Abstract
With advancements in biomarkers and momentum in precision medicine, biomarker‐guided trials such as basket trials and umbrella trials have been developed under the master protocol framework. A master protocol refers to a single, overarching design developed to evaluate multiple hypotheses with the general goal of improving the efficiency of trial evaluation. One type of master protocol is the basket trial, in which a targeted therapy is evaluated for multiple diseases that share common molecular alterations or risk factors that may help predict whether the patients will respond to the given therapy. Another variant of a master protocol is the umbrella trial, in which multiple targeted therapies are evaluated for a single disease that is stratified into multiple subgroups based on different molecular or other predictive risk factors. Both designs follow the core principle of precision medicine—to tailor intervention strategies based on the patient's risk factor(s) that can help predict whether they will respond to a specific treatment. There have been increasing numbers of basket and umbrella trials, but they are still poorly understood. This article reviews common characteristics of basket and umbrella trials, key trials and recent US Food and Drug Administration approvals for precision oncology, and important considerations for clinical readers when critically evaluating future publications on basket trials and umbrella trials and for researchers when designing these clinical trials.
Keywords: basket trials, master protocols, precision medicine, precision oncology, umbrella trials
Introduction
With increasing advancements in genomics, there has been growing interest in precision‐based medicine, which aims to improve the treatment of disease by identifying therapies that can specifically affect disease targets based on their genetic make‐up (ie, targeted therapies).1, 2, 3, 4, 5 In the United States, there is important momentum toward the implementation of precision medicine. In May 2018, the National Institutes of Health launched their “All of Us” Initiative, which aims to gather demographic and biological data from at least one million people living in the United States to be used for precision care in oncology and other areas of medicine.6 In the United Kingdom, the National Health Service's 2019 Long‐Term Plan shows momentum specifically toward realizing precision oncology.7 The National Health Service is currently preparing to offer whole genomic sequencing to all children with cancer and to fast‐track the introduction of personalized treatments based on patients' genetic alterations.7
With these rising interests and efforts toward precision care, it is vital to recognize the importance of biomarkers and how they are used to develop targeted therapies in clinical research.8, 9, 10 Notable methodological advancements that have recently been made toward biomarker‐guided clinical trials include the development of basket and umbrella design trials under the master protocol framework.11, 12, 13, 14, 15, 16, 17, 18, 19 A master protocol refers to a single, overarching design developed to evaluate multiple hypotheses with the general goal of improving efficiency through standardized trial procedures.11, 12, 13, 14 Two types of master protocols are basket and umbrella trials. Basket trials refer to designs in which a targeted therapy is evaluated for multiple diseases that share common molecular alterations, and umbrella trials refer to designs in which multiple targeted therapies are evaluated for a single disease that is stratified into multiple subgroups.11, 12, 13, 14 The US Food and Drug Administration (FDA) released its draft guidance document outlining recommendations for basket trials and umbrella trials on September 2018, highlighting their support for a wider dissemination of these master protocols.12 A recent landscape analysis using a comprehensive literature search has found a rapidly increasing number of these master protocols, as the number increased from 2 (a basket trial and an umbrella trial) to 67 (49 basket trials and 18 umbrella trials) over 10 years between 2009 and 2019.13
Despite these rapidly increasing numbers of basket and umbrella trials, currently, they are still poorly understood.14 Most basket and umbrella trials have been conducted in oncology and were led by investigators from the US National Cancer Institute (NCI), industry, and contract research organizations.13, 14 Understanding of basket and umbrella trials is not widespread among clinicians and researchers outside of the NCI and the private sector. With increasing numbers of basket and umbrella trials being conducted, it is critical to improve the literacy of these research approaches, thus motivating the current article. Herein, we outline and discuss common characteristics of basket and umbrella trials, key precision oncology trials, recent key regulatory FDA approvals in precision oncology, and key considerations that clinicians should make when reading these basket and umbrella trial publications.
Introduction to Basket and Umbrella Trials
Illustrative examples of basket and umbrella trials are provided in Figures 1 and 2, respectively. Basket trials are prospective clinical trials that test one or more targeted interventions across multiple types of diseases.11, 14, 15, 20 In basket trials, there are unifying eligibility criteria that combine patients with different diseases (eg, multihistological cancers) into a single trial. These unifying eligibility criteria usually are based on a patient's predictive risk factor. The predictive risk factor is generally based on the intervention's mechanism of action because it can help predict whether the patient will respond to a specific intervention. For instance, Li et al recently conducted a phase 2 basket trial that evaluated whether ado‐trastuzumab emtasine could achieve an antitumor response in HER2 (human epidermal growth factor receptor 2)‐amplified or HER2‐mutant cancers of multiple histologies (clinicaltrials.gov identifier NCT02675829).21, 22 Ado‐trastuzumab emtasine, which is an FDA‐approved drug for HER2‐positive metastatic breast cancer,23, 24 was hypothesized to produce an antitumor response in HER2‐amplified or HER2‐mutant cancers regardless of their histology based on its biological mechanistic pathway.22 In their basket trial, Li et al used HER2 amplification or mutation in advanced lung, endometrial, salivary gland, biliary tract, ovarian, bladder, colorectal, and other cancers as a common eligibility criterion to evaluate the role of this HER2‐targeting drug.21 In other words, HER2 amplification or mutation was the common predictive biomarker risk factor that was hypothesized to predict whether patients who had different histological types of cancers would respond to this targeted therapy for HER2‐positive disease.
Figure 1.
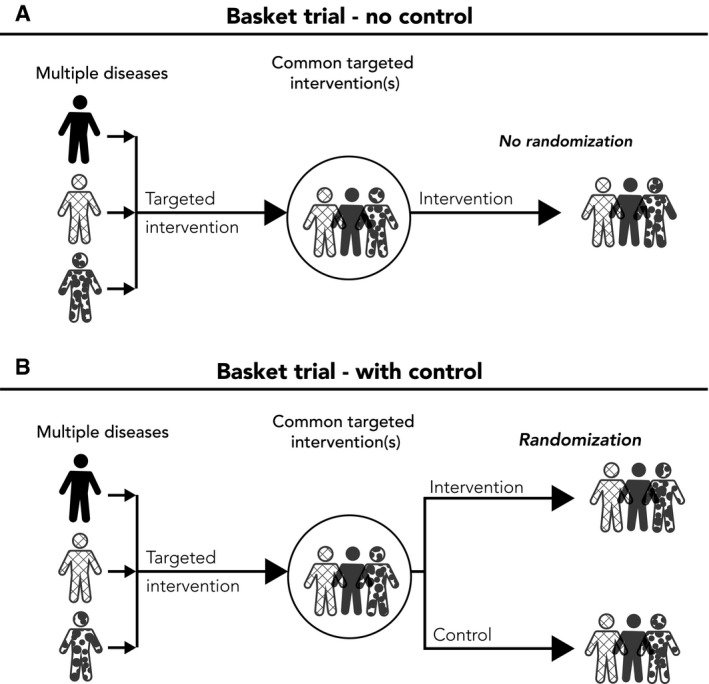
Illustrative Examples of a Basket Trial. (A) A single‐arm basket trial with a single targeted intervention without a control group is illustrated. (B) A 2‐arm randomized basket trial is shown.
Figure 2.
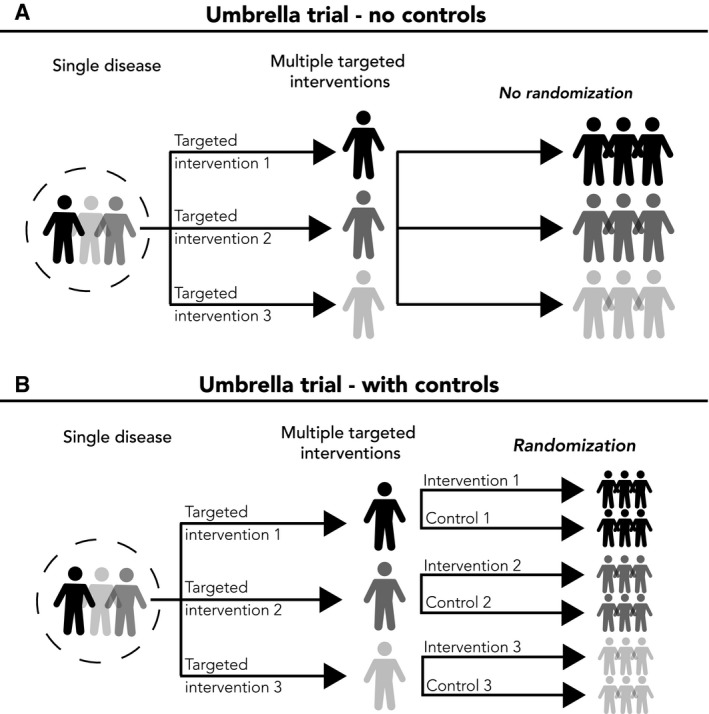
Illustrative Examples of an Umbrella Trial. (A) A nonrandomized umbrella trial with 3 targeted interventions is illustrated. (B) A randomized umbrella trial that includes 3 subgroups, each with a targeted intervention and a control group.
Umbrella trials, conversely, are prospective clinical trials that test multiple targeted interventions for a single disease based on predictive biomarkers or other predictive patient risk factors.11, 13, 14, 19, 25 In umbrella trials, a single disease (eg, advanced breast cancer) is stratified into multiple subgroups, with eligibility for each intervention arm defined by the intervention's mechanism of action. For example, plasmaMATCH is an umbrella trial that evaluated 5 different therapies for advanced breast cancer. The therapies were stratified as 5 treatment groups based on their molecular signatures (clinicaltrials.gov identifier NCT03182634).26, 27 These 5 subgroups included patients who had breast cancer with an ESR1 (estrogen receptor gene 1) mutation (group A), an HER2 mutation (group B), an AKT (serine/threonine‐specific protein kinase B) mutation (group C), AKT activation (group D), or triple‐negative status (group E).26, 27 Patients with ESR1 mutations in group A received an extended dose of the estrogen receptor downregulator fulvestrant (500 mg every 2 weeks).26, 27, 28 Patients with HER2 mutations in group B received an HER tyrosine kinase inhibitor (neratinib) and also received fulvestrant if they had an estrogen receptor co‐mutation.26, 27, 29 Patients with AKT mutations in group C received the AKT inhibitor AZD5364 plus fulvestrant, whereas patients with AKT activation in group D received AZD5364 only.27 For group E, patients with triple‐negative breast cancer received the poly(ADP‐ribose) polymerase inhibitor olaparib plus AZD5364.26, 27, 30 In that umbrella trial, multiple biomarker assays were applied to a single tumor histology, and patients were assigned to 1 of the 5 subgroups based on their biomarker status to evaluate the clinical utility of 5 different targeted therapy strategies for advanced breast cancer.
Characteristics of Basket and Umbrella Trials: Eligibility Criteria, Patient Subgroups, Intervention Assignment, and Choice in a Control Group
There are important similarities and differences between basket and umbrella trials that should be noted in terms of their eligibility criteria, patient subgrouping, and intervention assignment (Table 1). In both basket and umbrella trials, a common screening protocol is used to determine whether the patient is eligible. The majority of basket and umbrella trials conducted so far come from oncology and have been biomarker‐guided, so it is important to note that there was a common molecular screening protocol with standardized biomarker assays in these biomarker‐guided trials.14 Patients enrolled in a basket trial will represent multiple diseases with a common, unifying predictive risk factor; and, in umbrella trials, patients with a common disease (a single disease) will be recruited and enrolled.14
Table 1.
Basket and Umbrella Trials: Eligibility Criteria, Patient Subgroups, Intervention Assignment, and Choice in a Control Group
| KEY CHARACTERISTICS | BASKET TRIALS | UMBRELLA TRIALS |
|---|---|---|
| Eligibility criteria |
|
|
| Patient subgroups |
|
|
| Intervention assignment |
|
|
|
|
|
| Choice in a control group |
|
|
|
|
Umbrella trials have an inherent key methodological characteristic of using multiple predictive risk factors to determine patient subgroups. Naturally, the use of patient subgroups is more common in these trials. In basket trials, patient subgroups may be defined based on their disease subtypes given that the disease subtype is often a prognostic factor; however, because patients with multiple disease types are recruited into a common cohort, “unification of diseases” is a more appropriate way to describe the inherent nature of basket trials. These patient grouping strategies are important to highlight because they are used to determine the types of patients that are recruited and how patients with different predictive risk factors are allocated to different interventions in basket and umbrella trials. Although it is common for basket trials to have a targeted, single intervention based on a common unifying predictive risk factor, it is possible for basket trials to have more than one targeted intervention. However, even in multiarm basket trials, it is important to note that the clinical utilities of these interventions are usually assessed separately for each intervention. Umbrella trials all have multiple targeted interventions (ie, more than one intervention), and, like basket trials, the clinical utilities of these interventions are usually assessed separately.
For both basket trials and umbrella trials, they may be conducted with or without a control group (ie, randomized vs single‐arm nonrandomized designs). A landscape analysis recently showed that it is more common for umbrella trials to use a control group (n = 8 of 18 trials; 44.4%) than basket trials (n = 5 of 49 trials; 10.2%).13 For umbrella trials, the control group may be placebo if there is no established care, or the existing standard of care for the disease being studied may be used for all of the subgroups. Determining the control group can be more difficult for basket trials because there are multiple diseases being studied. If there is no established care for all diseases being studied, the placebo may be used as the control group; if the diseases being studied in the basket trials have different standards of care, it is possible that there may be different care being provided in the common control group. Of the 4 randomized basket trials that used a control group, 2 of them received no therapy (eg, saline injection) or placebo (Study of Efficacy and Safety of Canakinumab in Patients With Hereditary Periodic Fevers [Canakinumab Pivotal Umbrella Study in Three Hereditary Periodic Fevers (CLUSTER)], clinicaltrials.gov identifier NCT0205929131; and TNT0009 Basket Trial [Safety, Tolerability and Activity of BIVV009 in Healthy Volunteers and Patients With Complement Mediated Disorders], NCT0250290332), and the control group in the other 2 basket trials (IMPACT II [Molecular Profiling and Targeted Therapy in Treating Patients With Metastatic Cancer], NCT0215225433; and SHIVA [A Randomized Phase II Trial Comparing Therapy Based on Tumor Molecular Profiling Versus Conventional Therapy in Patients With Refractory Cancer], NCT0177145834) received the standard of care, which was left to the discretion of the treating physician.
Basket and Umbrella Trials Versus Other Biomarker‐Guided Trials
Basket and umbrella trials share many similarities with other nonmaster protocol biomarker‐guided trials, but there are key differences that should be noted. In accord with other biomarker‐guided trials, the aim of basket and umbrella trial approaches is to use genomics and other “omic” technologies to define disease and eligibility criteria for improved characterization and identification of predictive biomarkers and targeted therapies. The use of a single master protocol with standardized operating procedures is a key difference from other types of nonmaster protocol biomarker‐guided trials. Under the master protocol framework, basket and umbrella trials usually establish a large trial network and a common infrastructure established across and through multiple institutions. Between these institutions, standardized operating procedures, including a common screening mechanism, are instituted for patient identification. Adopting a common molecular screening mechanism under a master protocol can help achieve screening efficiency for biomarker‐guided trials.
In basket trials, multiple cancers of different histopathologies are recruited into a single cohort when these cancers have common molecular alteration(s). Basket trials try to aim to identify histology‐agnostic therapies. Traditionally, it is not uncommon for phase 1 cancer clinical trials to recruit multiple different types of histopathologies to test for the existence of signal, but basket trials and their histology‐agnostic approaches are now being considered for phase 2 and even some phase 3 evaluations.13 In umbrella trials, multiple histology‐dependent targeted therapies are evaluated for multiple subgroups of single histopathology that are molecularly differentiated through common screening mechanism. Screening for given biomarker(s) is an important consideration for biomarker‐guided trials, as the number of patients required for the enrollment target ultimately will depend on the biomarker prevalence of interest. For example, if the group of interest is patients who have advanced breast cancer with an ESR1 mutation, as in the case of aforementioned umbrella trial example of plasmaMATCH, the number of patients that will need to be screened will dependent on the biomarker prevalence of interest. If we assume that 10% of patients with advanced breast cancer will have an ESR1 mutation, an expected 1000 patients will need to be screened to reach the recruitment target size of 100 patients. Of course, umbrella trials (eg, plasmaMATCH) could be conducted as multiple, independent trials for each of the molecular subgroups of interest; however, it is important to note that conducting these trials independently would require a much larger number of patients that would need to be screened collectively.
Key Basket and Umbrella Trials in Precision Oncology
NCI Molecular Analysis for Therapy Choice
The NCI‐MATCH Trial (the NCI's Molecular Analysis for Therapy Choice) is a phase 2 basket trial for patients with advanced refractory solid tumors, lymphomas, or multiple myeloma who have progressed on their previous treatment. This trial started in 2015 with an overall goal of evaluating tumor‐agnostic approaches in treatment selection by matching targeted therapies based on genetic make‐up across multiple tumors. As a nonrandomized trial, NCI‐MATCH would assign a specific, targeted therapy for each of the molecular subgroups, where 31 patients would be enrolled to each group and assessed for their objective response (OR); if a targeted therapy shows an OR ≥16% (n ≥ 5 of 31), then the protocol has specified that targeted therapy would be deemed promising and worthy of further testing.35 According to clinicaltrials.gov (identifier NCT02465060), as of November 22, 2019, there are 37 molecular subgroups that either already have been tested or currently are being tested.
NCI Molecular Profiling‐Based Assignment of Cancer Therapy
NCI‐MPACT (the NCI's Molecular Profiling‐Based Assignment of Cancer Therapy) is another basket trial that aims to test tumor‐agnostic approaches in targeted treatment selection.36, 37 NCI‐MPACT is a phase 2 randomized clinical trial for patients with advanced refractory solid tumors. In this basket trial, 20 genes belonging to 3 pathways of RAS/RAF/MEK (5 genes), PI3K/mTOR/AKT (5 genes), and DNA repair (10 genes) are being evaluated as treatment selection approaches of 4 potential targeted therapies.36, 37 For the RAS pathway, trametinib (an MEK inhibitor) is being evaluated as a targeted therapy; everolimus (an mTOR inhibitor) is being evaluated for the PI3K pathway; and, for the DNA repair pathway, 2 regimens of veliparib (a poly[ADP‐ribose] polymerase inhibitor) plus temozolomide (an alkylating agent) and adavosertib (a tyrosine kinase WEE1 inhibitor) plus carboplatin are being evaluated.38 For each regimen, patients harboring the corresponding genetic mutations are being randomized 2:1 into the targeted therapy or a different therapy that does not target the respective genetic pathway.38 The target recruitment is 180 evaluable patients (120 to experimental intervention and 60 to control), for 88% statistical power with a 4% one‐sided type I error rate to detect an overall difference of 20% versus 5% for objective response outcome and 90% statistical power and a 1% one‐sided type I error rate for an 80% increase in median progression‐free survival.38 This trial has been ongoing since 2013 and is expected to finish by May 2020 (clinicaltrials.gov identifier NCT01827384).
Lung Cancer Master Protocol
Lung‐MAP (Lung Cancer Master Protocol) is an umbrella trial for patients with advanced squamous non–small‐cell lung cancer (NSCLC) that started in June 2014 with 5 sub‐studies (S1400; Biomarker‐Targeted Second‐Line Therapy in Treating Patients With Recurrent Stage IV Squamous Cell Lung Cancer, clinicaltrials.gov identifier NCT02154490).39 Initially, patients were assigned to the first sub‐study (S1400A) and received durvalumab, an anti–PD‐L1 (antiprogrammed cell death‐ligand 1) monoclonal antibody, if their tumors did not have actionable molecular alterations of interest that were used to assign patients into the other biomarker‐driven sub‐studies.40 The first sub‐study was a single‐arm, nonmatch study that did not involve investigation of a targeted therapy, whereas the other 4 sub‐studies were biomarker‐driven and investigated targeted therapies with 2‐arm, seamless phase 2 and 3 randomized clinical trial designs.39, 40 The second sub‐study (S1400B), investigating the PI3K inhibitor taselisib, included patients with a PIK3CA mutation41; the third sub‐study (S1400C), investigating the selective CDK4/CDK6 inhibitor palbociclib, included patients with CDK4, CCND1, CCND2, or CCND3 amplification42; and the fourth sub‐study (S1400D) investigated AZD4547 (an FGFR inhibitor) among patients with FGFR1, FGFR2, or FGFR3 mutation, fusion, or amplification.39 These 3 sub‐studies used docetaxel as the control.39 The fifth sub‐study (S1400E) was designed to investigate rilotumumab versus erlotinib for patients with MET mutations, but it closed because of withdrawal by the manufacturer, who observed toxicity of rilotumumab from other independent phase 3 trials.39 Lung‐MAP later added 2 more nonmatch sub‐studies (S1400F and S1400I) and 2 other biomarker‐driven sub‐studies (S1400G and S1400K) for patients with squamous NSCLC; and, on January 28, 2019, Lung‐MAP was expanded to all histologic types of NSCLC under a new screening protocol (Lung‐MAP: A Master Screening Protocol for Previously‐Treated Non–Small‐Cell Lung Cancer, NCT03851445) that aims to test other targeted and nontargeted therapies under a single master protocol.43
Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trial
ALCHEMIST (the Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trial) is an umbrella trial that started in August 2014 for patients with operable, early stage (Stage IB‐IIIA) lung adenocarcinoma, which is a common histological subtype of nonsquamous NSCLC.44 There are 4 subprotocols of ALCHEMIST: 1) ALCHEMIST Screening (A151216; clinicaltrials.gov identifier NCT02194738), 2) ALCHEMIST‐EGFR (A081105; NCT02193282), 3) ALCHEMIST‐ALK subprotocol (E4512; NCT02201992), and 4) ALCHEMIST‐Immunotherapy Treatment Trial (ANVIL) (EA5142; NCT02595944).
ALCHEMIST Screening has been established for the centralized screening of patients with adenocarcinoma across the United States for a genomic analysis of EGFR mutations and ALK rearrangements. ALCHEMIST Screening will screen up to 8000 patients with adenocarcinoma before or after surgical resection, and, based on their genomic analysis, they will be assigned to other ALCHEMIST subprotocols. The patients with EGFR mutations will be assigned to the ALCHEMIST‐EGFR subprotocol for an assessment of the clinical efficacy of tarceva (an EGFR inhibitor), and the patients with ALK rearrangements will be assigned to the ALCHEMIST‐ALK subprotocol for an assessment of crizotinib (an ALK and ROS1 inhibitor). Finally, patients who do not have EGFR or ALK mutations will be assigned to the forth subprotocol ANVIL, studying nivolumab (a PD‐L1 inhibitor).45 These treatment trials are phase 3, randomized clinical trials with the primary endpoint of overall survival (OS). ALCHEMIST‐EGFR and ALCHEMIST‐ALK will use placebo as the control, and ANVIL will use an observation arm as the control. ALCHEMIST‐EGFR plans to enroll 410 patients for 85% statistical power and a 5% one‐sided type I error rate to detect an OS hazard ratio of 0.67; ALCHEMIST‐ALK plans to enroll 378 patients for 80% statistical power and a 5% one‐sided type I error rate for the same treatment effect size.46 ANVIL, conversely, plans to recruit 714 patients to detect a 30% improvement in OS and/or a 33% reduction in disease‐free survival favoring nivolumab.45
Key Recent FDA Approvals for Precision Oncology
Table 2, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 highlights several of the key targeted precision oncology therapies that have been recently approved by the FDA. Currently, there are 3 FDA‐approved tumor‐agnostic therapies. These tumor‐agnostic therapies have not been approved solely based on evidence generated from basket trials. However, it is important to note that the evidence used for these approvals closely followed the core assumption of basket trials: the unification of disease. For example, pembrolizumab, a programmed cell death protein 1 inhibitor, is the first tumor‐agnostic therapy that received an accelerated approval from the FDA in March 2017 for adult and pediatric patients who have unresectable or metastatic solid tumors with microsatellite instability‐high (MSI‐H) or deficient mismatch repair (dMMR).47 This approval was based on a pooled analysis of tumor response rate and duration observed from 149 patients with 15 different tumors from 5 single‐arm trials: KEYNOTE‐016 (Study of MK‐3475 in Patients With Microsatellite Unstable [MSI] Tumors [Cohorts A, B, and C]; clinicaltrials.gov identifier NCT01876511), KEYNOTE‐164 (Study of Pembrolizumab [MK‐3475] as Monotherapy in Participants With Previously Treated Locally Advanced Unresectable or Metastatic Colorectal Cancer; NCT02460198), KEYNOTE‐158 (Study of Pembrolizumab [MK‐3475] in Participants With Advanced Solid Tumors; NCT02628067), KEYNOTE‐012 (Study of Pembrolizumab [MK‐3475] in Participants With Advanced Solid Tumors; NCT01848834), and KEYNOTE‐028 (Study of Pembrolizumab [MK‐3475] in Participants With Advanced Solid Tumors; NCT02054806).47, 48 The patients across these trials had common unifying risk factors in the form of MSI‐H and dMMR mutations.
Table 2.
Key Recent US Food and Drug Administration Approvals for Precision Oncology Targeted Therapies
| THERAPY NAME (APPROVAL DATE) | REFERENCES | MECHANISM OF ACTION | INDICATION | APPROVAL TYPE | CLINICAL TRIAL EVIDENCE | SUMMARY OF TRIAL EVIDENCE |
|---|---|---|---|---|---|---|
| Pembrolizumab (March 2017) | FDA 2017,47 Marcus 2019,48 Le 201549 | PD‐1 inhibitor | Adult and pediatric patients with unresectable or metastatic solid tumors with MSI‐H or dMMR | Accelerated approval based on tumor response rate and duration | KEYNOTE‐016 (NCT01876511), KEYNOTE‐164 (NCT02460198), KEYNOTE‐158 (NCT02628067), KEYNOTE‐012 (NCT01848834), and KEYNOTE‐028 (NCT02054806) | Approval based on a pooled analysis of 5 single‐arm trials |
| Larotrectinib (November 2018) | FDA 2018,50 Chen & Chi 2019,51 Drilon 201852 | TRK inhibitor | Adult and pediatric patients with unresectable or metastatic solid tumors with neurotropic TRK fusion | Accelerated approval based on tumor response rate and duration | LOXO‐TRK‐14001 (NCT02122913), NAVIGATE (NCT02576431), and SCOUT (NCT02637687) | Approval based on a pooled analysis of 3 single‐arm trials |
| Entrectinib (August 2019)a | FDA 2019,53 Demetri 201854 | TKI inhibitor | Adult and pediatric patients with unresectable or metastatic solid tumors with neurotropic TRK fusion without treatment options | Accelerated approval based on tumor response rate and duration | ALKA‐372‐001 (EudraCT 2012‐000148‐88), STARTRK‐1 (NCT02097810), and STARTRK‐2 (NCT02568267) | Approval based on a pooled analysis of 3 single‐arm trials |
| Vemurafenib (November 2017)b | FDA 2017,55 Hyman 2015,56 Diamond 201857 | BRAF enzyme inhibitor | Erdheim‐Chester disease with BRAF V600 mutation | Regular approval | VE‐Basket (NCT01524978) | Approval based on “other solid tumor” cohort of a multicohort phase 2 basket trial |
Abbreviations: ALKA‐372‐001, phase 1/2 entrectinib trial; EudraCT, European Union Drug Regulating Authorities Clinical Trials Database; FDA, US Food and Drug Administration; dMMR, deficient mismatch repair; KEYNOTE‐012, Study of Pembrolizumab (MK‐3475) in Participants With Advanced Solid Tumors; KEYNOTE‐016, Study of MK‐3475 in Patients With Microsatellite Unstable (MSI) Tumors (Cohorts A, B, and C); KEYNOTE‐028, Study of Pembrolizumab (MK‐3475) in Participants With Advanced Solid Tumors; KEYNOTE‐158, Study of Pembrolizumab (MK‐3475) in Participants With Advanced Solid Tumors; KEYNOTE‐164, Study of Pembrolizumab (MK‐3475) as Monotherapy in Participants With Previously Treated Locally Advanced Unresectable or Metastatic Colorectal Cancer; LOXO‐TRK‐14001, A Study to Test the Safety of the Investigational Drug Larotrectinib in Adults That May Treat Cancer; MSI‐H, microsatellite instability‐high; NAVIGATE, A Study to Test the Effect of the Drug Larotrectinib in Adults and Children With NTRK‐fusion Positive Solid Tumors; NCT, National Clinical Trials (clinicaltrials.gov identifier); PD‐1, programmed cell death protein 1; SCOUT, A Study to Test the Safety and Efficacy of the Drug Larotrectinib for the Treatment of Tumors With NTRK‐fusion in Children; STARTRK‐1, A Study of Oral RXDX‐101 in Adult Patients With Locally Advanced or Metastatic Cancer Targeting NTRK1, NTRK2, NTRK3, ROS1, or ALK Molecular Alterations; STARTRK‐2, Basket Trial of Entrectinib (RXDX‐101) for the Treatment of Patients With Solid Tumors Harboring NTRK 1/2/3 (Trk A/B/C), ROS1, or ALK Gene Rearrangements (Fusions); TKI, tyrosine kinase inhibitor; TRK, tropomyosin kinase receptor; VE‐Basket, A Study of Vemurafenib in Participants With BRAF V600 Mutation‐Positive Cancers.
Entrectinib was also approved for an indication of adults with metastatic non–small‐cell lung cancer with receptor tyrosine kinase 1 (ROS1) positivity.
Vemurafenib was approved in August 2011 for the treatment of unresectable or metastatic melanoma patients with BRAF V600E mutation (see Kim 201458).
Larotrectinib, a tropomyosin kinase receptor (TRK) inhibitor, is the second tumor‐agnostic therapy to be approved by the FDA.50 On November 2018, the FDA granted an accelerated approval to larotrectinib for adult and pediatric patients with unresectable or metastatic solid tumors with neurotropic TRK fusion.50 Larotrectinib was approved based on tumor response rates and duration observed from a pooled analysis of 55 patients with 16 different tumors from three single‐arm clinical trials: LOXO‐TRK‐14001 (A Study to Test the Safety of the Investigational Drug Larotrectinib in Adults That May Treat Cancer; clinicaltrials.gov identifier NCT02122913), SCOUT (A Study to Test the Safety and Efficacy of the Drug Larotrectinib for the Treatment of Tumors With NTRK‐fusion in Children; NCT02637687), and NAVIGATE (A Study to Test the Effect of the Drug Larotrectinib in Adults and Children With NTRK‐fusion Positive Solid Tumors; NCT02576431).50, 51, 52 TRK fusions in these trials were identified by either next‐generation sequencing or by fluorescence in situ hybridization according to the procedures and analytic pipelines established by each laboratory.52 Entrectinib, a tyrosine kinase inhibitor (TKI) is the third tumor‐agnostic therapy to receive an accelerated approval from the FDA on August 2019.53 Similar to the two other tumor‐agnostic therapies, entrectinib was approved based on a pooled analysis of three single‐arm clinical trials: ALKA (European Union Drug Regulating Authorities Clinical Trials Database, 2012‐000148‐88), STARTRK‐1 (A Study of Oral RXDX‐101 in Adult Patients With Locally Advanced or Metastatic Cancer Targeting NTRK1, NTRK2, NTRK3, ROS1, or ALK Molecular Alterations; clinicaltrials.gov identifier NCT02097810), and STARTRK‐2 (Basket Trial of Entrectinib [RXDX‐101] for the Treatment of Patients With Solid Tumors Harboring NTRK 1/2/3 [Trk A/B/C], ROS1, or ALK Gene Rearrangements [Fusions]; NCT02568267).53, 54
Vemurafenib is another key FDA‐approved targeted therapy for precision oncology. This BRAF kinase inhibitor was first approved in 2011 for the treatment of metastatic melanoma with BRAF V600 mutation.58 Recently in November 2017, vemurafenib was approved for adult patients with Erdheim‐Chester disease (ECD) who have BRAF V600 mutations, making it the first FDA‐approved therapy for this rare blood cancer.55 This approval was based on the results of VE‐BASKET (A Study of Vemurafenib in Participants With BRAF V600 Mutation‐Positive Cancers; clinicaltrials.gov identifier NCT01524978), a multicohort phase 2 basket trial with a nonrandomized design.55 In VE‐BASKET, patients who had nonmelanoma cancers with BRAF V600 mutations were enrolled in 7 cohorts, and the objective response rate was the primary outcome of the trial.56 Patients with ECD (n = 22 of 26) and Langerhans cell histiocytosis (n = 4 of 26) who had BRAF mutations were enrolled in an “other solid tumor” cohort this basket trial.57 An objective response rate of 54.5% (95% CI, 32.2%‐76.6%) was observed among the enrolled patients who had ECD with BRAF mutations in this trial.57
To our knowledge, no umbrella trials have led to FDA approvals currently (February 2020), and vemurafenib is the only targeted therapy that has been approved by the FDA using a basket trial design. This is not surprising because many basket and umbrella trials are currently ongoing, and they are largely exploratory in nature, highlighting how master protocol designs are at an early stage of development and adoption.13 For these reasons, it is currently difficult to assess the successes and failures of these designs that have been used to date.
Key Considerations for Basket and Umbrella Trials
Biologic Plausibility
There are several key design considerations for basket and umbrella trials (Table 3). First and foremost, it is important to consider the biological plausibility of the targeted intervention strategies being evaluated. Basket and umbrella trial designs have both been developed under the core principle of precision medicine, which aims to tailor medical intervention based on the patient's characteristics that make them more likely to respond to that intervention (ie, predictive risk factors). Therefore, the underlying biological plausibility assumption is critical for these trials because information on the diseases being studied and the treatment's mechanisms of actions will be used to derive targeted intervention strategies. For instance, it is common for cancers to have multiple genetic mutations; however, it is important to note that only some of these may be driver mutations for the carcinogenic process, and most mutations are passenger mutations that do not affect the underlying carcinogenic process.59 Intervention strategies, of course, should be targeting driver mutations, but it can be difficult to differentiate driver mutations from passenger mutations.59, 60 Careful consideration of the preclinical evidence and underlying biological models informing the targeted intervention strategies will need to be made for critical appraisals of both basket and umbrella trials.
Table 3.
Key Considerations Required for Basket and Umbrella Trials
| KEY CONSIDERATIONS | DETAILS |
|---|---|
| Biologic plausibility |
|
| Accuracy of biomarker tests |
|
| |
| Biospecimen collection |
|
| Biomarker prevalence |
|
| Sample size and assumptions |
|
| |
| |
| |
| Randomization |
|
| |
| |
|
Accuracy of Biomarker Assays
In addition to biomarker plausibility, it is critical to consider the accuracy of biomarker assays that are used in basket and umbrella trials. Conceptually, a targeted intervention should be more efficacious against diseases that demonstrate characteristics of the biomarker target versus diseases that do not possess the target. However, all medical tests will have some degree of diagnostic inaccuracy, so a proportion of biomarker false‐negative and false‐positive patients are expected in biomarker‐guided basket and umbrella trials. It has been shown that increasing false‐positive rates of biomarker tests will reduce the statistical power in early exploratory biomarker‐guided trials; although it has not been a common practice, false‐positive rates of biomarker tests should be incorporated into the planning of these biomarker‐guided trials.61 Careful considerations of the accuracy of biomarker assays are important for exploratory (ie, phase 2) basket and umbrella trials to improve the probability of selecting suitable candidates for further testing (ie, phase 3).61 For basket trials, it is important that the accuracies of biomarker assays are similar between different tumor types.
Biospecimen Collection
Careful considerations for biospecimen collection procedures will be important, particularly for basket trials that involve multiple histological tumors. Ease of biospecimen collection, biospecimen quality, and biospecimen yield should be similar between different tumors. Outside of direct measures of test performance, appropriate biopsy yield can be challenging.62 Even at high‐throughput centers with skilled technicians, a yield as low as 70% for adequate lung adenocarcinoma molecular profiling by biopsy has been reported.63 Advances in sampling methodologies and techniques such as liquid biopsy64 may represent less invasive ways to investigate molecular profiling, but their test performance must be carefully considered against their benefits to patient experience, and the importance of histopathological examination should not be underestimated.65
Biomarker Prevalence
Patient recruitment and statistical power are critical for any clinical trials.66 Thus, it is important to consider the prevalence of biomarkers that will be used in both basket and umbrella trials because the biomarker prevalence will affect the size of the patient pool eligible for these biomarker‐guided trials. A low biomarker prevalence would translate into a small pool of eligible patients, and a high prevalence would translate into a larger patient pool. If the biomarker prevalence is low for basket trials, there may be serious recruitment challenges in that it may not be feasible to recruit the planned sample size within the planned duration of the trial. Similarly, if the biomarker prevalence is low in one or more arms in umbrella trials, it will be difficult to recruit patients harboring the specific mutation for the intervention arm(s). Planning for comprehensive recruitment strategies to reach the target sample size within the trial duration therefore will be especially vital for biomarker‐guided basket and umbrella trials in which the prevalence of biomarker‐positive patients is low.
Sample Sizes and Assumptions
As with all types of clinical trials, sample size considerations are important for basket and umbrella trials. The sample size for a given basket or umbrella trial will depend on its clinical phase. Sample size requirements for exploratory (phase 2) trials are smaller than those for confirmatory (phase 3) trials because exploratory trials act as a screening tool to assess whether an intervention warrants further investigation.67 In exploratory trials, single‐arm designs without a control arm (nonrandomized design, phase 2A) or randomized designs (phase 2B) may be used.61, 67 For single‐arm designs, the FDA recommends that the planned sample size should be sufficient to rule out a clinically unimportant treatment effect in nonrandomized designs, and they recommend designs such as the Simon 2‐stage design to limit exposure to an ineffective intervention.12, 68
For basket trials, sample size calculations may be done for the overall cohort that consists of multiple diseases. If this is the case, one treatment effect size will be used as an input for the sample size calculation. That is, the underlying assumption of the common predictive risk factor being used for the unification of diseases in a given basket trial must be valid. For instance, if the targeted therapy being studied in a multihistology basket trial has different treatment effects between different tumors (eg, the treatment only works well in one tumor), then the clinical efficacy of the therapy may be underestimated because the overall treatment effect observed may be diluted due to nonresponding tumors that were included in the trial. In basket trials that show nonpromising results overall, it can be difficult to determine whether and which disease subtype(s) may respond to the therapy being studied because they are subgroups.
The sample size calculations for umbrella trials, conversely, may be done for each of the subgroups because there are multiple targeted interventions being evaluated in umbrella trials. If possible, the FDA has recommended the use of a common control arm for umbrella trials.12 Regardless, similar to basket trials, the assumptions that the risk factors being used are predictive are also critical for umbrella trials. For example, if a given intervention is truly effective and the predictive risk factor assumption is erroneous, the clinical efficacy of the given targeted therapy will only be investigated in a subpopulation instead of the general population (eg, all comers). Therefore, an all‐comers design strategy instead of the enrichment trial design strategy used in umbrella trials may be better if the predictive risk factor assumption is erroneous, because the all‐comers strategy would have found the intervention to be effective for the entire general disease population.
If the predictive risk factor assumption is valid (or reasonable), the basket and umbrella trial designs would be more favorable than the all‐comers designs. The sample size requirements for a given treatment effect size will be similar between the enrichment and all‐comers design approaches. However, recruitment will be more favorable for basket trials that can recruit from pools of patients with multiple diseases; conversely, it may be difficult to recruit patients for umbrella trials given that recruitment can only be done from a subset of one patient disease pool.
Randomization: Predictive Versus Prognostic Factors
Predictive risk factors refer to patient characteristics that are associated with their response (or lack of response) to a particular intervention.69, 70 Predictive risk factors are used in both basket and umbrella trials to inform their targeted intervention strategies. Prognostic factors, conversely, refer to patient characteristics that affect the clinical outcome independent of the intervention.69, 70 The patient's clinical outcome is determined by their prognostic risk factors, regardless whether they were treated or not, so it is important to distinguish between predictive and prognostic risk factors when assessing intervention utility. However, in basket and umbrella trials that do not use randomization, it may be difficult to determine whether the biomarker used for a given intervention strategy is a predictive factor or a prognostic factor.
Randomization removes selection bias and tends to produce groups that are comparable in terms of both measurable and unmeasurable factors.71 Randomization therefore helps to ascribe the observed difference in the treatment effects to the interventions that are being compared in a given trial, allowing for the establishment of causality.71, 72 In single‐arm basket and umbrella trials, it can be difficult to differentiate between predictive and prognostic factors. For instance, the clinical efficacy of a given experimental intervention may be overestimated in single‐arm basket trials with a unifying risk factor that has a favorable prognosis. Similarly, in umbrella trials, the subgroup arm(s) with a favorable prognostic risk factor may result in overestimated treatment effects. Conversely, if an unfavorable prognostic risk factor is used as the predictive intervention strategy, the treatment effects may be underestimated in both basket and umbrella trials. In these trials, randomization can help determine whether the risk factors being used as part of the targeted intervention strategies are indeed predictive, because randomization can help achieve a balance of measurable and unmeasurable prognostic factors between the experimental and control groups.
If randomization is not feasible, it may be possible to make statistical adjustments to ameliorate potential biases and imbalances to differentiate predictive risk factors versus prognostic risk factors. However, statistical adjustments are difficult in smaller data sets, so even adjusted analyses cannot lead to unbiased estimates if the sample size is small.73 Even if there is adequate sample size, it is important to note that statistical adjustments can only account for measurable factors. Thus the use of randomization is generally preferable.
Bias Assessment of Basket and Umbrella Trials
Currently, there is no existing risk‐of‐bias assessment tool that is specific for basket and umbrella trials. As these trials may be conducted with randomized or nonrandomized trial designs, the readers can use 2 of Cochrane's existing risk‐of‐bias assessment tools: Cochrane's revised risk of bias assessment tool (RoB 2)74 and risk of bias in nonrandomized studies of interventions (ROBINS‐I).75 The RoB 2 tool, which is intended for bias assessment of individually randomized clinical trials, covers important bias domains of randomization, deviation from intended interventions, missing outcome, measurement of outcome, and selective reporting.74 The ROBINS‐1 tool, which has been developed for bias assessment of nonrandomized intervention studies, covers similar bias domains as the RoB 2 tool (ie, deviation of intended interventions, missing outcome, measurement of outcome, and selective reporting) and 3 additional bias domains: confounding prognostic variables, selection of participants, and misclassification of interventions that are related and unrelated to the outcome.75 Table 3 outlines important additional points that the readers should consider for basket and umbrella trial publications. For instance, as it is important to consider the accuracy of biomarker assays, the readers should consider the assay's accuracy for possible misclassification of targeted interventions for randomized basket or umbrella trials, even if the available bias assessment tools do not specifically cover this bias domain.
Conclusion
Methodological advancements in basket and umbrella trials will help catalyze the adoption of precision medicine and oncology into clinical practice. This primer on basket and umbrella trials outlines key characteristics and examples of basket and umbrella trials as well as several important considerations that need to be made for these clinical trials in terms of biological plausibility, biomarker test accuracy and prevalence, sample sizes, and predictive or prognostic significance of the biomarker used for the targeted intervention strategies. This primer can also be used by clinical readers to critically evaluate future publications on basket and umbrella trials. Because increasing numbers of basket and umbrella trials will continue to be published, the current review serves to improve the general scientific literacy regarding these clinical trial designs.
DISCLOSURES : The authors report no conflicts of interest.
References
- 1. Kumar‐Sinha C, Chinnaiyan AM. Precision oncology in the age of integrative genomics. Nat Biotechnol. 2018;36:46‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abrams J, Conley B, Mooney M, et al. National Cancer Institute's precision medicine initiatives for the new national clinical trials network. Am Soc Clin Oncol Educ Book. 2014;34:71‐76. [DOI] [PubMed] [Google Scholar]
- 3. Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ashley EA. The precision medicine initiative: a new national effort. JAMA. 2015;313:2119‐2120. [DOI] [PubMed] [Google Scholar]
- 5. Ashley EA. Towards precision medicine. Nat Rev Genet. 2016;17:507‐522. [DOI] [PubMed] [Google Scholar]
- 6. National Institutes of Health . All of Us Research Program Overview. All of Us and Precision Medicine. Accessed December 19, 2019. http://allofus.nih.gov/about/about-all-us-research-program
- 7. The National Health Service (NHS) . The NHS Long Term Plan. NHS; 2019. Accessed June 20, 2019. http://longtermplan.nhs.uk/wp-content/uploads/2019/01/nhs-long-term-plan-june-2019.pdf [Google Scholar]
- 8. Heckman‐Stoddard BM, Smith JJ. Precision medicine clinical trials: defining new treatment strategies. Semin Oncol Nurs. 2014;30:109‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berry DA. The Brave New World of clinical cancer research: adaptive biomarker‐driven trials integrating clinical practice with clinical research. Mol Oncol. 2015;9:951‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee JJ, Liu S, Chen N. Biomarker‐based Bayesian adaptive designs for targeted agent development—implementation and lessons learned from the BATTLE Trial. Accessed December 19, 2019? http://www.newton.ac.uk/files/seminar/20110815140014452-152818.pdf
- 11. Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377:62‐70. [DOI] [PubMed] [Google Scholar]
- 12. US Department of Health and Human Services, Food and Drug Administration (FDA) . Guidance Document. Master Protocols: Efficient Clinical Trial Design Strategies to Expedite Development of Oncology Drugs and Biologics Guidance for Industry. Guidance for Industry. FDA; 2018. Accessed December 19, 2019. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM621817.pdf [Google Scholar]
- 13. Park JJH, Siden E, Zoratti MJ, et al. Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials. 2019;20:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siden EG, Park JJ, Zoratti MJ, et al. Reporting of master protocols towards a standardized approach: a systematic review. Contemp Clin Trials Commun. 2019;15:100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirakawa A, Asano J, Sato H, Teramukai S. Master protocol trials in oncology: review and new trial designs. Contemp Clin Trials Commun. 2018;12:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lam VK, Papadimitrakopoulou V. Master protocols in lung cancer: experience from Lung Master Protocol. Curr Opin Oncol. 2018;30:92‐97. [DOI] [PubMed] [Google Scholar]
- 17. Ledford H. “Master protocol” aims to revamp cancer trials: pilot project will bring drug companies together to test targeted lung‐cancer therapies. Nature. 2013;498:146‐148. [DOI] [PubMed] [Google Scholar]
- 18. Redman MW, Allegra CJ. The master protocol concept. Semin Oncol. 2015;42:724‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Renfro LA, Sargent DJ. Statistical controversies in clinical research: basket trials, umbrella trials, and other master protocols: a review and examples. Ann Oncol. 2017;28:34‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ornes S. Core concept: basket trial approach capitalizes on the molecular mechanisms of tumors. Proc Natl Acad Sci U S A. 2016;113:7007‐7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li BT, Makker V, Buonocore DJ, et al. A multi‐histology basket trial of ado‐trastuzumab emtansine in patients with HER2 amplified cancers [abstract]. J Clin Oncol. 2018;36(15 suppl):2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li BT, Shen R, Buonocore D, et al. Ado‐Trastuzumab emtansine for patients with HER2‐mutant lung cancers: results from a phase II basket trial. J Clin Oncol. 2018;36:2532‐2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2‐positive advanced breast cancer. N Engl J Med. 2012;367:1783‐1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amiri‐Kordestani L, Blumenthal GM, Xu QC, et al. FDA approval: ado‐trastuzumab emtansine for the treatment of patients with HER2‐positive metastatic breast cancer. Clin Cancer Res. 2014;20:4436‐4441. [DOI] [PubMed] [Google Scholar]
- 25. Parmar MK, Sydes MR, Cafferty FH, et al. Testing many treatments within a single protocol over 10 years at MRC Clinical Trials Unit at UCL: multi‐arm, multi‐stage platform, umbrella and basket protocols. Clin Trials. 2017;14:451‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Turner N, Bye H, Kernaghan S, et al. Abstract OT1‐06‐03: The plasmaMATCH trial: a multiple parallel cohort, open‐label, multi‐centre phase II clinical trial of ctDNA screening to direct targeted therapies in patients with advanced breast cancer (CRUK/15/010). Cancer Res. 2018;78(4 suppl):OT1‐06‐03. [Google Scholar]
- 27. Cancer Research UK . A trial using a blood test to find certain gene changes and decide treatment for advanced breast cancer (plasmaMATCH). Cancer Research UK; 2019. Accessed September 9, 2019. https://www.cancerresearchuk.org/about-cancer/find-a-clinical-trial/a-trial-using-a-blood-test-to-find-certain-gene-changes-and-decide-treatment-for-advanced-breast#undefined [Google Scholar]
- 28. Covens AL, Filiaci V, Gersell D, Lutman CV, Bonebrake A, Lee YC. Phase II study of fulvestrant in recurrent/metastatic endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2011;120:185‐188. [DOI] [PubMed] [Google Scholar]
- 29. Rabindran SK, Discafani CM, Rosfjord EC, et al. Antitumor activity of HKI‐272, an orally active, irreversible inhibitor of the HER‐2 tyrosine kinase. Cancer Res. 2004;64:3958‐3965. [DOI] [PubMed] [Google Scholar]
- 30. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP‐ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123‐134. [DOI] [PubMed] [Google Scholar]
- 31. De Benedetti F, Gattorno M, Anton J, et al. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N Engl J Med. 2018;378:1908‐1919. [DOI] [PubMed] [Google Scholar]
- 32. Muhlbacher J, Jilma B, Wahrmann M, et al. Blockade of HLA antibody‐triggered classical complement activation in sera from subjects dosed with the anti‐C1s monoclonal antibody TNT009—results from a randomized first‐in‐human phase 1 trial. Transplantation. 2017;101:2410‐2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. The ASCO Post . IMPACT Trial Matches Treatment to Genetic Changes in the Tumor to Improve Survival Across Multiple Cancer Types. HSP News Service, LLC; 2018. Accessed December 19, 2019. http://ascopost.com/News/58897 [Google Scholar]
- 34. Le Tourneau C, Delord JP, Goncalves A, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open‐label, proof‐of‐concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16:1324‐1334. [DOI] [PubMed] [Google Scholar]
- 35. Moore KN, Mannel RS. Is the NCI MATCH trial a match for gynecologic oncology? Gynecol Oncol. 2016;140:161‐166. [DOI] [PubMed] [Google Scholar]
- 36. Lih CJ, Sims DJ, Harrington RD, et al. Analytical validation and application of a targeted next‐generation sequencing mutation‐detection assay for use in treatment assignment in the NCI‐MPACT Trial. J Mol Diagn. 2016;18:51‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lih CJ, Takebe N. Considerations of developing an NGS assay for clinical applications in precision oncology: the NCI‐MATCH NGS assay experience. Curr Probl Cancer. 2017;41:201‐211. [DOI] [PubMed] [Google Scholar]
- 38. Kummar S, Williams M, Lih C‐J, et al. NCI mpact: National Cancer Institute molecular profiling‐based assignment of cancer therapy [abstract]. J Clin Oncol. 2014;32(15 suppl):tps2642. [Google Scholar]
- 39. Ferrarotto R, Redman MW, Gandara DR, Herbst RS, Papadimitrakopoulou VA. Lung‐MAP—framework, overview, and design principles. Chin Clin Oncol. 2015;4:36. [DOI] [PubMed] [Google Scholar]
- 40. Papadimitrakopoulou V, Redman M, Borghaei H, et al. 83O A phase II study of durvalumab (MEDI4736) for previously treated patients with stage IV squamous NSCLC (SqNSCLC): Lung‐MAP sub‐study SWOG S1400A. Ann Oncol. 2017;28(suppl 2):mdx091.003. [Google Scholar]
- 41. Wade JL, Langer CJ, Redman M, et al. A phase II study of GDC‐0032 (taselisib) for previously treated PI3K positive patients with stage IV squamous cell lung cancer (SqNSCLC): LUNG‐MAP sub‐study SWOG S1400B. [abstract]. J Clin Oncol. 2017;35(15 suppl):9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Edelman MJ, Redman MW, Albain KS, et al. A phase II study of palbociclib (P) for previously treated cell cycle gene alteration positive patients (pts) with stage IV squamous cell lung cancer (SCC): Lung‐MAP sub‐study SWOG S1400C [abstract]. J Clin Oncol. 2017;35(15 suppl):9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Southwest Oncology Group (SWOG) Cancer Research Network . A Master Protocol to Evaluate Biomarker‐driven Therapies and Immunotherapies in Previously‐Treated Non‐Small Cell Lung Cancer (Lung‐MAP Screening Study). SWOG; 2019. Accessed November 19, 2019. http://swog.org/clinical-trials/lungmap [Google Scholar]
- 44. Govindan R, Mandrekar SJ, Gerber DE, et al. ALCHEMIST trials: a golden opportunity to transform outcomes in early‐stage non‐small cell lung cancer. Clin Cancer Res. 2015;21:5439‐5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chaft JE, Dahlberg SE, Khullar OV, et al. EA5142 adjuvant nivolumab in resected lung cancers (ANVIL) [abstract]. J Clin Oncol. 2018;36(15 suppl):TPS8581. [Google Scholar]
- 46. Gerber DE, Oxnard GR, Mandrekar SJ, et al. ALCHEMIST: a clinical trial platform to bring genomic discovery and molecularly targeted therapies to early‐stage lung cancer [abstract]. J Clin Oncol. 2015;33(15 suppl):TPS7583. [Google Scholar]
- 47. US Food and Drug Administration (FDA) . FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. FDA; 2017. Accessed December 19, 2019. http://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication [Google Scholar]
- 48. Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability‐high solid tumors. Clin Cancer Res. 2019;25:3753‐3758. [DOI] [PubMed] [Google Scholar]
- 49. Le DT, Uram JN, Wang H, et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med. 2015;372:2509‐2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. US Food and Drug Administration (FDA) . FDA approves larotrectinib for solid tumors with NTRK gene fusions. FDA; 2018. Accessed December 19, 2019. http://www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions-0 [Google Scholar]
- 51. Chen Y, Chi P. Basket trial of TRK inhibitors demonstrates efficacy in TRK fusion‐positive cancers. J Hematol Oncol. 2018;11:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion‐positive cancers in adults and children. N Engl J Med. 2018;378:731‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. US Food and Drug Administration (FDA) . FDA approves entrectinib for NTRK solid tumors and ROS‐1 NSCLC. FDA; 2019. Accessed December 19, 2019. http://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-entrectinib-ntrk-solid-tumors-and-ros-1-nsclc [Google Scholar]
- 54. Demetri GD, Paz‐Ares L, Farago AF, et al. LBA17. Efficacy and safety of entrectinib in patients with NTRK fusion‐positive (NTRK‐fp) tumors: pooled analysis of STARTRK‐2, STARTRK‐1 and ALKA‐372‐001 [abstract]. Ann Oncol. 2018;29(suppl 8):mdy424.017. [Google Scholar]
- 55. US Food and Drug Administration (FDA) . FDA approves first treatment for certain patients with Erdheim‐Chester Disease, a rare blood cancer. FDA; 2017. Accessed December 19, 2019. http://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-certain-patients-erdheim-chester-disease-rare-blood-cancer [Google Scholar]
- 56. Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373:726‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Diamond EL, Subbiah V, Lockhart AC, et al. Vemurafenib for BRAF V600‐mutant Erdheim‐Chester disease and Langerhans cell histiocytosis: analysis of data from the histology‐independent, phase 2, open‐label VE‐BASKET Study. JAMA Oncol. 2018;4:384‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim G, McKee AE, Ning YM, et al. FDA approval summary: vemurafenib for treatment of unresectable or metastatic melanoma with the BRAFV600E mutation. Clin Cancer Res. 2014;20:4994‐5000. [DOI] [PubMed] [Google Scholar]
- 59. Pon JR, Marra MA. Driver and passenger mutations in cancer. Annu Rev Pathol. 2015;10:25‐50. [DOI] [PubMed] [Google Scholar]
- 60. Brown AL, Li M, Goncearenco A, Panchenko AR. Finding driver mutations in cancer: elucidating the role of background mutational processes. PLoS Comput Biol. 2019;15:e1006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Park JJ, Harari O, Dron L, Mills EJ, Thorlund K. Effects of biomarker diagnostic accuracy on biomarker‐guided phase 2 trials. Contemp Clin Trials Commun. 2019;15:100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bubendorf L, Lantuejoul S, de Langen AJ, Thunnissen E. Nonsmall cell lung carcinoma: diagnostic difficulties in small biopsies and cytological specimens: number 2 in the series “Pathology for the Clinicia” edited by Peter Dorfmuller and Alberto Cavazza. Eur Respir Rev. 2017;26:170007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Iding JS, Krimsky W, Browning R. Tissue requirements in lung cancer diagnosis for tumor heterogeneity, mutational analysis and targeted therapies: initial experience with intra‐operative Frozen Section Evaluation (FROSE) in bronchoscopic biopsies. J Thorac Dis. 2016;8(suppl 6):S488‐S493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Arneth B. Update on the types and usage of liquid biopsies in the clinical setting: a systematic review. BMC Cancer. 2018;18:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112‐123. [DOI] [PubMed] [Google Scholar]
- 66. Huang GD, Bull J, Johnston McKee K, et al. Clinical trials recruitment planning: a proposed framework from the Clinical Trials Transformation Initiative. Contemp Clin Trials. 2018;66:74‐79. [DOI] [PubMed] [Google Scholar]
- 67. Brown SR, Gregory WM, Twelves CJ, et al. Designing phase II trials in cancer: a systematic review and guidance. Br J Cancer. 2011;105:194‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Simon R. Optimal two‐stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1‐10. [DOI] [PubMed] [Google Scholar]
- 69. Clark GM, Zborowski DM, Culbertson JL, et al. Clinical utility of epidermal growth factor receptor expression for selecting patients with advanced non‐small cell lung cancer for treatment with erlotinib. J Thorac Oncol. 2006;1:837‐846. [PubMed] [Google Scholar]
- 70. Clark GM. Prognostic factors versus predictive factors: examples from a clinical trial of erlotinib. Mol Oncol. 2008;1:406‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Friedman LM, Furberg C, DeMets DL, Reboussin D, Granger CB. Fundamentals of Clinical Trials. Springer; 2015. [Google Scholar]
- 72. Cartwright N. What are randomised controlled trials good for? Philos Stud. 2010;147:59. [Google Scholar]
- 73. Roberts C, Torgerson DJ. Understanding controlled trials: baseline imbalance in randomised controlled trials. BMJ. 1999;319:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 75. Sterne JA, Hernan MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]


