Abstract
The effect of steady‐state supratherapeutic sertraline (Zoloft) on QT interval was assessed in a single‐center, randomized, 3‐way crossover, double‐blind, placebo‐ and moxifloxacin‐controlled thorough QT study. Healthy adults received sertraline 400 mg/day, moxifloxacin 400 mg, and placebo, with a washout period (≥14 days) between treatments. A 12‐lead electrocardiogram was recorded in triplicate before dosing and at selected time points up to 72 hours after dosing. Analysis of covariance using a mixed‐effect model with sequence, period, treatment, time, and treatment‐by‐time interaction as fixed effects; subject within sequence as a random effect; and baseline QT corrected for heart rate using Fridericia formula (QTcF) as a covariate was conducted. A 90% confidence interval for the least squares (LS) mean difference in QTcF between active treatment and placebo was computed for each postdose time point. Exposure‐response was assessed using linear mixed‐effect modeling. Fifty‐four subjects were enrolled. Over 24 hours after dosing, the LS mean difference in QTcF for sertraline versus placebo ranged from 5.597 milliseconds to 9.651 milliseconds. The upper bound of the 90% confidence interval for the LS mean difference exceeded a predefined 10‐millisecond significance threshold at the 4‐hour postdose time point only (LS mean, 9.651 milliseconds [90% confidence interval, 7.635‐11.666]). In the exposure‐response analysis, QTcF values increased significantly with increasing sertraline concentration (slope = 0.036 milliseconds/ng/mL; P < .0001). Predicted change from baseline in QTcF at therapeutic maximum plasma sertraline concentration was 3.57 milliseconds. This thorough QTc study demonstrated a positive signal for QTc prolongation for sertraline at the steady‐state 400‐mg/day dose.
Keywords: electrocardiogram, QT interval prolongation, sertraline, thorough QTc study
Sertraline (Zoloft, Pfizer Inc, New York, New York) is a selective serotonin reuptake inhibitor (SSRI) with multiple indications, including major depressive disorder and multiple anxiety‐spectrum disorders.1 The drug undergoes extensive first‐pass metabolism, primarily N‐demethylation, via multiple cytochrome P450 (CYP) isozymes (2C9, 3A4, 2C19, 2D6, and 2B6).2, 3 Sertraline and its principal metabolite N‐desmethylsertraline subsequently undergo oxidative deamination, hydroxylation, and glucuronide conjugation.2 Based on its lack of effect on extracellular 5‐HT levels compared with sertraline, N‐desmethylsertraline is unlikely to contribute significantly to the blockade of central serotonin reuptake and is thought to play a negligible role in clinical activity.4 The elimination half‐life of sertraline and N‐desmethylsertraline is 26 hours and 62 to 104 hours, respectively.5, 6 Sertraline has potential for drug‐drug interaction with drugs metabolized by CYP2D6, for which it is a weak to moderate inhibitor; additionally, sertraline is a weak inhibitor of CYP1A2, CYP2C9, CYP2C19, and CYPBA4.7 There is also potential for drug‐drug interaction involving P‐glycoprotein, as sertraline is both a possible substrate of P‐glycoprotein and a potent inhibitor of its function.8, 9
The maximum recommended sertraline dose for US adult patients is 200 mg/day.1 Sertraline is considered generally safe and well tolerated, and the benefit‐risk profile is well known.10 Preclinical data, however, suggest a potential dose‐dependent risk of QT interval prolongation after sertraline administration. Sertraline acts on multiple voltage‐dependent channels (Na+, Ca2+, and K+ channels) including Kv1.5 and human Ether‐à‐go‐go–related gene (hERG) cardiac ion channels; the activity of N‐desmethylsertraline at these channels has not been examined.11, 12, 13, 14, 15, 16 Sertraline induced voltage‐dependent inhibition of Kv1.5 expressed in Chinese hamster ovary cells15 and inhibited delayed rectifier potassium current associated with hERG cardiac K+channels expressed in human embryonic kidney 293 cells by 8% to 86% in a concentration‐dependent manner (1‐30 µM).16 Although not a direct relationship, reduction of delayed rectifier potassium/hERG current is considered a surrogate marker of delayed cardiac repolarization.17 No significant effect of sertraline on QTc was observed in a population‐based cohort study comparing SSRI‐treated adults with untreated individuals or in either of 2 retrospective analyses of electrocardiogram (ECG) records.18, 19, 20 Nonetheless, pharmacovigilance assessments indicate that QTc prolongation may occur after ingestion of suprathreshold sertraline doses.21
Although no clinically significant effects of sertraline on QTc prolongation have been identified to date, no thorough QTc (TQTc) study for sertraline has been reported. We therefore conducted a phase 1, single‐center, randomized, double‐blind, placebo‐ and moxifloxacin‐controlled, 3‐period, 6‐sequence, 3‐treatment (sertraline and placebo blinded; moxifloxacin open label), 3‐way crossover TQTc study examining the effects of sertraline on cardiac repolarization in healthy subjects to satisfy a regulatory commitment. Because sertraline and N‐desmethylsertraline have prolonged terminal half‐lives,5, 6 and a supratherapeutic sertraline dose is assessed, a unique study design was developed for this investigation in accordance with the US Food and Drug Administration guidelines for conducting TQTc studies.22 The study included a slow escalation of sertraline dose over a 2‐week period to achieve a supratherapeutic dose and steady‐state concentrations, with a washout period of at least 2 weeks between successive treatment periods. The study objective was to assess the effect of a supratherapeutic dose of sertraline on QT interval relative to time‐matched placebo in healthy subjects.
Methods
The study protocol was reviewed and approved by the study site's Independent Ethics Committee (Comite d'Ethique Hospitalo Facultaire Saint‐Luc–UCL, Brussels, Belgium). The study was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with all International Conference on Harmonisation Good Clinical Practice Guidelines and local regulatory requirements protecting the safety of study participants. All subjects provided written informed consent before screening.
Study Design and Subjects
This 3‐period, 6‐sequence, 3‐treatment, randomized, double‐blind, steady‐state, crossover study was conducted at a single center (Pfizer Clinical Research Unit, Erasme Hospital) in Brussels, Belgium, between January and September 2016. Study treatments were double‐blind sertraline 400 mg/day and placebo, and open‐label moxifloxacin 400 mg (Avelox, Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey), administered in a randomized sequence in study period 1, 2, or 3 with a washout period of ≥14 days after each treatment. Each study period included a 17‐night inpatient stay. Participants were discharged from the study site on day 17 (or at early termination).
A supratherapeutic dose of 400 mg/day was used to adequately assess potential QTc prolongation following sertraline administration on day 14. Dose selection for this study balanced the need to assess plasma concentrations higher than those achieved with therapeutic doses, in accordance with US Food and Drug Administration guidance, with safety and tolerability. Sertraline 400 mg/day is the maximum tolerated dose; nausea and vomiting are among the most common adverse events, with greater rates of occurrence at higher doses (200 and 400 mg/day).23 The 400‐mg/day dose was expected to produce plasma sertraline concentrations in excess of those resulting from the recommended therapeutic dose range. In an unpublished single‐ and multiple‐dose study of sertraline 200 mg/day or 400 mg/day in healthy subjects (Pfizer Inc, data on file), the mean maximum plasma concentration (Cmax) for sertraline at the 400‐mg/day dose on day 14 (282 ng/mL; range, 161‐500 ng/mL) was approximately 3 times the Cmax for the maximum approved therapeutic dose of 200 mg/day (mean Cmax, 86.1 ng/mL) and exceeded the maximum Cmax observed for that dose (range, 27‐165 ng/mL). Hepatic impairment has been associated with a reduced rate of sertraline clearance and a 1.7‐fold increase in exposure.24 However, a dosage reduction (50 mg/day) or increased dosing interval is recommended for patients with mild hepatic impairment, and sertraline is not recommended for those with moderate or severe impairment.25, 26
Dose escalation from a recommended starting dose of 50 mg was used to achieve the supratherapeutic sertraline 400‐mg/day dose by day 14 so that subjects could tolerate the study treatment while minimizing nausea and vomiting. The study design therefore included a double‐blind dose titration for sertraline to supratherapeutic doses. The sertraline dose was titrated on days 1 to 7, starting with a single dose of 50 mg on day 1 followed by twice‐daily (BID, given approximately 12 hours apart) escalating doses of sertraline administered on days 2 through 6 (Table 1). On days 7 to 13, subjects received sertraline 400 mg/day (administered 200 mg BID). On day 14, subjects received only the morning dose of sertraline 200 mg/day, after which the day 14 assessments were completed. The evening dose was not administered on day 14. A single dose of moxifloxacin 400 mg was administered open label on day 14 preceded by double‐blinded placebo, which matched the dosing schema for sertraline titration from day 1 to day 13. Moxifloxacin was used to confirm assay sensitivity, as it is typically associated with a peak increase in QTc of 8 to 15 milliseconds occurring between 1 and 3 hours after single‐dose administration.22, 27 Placebo was administered on days 1 to 14, according to the sertraline dose‐titration scheme.
Table 1.
Sertraline Dosing Schema
| Double‐Blind Sertraline Dose | ||
|---|---|---|
| Study Period Day | Total Daily Dose | Dosing Regimen |
| 1 | 50 mg QD | |
| 2 | 100 mg | 50 mg BID |
| 3 | 150 mg |
50 mg am 100 mg pm |
| 4‐5 | 200 mg | 100 mg BID |
| 6 | 300 mg |
100 mg am 200 mg pm |
| 7‐14a | 400 mg | 200 mg BID |
BID, twice a day; QD, once daily.
On day 14, only the morning dose of 200 mg was administered, and no evening dose was administered.
The study enrolled healthy adult subjects aged 18 to 55 years. Eligible subjects had a body mass index between 17.5 and 30.5 kg/m2, inclusive, and a total body weight >50 kg. Excluded from the study were subjects with evidence or history of clinically significant disease, including drug allergies; other severe, acute, or chronic medical or psychiatric conditions; laboratory abnormalities that might increase the risk associated with study participation; or abnormalities in clinical laboratory tests at screening. Also excluded were those deemed by the investigator to be at significant risk of suicidal or violent behavior. Subjects were excluded if they had a history of sensitivity to sertraline, SSRIs, moxifloxacin, quinolones, or heparin; if they had had heparin‐induced thrombocytopenia or a positive urine drug screen; or if they had any condition possibly affecting drug absorption. Subjects were also excluded if their screening supine systolic blood pressure was ≥140 mm Hg or diastolic blood pressure was ≥90 mm Hg; if they had a history of known QT interval prolongation or ECG abnormalities; if they had a self‐reported history of sick sinus syndrome; if they had first‐, second‐, or third‐degree atrioventricular block, myocardial infarction, pulmonary congestion, cardiac arrhythmia, conduction abnormalities, or any other clinically significant cardiovascular disease history; or if they had a QT interval >450 milliseconds or a QRS interval >120 milliseconds at screening.
Individuals were not enrolled if they had taken sertraline or moxifloxacin within 30 days, herbal supplements or hormone replacement therapy within 28 days, or nonprescription drugs and dietary supplements within 7 days (with the exception of acetaminophen/paracetamol, <1 g/day) before the first dose of study drug. They were not enrolled if they had used tobacco‐ or nicotine‐containing products in excess of the equivalent of 5 cigarettes per day, or if they had a history of regular alcohol consumption exceeding 14 drinks/week for females or 21 drinks/week for males within 6 months of screening. Individuals who had donated blood (≥500 mL, excluding plasma donations) within 56 days before dosing were excluded. Women of childbearing potential and men able to father children were required to use ≥1 highly effective method of contraception for the duration of the study and for ≥28 days after the last study dose.
Pharmacokinetics
In each study period, blood samples (4 mL) for pharmacokinetic (PK) analysis were collected into sodium heparin‐treated tubes before dosing and 1, 2, 3, 4, 5, 6, 8, 12, and 24 hours after single‐dose administration (day 1), and before dosing and 1, 2, 3, 4, 5, 6, 8, 12, 24, 48, and 72 hours after dosing following multiple dosing (day 14). Blood samples were centrifuged at 1700 × g for 10 minutes at 4°C and plasma was separated; plasma samples were stored at approximately −70°C within 1 hour of collection and shipped to Covance Inc (West Trenton, New Jersey) for analysis.
Plasma samples were assayed for sertraline hydrochloride (HCl) and N‐desmethylsertraline HCl using a validated liquid chromatography–tandem mass spectrometric method. According to the study protocol, moxifloxacin plasma concentrations were to be determined only if a positive signal for QTc prolongation was not observed; plasma samples for moxifloxacin assay were to be held until notification by the clinical team. The analytes were extracted from plasma using a protein precipitation procedure in a 96‐well format. Briefly, plasma samples (100 µL) were fortified with 100 µL of working solution containing internal standards, sertraline‐D3 HCl (25.0 ng/mL in methanol:water, 50:50) and N‐desmethylsertraline 13C6 HCl (50.0 ng/mL in methanol:water, 50:50) followed by the addition of 500 µL of 1% formic acid in acetonitrile. The samples were vortexed and centrifuged and the supernatant (500 µL) dried under heated nitrogen and then reconstituted with 200 µL methanol:water, 50:50. A 5‐ to 50‐µL aliquot of extract was injected into an API 4000 MDS Sciex (Thornhill, Ontario, Canada) with a TurboIonSpray source and set up with a Phenomenex, Gemini C6‐Phenyl, 2.1 × 50 mm, 5‐µm column (Torrance, California). The mobile phase consisted of methanol in water plus 0.2% tetrahydrofuran using a gradient program and a flow rate of 0.300 mL/min. The mass spectrometer was operated in multiple reaction monitoring mode and the transitions monitored were m/z 306.2→159.2 (sertraline HCl), m/z 309.2→159.2 (sertraline‐D3 HCl), m/z 292.2→159.2 (N‐desmethylsertraline HCl), m/z 300.2→161.2 (N‐desmethylsertraline 13C6 HCl).
The peak area ratios of analytes and internal standards were determined using Analyst Version 1.6.1 (AB Sciex, Framingham, Massachusetts), and sertraline and N‐desmethylsertraline concentrations were calculated by Watson LIMS Version 7.4.2 (Thermo Electron Corp, Bellefonte, Pennsylvania). Calibration standard responses were linear over the range of 0.500 to 250 ng/mL using a weighted (1/concentration2) linear least squares (LS) regression. Samples with concentrations above the upper limit of quantification were diluted into calibration range. The lower limit of quantification for sertraline and N‐desmethylsertraline was 0.500 ng/mL; concentrations below that limit were reported as below lower limit of quantification. The concentrations of the quality control samples were 1.50, 15.0, 100, 200 and 1000 (diluted quality control) ng/mL for sertraline and N‐desmethylsertraline. The between‐day assay accuracy (expressed as percent relative error) of the quality control samples used during sample analysis ranged from −3.5% to 0.0% for sertraline and −1.6% to 0.7% for N‐desmethylsertraline, and the between‐day precision (expressed as the percent coefficient of variation) was ≤4.5% for sertraline and ≤9.3% for N‐desmethylsertraline. Incurred sample reproducibility for sertraline and N‐desmethylsertraline was tested on at least 10% of the study samples, and results met the acceptance criteria; there were 97.4% samples that had a percent difference, relative to the average of the original value and the reassayed value, within ±20%.
PK parameters were calculated using validated, internal Pfizer electronic noncompartmental analysis software (eNCA, Version 2.2.4). Concentrations below the lower limit of quantitation were set to 0 for analysis. Following single‐ and multiple‐dose sertraline administration, Cmax and time of Cmax (tmax) were observed directly from data; area under the plasma concentration–time curve from time 0 to 24 hours after dosing (AUC24) and from time 0 to the time of the last quantifiable concentration (AUClast; multiple‐dose only) were determined using the log/linear trapezoidal method. For multiple‐dose administration, plasma terminal elimination half‐lifewas calculated as loge(2)/kel, where kel is the terminal phase rate constant calculated by a linear regression of the log‐linear concentration‐time curve. Minimum plasma concentration over the 0‐ to 24‐hour period after the last dose (Cmin) was observed directly from the data, and Rac and Rac,Cmax were calculated as the observed accumulation ratios for AUC24 and Cmax, respectively. Metabolite‐to‐parent ratio for AUC24 was the ratio of N‐desmethylsertraline AUC24 to sertraline AUC24, corrected for molecular weight.
Electrocardiogram
Subjects rested quietly for ≥10 minutes and abstained from fluids for approximately 0.5 hour prior to any ECG measurement. Twelve‐lead ECGs were obtained with the patient in a supine position. On days 1 and 14 of each study period, ECGs were collected in triplicate approximately 2 minutes apart before dosing (−1, −0.5, and 0 hours) and at 1, 2, 3, 4, 5, 6, 8, 12, and 24 hours after single‐dose administration (day 1), and before dosing (0 hours) and at 1, 2, 3, 4, 5, 6, 8, 12, 24, 48, and 72 hours after dosing following multiple dosing (day 14). For each period, the average of the day 1 predose triplicate ECGs collected in that period served as the subject's baseline QTc value. QT, PR, QRS, and RR intervals and heart rate were collected from the ECGs. QT was corrected for heart rate using Fridericia's correction (QTcF), Bazett's correction (QTcB), and the individual QT correction method. QTcF was determined to be sufficient to evaluate effect of sertraline on QTc based on an assessment of scatter plots comparing predose QT and RR data; therefore, results for QTcF are reported. If the QTc interval was increased ≥45 milliseconds from baseline, or if an absolute QTc value was ≥500 milliseconds, 2 additional ECGs were taken to confirm the original measurement. If any repeated ECGs continued to show prolonged QTc at or above those thresholds, then hourly ECGs were obtained until 2 successive ECGs fell below the threshold. If QTc values remained above threshold levels for >4 hours, or became progressively longer, the subject underwent continuous ECG monitoring. If QTc intervals did not return to <500 milliseconds (or to <45 milliseconds above the baseline) after 8 hours, a cardiologist was to be consulted.
ECG measurements on day 14 up to 24 hours were used for the primary analysis of postdose QTcF intervals. All ECG data, collected on days 1 and 14, were used for PK/pharmacodynamic (PD) analysis.
Pharmacokinetic/Pharmacodynamic Analysis
The PK/PD data set included data from 54 subjects and provided 1066 sertraline‐QTcF pairs (1050 QTcF records on placebo treatment). PK samples reported as below the limit of quantitation (n = 80) were set to zero; 52 samples reported as below the limit of quantitation were predose samples, 27 were collected at initial time points, 1 or 2 hours after dosing, and 1 record on day 1 postdose. A total of 10 records were excluded due to missing concentrations or ECG measurements.
Before conducting model‐based analysis, graphical assessments were conducted to assess key assumptions about the relationship between plasma sertraline and N‐desmethylsertraline concentration and QTc interval data, including drug effect on heart rate, the potential effect of drug treatment on the QT‐RR interval relationship, lag between parent‐drug concentration and change in QTc interval, and shape of the response curve (linear vs nonlinear). The relationship between QTc interval and plasma sertraline/N‐desmethylsertraline concentration was examined using linear mixed‐effect modeling with an unstructured random effects covariance matrix, implemented in R Version 3.2.2 (http://www.R-project.org) using the lme function in nlme package version 3.1‐128 and restricted maximum likelihood estimation. The dependent variable was defined as baseline corrected QTcF (∆QTcF), and its relationship with plasma concentrations of sertraline/N‐desmethylsertraline was modeled using the following linear mixed‐effect model:
where i is the ith subject, j is the jth treatment, k is the kth time point relative to dosing, μ is the intercept, TRT is the jth treatment effect, tk is the kth time effect, BQTc,i is the baseline QTc for the ith subject for the jth treatment, is the population mean baseline QTc, Cijk is the concentration at the kth time point for treatment j for subject i. The intercept was treatment‐specific for each subject. ημ,I and ηC,I were the subject‐specific random effects for the intercept and slope, respectively, having mean [0, 0] and unstructured covariance. ε are independent residuals with mean 0 and variance σ2.
Sertraline and N‐desmethylsertraline concentrations were modeled separately using the ∆QTc values from individual ECG measurements at each time point. Visual and statistical metrics were used to evaluate goodness of fit. PK/PD hysteresis between placebo‐corrected ΔQTcF (ΔΔQTcF) and sertraline/N‐desmethylsertraline plasma concentrations was evaluated graphically.
Safety Assessments
Adverse events (AEs), classified using the Medical Dictionary for Regulatory Activities, version 19.0, were collected throughout the study. Serious AEs (SAEs) were defined as AEs that resulted in death or immediate risk of death, inpatient hospitalization or prolongation of existing hospitalization, or congenital anomaly/birth defect. Laboratory evaluations, including hematology, chemistry, and urinalysis, were performed at screening, baseline, and days 7, 14, and 17 (or early termination). Liver function tests were conducted when deemed necessary by the investigator, based on clinical sign/symptom presentation in a subject. Physical examinations were performed at baseline and day 17 (or early termination); vital sign measurements were taken on days 1, 2, and 17 (or early termination); and suicide assessments (Columbia Suicide Severity Rating Scale) were administered at baseline, day 14, and day 17 (or early termination).
Statistical Analysis
Assuming that the expected mean difference between sertraline and placebo was ≤5 milliseconds at each time point and that intrasubject variability was 5.36 milliseconds, a sample size of 42 subjects (7 per treatment sequence) provided ≥99% power to exclude that the upper bound of a 2‐sided 90% confidence interval (CI) of time‐matched difference between sertraline and placebo was >10 milliseconds at each time point.
The effect of sertraline versus placebo administration on postdose QTcF was evaluated in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) E14 guidance.22 An analysis of covariance was performed using a mixed‐effect model with sequence, study period, treatment, time, and treatment‐by‐time interaction as fixed effects; subject within sequence as a random effect; and baseline QTcF as a covariate. A 2‐sided 90%CI (equivalent to a 1‐sided 95%CI) for the LS mean difference in QTcF between sertraline and placebo was computed for each postdose time point. The 90%CIs were compared with a predefined significance threshold of 10 milliseconds; a lack of effect of sertraline was to be concluded if the upper bound of the CIs for mean differences between sertraline and placebo fell below the 10‐millisecond threshold at each time point. In accordance with the ICH E14 guidance,22 assay sensitivity was confirmed if the lower bound of a 2‐sided 90%CI for the mean difference between moxifloxacin and placebo was >5 milliseconds at 3 hours after dosing (population tmax for moxifloxacin28). Additionally, change from baseline QTcF, QTcB, uncorrected QT, PR, QRS, and heart rate were calculated for each subject and treatment at each time point.
Sertraline and metabolite PK parameters were summarized descriptively by treatment and day. For each time point, the mean of the triplicate QTc values was used.
Safety data were summarized during the study to assess ongoing subject safety. AEs and treatment‐emergent AEs (TEAEs) were summarized descriptively by treatment and severity; SAEs and discontinuations due to AEs were summarized by treatment. For vital sign measurements, baseline values and change from baseline were summarized using descriptive statistics.
Results
Subjects
A total of 54 subjects were enrolled in the study. The ECG analysis population included subjects who received treatment in all 3 treatment periods (sertraline, n = 52; moxifloxacin, n = 50; placebo, n = 50); sertraline, moxifloxacin, and placebo treatment periods were completed by 47, 49, and 47 subjects, respectively. Nine subjects discontinued during the double‐blind period (5 subjects while receiving sertraline, 1 subject while receiving moxifloxacin, and 3 subjects while receiving placebo treatments). Subjects ranged in age from 20 to 54 years; 80% of subjects were male, and 85% were white (Table 2). The subjects’ body mass index ranged from 18.1 to 30.5 kg/m2.
Table 2.
Baseline and Demographic Characteristics of Healthy Adult Volunteers
| Characteristic | Enrolled Population (n = 54) |
|---|---|
| Age, y, mean ± SD | 36.5 ± 9.7 |
| Sex, n (%) | |
| Male | 43 (80) |
| Female | 11 (20) |
| Race, n (%) | |
| White | 46 (85.2) |
| Black | 3 (5.6) |
| Asian | 1 (1.9) |
| Other | 4 (7.4) |
| Height, cm, mean ± SD | 173.3 ± 7.6 |
| Weight, kg, mean ± SD | 74.4 ± 10.3 |
| Body mass index, kg/m2, mean ± SD | 24.8 ± 3.0 |
SD, standard deviation.
Pharmacokinetics
Mean (standard deviation) plasma sertraline and N‐desmethylsertraline concentration‐time profiles for a single 50‐mg dose (day 1) and multiple dose (400 mg/day, administered as 200 mg BID) administration (day 14) are shown in Figure 1. Geometric mean sertraline Cmax was 11.4 ng/mL and 234.2 ng/mL for the 50 mg and 400 mg/day doses, respectively, and was achieved at 5.06 hours and 7.15 hours after dosing on days 1 and 14, respectively. N‐desmethylsertraline Cmax was reached at 12.0 and 8.0 hours following the 50 mg (day 1) and 400 mg/day doses (day 14), respectively. Mean t½ for sertraline following the last dose of 400 mg/day sertraline was approximately 27 hours; t½ for N‐desmethylsertraline after dosing on day 14 was not reportable for any subjects due to lack of a well‐characterized terminal phase given the 72‐hour sampling employed in this study. Sertraline and N‐desmethylsertraline PK values for the 50‐mg and 400‐mg/day doses are summarized in Table 3.
Figure 1.
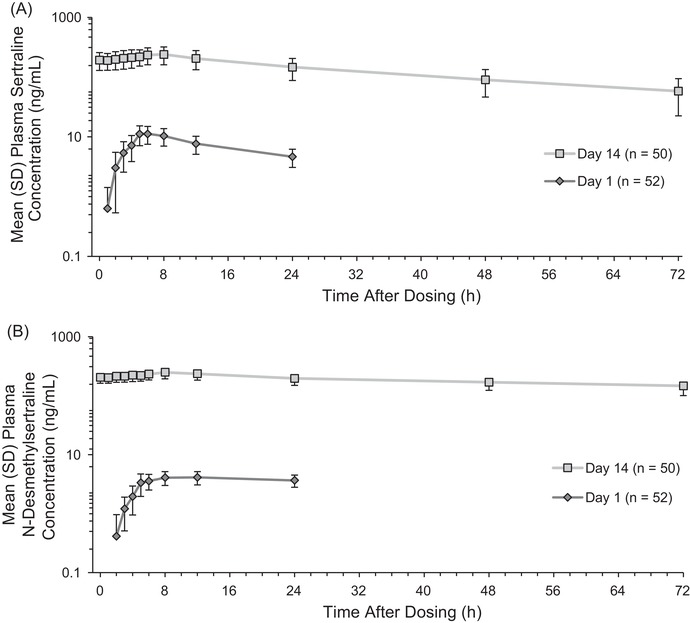
Mean (standard deviation [SD]) plasma (A) sertraline and (B) N‐desmethylsertraline concentration‐time profiles on day 1 (dose = 50 mg) and day 14 (dose = 400 mg/daya). aDosing was 200 mg BID; postdose blood draws for pharmacokinetic analyses were completed after the morning 200‐mg dose, and no further doses were administered.
Table 3.
Descriptive Summary of Plasma Sertraline and N‐desmethylsertraline Pharmacokinetic Parameter Values on Day 1 and Day 14, PK Population
| Parameter, Units | Day 1, 50 mg (Single Dose) n = 52 | Day 14, 400 mg/daya (Multiple Dose) n = 50b |
|---|---|---|
| Sertraline | ||
| Cmax, ng/mL, geometric mean (%CV) | 11.39 (37) | 234.2 (34) |
|
Arithmetic mean ± SD Range |
12.11 ± 4.3387 4.26‐27.5 |
246.5 ± 80.561 94.1‐502 |
| tmax, h, median (range) | 5.06 (4.02‐8.05) | 7.15 (3.00‐8.08) |
| AUC24, ng • h/mL, geometric mean (%CV) | 154.3 (34) | 4388 (36) |
| Arithmetic mean ± SD | 162.3 ± 50.257 | 4648 ± 1575.7 |
| AUClast, ng • h/mL, geometric mean (%CV) | NA | 8489 (43) |
| Arithmetic mean ± SD | NA | 9194 ± 3724.1 |
| t1/2, h, arithmetic mean ± SD | NA | 26.93 ± 2.71 |
| Cmin, ng/mL, geometric mean (%CV) | NA | 135.3 (43) |
| Arithmetic mean ± SD | NA | 146.1 ± 57.093 |
| Rac, geometric mean (%CV) | NA | 3.62 (22.79) |
| Arithmetic mean ± SD | NA | 3.71 ± 0.82 |
| N‐desmethylsertraline | ||
| Cmax, ng/mL, geometric mean (%CV) | 4.269 (27) | 249.6 (23) |
|
Arithmetic mean ± SD Range |
4.428 ± 1.3189 2.75‐9.99 |
255.8 ± 58.383 134‐438 |
| tmax, h, median (range) | 12.0 (5.00‐23.9) | 8.00 (1.07‐12.1) |
| AUC24, ng • h/mL, geometric mean (%CV) | 76.87 (24) | 5227 (22) |
| Arithmetic mean ± SD | 79.03 ± 19.199 | 5342 ± 1109.5 |
| AUClast, ng • h/mL, geometric mean (%CV) | NA | 13110 (25) |
| Arithmetic mean ± SD | NA | 13500 ± 3225.2 |
| t1/2, h, arithmetic mean ± SD | NA | NC |
| Cmin, ng/mL, geometric mean (%CV) | NA | 184.1 (23) |
| Arithmetic mean ± SD | NA | 188.4 ± 39.567 |
| MR, geometric mean (%CV) | 0.5222 (34) | 1.248 (24) |
| Arithmetic mean ± SD | 0.5493 ± 0.17427 | 1.283 ± 0.30751 |
AUC24, area under the plasma concentration–time curve from time 0 to 24 hours; AUClast, area under the plasma concentration–time curve to the last quantifiable concentration; Cmax, maximum plasma concentration; Cmin, minimum observed plasma concentration during the dosing interval; %CV, percent coefficients of variation; MR, metabolite‐to‐parent ratio; NA, not applicable; NC, not calculated; Rac, observed accumulation ratio of AUC; Rac,Cmax, observed accumulation ratio for Cmax; t1/2, plasma terminal elimination half‐life; tmax, time to reach maximum plasma concentration.
Dosing was 200 mg BID; postdose blood draws for pharmacokinetic analyses were completed after the morning 200‐mg dose on day 14; no further doses were administered.
N = 49 for all AUC parameters, Cmin, and Rac due to an incomplete profile for 1 subject; number of subjects with reportable sertraline t½ was 23.
Electrocardiography
Mean placebo‐corrected heart rate for 400‐mg/day sertraline and 400‐mg moxifloxacin on day 14 are shown in Figure S1. Based on observed predose QT and RR data, the QTcF for heart rate was determined to be sufficient to evaluate effect of sertraline on QTc. No significant period or sequence effect was observed. Mean (standard deviation) day 1 predose (time 0) QTcF values were 414.7 (16.79) milliseconds for the sertraline treatment period, 413.3 (17.30) milliseconds for the moxifloxacin treatment period, and 414.5 (16.51) milliseconds for the placebo treatment period. On day 14, predose (time 0) QTcF values were 414.9 (14.89) milliseconds for the sertraline treatment period, 407.6 (15.20) milliseconds for the moxifloxacin treatment period, and 407.2 (16.85) milliseconds for the placebo treatment period. Figure 2 shows the LS mean difference in QTcF after supratherapeutic sertraline (400 mg/day) versus placebo at each time point after dosing on day 14. In the primary analysis, at the 4‐hour postdose (day 14) time point only, the upper bound of the 90%CI for the LS mean difference in QTcF at the supratherapeutic sertraline 400 mg/day dose vs placebo exceeded the predefined threshold of 10 milliseconds (LS mean = 9.651 milliseconds [90%CI, 7.635‐11.666]; Table 4). The upper bound of the 90%CI did not exceed the predefined threshold of 10 milliseconds at any of the remaining 8 postdose time points on day 14.
Figure 2.
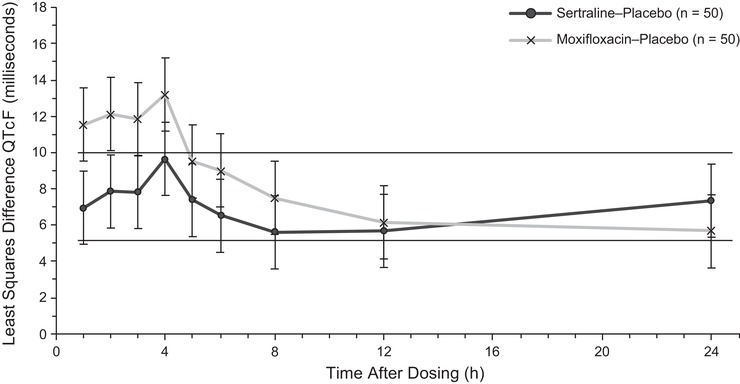
Least squares mean difference of QTcF (milliseconds) for 400 mg/daya sertraline versus placebo and 400 mg moxifloxacin versus placebo at each time point after dosing on day 14 for the primary ECG analysis population. BID, twice daily; ECG, electrocardiogram; QTcF, QT corrected for heart rate using Fridericia formula. aDosing was 200 mg BID; postdose blood draws for pharmacokinetic analyses were completed after the morning 200‐mg dose, and no further doses were administered.
Table 4.
Statistical Comparisons of QTcF Between Sertraline and Placebo at Each Time Point After Dosing on Day 14
| Least Squares Mean, ms | ||||
|---|---|---|---|---|
| Nominal Time After Dosing, h | Sertraline N = 50 | Placebo N = 50 | Least Squares Difference (Sertraline‐Placebo), ms | 90% Confidence Interval |
| 1 | 414.299 | 407.341 | 6.957 | 4.942‐8.973 |
| 2 | 415.625 | 407.775 | 7.851 | 5.835‐9.866 |
| 3 | 416.465 | 408.641 | 7.824 | 5.808‐9.840 |
| 4 | 418.252 | 408.601 | 9.651 | 7.635‐11.666 |
| 5 | 411.285 | 403.908 | 7.377 | 5.362‐9.393 |
| 6 | 408.985 | 402.475 | 6.511 | 4.495‐8.526 |
| 8 | 408.732 | 403.135 | 5.597 | 3.582‐7.613 |
| 12 | 411.019 | 405.341 | 5.677 | 3.662‐7.693 |
| 24 | 414.294 | 406.948 | 7.346 | 5.331‐9.360 |
Baseline was defined as the mean of the 3 average triplicate measurements taken at −1 h, −0.5 h, and 0 h before dosing within each period.
Assay sensitivity was demonstrated according to ICH guidance in the time‐matched comparison of moxifloxacin vs placebo. QTcF increased significantly versus placebo after moxifloxacin administration (Figure 2). The lower bound of the 90%CI for the LS mean difference in QTcF for moxifloxacin versus placebo was 9.813 milliseconds at 3 hours after dosing (LS mean difference = 11.831 [90%CI, 9.813‐13.849]), which exceed the threshold for assay sensitivity of 5 milliseconds.
In a categorical analysis of maximum postdose QTcF, no participant had an absolute QTcF interval of ≥480 milliseconds or a maximum increase from baseline in QTcF of ≥60 milliseconds after receiving sertraline, moxifloxacin, or placebo. A maximum postdose QTcF interval of 450 milliseconds to <480 milliseconds was observed in 1 (1.9%) subject after receiving sertraline on day 14, and in 3 (6.0%) subjects each after day 14 dosing of moxifloxacin and placebo. A maximum change from baseline in QTcF interval of ≥30 milliseconds to <60 milliseconds was observed in a single subject, after receiving moxifloxacin on day 14. No abnormalities in T‐wave morphology were reported in this study.
Pharmacokinetic/Pharmacodynamic Analysis
The exposure‐response analysis based on separate PK/PD models for sertraline and its active metabolite showed that ∆QTcF values increased with increasing sertraline (P < .0001) and N‐desmethylsertraline (P < .0001) concentrations. In Figure 3, ∆QTcF is plotted against individual sertraline and N‐desmethylsertraline plasma concentrations on day 14. The estimated slope of the regression for ∆QTcF versus sertraline plasma concentration was 0.036 ms/ng/mL (90%CI, 0.029‐0.043; P < .0001); for ∆QTcF versus N‐desmethylsertraline plasma concentration, the estimated slope was 0.036 ms/ng/mL (90%CI, 0.029‐0.042; P < .0001). Predicted mean change from baseline QTcF adjusted for placebo (∆ΔQTcF) at mean therapeutic maximum plasma sertraline concentration of 86 ng/mL (Pfizer Inc, data on file) is 3.57 milliseconds (90%CI, 2.92‐4.23), and for a mean supratherapeutic Cmax of 234 ng/mL on day 14 (current study), predicted mean ∆∆QTcF was 8.93 milliseconds (90%CI, 7.42‐10.45). The upper bound of 2‐sided 90%CI is predicted to cross the threshold of 10 milliseconds for peak plasma sertraline concentrations >223.5 ng/mL (predicted mean ∆∆QTcF, 8.55 milliseconds [90% CI, 7.11‐9.99]). Based on this PK/PD model, plasma sertraline concentration value has to be greater than approximately 2.6 times the mean therapeutic Cmax (86 ng/mL) for the predicted upper bound of 2‐sided 90%CI to cross the 10‐millisecond threshold.
Figure 3.
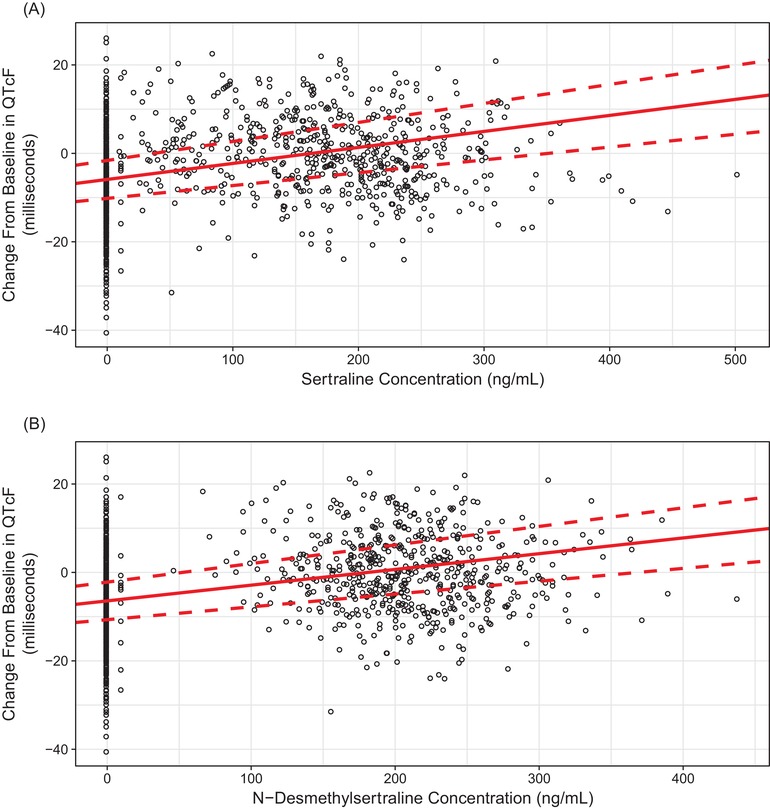
Observed and predicted (regression line) change from baseline in QTcF versus sertraline (P < .0001) (A) and N‐desmethylsertraline (P < .0001) (B) plasma concentrations on day 14 after administration of sertraline 400 mg/day.a QTcF, QT corrected for heart rate using Fridericia formula. Open circle represents observed data, solid red line represents regression line, and dotted red line represents 90% confidence interval. aDosing was 200 mg BID; postdose blood draws for pharmacokinetic analyses were completed after the morning 200‐mg dose, and no further doses were administered.
Graphical evaluation of the time course of mean plasma sertraline concentration on day 14 compared with mean placebo‐adjusted QTcF for sertraline indicated PK/PD proteresis (Figure 4A). The longest placebo‐adjusted QTc interval was observed at 4 hours after dosing, whereas median tmax for sertraline was 7.15 hours. Mean (standard deviation) plasma sertraline concentration was 213.1 (72.835) ng/mL at 4 hours after dosing compared with the Cmax of 234.2 ng/mL at tmax. A similar temporal relationship was observed between placebo‐adjusted QTcF and plasma N‐desmethylsertraline concentration on day 14 (Figure 4B).
Figure 4.
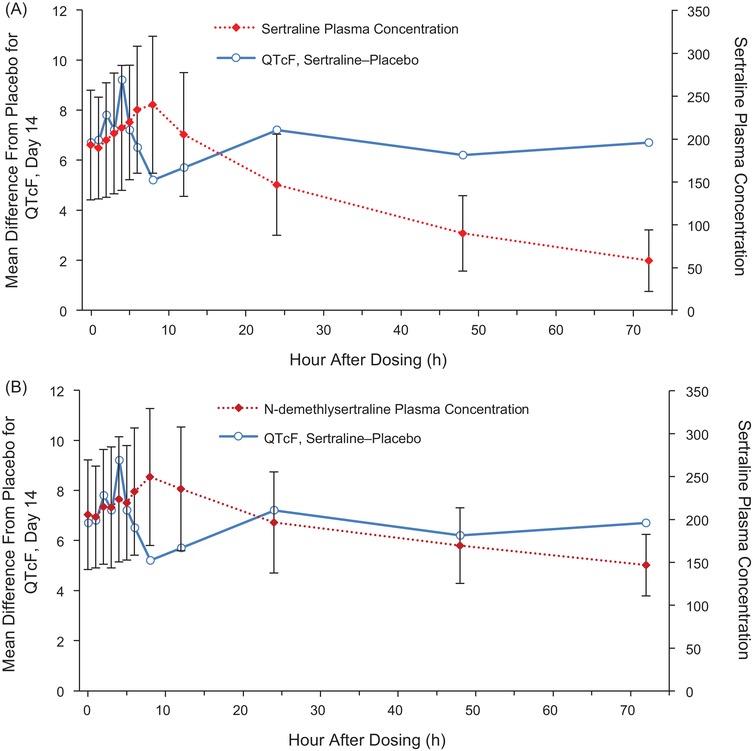
Overlay plots of time course of mean (A) sertraline and (B) N‐desmethylsertraline concentrations and differences from placebo in QTcF on day 14.
Safety
A total of 52 subjects received ≥1 dose of sertraline, 50 received ≥1 dose of moxifloxacin, and 50 received ≥1 dose of placebo. Of those, 45 reported ≥1 AE during sertraline treatment, 42 reported ≥1 AE during moxifloxacin treatment, and 33 reported ≥1 AE during placebo treatment. A total of 251 TEAEs were reported during sertraline treatment, 238 of which were considered treatment related (moxifloxacin, 105 TEAEs, 92 treatment related; placebo, 121 TEAEs, 106 treatment related). The most common AEs reported after supratherapeutic sertraline administration were headache (18 subjects; moxifloxacin, 11 subjects; placebo, 13 subjects), insomnia (22 subjects; moxifloxacin, 11 subjects; placebo, 6 subjects), and fatigue (17 subjects; moxifloxacin, 7 subjects; placebo, 7 subjects), all of which were considered treatment related.
TEAEs were generally mild (90%) or moderate (10%) in severity. A single severe AE was reported: psychiatric decompensation, reported in a subject receiving sertraline. That AE was reported as an SAE, the patient was withdrawn from the study, and the SAE resolved the same day. No other SAEs were reported during the study. One additional subject discontinued study treatment due to an AE (asthma), also while receiving sertraline treatment. No deaths were reported during the study.
No clinically significant laboratory test results or vital sign changes were observed during the study, and no laboratory or vital sign findings were reported as AEs.
Discussion
In this TQTc study, sertraline administered at twice the maximum therapeutic dose (400 mg/day) increased QTcF compared with placebo at 4 hours after dosing in healthy subjects. The upper bound of the 90%CI for the LS mean difference in QTcF for sertraline vs placebo did not exceed the predefined 10‐millisecond significance threshold at any other time points. In this study, the geometric mean Cmax for sertraline following day 14 administration of the 400‐mg/day dose was approximately 3‐fold higher than the steady‐state Cmax value observed at the highest approved therapeutic dosing level of 200 mg/day in a previous unpublished multiple‐dose PK study in healthy volunteers.
At plasma sertraline and N‐desmethylsertraline levels achieved with the maximum therapeutic dose of 200 mg/day, predicted QTcF prolongation is 3.57 milliseconds (90%CI, 2.92‐4.23 milliseconds) based on the PK/PD analysis, and does not cross the 10‐millisecond threshold of regulatory concern. Significant QTcF prolongation is expected at plasma sertraline concentrations exceeding 223.5 ng/mL, a value >2.5 times that of the mean therapeutic Cmax observed in the previous PK study. It is notable that PK/PD proteresis was observed, such that the greatest QTcF interval preceded peak plasma sertraline and N‐desmethylsertraline concentrations. The proteresis may have been related to the conduct of the study at steady state: the mean plasma drug concentrations at 4 hours were fairly close to Cmax values seen at 7 hours, and the prolonged high concentrations seen around Cmax likely influenced the proteresis. There also may be a food effect contribution, as lunch was provided to study subjects approximately 4 hours after morning dosing. A food effect might be expected to reduce QTc interval for up to 4 hours after a carbohydrate‐rich meal,29 the time frame during which a sharp decline in QTcF was observed in the current study.
The current results are generally consistent with previously published findings based on ECG recordings in clinical trials of sertraline at therapeutic doses (50‐200 mg/day).19, 30 No statistically significant increase in QT/QTc interval was observed in an analysis of safety data from 4 amitriptyline‐ and/or placebo‐controlled sertraline trials enrolling patients with major depression (sertraline 50‐200 mg/day; 1 study included a 400‐mg dose arm)19 or in a trial enrolling patients with major depression and acute myocardial infarction or unstable angina who were administered sertraline (50‐200 mg/day) or placebo.30 A meta‐analysis of study‐level data from those trials reported a mean 3.0‐millisecond (95%CI, 2.95‐3.05) increase in QTc interval for sertraline compared with placebo (P < .0001).31 In a prospective, cross‐sectional population‐based study (n = 42), sertraline was associated with a mean QTcF increase of 1.7 milliseconds (90%CI, −3.4 to 6.9).18 At suprathreshold doses, however, sertraline has been associated with QTc prolongation. In patients admitted for sertraline overdose (>200 mg), incidence of QTc interval >500 ms was 6% (6/103); incidence of QTc interval greater than 440 ms was 40% (41/103).21 Incidence of maximum QTcB interval prolongation (males, QTcB >450 ms; females, QTcB >460 ms) was 7.9% (3/38) in an analysis of recordings from the Electrocardiogram Vigilance With Electronic Data Warehouse database in which dosing and concomitant medications were unknown.20 A case study describing a patient admitted to intensive care after a multiple‐drug overdose including 2250 mg of sertraline, 200 mg of diazepam, and 400 mg of temazepam reported an increase in QTc from 370 milliseconds at admission to 525 milliseconds 1 day later.32 QTc normalized after sertraline withdrawal, suggesting that sertraline contributed to the increase in QTc interval.
Although the sertraline exposure‐response model suggests that an increase in QTc interval is not expected to cross the 10‐millisecond threshold at doses ≤200 mg/day, the risk of QTc prolongation could be increased at therapeutic doses if sertraline is administered with other drugs that increase plasma concentrations of sertraline. For example, because sertraline is highly bound to plasma protein (fraction unbound in plasma = 1.34%−1.61%33), concomitant use of another drug that is highly protein bound may increase free plasma concentrations of sertraline to supratherapeutic levels, which could, in turn, increase QTc interval. Alternatively, sertraline could potentially exacerbate an increase in QTc if administered concomitantly with other drugs associated with a risk of QTc prolongation. In a case report, a QTc interval of 826 milliseconds was reported in a patient with mild hypokalemia receiving vorinostat, sertraline, and doxepin, the latter of which is associated with an increased risk for QTc prolongation.34 The QTc interval decreased to 559 milliseconds after the patient was discontinued from all 3 drugs, and the authors concluded that none of the drugs on their own were likely to have accounted for the extreme QTc value.
The current sertraline TQTc study was designed in accordance with the ICH E14 guidance for TQTc studies but was also customized to address specific treatment characteristics that were operant: the prolonged half‐life of sertraline and its active metabolite (26 hours and 62‐104 hours, respectively5, 6) and the supratherapeutic dose assessed. This study used the highest tolerable dose of sertraline, which necessitated a weeklong, double‐blind titration period to attain the supratherapeutic concentration while minimizing nausea and vomiting. Steady‐state concentration was achieved by administering multiple‐dose sertraline at the target dose for 7 days before ECG recordings were administered starting on day 14.
As the primary metabolite of sertraline, N‐desmethylsertraline was included in this TQTc study, and its extended half‐life was a factor in the study design. Whereas parallel‐group studies might be preferred for drugs with long elimination half‐lives, the crossover design offers the advantage of having subjects serve as their own controls, allowing for smaller numbers of subjects, reducing intersubject variability, and facilitating heart rate correction approaches based on individual subject data. However, the use of a crossover design in this study, coupled with the need to achieve supratherapeutic levels by escalating doses to steady state, necessitated a lengthy washout period (≥2 weeks) between successive treatment periods. A multiple‐dose, TQTc study of indacaterol (half‐life, 45.5‐126 hours) used a parallel‐group design,35 whereas several other previous TQTc studies for medications with long half‐lives have used a crossover design with 7‐ to 28‐day washout periods.36, 37, 38 These crossover design studies were single‐dose trials; steady‐state concentrations were not achieved. The impact of a study design that uses such a lengthy study period on observed TQTc results is unclear. In the current study, the preliminary mixed‐effect model fit of QTcF data indicated that period effect was not statistically significant; therefore, a full model with carryover effect was not analyzed. However, it should be noted that there appeared to be a reduction in the QTcF observed for the placebo group over the course of the study period (mean predose QTc: day 1, 414.5 milliseconds vs day 14, 407.2 milliseconds). This trend potentially accentuated the magnitude of the effect of sertraline on QTc interval relative to time‐matched placebo.
Limitations of the current study should be noted. The study enrolled healthy volunteers; results may not generalize to a patient population that includes individuals with underlying conditions or taking medications that could affect cardiac repolarization.
Conclusions
This TQTc study achieved a steady‐state supratherapeutic sertraline concentration that is approximately 3‐fold higher than the steady‐state Cmax value observed at the highest approved therapeutic dosing level of 200 mg/day. In healthy subjects, the upper bound of the 90%CI for the time‐matched LS mean difference in QTcF for sertraline vs placebo exceeded the predefined 10‐millisecond significance threshold at 4 hours after dosing, demonstrating a positive signal for QTc prolongation for sertraline at the steady‐state 400‐mg/day dose.
Disclosures
The authors are employees of Pfizer Inc.
Funding
This study was sponsored by Pfizer Inc.
Data Sharing Statement
Upon request, and subject to certain criteria, conditions, and exceptions see (https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Supporting information
Supplemental Figure 1
Additional supplemental information can be found by clicking the Supplements link in the PDF toolbar or the Supplemental Information section at the end of the web‐based version of this article.
Acknowledgments
Medical writing support was provided by Kathleen M. Dorries, PhD, of Peloton Advantage LLC, an Open Health company, and was funded by Pfizer. The authors would like to thank Michael Popovitz, clinical operation study manager, and the clinical personnel, biostatisticians, and all study participants.
References
- 1. Sheehan DV, Kamijima K. An evidence‐based review of the clinical use of sertraline in mood and anxiety disorders. Int Clin Psychopharmacol. 2009;24(2):43‐60. [DOI] [PubMed] [Google Scholar]
- 2. Murdoch D, McTavish D. Sertraline. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in depression and obsessive‐compulsive disorder. Drugs. 1992;44(4):604‐624. [DOI] [PubMed] [Google Scholar]
- 3. Greenblatt DJ, von Moltke LL, Harmatz JS, Shader RI. Human cytochromes mediating sertraline biotransformation: seeking attribution. J Clin Psychopharmacol. 1999;19(6):489‐493. [DOI] [PubMed] [Google Scholar]
- 4. Sprouse J, Clarke T, Reynolds L, Heym J, Rollema H. Comparison of the effects of sertraline and its metabolite desmethylsertraline on blockade of central 5‐HT reuptake in vivo. Neuropsychopharmacology. 1996;14(4):225‐231. [DOI] [PubMed] [Google Scholar]
- 5. Catterson ML, Preskorn SH. Pharmacokinetics of selective serotonin reuptake inhibitors: clinical relevance. Pharmacol Toxicol. 1996;78(4):203‐208. [DOI] [PubMed] [Google Scholar]
- 6. Perry CM, Benfield P. Sertraline. An overview of its pharmacological properties and a review of its therapeutic efficacy in obsessive‐compulsive disorder. CNS Drugs. 1997;7(6):480‐500. [Google Scholar]
- 7. Spina E, Santoro V, D'Arrigo C. Clinically relevant pharmacokinetic drug interactions with second‐generation antidepressants: an update. Clin Ther. 2008;30(7):1206‐1227. [DOI] [PubMed] [Google Scholar]
- 8. Wang JS, Zhu HJ, Gibson BB, Markowitz JS, Donovan JL, DeVane CL. Sertraline and its metabolite desmethylsertraline, but not bupropion or its three major metabolites, have high affinity for P‐glycoprotein. Biol Pharm Bull. 2008;31(2):231‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kapoor A, Iqbal M, Petropoulos S, Ho HL, Gibb W, Matthews SG. Effects of sertraline and fluoxetine on P‐glycoprotein at barrier sites: in vivo and in vitro approaches. PLoS One. 2013;8(2):e56525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. MacQueen G, Born L, Steiner M. The selective serotonin reuptake inhibitor sertraline: its profile and use in psychiatric disorders. CNS Drug Rev. 2001;7(1):1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang GK, Mitchell J, Wang SY. Block of persistent late Na+ currents by antidepressant sertraline and paroxetine. J Membr Biol. 2008;222(2):79‐90. [DOI] [PubMed] [Google Scholar]
- 12. Aldana BI, Sitges M. Sertraline inhibits pre‐synaptic Na(+) channel‐mediated responses in hippocampus‐isolated nerve endings. J Neurochem. 2012;121(2):197‐205. [DOI] [PubMed] [Google Scholar]
- 13. Becker B, Morel N, Vanbellinghen AM, Lebrun P. Blockade of calcium entry in smooth muscle cells by the antidepressant imipramine. Biochem Pharmacol. 2004;68(5):833‐842. [DOI] [PubMed] [Google Scholar]
- 14. Ohno Y, Hibino H, Lossin C, Inanobe A, Kurachi Y. Inhibition of astroglial Kir4.1 channels by selective serotonin reuptake inhibitors. Brain Res. 2007;1178:44‐51. [DOI] [PubMed] [Google Scholar]
- 15. Lee HM, Hahn SJ, Choi BH. Blockade of Kv1.5 channels by the antidepressant drug sertraline. Korean J Physiol Pharmacol. 2016;20(2):193‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee HA, Kim KS, Hyun SA, Park SG, Kim SJ. Wide spectrum of inhibitory effects of sertraline on cardiac ion channels. Korean J Physiol Pharmacol. 2012;16(5):327‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gintant G. An evaluation of hERG current assay performance: Translating preclinical safety studies to clinical QT prolongation. Pharmacol Ther. 2011;129(2):109‐119. [DOI] [PubMed] [Google Scholar]
- 18. Maljuric NM, Noordam R, Aarts N, et al. Use of selective serotonin re‐uptake inhibitors and the heart rate corrected QT interval in a real‐life setting: the population‐based Rotterdam Study. Br J Clin Pharmacol. 2015;80(4):698‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fisch C, Knoebel SB. Electrocardiographic findings in sertraline depression trials. Drug Investig. 1992;4(4):305‐312. [Google Scholar]
- 20. Park SI, An H, Kim A, Jang IJ, Yu KS, Chung JY. An analysis of QTc prolongation with atypical antipsychotic medications and selective serotonin reuptake inhibitors using a large ECG record database. Expert Opin Drug Saf. 2016;15(8):1013‐1019. [DOI] [PubMed] [Google Scholar]
- 21. Isbister GK, Bowe SJ, Dawson A, Whyte IM. Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose. J Toxicol Clin Toxicol. 2004;42(3):277‐285. [DOI] [PubMed] [Google Scholar]
- 22. Guidance for Industry E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non‐Antiarrhythmic Drugs. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073153.pdf. Published 2005. Accessed May 1, 2018.
- 23. Saletu B, Grunberger J, Linzmayer L. On central effects of serotonin re‐uptake inhibitors: quantitative EEG and psychometric studies with sertraline and zimelidine. J Neural Transm. 1986;67(3‐4):241‐266. [DOI] [PubMed] [Google Scholar]
- 24. Demolis JL, Angebaud P, Grange JD, Coates P, Funck‐Brentano C, Jaillon P. Influence of liver cirrhosis on sertraline pharmacokinetics. Br J Clin Pharmacol. 1996;42(3):394‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Preskorn SH, Lane RM. Sertraline 50 mg daily: the optimal dose in the treatment of depression. Int Clin Psychopharmacol. 1995;10(3):129‐141. [DOI] [PubMed] [Google Scholar]
- 26. DeVane CL, Liston HL, Markowitz JS. Clinical pharmacokinetics of sertraline. Clin Pharmacokinet. 2002;41(15):1247‐1266. [DOI] [PubMed] [Google Scholar]
- 27. Darpo B. The thorough QT/QTc study 4 years after the implementation of the ICH E14 guidance. Br J Pharmacol. 2010;159(1):49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Damle B, Labadie RR, Cuozzo C, et al. Lack of an effect of standard and supratherapeutic doses of linezolid on QTc interval prolongation. Antimicrob Agents Chemother. 2011;55(9):4302‐4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taubel J, Wong AH, Naseem A, Ferber G, Camm AJ. Shortening of the QT interval after food can be used to demonstrate assay sensitivity in thorough QT studies. J Clin Pharmacol. 2012;52(10):1558‐1565. [DOI] [PubMed] [Google Scholar]
- 30. Glassman AH, O'Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701‐709. [DOI] [PubMed] [Google Scholar]
- 31. Beach SR, Kostis WJ, Celano CM, et al. Meta‐analysis of selective serotonin reuptake inhibitor‐associated QTc prolongation. J Clin Psychiatry. 2014;75(5):e441‐e449. [DOI] [PubMed] [Google Scholar]
- 32. de Boer RA, van Dijk TH, Holman ND, van Melle JP. QT interval prolongation after sertraline overdose: a case report. BMC Emerg Med. 2005;5:1‐4. 10.1186/1471-227X-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ronfeld RA, Tremaine LM, Wilner KD. Pharmacokinetics of sertraline and its N‐demethyl metabolite in elderly and young male and female volunteers. Clin Pharmacokinet. 1997;32(Suppl 1):22‐30. [DOI] [PubMed] [Google Scholar]
- 34. Lynch DR Jr, Washam JB, Newby LK. QT interval prolongation and torsades de pointes in a patient undergoing treatment with vorinostat: a case report and review of the literature. Cardiol J. 2012;19(4):434‐438. [PubMed] [Google Scholar]
- 35. Khindri S, Sabo R, Harris S, Woessner R, Jennings S, Drollmann AF. Cardiac safety of indacaterol in healthy subjects: a randomized, multidose, placebo‐ and positive‐controlled, parallel‐group thorough QT study. BMC Pulm Med. 2011;11:1‐7. 10.1186/1471-2466-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klamerus KJ, Watsky E, Moller R, Wang R, Riley S. The effect of tafamidis on the QTc interval in healthy subjects. Br J Clin Pharmacol. 2015;79(6):918‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tatosian DA, Cardillo Marricco N, Glasgow XS, et al. A thorough QTc study confirms early pharmacokinetics/QTc modeling: a supratherapeutic dose of omarigliptin, a once‐weekly DPP‐4 inhibitor, does not prolong the QTc interval. Clin Pharmacol Drug Dev. 2016;5(5):383‐392. [DOI] [PubMed] [Google Scholar]
- 38. Zhao C, Lv Y, Li X, et al. Effects of nemonoxacin on thorough ECG QT/QTc interval: a randomized, placebo‐ and positive‐controlled crossover study in healthy Chinese adults. Clin Ther. 2018;40(6):983‐992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1
Additional supplemental information can be found by clicking the Supplements link in the PDF toolbar or the Supplemental Information section at the end of the web‐based version of this article.


