Abstract
It is becoming increasingly appreciated that intermediates of metabolic pathways, besides their anabolic and catabolic functions, can act as signaling molecules and influence the outcome of immune responses. Although lactate was previously considered as a waste product of glucose metabolism, accumulating evidence has highlighted its pivotal role in regulating diverse biological processes, including immune cell polarization, differentiation and effector functions. In addition, lactate is a key player in modulating tumor immune surveillance. Hence, targeting lactate‐induced signaling pathways is a promising tool to reduce inflammation, to prevent autoimmunity and to restore anti‐tumor immune response.
This article is characterized under:
Biological Mechanisms > Metabolism
Keywords: immunity, inflammation, lactate, metabolism, tumor
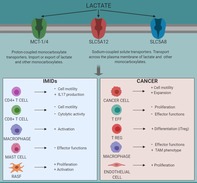
Immune cells ‘sense’ high concentration of lactate which accumulates at the site of inflammation or tumor as result of accelerated metabolism of immune, stromal, or cancer cells.
1. INTRODUCTION
Lactate, produced at the end of glycolysis, is a metabolite described for the first time at the beginning of the 20th century (Corbet & Feron, 2017; Figure 1). Its physiological concentration is in the range of 1.5–3 mM in blood and tissues. However, lactate levels can increase to 10 mM in several inflammatory conditions and to a concentration of 30–40 mM in tumors (Colegio et al., 2014; Haas et al., 2015; Hirschhaeuser, Sattler, & Mueller‐Klieser, 2011). Elevated amounts of lactate are produced as a consequence of increased glycolysis in proliferating cells (Warburg effect) via lactate dehydrogenase (LDH), an enzyme that also regenerates NAD+ from the reduced form of nicotinamide adenine dinucleotide (NADH), allowing a steady flow of glycolysis (Bonuccelli et al., 2010; Warburg, 1956). Lactate is present in solution either as lactic acid at low pH or as a salt (i.e., sodium lactate) at higher pH, with a pKa of 3.83 (Haas et al., 2015). During hypoxia (i.e., inflammation and cancer) lactate accumulates as a result of increased cell turnover and glycolytic engagement subsequent to the increased hypoxia‐inducible factor (HIF)‐1α response to low oxygen tension (Corcoran & O'Neill, 2016; Lee et al., 2015; Semenza, Roth, Fang, & Wang, 1994). Hypoxic conditions result in prolyl hydroxylase domain (PHD) inhibition and an attenuation of HIF‐1α hydroxylation and proteasomal degradation. HIF‐1α accumulates, translocates to the nucleus, and increases transcription of glycolytic genes. HIF‐1α activates pyruvate dehydrogenase kinase‐1 which phosphorylates and inactivates pyruvate dehydrogenase (PDH) (Kim, Tchernyshyov, Semenza, & Dang, 2006). PDH is normally responsible for the oxidation of pyruvate to acetyl‐CoA for mitochondrial oxidation and its inactivation limits the oxidative disposal of pyruvate favoring the diversion of the glycolytic flux to lactate. Inactivation of PDH by HIF‐1α is well known in exercise metabolism characterized by high glycolytic flux and lactate production (Brooks, 1986, 2009). During high‐intensity intermittent physical exercise, muscle can cope with glycolytic stress by shuttling lactate among tissues and using it as carbon source for energy production. For instance, lactate is a major gluconeogenic precursor acting as a shuttle in both muscle and liver (Cori & Cori, 1946). Indeed, lactate flux is metabolically beneficial through supplying carbons to gluconeogenesis during exercise (Brooks, 2009, 2018). In cancer and chronic inflammation, the persistent stimulation of glycolysis for the necessity of carbon sources is maladaptive and leads to an unbalanced shuttle mechanism between the tissues (Brooks, 2018). Lactate has also been shown to be important for normal brain physiology, through the astrocyte‐neuron lactate shuttle. In this model, described in 1994, the neurotransmitter glutamate released in the synapse, triggers glucose uptake and therefore lactate production by astrocytes; lactate so produced is then utilized by neurons as a source of energy (Magistretti & Allaman, 2018; Pellerin & Magistretti, 1994).
Figure 1.
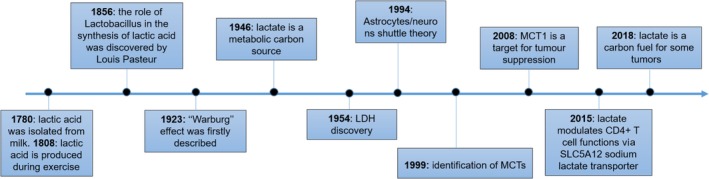
The history of lactate. Lactic acid was isolated for the first time by the chemist Carl Wilhelm Scheele in 1780 from sour milk. Indeed, the name lactate originates from the Latin word lac, which means milk. In 1808, Jöns Jacob Berzelius discovered that lactic acid (l‐lactate) is produced in muscles during exercise. In 1856, the role of Lactobacillus in the synthesis of lactate was discovered by Pasteur (1861) and only 20 years later in 1873 lactate molecular structure was resolved by Johannes Wislicenus (Nalbandian & Takeda, 2016). In 1923, Otto Warburg observed that cancer cells were characterized by accelerated glycolysis and excessive lactate generation even under fully oxygenated conditions (Warburg, 1956). His discovery was subsequently named the “Warburg Effect” by Efraim Racker in 1972. In 1946 lactate was identified as a major gluconeogenic precursor acting as shuttle in both muscle and liver (Cori & Cori, 1946). Later in 1954, LDH, the enzyme responsible for lactate production was found elevated in cancer (Hill & Levi, 1954) and in 1994 lactate was identified as major carbon source in the brain facilitating the interconnections between astrocytes and neurons (Pellerin & Magistretti, 1994). MCTs were firstly described as lactate transporters (Halestrap & Price, 1999) and MCT1 was later identified as target for tumor suppression (Sonveaux et al., 2008). Recent studies have emerged describing lactate as immune modulator in inflammatory disorders and as key carbon fuel for some tumors (Colegio et al., 2014; Faubert et al., 2017; Haas et al., 2015; Hui et al., 2017; Pucino et al., 2019)
2. LACTATE TRANSPORT
Lactate uptake and release requires the presence of transporters on the cell plasma membrane. Four members of the solute carrier 16a family of 12‐membrane pass, proton‐linked monocarboxylic acid symporters (i.e., monocarboxylic transporter 1 [MCT1, SLC16A1], MCT2 [SLC16A7], MCT3 [SLC16A8], and MCT4 [SLC16A3]), and two sodium‐coupled lactate cotransporters (SLC5A12, SLC5A8) have been studied in more detail (Table 1, Figure 2; Halestrap & Price, 1999; Pucino, Cucchi, & Mauro, 2018; Srinivas et al., 2005). These channels share conserved sequence motifs but display different affinity for lactate and other monocarboxylates (Doherty & Cleveland, 2013; Hirschhaeuser et al., 2011). The transport depends on pH, intra‐ and extracellular lactate concentration as well as other substrates such as pyruvate, butyrate, etc. (Doherty & Cleveland, 2013; Hirschhaeuser et al., 2011).
Table 1.
Lactate transporters
| Transporter | Function | High affinity substrates | Cell expression |
|---|---|---|---|
| SLC16A1/MCT1 | H+‐coupled electroneutral transporter | Lactate, pyruvate, ketone bodies | Epithelial cells, macrophages, CD8+ lymphocytes, cancer cells |
| SLC16A7/MCT2 | H+‐coupled electroneutral transporter | Pyruvate, lactate, ketone bodies | Epithelial cells, cancer cells |
| SLC16A8/MCT3 | H+‐coupled electroneutral transporter | Lactate | Epithelial cells (highly expressed in the retina) |
| SLC16A3/MCT4 | H+‐coupled electroneutral transporter | Lactate, pyruvate, ketone bodies | Epithelial cells, fibroblasts, macrophages, cancer cells |
| SLC5A8/SMCT1 | Na+‐coupled electroneutral transporter | Lactate, pyruvate, propionate, butyrate, nicotinate, and short‐chain fatty acids | Epithelial cells (mainly kidney, intestine, brain) |
| SLC5A12/SMCT2 | Na+‐coupled electroneutral transporter | Lactate, pyruvate, propionate, butyrate, nicotinate, and short‐chain fatty acids | Epithelial cells (mainly kidney, intestine, brain), CD4+ lymphocytes |
Figure 2.
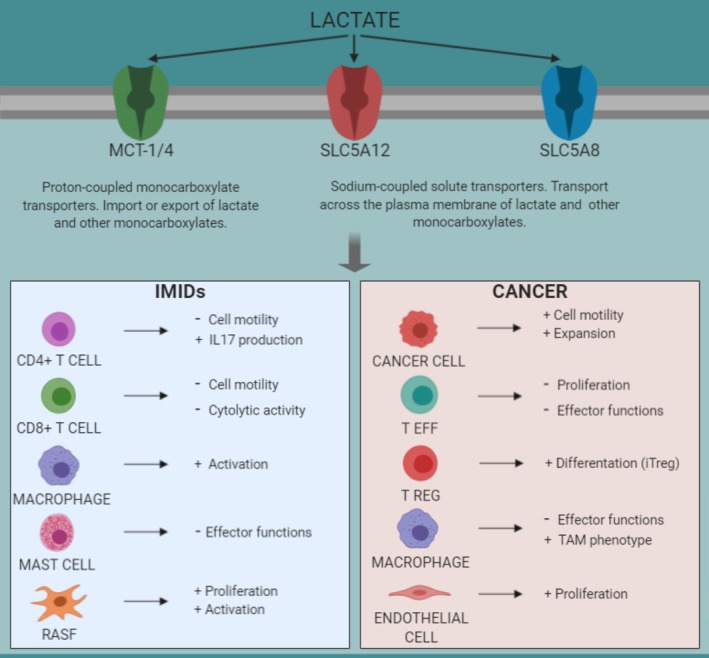
Lactate modulates immune cell functions in immune‐mediated inflammatory disorders and cancer. Immune cells “sense” high concentration of lactate which accumulates at the site of inflammation or tumor as result of accelerated metabolism of immune, stromal, or cancer cells. Lactate is taken up through specific transporters expressed on the cell membrane and modulates immune responses, including activation, differentiation, proliferation, migration, and cytokine production. These events promote the establishment of a chronic inflammatory process in IMIDs and induce tumor growth and metastatic spread in cancer
MCTs have been identified in all eukaryotic organisms and catalyze the proton‐linked transport of a wide variety of substrates (Perez‐Escuredo, Dadhich, et al., 2016) such as pyruvate, lactate and ketone bodies (acetoacetate and d‐β‐hydroxybutyrate), across the plasma membrane (Halestrap & Meredith, 2004; Halestrap & Price, 1999). Other identified MCTs are MCT5‐9, which show high affinity for other substrates such as thyroid hormones, ketone bodies, bumetanide and small aminoacids (Halestrap & Meredith, 2004; Hugo et al., 2012; Murakami et al., 2005; Suhre et al., 2011; Visser, van Mullem, Jansen, & Visser, 2011). The substrates and functions of the other MCT family members are still under investigation.
MCTs are expressed in a wide range of tissues (such as brain, skeletal muscle, heart, bowel, and liver) and display many physiological functions. In particular, they play a pivotal role in the control of glucose metabolism, and in the regulation of many biological functions including spermatogenesis, pancreatic β cell activity, thyroid hormone metabolism and drug transport (Perez‐Escuredo, Van Hee, et al., 2016).
MCTs are important regulators of intracellular lactate and pH; in particular lactate is one of the main substrates of MCT1–4. Indeed, highly glycolytic cells (i.e., during inflammation or tumors) utilize MCT transporters to export or import lactate (Bonen, 2001; Halestrap & Meredith, 2004; Huang et al., 2017; Sonveaux et al., 2008; Van Hee, Perez‐Escuredo, Cacace, Copetti, & Sonveaux, 2015).
All MCTs are capable of facilitating either import or export of lactate with substrate gradient being the main factor driving directionality. However, several studies have identified MCT4 to be responsible for its export in to the extracellular space while MCT1 regulates lactate import across the plasma membrane in the majority of the tissues where lactate is produced. Here, lactate can be used as a substrate to fuel other metabolic pathways (Faubert et al., 2017; Halestrap, 2012; Halestrap & Meredith, 2004; Perez‐Escuredo, Dadhich, et al., 2016).
In many cancer cells with an oxidative metabolic fingerprint, MCT1 is the most expressed MCT isoform (Kennedy & Dewhirst, 2010; Sonveaux et al., 2008). However, in glycolytic cancer cells or white muscle fibers and astrocytes, MCT4 is expressed at higher level than MCT1 (Baltazar et al., 2014; Sonveaux et al., 2008; Ullah, Davies, & Halestrap, 2006). This different MCTs expression allows the shuttling of this metabolite between cells and tissues with different metabolic behaviors. Such phenomenon has been described in the skeletal muscle where glycolytic/white fibers export lactate through MCT4 and oxidative/red fibers import lactate through MCT1 to fuel the tricarboxylic acid cycle (TCA) (Juel & Halestrap, 1999). A similar mechanism has been reported in cancer where lactate is exchanged between glycolytic/hypoxic and oxidative/oxygenated malignant cells (Sonveaux et al., 2008). In the brain, glycolytic oligodendrocytes and astrocytes export lactate through MCT1 and MCT4 to fuel oxidative neurons expressing MCT2 (Brooks, 2009; Funfschilling et al., 2012; Pellerin & Magistretti, 2012; Saab, Tzvetanova, & Nave, 2013). MCT2 and MCT4 show a high intracellular expression suggesting a possible role in mediating monocarboxylate transport across the membranes of intracellular vesicles or organelles (Afonso et al., 2015; Baltazar et al., 2014). In contrast to MCTs, which function as H+‐coupled electroneutral transporters, sodium‐coupled monocarboxylate transporters (SMCTs) function as Na+‐coupled transporters with a substrate ratio Na+/monocarboxylate ≥2. Two members of the SMCT family have been identified, the high‐affinity transporter SMCT1 (SLC5A8) and the low‐affinity SMCT2 (SLC5A12) (Rodriguez et al., 2002; Srinivas et al., 2005). The SLC5A8 gene was originally identified in the kidney (Rodriguez et al., 2002) and subsequently detected in the intestine, salivary gland, thyroid gland, brain, and retina (Ganapathy et al., 2008). In these tissues, SLC5A8 mediates the transport of monocarboxylic acids such as lactate, pyruvate, propionate, butyrate, nicotinate, and short‐chain fatty acids similar to MCTs with a high affinity (Gopal et al., 2005; Morris & Felmlee, 2008).
SLC5A12 is expressed in the kidney, small intestine, and skeletal muscle and to a lesser level in brain and retina. SLC5A12 substrate specificity is similar to that of SLC5A8. However, the affinity of SLC5A12 for monocarboxylate substrates is lower than SLC5A8 (Srinivas et al., 2005).
Notably, the localization of these transporters is different at the tissue level suggesting different functions. In the kidney, SLC5A8 is expressed in the apical membrane of tubular epithelial cells in the S2–S3 proximal tubule segments where it is involved in renal reabsorption of lactate and pyruvate (Barac‐Nieto, Murer, & Kinne, 1980; Ganapathy et al., 2008; Gopal, Miyauchi, et al., 2007; Yanase, Takebe, Nio‐Kobayashi, Takahashi‐Iwanaga, & Iwanaga, 2008). Indeed, SLC5A8‐knockout mice exhibit increased urinary excretion of lactate (Frank et al., 2008). By contrast, renal SLC5A12 is highly expressed in the initial part of the proximal tubules and decreases toward the S3 segment. Thus, the proximal convoluted tubules provide low and high affinity transporters in the upper and lower proximal tubules, respectively (Gopal, Umapathy, et al., 2007).
In the brain, SLC5A8 mediates cellular uptake of lactate and ketone bodies by neurons (Martin et al., 2006), while SLC5A12 is specifically expressed by astrocytes. Besides the physiological functions, SLC5A8 has been reported as tumor‐suppressing molecule; its expression is in fact decreased in several tumors such as human colon cancer, papillary thyroid carcinomas, pancreatic cancer, prostate tumor, acute myeloid leukemia, and glioma formation (Ganapathy et al., 2008; Li et al., 2003; Park et al., 2008).
In the bowel, SLC5A8 is expressed in the lumen‐facing apical membrane of colonic and intestinal epithelial cells, while SLC5A12 is expressed primarily in the small intestinal tract (Gopal, Miyauchi, et al., 2007; Teramae et al., 2010).
The different tissue localization may indicate a distinct role of these transporters in regulating cell functions with important therapeutic implications.
3. LACTATE MODULATES IMMUNE CELL FUNCTIONS
It is becoming particularly attractive the concept that metabolites can act as immunomodulatory molecules regulating several immune cell functions (Figure 2; Haas et al., 2016). For instance, lactic acid has been reported to suppress the proliferation and cytokine production of human cytotoxic T lymphocytes (Fischer et al., 2007) via a mechanism that involves T cell receptor‐triggered phosphorylation of JNK, c‐Jun and p38, which is implicated in interferon‐γ (IFN‐γ) production (Mendler et al., 2012). In this context, our group has reported that lactic acid is able to modulate CD8+ but not CD4+ T cell responses (Haas et al., 2015; Pucino, Bombardieri, Pitzalis, & Mauro, 2017), whereas sodium lactate regulates CD4+ T cell migration and cytokine production without affecting CD8+ T cells.
It is well recognized that T cells display different metabolic requirements; with cytotoxic and effector T cells being more dependent on glycolysis for proliferation and cytokine production (Macintyre et al., 2014), while regulatory T cells (Tregs) rely more on oxidative phosphorylation (Gerriets et al., 2015; Michalek et al., 2011). Recently, Angelin et al. (2017) showed that the Treg transcription factor forkhead box P3 (FOXP3) is able to reprogram the metabolism of Tregs, allowing them to cope with low‐glucose and high‐lactate microenvironments, therefore escaping the anti‐tumor immune surveillance. This elegant study demonstrates that Tregs can withstand sustained exposure to elevated levels of lactate, whereas effector T cells are impaired. This is due to lactate‐mediated reduction of NAD+ availability that is crucial for effector T cells for their functions. Indeed cytotoxic and effector T cells reduce NAD+ to NADH via glyceraldehyde‐3‐phosphate dehydrogenase and require constant NAD+ recycling through LDH to sustain glycolysis. In conditions where extracellular lactate is abundant (i.e., during chronic inflammation) this metabolite is re‐converted to pyruvate (reversed LDH reaction) with generation of NADH and impairment of glycolytic flux (Leite et al., 2011; Pucino et al., 2019). Reduced glycolysis is particularly detrimental for effector and cytotoxic T cells, as it affects the ability to produce IFN‐γ (Chang et al., 2013) as well as their migratory capabilities (Haas et al., 2015; Pucino et al., 2019). On the other side, Tregs are less affected by reduced NAD+/glycolysis. In addition, FOXP3 is also able to regulate the direction of the LDH reaction in favor of the oxidation of lactate to pyruvate, leading to a decreased production of lactate by Tregs (as compared to conventional T cells) (Angelin et al., 2017). This is mechanistically related to FOXP3‐mediated suppression of the transcription factor Myc which in turn increases pyruvate dehydrogenase activity enhancing oxidative phosphorylation and NAD+ regeneration (Angelin et al., 2017).
LDH is required for CD4+ T cells to sustain aerobic glycolysis and for the production of IFN‐γ, enabling a proper differentiation to T helper 1 (Th1) cells (Peng et al., 2011). Genetic deletion of LDH isoform A (LDHA) in CD4+ T cells significantly reduced glucose consumption with a shift toward an oxidative metabolism, and decreased IFN‐γ expression. Moreover, the deletion of LDHA caused a reduction in the available pool of acetyl‐coenzyme A (acetyl‐CoA), which is important for histone acetylation. These data demonstrate that LDHA regulates INF‐γ production in Th1 cells, through an epigenetic mechanism of histone acetylation coupled with cellular metabolism (Peng et al., 2011).
Lactate has been shown to regulate immune responses during infections. Indeed, lactate serves as a key metabolite responsible for glycolysis‐mediated retinoic‐acid‐inducible gene I (RIG‐I)‐like receptors (RLRs), which is implicated in type 1 IFN production and viral clearance. Specifically, lactate induces an inhibitory signal by directly binding to mitochondrial antiviral‐signaling (MAVS) protein and preventing MAVS aggregation. Notably, lactate restoration reverses increased IFN production caused by lactate deficiency. LDHA inactivation and subsequent lactate reduction, using pharmacological and genetic approaches, increases type I IFN production and protects mice from viral infection (W. Zhang et al., 2019).
4. LACTATE: A KEY PLAYER IN CANCER
The tumor‐associated microenvironment consists of malignant cells, several immune cells, non‐cancer stromal cells, fibroblasts as well as increased angiogenesis and lymphangiogenesis (Galdiero, Varricchi, Loffredo, Mantovani, & Marone, 2018; Hanahan & Weinberg, 2011; Varricchi et al., 2018). A typical feature of the tumor microenvironment is its reduced pH to levels of ~6.5 (Corbet & Feron, 2017). Lactate is the main metabolite responsible for this acidosis as a consequence of high cellular glycolytic rate. Here lactate, transported through specific carriers expressed on the cell membrane of cancer and immune cells, and shuttling from cancer cells to the extracellular space, rewires intracellular metabolic pathways and suppresses immune responses enhancing tumor growth (Figure 2; Liu et al., 2019). Lactate is in fact an energy substrate for cancer cells in conditions of glucose deprivation. For instance, lactate is a carbon fuel molecule for the TCA cycle in some tumors such as the human non‐small‐cell lung cancers (NSCLCs), contributing to biomass synthesis for tumor growth (Faubert et al., 2017). Infusing human NSCLCs patients with 13C‐lactate has indeed revealed extensive labeling of TCA cycle metabolites. Deleting MCT1 from tumor cells suppressed lactate‐dependent metabolite labeling in mice. In addition, lactate contribution to the TCA cycle was predominant in comparison to glucose (Faubert et al., 2017). These data were further confirmed in the same year by Hui et al. who identified lactate as carbon fuel for TCA cycle, both in normal and cancerous tissues. This study showed that the infusion of 13C‐lactate, in fed and fasted mice, induced extensive labeling of TCA cycle metabolites in all tissues, and in lung and pancreatic tumors the contribution of circulating lactate to the activity of TCA cycle was greater than that of glucose. In addition, lactate promotes glutamate uptake and catabolism by increasing the expression of glutamine transporter ASCT2 and glutaminase 1 in cancer cells (Perez‐Escuredo, Dadhich, et al., 2016). These findings demonstrate that lactate is a main carbon supplier of the TCA cycle both in normal tissues and cancer, and that glycolysis and the TCA cycle are uncoupled at the level of lactate, allowing the independent regulation of the two processes (Hui et al., 2017).
The effects of lactate are not only due to its ability to feed metabolic pathways, but also to its ability to trigger a signaling pathway via its receptor G protein‐coupled receptor 81 (GPR81); a surface lactate receptor involved in the regulation of lipolysis (Lafontan & Langin, 2009) and in cancer cell survival (Roland et al., 2014). In this context, Feng et al. (2017) showed that lactate can regulate the expression of PD‐L1 in human lung cancer cells via GPR81. PD‐L1 is the ligand of PD1, a receptor expressed on the membrane of activated T cells, responsible for reduced proliferation and effector function of T cells, and a major target for cancer immunotherapy. The authors showed that lactate, via GPR81, upregulates the expression of PD‐L1 at a transcriptional level leading to suppression of the T cells effector functions in co‐culture experiments (Feng et al., 2017).
Lactate influences macrophage functions in the context of tumor environment. Tumor‐associated macrophages (TAMs) “sense” metabolic changes typical of the tumor microenvironment via the expression of MCTs. Cancer cells produce high amount of lactate, which is extruded in the intercellular space via MCT4. Released lactate is then taken up by tumor‐associated macrophages via MCT1, promoting in turn macrophage polarization toward a TAM (M2) phenotype with high expression of arginase 1 (Arg1), vascular endothelial growth factor (VEGF) production and subsequently tumor growth (Colegio et al., 2014; Figure 2). The authors found that lactate‐induced VEGF and Arg1 expression was dependent on HIF‐1α, as HIF‐1α‐deficient macrophages were not able to upregulate both VEGF and Arg1 upon lactate stimulation (Colegio et al., 2014). Notably, TNF‐α secretion by human monocytes was found to be suppressed in the presence of high lactate concentration and reduced pH in tumor microenvironment (Mendler et al., 2012). Neutralizing tumor acidity with bicarbonate monotherapy was able to impair the growth of some cancer types (Pilon‐Thomas et al., 2016). Similarly, another study demonstrated that lactate plays an important role in the communication between TAM and cancer cells undergoing epithelial‐to‐mesenchymal transition (Su et al., 2014).
Recently, it has been reported that lactate modulates the expression of macrophage‐specific vascular ATPase subunit ATP6V0d2, whose levels are regulated by transcription factor EB. The authors found increased mammalian target of rapamycin (mTOR) activation upon lactate stimulation suggesting that lactate could modulate ATP6V0d2 expression via this pathway (Liu et al., 2019). However, further investigation is needed to establish a key role of mTOR in lactate‐mediated ATP6V0d2 expression. Interestingly, Atp6v0d2−/− mice were susceptible to tumor growth, with enhanced HIF‐2α‐mediated VEGF production in macrophages that display a pro‐tumorigenic phenotype. These findings were also confirmed in humans. Indeed, in a cohort of patients with lung adenocarcinoma, expression of ATP6V0d2 and HIF‐2α was positively and negatively correlated with survival, respectively (Liu et al., 2019). The latter finding suggests a critical role of the macrophage lactate/ATP6V0d2/HIF‐2α axis in maintaining tumor growth in human patients. Furthermore, lactate can directly modulate angiogenesis allowing tumor growth. The lactate‐signaling pathway was reported to drive HIF‐1/VEGF activation independently of hypoxia in endothelial cells. MCT1 blockade was shown to have anti‐angiogenic effects together with reduced tumor growth in a model of colon cancer xenograft involving the co‐injection of human umbilical vein endothelial cells HUVECs (Vegran, Boidot, Michiels, Sonveaux, & Feron, 2011). Lactate has also an established role in the biology of cancer‐associated fibroblasts (CAFs) that are the major cellular stromal component of many solid tumors.
Ippolito et al. (2019) recently found that lactate uptake alters the NAD+/NADH ratio in the prostate cancer cells, which leads to increased mitochondrial mass and activity via an intracellular mechanism involving SIRT1‐dependent peroxisome proliferator‐activated receptor γ coactivator‐1 activation. The high exploitation of mitochondria results in tricarboxylic acid cycle deregulation, accumulation of oncometabolites and reactive oxygen species (ROS) generation. Additionally, using both in vitro and in vivo prostate cancer models the authors found that cancer cells hijack CAF‐derived functional mitochondria through the formation of cellular bridges.
In pancreatic cancer, lactate has been reported to promote posttranscriptional modifications that can contribute to tumor growth (Bhagat et al., 2019). Indeed, lactate produced by cancer cells increases the production of alpha‐ketoglutarate (aKG) within mesenchymal stem cells (MSCs). In turn, aKG‐mediated activation of the demethylase TET is responsible for decreased cytosine methylation and increased hydroxymethylation during de novo differentiation of MSCs to CAF. Notably, the co‐injection of neoplastic cells with TET‐deficient MSCs inhibited tumor growth in vivo proposing a new anti‐cancer therapeutic option (Bhagat et al., 2019).
4.1. MCTs: Expression in cancer
MCT1 expression has been reported in a variety of human malignancies including head and neck, lung, stomach, colon, prostate and cervix cancers, as well as gliomas (Afonso et al., 2015; Kennedy & Dewhirst, 2010; Miranda‐Goncalves et al., 2013; Pinheiro et al., 2012; Sonveaux et al., 2008). MCT1 has also been proposed to be the most important isoform responsible for lactate transport across the plasma membrane in breast and bladder cancer, NSCLCs and ovarian carcinomas (Afonso et al., 2015).
MCT4 is also widely distributed in different cancer types. Its expression has indeed been reported in breast, colon, bladder and prostate cancers, as well as in cancers of the gynecologic tract and gliomas (Afonso et al., 2015; Miranda‐Goncalves et al., 2013; Pinheiro et al., 2012).
Pertega‐Gomes and Baltazar (2014) reported a correlation between the expression of MCT1, MCT2 and MCT4, and the different stages of prostate cancer progression (Pertega‐Gomes & Baltazar, 2014). In another study focused at NSCLC, Eilertsen et al. (2014) described MCT1 as a prognostic and survival biomarker. In the same vein, the co‐expression of GLUT1 and MCT1 and of GLUT1 and MCT4 was found to be a negative prognostic factor associated with poor disease‐specific survival.
Besides MCTs, proton‐sensing GPRs such as T cell death‐associated gene 8 have also been shown to be important for the modulation of T cells in an acidic tumor environment and during inflammation suggesting that an interaction between MCTs and other sensing molecules is necessary to facilitate a proper shuttling activity (Ishii, Kihara, & Shimizu, 2005; Pilon‐Thomas et al., 2016). In line with this, it has been reported that intracellular carbonic anhydrase CAII can act as proton antennae facilitating proton‐driven lactate flux through MCTs in Xenopus oocytes. The knockdown of CAII with siRNA reduced MCT1‐mediated lactate transport in MCF‐7 breast cancer cells revealing a new potential therapeutic strategy (Noor et al., 2018).
5. LACTATE SUSTAINS CHRONIC INFLAMMATION IN IMMUNE‐MEDIATED INFLAMMATORY DISORDERS
While in tumor cells lactate plays a key role in suppressing T cell effector functions, in the inflammatory context lactate activates a “stop migration” signal in T cells leading to their accumulation at the inflammatory site (Figure 2). These events are mediated by sodium lactate and lactic acid uptake through the transporters SLC5A12 and SLC16A1, which are selectively expressed on the surface of CD4+ and CD8+ T cells, respectively (Haas et al., 2015). Interestingly, the inhibition of T cell migration is regulated via lactate interference with the glycolytic pathway (Droge, Roth, Altmann, & Mihm, 1987; Haas et al., 2015; Pucino et al., 2019). Indeed, CD4+ T cells, in the presence of sodium lactate, display a downregulation of several glycolytic enzymes and glucose flux (Haas et al., 2015; Pucino et al., 2019). Lactate can also modulate cytokine production. For instance, tumor derived lactate has been shown to enhance the IL‐23/IL‐17 pathway acting as a pro‐inflammatory signal (Shime et al., 2008; Yabu et al., 2011). Subsequently, it was found by our group that sodium lactate can directly modulate IL‐17 production by driving the plastic differentiation of CD4+ T cells in a Th17 subset in the inflamed tissue (Haas et al., 2015; Pucino et al., 2019). These findings have an important impact on the understanding of the role of lactate in the context of immune‐mediated inflammatory disorders (IMIDs, i.e., rheumatoid arthritis [RA]) where lactate may act as an inflammatory signal leading to the inhibition of CD4+ T cell migratory capability and to their differentiation in a Th17 subset thus sustaining the chronic inflammatory process (Figure 2). In addition, SLC5A12 has been found to correlate with the inflammatory score and T cell infiltration in RA synovial tissue, suggesting its possible role in the pathogenesis of this disease (Haas et al., 2015; Pucino et al., 2019).
Accumulation of lactate in the synovial fluid of RA patients is the result of the high metabolic demand of synovial cells (Garcia‐Carbonell et al., 2016). Even though there is not a clear correlation between lactate concentration in the synovial fluid and the disease activity, some authors found that synovial lactate level could be a reliable indicator of differentiating inflammatory arthritis (Gobelet & Gerster, 1984). Similarly, LDH isoenzymes were found to be higher in serum and synovial fluid of RA compared to osteoarthritis (OA) patients (Pejovic, Stankovic, & Mitrovic, 1992) and LDH activity was found to be increased in RA synovial tissues compared to healthy controls (Lindy, Uitto, Turto, Rokkanen, & Vainio, 1971).
The abundance of lactate in inflammatory sites, as result of the hypoxic environment, is responsible, at least in part, for the hypoxic‐induced signaling. Notably, lactate signaling and subsequent biologic responses appear to be functionally uncoupled from HIF‐1α‐induced metabolic reprogramming, by employing NDRG family member 3 (NDRG3) as a critical link (Lee et al., 2015). The authors found that the NDRG3 protein is degraded in a PHD2/VHL‐dependent manner in normoxia, similarly to HIF‐1α, but is protected from degradation by binding to lactate that accumulates under hypoxia. Indeed NDRG3 levels increase upon lactate stimulation irrespective of HIF‐1α. In addition, suppression of lactate production with either a LDHA pharmacologic inhibitor or LDHA silencing (siRNA) specifically inhibited the NDRG3 protein accumulation in a dose‐dependent manner. These events were reversed by the addition of lactate to the culture medium without affecting HIF‐1α protein levels. Moreover, NDRG3 promotes the switch toward a Th17 phenotype while it inhibits regulatory T cell differentiation (Shi et al., 2011). This might explain a possible mechanism through which lactate can modulate the balance between Th17 and Treg cells during hypoxia.
Opposite to T cells where it acts as a pro‐inflammatory molecule, on mast cells lactate has been reported to exhibit an anti‐inflammatory effect. Indeed, IL‐33‐mediated mast cell inflammatory cytokine production is inhibited upon lactate treatment (Abebayehu et al., 2016). These effects of lactate on mast cell activation were dependent on acidic pH and the MCT1 carrier. More recently, the same group reported that lactate inhibited lipopolysaccharide (LPS)‐induced cytokine and chemokine production by mast cells in vitro (Caslin et al., 2019). These events were linked to HIF‐1α‐dependent miR‐155 suppression. Indeed, HIF‐1α siRNA transfection in bone marrow‐derived mast cells prevented lactate‐mediated miR‐155‐5 suppression. Interestingly, lactate effects were mimicked by glycolytic inhibitors and reversed by increasing ATP availability (Abebayehu et al., 2016; Caslin et al., 2019).
The role that lactate may play in modulating macrophages in the context of IMIDs is controversial. It has been reported that LPS‐induced inflammatory responses in macrophages is reduced in the presence of lactate in a GPR81‐independent manner (Errea et al., 2016). While another study showed that lactate promoted macrophage activation upon TLR2 and TLR4 stimulations via MCT4, which regulates lactate export through the cell membrane. This was supported by observations that MCT4 knockdown attenuated the expression of pro‐inflammatory mediators in murine macrophages (Tan et al., 2015). This evidence indicates that lactate can modulate macrophage functions and fate engaging different signaling pathways. It would be interesting to investigate how these observations can differ in macrophages from healthy versus pathological conditions.
In this context, lactate‐derived lactylation of histone lysine residues has been recently identified as a new epigenetic modification that directly stimulates gene transcription from chromatin both in mouse and human. Using M1 macrophages that have been exposed to bacteria, the authors showed that histone lactylation has different temporal dynamics from acetylation. In the late phase of M1 macrophage polarization, increased histone lactylation induces homeostatic genes that are involved in wound healing, including Arg1. These findings highlight a homeostatic role for lactate during infections (D. Zhang et al., 2019).
Taken together these data support a role for lactate in the modulation of immune cell functions during inflammation. While the role of lactate in macrophage‐directed inflammation is still being debated, lactate behaves as a pro‐inflammatory molecule on T cells, leading to the production of cytokines necessary for the differentiation of specific T cell subsets and regulating their ability to migrate. Targeting lactate transporters, LDH, and HIF‐1α may represent a novel therapeutic intervention in IMIDs elicited by pro‐inflammatory T cells.
6. THERAPEUTIC STRATEGIES TARGETING LACTATE SIGNALING PATHWAY
Different approaches modulating lactate signaling pathway, such as MCTs and LDH inhibitors, have been demonstrated to be successful both in vitro and in vivo. The blockade of the lactate transporter MCT1 has shown to reduce the proliferation of breast cancer cells co‐expressing MCT1 and MCT4 (Hong et al., 2016), and decrease HIF‐1α‐induced angiogenesis in cervix squamous carcinoma (Sonveaux et al., 2008, 2012). In addition, MCTs inhibition with α‐cyano‐4‐hydroxycinnamic acid was reported to reduce lactate‐mediated inhibition of inflammatory gene expression and NF‐kappaB activity in human macrophages, indicating that lactate transport through monocarboxylate transporters is required for macrophage effector functions (Samuvel, Sundararaj, Nareika, Lopes‐Virella, & Huang, 2009).
In this context, AZD3965 (MCT‐1 inhibitor) has shown to be safe in a Phase 1 clinical study in the United Kingdom where it has been tested in solid tumor and B cell lymphoma patients (Curtis et al., 2017).
LDH has also been reported to be increased in tumor cells (Fantin, St‐Pierre, & Leder, 2006; Husain, Huang, Seth, & Sukhatme, 2013). Knocking down LDHA by short hairpin RNAs determined a reduced ability of tumor cells to proliferate under hypoxic conditions. This event was combined with increased mitochondrial respiration and decreased mitochondrial membrane potential. The tumorigenicity of the LDHA‐deficient cells was strongly reduced, and this phenotype was reversed by supplementation with the human LDHA (Fantin et al., 2006). LDHA‐depleted tumors display a decreased frequency of myeloid‐derived suppressor cells (MDSCs) and natural killer cells improved cytolytic function. The addition of exogenous lactate increased the frequency of MDSCs generated from murine bone marrow cells and inhibited cytolytic function of both human and murine NK cells in vitro, supporting the role of lactate in tumor immune surveillance escape (Husain et al., 2013). This evidence was also supported by Brand et al. (2016) showing that lactate leads to tumor immune escape by inhibiting the function and survival of T and NK cells. In the same context, another study has shown that tumor‐infiltrating liver‐resident NK cells display signs of mitochondrial stress, which are recapitulated in vitro by treating liver‐resident NK cells with lactate. Lactate‐mediated apoptosis and mitochondrial dysfunction in these cells was reverted by blocking mitochondrial ROS accumulation (Harmon et al., 2019).
Due to the emerging role of lactate in the field of inflammation and autoimmunity, lactate transporters are gaining much attention as novel therapeutic targets even in these contexts. In this regard, it has been found that MCT4 is upregulated by RA synovial fibroblast (RASFs) compared to OA SF (Fujii et al., 2015). Silencing of MCT4‐inhibited proliferation of RASFs in vitro and reduced the severity of arthritis in a mouse model of collagen‐induced arthritis in vivo (Fujii et al., 2015). Similarly, MCT4 is required for macrophage activation upon TLR2 and TLR4 stimulations as confirmed by reduced expression of pro‐inflammatory molecules, such as TNF‐α and IL‐6, in MCT4 knockdown macrophages. In addition, these events were accompanied by intracellular accumulation of lactate and decreased glycolysis (Tan et al., 2015).
In addition, the blockade of SLC5A12 (with shRNA or antibody) restored T cell functions in vitro as well as ameliorated disease severity in vivo in mouse models of peritonitis and arthritis (Haas et al., 2015; Pucino et al., 2019) suggesting a novel therapeutic target to reduce inflammation in IMIDs.
7. CONCLUDING REMARKS
The mechanisms regulating the crosstalk between metabolism and the immune system have been investigated in some depth in the last few years (Buck, Sowell, Kaech, & Pearce, 2017; Geltink, Kyle, & Pearce, 2018). In this context, lactate has emerged as an important signaling and immunomodulatory molecule, able to promote inflammatory responses and suppress anti‐tumor response. The discovery of lactate transporters on the surface of immune cells has opened novel and important perspectives for the understanding of the pathogenesis of inflammatory disorders and tumors. Targeting these molecules might prove crucial in the modulation of immune cell functions to achieve beneficial therapeutic effects.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
AUTHOR CONTRIBUTIONS
Michelangelo Certo: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing‐original draft, review and editing. Giancarlo Marone: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing‐original draft, review and editing. Amato de Paulis: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; software; supervision; validation; visualization; writing‐original draft, review and editing. Claudio Mauro: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing‐original draft, review and editing. Valentina Pucino: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing‐original draft, review and editing.
RELATED WIREs ARTICLE
ACKNOWLEDGMENTS
The research underlying this review was supported by Versus Arthritis (Clinical Research Fellowship 21386) to V.P. and by British Heart Foundation Intermediate Basic Science Research Fellowship (FS/12/38/29640) and a University of Birmingham Professorial Research Fellowship to C.M. G.M. and A.D.P. are supported by Research Centre and Regione Campania CISI‐Lab and TIMING Project. We would like to thank John D. O'Neil for reading and commenting the manuscript.
Certo M, Marone G, de Paulis A, Mauro C, Pucino V. Lactate: Fueling the fire starter. WIREs Syst Biol Med. 2020;12:e1474 10.1002/wsbm.1474
Claudio Mauro and Valentina Pucino are joint last authors.
Funding information TIMING Project; Research Centre and Regione Campania CISI‐Lab; University of Birmingham Professorial Research Fellowship; British Heart Foundation Intermediate Basic Science Research Fellowship, Grant/Award Number: FS/12/38/29640; Versus Arthritis, Grant/Award Number: 21386
REFERENCES
- Abebayehu, D. , Spence, A. J. , Qayum, A. A. , Taruselli, M. T. , McLeod, J. J. , Caslin, H. L. , … Ryan, J. J. (2016). Lactic acid suppresses IL‐33‐mediated mast cell inflammatory responses via hypoxia‐inducible factor‐1alpha‐dependent miR‐155 suppression. Journal of Immunology, 197(7), 2909–2917. 10.4049/jimmunol.1600651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso, J. , Santos, L. L. , Miranda‐Goncalves, V. , Morais, A. , Amaro, T. , Longatto‐Filho, A. , & Baltazar, F. (2015). CD147 and MCT1‐potential partners in bladder cancer aggressiveness and cisplatin resistance. Molecular Carcinogenesis, 54(11), 1451–1466. 10.1002/mc.22222 [DOI] [PubMed] [Google Scholar]
- Angelin, A. , Gil‐de‐Gomez, L. , Dahiya, S. , Jiao, J. , Guo, L. , Levine, M. H. , … Beier, U. H. (2017). Foxp3 reprograms T cell metabolism to function in low‐glucose, high‐lactate environments. Cell Metabolism, 25(6), 1282–1293.e1287. 10.1016/j.cmet.2016.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltazar, F. , Pinheiro, C. , Morais‐Santos, F. , Azevedo‐Silva, J. , Queiros, O. , Preto, A. , & Casal, M. (2014). Monocarboxylate transporters as targets and mediators in cancer therapy response. Histology and Histopathology, 29(12), 1511–1524. 10.14670/hh-29.1511 [DOI] [PubMed] [Google Scholar]
- Barac‐Nieto, M. , Murer, H. , & Kinne, R. (1980). Lactate‐sodium cotransport in rat renal brush border membranes. The American Journal of Physiology, 239(5), F496–F506. 10.1152/ajprenal.1980.239.5.F496 [DOI] [PubMed] [Google Scholar]
- Bhagat, T. D. , Von Ahrens, D. , Dawlaty, M. , Zou, Y. , Baddour, J. , Achreja, A. , … Verma, A. (2019). Lactate‐mediated epigenetic reprogramming regulates formation of human pancreatic cancer‐associated fibroblasts. eLife, 8, e50663 10.7554/eLife.50663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen, A. (2001). The expression of lactate transporters (MCT1 and MCT4) in heart and muscle. European Journal of Applied Physiology, 86(1), 6–11. 10.1007/s004210100516 [DOI] [PubMed] [Google Scholar]
- Bonuccelli, G. , Whitaker‐Menezes, D. , Castello‐Cros, R. , Pavlides, S. , Pestell, R. G. , Fatatis, A. , … Lisanti, M. P. (2010). The reverse Warburg effect: Glycolysis inhibitors prevent the tumor promoting effects of caveolin‐1 deficient cancer associated fibroblasts. Cell Cycle, 9(10), 1960–1971. 10.4161/cc.9.10.11601 [DOI] [PubMed] [Google Scholar]
- Brand, A. , Singer, K. , Koehl, G. E. , Kolitzus, M. , Schoenhammer, G. , Thiel, A. , … Kreutz, M. (2016). LDHA‐associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metabolism, 24(5), 657–671. 10.1016/j.cmet.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Brooks, G. A. (1986). Lactate production under fully aerobic conditions: The lactate shuttle during rest and exercise. Federation Proceedings, 45(13), 2924–2929. [PubMed] [Google Scholar]
- Brooks, G. A. (2009). Cell–cell and intracellular lactate shuttles. The Journal of Physiology, 587(Pt. 23), 5591–5600. 10.1113/jphysiol.2009.178350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, G. A. (2018). The science and translation of lactate shuttle theory. Cell Metabolism, 27(4), 757–785. 10.1016/j.cmet.2018.03.008 [DOI] [PubMed] [Google Scholar]
- Buck, M. D. , Sowell, R. T. , Kaech, S. M. , & Pearce, E. L. (2017). Metabolic instruction of immunity. Cell, 169(4), 570–586. 10.1016/j.cell.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caslin, H. L. , Abebayehu, D. , Abdul Qayum, A. , Haque, T. T. , Taruselli, M. T. , Paez, P. A. , … Ryan, J. J. (2019). Lactic acid inhibits lipopolysaccharide‐induced mast cell function by limiting glycolysis and ATP availability. Journal of Immunology, 203(2), 453–464. 10.4049/jimmunol.1801005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C. H. , Curtis, J. D. , Maggi, L. B., Jr. , Faubert, B. , Villarino, A. V. , O'Sullivan, D. , … Pearce, E. L. (2013). Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell, 153(6), 1239–1251. 10.1016/j.cell.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegio, O. R. , Chu, N. Q. , Szabo, A. L. , Chu, T. , Rhebergen, A. M. , Jairam, V. , … Medzhitov, R. (2014). Functional polarization of tumour‐associated macrophages by tumour‐derived lactic acid. Nature, 513(7519), 559–563. 10.1038/nature13490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbet, C. , & Feron, O. (2017). Tumour acidosis: From the passenger to the driver's seat. Nature Reviews. Cancer, 17(10), 577–593. 10.1038/nrc.2017.77 [DOI] [PubMed] [Google Scholar]
- Corcoran, S. E. , & O'Neill, L. A. (2016). HIF1alpha and metabolic reprogramming in inflammation. The Journal of Clinical Investigation, 126(10), 3699–3707. 10.1172/jci84431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cori, C. F. , & Cori, G. T. (1946). Carbohydrate metabolism. Annual Review of Biochemistry, 15, 193–218. [DOI] [PubMed] [Google Scholar]
- Curtis, N. J. , Mooney, L. , Hopcroft, L. , Michopoulos, F. , Whalley, N. , Zhong, H. , … Critchlow, S. E. (2017). Pre‐clinical pharmacology of AZD3965, a selective inhibitor of MCT1: DLBCL, NHL and Burkitt's lymphoma anti‐tumor activity. Oncotarget, 8(41), 69219–69236. 10.18632/oncotarget.18215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty, J. R. , & Cleveland, J. L. (2013). Targeting lactate metabolism for cancer therapeutics. The Journal of Clinical Investigation, 123(9), 3685–3692. 10.1172/jci69741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge, W. , Roth, S. , Altmann, A. , & Mihm, S. (1987). Regulation of T‐cell functions by l‐lactate. Cellular Immunology, 108(2), 405–416. 10.1016/0008-8749(87)90223-1 [DOI] [PubMed] [Google Scholar]
- Eilertsen, M. , Andersen, S. , Al‐Saad, S. , Kiselev, Y. , Donnem, T. , Stenvold, H. , … Bremnes, R. M. (2014). Monocarboxylate transporters 1‐4 in NSCLC: MCT1 is an independent prognostic marker for survival. PLoS One, 9(9), e105038 10.1371/journal.pone.0105038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errea, A. , Cayet, D. , Marchetti, P. , Tang, C. , Kluza, J. , Offermanns, S. , … Rumbo, M. (2016). Lactate inhibits the pro‐inflammatory response and metabolic reprogramming in murine macrophages in a GPR81‐independent manner. PLoS One, 11(11), e0163694 10.1371/journal.pone.0163694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin, V. R. , St‐Pierre, J. , & Leder, P. (2006). Attenuation of LDH‐A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell, 9(6), 425–434. 10.1016/j.ccr.2006.04.023 [DOI] [PubMed] [Google Scholar]
- Faubert, B. , Li, K. Y. , Cai, L. , Hensley, C. T. , Kim, J. , Zacharias, L. G. , … DeBerardinis, R. J. (2017). Lactate metabolism in human lung tumors. Cell, 171(2), 358–371.e359. 10.1016/j.cell.2017.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, J. , Yang, H. , Zhang, Y. , Wei, H. , Zhu, Z. , Zhu, B. , … Wu, Z. (2017). Tumor cell‐derived lactate induces TAZ‐dependent upregulation of PD‐L1 through GPR81 in human lung cancer cells. Oncogene, 36(42), 5829–5839. 10.1038/onc.2017.188 [DOI] [PubMed] [Google Scholar]
- Fischer, K. , Hoffmann, P. , Voelkl, S. , Meidenbauer, N. , Ammer, J. , Edinger, M. , … Kreutz, M. (2007). Inhibitory effect of tumor cell‐derived lactic acid on human T cells. Blood, 109(9), 3812–3819. 10.1182/blood-2006-07-035972 [DOI] [PubMed] [Google Scholar]
- Frank, H. , Groger, N. , Diener, M. , Becker, C. , Braun, T. , & Boettger, T. (2008). Lactaturia and loss of sodium‐dependent lactate uptake in the colon of SLC5A8‐deficient mice. The Journal of Biological Chemistry, 283(36), 24729–24737. 10.1074/jbc.M802681200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii, W. , Kawahito, Y. , Nagahara, H. , Kukida, Y. , Seno, T. , Yamamoto, A. , … Ashihara, E. (2015). Monocarboxylate transporter 4, associated with the acidification of synovial fluid, is a novel therapeutic target for inflammatory arthritis. Arthritis & Rhematology, 67(11), 2888–2896. 10.1002/art.39270 [DOI] [PubMed] [Google Scholar]
- Funfschilling, U. , Supplie, L. M. , Mahad, D. , Boretius, S. , Saab, A. S. , Edgar, J. , … Nave, K. A. (2012). Glycolytic oligodendrocytes maintain myelin and long‐term axonal integrity. Nature, 485(7399), 517–521. 10.1038/nature11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdiero, M. R. , Varricchi, G. , Loffredo, S. , Mantovani, A. , & Marone, G. (2018). Roles of neutrophils in cancer growth and progression. Journal of Leukocyte Biology, 103(3), 457–464. 10.1002/jlb.3mr0717-292r [DOI] [PubMed] [Google Scholar]
- Ganapathy, V. , Thangaraju, M. , Gopal, E. , Martin, P. M. , Itagaki, S. , Miyauchi, S. , & Prasad, P. D. (2008). Sodium‐coupled monocarboxylate transporters in normal tissues and in cancer. The AAPS Journal, 10(1), 193–199. 10.1208/s12248-008-9022-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Carbonell, R. , Divakaruni, A. S. , Lodi, A. , Vicente‐Suarez, I. , Saha, A. , Cheroutre, H. , … Guma, M. (2016). Critical role of glucose metabolism in rheumatoid arthritis fibroblast‐like synoviocytes. Arthritis & Rhematology, 68(7), 1614–1626. 10.1002/art.39608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geltink, R. I. K. , Kyle, R. L. , & Pearce, E. L. (2018). Unraveling the complex interplay between T cell metabolism and function. Annual Review of Immunology, 36, 461–488. 10.1146/annurev-immunol-042617-053019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerriets, V. A. , Kishton, R. J. , Nichols, A. G. , Macintyre, A. N. , Inoue, M. , Ilkayeva, O. , … Rathmell, J. C. (2015). Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. The Journal of Clinical Investigation, 125(1), 194–207. 10.1172/jci76012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobelet, C. , & Gerster, J. C. (1984). Synovial fluid lactate levels in septic and non‐septic arthritides. Annals of the Rheumatic Diseases, 43(5), 742–745. 10.1136/ard.43.5.742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal, E. , Fei, Y. J. , Miyauchi, S. , Zhuang, L. , Prasad, P. D. , & Ganapathy, V. (2005). Sodium‐coupled and electrogenic transport of B‐complex vitamin nicotinic acid by slc5a8, a member of the Na/glucose co‐transporter gene family. The Biochemical Journal, 388(Pt. 1), 309–316. 10.1042/bj20041916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal, E. , Miyauchi, S. , Martin, P. M. , Ananth, S. , Roon, P. , Smith, S. B. , & Ganapathy, V. (2007). Transport of nicotinate and structurally related compounds by human SMCT1 (SLC5A8) and its relevance to drug transport in the mammalian intestinal tract. Pharmaceutical Research, 24(3), 575–584. 10.1007/s11095-006-9176-1 [DOI] [PubMed] [Google Scholar]
- Gopal, E. , Umapathy, N. S. , Martin, P. M. , Ananth, S. , Gnana‐Prakasam, J. P. , Becker, H. , … Prasad, P. D. (2007). Cloning and functional characterization of human SMCT2 (SLC5A12) and expression pattern of the transporter in kidney. Biochimica et Biophysica Acta, 1768(11), 2690–2697. 10.1016/j.bbamem.2007.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas, R. , Cucchi, D. , Smith, J. , Pucino, V. , Macdougall, C. E. , & Mauro, C. (2016). Intermediates of metabolism: from bystanders to signalling molecules. Trends in Biochemical Sciences, 41(5), 460–471. 10.1016/j.tibs.2016.02.003 [DOI] [PubMed] [Google Scholar]
- Haas, R. , Smith, J. , Rocher‐Ros, V. , Nadkarni, S. , Montero‐Melendez, T. , D'Acquisto, F. , … Mauro, C. (2015). Lactate regulates metabolic and pro‐inflammatory circuits in control of T cell migration and effector functions. PLoS Biology, 13(7), e1002202 10.1371/journal.pbio.1002202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap, A. P. (2012). The monocarboxylate transporter family—Structure and functional characterization. IUBMB Life, 64(1), 1–9. 10.1002/iub.573 [DOI] [PubMed] [Google Scholar]
- Halestrap, A. P. , & Meredith, D. (2004). The SLC16 gene family‐from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Archiv, 447(5), 619–628. 10.1007/s00424-003-1067-2 [DOI] [PubMed] [Google Scholar]
- Halestrap, A. P. , & Price, N. T. (1999). The proton‐linked monocarboxylate transporter (MCT) family: Structure, function and regulation. The Biochemical Journal, 343(Pt. 2), 281–299. [PMC free article] [PubMed] [Google Scholar]
- Hanahan, D. , & Weinberg, R. A. (2011). Hallmarks of cancer: The next generation. Cell, 144(5), 646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Harmon, C. , Robinson, M. W. , Hand, F. , Almuaili, D. , Mentor, K. , Houlihan, D. D. , … O'Farrelly, C. (2019). Lactate‐mediated acidification of tumor microenvironment induces apoptosis of liver‐resident NK cells in colorectal liver metastasis. Cancer Immunology Research, 7(2), 335–346. 10.1158/2326-6066.Cir-18-0481 [DOI] [PubMed] [Google Scholar]
- Hill, B. R. , & Levi, C. (1954). Elevation of a serum component in neoplastic disease. Cancer Research, 14(7), 513–515. [PubMed] [Google Scholar]
- Hirschhaeuser, F. , Sattler, U. G. , & Mueller‐Klieser, W. (2011). Lactate: A metabolic key player in cancer. Cancer Research, 71(22), 6921–6925. 10.1158/0008-5472.Can-11-1457 [DOI] [PubMed] [Google Scholar]
- Hong, C. S. , Graham, N. A. , Gu, W. , Espindola Camacho, C. , Mah, V. , Maresh, E. L. , … Christofk, H. R. (2016). MCT1 modulates cancer cell pyruvate export and growth of tumors that co‐express MCT1 and MCT4. Cell Reports, 14(7), 1590–1601. 10.1016/j.celrep.2016.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. K. , Chang, P. H. , Kuo, W. H. , Chen, C. L. , Jeng, Y. M. , Chang, K. J. , … Lee, W. H. (2017). Adipocytes promote malignant growth of breast tumours with monocarboxylate transporter 2 expression via beta‐hydroxybutyrate. Nature Communications, 8, 14706 10.1038/ncomms14706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo, S. E. , Cruz‐Garcia, L. , Karanth, S. , Anderson, R. M. , Stainier, D. Y. , & Schlegel, A. (2012). A monocarboxylate transporter required for hepatocyte secretion of ketone bodies during fasting. Genes & Development, 26(3), 282–293. 10.1101/gad.180968.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui, S. , Ghergurovich, J. M. , Morscher, R. J. , Jang, C. , Teng, X. , Lu, W. , … Rabinowitz, J. D. (2017). Glucose feeds the TCA cycle via circulating lactate. Nature, 551(7678), 115–118. 10.1038/nature24057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain, Z. , Huang, Y. , Seth, P. , & Sukhatme, V. P. (2013). Tumor‐derived lactate modifies antitumor immune response: Effect on myeloid‐derived suppressor cells and NK cells. Journal of Immunology, 191(3), 1486–1495. 10.4049/jimmunol.1202702 [DOI] [PubMed] [Google Scholar]
- Ippolito, L. , Morandi, A. , Taddei, M. L. , Parri, M. , Comito, G. , Iscaro, A. , … Giannoni, E. (2019). Cancer‐associated fibroblasts promote prostate cancer malignancy via metabolic rewiring and mitochondrial transfer. Oncogene, 38(27), 5339–5355. 10.1038/s41388-019-0805-7 [DOI] [PubMed] [Google Scholar]
- Ishii, S. , Kihara, Y. , & Shimizu, T. (2005). Identification of T cell death‐associated gene 8 (TDAG8) as a novel acid sensing G‐protein‐coupled receptor. The Journal of Biological Chemistry, 280(10), 9083–9087. 10.1074/jbc.M407832200 [DOI] [PubMed] [Google Scholar]
- Juel, C. , & Halestrap, A. P. (1999). Lactate transport in skeletal muscle—Role and regulation of the monocarboxylate transporter. The Journal of Physiology, 517(Pt. 3), 633–642. 10.1111/j.1469-7793.1999.0633s.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, K. M. , & Dewhirst, M. W. (2010). Tumor metabolism of lactate: The influence and therapeutic potential for MCT and CD147 regulation. Future Oncology, 6(1), 127–148. 10.2217/fon.09.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. W. , Tchernyshyov, I. , Semenza, G. L. , & Dang, C. V. (2006). HIF‐1‐mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metabolism, 3(3), 177–185. 10.1016/j.cmet.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Lafontan, M. , & Langin, D. (2009). Lipolysis and lipid mobilization in human adipose tissue. Progress in Lipid Research, 48(5), 275–297. 10.1016/j.plipres.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Lee, D. C. , Sohn, H. A. , Park, Z. Y. , Oh, S. , Kang, Y. K. , Lee, K. M. , … Yeom, Y. I. (2015). A lactate‐induced response to hypoxia. Cell, 161(3), 595–609. 10.1016/j.cell.2015.03.011 [DOI] [PubMed] [Google Scholar]
- Leite, T. C. , Coelho, R. G. , Da Silva, D. , Coelho, W. S. , Marinho‐Carvalho, M. M. , & Sola‐Penna, M. (2011). Lactate downregulates the glycolytic enzymes hexokinase and phosphofructokinase in diverse tissues from mice. FEBS Letters, 585(1), 92–98. 10.1016/j.febslet.2010.11.009 [DOI] [PubMed] [Google Scholar]
- Li, H. , Myeroff, L. , Smiraglia, D. , Romero, M. F. , Pretlow, T. P. , Kasturi, L. , … Markowitz, S. D. (2003). SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proceedings of the National Academy of Sciences of the United States of America, 100(14), 8412–8417. 10.1073/pnas.1430846100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindy, S. , Uitto, J. , Turto, H. , Rokkanen, P. , & Vainio, K. (1971). Lactate dehydrogenase in the synovial tissue in rheumatoid arthritis: Total activity and isoenzyme composition. Clinica Chimica Acta, 31(1), 19–23. 10.1016/0009-8981(71)90357-3 [DOI] [PubMed] [Google Scholar]
- Liu, N. , Luo, J. , Kuang, D. , Xu, S. , Duan, Y. , Xia, Y. , … Yang, X. P. (2019). Lactate inhibits ATP6V0d2 expression in tumor‐associated macrophages to promote HIF‐2alpha‐mediated tumor progression. The Journal of Clinical Investigation, 129(2), 631–646. 10.1172/jci123027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre, A. N. , Gerriets, V. A. , Nichols, A. G. , Michalek, R. D. , Rudolph, M. C. , Deoliveira, D. , … Rathmell, J. C. (2014). The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metabolism, 20(1), 61–72. 10.1016/j.cmet.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti, P. J. , & Allaman, I. (2018). Lactate in the brain: From metabolic end‐product to signalling molecule. Nature Reviews. Neuroscience, 19(4), 235–249. 10.1038/nrn.2018.19 [DOI] [PubMed] [Google Scholar]
- Martin, P. M. , Gopal, E. , Ananth, S. , Zhuang, L. , Itagaki, S. , Prasad, B. M. , … Ganapathy, V. (2006). Identity of SMCT1 (SLC5A8) as a neuron‐specific Na+‐coupled transporter for active uptake of l‐lactate and ketone bodies in the brain. Journal of Neurochemistry, 98(1), 279–288. 10.1111/j.1471-4159.2006.03878.x [DOI] [PubMed] [Google Scholar]
- Mendler, A. N. , Hu, B. , Prinz, P. U. , Kreutz, M. , Gottfried, E. , & Noessner, E. (2012). Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c‐Jun activation. International Journal of Cancer, 131(3), 633–640. 10.1002/ijc.26410 [DOI] [PubMed] [Google Scholar]
- Michalek, R. D. , Gerriets, V. A. , Jacobs, S. R. , Macintyre, A. N. , MacIver, N. J. , Mason, E. F. , … Rathmell, J. C. (2011). Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. Journal of Immunology, 186(6), 3299–3303. 10.4049/jimmunol.1003613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda‐Goncalves, V. , Honavar, M. , Pinheiro, C. , Martinho, O. , Pires, M. M. , Pinheiro, C. , … Baltazar, F. (2013). Monocarboxylate transporters (MCTs) in gliomas: Expression and exploitation as therapeutic targets. Neuro‐Oncology, 15(2), 172–188. 10.1093/neuonc/nos298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, M. E. , & Felmlee, M. A. (2008). Overview of the proton‐coupled MCT (SLC16A) family of transporters: Characterization, function and role in the transport of the drug of abuse gamma‐hydroxybutyric acid. The AAPS Journal, 10(2), 311–321. 10.1208/s12248-008-9035-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami, Y. , Kohyama, N. , Kobayashi, Y. , Ohbayashi, M. , Ohtani, H. , Sawada, Y. , & Yamamoto, T. (2005). Functional characterization of human monocarboxylate transporter 6 (SLC16A5). Drug Metabolism and Disposition, 33(12), 1845–1851. 10.1124/dmd.105.005264 [DOI] [PubMed] [Google Scholar]
- Nalbandian, M. , & Takeda, M. (2016). Lactate as a signaling molecule that regulates exercise‐induced adaptations. Biology, 5(4), 38 10.3390/biology5040038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor, S. I. , Jamali, S. , Ames, S. , Langer, S. , Deitmer, J. W. , & Becker, H. M. (2018). A surface proton antenna in carbonic anhydrase II supports lactate transport in cancer cells. eLife, 7, e35176 10.7554/eLife.35176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. Y. , Helm, J. F. , Zheng, W. , Ly, Q. P. , Hodul, P. J. , Centeno, B. A. , & Malafa, M. P. (2008). Silencing of the candidate tumor suppressor gene solute carrier family 5 member 8 (SLC5A8) in human pancreatic cancer. Pancreas, 36(4), e32–e39. 10.1097/MPA.0b013e3181630ffe [DOI] [PubMed] [Google Scholar]
- Pasteur, L. (1861). Expériences et vues nouvelles sur la nature des fermentations. C. R. Hebd. Seances Acad. Sci., Paris, 53, 1260–1264. [Google Scholar]
- Pejovic, M. , Stankovic, A. , & Mitrovic, D. R. (1992). Lactate dehydrogenase activity and its isoenzymes in serum and synovial fluid of patients with rheumatoid arthritis and osteoarthritis. The Journal of Rheumatology, 19(4), 529–533. [PubMed] [Google Scholar]
- Pellerin, L. , & Magistretti, P. J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proceedings of the National Academy of Sciences of the United States of America, 91(22), 10625–10629. 10.1073/pnas.91.22.10625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin, L. , & Magistretti, P. J. (2012). Sweet sixteen for ANLS. Journal of Cerebral Blood Flow and Metabolism, 32(7), 1152–1166. 10.1038/jcbfm.2011.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, C. , Lu, Z. , Xie, Z. , Cheng, Z. , Chen, Y. , Tan, M. , … Zhao, Y. (2011). The first identification of lysine malonylation substrates and its regulatory enzyme. Molecular & Cellular Proteomics, 10(12), M111.012658 10.1074/mcp.M111.012658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Escuredo, J. , Dadhich, R. K. , Dhup, S. , Cacace, A. , Van Hee, V. F. , De Saedeleer, C. J. , … Sonveaux, P. (2016). Lactate promotes glutamine uptake and metabolism in oxidative cancer cells. Cell Cycle, 15(1), 72–83. 10.1080/15384101.2015.1120930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Escuredo, J. , Van Hee, V. F. , Sboarina, M. , Falces, J. , Payen, V. L. , Pellerin, L. , & Sonveaux, P. (2016). Monocarboxylate transporters in the brain and in cancer. Biochimica et Biophysica Acta, 1863(10), 2481–2497. 10.1016/j.bbamcr.2016.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertega‐Gomes, N. , & Baltazar, F. (2014). Lactate transporters in the context of prostate cancer metabolism: What do we know? International Journal of Molecular Sciences, 15(10), 18333–18348. 10.3390/ijms151018333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon‐Thomas, S. , Kodumudi, K. N. , El‐Kenawi, A. E. , Russell, S. , Weber, A. M. , Luddy, K. , … Gillies, R. J. (2016). Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Research, 76(6), 1381–1390. 10.1158/0008-5472.Can-15-1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro, C. , Longatto‐Filho, A. , Azevedo‐Silva, J. , Casal, M. , Schmitt, F. C. , & Baltazar, F. (2012). Role of monocarboxylate transporters in human cancers: State of the art. Journal of Bioenergetics and Biomembranes, 44(1), 127–139. 10.1007/s10863-012-9428-1 [DOI] [PubMed] [Google Scholar]
- Pucino, V. , Bombardieri, M. , Pitzalis, C. , & Mauro, C. (2017). Lactate at the crossroads of metabolism, inflammation, and autoimmunity. European Journal of Immunology, 47(1), 14–21. 10.1002/eji.201646477 [DOI] [PubMed] [Google Scholar]
- Pucino, V. , Certo, M. , Bulusu, V. , Cucchi, D. , Goldmann, K. , Pontarini, E. , … Mauro, C. (2019). Lactate buildup at the site of chronic inflammation promotes disease by inducing CD4(+) T cell metabolic rewiring. Cell Metabolism, 30, 1055–1074.e8. 10.1016/j.cmet.2019.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucino, V. , Cucchi, D. , & Mauro, C. (2018). Lactate transporters as therapeutic targets in cancer and inflammatory diseases. Expert Opinion on Therapeutic Targets, 22(9), 735–743. 10.1080/14728222.2018.1511706 [DOI] [PubMed] [Google Scholar]
- Rodriguez, A. M. , Perron, B. , Lacroix, L. , Caillou, B. , Leblanc, G. , Schlumberger, M. , … Pourcher, T. (2002). Identification and characterization of a putative human iodide transporter located at the apical membrane of thyrocytes. The Journal of Clinical Endocrinology and Metabolism, 87(7), 3500–3503. 10.1210/jcem.87.7.8797 [DOI] [PubMed] [Google Scholar]
- Roland, C. L. , Arumugam, T. , Deng, D. , Liu, S. H. , Philip, B. , Gomez, S. , … Logsdon, C. D. (2014). Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Research, 74(18), 5301–5310. 10.1158/0008-5472.Can-14-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab, A. S. , Tzvetanova, I. D. , & Nave, K. A. (2013). The role of myelin and oligodendrocytes in axonal energy metabolism. Current Opinion in Neurobiology, 23(6), 1065–1072. 10.1016/j.conb.2013.09.008 [DOI] [PubMed] [Google Scholar]
- Samuvel, D. J. , Sundararaj, K. P. , Nareika, A. , Lopes‐Virella, M. F. , & Huang, Y. (2009). Lactate boosts TLR4 signaling and NF‐kappaB pathway‐mediated gene transcription in macrophages via monocarboxylate transporters and MD‐2 up‐regulation. Journal of Immunology, 182(4), 2476–2484. 10.4049/jimmunol.0802059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza, G. L. , Roth, P. H. , Fang, H. M. , & Wang, G. L. (1994). Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia‐inducible factor 1. The Journal of Biological Chemistry, 269(38), 23757–23763. [PubMed] [Google Scholar]
- Shi, L. Z. , Wang, R. , Huang, G. , Vogel, P. , Neale, G. , Green, D. R. , & Chi, H. (2011). HIF1alpha‐dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. The Journal of Experimental Medicine, 208(7), 1367–1376. 10.1084/jem.20110278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shime, H. , Yabu, M. , Akazawa, T. , Kodama, K. , Matsumoto, M. , Seya, T. , & Inoue, N. (2008). Tumor‐secreted lactic acid promotes IL‐23/IL‐17 proinflammatory pathway. Journal of Immunology, 180(11), 7175–7183. 10.4049/jimmunol.180.11.7175 [DOI] [PubMed] [Google Scholar]
- Sonveaux, P. , Copetti, T. , De Saedeleer, C. J. , Vegran, F. , Verrax, J. , Kennedy, K. M. , … Feron, O. (2012). Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate‐induced HIF‐1 activation and tumor angiogenesis. PLoS One, 7(3), e33418 10.1371/journal.pone.0033418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonveaux, P. , Vegran, F. , Schroeder, T. , Wergin, M. C. , Verrax, J. , Rabbani, Z. N. , … Dewhirst, M. W. (2008). Targeting lactate‐fueled respiration selectively kills hypoxic tumor cells in mice. The Journal of Clinical Investigation, 118(12), 3930–3942. 10.1172/jci36843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas, S. R. , Gopal, E. , Zhuang, L. , Itagaki, S. , Martin, P. M. , Fei, Y. J. , … Prasad, P. D. (2005). Cloning and functional identification of slc5a12 as a sodium‐coupled low‐affinity transporter for monocarboxylates (SMCT2). The Biochemical Journal, 392(Pt. 3), 655–664. 10.1042/bj20050927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, S. , Liu, Q. , Chen, J. , Chen, J. , Chen, F. , He, C. , … Song, E. (2014). A positive feedback loop between mesenchymal‐like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell, 25(5), 605–620. 10.1016/j.ccr.2014.03.021 [DOI] [PubMed] [Google Scholar]
- Suhre, K. , Shin, S. Y. , Petersen, A. K. , Mohney, R. P. , Meredith, D. , Wagele, B. , … Gieger, C. (2011). Human metabolic individuality in biomedical and pharmaceutical research. Nature, 477(7362), 54–60. 10.1038/nature10354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, Z. , Xie, N. , Banerjee, S. , Cui, H. , Fu, M. , Thannickal, V. J. , & Liu, G. (2015). The monocarboxylate transporter 4 is required for glycolytic reprogramming and inflammatory response in macrophages. The Journal of Biological Chemistry, 290(1), 46–55. 10.1074/jbc.M114.603589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramae, H. , Yoshikawa, T. , Inoue, R. , Ushida, K. , Takebe, K. , Nio‐Kobayashi, J. , & Iwanaga, T. (2010). The cellular expression of SMCT2 and its comparison with other transporters for monocarboxylates in the mouse digestive tract. Biomedical Research, 31(4), 239–249. [DOI] [PubMed] [Google Scholar]
- Ullah, M. S. , Davies, A. J. , & Halestrap, A. P. (2006). The plasma membrane lactate transporter MCT4, but not MCT1, is up‐regulated by hypoxia through a HIF‐1alpha‐dependent mechanism. The Journal of Biological Chemistry, 281(14), 9030–9037. 10.1074/jbc.M511397200 [DOI] [PubMed] [Google Scholar]
- Van Hee, V. F. , Perez‐Escuredo, J. , Cacace, A. , Copetti, T. , & Sonveaux, P. (2015). Lactate does not activate NF‐kappaB in oxidative tumor cells. Frontiers in Pharmacology, 6, 228 10.3389/fphar.2015.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varricchi, G. , Pecoraro, A. , Marone, G. , Criscuolo, G. , Spadaro, G. , Genovese, A. , & Marone, G. (2018). Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer. Frontiers in Immunology, 9, 1595 10.3389/fimmu.2018.01595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegran, F. , Boidot, R. , Michiels, C. , Sonveaux, P. , & Feron, O. (2011). Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF‐kappaB/IL‐8 pathway that drives tumor angiogenesis. Cancer Research, 71(7), 2550–2560. 10.1158/0008-5472.Can-10-2828 [DOI] [PubMed] [Google Scholar]
- Visser, W. E. , van Mullem, A. A. , Jansen, J. , & Visser, T. J. (2011). The thyroid hormone transporters MCT8 and MCT10 transport the affinity‐label N‐bromoacetyl‐[(125)I]T3 but are not modified by it. Molecular and Cellular Endocrinology, 337(1–2), 96–100. 10.1016/j.mce.2011.02.003 [DOI] [PubMed] [Google Scholar]
- Warburg, O. (1956). Origin of cancer cells. Oncologia, 9(2), 75–83. [PubMed] [Google Scholar]
- Yabu, M. , Shime, H. , Hara, H. , Saito, T. , Matsumoto, M. , Seya, T. , … Inoue, N. (2011). IL‐23‐dependent and ‐independent enhancement pathways of IL‐17A production by lactic acid. International Immunology, 23(1), 29–41. 10.1093/intimm/dxq455 [DOI] [PubMed] [Google Scholar]
- Yanase, H. , Takebe, K. , Nio‐Kobayashi, J. , Takahashi‐Iwanaga, H. , & Iwanaga, T. (2008). Cellular expression of a sodium‐dependent monocarboxylate transporter (Slc5a8) and the MCT family in the mouse kidney. Histochemistry and Cell Biology, 130(5), 957–966. 10.1007/s00418-008-0490-z [DOI] [PubMed] [Google Scholar]
- Zhang, D. , Tang, Z. , Huang, H. , Zhou, G. , Cui, C. , Weng, Y. , … Zhao, Y. (2019). Metabolic regulation of gene expression by histone lactylation. Nature, 574(7779), 575–580. 10.1038/s41586-019-1678-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Wang, G. , Xu, Z. G. , Tu, H. , Hu, F. , Dai, J. , … Lin, H. K. (2019). Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell, 178(1), 176–189.e115. 10.1016/j.cell.2019.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]


