developing countries also show poor achievement of glycaemic targets, with 85.8% of people using insulin in combination with OADs showing inadequate glycaemic control.14
Therapeutic inertia of BI therapy includes initiation inertia (delayed initiation of BI), titration inertia (lack of BI dose adjustment) and intensification inertia (delayed intensification of BI with additional antihyperglycaemic drugs).15 Inertia in initiating and titrating BI probably reflects a number of patient‐, physician‐ and healthcare system‐related barriers. These barriers include fear of hypoglycaemia (highlighted by the fact that 75.5% of physicians would treat more aggressively were it not for the risk of hypoglycaemia with insulin16, 17), weight gain, lack of healthcare provider time or resources, lack of perceived therapy efficacy, limited patient motivation, lack of experience in diabetes self‐management, and the perception of burdensome or complex treatment regimens (Figure 1).15, 16, 17, 18 The healthcare system‐related issue of lack of resources and time for individual physicians may also make it difficult to determine optimal dose adjustments.15 Other system‐level barriers, particularly in developing countries, may include lack of access to appropriate therapies or reimbursement policies.19 Many of the barriers to optimal insulin titration also contribute to an increased probability of overall poor glycaemic control.
Figure 1.
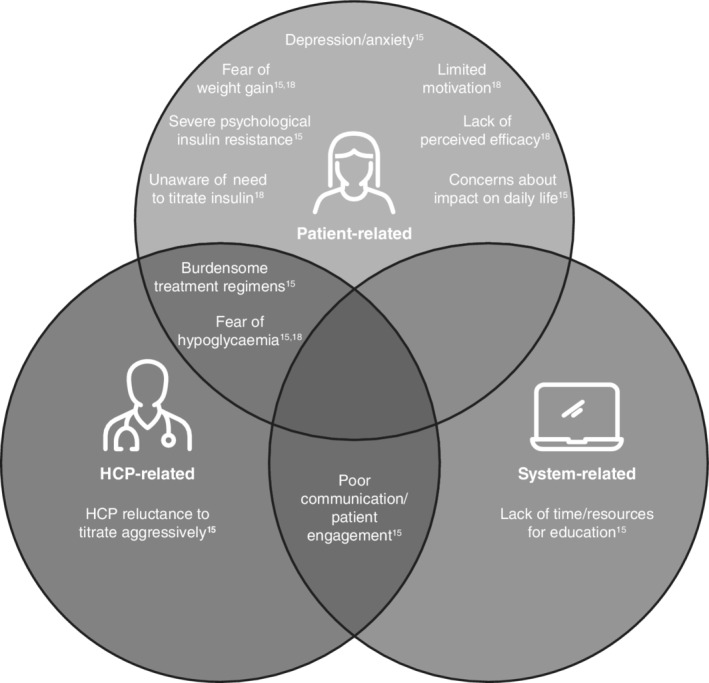
Factors contributing to therapeutic inertia of insulin. HCP, healthcare professional
Historically, clinical practice guidelines have emphasized a stepwise approach to antihyperglycaemic therapy. Recently, to reduce therapeutic inertia, the American Diabetes Association (ADA) and joint ADA/European Association for the Study of Diabetes (EASD) consensus recommendations have suggested regular (every 3–6 months) re‐evaluation of antihyperglycaemic regimens, to ensure that intensification of antihyperglycaemic regimens is considered in patients who are not achieving glycaemic targets.9, 10 Other recommendations to help alleviate therapeutic inertia and avoid suboptimal therapy include ongoing monitoring of metabolic variables and cardiovascular risk factors (eg, HbA1c, self‐monitoring of blood glucose, weight, blood pressure and lipids), self‐management education programmes, support for emotional well‐being, and the use of regular self‐titration of BI with an evidence‐based titration algorithm to achieve a fasting plasma glucose (FPG) target.9, 10 Insulin titration can be facilitated using simplified and, ideally, patient‐directed titration algorithms. The optimal titration algorithm should be simple to follow, effective, and should minimize the risk of hypoglycaemia, particularly during the initial titration stage.
This review details the clinical evidence for the benefits of titrating BI to achieve early glycaemic control and minimizing hypoglycaemia risk, with examples from both randomized controlled trials (RCTs) and real‐world evidence studies of BI therapy. The importance of facilitating patients’ self‐management of BI therapy to further improve adherence and early glycaemic control attainment is also explored.
2. HOW IS THE TITRATION PERIOD DEFINED?
RCTs of BI treatment are typically separated into two phases: (i) the initiation and titration phase, defined in many clinical trials as the first 8–12 weeks of treatment; and (ii) the maintenance phase, where insulin dose is more stable, with fewer adjustments required.20 Recent guidelines recommend that once BI therapy is initiated (at 10 units [U]/day or 0.1 to 0.2 U/kg/day), titration is performed every 3 days to reach a suitable FPG target.10
In a pooled analysis of 15 treat‐to‐target studies of insulin glargine 100 U/mL (Gla‐100) initiation in insulin‐naïve people with diabetes, 89% of the overall increase in BI dose occurred in the first 12 weeks (BI doses: at baseline, 0.16; at 12 weeks, 0.40; at 24 weeks, 0.45 U/kg; Figure 2).21 In addition, most of the overall improvements in HbA1c and FPG were achieved by week 12.21 Data from the SOLVE study are also in agreement with these results, showing that the greatest changes in insulin dose and the greatest HbA1c reductions occurred during the initial 12 weeks of BI therapy with insulin detemir (IDet).22
Figure 2.
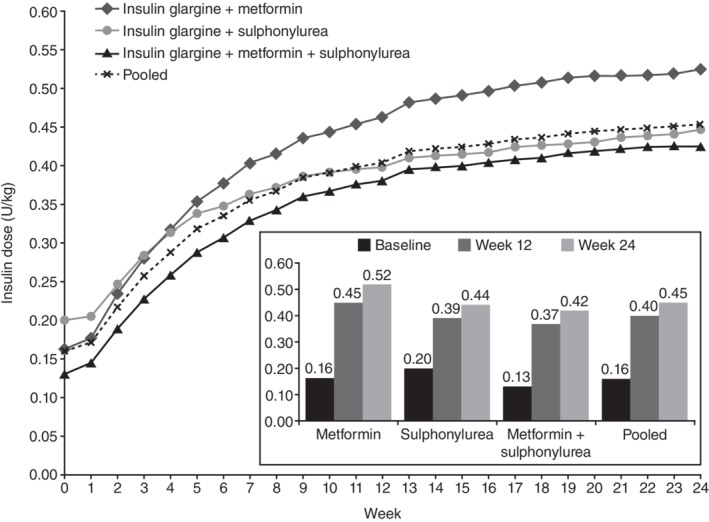
Insulin dose titration with insulin glargine. Figure reproduced from Owens DR, et al. Diab Res Clin Pract 2014;106:264–274
Although less frequent, insulin dose adjustments are still important after the titration period. During the maintenance period, which may resemble day‐to‐day reality for most patients using BI therapy, patients usually need to make slight adjustments to their insulin dose to maintain glycaemic targets while continuing to avoid hypoglycaemia.
3. THE CLINICAL IMPORTANCE OF THE TITRATION PERIOD
As described previously, there is evidence suggesting that early glycaemic control promotes improved glycaemic outcomes in the longer term, and fewer vascular complications in people with diabetes,1, 2, 3, 4, 5 suggesting that prompt titration is important for people with T2D initiating BI therapy.
One real‐world study in insulin‐naïve people with T2D newly initiating BI therapy showed that failure to achieve HbA1c ≤7% during the initial 3 months was associated with increased risk of failure to achieve glycaemic targets at 2 years (odds ratio, 3.70 [95% CI: 3.41–4.00]).12 In addition, an observational study of electronic health record data showed that many previously insulin‐naïve people with diabetes discontinue insulin shortly after initiation: 41% in the first month, 62% in the first 3 months and 82% in the first year.23 Similarly, another real‐world study looking at data from the UK Clinical Practice Research Datalink database showed that, in people with T2D with inadequate HbA1c control (≥7.5%) while receiving BI treatment, discontinuation of BI therapy was 32.1%.24 Only 30.9% of those individuals had their treatment intensified, and the median time to intensification was 3.7 years.24 BI discontinuation is associated with increased acute care costs (hospitalization and emergency room visits) and may be associated with poorer long‐term outcomes.23
While pursuing glycaemic targets in the early stages of treatment is important, it is also key to minimize the risk of hypoglycaemia to encourage treatment adherence, reduce hypoglycaemia in the long term, and reduce the risks of hypoglycaemia‐associated morbidity and mortality.12, 16, 25 In a retrospective study of over 55 000 insulin‐naïve individuals with T2D, ~ 5% experienced hypoglycaemia that required medical assistance during the first 6 months after starting BI therapy, and over 33% experienced a hypoglycaemic event in the first month after initiation.16 Those who experienced hypoglycaemia within 6 months of initiating BI were more probable to discontinue therapy within the first 12 months of treatment compared with those who did not (hazard ratio 1.16 [95% CI: 1.03–1.32]; P = 0.016).16 Although there were several limitations to the study, being a retrospective analysis of US claims data, the authors highlighted that the presence of comparable results across several databases emphasized the trend for discontinuation being more probable among those who experienced hypoglycaemia within 6 months of initiating BI therapy.16 Another observational real‐world study in insulin‐naïve people with T2D in Europe and the USA showed that hypoglycaemia during the initial 3‐month period after starting BI therapy was strongly associated with longer‐term risk of these events between 3 months and 2 years (odds ratio: 5.71 [95% CI: 4.67–6.99]).12 Similar findings have also been reported in a post hoc analysis of the BRIGHT RCT, a 24‐week, treat‐to‐target study, which compared the two second‐generation BI analogues insulin glargine 300 U/mL (Gla‐300) and insulin degludec (IDeg; 100 U/mL) in insulin‐naïve people with T2D.26 Results showed that the incidence of anytime (24 hours) hypoglycaemic events within the maintenance period (weeks 13–24) was lower for individuals who did not experience hypoglycaemia within the initial 12‐week titration period compared with those who did (38.2% vs. 75.4%).26 Additionally, an analysis of 16 RCTs of Gla‐100 initiation showed that a higher risk of hypoglycaemia during titration was associated with a continued higher risk of non‐severe and severe hypoglycaemia for up to 6 months.27
4. CAN NEWER BI ANALOGUES ASSIST WITH INSULIN TITRATION AND TREATMENT ADHERENCE?
First‐generation BI analogues, such as Gla‐100 and IDet, have a prolonged and more stable duration of action compared with previous intermediate‐acting insulins, such as twice‐daily neutral protamine Hagedorn (NPH) insulin, with Gla‐100 (0.3 U/kg) showing glucose‐lowering activity of up to 22 ± 4 hours and IDet (0.4 U/kg) possessing a slightly shorter duration of 21.5 ± 3.3 hours under single‐dose conditions.28, 29, 30 As such, first‐generation BI analogues helped to simplify treatment regimens, with once‐daily dosing becoming possible for a large proportion of patients.30 Additional benefits of first‐generation BI analogues include lower glycaemic variability and lower risk of hypoglycaemia compared with NPH insulin.30
Second‐generation BI analogues, such as Gla‐300 and IDeg, have a more prolonged duration of action, flatter pharmacokinetic and pharmacodynamic profiles, and a lower risk of hypoglycaemia compared with first‐generation BIs.30, 31 The prolonged duration of action results in only once‐daily dosing,30, 31 which can help to alleviate the treatment burdens in people otherwise requiring more frequent dosing with older intermediate‐acting insulins, such as NPH insulin, or first‐generation BIs, Gla‐100 or IDet.
Fear of hypoglycaemia is also a key factor in titration inertia; the lower risk of hypoglycaemia observed in clinical trials with second‐generation BI analogues compared with first‐generation BI analogues may help to alleviate such concerns during insulin initiation or titration. Given that burden of treatment is a factor influencing adherence,32 these second‐generation BI analogues could help further improve patient adherence, although there are no studies that have directly examined this relationship.
4.1. Evidence from randomized controlled trials
The EDITION clinical trial programme compared Gla‐300 with Gla‐100 in people with T2D using the same titration algorithm (Figure 3).33, 34, 35 While EDITION 1 (prior BI and mealtime insulin) and EDITION 2 (prior BI) included people with T2D who were already receiving BI therapy and provided insights into the efficacy and safety of up‐titration of BI in BI‐switcher populations,33, 34 EDITION 3 included previously insulin‐naïve individuals with T2D, and was representative of BI initiation and titration.35 In EDITION 3, the mean change in HbA1c from baseline to month 6 and proportion of participants reaching their target HbA1c or FPG at month 6 was similar between the two treatment groups. Additionally, the percentage of participants experiencing ≥1 nocturnal (00:00–05:59 hours) confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemic event during the maintenance period (week 9 to month 6; main secondary efficacy endpoint) was similar in both groups (relative risk [RR] 0.89 [95% CI: 0.66–1.20]). However, over the full 6‐month treatment period, the incidence of nocturnal confirmed (≤3.9 mmol/L [≤70 mg/dL]) hypoglycaemia was significantly lower with Gla‐300 versus Gla‐100 (RR: 0.76 [95% CI: 0.59–0.99]). Furthermore, rates of anytime (24 hours) hypoglycaemia were significantly lower with Gla‐300 versus Gla‐100 over the full 6‐month treatment period, particularly during the initial 8‐week titration period.35 However, it should be noted that FPG at month 6 was higher and prebreakfast self‐measured plasma glucose (SMPG) decreased more gradually with Gla‐300 versus Gla‐100, which may indicate that while the insulin dose increase was greater with Gla‐300 versus Gla‐100 throughout the study, the increase in circulating Gla‐300 may still have been more gradual as Gla‐300 and Gla‐100 are not bioequivalent.36 It may be relevant to consider these results when interpreting the hypoglycaemia risk profiles of Gla‐300 and Gla‐100. Similar results to those described for EDITION 3 were observed in the EDITION 1 and 2 trials.33, 34
Figure 3.
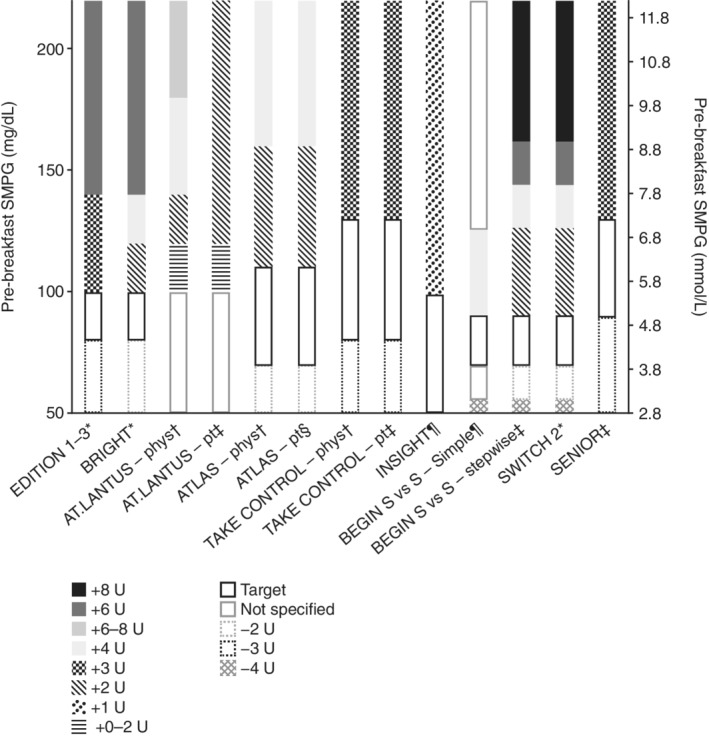
Titration algorithms in randomized controlled trials of basal insulins. For the EDITION 1–3 studies, if a blood glucose (BG) of <3.3 mmol/L (<60 mg/dL) was recorded, the dose could be reduced by 3 units (U) or at the investigator's discretion. In BRIGHT, if a BG of <4.4 mmol/L (<80 mg/dL) was recorded, the dose could be reduced by either 2 U or at the investigator's discretion. In ATLAS, if a BG of ≤3.1 mmol/L (≤56 mg/dL) was recorded, the dose was reduced at the physician's discretion. In TAKE CONTROL, the dose was reduced by 3 U in both the physician‐managed or self‐managed titration groups if either a BG of <4.4 mmol/L (<80 mg/dL) or ≥2 symptomatic or 1 severe hypoglycaemic event(s) in the preceding week were recorded. In SENIOR, the dose was reduced by 3 U if either a BG of <5.0 mmol/L (<90 mg/dL) or ≥2 symptomatic or 1 severe hypoglycaemic event(s) in the preceding week were recorded. Phys, physician‐managed titration group; pt, patient‐managed titration group; S vs. S, simple versus stepwise titration; SMPG, self‐measured plasma glucose.
*Doses were adjusted weekly; †doses were adjusted at every visit (AT.LANTUS: weekly during clinic visits or telephone contact visits; ATLAS: clinic visits at baseline, weeks 2, 6, 12, 16 and 24; telephone contact visits were made at weeks 1, 3, 4, 5, 8, 10 and 20; TAKE CONTROL: weekly for the first 8 weeks, biweekly until week 12, monthly until week 24); ‡doses were adjusted every 3 days; §doses were adjusted twice a week; ¶doses were adjusted daily
A pooled meta‐analysis that compared the efficacy and safety of IDeg versus Gla‐100 across BEGIN RCTs, which included both insulin‐pretreated and insulin‐naïve people with T2D, showed similar glycaemic control with IDeg and Gla‐100 and a lower risk of hypoglycaemia with IDeg versus Gla‐100 during the overall study period (rate ratio: 0.83 [95% CI: 0.74–0.94]), particularly during the maintenance period (0.75 [0.66–0.87]).37 However, the lower risk of hypoglycaemia observed with IDeg versus Gla‐100 was not observed during the titration period.37 It should be noted that the BEGIN RCTs adopted a very stringent FPG target of >4.0 to <5.0 mmol/L (>70 to <90 mg/dL),37 which is seldom used in adults with T2D in routine clinical practice, and this may have increased the risk of hypoglycaemia observed in these trials.
Results of the previously mentioned BRIGHT study showed similar HbA1c reductions between Gla‐300 and IDeg over 24 weeks and comparable incidence and rates of hypoglycaemia during the overall 24‐week study period.38 There were, however, statistically significantly lower incidences and rates of anytime confirmed (≤3.9 mmol/L [≤70 mg/dL] and <3.0 mmol/L [<54 mg/dL]) hypoglycaemia and significantly lower rates of nocturnal confirmed (≤3.9 mmol/L [≤70 mg/dL]) hypoglycaemia during the titration period (0–12 weeks) with Gla‐300 versus IDeg.38 The lower hypoglycaemia risk appears not to reflect differences in glycaemic control, as HbA1c reductions and target achievement (<7.0%) were similar between treatment groups at week 12.39, 40 However, as seen with EDITION 3, mean change in FPG appeared to decrease more gradually with Gla‐300 than IDeg, and the impact this may have on hypoglycaemia risk is unknown.
As previously mentioned, a post hoc analysis of BRIGHT showed that the incidence of participants who experienced hypoglycaemic events within the maintenance phase (weeks 13–24) was lower for individuals who did not experience hypoglycaemia within the initial 12‐week titration period compared with those who did (38.2% vs. 75.4%, respectively).26 However, HbA1c reductions during the titration period (baseline to week 12) were greater in the subgroup that experienced early hypoglycaemia (−1.46%) compared with those who did not (−1.28%). A second trial comparing Gla‐300 and IDeg has been completed (NCT03078478) and full results are expected to be published soon. However, this study required an important protocol amendment to change the glucose meter used for titration; as such, comparisons of the two second‐generation BI analogues within the titration period are not possible.41, 42
4.2. Real‐world evidence
Real‐world evidence (RWE) is an important source of information on how people titrate BI in everyday clinical practice. Despite the clinical benefits favouring second‐generation versus first‐generation BIs, including lower rates of hypoglycaemia, RWE suggests that patients are still not achieving glycaemic targets during the initial 12 weeks of treatment. For example, the DUNE study, a prospective, 12‐week real‐world observational study in insulin‐naïve and pretreated (within the preceding 12 months) individuals with T2D who initiated or switched BI therapy, showed that only 28% and 27% of these patients, respectively, achieved their individualized HbA1c targets.43 Failure to achieve these targets could have been because of insufficient dose titration, as only modest increases in dose were observed (mean 0.10 U/kg in insulin‐naïve patients and 0.06 U/kg in pretreated patients). By comparison, a pooled analysis of 15 RCTs examining the initiation of Gla‐100 in insulin‐naïve individuals showed mean increases of >0.24 U/kg.21
A recent retrospective observational study of 25 489 people with T2D in the USA assessed hypoglycaemia‐related emergency department (ED) visits or hospital admissions after initiation of long‐acting insulin analogues (1928; 8%) compared with NPH insulin (n = 23 561; 92%).44 Results indicated no significant differences in rates of hypoglycaemia‐related ED visits between individuals using NPH insulin (8.8 events per 1000 person‐years) or long‐acting insulin analogues (11.9 events per 1000 person‐years; P = 0.07).44 However, this study was limited in that it compared first‐generation BI analogues, insulin detemir or Gla‐100, and utilized data from a time period preceding clinical use of Gla‐300 (January 2006–December 2014).44 As such, the impact of second‐generation BIs Gla‐300 and IDeg cannot be determined from these data. The study also did not assess the proportion of people in each treatment group who achieved their glycaemic target.
A second recent study assessed the clinical and economic impact of switching from insulin analogue treatment (unspecified basal or prandial insulin analogues and secretagogues) to human insulin (premixed human 70/30 or NPH) in 14 635 older individuals (mean age: 72.2 years) with diabetes, filling in a total of 221 866 prescriptions.45 Results showed that the change in insulin use did not affect the rate of serious hypo‐ or hyperglycaemic events (defined by hospitalization or ED admissions) and was associated with a decrease in total cost of ~ $2 million per month at the end of the second year following implementation of the intervention. Furthermore, while there was a small but significant increase in HbA1c of 0.14% (95% CI: 0.05–0.23; P = 0.003) within the first year of the intervention, mean HbA1c remained stable in the following year.45
Results of these two studies suggest that while RCTs might show lower hypoglycaemia risk and improved pharmacokinetic/pharmacodynamic profiles of long‐acting insulins compared with intermediate‐acting insulins, such as NPH,30 these benefits may not translate to real‐life clinical practice. Furthermore, the higher cost of long‐acting BI analogues compared with human insulins may be an important factor to consider when selecting insulin therapies; for instance, one USA‐based survey conducted in an urban diabetes centre indicated that 25% of individuals with diabetes reported cost‐related underuse of insulin, and this was associated with significantly poorer glycaemic control.46
Real‐world studies comparing first‐ and second‐generation BIs using electronic health record data have also been conducted. The CONFIRM and DELIVER‐D+ studies compared HbA1c reduction and hypoglycaemia risk in US patient records for individuals using either Gla‐300 or IDeg, with both studies using propensity score matching (PSM) to account for potential confounders; however, these studies showed conflicting results.47, 48 DELIVER‐D+ showed similar HbA1c reductions (Gla‐300: 0.63 [SD 1.7]%, IDeg‐100: 0.58 [1.6]%; P = 0.488) and HbA1c <7% target achievement (Gla‐300: 15.1%, IDeg: 16.1%; P = 0.628) from baseline to 3–6 months’ follow‐up.48 No significant between‐treatment differences in hypoglycaemia incidence or rates (hospitalizations or ED visits) were observed between Gla‐300 or IDeg following adjustment for baseline hypoglycaemia.48 By contrast, CONFIRM showed significantly greater HbA1c reductions with IDeg than Gla‐300 (−1.48% vs. −1.22%; P = 0.03).47 Similarly, over the initial 180 days, the proportion of patients experiencing ≥1 hypoglycaemic event was significantly lower with IDeg than Gla‐300 (P <0.01) and fewer patients discontinued treatment with IDeg than Gla‐300 (13% vs. 21%; P <0.001).47
The LIGHTNING study also compared the safety and effectiveness of first‐generation (Gla‐100 and IDet) and second‐generation (Gla‐300 and IDeg) BI analogues using data from US electronic health records.49 Potential confounders were controlled using either PSM or predictive modelling with machine learning techniques. Results of predictive modelling indicated that rates of severe hypoglycaemia with Gla‐300 (0.07 events per patient‐year) were significantly lower than with Gla‐100 (0.14 events per patient‐year; P <0.05) or IDet (0.15 events per patient‐year; P <0.05) in insulin‐naïve individuals, but were not significantly lower versus IDeg (0.10 events per patient‐year, P >0.05).49 Similar results were observed in the PSM analysis.49 It should be noted that CONFIRM, DELIVER‐D+ and LIGHTNING did not specifically compare the safety and efficacy of different BI formulations within the initial period of insulin titration, probably because of the limitations of the electronic healthcare records datasets. Nevertheless, data from these RWE studies add to the results observed in RCTs.
There are limitations to consider when interpreting results from RWE studies. The lack of randomization and unselected patient populations may lead to potential biases and confounders.50 Furthermore, the data used are not collected or organized with the purpose of supporting research, and as such, data may be inaccurate or incomplete50; this could lead to underreporting of events (eg, hypoglycaemia episodes). Furthermore, the studies detailed above did not capture data on how titration was performed, or the insulin doses used, and did not present results by separate titration and maintenance periods. Therefore, comparisons of clinical outcomes specifically within the titration period are not possible. Additionally, many RWE studies assessing hypoglycaemia utilize ED visits and hospitalizations to define hypoglycaemic events. However, this approach would not capture non‐severe hypoglycaemia events, which also have a large impact on people with diabetes,51 or severe episodes where the individual was not hospitalized or did not require an ED visit.
4.3. Self‐titration and patient empowerment
Patient empowerment can also be achieved with titration tools that enable people to self‐titrate their BI, with guidelines recommending the use of evidence‐based titration algorithms to facilitate self‐titration.9, 10 There have been several trials in which self‐ and physician‐managed titration utilizing simple paper‐based titration algorithms have been compared (Table 1). Three such trials are ATLAS, AT.LANTUS and TAKE CONTROL, which compared self‐ versus physician‐managed titration with either Gla‐100 or Gla‐300. All three trials showed significantly greater reductions in HbA1c with self‐ versus physician‐led insulin titration (Table 1).52, 53, 54 While the incidence of hypoglycaemia was slightly higher with the self‐ versus physician‐managed groups in the two trials that investigated Gla‐100 titration (ATLAS and AT.LANTUS), both titration arms showed a similar incidence of hypoglycaemia in TAKE CONTROL, where participants received Gla‐300.52 However, it should be noted that the definitions of hypoglycaemia varied between these studies. Results from TAKE CONTROL also showed that a greater proportion of participants in the self‐ versus physician‐managed group achieved SMPG targets without hypoglycaemia and had slightly greater improvements in emotional burden.52
Table 1.
Summary of randomized controlled trials comparing self‐management versus physician‐led management of titration
| TAKE CONTROL52 | AT.LANTUS54 | ATLAS53 | INNOVATE55 | AUTOMATIX56 | |
|---|---|---|---|---|---|
| Study length, weeks | 24 | 24 | 24 | 12 | 16 |
| Location | 10 European countries | Europe, South America, Asia, Africa/Middle East | Asia | Canada | Germany/UK |
| Population | Insulin‐naïve and previously treated, age ≥18 years, HbA1c ≥7.0% and ≤10.0% (prior BI) or ≥7.5% and ≤11.0% (BI‐naïve) | Insulin‐naïve and previously treated, age ≥18 years, BMI <40 kg/m2, HbA1c >7.0% and <12.0% | Insulin‐naïve, age 40–75 years, HbA1c ≥7.0% and ≤11.0% | Insulin‐naïve and previously treated, age ≥18 and ≤75 years, BMI ≤45 kg/m2, HbA1c >7.0% | Insulin‐naïve and previously treated, age ≥18 years, HbA1c ≥7.5% and ≤11.0% |
| Type of titration support | Paper‐based titration algorithm | Paper‐based titration algorithm | Paper‐based titration algorithm | Self‐titration web tool (LTHome) | Self‐titration device (MyStar DoseCoach) |
| Study drug | Gla‐300 | Gla‐100 | Gla‐100 | Gla‐100 | Gla‐300 |
| Key results | Significantly greater HbA1c reductions with self‐ vs. physician‐managed titration and a higher proportion of people achieving HbA1c of <7.0%. Similar incidence of hypoglycaemia (all categories and BG thresholds reported)a in both groups. Slightly greater increase in insulin dose with self‐ vs. physician‐managed titration. | Significantly greater HbA1c reductions with self‐ vs. physician‐managed titration and a higher proportion of people achieving HbA1c of <7.0%. Significantly greater incidence of any hypoglycaemia and symptomatic (<2.8 mmol/L [<50 mg/dL]) hypoglycaemia in the self‐ vs. physician‐managed titration group, but similar incidence of nocturnal hypoglycaemia (occurring during sleep with BG of <2.8 mmol/L [<50 mg/dL]). | Significantly greater HbA1c reductions with self‐ vs. physician‐managed titration and a numerically higher proportion of patients achieving HbA1c of <7.0% without severe hypoglycaemia. Similar incidence of severe hypoglycaemia, but significantly greater incidence of symptomatic (clinical symptoms of hypoglycaemia regardless of BG measurement) hypoglycaemia and nocturnal hypoglycaemia (occurring during sleep with BG of <2.8 mmol/L [<50 mg/dL]) with self‐ vs. physician‐managed titration. Significantly greater increase in insulin dose with self‐ vs. physician‐managed titration. | Similar HbA1c reductions observed between groups and a greater proportion of patients achieving HbA1c of ≤7.0% in the self‐ vs. physician‐managed titration group. Lower achievement of the composite primary outcome‡ in the self‐ vs. physician‐managed titration arm. Incidence of hypoglycaemia (all categories reported)§ was similar in both groups. | Similar HbA1c reductions observed between groups. Numerically greater achievement of fasting SMPG target without severe hypoglycaemia with self‐ vs. physician‐managed titration (not significant). Slightly lower incidence of any hypoglycaemia and confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemia, occurring either at night (00:00–05:59 hours) or any time of day (24 hours) with self (device)‐ vs. physician (routine)‐managed titration. Greater dose increases with self‐ vs. physician‐managed titration. |
Abbreviations: BG, blood glucose; BI, basal insulin; BMI, body mass index; FPG, fasting plasma glucose; SMPG, self‐measured plasma glucose.
Any hypoglycaemia, confirmed or severe at BG thresholds of ≤3.9 mmol/L (≤70 mg/dL) or <3.0 mmol/L (<54 mg/dL) and symptomatic, with all categories reported at any time of day (24 hours) or at night (00:00–05:59 hours); ‡primary composite outcome: (i) at least four out of seven FPG values within a 10‐day period in the target range (5.0–7.2 mmol/L [90–130 mg/dL]); (ii) mean FPG for three consecutive prior FPG values within a 10‐day period in the target range; (iii) no severe hypoglycaemia during the 7‐10‐day period; §overall, nocturnal, daytime, symptomatic or asymptomatic hypoglycaemia, time intervals and BG thresholds were not specified.
Providing enhanced digital educational tools or titration‐support devices can further empower patients to self‐titrate their BI, as shown with the INNOVATE and AUTOMATIX trials (Table 1). INNOVATE compared the efficacy and safety of Gla‐100 administered with physician‐led titration versus self‐titrated insulin titration facilitated with a home‐based web tool (LTHome).55 While the primary endpoint in INNOVATE was not met (non‐inferiority was not shown for LTHome self‐titration vs. physician‐managed titration for the composite endpoint: four out of seven FPG readings within 5.0–7.2 mmol/L, mean of three consecutive FPG measurements within 5.0–7.2 mmol/L, and no severe hypoglycaemia), HbA1c reductions and incidence of hypoglycaemia were similar between groups. Additionally, LTHome self‐titration was associated with greater reductions in hypoglycaemia fear scores and diabetes distress scores versus physician‐managed titration.55 The AUTOMATIX trial compared physician‐led titration with a device‐supported titration system to facilitate self‐titration for Gla‐300. The device‐supported titration system provided automated titration suggestions based on device‐measured fasting SMPG readings from a minimum of 3 consecutive days.56 Results showed that the device‐supported titration was non‐inferior to physician‐led titration for the primary endpoint (fasting SMPG target achievement of 5.0–7.2 mmol/L [90–130 mg/dL] after 16 weeks without severe hypoglycaemia). Additionally, a greater proportion of patients achieved target fasting SMPG without confirmed (≤3.9 mmol/L [≤70 mg/dL]) hypoglycaemia in the device‐supported versus physician‐led titration arm. Importantly, patient‐reported outcome scores were similar between groups, and the device‐supported titration was rated as easy to use.56 A similar trial comparing device‐supported versus physician‐managed titration is currently ongoing in people with T2D receiving IDeg BI therapy.57
Providing simpler titration algorithms can also facilitate patients in self‐titrating their BI. For example, the TITRATION study, which compared self‐titration of Gla‐300 using either a simple insulin titration algorithm of 1 U/day dose increases (the INSIGHT study BI titration algorithm, Figure 3)58 or physician‐titration with the EDITION algorithm33, 34, 35 in both insulin‐pretreated or insulin‐naïve people with T2D (Figure 3), showed that both titration groups achieved similar glycaemic outcomes without differences in hypoglycaemia or insulin dose increases.59 Additionally, the BEGIN: ONCE Simple Use trial compared simple versus stepwise titration algorithms using IDeg in insulin‐naïve people with T2D (Figure 3).60 Both titration algorithms provided similar reductions in HbA1c (−1.09% and −0.93% with simple and stepwise titration, respectively) with comparable confirmed (<3.1 mmol/L [<56 mg/dL]) hypoglycaemia rates (1.60 and 1.17 events/patient‐year of exposure, P = 0.43), comparable confirmed nocturnal hypoglycaemia rates (0.21 and 0.10 events/patient‐year of exposure, P = 0.20), similar insulin doses, and similar weight changes observed over the 26‐week study.60
The results of these studies show that self‐titration did not increase the risk of hypoglycaemia and was still effective in achieving glycaemic control, while providing similar or better patient satisfaction compared with physician‐managed titration. Use of digital education tools, titration‐support devices or providing simpler titration algorithms could further facilitate patients to self‐titrate their BI.
4.4. Insulin titration in special populations
Hypoglycaemia risk is increased in certain populations, such as those with renal impairment, established cardiovascular disease, long duration of diabetes, impaired awareness of hypoglycaemia, and those with cognitive impairment.25, 61 Additionally, certain populations (such as older individuals) may be at increased risk from the consequences of hypoglycaemia, such as falls/fractures and hospitalizations.62 While insulin therapy must always be carefully balanced between optimizing glycaemic control and minimizing the risk of hypoglycaemia, it is an especially sensitive issue in these high‐risk populations.
Studies comparing BI therapy use in high‐risk populations have showed that simple titration algorithms can be effective in these patients.
The SWITCH 2 RCT investigated the use of IDeg versus Gla‐100 in people with poorly controlled T2D despite prior treatment with BI with or without OADs and at least one hypoglycaemia risk factor: ≥1 severe hypoglycaemic episode within the last year (ADA definition); moderate chronic renal failure (estimated glomerular filtration rate of 30–59 mL/min/1.73 m2); hypoglycaemic symptom unawareness; exposure to insulin for >5 years; episode of hypoglycaemia (symptoms and/or blood glucose level ≤3.9 mmol/L [≤70 mg/dL]) within the last 12 weeks.63 Insulin was adjusted weekly based on the mean of 3 days prebreakfast blood glucose with a target of 4.0–5.0 mmol/L (71–90 mg/dL) (Figure 3). In this population with a high risk of hypoglycaemia, IDeg was associated with a significant absolute 9% reduction in overall symptomatic (<3.1 mmol/L [<56 mg/dL]) hypoglycaemia (P < 0.001) and a 5.1% reduction in nocturnal symptomatic (<3.1 mmol/L [<56 mg/dL]) hypoglycaemia (P = 0.001) compared with Gla‐100.63 However, it should be noted that SWITCH 2 included patients who were previously treated with insulin; as such, extrapolation of the results to an insulin‐naïve population may not be possible. Additionally, the SWITCH 2 trials employed a stringent fasting SMPG titration target that is often not used in routine clinical practice, particularly in patients with an increased risk of hypoglycaemia.
The SENIOR study compared Gla‐300 versus Gla‐100 in both insulin‐pretreated and insulin‐naïve older individuals with T2D aged ≥65 years (and also in a subgroup aged ≥75 years).64 A more relaxed fasting SMPG target of 5.0–7.2 mmol/L (90–130 mg/dL) was used in SENIOR, in line with ADA guidelines for recommended targets in older individuals (Figure 3).9 Glycaemic control was comparable between treatment groups, and incidence of anytime (24 hours) and nocturnal (00:00–05:59 hours) hypoglycaemia, and annualized event rates of confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemia at any time or at night, were low and similar between treatment groups. Overall, a trend towards lower annualized event rates of hypoglycaemia with Gla‐300 was observed; this between‐treatment difference was more pronounced in the ≥75 years subgroup.64
A post hoc analysis of TAKE CONTROL compared the efficacy and safety of self‐ versus physician‐managed titration of Gla‐300 in older (≥65 years of age) versus younger (<65 years of age) people with T2D receiving treatment with ≥1 non‐insulin antihyperglycaemic therapy with or without concomitant BI.52, 65 HbA1c and SMPG reductions were similar between age groups and the proportions achieving the SMPG target (4.4–7.2 mmol/L [80–130 mg/dL]) without confirmed (<3.0 mmol/L [<54 mg/dL]) or severe hypoglycaemia were higher in the older age group. The incidence and rate of hypoglycaemia was also similar between groups, showing that self‐titration with Gla‐300 was effective in this older population without increasing the risk of hypoglycaemia.65 Similar results were observed in a post hoc analysis of the BRIGHT study, where Gla‐300 provided similar HbA1c reduction versus IDeg‐100 in insulin‐naïve older people with T2D, and greater reductions versus IDeg‐100 in those aged ≥70 years with no increased hypoglycaemia risk.66 These results suggest that the simple titration algorithm used in BRIGHT, along with the more stringent fasting SMPG titration target (4.4–5.6 mmol/L [80–100 mg/dL]), was effective in this older population, without increasing their risk of hypoglycaemia.66
While these studies provide reassurance that simple titration algorithms can be used to titrate second‐generation BI analogues in populations at high risk of hypoglycaemia, it should be noted that minimizing hypoglycaemia requires a multifaceted approach that reaches beyond BI dose adjustments. Indeed, the consensus report of the ADA and EASD proposed that the appropriate use of insulin (dose, timing, targets) impacts the effectiveness and adverse effects of insulin therapy more than the differences between formulations.10 Factors that should be considered include treatment choices, patient education, and individualized glucose targets that account for hypoglycaemia risk, lifestyle, activity levels and concomitant medications.10, 67 Patient education should include awareness of the times (eg, during the overnight fast and prebreakfast), daily situations (eg, missed meals, exercise, alcohol intake) and other factors (eg, vulnerable/special populations, co‐morbidities) that raise hypoglycaemia risk, so that glucose monitoring can be increased and countermeasures taken, which in a busy practice may be best achieved by dedicated diabetes nurses and educators.25
5. CONCLUSIONS
The initial period during insulin initiation and titration is a critical time for successful glycaemic target achievement. Despite the well‐evidenced benefit of early tight glycaemic control, there are still a number of barriers at the patient, physician and healthcare system levels to initiating, intensifying and up‐titrating BI therapy. There are also different approaches to optimizing BI titration in people with T2D. It should be noted that this article was not a systematic review and, as such, may have omitted some trials showing use of novel titration algorithms or methods to improve self‐management. Additionally, for the purposes of brevity, this review focuses on the recent ADA and EASD guidelines; however, there are other global guidelines which provide similar recommendations.
Helping patients to effectively self‐manage their own diabetes can provide similar or greater reductions in HbA1c with comparable safety profiles to physician‐managed care, often accompanied by improvements in treatment satisfaction, perceived self‐management efficacy, and reduced diabetes‐related distress. Consequently, empowering patients to self‐manage their diabetes may help alleviate many of the patient‐related barriers to insulin therapy inertia and thus may improve therapy adherence. Self‐management can also be improved through the implementation of simple and easy titration algorithms (daily or weekly), which have been proven to result in glycaemic efficacy while helping to minimize hypoglycaemia risk.
There is currently no evidence for second‐generation BI analogues improving treatment adherence compared with first‐generation BI analogues. Further research is required to directly assess this relationship and to assess the cost‐effectiveness of second‐generation versus first‐generation BIs. However, given that fear of hypoglycaemia is a major barrier that leads to insulin therapy inertia in patients and their healthcare providers, by reducing the risk of hypoglycaemia overall—particularly during the titration period when most insulin dose change occurs—second‐generation BI analogues may potentially help to improve treatment adherence and long‐term glycaemic outcomes compared with first‐generation BI analogues.
CONFLICT OF INTEREST
S. B. H. — advisory board/consultant: Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi; research support: Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk, Sanofi. D. M. — advisory board: AstraZeneca, Ferrer, Merck, Novo Nordisk, Praxis Pharmaceutical, Sanofi; speakers bureau: Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Menarini, Merck, Novartis, Novo Nordisk, Sanofi. K. K. — advisory board, consultant, and speakers bureau: Berlin‐Chemie AG / Menarini Group, Novartis, Novo Nordisk, Sanofi, Eli Lilly, Servier, MSD; research support: Novartis, Novo Nordisk, Sanofi, Eli Lilly, Pfizer, Boehringer Ingelheim, MSD, Roche. L. B. — consultancy: Eli Lilly, Sanofi, Novo Nordisk, LifeScan, Abbott, BD, MontMed, Merck, Janssen, AstraZeneca, Boehringer Ingelheim; grants: MontMed; Honoraria: Eli Lilly, Sanofi, Novo Nordisk, LifeScan, Abbott, BD, Merck, Janssen, AstraZeneca; lecture/other fees: Eli Lilly, Sanofi, Novo Nordisk, LifeScan, Abbott, BD, Merck, Janssen, AstraZeneca. F. G. — advisory board: AstraZeneca, Eli Lilly, Novo Nordisk, Roche Diabetes Care, Sanofi; consultant: Boehringer Ingelheim, LifeScan, Merck Sharp & Dohme, Sanofi, AstraZeneca, MedImmune, Roche Diabetes Care, Sanofi; research support: Eli Lilly; LifeScan, Takeda.
AUTHOR CONTRIBUTIONS
The authors were involved from the conception of the review article, the generation of the review outline and all subsequent drafts. All authors critically reviewed the manuscript and approved the final version for submission.
FUNDING INFORMATION
This review article was supported by Sanofi.
ACKNOWLEDGMENTS
K. K. acknowledges support from the NIHR Collaboration for Leadership in Applied Health Research and Care East Midlands (CLAHRC‐EM) to NIHR Applied Research Collaboration East Midlands (NIHR ARC‐EM). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
This review article was supported by Sanofi. The authors received editorial/writing support in the preparation of this manuscript provided by Hannah Brown, PhD, of Fishawack Communications Ltd, funded by SANOFI.
Khunti K, Giorgino F, Berard L, Mauricio D, Harris SB. The importance of the initial period of basal insulin titration in people with diabetes. Diabetes Obes Metab. 2020;22:722–733. 10.1111/dom.13946
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13946.
Funding information Sanofi
REFERENCES
- 1. Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32:187‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Action to Control Cardiovascular Risk in Diabetes Study Group , Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545‐2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129‐139. [DOI] [PubMed] [Google Scholar]
- 4. Hayward RA, Reaven PD, Wiitala WL, et al. Follow‐up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197‐2206. [DOI] [PubMed] [Google Scholar]
- 5. ADVANCE Collaborative Group , Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560‐2572. [DOI] [PubMed] [Google Scholar]
- 6. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577‐1589. [DOI] [PubMed] [Google Scholar]
- 7. Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: A systematic review. Diabetes Obes Metab. 2018;20:427‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Diabetes Association . Standards of Medical Care in Diabetes 2019. Diabetes Care. 2019;42:S1‐S193. [DOI] [PubMed] [Google Scholar]
- 10. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khunti K, Damci T, Meneghini L, Pan CY, Yale JF, Group SS . Study of Once Daily Levemir (SOLVE): insights into the timing of insulin initiation in people with poorly controlled type 2 diabetes in routine clinical practice. Diabetes Obes Metab. 2012;14:654‐661. [DOI] [PubMed] [Google Scholar]
- 12. Mauricio D, Meneghini L, Seufert J, et al. Glycaemic control and hypoglycaemia burden in patients with type 2 diabetes initiating basal insulin in Europe and the USA. Diabetes Obes Metab. 2017;19:1155‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stone MA, Charpentier G, Doggen K, et al. Quality of care of people with type 2 diabetes in eight European countries: findings from the Guideline Adherence to Enhance Care (GUIDANCE) study. Diabetes Care. 2013;36:2628‐2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aschner P, Gagliardino JJ, Ilkova HM, et al. Poor Glycemic Control in People with T1D and T2D—Results from the International Diabetes Management Practices Study (IDMPS). Diabetes. 2018;67:1656. [Google Scholar]
- 15. Russell‐Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab. 2018;20:488‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dalal MR, Kazemi M, Ye F, Xie L. Hypoglycemia after initiation of basal insulin in patients with type 2 diabetes in the United States: implications for treatment discontinuation and healthcare costs and utilization. Adv Ther. 2017;34:2083‐2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peyrot M, Barnett AH, Meneghini LF, Schumm‐Draeger PM. Factors associated with injection omission/non‐adherence in the Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabetes Obes Metab. 2012;14:1081‐1087. [DOI] [PubMed] [Google Scholar]
- 18. Berard L, Bonnemaire M, Mical M, Edelman S. Insights into optimal basal insulin titration in type 2 diabetes: Results of a quantitative survey. Diabetes Obes Metab. 2018;20:301‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pastakia SD, Pekny CR, Manyara SM, Fischer L. Diabetes in sub‐Saharan Africa ‐ from policy to practice to progress: targeting the existing gaps for future care for diabetes. Diabetes Metab Syndr Obes. 2017;10:247‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Medicines Agency. Guidelines on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus 2012. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-prevention-diabetes-mellitus-revision_en.pdf. Accessed April 29, 2019.
- 21. Owens DR, Traylor L, Dain MP, Landgraf W. Efficacy and safety of basal insulin glargine 12 and 24 weeks after initiation in persons with type 2 diabetes: a pooled analysis of data from treatment arms of 15 treat‐to‐target randomised controlled trials. Diabetes Res Clin Pract. 2014;106:264‐274. [DOI] [PubMed] [Google Scholar]
- 22. Ross SA. Breaking down patient and physician barriers to optimize glycemic control in type 2 diabetes. Am J Med. 2013;126:S38‐S48. [DOI] [PubMed] [Google Scholar]
- 23. Ascher‐Svanum H, Lage MJ, Perez‐Nieves M, et al. Early discontinuation and restart of insulin in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2014;5:225‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18:401‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amiel SA, Aschner P, Childs B, et al. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019;7:385‐396. [DOI] [PubMed] [Google Scholar]
- 26. Harris SB, Berard L, Westerbacka J, et al. Early hypoglycemia after initiation of second‐generation basal insulin (BI) analogs: patient characteristics and clinical outcomes. Diabetes. 2019;68:1095. [Google Scholar]
- 27. Frier BM, Landgraf W, Zhang M, Bolli GB, Owens DR. Hypoglycaemia risk in the first 8 weeks of titration with insulin glargine 100 U/mL in previously insulin‐naive individuals with type 2 diabetes mellitus. Diabetes Obes Metab. 2018;20:2894‐2898. [DOI] [PubMed] [Google Scholar]
- 28. Plank J, Bodenlenz M, Sinner F, et al. A double‐blind, randomized, dose‐response study investigating the pharmacodynamic and pharmacokinetic properties of the long‐acting insulin analog detemir. Diabetes Care. 2005;28:1107‐1112. [DOI] [PubMed] [Google Scholar]
- 29. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long‐acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142‐2148. [DOI] [PubMed] [Google Scholar]
- 30. Heise T, Mathieu C. Impact of the mode of protraction of basal insulin therapies on their pharmacokinetic and pharmacodynamic properties and resulting clinical outcomes. Diabetes Obes Metab. 2017;19:3‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chatterjee S, Khunti K, Davies MJ. Achieving glycaemic control with concentrated insulin in patients with type 2 diabetes. Drugs. 2019;79:173‐186. [DOI] [PubMed] [Google Scholar]
- 32. Vijan S, Hayward RA, Ronis DL, Hofer TP. Brief report: the burden of diabetes therapy: implications for the design of effective patient‐centered treatment regimens. J Gen Intern Med. 2005;20:479‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Riddle MC, Bolli GB, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6‐month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37:2755‐2762. [DOI] [PubMed] [Google Scholar]
- 34. Yki‐Jarvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6‐month randomized controlled trial (EDITION 2). Diabetes Care. 2014;37:3235‐3243. [DOI] [PubMed] [Google Scholar]
- 35. Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin‐naive people with type 2 diabetes on oral glucose‐lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17:386‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.European Medicines Agency. Toujeo 300 units/mL Summary of Product Characteristics. https://www.medicines.org.uk/emc/product/6938/smpc. Accessed October 24, 2019.
- 37. Ratner RE, Gough SC, Mathieu C, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre‐planned meta‐analysis of phase 3 trials. Diabetes Obes Metab. 2013;15:175‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosenstock J, Cheng A, Ritzel R, et al. More similarities than differences testing insulin glargine 300 units/mL versus insulin degludec 100 units/mL in insulin‐naive type 2 diabetes: the randomized head‐to‐head BRIGHT trial. Diabetes Care. 2018;41:2147‐2154. [DOI] [PubMed] [Google Scholar]
- 39. Ritzel R, Cheng A, Rosenstock J, et al. The BRIGHT randomised study: similar glycaemic control and less confirmed hypoglycaemia with Gla‐300 vs IDeg‐100 during the initial titration period. Diabetes Technol Ther. 2019;21:A‐42. [Google Scholar]
- 40. Roussel R, Frias J, Westerbacka J, et al. Reaching ADA glycemic targets without hypoglycemia during titration with Gla‐300 and IDeg in T2DM: exploratory analyses from BRIGHT. Diabetes. 2019;68:1090. [Google Scholar]
- 41. https://ClinicalTrials.gov. A Trial Comparing the Efficacy and Safety of Insulin Degludec and Insulin Glargine 300 Units/mL in Subjects With Type 2 Diabetes Mellitus Inadequately Treated With Basal Insulin With or Without Oral Antidiabetic Drugs. https://clinicaltrials.gov/ct2/show/NCT03078478. Accessed April 29, 2019.
- 42. Philis‐Tsimikas A, Stratton I, Norgard Troelsen L, Anker Bak B, Leiter LA. Efficacy and safety of degludec compared to glargine 300 units/mL in insulin‐experienced patients with type 2 diabetes: trial protocol amendment (NCT03078478). J Diabetes Sci Technol. 2019;13:498‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meneghini LF, Mauricio D, Orsi E, et al. The Diabetes Unmet Need with Basal Insulin Evaluation (DUNE) study in type 2 diabetes: Achieving HbA1c targets with basal insulin in a real‐world setting. Diabetes Obes Metab. 2019;21:1429‐1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lipska KJ, Parker MM, Moffet HH, Huang ES, Karter AJ. Association of initiation of basal insulin analogs vs neutral protamine hagedorn insulin with hypoglycemia‐related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Luo J, Khan NF, Manetti T, et al. Implementation of a health plan program for switching from analogue to human insulin and glycemic control among medicare beneficiaries with type 2 diabetes. JAMA. 2019;321:374‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Herkert D, Vijayakumar P, Luo J, et al. Cost‐related insulin underuse among patients with diabetes. JAMA Intern Med. 2019;179:112‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tibaldi J, Hadley‐Brown M, Liebl A, et al. A comparative effectiveness study of degludec and insulin glargine 300 U/mL in insulin‐naive patients with type 2 diabetes. Diabetes Obes Metab. 2019;21:1001‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sullivan SD, Bailey TS, Roussel R, et al. Clinical outcomes in real‐world patients with type 2 diabetes switching from first‐ to second‐generation basal insulin analogues: Comparative effectiveness of insulin glargine 300 units/mL and insulin degludec in the DELIVER D+ cohort study. Diabetes Obes Metab. 2018;20:2148‐2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pettus J, Roussel R, Liz Zhou F, et al. Rates of hypoglycemia predicted in patients with type 2 diabetes on insulin glargine 300 U/ml versus first‐ and second‐generation basal insulin analogs: the real‐world LIGHTNING study. Diabetes Ther. 2019;10:617‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sherman RE, Anderson SA, Dal Pan GJ, et al. Real‐world evidence ‐ What is it and what can it tell us? N Engl J Med. 2016;375:2293‐2297. [DOI] [PubMed] [Google Scholar]
- 51. Frier BM, Jensen MM, Chubb BD. Hypoglycaemia in adults with insulin‐treated diabetes in the UK: self‐reported frequency and effects. Diabet Med. 2016;33:1125‐1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Russell‐Jones D, Dauchy A, Delgado E, et al. Take Control: a randomized trial evaluating the efficacy and safety of self‐ versus physician‐managed titration of insulin glargine 300 U/mL in patients with uncontrolled type 2 diabetes. Diabetes Obes Metab. 2019;21:1615‐1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Garg SK, Admane K, Freemantle N, et al. Patient‐led versus physician‐led titration of insulin glargine in patients with uncontrolled type 2 diabetes: a randomized multinational ATLAS study. Endocr Pract. 2015;21:143‐157. [DOI] [PubMed] [Google Scholar]
- 54. Davies M, Lavalle‐Gonzalez F, Storms F, Gomis R, Group ALS . Initiation of insulin glargine therapy in type 2 diabetes subjects suboptimally controlled on oral antidiabetic agents: results from the AT.LANTUS trial. Diabetes Obes Metab. 2008;10:387‐399. [DOI] [PubMed] [Google Scholar]
- 55. Bajaj HS, Venn K, Ye C, Aronson R. Randomized trial of long‐acting insulin glargine titration web tool (LTHome) versus enhanced usual therapy of glargine titration (INNOVATE trial). Diabetes Technol Ther. 2016;18:610‐615. [DOI] [PubMed] [Google Scholar]
- 56. Davies M, Bain S, Charpentier G, et al. A randomized controlled, treat‐to‐target study evaluating the efficacy and safety of insulin glargine 300 U/mL (Gla‐300) administered using either device‐supported or routine titration in people with type 2 diabetes. J Diabetes Sci Technol. 2019;13:881‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. https://ClinicalTrials.gov. Insulin Degludec Titration Using Mobile Insulin Dosing System. https://clinicaltrials.gov/ct2/show/NCT03091712. Accessed April 29, 2019.
- 58. Gerstein HC, Yale JF, Harris SB, Issa M, Stewart JA, Dempsey E. A randomized trial of adding insulin glargine vs. avoidance of insulin in people with type 2 diabetes on either no oral glucose‐lowering agents or submaximal doses of metformin and/or sulphonylureas. The Canadian INSIGHT (Implementing New Strategies with Insulin Glargine for Hyperglycaemia Treatment) study. Diabet Med. 2006;23:736‐742. [DOI] [PubMed] [Google Scholar]
- 59. Yale JF, Berard L, Groleau M, Javadi P, Stewart J, Harris SB. TITRATION: a randomized study to assess 2 treatment algorithms with new insulin glargine 300 units/mL. Can J Diabetes. 2017;41:478‐484. [DOI] [PubMed] [Google Scholar]
- 60. Philis‐Tsimikas A, Brod M, Niemeyer M, Ocampo Francisco AM, Rothman J. Insulin degludec once‐daily in type 2 diabetes: simple or step‐wise titration (BEGIN: once simple use). Adv Ther. 2013;30:607‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee AK, Lee CJ, Huang ES, Sharrett AR, Coresh J, Selvin E. Risk factors for severe hypoglycemia in black and white adults with diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2017;40:1661‐1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Abdelhafiz AH, Rodriguez‐Manas L, Morley JE, Sinclair AJ. Hypoglycemia in older people ‐ a less well recognized risk factor for frailty. Aging Dis. 2015;6:156‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wysham C, Bhargava A, Chaykin L, et al. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 2 diabetes: the SWITCH 2 randomized clinical trial. JAMA. 2017;318:45‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ritzel R, Harris SB, Baron H, et al. A randomized controlled trial comparing efficacy and safety of insulin glargine 300 units/mL versus 100 units/mL in older people with type 2 diabetes: results from the SENIOR study. Diabetes Care. 2018;41:1672‐1680. [DOI] [PubMed] [Google Scholar]
- 65. Strojek K, Bigot G, Bonnemaire M, et al. Self‐ vs. physician‐led titration of insulin glargine 300 U/mL (Gla‐300)—improved or comparable efficacy at week 24 without increased risk of hypoglycemia, irrespective of age (<65 or =65 years)—TAKE CONTROL. Diabetes. 2018;67:303‐OR. [Google Scholar]
- 66. Charbonnel B, Aroda V, Westerbacka J, et al. Differences in HbA1c reduction between insulin glargine 300 U/mL (Gla‐300) and insulin degludec 100 U/mL (IDeg‐100) in adults ≥70 years of age with T2DM in the BRIGHT trial. Diabetes. 2019;68:131‐LB.30305366 [Google Scholar]
- 67. International Hypoglycaemia Study Group . Minimizing hypoglycemia in diabetes. Diabetes Care. 2015;38:1583‐1591. [DOI] [PubMed] [Google Scholar]


