Summary
Analgesic protocols used to treat pain after breast surgery vary significantly. The aim of this systematic review was to evaluate the available literature on this topic and develop recommendations for optimal pain management after oncological breast surgery. A systematic review using preferred reporting items for systematic reviews and meta‐analysis guidance with procedure‐specific postoperative pain management (PROSPECT) methodology was undertaken. Randomised controlled trials assessing postoperative pain using analgesic, anaesthetic or surgical interventions were identified. Seven hundred and forty‐nine studies were found, of which 53 randomised controlled trials and nine meta‐analyses met the inclusion criteria and were included in this review. Quantitative analysis suggests that dexamethasone and gabapentin reduced postoperative pain. The use of paravertebral blocks also reduced postoperative pain scores, analgesia consumption and the incidence of postoperative nausea and vomiting. Intra‐operative opioid requirements were documented to be lower when a pectoral nerves block was performed, which also reduced postoperative pain scores and opioid consumption. We recommend basic analgesics (i.e. paracetamol and non‐steroidal anti‐inflammatory drugs) administered pre‐operatively or intra‐operatively and continued postoperatively. In addition, pre‐operative gabapentin and dexamethasone are also recommended. In major breast surgery, a regional anaesthetic technique such as paravertebral block or pectoral nerves block and/or local anaesthetic wound infiltration may be considered for additional pain relief. Paravertebral block may be continued postoperatively using catheter techniques. Opioids should be reserved as rescue analgesics in the postoperative period. Research is needed to evaluate the role of novel regional analgesic techniques such as erector spinae plane or retrolaminar plane blocks combined with basic analgesics in an enhanced recovery setting.
Keywords: analgesia, breast surgery, evidence‐based medicine, pain, systematic review
Recommendations
Systemic analgesia should include paracetamol and non‐steroidal anti‐inflammatory drugs (NSAID) administered pre‐operatively or intra‐operatively and continued postoperatively.
Pre‐operative gabapentin is recommended.
A single dose of intravenous (i.v.) dexamethasone is recommended for its ability to increase the analgesic duration of peripheral nerve blocks, decrease analgesia use and anti‐emetic effects.
Opioids should be reserved as rescue analgesia in the postoperative period.
Paravertebral blockade is recommended as the first‐choice regional analgesic technique. Pectoral nerves block may be used as an alternative to paravertebral block. Local anaesthetic wound infiltration may be added to regional analgesia techniques.
Why was this guideline developed?
Oncological breast surgery is associated with significant acute and chronic postoperative pain. The aim of this guideline is to provide clinicians with an evidence‐based approach to pain management after oncological breast surgery, which may improve postoperative pain relief.
What other guidelines are available on this topic?
A systematic review was performed by the PROSPECT collaboration in 2006; however, several new analgesic regimens, particularly regional analgesia techniques have been introduced since the previous systematic review.
How does this guideline differ from other guidelines?
The procedure‐specific postoperative pain management (PROSPECT) approach to developing guidelines is unique such that the available evidence is critically assessed for current clinical relevance. This approach reports true clinical effectiveness by balancing the invasiveness of the analgesic interventions and the degree of pain after surgery, as well as balancing efficacy and adverse effects.
Introduction
Oncological breast surgery, hereafter simply referred to as breast surgery, is associated with significant acute and chronic postoperative pain 1. A systematic review of analgesic strategies was performed in 2006 2, however, several new analgesic regimens, particularly regional analgesic techniques (e.g. pectoral nerves and erector spinae plane blocks) have been introduced since 3. An updated systematic review on analgesic interventions dedicated to breast surgery was consequently required.
The PROSPECT Working Group is a collaboration of surgeons and anaesthetists working to formulate procedure‐specific recommendations for pain management after common but potentially painful operations 4, 5.The recommendations are based on a procedure‐specific systematic review of randomised controlled trials (RCT). The methodology considers clinical practice, efficacy and adverse effects of analgesic techniques 6.
The aim of this systematic review was to evaluate the available literature on the effects of analgesic, anaesthetic and surgical interventions on pain after breast surgery. The primary outcome included postoperative pain scores. Other recovery outcomes, including opioid requirements and adverse effects, were also assessed when reported and the limitations of the data were reviewed. The ultimate aim was to develop recommendations for pain management after oncological breast surgery.
Methods
The methods of this review adhered to the PROSPECT methodology as previously reported 7. Specific to this study, the EMBASE, MEDLINE, PubMed and Cochrane Databases (Cochrane Central Register of Controlled Trials, Cochrane Database of Abstracts or Reviews of Effects, Cochrane Database of Systematic Reviews) were searched for RCTs published between 31 May 2006 and 15 October 2019. Search terms related to pain and interventions for radical mastectomy OR mastectomy OR mammectomy OR lumpectomy OR axillary node dissection OR axillary node clearance OR wedge resection OR skin‐sparing mastectomy OR breast reconstruction OR implant reconstruction OR breast surgery AND pain OR analgesi* OR anaesthe* OR vas OR visual analog* OR VRS OR McGill OR epidural OR neuraxial OR intrathecal OR spinal OR caudal OR peripheral nerve OR peripheral block OR regional nerve OR paravertebral block OR intercostal nerve OR infiltration OR instillation OR NSAID OR COX‐2 OR paracetamol OR acetaminophen OR gabapentin OR pregabalin OR clonidine OR opioid OR ketamine OR corticosteroid OR dexamethasone OR patient controlled analgesia OR PCA OR PEC* block OR serratus block.
Quality assessment, data extraction and data analysis adhered to the PROSPECT methodology 7. Studies that reported pooled data from patients undergoing mixed procedures of cancer and non‐cancer breast surgery were excluded. Pain intensity scores were used as the primary outcome measure. In this study, we defined a change of more than 10 mm on the visual analogue scale (VAS) or numerical rating score (NRS) as clinically‐relevant 8. The effectiveness of each intervention for each outcome was evaluated qualitatively by assessing the number of studies showing a significant difference between treatment arms as reported in the study publication. A meta‐analysis was not performed due to heterogeneity in study design and result reporting, restricting pooled analysis.
Recommendations were made according to PROSPECT methodology 7. In brief, this involved a grading of A–D according to the overall level of evidence, as determined by the quality of studies included, consistency of evidence and study design. The proposed recommendations were sent to the PROSPECT Working Group for review and comments and a modified Delphi approach was utilised as previously described. Once a consensus was achieved, the lead authors drafted the final document, which was ultimately approved by the Working Group.
Results
The preferred reporting items for systematic review and meta‐analysis (PRISMA) flow chart demonstrating the search data is presented in Fig. 1. The methodological quality assessments of the 53 RCTs and nine meta‐analyses included for the final qualitative analysis are summarised in Table S1. The characteristics of the included studies are shown in Table S2.
Figure 1.
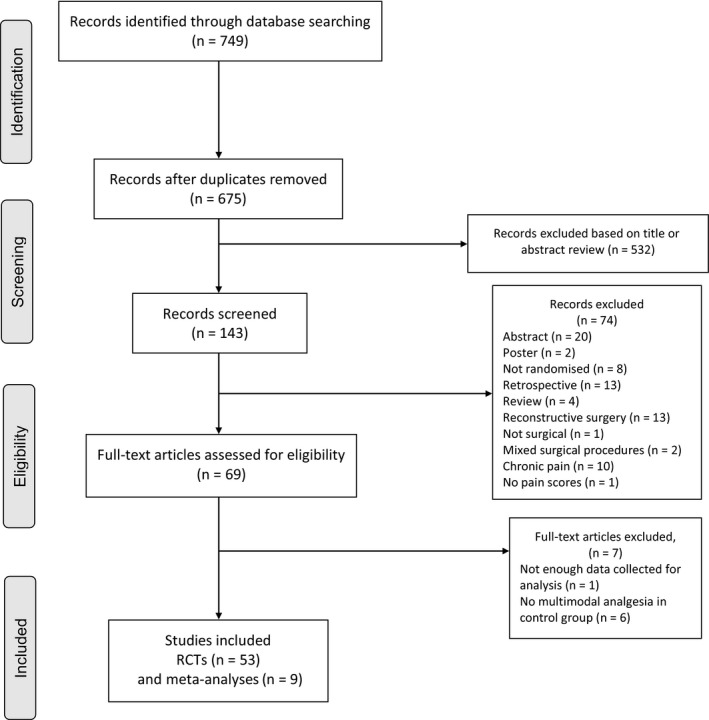
Flow diagram of studies included in this systematic review.
Pre‐operative interventions
Two meta‐analyses of parallel group, placebo‐controlled RCTs have evaluated the efficacy of the pre‐operative use of gabapentin for the treatment of acute and chronic postoperative pain following breast cancer surgery 9, 10. The meta‐analysis by Rai et al. 10 included all the studies of the meta‐analysis by Jiang et al. 9 but also included one study concerning gabapentin (516 patients) and four studies concerning pregabalin (209 patients). Doses of gabapentin ranged between 300 mg and 1200 mg. Doses of pregabalin ranged between 150 mg and 900 mg. Gabapentin reduced pain scores in the post‐anaesthetic care unit (PACU) and 24 h postoperatively 9, 10. Rai et al. also reported that six studies demonstrated that gabapentin also significantly reduced 24‐h morphine consumption. A sub‐group analysis was conducted according to the dose of gabapentin (< 900 mg or ≥ 900 mg daily) and documented that a high dose of gabapentin (> 900 mg daily) was superior in terms of pain scores 9. Rai et al. demonstrated that pre‐operative pregabalin reduced pain scores and morphine consumption in the PACU 10. However, no significant difference was observed in pain scores at 24 h with the addition of pregabalin 10. No significant differences in gabapentin‐related side‐effects were observed. The incidence of sedation was increased with pregabalin but not that of blurred vision or dizziness 9, 10.
Intra‐operative Interventions
Two placebo‐controlled studies have investigated the effect of systemic dexamethasone on postoperative pain, nausea and vomiting after conservative oncologic breast surgery and mastectomy 11, 12. Both studies reported similar results. In particular, patients receiving i.v. dexamethasone had significantly less pain up to 24 h after surgery. Rescue analgesics were required in more patients in the control group than in the dexamethasone groups. Intravenous dexamethasone 8 mg given 1 h before surgery also reduced postoperative nausea and vomiting at 6 h postoperatively. Consequently, the frequency of use of anti‐emetic medications was higher in the control groups.
Postoperative interventions
In 39 out of the 62 included studies and meta‐analyses, paracetamol, NSAIDs or cyclo‐oxygenase‐2 (COX‐2) inhibitors were given postoperatively. We retrieved one study comparing paracetamol, metamizole and placebo. The authors reported analgesic superiority of paracetamol (4 g daily) as 42% of patients did not require rescue analgesia compared with 4% in the placebo group and 4% in the metamizole group 13. Another study documented that paracetamol 1 g administered at the end of surgery improved pain control during recovery 14. In yet another study, paracetamol was documented to be equivalent to metamizole but the number of patients included (n = 40) was insufficient to draw definite conclusions 15. The combination of postoperative paracetamol with codeine was found to be as effective as the combination of paracetamol and ibuprofen 16. One study compared the combination of paracetamol and ibuprofen with the combination of paracetamol, codeine and caffeine in ambulatory patients. Both analgesic protocols had comparable effect on pain intensity but the incidence of nausea and constipation was reduced in the group receiving paracetamol and ibuprofen 16.
Only one study in the search period specifically examined postoperative pain control with NSAIDs after oncologic breast surgery. The addition of systemic COX‐2 inhibitors (parecoxib and celecoxib) to paravertebral block has been demonstrated to decrease the intensity of pain on movement on day five 17 but has no effect on the occurrence of postoperative hyperalgesia.
Regional analgesic interventions
Thoracic paravertebral blocks are commonly performed before surgery. The efficacy of paravertebral blocks in breast cancer surgery has been reported in four meta‐analyses including 32 studies 18, 19, 20, 21. Our literature search identified 21 studies over our search period, nine of which were included in those meta‐analyses 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43. The use of thoracic paravertebral blocks resulted in lower postoperative pain scores (p < 0.001) 18, lower opioid consumption compared with general anaesthesia (relative risk (RR) 0.23; 95%CI 0.15–0.37) and a lower incidence of postoperative nausea and vomiting (RR 0.27; 95%CI 0.12–0.61) 19 compared with a control group receiving systemic analgesia alone. In one of the meta‐analyses, the average length of hospital stay was shorter in patients who received paravertebral blocks, but the difference was small and probably not clinically relevant (−0.60 h; 95%CI −1.13 to −0.06) 20. Two studies have compared single‐level and multiple‐level paravertebral blocks. Multiple injections resulted in less postoperative analgesia consumption but no significant difference in the incidence of postoperative nausea and vomiting was reported 27. The time required to perform a single‐injection paravertebral block was shorter compared with the multiple‐injection group (5 min vs. 10 min; median difference 4 min; 95%CI ‐6 to ‐3 min; p < 0.001) 37. Ultrasound guidance was used to perform paravertebral injections or catheter positioning in eight studies 21, 26, 27, 36, 37, 39, 41, 42, whereas 16 other studies did not use ultrasound 22, 23, 24, 25, 28, 30, 31, 32, 34, 35, 38, 43, 44, 45, 46, 47. The results were not different depending on the use of ultrasound guidance or not.
Nine studies have evaluated the use of continuous paravertebral block after mastectomy with or without axillary clearance 40, 41, 43, 44, 45, 46, 47, 48, 49. Continuous paravertebral block resulted in lower pain scores up to the fifth postoperative day 45. Similarly, Ilfeld et al. reported that a continuous paravertebral infusion administered on an ambulatory basis was associated with lower pain scores and improved functional outcomes over three postoperative days 41. Another study reported a similar incidence (95%CI) of chronic pain in patients who received a continuous paravertebral block (57% (44–70%)) to those that did not (73% (62–85%); p = 0.13), although paravertebral block was associated with a reduced severity of chronic pain symptoms 46. Bouman et al. compared continuous paravertebral blockade with local anaesthetic infiltration, and reported that continuous paravertebral block was equally effective in terms of analgesia consumption during the first 24 h 48.
The pectoral nerves (PECS)‐1 block is performed by injecting local anaesthesia between the pectoralis major and minor muscles, whereas the PECS‐2 block includes the PECS‐1 interpectoral injection along with infiltration of local anaesthesia between the pectoralis minor and the serratus anterior muscles. Twelve studies have investigated the analgesic effects of intra‐operative PECS blocks in minor and major breast surgery 38, 42, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60. Intra‐operative opioid requirements were found to be significantly lower after a PECS‐2 block compared with no block 50. Lower pain scores and a reduction in postoperative opioid consumption have been reported in patients having PECS‐2 blocks compared with no block or placebo 54, 57, 60.
Two meta‐analyses comparing PECS to paravertebral blocks reported similar results on pain reIief and analgesia consumption 61, 62. A third meta‐analysis also demonstrated the efficacy of PECS blocks to reduce pain and morphine consumption when compared with no block or placebo 61, 62, 63.
Three studies have compared the paravertebral block to PECS‐2 block and have documented lower pain scores during the first two postoperative hours in patients having a PECS‐2 block 38, 42, 52. There was no significant difference in pain scores between the paravertebral block and the PECS block groups between 2 and 18 postoperative hours 42. However, after 18 h the paravertebral block group had lower pain scores 38. One study evaluated the effect of adding a PECS‐1 block to multimodal analgesia, including local anaesthetic infiltration 55. No reduction in pain scores was documented postoperatively in patients having a PECS‐1 block but the sub‐group of patients scheduled for major breast surgery had lower pain scores after a PECS block. One study compared PECS block with erector spinae plane block 58. Initial pain scores were similar with both blocks, but became quickly inferior in the PECS block group and postoperative opioid consumption was also inferior in the PECS block group.
In one study, the combination of a transversus thoracic muscle plane block with PECS‐2 blocks provided lower pain scores and less rescue analgesia consumption than PECS block alone 56.
Compared with placebo, serratus plane block provided lower pain scores at rest, but there was no significant difference in reducing postoperative opioid consumption 64. In two studies 26, 65, serratus plane block was compared with paravertebral block and was documented to be less effective. In both studies, pain intensity was not different, but total rescue morphine consumption was significantly higher in the serratus plane block group. In another study, different volumes of the same local anaesthetic solution (40 ml vs. 20 ml ropivacaine 0.375%) used for serratus plane block provided no difference in pain relief 66.
Three studies compared erector spinae plane block with general anaesthesia alone 67, 68 or with PECS block 58. The patients receiving erector spinae plane block reported no difference in pain scores, but had decreased morphine consumption compared with general anaesthesia alone 67, 68. Compared with PECS block, erector spinae plane block initially provided no difference in pain scores 58. But after the first postoperative hour, pain scores and opioid consumption were both lower in the PECS block group 58.
Local anaesthetic wound infiltration
A systematic review assessing local anaesthetic wound infiltration in breast surgery included 10 randomised controlled studies (699 patients, 350 receiving local infiltration) 69. Of those, eight studies were related to oncologic surgery. Three different local anaesthetic solutions were used: ropivacaine; bupivacaine; and lidocaine. Local anaesthetic wound infiltration resulted in a reduction in pain scores in four trials and reduced rescue opioid consumption in two trials. The effect never lasted more than 24 h and was most commonly limited to the sixth hour.
In three studies comparing local anaesthetic wound infiltration with paravertebral block, analgesia was improved in the group who had a paravertebral block, with lower pain scores, and in one study, a reduction in rescue analgesic requirement 42, 43, 47. In one of those studies 47, pain scores appeared to be lower in the paravertebral block group up to 4 h postoperatively, but at 16 h and 24 h, wound infiltration was associated with lower pain scores.
Compared with continuous paravertebral block, one study reported that continuous local anaesthetic wound infiltration was equally effective in terms of pain control 48.
Discussion
This systematic review included a total of 62 studies, representing 53 RCTs and nine meta‐analyses, with the majority of studies being of high quality. All but three studies showed statistically significant results for the intervention tested. Based on the PROSPECT approach, basic analgesia including the combination of paracetamol and conventional NSAIDs or COX‐2‐selective inhibitors should be administered pre‐operatively or intra‐operatively and continued into the postoperative period, unless there are contraindications (Table 1). The use of NSAIDs is supported by studies performed before 2006 in breast surgery 70, 71, 72, although there are no recent data. In addition, the analgesic benefits and opioid‐sparing effects of paracetamol and NSAIDs are well described in a range of peri‐operative settings 73, 74. A balance of the analgesic efficacy and potential risks of the analgesic intervention determine these recommendations.
Table 1.
Overall recommendations for pain management in patients undergoing oncological breast surgery
| Recommendations | Minor breast surgery | Major breast surgery |
|---|---|---|
| Pre‐operative and intra‐operative interventions |
|
|
| Postoperative Interventions |
|
|
NSAIDs, non‐steroidal anti‐inflammatory drugs; COX, cyclo‐oxygenase; PECS, pectoral nerves.
Pre‐operative gabapentin is recommended as it has been shown to reduce postoperative pain scores and opioid consumption. However, a wide range of doses were administered in different clinical trials without any documented dose–response effect. Surprisingly, the well‐documented side‐effects of gabapentinoids, such as dizziness, blurred vision or sedation, were not reported in the studies dedicated to postoperative analgesia after breast surgery, even in patients receiving high doses. Overall, gabapentin is recommended but with caution, as high doses may induce side‐effects that could be especially concerning in ambulatory patients 75. Pregabalin is not recommended as the observed pain relief did not last up to 24 h. Intravenous dexamethasone administration is recommended as it provides additional pain relief as well as reducing postoperative nausea and vomiting 76.
Local anaesthetic wound infiltration should be considered in patients scheduled for minor‐to‐moderately invasive surgical procedures (e.g. lumpectomy and partial mastectomy), although it provides postoperative analgesia of a limited duration. Postoperative pain after these procedures is typically mild‐to‐moderate and the intensity decreases over the first couple of postoperative days. Paravertebral blocks should be considered for major breast surgery (e.g. mastectomy with or without axillary node dissection). Studies demonstrated that this intervention was associated with: lower postoperative pain scores; lower systemic analgesia consumption; reduced postoperative nausea and vomiting; and a shorter length of hospital stay than general anaesthesia alone, although the studies did not follow an enhanced recovery programme. A single‐injection paravertebral block requires a shorter time to perform and is less labour intensive as compared with the multiple‐injection technique or placement of a paravertebral catheter. Some studies report an improvement in functional outcomes and less severe chronic pain after the use of continuous paravertebral block. These findings should be interpreted with caution as these studies did not use ‘basic’ simple non‐opioid analgesics (i.e. paracetamol, NSAIDs or COX‐2 selective inhibitors) in a fully implemented multimodal analgesia programme. Not surprisingly, a cost effectiveness study reported higher costs of continuous paravertebral block 77; however, these costs would be offset by the reduced duration of hospital stay 20, 21, 25, 30. Interfascial plane blocks also improve postoperative analgesia with lower pain scores and opioid consumption than general anaesthesia alone. However, data concerning the use of these bocks are limited and the choice of the appropriate block (i.e. PECS 1, PECS 2 and serratus plane blocks) remains to be validated. It should also be noted that for anatomical reasons neither the PECS nor paravertebral blocks can reliably provide sufficient analgesia to the axilla (i.e. T1 nerve distribution) 78. Thus, supplemental local anaesthetic wound infiltration may be beneficial for these cases.
Interfascial plane bocks such as the retrolaminar block or erector spinae plane block have been recently described and could potentially provide an alternative to paravertebral block. However, retrolaminar block has only been studied in one RCT in the context of breast surgery 32. Three studies compared erector spinae plane block with general anaesthesia alone 67, 68 or to PECS block 58 with conflicting results. These blocks need further studies and should be compared with paravertebral and PECS blocks in the context of breast surgery for them to be included in pain management strategies.
Our search did not find evidence regarding the choice of surgical technique that could prevent or reduce pain after breast surgery. There is a need for further study.
The limitations of this review are related to those of the included studies. There was considerable heterogeneity between studies such as: variable dosing regimens; variable methods of administration; a difference in the baseline analgesic management of control groups; and variable time‐points of pain measurement. The sample sizes of most studies were small and therefore it is hard to draw firm conclusions regarding the side‐effect profile of many proposed interventions. Future adequately powered studies should assess the effects of analgesic interventions not only on pain, opioid consumption, opioid‐related adverse events and complications associated with the intervention but also outcome measures such as: time to ambulation; length of hospital stay; occurrence of opioid dependence; and patient‐centred outcomes such as patient satisfaction or quality of recovery.
In summary, this review has identified analgesic regimens for optimal pain management after breast surgery (Table 1). In addition, we also identified analgesic interventions that are not recommended for pain management in patients undergoing breast surgery (Table 2). Peri‐operative pain management for breast surgery should include, unless contraindicated, paracetamol and a conventional NSAID or COX‐2‐selective inhibitor continued into the postoperative period. Other recommended analgesic adjuncts include pre‐operative gabapentin and a single intra‐operative dose of i.v. dexamethasone. For major breast surgery, additional pain management can be achieved by performing an ultrasound‐guided paravertebral block while minor surgery may benefit from local anaesthetic wound infiltration. Interfascial plane blocks such as PECS blocks could be performed in major breast surgery as an alternative to paravertebral blocks. Importantly, opioids should be used only as a rescue medication if non‐opioid analgesics and regional analgesic techniques are do not provide effective pain control. Future studies are necessary to assess the role of novel regional analgesic techniques such as erector spinae plane blocks when combined with basic analgesics in an enhanced recovery setting.
Table 2.
Analgesic interventions that are not recommended for pain management in patients undergoing oncological breast surgery
| Intervention | Reason for not recommending |
|---|---|
| Intra‐operative | |
| Retrolaminar block | Limited procedure‐specific evidence |
| Erector spinae plane block | Limited procedure‐specific evidence |
| Perineural adjuncts: opioids (fentanyl, tramadol), alpha‐2‐adrenoceptor agonists (clonidine, dexmedetomidine), catecholamines (adrenaline) or N‐methyl‐D‐aspartate antagonists (ketamine) added to the local anaesthetic solution | Limited procedure‐specific evidence |
| Postoperative | |
| Transversus thoracic muscle plane block | Limited procedure‐specific evidence |
Appendix 1 PROSPECT Working Group
G. P. Joshi, E. Pogatzki‐Zahn, M. Van de Velde, S. Schug, H. Kehlet, F. Bonnet, N. Rawal, A. Delbos, P. Lavand'homme, H. Beloeil, J. Raeder, A. Sauter, E. Albrecht, P. Lirk, S. Freys and D. Lobo.
Supporting information
Table S1. Quality assessment and level of evidence assigned to the randomised trials included in this review for analgesia in oncological breast surgery.
Table S2. Summary of key results from studies evaluating systemic analgesics, systemic analgesic adjuncts, regional analgesia and surgical procedures in patients undergoing oncological breast surgery. This is not part of the table title, therefore it can be removed from here, and only kept as explaination in the supporting information section.
Table S3. Summary of key results from studies evaluating regional anaesthetic techniques and adjuncts used to support interventions that are not recommended for analgesic benefit in patients undergoing oncological breast surgery.
Acknowledgements
AJ and AL equally contributed to this manuscript and therefore share first authorship. PROSPECT is supported by an unrestricted grant from the European Society of Regional Anaesthesia and Pain Therapy (ESRA). In the past, PROSPECT has received unrestricted grants from Pfizer Inc. New York, NY, USA and Grunenthal, Aachen, Germany. GJ has received honoraria from Baxter and Pacira Pharmaceuticals. FB has received honoraria from Pfizer, The Medicine Company, Abbott France, Nordic Pharma France, Heron therapeutics, AMBU and Grunenthal. MVdV has received honoraria from Sintetica, Grunenthal, Vifor Pharma, MSD, Nordic Pharma, Janssen Pharmaceuticals, Heron Therapeutics and Aquettant. No other competing interests declared.
Contributor Information
A. Lemoine, Email: adrien.lemoine@aphp.fr.
the PROSPECT Working Group collaborators#:
E. Pogatzki‐Zahn, S. Schug, H. Kehlet, N. Rawal, A. Delbos, P. Lavand'homme, H. Beloeil, J. Raeder, A. Sauter, E. Albrecht, P. Lirk, S. Freys, and D. Lobo
References
- 1. Vadivelu N, Schreck M, Lopez J, Kodumudi G, Narayan D. Pain after mastectomy and breast reconstruction. American Surgeon 2008; 74: 285–96. [PubMed] [Google Scholar]
- 2. Bonnet F, Camu F, Barranger E. Non cosmetic breast surgery analgesia ‐ Prospect review 2006; https://esraeurope.org/wp-content/uploads/2019/03/Summary-recommendations_Breast-surgery_EN.pdf (accessed 27/11/2019).
- 3. Elsharkawy H, Pawa A, Mariano ER. Interfascial plane blocks: back to basics. Regional Anesthesia and Pain Medicine 2018; 43: 341–6. [DOI] [PubMed] [Google Scholar]
- 4. Joshi GP, Schug SA, Kehlet H. Procedure‐specific pain management and outcome strategies. Best Practice and Research Clinical Anaesthesiology 2014; 28: 191–201. [DOI] [PubMed] [Google Scholar]
- 5. Lee B, Schug SA, Joshi GP, Kehlet H. Procedure‐specific pain management (PROSPECT) – an update. Best Practice and Research. Clinical Anaesthesiology 2018; 32: 101–11. [DOI] [PubMed] [Google Scholar]
- 6. Joshi GP, Kehlet H, Beloeil H, et al. Guidelines for perioperative pain management: need for re‐evaluation. British Journal of Anaesthesia 2017; 119: 720–2. [DOI] [PubMed] [Google Scholar]
- 7. Joshi GP, Van de Velde M, Kehlet H, et al. Development of evidence‐based recommendations for procedure‐specific pain management: PROSPECT methodology. Anaesthesia 2019; 74: 1298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Myles PS, Myles DB, Galagher W, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. British Journal of Anaesthesia 2017; 118: 424–9. [DOI] [PubMed] [Google Scholar]
- 9. Jiang Y, Li J, Lin H, et al. The efficacy of gabapentin in reducing pain intensity and morphine consumption after breast cancer surgery: a meta‐analysis. Medicine 2018; 97: e11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rai AS, Khan JS, Dhaliwal J, et al. Preoperative pregabalin or gabapentin for acute and chronic postoperative pain among patients undergoing breast cancer surgery: a systematic review and meta‐analysis of randomized controlled trials. Journal of Plastic, Reconstructive and Aesthetic Surgery 2017; 70: 1317–28. [DOI] [PubMed] [Google Scholar]
- 11. Gómez‐Hernández J, Orozco‐Alatorre AL, Domínguez‐Contreras M, et al. Preoperative dexamethasone reduces postoperative pain, nausea and vomiting following mastectomy for breast cancer. BMC Cancer 2010; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cortés‐Flores AO, Jiménez‐Tornero J, Morgan‐Villela G, et al. Effects of preoperative dexamethasone on postoperative pain, nausea, vomiting and respiratory function in women undergoing conservative breast surgery for cancer: results of a controlled clinical trial. European Journal of Cancer Care 2018; 27: e12686. [DOI] [PubMed] [Google Scholar]
- 13. Ohnesorge H, Bein B, Hanss R, et al. Paracetamol versus metamizol in the treatment of postoperative pain after breast surgery: a randomized, controlled trial. European Journal of Anaesthesiology 2009; 26: 648–53. [DOI] [PubMed] [Google Scholar]
- 14. De Oliveira GS, Rodes ME, Bialek J, Kendall MC, McCarthy RJ. Single dose systemic acetaminophen to improve patient reported quality of recovery after ambulatory segmental mastectomy: a prospective, randomized, double‐blinded, placebo controlled, clinical trial. Breast Journal 2018; 24: 240–4. [DOI] [PubMed] [Google Scholar]
- 15. Kampe S, Warm M, Landwehr S, et al. Clinical equivalence of IV paracetamol compared to IV dipyrone for postoperative analgesia after surgery for breast cancer. Current Medical Research and Opinion 2006; 22: 1949–54. [DOI] [PubMed] [Google Scholar]
- 16. Mitchell A, McCrea P, Inglis K, Porter G. A randomized, controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery. Annals of Surgical Oncology 2012; 19: 3792–800. [DOI] [PubMed] [Google Scholar]
- 17. van Helmond N, Steegers MA, Filippini‐de Moor GP, Vissers KC, Wilder‐Smith OH. Hyperalgesia and persistent pain after breast cancer surgery: a prospective randomized controlled trial with perioperative COX‐2 inhibition. PLoS ONE 2016; 11: e0166601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schnabel A, Reichl SU, Kranke P, Pogatzki‐Zahn EM, Zahn PK. Efficacy and safety of paravertebral blocks in breast surgery: a meta‐analysis of randomized controlled trials. British Journal of Anaesthesia 2010; 105: 842–52. [DOI] [PubMed] [Google Scholar]
- 19. Tahiri Y, Tran DQH, Bouteaud J, et al. General anaesthesia versus thoracic paravertebral block for breast surgery: a meta‐analysis. Journal of Plastic, Reconstructive and Aesthetic Surgery 2011; 64: 1261–9. [DOI] [PubMed] [Google Scholar]
- 20. Terkawi AS, Tsang S, Sessler DI, et al. Improving analgesic efficacy and safety of thoracic paravertebral block for breast surgery: a mixed‐effects meta‐analysis. Pain Physician 2015; 18: E757–80. [PubMed] [Google Scholar]
- 21. Abdallah FW, Morgan PJ, Cil T, et al. Ultrasound‐guided multilevel paravertebral blocks and total intravenous anesthesia improve the quality of recovery after ambulatory breast tumor resection. Anesthesiology 2014; 120: 703–13. [DOI] [PubMed] [Google Scholar]
- 22. Boughey JC, Goravanchi F, Parris RN, et al. Improved postoperative pain control using thoracic paravertebral block for breast operations. Breast Journal 2009; 15: 483–8. [DOI] [PubMed] [Google Scholar]
- 23. Bhuvaneswari V, Wig J, Mathew PJ, Singh G. Post‐operative pain and analgesic requirements after paravertebral block for mastectomy: a randomized controlled trial of different concentrations of bupivacaine and fentanyl. Indian Journal of Anaesthesia 2012; 56: 34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Das S, Bhattacharya P, Mandal MC, Mukhopadhyay S, Basu SR, Mandol BK. Multiple‐injection thoracic paravertebral block as an alternative to general anaesthesia for elective breast surgeries: a randomised controlled trial. Indian Journal of Anaesthesia 2012; 56: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fallatah S, Mousa WF. Multiple levels paravertebral block versus morphine patient‐controlled analgesia for postoperative analgesia following breast cancer surgery with unilateral lumpectomy, and axillary lymph nodes dissection. Saudi Journal of Anaesthesia 2016; 10: 13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gupta K, Srikanth K, Girdhar KK, Chan V. Analgesic efficacy of ultrasound‐guided paravertebral block versus serratus plane block for modified radical mastectomy: a randomised, controlled trial. Indian Journal of Anaesthesia 2017; 61: 381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kasimahanti R, Arora S, Bhatia N, Singh G. Ultrasound‐guided single‐ vs double‐level thoracic paravertebral block for postoperative analgesia in total mastectomy with axillary clearance. Journal of Clinical Anesthesia 2016; 33: 414–21. [DOI] [PubMed] [Google Scholar]
- 28. Kundra P, Varadharajan R, Yuvaraj K, Vinayagam S. Comparison of paravertebral and interpleural block in patients undergoing modified radical mastectomy. Journal of Anaesthesiology, Clinical Pharmacology 2013; 29: 459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mohamed SA, Fares KM, Mohamed AA, Alieldin NH. Dexmedetomidine as an adjunctive analgesic with bupivacaine in paravertebral analgesia for breast cancer surgery. Pain Physician 2014; 17: E589–98. [PubMed] [Google Scholar]
- 30. Mohta M, Kalra B, Sethi AK, Kaur N. Efficacy of dexmedetomidine as an adjuvant in paravertebral block in breast cancer surgery. Journal of Anesthesia 2016; 30: 252–60. [DOI] [PubMed] [Google Scholar]
- 31. Moller JF, Nikolajsen L, Rodt SA, Ronning H, Carlsson PS. Thoracic paravertebral block for breast cancer surgery: a randomized double‐blind study. Anesthesia and Analgesia 2007; 105: 1848–51. [DOI] [PubMed] [Google Scholar]
- 32. Murouchi T, Yamakage M. Retrolaminar block: analgesic efficacy and safety evaluation. Journal of Anesthesia 2016; 30: 1003–7. [DOI] [PubMed] [Google Scholar]
- 33. Naja ZM, Ziade FM, El‐Rajab MA, Naccash N, Ayoubi J‐M. Guided paravertebral blocks with versus without clonidine for women undergoing breast surgery: a prospective double‐blinded randomized study. Anesthesia and Analgesia 2013; 117: 252–8. [DOI] [PubMed] [Google Scholar]
- 34. Omar AM, Mansour MA, Abdelwahab HH, Aboushanab OH. Role of ketamine and tramadol as adjuncts to bupivacaine 0.5% in paravertebral block for breast surgery: a randomized double‐blind study. Egyptian Journal of Anaesthesia 2011; 27: 101–5. [Google Scholar]
- 35. Sahu A, Kumar R, Hussain M, Gupta A, Raghwendra KH. Comparisons of single‐injection thoracic paravertebral block with ropivacaine and bupivacaine in breast cancer surgery: a prospective, randomized, double‐blinded study. Anesthesia, Essays and Researches 2016; 10: 655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sundarathiti P, von Bormann B, Suvikapakornkul R, Lertsithichai P, Arnuntasupakul V. Paravertebral catheter for three‐level injection in radical mastectomy: a randomised controlled study. PLoS One 2015; 10: e0129539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uppal V, Sondekoppam RV, Sodhi P, Johnston D, Ganapathy S. Single‐injection versus multiple‐injection technique of ultrasound‐guided paravertebral blocks: a randomized controlled study comparing dermatomal spread. Regional Anesthesia and Pain Medicine 2017; 42: 575–81. [DOI] [PubMed] [Google Scholar]
- 38. Wahba SS, Kamal SM. Thoracic paravertebral block versus pectoral nerve block for analgesia after breast surgery. Egyptian Journal of Anaesthesia 2014; 30: 129–35. [Google Scholar]
- 39. Župčić M, Graf Župčić S, Duzel V, et al. A combination of levobupivacaine and lidocaine for paravertebral block in breast cancer patients undergoing quadrantectomy causes greater hemodynamic oscillations than levobupivacaine alone. Croatian Medical Journal 2017; 58: 270–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu J, Buggy D, Fleischmann E, et al. Thoracic paravertebral regional anesthesia improves analgesia after breast cancer surgery: a randomized controlled multicentre clinical trial. Canadian Journal of Anesthesia 2015; 62: 241–51. [DOI] [PubMed] [Google Scholar]
- 41. Ilfeld BM, Madison SJ, Suresh PJ, et al. Treatment of postmastectomy pain with ambulatory continuous paravertebral nerve blocks: a randomized, triple‐masked, placebo‐controlled study. Regional Anesthesia and Pain Medicine 2014; 39: 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Syal K, Chandel A. Comparison of the post‐operative analgesic effect of paravertebral block, pectoral nerve block and local infiltration in patients undergoing modified radical mastectomy: a randomised double‐blind trial. Indian Journal of Anaesthesia 2017; 61: 643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bansal P, Saxena KN, Taneja B, Sareen B. A comparative randomized study of paravertebral block versus wound infiltration of bupivacaine in modified radical mastectomy. Journal of Anaesthesiology, Clinical Pharmacology 2012; 28: 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Burlacu CL, Frizelle HP, Moriarty DC, Buggy DJ. Fentanyl and clonidine as adjunctive analgesics with levobupivacaine in paravertebral analgesia for breast surgery. Anaesthesia 2006; 61: 932–7. [DOI] [PubMed] [Google Scholar]
- 45. Iohom G, Abdalla H, O'Brien J, et al. The associations between severity of early postoperative pain, chronic postsurgical pain and plasma concentration of stable nitric oxide products after breast surgery. Anesthesia and Analgesia 2006; 103: 995–1000. [DOI] [PubMed] [Google Scholar]
- 46. Karmakar MK, Samy W, Li JW, et al. Thoracic paravertebral block and its effects on chronic pain and health‐related quality of life after modified radical mastectomy. Regional Anesthesia and Pain Medicine 2014; 39: 289–98. [DOI] [PubMed] [Google Scholar]
- 47. Sidiropoulou T, Buonomo O, Fabbi E, et al. A prospective comparison of continuous wound infiltration with ropivacaine versus single‐injection paravertebral block after modified radical mastectomy. Anesthesia and Analgesia 2008; 106: 997–1001. [DOI] [PubMed] [Google Scholar]
- 48. Bouman EAC, Theunissen M, Kessels AG, et al. Continuous paravertebral block for postoperative pain compared to general anaesthesia and wound infiltration for major oncological breast surgery. SpringerPlus 2014; 3: 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buckenmaier CC, Kwon KH, Howard RS, et al. Double‐blinded, placebo‐controlled, prospective randomized trial evaluating the efficacy of paravertebral block with and without continuous paravertebral block analgesia in outpatient breast cancer surgery. Pain Medicine 2010; 11: 790–9. [DOI] [PubMed] [Google Scholar]
- 50. Bashandy GMN, Abbas DN. Pectoral nerves I and II blocks in multimodal analgesia for breast cancer surgery: a randomized clinical trial. Regional Anesthesia and Pain Medicine 2015; 40: 68–74. [DOI] [PubMed] [Google Scholar]
- 51. ELdeen HMS. Ultrasound guided pectoral nerve blockade versus thoracic spinal blockade for conservative breast surgery in cancer breast: a randomized controlled trial. Egyptian Journal of Anaesthesia 2016; 32: 29–35. [Google Scholar]
- 52. Kulhari S, Bharti N, Bala I, Arora S, Singh G. Efficacy of pectoral nerve block versus thoracic paravertebral block for postoperative analgesia after radical mastectomy: a randomized controlled trial. British Journal of Anaesthesia 2016; 117: 382–6. [DOI] [PubMed] [Google Scholar]
- 53. Othman AH, El‐Rahman AMA, El Sherif F. Efficacy and safety of ketamine added to local anesthetic in modified pectoral block for management of postoperative pain in patients undergoing modified radical mastectomy. Pain Physician 2016; 19: 485–94. [PubMed] [Google Scholar]
- 54. Versyck B, van Geffen G‐J, Van Houwe P. Prospective double blind randomized placebo‐controlled clinical trial of the pectoral nerves (Pecs) block type II. Journal of Clinical Anesthesia 2017; 40: 46–50. [DOI] [PubMed] [Google Scholar]
- 55. Cros J, Sengès P, Kaprelian S, et al. Pectoral I block does not improve postoperative analgesia after breast cancer surgery: a randomized, double‐blind, dual‐centered controlled trial. Regional Anesthesia and Pain Medicine 2018; 43: 596–604. [DOI] [PubMed] [Google Scholar]
- 56. Ueshima H, Otake H. Addition of transversus thoracic muscle plane block to pectoral nerves block provides more effective perioperative pain relief than pectoral nerves block alone for breast cancer surgery. British Journal of Anaesthesia 2017; 118: 439–43. [DOI] [PubMed] [Google Scholar]
- 57. Al Ja’bari A, Robertson M, El‐Boghdadly K, Albrecht E. A randomised controlled trial of the pectoral nerves‐2 (PECS‐2) block for radical mastectomy. Anaesthesia 2019; 74: 1277–81. [DOI] [PubMed] [Google Scholar]
- 58. Altiparmak B, Korkmaz Toker M, Uysal AI, Turan M, Gumus Demirbilek S. Comparison of the effects of modified pectoral nerve block and erector spinae plane block on postoperative opioid consumption and pain scores of patients after radical mastectomy surgery: a prospective, randomized, controlled trial. Journal of Clinical Anesthesia 2019; 54: 61–5. [DOI] [PubMed] [Google Scholar]
- 59. Kamiya Y, Hasegawa M, Yoshida T, Takamatsu M, Koyama Y. Impact of pectoral nerve block on postoperative pain and quality of recovery in patients undergoing breast cancer surgery: a randomised controlled trial. European Journal of Anaesthesiology 2018; 35: 215–23. [DOI] [PubMed] [Google Scholar]
- 60. Senapathi TGA, Widnyana IMG, Aribawa IGNM, Jaya AAGPS, Junaedi IMD. Combined ultrasound‐guided Pecs II block and general anesthesia are effective for reducing pain from modified radical mastectomy. Journal of Pain Research 2019; 12: 1353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Versyck B, Geffen G‐J, Chin K‐J. Analgesic efficacy of the Pecs II block: a systematic review and meta‐analysis. Anaesthesia 2019; 74: 663–73. [DOI] [PubMed] [Google Scholar]
- 62. Hussain N, Brull R, McCartney CJL, et al. Pectoralis‐II myofascial block and analgesia in breast cancer surgery: a systematic review and meta‐analysis. Anesthesiology 2019; 1. [DOI] [PubMed] [Google Scholar]
- 63. Zhao J, Han F, Yang Y, Li H, Li Z. Pectoral nerve block in anesthesia for modified radical mastectomy: a meta‐analysis based on randomized controlled trials. Medicine 2019; 98: e15423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yao Y, Li J, Hu H, Xu T, Chen Y. Ultrasound‐guided serratus plane block enhances pain relief and quality of recovery after breast cancer surgery: a randomised controlled trial. European Journal of Anaesthesiology 2019; 36: 436–41. [DOI] [PubMed] [Google Scholar]
- 65. Hetta DF, Rezk KM. Pectoralis‐serratus interfascial plane block vs thoracic paravertebral block for unilateral radical mastectomy with axillary evacuation. Journal of Clinical Anesthesia 2016; 34: 91–7. [DOI] [PubMed] [Google Scholar]
- 66. Kunigo T, Murouchi T, Yamamoto S, Yamakage M. Injection volume and anesthetic effect in serratus plane block. Regional Anesthesia and Pain Medicine 2017; 42: 737–40. [DOI] [PubMed] [Google Scholar]
- 67. Gurkan Y, Aksu C, Kus A, Yorukoglu UH, Kilic CT. Ultrasound guided erector spinae plane block reduces postoperative opioid consumption following breast surgery: a randomized controlled study. Journal of Clinical Anesthesia 2018; 50: 65–8. [DOI] [PubMed] [Google Scholar]
- 68. Singh S, Kumar G. Ultrasound‐guided erector spinae plane block for postoperative analgesia in modified radical mastectomy: a randomised control study. Indian Journal of Anaesthesia 2019; 63: 200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Byager N, Hansen MS, Mathiesen O, Dahl JB. The analgesic effect of wound infiltration with local anaesthetics after breast surgery: a qualitative systematic review: wound infiltration after breast surgery. Acta Anaesthesiologica Scandinavica 2014; 58: 402–10. [DOI] [PubMed] [Google Scholar]
- 70. Legeby M, Jurell G, Beausang‐Linder M, Olofsson C. Placebo‐controlled trial of local anaesthesia for treatment of pain after breast reconstruction. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery 2009; 43: 315–19. [DOI] [PubMed] [Google Scholar]
- 71. Chan A, Dore CJ, Ramachandra V. Analgesia for day surgery. Evaluation of the effect of diclofenac given before or after surgery with or without bupivacaine infiltration. Anaesthesia 1996; 51: 592–5. [DOI] [PubMed] [Google Scholar]
- 72. Priya V, Divatia JV, Sareen R, Upadhye S. Efficacy of intravenous ketoprofen for pre‐emptive analgesia. Journal of Postgraduate Medicine 2002; 48: 109–12. [PubMed] [Google Scholar]
- 73. Martinez V, Beloeil H, Marret E, Fletcher D, Ravaud P, Trinquart L. Non‐opioid analgesics in adults after major surgery: systematic review with network meta‐analysis of randomized trials. British Journal of Anaesthesia 2017; 118: 22–31. [DOI] [PubMed] [Google Scholar]
- 74. Ong CKS, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesthesia and Analgesia 2010; 110: 1170–9. [DOI] [PubMed] [Google Scholar]
- 75. Tiippana EM, Hamunen K, Kontinen VK, Kalso E. Do surgical patients benefit from perioperative gabapentin/pregabalin? a systematic review of efficacy and safety. Anesthesia and Analgesia 2007; 104: 1545–56. [DOI] [PubMed] [Google Scholar]
- 76. Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesthesia and Analgesia 2014; 118: 85–113. [DOI] [PubMed] [Google Scholar]
- 77. Offodile AC, Sheckter CC, Tucker A, et al. Preoperative paravertebral blocks for the management of acute pain following mastectomy: a cost‐effectiveness analysis. Breast Cancer Research and Treatment 2017; 165: 477–84. [DOI] [PubMed] [Google Scholar]
- 78. Pawa A, Wight J, Onwochei DN, et al. Combined thoracic paravertebral and pectoral nerve blocks for breast surgery under sedation: a prospective observational case series. Anaesthesia 2018; 73: 438–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Quality assessment and level of evidence assigned to the randomised trials included in this review for analgesia in oncological breast surgery.
Table S2. Summary of key results from studies evaluating systemic analgesics, systemic analgesic adjuncts, regional analgesia and surgical procedures in patients undergoing oncological breast surgery. This is not part of the table title, therefore it can be removed from here, and only kept as explaination in the supporting information section.
Table S3. Summary of key results from studies evaluating regional anaesthetic techniques and adjuncts used to support interventions that are not recommended for analgesic benefit in patients undergoing oncological breast surgery.


