Abstract
Shati/Nat8l is a novel N‐acetyltransferase identified in the brain of mice treated with methamphetamine (METH). Shati/Nat8l mRNA is expressed in various brain areas, including the prefrontal cortex (PFC), where the expression level is higher than that in other brain regions. Shati/Nat8l overexpression in the nucleus accumbens (NAc) attenuates the pharmacological response to METH via mGluR3. Meanwhile, dopamine (DA) and glutamate dysregulations have been reported in the medial prefrontal cortex (mPFC) and NAc after METH self‐administration and during reinstatement. However, the mechanism, the reward system, and function of Shati/Nat8l in the mPFC is unclear. Here, we injected an adeno‐associated virus (AAV) vector containing Shati/Nat8l into the mPFC of mice, to overexpress Shati/Nat8l in the mPFC (mPFC‐Shati/Nat8l). Interestingly, the METH‐induced conditioned place preference (CPP) was attenuated in the mPFC‐Shati/Nat8l mice, but locomotor activity was not. Additionally, immunohistochemical results from mice that were injected with AAV‐GFP showed fluorescence in the mPFC and other brain regions, mainly the NAc, indicating an mPFC‐NAc top‐down connection. Finally, in vivo microdialysis experiments revealed that Shati/Nat8l overexpression in the mPFC reduced extracellular DA levels and suppressed the METH‐induced DA increase in the NAc. Moreover, decreased extracellular glutamate levels were observed in the NAc. These results indicate that Shati/Nat8l overexpression in the mPFC attenuates METH‐induced CPP by decreasing extracellular DA in the NAc. In contrast, Shati/Nat8l‐mPFC overexpression did not alter METH‐induced hyperlocomotion. This study demonstrates that Shati/Nat8l in the mPFC attenuates METH reward‐seeking behaviour but not the psychomotor activity of METH.
Keywords: dopamine, drug addiction, glutamate, mPFC, NAc, Shati/Nat8l
Shati/Nat8l overexpression in the medial prefrontal cortex attenuates methamphetamine‐induced reward behaviour by decreasing extracellular glutamate and dopamine levels in the nucleus accumbens. We consider that extracellular dopamine was reduced by the decrease of glutamate in the nucleus accumbens. Indeed, glutamate acts on medium spiny neurons that project to GABA interneurons in the ventral tegmental area. These interneurons inhibit the dopaminergic neurons projecting to the nucleus accumbens.
1. INTRODUCTION
Methamphetamine (METH) addiction is a serious neuropsychiatric disease that does not only reduce the quality of life of addicted people but also greatly affects their health.1 However, the currently available treatments for METH‐addiction are not fully effective.2 Indeed, patients may experience relapse even after a long period of abstinence.3 A novel N‐acetyltransferase, Shati/Nat8l, which generates N‐acetylsapartate from aspartate and acetyl‐CoA, has been identified in the nucleus accumbens (NAc) of mice following exposure to METH.4, 5, 6 Recent study has shown that Shati/Nat8l overexpression in the NAc attenuates the METH response.7 Shati/Nat8l mRNA is expressed in various brain regions, including the prefrontal cortex (PFC), which shows a higher level of Shati/Nat8l mRNA than other brain regions.8
The medial prefrontal cortex (mPFC) is a brain region that is considered to be fundamental for psychological processes and is implicated in several psychiatric disorders. The mPFC dynamically interacts with other cortical and subcortical regions.9 Thus, the mPFC is described as a control board that integrates information that is received from inputs, processes them and conveys them to other brain regions.10, 11
In mice experiments, METH induces hyperlocomotion and conditioned place preference (CPP). These behavioural alterations are mediated by an increase in the levels of DA in the NAc and striatum (STR).
After self‐administration of METH and during reinstatement, dopamine (DA) and glutamate dysregulation is observed in the mPFC and NAc of rats.12
It has also been established that both the mPFC and NAc receive DA projections from the ventral tegmental area (VTA), another key effector brain region in drug addiction. In addition, it is known that the level of DA in the NAc is considered to have an important role in motivation behaviour.13
Interestingly, a previous study that investigated the effects of cocaine reported that intra‐mPFC injection of cocaine reinstated the cocaine‐seeking behaviour that is blocked by an AMPAR antagonist.14 Many reports have demonstrated the involvement of glutamate in drug addiction through its action in the NAc.15 Glutamatergic neurons of the mPFC project to the NAc core and shell.16, 17
However, the mechanism underlying mPFC control over the reward system and the function of Shati/Nat8l in the mPFC is not clear. The purpose of this study is to clarify the function of Shati/Nat8l in the mPFC in METH addiction. We assessed the role of Shati/Nat8l overexpression in the mPFC using experimental models of METH addiction, including CPP and locomotor activity. Next, we investigated the mechanism underlying the behavioural changes by evaluating DA and glutamate in the NAc of Shati/Nat8l overexpressed mice.
2. MATERIALS AND METHODS
2.1. Animals and drugs
Male C57BL/6 J mice (8 weeks old; 22 to 27 g; Nihon SLC, Inc. Hamamatsu, Japan) were housed in a room with a 12‐hour light/dark cycle. Lights were turned on at 7 am (Zeitgeber time ZT 0) and turned off at 7 pm (ZT 12). All procedures followed the National Institute of Health Guideline for the Care and Use of Laboratory Animals and were approved by the Animal Experiments Committee of the University of Toyama (Permission Number A2015pha‐21 and A2018pha10). METH was purchased from Dainippon Sumitomo Pharmaceutical Co. Ltd. (Osaka, Japan) and was dissolved in saline (0.1 mg/mL). All other reagents were obtained from standard commercial sources.
2.2. AAV vector production and microinjection
The method used for the production and microinjection of AAV vector has been reported previously.18, 19 Briefly, the AAV vector plasmids contained an expression cassette that included a CMV promoter and cDNA encoding either 3′‐6xHis tagged Shati/Nat8l (NM_001001985) or EGFP. The recombinant AAV‐Shati/Nat8l or AAV‐Mock vectors were produced by cotransfection of the AAV plasmid, AAV3 rep/AAV9 vp expression plasmid, and pHelper (Agilent Technologies) in HEK293 cells. The study was approved by the Board of Safety Committee for Recombination DNA Experiments of the University of Toyama (G2015PHA‐12).
Mice were anesthetised with a combination of anaesthetics (medetomidine [0.3 mg/kg], midazolam [4.0 mg/kg], and butorphanol [5.0 mg/kg]) and were fixed in a stereotaxic frame (SR‐5M, Narishige, Tokyo, Japan). AAV9 CMV 6xHis Shati/Nat8l or CMV 6xHis GFP vector were injected (1010 to 1012 unit/0.7 μL/side for mPFC‐Shati/Nat8l and mPFC‐Mock, respectively). The suspension was injected bilaterally into the mPFC (AP 1.7; ML +/−0.3; DV 1.5) according to the mouse brain atlas.7, 20 The injection volume was set similarly to that used in previous studies.19 The injection rate was 0.05 μL/min, and the needle remained at rest at the injection site for 10 minutes after the end of the injection. Experiments were performed on mice 3 weeks after the injection procedure.
2.3. Quantitative RT‐PCR
Whole brains were removed and divided into 1‐mm‐thick sections using the mouse brain matrix. The tissue that corresponded to the mPFC was collected from the section. The accurate location of the brain structures was based on visual inspection of each section using a stereomicroscope and comparison with the stereotaxic atlas of mouse brain.20 The qRT‐PCR was conducted according to a previous report.7 The total RNA from the tissue sample was transcribed into cDNA using the Prime Script RT reagent kit (Takara, Otsu, Japan) according to the manufacturer's recommendation. Quantitative real‐time PCR was run in a Thermal Cycler Dice Real Time System (Takara) using Thunderbird Syber qPCR Mix (Toyobo, Osaka, Japan).
2.4. Immunohistochemistry
Using the floating method, sections of 50 μm were placed in 4% PFA for 20 minutes, washed with PBS and incubated with 0.25% Triton X‐100 for 15 minutes. Sections were treated with 10‐mM citrate buffer (pH 6.00) for antigen retrieval at 100°C for 3 minutes, washed with Tris buffered saline with Tween‐20 (TBS‐T), and then blocked in 10% goat serum for 1 hour. Sections were incubated with mouse antibody against GFP IgG (MBL) at 4°C overnight, washed with TBS‐T, and then incubated with CF488A goat anti‐mouse IgG (H + L) (Biotium, Hayward, CA) at room temperature for 2 hours. After being washed and mounted, sections were observed under a ZEISS fluorescence microscope, model BZ‐X700 (Keyence Co., Osaka, Japan)
2.5. Behavioural experiments
2.5.1. Locomotor activity
Locomotor activity was evaluated as in previous reports.7, 21 Briefly, mice were placed individually in a transparent acrylic cage with a black frosting Plexiglas floor (45 × 25 × 40 cm). Locomotor activity was measured every 5 minutes for 60 minutes using digital counters with infrared sensors (Scanet MV‐40; MELQEST, Japan). Mice were subcutaneously (s.c.) injected with METH (0.5 mg/kg) or saline immediately before the measurement of locomotor activity.
2.5.2. CPP test
The place conditioning test was performed according to a previously used method (Miyamoto et al., 201721;). Briefly, the apparatus was divided into two compartments (dark and light). The experiment was performed over three sessions, the first session consisted of a 3‐day habituation period. On the third day (preconditioning) the time spent in each compartment was measured using Scanet MV‐40 (MILQEST). The next day, the conditioning session was performed during which the mice were injected with METH (0.5 mg/kg) or saline (day 4 to 9). Finally, on day 10 (postconditioning session), the time spent in each compartment was measured in the same way as in the preconditioning session. In both CPP and locomotor activity experiments, mice were randomly tested in the interval time between ZT 2 and ZT 11.
2.6. In vivo microdialysis
2.6.1. DA measurement
In vivo microdialysis was performed as has been described previously (7, 21, 22 and 2017). The cannula was placed into the NAc shell (1.4‐mm anterior and 0.5‐mm lateral from the bregma, 3.2 mm below the skull surface) according to the atlas (Paxions and Franklin, 2008). The day after surgery, a dialysis probe (AI‐4‐1; 1‐mm membrane length, Eicom, Japan) was inserted through the guide cannula and perfused with a ringer's solution (147‐mM NaCl, 4‐mM KCl, and 2.3‐mM CaCl2) at a flow rate of 0.5 μL/min by a syringe pump (ESP‐64, EICOM). The dialysis probe was inserted from 9 am (ZT 2). DA was allowed to stabilize for about 3 hours. DA baseline levels were measured for 1 hour after stabilization.
2.6.2. METH stimulation
After DA baseline levels assessment, mice were injected with 0.5 mg/kg METH (s.c.) and the DA levels were measured during the 120 minutes after injection using in vivo microdialysis. DA standard was purchased from SIGMA (Switzerland).
2.6.3. Glutamate measurement
Extracellular glutamate levels were measured in the same position as DA microdialysis and according to the EICOM protocol with a GU‐GEL column and an enzymatic column at a flow rate of 1 μL/min. Glutamate standard was purchased from Sigma (France).
We used separate groups of mice to measure DA and glutamate extracellular levels. Both groups of mice were put in the same conditions.
In these experiments, we selected the dose of 0.5 mg/kg for METH treatment because we previously demonstrated that METH at the dose of 0.3 mg/kg was enough to induce significant CPP.5 Moreover, Kitanaka,23 used 0.5 mg/kg to induce significant CPP. Another group reported that METH administration at 0.5 mg/kg induce greater effect on CPP.24
2.7. Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). The statistical differences between the two groups were determined using Student's t tests. The statistical differences between the two groups after different drug administrations were determined by a two‐way analysis of variance (ANOVA), followed by the Bonferroni's post hoc tests when the F ratios were significant (P < 0.05). To analyse the development of in vivo microdialysis, statistical differences were determined by a two‐way ANOVA with repeated measurement, followed by the Bonferroni's post hoc tests (using Prism version 5).
3. RESULTS
3.1. Microinjection of AAV‐Shati/Nat8l vector enhanced the expression levels of Shati/Nat8l in the mPFC
Eight‐week old mice were divided into two groups; in one group, the mPFC was injected with AAV containing Shati/Nat8l and the other group the mPFC was injected with the GFP containing vector as a control group (mPFC‐Mock). This process was described in the methods section. Three weeks after the injections, the mice brains were collected and qRT‐PCR was performed for the Shati/Nat8l mRNA level, as compared with the house keeping gene 36B4 level. As expected, in the mPFC of the mice that were injected with the Shati/Nat8l AAV containing vector, the Shati/Nat8l mRNA level was more than nine‐fold (9.1+/−3.6) higher than in the Mock group (P < 0.05; Figure 1A).
Figure 1.
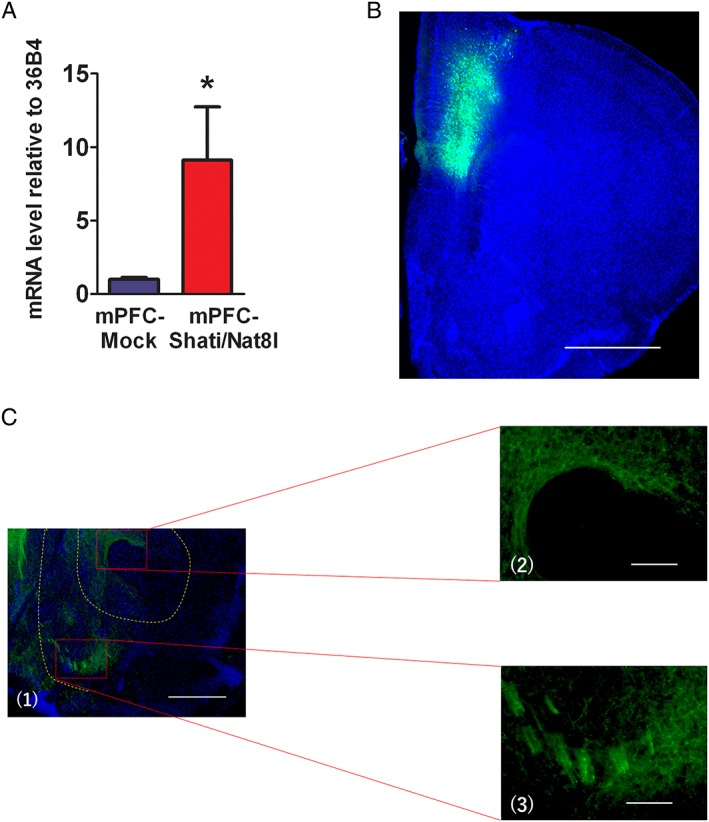
The effect of AAV‐Shati/Nat8l and AAV‐GFP vectors microinjection in the mPFC. (A) Shati/Nat8l mRNA level expression in the mPFC of Mock and Shati/Nat8l overexpressed mice. N = 5 and 4 (mPFC‐Mock and mPFC‐shati/Nat8l, respectively) *P < 0.05 (Student t test). (B, C) Tissue from the mice that were injected with the AAV‐GFP vector (mPFC‐Mock) were cut into 50‐μm coronal slices and stained with a GFP antibody; B, GFP is strongly expressed in the injected site of the mPFC; C, GFP staining can also be seen in the NAc. Scale bar in B: 1000 μm; C1: 500 μm; C2 and 3: 100 μm.
3.2. Microinjection of AAV‐GFP vector in the mPFC‐induced expression of GFP in both mPFC and NAc
Next, we attempted to confirm the injection site of the AAV vectors in the mPFC through immunostaining. The immunohistochemical results following the injection of the AAV vector containing GFP into the mPFC (mPFC‐Mock) showed strong green staining in the injection site (Figure 1B). This result confirmed the location of the injection site. Additionally, we evaluated the expression of GFP in the NAc, because the NAc has a key role in drug addiction. As expected, green fluorescence was also clearly visible in the NAc (Figure 1C). According to these results, the mPFC is directly connected to the NAc. This connection suggests a possible influence of the mPFC on the NAc.
3.3. Overexpression of Shati/Nat8l in the mPFC‐attenuated METH‐induced CPP
Next, we tried to understand the role of Shati/Nat8l in the mPFC in METH drug dependence. For this purpose, we conducted a CPP test on the mPFC‐Shati/Nat8l and mPFC‐Mock mice, using a protocol that was described in the methods section and is outlined in Figure 2A. In this experiment and during the conditioning session, mice were injected with 0.5 mg/kg of METH or saline immediately before the beginning of the experiment. In the postconditioning session, CPP was induced in the mPFC‐Mock group that were treated with METH (251.6+/−37.1 s). Interestingly, Shati/Nat8l overexpression in the mPFC significantly decreased the pre‐post value in CPP (51.3+/−36.6 s). This means that the preference for METH was attenuated (Figure 2B): F Interaction(1,49) = 7.96; P < 0.01, F Genotype(1,49) = 4.596; P < 0.05, F Drug(1,49) = 1.249; P > 0.05.
Figure 2.
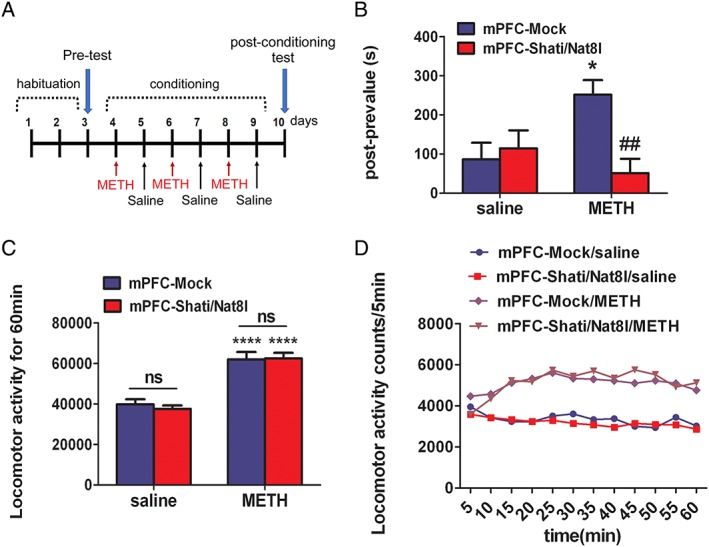
Effect of overexpression of Shati/Nat8l in the mPFC on METH‐induced behavioral alterations. A, CPP paradigm protocol; mice received METH (0.5 mg/kg s.c.) or saline injections immediately before exposure to one of the light/dark box compartments. B, In mPFC‐Mock mice and mPFC‐Shati/Nat8l mice, animals were trained for place preference with METH. Place preference data are expressed as the proportion of time spent in the drug‐paired conditioning compartment. Columns indicate means ± S.E.M.; N = 13 (Mock saline), 13 (Mock METH), 15 (Shati/Nat8l saline), and 15 (Shati/Nat8l METH). *P < 0.05 vs saline group, ## P < 0.01 vs mPFC‐Mock (METH) (two‐way ANOVA followed by the Bonferroni's post hoc test). (B and C) Locomotor activities in mPFC‐Mock mice and mPFC‐Shati/Nat8l mice were measured for 60 min after METH administration (0.5 mg/kg, s.c.). Columns indicate means ± S.E.M.; N = 8 (mPFC‐Mock saline), 9 (mPFC‐Mock METH), 9 (mPFC‐Shati/Nat8l saline), and 10 (mPFC‐Shati/Nat8l METH); ****P < 0.0001 vs mPFC‐Mock (saline); ns P > 0.05 (nonsignificant) vs mPFC‐Mock (METH) (two‐way ANOVA followed by the Bonferroni's post hoc test).
3.4. Overexpression of Shati/Nat8l in the mPFC had no effect on METH‐induced hyperlocomotion
After evaluating the CPP, we assessed the role of Shati/Nat8l in METH induced hyperlocomotion. A locomotor activity test was conducted using the same dose of METH as in the previous experiment (0.5 mg/kg). The locomotor activity was significantly higher in the METH group than in the saline group in both the mPFC‐Mock and the mPFC‐Shati/Nat8l groups (61934.89+/−3666.723 and 62461.3+/−3123.833 counts/1 h, respectively). However, the mPFC‐Shati/Nat8l did not attenuate the METH‐induced psychomotor activity (Figure 2C and 2D). F Interaction(1,32) = 0.23; P > 0.05, F Genotype(1,32) = 0.08; P > 0.05, F drug(1,32) = 70.36; P < 0.0001. In these experiments, Shati/Nat8l attenuated the METH‐induced CPP but had no effect on the METH‐induced hyperlocomotion.
3.5. Overexpression of Shati/Nat8l in the mPFC reduced the basal levels of extracellular DA and attenuated METH‐induced elevation of extracellular DA levels
To clarify the mechanism through which Shati/Nat8l overexpression in the mPFC affects the METH‐induced CPP, we performed a microdialysis of DA in the NAc. We first measured the baseline DA levels, followed by a single s.c. injection of METH (0.5 mg/g; the same dose as was used in the behavioural experiments). The DA levels were measured for 2 hours after the injection. Interestingly, the DA baseline levels in the mPFC‐Shati/Nat8l mice were 50% lower than those in the Mock group (Figure 3A). The extracellular DA levels in the mPFC‐Mock and mPFC‐Shati/Nat8l groups were 0.49+/−0.07 pg/7.5 μL and 0.25+/−0.05 pg/7.5 μL, respectively.
Figure 3.
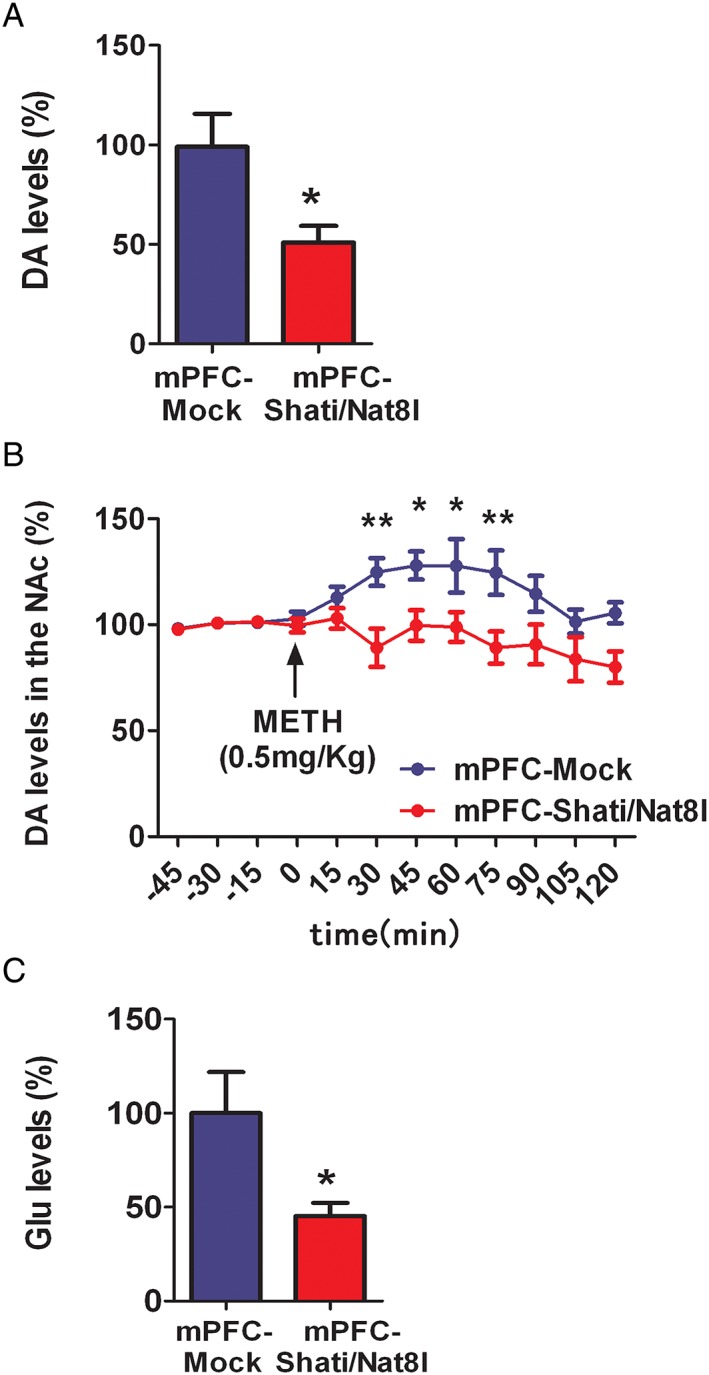
Effect of overexpression of Shati/Nat8l in the NAc on basal levels of extracellular DA, glutamate and METH‐induced elevation of DA. A, Basal levels of extracellular DA in the NAc of mPFC‐Mock and mPFC‐Shati/Nat8l. N = 10 (mPFC‐Mock) and 8 (mPFC‐Shati/Nat8l). *P < 0.05 vs. Mock group (Student t test). B, METH‐induced elevation of extracellular DA levels in the NAc of mPFC‐Mock and mPFC‐Shati/Nat8l mice. N = 10 (mPFC‐Mock) and 8 (mPFC‐Shati/Nat8l). *p < 0.05 and **P < 0.01 vs. METH‐treated Mock group (ANOVA with repeated measures followed by the Bonferroni's post‐hoc test). (C) In vivo microdialysis of glutamate in the NAc of mPFC‐Mock vs mPFC‐Shati/Nat8l. N = 9 (mPFC‐Mock) and 7 (mPFC‐Shati/Nat8l). *p < 0.05 (Student t test).
Additionally, the increase in the level of DA after METH stimulation was also significantly alleviated in mPFC‐Shati/Nat8l group (Figure 3B) F Interaction(11,204) = 2.23; P < 0.05, F Genotype(11,204) = 40.64; P < 0.0001, F time(11,204) = 2.15; P < 0.05. These results suggest that the mPFC not only effectively controls the NAc but that this effect could be mediated by Shati/Nat8l.
3.6. Overexpression of Shati/Nat8l in the mPFC reduced the basal levels of extracellular glutamate from NAc
To understand how Shati/Nat8l in the mPFC lowered the level of DA in the NAc, we measured the levels of glutamate in the NAc using in vivo microdialysis, targeting the same point that was targeted in the DA measurement. Interestingly, the microdialysis also showed that the extracellular glutamate levels in the NAc in the mPFC‐Shati/Nat8l mice (glutamate level was 45.32%) were significantly lower than in the mPFC‐Mock group (Figure 3C). Extracellular glutamate levels for mPFC‐Mock and mPFC‐Shati/Nat8l were 0.64+/−0.13 pmol/10 μL and 0.29+/−0.04 pmol/10 μL, respectively.
4. DISCUSSION
Shati/Nat8l is a novel molecule that has been proven to attenuate the METH response in mice. However, little is known about the mechanism that underlies this effect or the involvement of the mPFC in addiction. In this study, we demonstrated that Shati/Nat8l in the mPFC attenuated METH‐induced CPP through a top down control from the mPFC to the NAc, by decreasing the DA levels in the NAc and, thus, reducing the METH‐induced DA increase.
As has been previously described,7 we injected an AAV vector in to the mPFC to overexpress Shati/Nat8l. After 3 weeks, we observed a significantly higher expression of Shati/Nat8l in the mPFC of mice that were injected with the AAV‐Shati/Nat8l vector than in the Mock group (Figure 1A). This confirmed the effectiveness of the AAV‐Shati/Nat8l vector. Neurons from the mPFC project to different brain regions that are involved in reward, including the NAc. The immunohistochemical results of this study revealed the projections from the mPFC to the NAc (Figure 1B and 1C).
In the behavioural experiments, METH‐induced CPP was attenuated by Shati/Nat8l overexpression (Figure 2B). Hyperlocomotion is also induced by METH, as was demonstrated in the locomotor activity test. However, Shati/Nat8l overexpression in the mPFC had no effect on the psychomotor activity following exposure to METH (Figure 2C).
The discrepancy in these responses might be explained by the difference between the reward and motor systems, which are distinct entities that have different pathways and mechanisms. This distinction was reported by Kelley and colleagues,25 who used NMDA blockade to separate the reward and locomotor activity in a nonlearning context. Effectively, reward is mainly controlled by the NAc, while psychomotor activity is controlled by the STR.26 Additionally, DA terminals in the NAc shell and dorsolateral STR originate mainly from the VTA and substantia nigra, pars compacta, respectively.27 This theory is also supported by nicotine addiction experiments. Nicotine has been reported to differentially influence DA signalling in the dorsal STR and NAc shell.28 Furthermore, another report demonstrated that systemic treatment with ceftriaxone, to upregulate the expression of the excitatory amino acid transporter 2, a glutamate transporter, effectively blocked METH‐induced CPP but did not alter basal locomotor activity.29 Other studies have shown that the NAc shell is mainly related to the reward system, whereas the core is related to psychomotor activity.30
Time of the day (ZT 6‐8 vs ZT 19‐21) may affect methamphetamine‐induced CPP as observed in C3H/HeN mice.31 The latter showed that methamphetamine induced CPP during the day but not the night. Furthermore, deletion of melatonin (a hormone produced in the pineal gland) MT1 or MT2 receptors abolished methamphetamine induced‐CPP. In addition, serum and pineal melatonin vary during the day with a maximum during the dark part of the day (ZT 14 and ZT 22 respectively) as assessed in C3H/HeN mice.32 This suggests that methamphetamine‐induced CPP may be affected by melatonin expression. It is also shown that melatonin is involved in facilitating methamphetamine‐induced sensitization in C57BL/6 mice.33 Indeed, during the night, melatonin levels are higher suggesting that melatonin receptor activity is needed for methamphetamine‐induced sensitization instead of melatonin expression, since higher expression promote desensitization of receptors.31, 33 However, in our study we conducted both CPP and LA experiments in the light part of the day between 9 am to 6 pm (ZT 2‐11) on C57BL/6J mice as previously discussed in the material and methods section and performed these behavior experiments more than three times.
In our study, METH induced a psychostimulant effect by blocking the DA transporter, thus decreasing the reuptake of DA to the intracellular space and increasing the levels of DA in the synaptic cleft. Our results demonstrate that Shati/Nat8l overexpression in the mPFC decreases the baseline DA levels in the NAc shell (Figure 3B). This suggests that the mPFC has an important role in the regulation of DA from the NAc. The top‐down control of the NAc by the mPFC is not a new concept. However, in this study, we demonstrated that the mPFC can regulate the extracellular DA level in the NAc. Additionally, we demonstrated that Shati/Nat8l in the mPFC is able to decrease the DA levels in the NAc. Moreover, Shati/Nat8l overexpression diminishes the METH‐induced DA increase in the NAc (Figure 3C). The decrease in the DA level in the NAc is responsible for the reduction in the addictive effects of METH. This decrease in the DA level is caused by a modification in the mPFC that is mediated by Shati/Nat8l. This suggests that the projections from the mPFC to the NAc are responsible for this alteration, and as shown through in vivo microdialysis of glutamate in the NAc shell, a decrease in the glutamate baseline level was observed (Figure 3D). In other words, the mechanism underlying the Shati/Nat8l‐induced decrease in DA levels and the METH‐induced DA increase are probably because of the decrease in glutamate levels in the NAc. Indeed, several studies have reported that the inhibition of glutamate receptors has an attenuating effect on some drugs of addiction.34, 35 Another report assessed the modulating effect of mGluR group II on DA release.36
We are considering that the effects of Shati/Nat8l on the reduction of dopamine and glutamate is strongly related to the enzymatic activity of Shati/Nat8l via NAAG and mGluR2/3.37 Activation of presynaptic mGluR2/3 on glutamatergic terminals in the NAc by NAAG could decrease glutamate levels and in turn reduce dopamine in the NAc. We previously assessed that mGluR2/3 antagonist, LY341495, prevented action of Shati/Nat8l on the increased dopamine induced by methamphetamine.7 Effectively, Furthermore, intra‐NAc perfusion of NAAG reduced basic and potentiated (with methamphetamine) dopamine level.
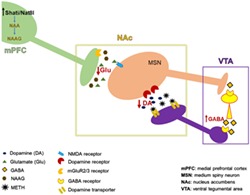
The hypothesised mechanism of Shati/Nat8l action on METH addiction through top‐down control of the mPFC is displayed in Figure 4. Shati/Nat8l in the mPFC decreases the level of glutamate in the NAc as shown in this paper, which acts on MSN neurons that projects to VTA GABAergic interneurons.38 It has been established that the activation of MSNs leads to the inhibition of VTA GABA cells and, consequently, disinhibition of DA neurons.39 Thus, reducing MSNs activation will increase VTA GABA interneurons activity, in turn, boosting the inhibitory action on dopaminergic neurons that project to the NAc. Finally, this leads to a decrease in the DA levels in the NAc.
Figure 4.
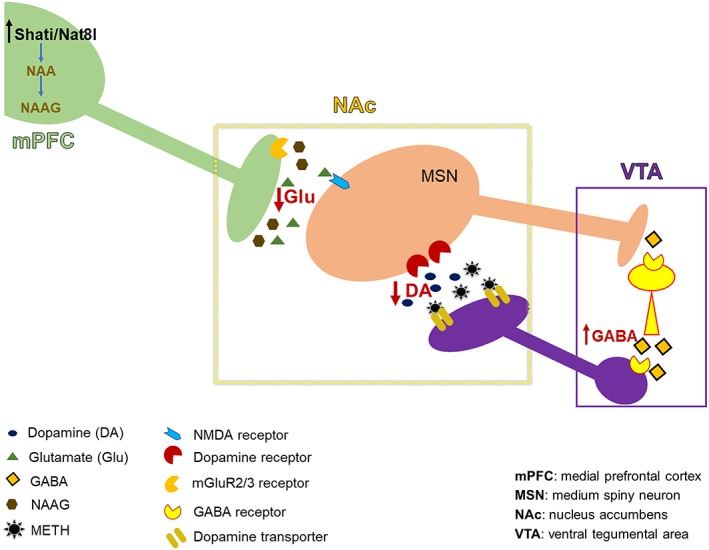
Hypothesised mechanism of action of Shati/Nat8l in the mPFC during establishment of METH dependence. Shati/Nat8l overexpression in the mPFC induces a decrease in the glutamate level in the NAc. Consequently, there is a decrease in the stimulation of MSN GABAergic neurons that project to the VTA GABAergic interneurons. This induces an increase in the inhibitory effect of VTA GABAergic interneurons on the dopaminergic neurons that project to the NAc. Finally, the DA level in the NAc is decreased.
In this study, we demonstrated that the mPFC affects the NAc via glutamatergic projections that can alter METH addiction. Furthermore, this effect is mediated by Shati/Nat8l, which reduces extracellular glutamate in the NAc and, consequently, decreases the DA levels. This mechanism of action is responsible for the attenuation of the METH‐induced CPP and DA increase but not METH‐induced locomotor activity.
AUTHORS CONTRIBUTION
M.H., K.U., and A.N. were responsible for the study concept and design. M.H. and K.A. contributed to the acquisition of data for behavioral experiments and in vivo microdialysis. K.U. and S.M. designed and produced AAV vectors, respectively. K.U. performed the proteomics analysis. M.H. assisted with data analysis and interpretation of findings. M.H. drafted the manuscript. A.N. provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
ACKNOWLEDGEMENTS
We thank Naomi Takino and Mika Ito for technical assistance in producing the Shati/Mat8l AAV vectors.
Haddar M, Uno K, Azuma K, Muramatsu S, Nitta A. Inhibitory effects of Shati/Nat8l overexpression in the medial prefrontal cortex on methamphetamine‐induced conditioned place preference in mice. Addiction Biology. 2020;25:e12749 10.1111/adb.12749.
REFERENCES
- 1. Grant KM, LeVan TD, Wells SM, et al. Methamphetamine‐associated psychosis. J Neuroimmune Pharmacol. 2012;7:113‐139. 10.1007/s11481-011-9288-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Courtney KE, Ray LA. Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend. 2014;143:11‐21. 10.1016/j.drugalcdep.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brecht ML, Herbeck D. Time to relapse following treatment for methamphetamine use: a long‐term perspective on patterns and predictors. Drug Alcohol Depend. 2014;139:18‐25. 10.1016/j.drugalcdep.2014.02.702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ariyannur PS, Moffett JR, Manickam P, et al. Methamphetamine‐induced neuronal protein NAT8L is the NAA biosynthetic enzyme: implications for specialized acetyl coenzyme A metabolism in the brain. Brain Res. 2010;1335:1‐13. 10.1016/j.brainres.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 5. Niwa M, Nitta A, Mizoguchi H, et al. A novel molecule “shati” is involved in methamphetamine‐induced hyperlocomotion, sensitization, and conditioned place preference. J Neurosci. 2007;27:7604‐7615. 10.1523/JNEUROSCI.1575-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wiame E, Tyteca D, Pierrot N, et al. Molecular identification of aspartate N‐acetyltransferase and its mutation in hypoacetylaspartia. Biochem J. 2010;425:127‐136. 10.1042/BJ20091024 [DOI] [PubMed] [Google Scholar]
- 7. Miyamoto Y, Ishikawa Y, Iegaki N, et al. Overexpression of Shati/Nat8l, an N‐acetyltransferase, in the nucleus accumbens attenuates the response to methamphetamine via activation of group II mGluRs in mice. Int J Neuropsychopharmacol. 2014;17:1283‐1294. 10.1017/S146114571400011X [DOI] [PubMed] [Google Scholar]
- 8. Furukawa‐Hibi Y, Nitta A, Fukumitsu H, et al. Absence of SHATI/Nat8l reduces social interaction in mice. Neurosci Lett. 2012;526:79‐84. 10.1016/j.neulet.2012.08.028 [DOI] [PubMed] [Google Scholar]
- 9. Groenewegen HJ, Wright CI, Uylings HB. The anatomical relationships of the prefrontal cortex with limbic structures and the basal ganglia. J Psychopharmacol. 1997;11:99‐106. 10.1177/026988119701100202 [DOI] [PubMed] [Google Scholar]
- 10. Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167‐202. 10.1177/026988119701100202 [DOI] [PubMed] [Google Scholar]
- 11. Riga D, Matos MR, Glas A, Smit AB, Spijker S, Van den Oever MC. Optogenetic dissection of medial prefrontal cortex circuitry. Front Syst Neurosci. 2014;8:230 10.3389/fnsys.2014.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parsegian A, See RE. Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self‐administration and during reinstatement in rats. Neuropsychopharmacology. 2014;39:811‐822. 10.1038/npp.2013.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309‐369. 10.1016/S0165-0173(98)00019-8 [DOI] [PubMed] [Google Scholar]
- 14. Park WK, Bari AA, Jey AR, et al. Cocaine administered into the medial prefrontal cortex reinstates cocaine‐seeking behavior by increasing AMPA receptor‐mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916‐2925. 10.1523/JNEUROSCI.22-07-02916.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scofield MD, Heinsbroek JA, Gipson CD, et al. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol Rev. 2016;68(3):816‐871. 10.1124/pr.116.012484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790‐803. 10.1016/j.neuron.2012.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suska A, Lee BR, Huang YH, Dong Y, Schlüter OM. Selective presynaptic enhancement of the prefrontal cortex to nucleus accumbens pathway by cocaine. Proc Natl Acad Sci U S A. 2013;110:713‐718. 10.1073/pnas.1206287110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iida A, Takino N, Miyauchi H, Shimazaki K, Muramatsu S. Systemic delivery of tyrosine‐mutant AAV vectors results in robust transduction of neurons in adult mice. Biomed Res Int. 2013;2013:974819 10.1155/2013/974819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krzyzosiak A, Szyszka‐Niagolov M, Wietrzych M, Gobaille S, Muramatsu S, Krezel W. Retinoid x receptor gamma control of affective behaviors involves dopaminergic signaling in mice. Neuron. 2010;66:908‐920. 10.1016/j.neuron.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 20. Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Compact. 3rd ed. Amsterdam: Elsevier; 2008. [Google Scholar]
- 21. Fu K, Lin H, Miyamoto Y, et al. Pseudoginsenoside‐F11 inhibits methamphetamine‐induced behaviors by regulating dopaminergic and GABAergic neurons in the nucleus accumbens. Psychopharmacology. 2016;233:831‐840. 10.1007/s00213-015-4159-8 [DOI] [PubMed] [Google Scholar]
- 22. Fu K, Miyamoto Y, Otake K, et al. Involvement of the accumbal osteopontin‐interacting transmembrane protein 168 in methamphetamine‐induced place preference and hyperlocomotion in mice. Sci Rep. 2017;7:13084 10.1038/s41598-017-13289-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kitanaka N, Kitanaka J, Tatsuta T, Watabe K, Morita Y, Takemura M. Methamphetamine reward in mice as assessed by conditioned place preference test with Supermex sensors: effect of subchronic clorgyline pretreatment. Neurochem Res. 2006;31:805‐813. 10.1007/s11064-006-9081-3 [DOI] [PubMed] [Google Scholar]
- 24. Shabani S, McKinnon CS, Reed C, Cunningham CL, Phillips TJ. Sensitivity to rewarding or aversive effects of methamphetamine determines methamphetamine intake. Genes Brain Behav. 2011;10:625‐636. 10.1111/j.1601-183X.2011.00700.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelley AE, Smith‐Roe SL, Holahan MR. Response‐reinforcement learning is dependent on N‐methyl‐d‐aspartate receptor activation in the nucleus accumbens core. Proc Natl Acad Sci. 1997;94:12174‐12179. 10.1073/pnas.94.22.12174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goodwin JS, Larson GA, Swant J, et al. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem. 2009;284:2978‐2989. 10.1074/jbc.M805298200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369‐2382. 10.1523/JNEUROSCI.20-06-02369.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang T, Zhang L, Liang Y, Siapas AG, Zhou FM, Dani JA. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J Neurosci. 2009;29:4035‐4043. 10.1523/JNEUROSCI.0261-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abulseoud OA, Miller JD, Wu J, Choi DS, Holschneider DP. Ceftriaxone upregulates the glutamate transporter in medial prefrontal cortex and blocks reinstatement of methamphetamine seeking in a condition place preference paradigm. Brain Res. 2012;1456:14‐21. 10.1016/j.brainres.2012.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sellings LHL, McQuade LE, Clarke PBS. Evidence for multiple sites within rat ventral striatum mediating cocaine‐conditioned place preference and locomotor activation. J Pharmacol Exp Ther. 2001;317:1178 http://jpet.aspetjournals.org/content/317/3/1178 [DOI] [PubMed] [Google Scholar]
- 31. Clough SJ, Hutchinson AJ, Hudson RL, Dubocovich ML. Genetic deletion of the MT1 or MT2 melatonin receptors abrogates methamphetamine‐induced reward in C3H/HeN mice. Physiol Behav. 2014;132:79‐86. 10.1016/j.physbeh.2014.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Masana MI, Benloucif S, Dubocovich ML. Circadian rhythm of mt1 melatonin receptor expression in the suprachiasmatic nucleus of the C3H/HeN mouse. J Pineal Res. 2000;28:185‐192. 10.1034/j.1600-079X.2001.280309.x [DOI] [PubMed] [Google Scholar]
- 33. Hutchinson AJ, Ma J, Liu J, Hudson RL, Dubocovich ML. Role of MT1 melatonin receptors in methamphetamine‐induced locomotor sensitization in C57BL/6 mice. Psychopharmacology. 2013;231:257‐267. 10.1007/s00213-013-3228-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allen RM, Carelli RM, Dykstra LA, Suchey TL, Everett CV. Effects of the competitive N‐methyl‐D‐aspartate receptor antagonist, LY235959 [(‐)‐6‐phosphonomethyl‐deca‐hydroisoquinoline‐3‐carboxylic acid], on responding for cocaine under both fixed and progressive ratio schedules of reinforcement. J Pharmacol Exp Ther. 2005;315:449‐457. 10.1124/jpet.105.086355 [DOI] [PubMed] [Google Scholar]
- 35. Pierce RC, Meil WM, Kalivas PW. The NMDA antagonist, dizocilpine, enhances cocaine reinforcement without influencing mesoaccumbens dopamine transmission. Psychopharmacology. 1997;133:188‐195. 10.1007/s002130050390 [DOI] [PubMed] [Google Scholar]
- 36. Chaki S, Yoshikawa R, Okuyama S. Group II metabotropic glutamate receptor‐mediated regulation of dopamine release from slices of rat nucleus accumbens. Neurosci Lett. 2006;404:182‐186. 10.1016/j.neulet.2006.05.043 [DOI] [PubMed] [Google Scholar]
- 37. Wroblewska B, Wrobleski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH. N‐acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem. 1997;69:174‐181. 10.1046/j.1471-4159.1997.69010174.x [DOI] [PubMed] [Google Scholar]
- 38. Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO. Nucleus accumbens medium spiny neurons target non‐dopaminergic neurons in the ventral tegmental area. J Neurosci. 2011;31:7811‐7816. 10.1523/JNEUROSCI.1504-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bocklisch C, Pascoli V, Wong JCY, et al. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science. 2013;341:1521‐1525. http://science.sciencemag.org/content/341/6153/1521 [DOI] [PubMed] [Google Scholar]


