Abstract
Lactic acid‐producing bacteria are important in many fermentations, such as the production of biobased plastics. Insight in the competitive advantage of lactic acid bacteria over other fermentative bacteria in a mixed culture enables ecology‐based process design and can aid the development of sustainable and energy‐efficient bioprocesses. Here we demonstrate the enrichment of lactic acid bacteria in a controlled sequencing batch bioreactor environment using a glucose‐based medium supplemented with peptides and B vitamins. A mineral medium enrichment operated in parallel was dominated by Ethanoligenens species and fermented glucose to acetate, butyrate and hydrogen. The complex medium enrichment was populated by Lactococcus, Lactobacillus and Megasphaera species and showed a product spectrum of acetate, ethanol, propionate, butyrate and valerate. An intermediate peak of lactate was observed, showing the simultaneous production and consumption of lactate, which is of concern for lactic acid production purposes. This study underlines that the competitive advantage for lactic acid‐producing bacteria primarily lies in their ability to attain a high biomass specific uptake rate of glucose, which was two times higher for the complex medium enrichment when compared to the mineral medium enrichment. The competitive advantage of lactic acid production in rich media can be explained using a resource allocation theory for microbial growth processes.
Keywords: enrichment cultures, kinetics, lactic acid bacteria, microbial ecology, resource allocation
The supplementation of peptides and B vitamins to an ecosystem where glucose was fermented directs the glucose to lactate and ethanol. Enriching with glucose and a mineral medium, acetate, butyrate and hydrogen was the dominant product pathway. This is likely caused by proteome optimisation of lactic acid bacteria. Also, lactate was only fermented in the presence of B vitamins and peptides. We demonstrate the rate efficiency trade off in fermentative ecosystems and pave the way for directing product formation.
1. INTRODUCTION
Lactic acid bacteria are key species in many fermentative processes (Axelsson & Ahrné, 2000), such as biogas production and food‐related fermentations (Leroy & De Vuyst, 2004). They also are essential in promoting human health, for example, a healthy human infant microbiome (Solís, de los Reyes‐Gavilan, Fernández, Margolles, & Gueimonde, 2010). In an industrial biotechnology setting, these microorganisms are applied in the production of lactic acid, which is used to preserve food and to produce the biobased and biodegradable plastic polylactic acid (Straathof, 2014). The lactic acid market is expected to reach 9.8 billion US dollars by 2025 which shows the economic significance of lactic acid.
Mixed culture biotechnology (Kleerebezem & van Loosdrecht, 2007) can aid in the development of more sustainable and energy‐efficient bioprocesses. Such processes rely on “ecology‐based design” of bioprocesses to perform the desired conversion, which contrasts with the traditional pure culture approach. Typically, enrichment cultures are used to function as a model system to develop such ecology‐based bioprocesses. Compared to pure culture processes, ecology‐based processes offer the advantage of (semi)‐continuous bioprocessing and omit the need for sterilisation of the feedstock and equipment (Kleerebezem & van Loosdrecht, 2007). Examples of successful ecology‐based bioprocesses are PHA production from Volatile fatty acids (VFAs; Johnson, Jiang, Kleerebezem, Muyzer, & Loosdrecht, 2009) or biological phosphorous removal (reviewed by Bunce, Ndam, Ofiteru, Moore, & Graham, 2018). To create a stable ecology‐based process, its design needs to be based on the competitive advantage of the concerned type of conversion. In the case of lactic acid to be produced from carbohydrates, the ecological question is which environmental conditions provide lactic acid bacteria with a competitive advantage over other carbohydrate fermenting microorganisms?
Lactic acid bacteria tend to dominate in anaerobic, carbohydrate‐containing environments characterised by acidic pH and abundant availability of compounds required for anabolism, such as in fermented milk, meats and vegetables (Axelsson & Ahrné, 2000). Most well‐studied lactic acid bacteria are part of the Bacilli class, such as Streptococcus, Lactococcus, Bacillus and Lactobacillus species. Lactic acid bacteria have high maximal biomass specific growth rates (µ max), for example, Streptococcus salivarius shows a µ max of 2.8 hr−1 in a complex medium at 37°C, at neutral pH (Roger, Delettre, Bouix, & Béal, 2011). This can be compared to µ max for Escherichia coli strain K12 of around 0.98 hr−1 at similar conditions (Kim, Ingram, & Shanmugam, 2007). Lactic acid bacteria seem to have a kinetic advantage over other species and have quite extraordinary growth rates while being anaerobic microorganisms.
Lactic acid bacteria only display fast growth when sufficient B vitamins and peptides are supplied to their medium environment. For example, Lactococcus lactis strains are auxotrophic for 14 of 20 amino acids (Cocaign‐Bousquet, Garrigues, Novak, Lindley, & Loublere, 1995). A comparative genome study predicted that of the 46 Lactobacillus species analysed all are auxotrophic for biotin, folate, pantothenate and thiamine (Magnúsdóttir, Ravcheev, de Crécy‐Lagard, & Thiele, 2015). Lactic acid bacteria grow poorly or do not grow at all in environments where such B vitamins or peptides are not available. We therefore suggest that auxotrophies are common among lactic acid bacteria, certainly under conditions of high growth rates.
Prototrophic fermentative microorganisms in general have lower µ max values when compared with lactic acid bacteria. These organisms can be found in the genus of Clostridium and the families of Enterobacteriaceae and Ruminococcaceae. The extensively studied Enterobacteriaceae species E. coli is a prototroph, and is reported to have a µ max of 0.31 hr−1 at 37°C and a pH of 7 in a mineral medium with glucose (Hasona, Kim, Healy, Ingram, & Shanmugam, 2004). E. coli here produced a mixture of acetate, ethanol and formate. We hypothesise that lactic acid bacteria will outcompete prototrophic fermenters by achieving a higher µ max in complex environments where there is an abundance of peptides and B vitamins.
The switch between lactate production on the one hand, and acetate and ethanol production on the other hand, has been reported for a single species under complex medium conditions. L. lactis (formerly known as Streptococcus lactis), switches its catabolism from lactate production to acetate, ethanol and formate or H2 production at lower dilution rates, that is, lower growth rates (Thomas, Ellwood, & Longyear, 1979). Lactate is produced from pyruvate with one enzyme and delivers and acetate and ethanol with five enzymes. Lactate delivers two ATP by substrate level phosphorylation, while acetate and ethanol deliver three ATP. This switch is thought to be caused by resource allocation, which essentially describes that a cell has a certain amount of functional protein available, and shorter catabolic pathways can evoke a higher biomass specific substrate uptake rate, q s max (de Groot, van Boxtel, Planqué, Bruggeman, & Teusink, 2018; Molenaar, van Berlo, de Ridder, & Teusink, 2009), often at the expense of less energy harvesting per unit of substrate.
Here, we tested the hypothesis that lactic acid‐producing enrichment cultures can be obtained by providing a complex medium and selecting on high growth rate. We compared two parallel anaerobic non‐axenic or open mixed culture sequencing batch reactors (SBRs) operated under mesophilic (T = 30°) and slightly acidic conditions (pH = 5), with either mineral or complex cultivation media. The mineral medium was replicated from the work of Temudo (2008) and containing 4 g L−1 of glucose. The complex medium consisted of the mineral medium, and also 0.8 g L−1 of tryptone and 9 B vitamins. The cultures were characterised for their stoichiometric, kinetic and thermodynamic properties and the microbial community structure was analysed.
2. MATERIALS AND METHODS
2.1. Bioreactor enrichment
Both enrichments were performed in 3 L jacketed bioreactors with working volumes of 2 L. pH was maintained at 5.0 ± 0.1 using NaOH at 4 mol L−1 and HCl at 1 mol L−1. Temperature was maintained at 30°C ± 0.1. The cultures were stirred constantly at 300 rpm. Anaerobic conditions were maintained by sparging the reactor with a flow of 576 mmol N2 hr−1. The off‐gas was cooled and dried at 5°C using a gas condenser. A hydraulic retention time (HRT) and solid retention time (SRT) of 12 hr was maintained by removing 1 L of culture per cycle under continuous stirring and a cycle time set to 6 hr.
The mineral cultivation medium was identical to the one used by Temudo, Kleerebezem, and van Loosdrecht (2007), while the complex medium was supplemented by 9 B vitamins and peptides according to Table S1 in the supplementary information. The carbon source, peptides and B vitamins and the ammonium, phosphate and trace elements were fed separately from 12.5 times concentrated stock solutions and diluted using N2‐sparged demineralised water. Connected to the base pump was a pump supplying 3% (v:v) antifoam C (Sigma‐Aldrich, Germany), which ensured a flow of 3–5 ml hr−1 or 14–17 ml cycle−1. The glucose solution was sterilised at 110°C for 20 min. For the complex medium, the peptides were sterilised separately at 110°C for 20 min and the B vitamins were added by filter sterilisation through 0.45 and 0.2 µm polyvinylidene fluoride filters.
The inoculum for all enrichments consisted of sludge taken from an anaerobic digester of the wastewater treatment plant (WWTP; Harnaschpolder, The Netherlands). The pH, temperature and HRT and SRT of the digester in the WWTP were 7–7.2, 36–38°C and 20 days, respectively. At the beginning of each experiment, the reactor was seeded with approximately 10 ml of 200 µm filtered inoculum (0.5% of the total volume). The reactors were gradually moving from 24‐ and 12‐hr cycles in 3 days to the final desired 6‐hr cycles to maintain a HRT of 12 hr. Steady state was assumed if during a period of at least 5 days little variation was detected in the product concentrations.
2.2. Analytical methods
Samples from the reactors were immediately filtered on 0.45 µm polyvinylidene fluoride membranes (Millipore) and stored at −20°C until analysis. Volatile fatty acids (VFAs; formate to valerate), lactate, succinate and glucose were analysed using high‐performance liquid chromatography (HPLC) method described previously (Rombouts, Mos, Weissbrodt, Kleerebezem, & van Loosdrecht, 2019). Ethanol was analysed using a gas chromatography (GC) method described previously (Rombouts et al., 2019). The off‐gases were monitored on‐line for H2 and CO2 by a connection to a NGA 2000 MLT 1 multicomponent analyser (Rosemount, Shakopee, MN). Data acquisition (base, H2, CO2) was made using a BBI systems MFCS/win 2.1 (Sartorius, Göttingen, Germany).
Methane was measured manually using GC with a Varian CP 3800 (Varian Medical Systems, Palo Alto, CA) equipped with a MolSieve capillary column (1.2 m × 1 mm; 13 × 80/100 mesh, 50°C) and a thermal conductivity detector (200°C) with N2 as a carrier gas (2 ml min−1).
Biomass concentration was measured using a standard method which relies on centrifugation of 150 ml to separate the cells from the medium, drying these solids to obtain the total suspended solids (TSS) and burning these solids at 550°C to determine the amount of volatile suspended solids (VSS; APHA, 1998). This analysis was coupled to absorbance measurement at 660 nm to establish a correlation. Absorbance values were used to calculate the biomass concentration during the cycle analysis and batch experiments.
The carbon, nitrogen and chemical oxygen demand (COD) content of tryptone was measured by measuring the carbon, nitrogen and COD in a concentration of 1.000 g L−1 of tryptone in demineralised water. Carbon and nitrogen content was measured by the total organic carbon (TOC) and total nitrogen (in g L−1) using a TOC‐L CPH/CPN analyser (Shimadzu Benelux, ‘s‐Hertogenbosch, The Netherlands). TOC was determined as total carbon (TC) subtracted by the inorganic carbon (IC) (TOC = TC – IC). COD was measured using a Hach Lange COD cuvette kit in the range of 20–1500 mgCOD L−1 (Hach, Loveland, CO).
2.3. Cycle analysis and batch experiments
To characterise one cycle, product and biomass concentrations were measured in parallel to H2 and CO2 in the off‐gas. Sampling and off‐gas analysis were carried out as described above. The biomass concentration was determined spectrophotometrically at 660 nm (OD660) and this value was correlated to the three previous measurements of VSS.
A batch test with lactate and a batch test with H2, and CO2 was performed in the complex medium enrichment. This went by adding the peptides and B vitamins and peptides together with the N, P, S, trace elements, and either 11 mmol of lactate or 0.46% of H2 and 1.00% of CO2. Sampling was conducted as in a cycle measurement.
2.4. Microbial community analysis
Genomic DNA was extracted using the Ultra Clean Soil DNA extraction kit (Qiagen, Hilden, Germany) following manufacturer's instructions, with the exception of heating the samples for 5 min at 65°C before bead beating. DNA extracts were checked on a 1% agarose gel. High molecular weight DNA was obtained (>10 kb) with a concentration of 10 ng µL−1 or higher. Extracted DNA was stored at −20°C until further use.
Analysis of the V3‐V4 region of the 16S rRNA gene was conducted using amplicon sequencing. The extracted DNA was sent for amplification and sequencing at a commercial company (Novogene, China). Amplification was achieved using the universal primer set 341 f (CCTAYGGGRBGCASCAG)/806r (GGACTACNNGGGTATCTAA T; Muyzer, de Waal, & Uitterlinden, 1993; Caporaso et al., 2011). All polymerase chain reactions (PCRs) were carried out in 30 µL reactions with 15 µL of Phusion® High_fidelity PCR Master Mix (New England Biolabs), 0.2 µmol L−1 of forward and reverse primers, and 10 ng template DNA. Thermal cycling started with denaturation at 98°C for 10 s, annealing at 50°C for 30 s, and elongation at 72°C for 60 s for 30 cycles, before ending with 72°C for 5 min. These pools of amplicons were sequenced using an Illumina HiSeq2500 platform. The sequencing data sets were cleaned and trimmed according to Jia et al. (2016) and processed with Qiime (Caporaso et al., 2010) using Uparse with a 97% stringency to yield operational taxonomic units (OTUs). OTUs were taxonomically classified using the Mothur classifier (Wang, Garrity, Tiedje, & Cole, 2007) with 0.8 confidence interval against the SILVA database 123 release of July 2015. The clean and trimmed sequences can be retrieved at NCBI using accession number SAMN11350619–SAMN11350630. The inoculum was sequenced twice as a technical replicate, this data can be retrieved at NCBI (data not presented in this paper).
Cell fixation and fluorescence in situ hybridisation (FISH) were carried out as described by Johnson et al. (2009) using the probes listed in Table S2, except that hybridisation was carried out overnight. Additionally, 4′,6‐diamidino‐2‐phenylindole (DAPI) staining was used to stain all microbial cells by incubating the multi‐wells microscopy slides of fixed cells with 10 µL of a solution of 10 mg DAPI mL−1 per well for 15 min. The samples were analysed using an epifluorescence microscope, Axioplan 2, (Zeiss, Oberkochen, Germany). Digital images were acquired using a Zeiss MRM camera together with Zeiss imaging software AxioVision version 4.7.
2.5. Parameter estimation of the kinetics of the enrichment cultures using minimisation of residual error
To estimate the kinetic parameters of the enrichments and to derive the stoichiometry of the process a kinetic model was built. The uptake rate was modelled using Monod equations and no biomass decay or pH inhibition was considered. The saturation constant of the Monod equations for the different microorganisms was assumed to be 0.1 g/L. It is assumed that there is a microbial group degrading glucose and other degrading lactate (only in the complex medium enrichment). In this last case, the measured biomass was initially divided between glucose degraders and lactate degraders at 35% and 65%. After the optimisation, it was based on the ATP yield on the substrate corresponding to that stoichiometry. Parameter estimation was performed following the method proposed by Gonzalez‐Gil, Mauricio‐iglesias, Carballa, and Lema (2018) and is further explained in Appendix B.
2.6. ATP yield estimation using the obtained parameters
The model estimates the distribution of substrate to select a set of catabolic pathways to obtain the lowest residual error with respect to the measured metabolic product distribution during a cycle analysis. These fractions are combined with the ATP yield per catabolic reaction to obtain the overall yield of ATP on substrate (Y ATP,S). This yield is then combined with the biomass yields on glucose (Y x,s) observed in time (n = 3) to obtain the biomass yield on ATP harvested (Y X,ATP). An argumentation in which catabolic routes were selected for both enrichments can be found in Appendix C.
2.7. Estimation of µ max from on‐line data collected from bioreactors
A script was developed in Matlab version 2014 (MathWorks, Natick, MA) to estimate the growth rate over each SBR cycle. This script is based on determining the last moment of base dosage in a cycle, which indicates the end of the fermentation, and thus the growth. This method is explained in detail in the supplementary information section published previously (Rombouts et al., 2019). The maximum biomass‐specific substrate conversion rate (q s max) is calculated using µ max and Y x,s using the Herbert‐Pirt equation (Pirt, 1965) and neglecting maintenance.
2.8. Carbon and COD balancing
During steady state, carbon and electron (as chemical oxygen demand, COD) balances were defined using the elemental metabolite matrix given in Table S3 multiplied by the in‐ and outgoing rates in the reactor. COD balances were set up for the cycle analyses by dividing the amount of COD present in the metabolic compounds measured at a time in the cycle by the measured COD at the start of the cycle. For tryptone, the carbon and COD balance were set up by assuming all nitrogen needed for biomass formation was obtained taking up tryptone, using a biomass composition of C1N0.2H1.8O0.5 (Roels, 1983).
3. RESULTS
3.1. A complex medium promotes fermentation to VFAs and ethanol with little hydrogen while a mineral medium promotes acetate–butyrate–hydrogen formation
Two anaerobic SBRs were operated with either a mineral or complex medium. The enrichments displayed distinct fermentation patterns after 20 SRTs. Initially, the mineral‐medium enrichment showed a 1:2:1 acetate:propionate:butyrate product spectrum with little ethanol and no lactate (Figure S1). This shifted after 10 SRTs to primarily acetate and butyrate, with a small amount of lactate and ethanol (Figure 1). Hydrogen was the major gaseous product in the mineral medium enrichment (13% ± 1% of incoming COD). Up to 94% ± 3% of the incoming COD could be recovered for this enrichment, which indicates that a minor by‐product might have been missed. Succinate, valerate or formate concentrations were below the detection limit of 50 µM.
Figure 1.
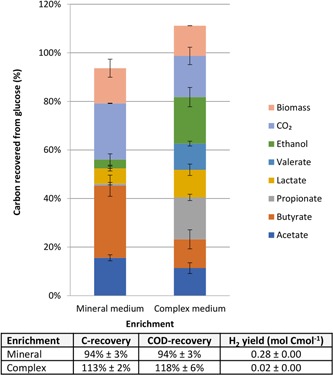
Observed product spectrum on glucose for the mineral and complex medium enrichment and calculated carbon and chemical oxygen demand (COD) recovery (assuming only glucose is consumed from the medium) [Color figure can be viewed at http://wileyonlinelibrary.com]
The complex medium enrichment showed a more dynamic product spectrum development. Initially lactate and acetate were the dominant products (Figure S1). After 3 SRTs, the product spectrum shifted to acetate, propionate, butyrate, valerate and lactate. After 31 SRTs, 0.19 Cmol ethanol Cmol−1 sugar was produced, and only a minor amount of hydrogen was detected in the off‐gas (1% ± 0% of the incoming COD; Figure 1). This product spectrum was more diverse than for the mineral medium enrichment. The carbon balance (based on glucose as the only substrate) displayed a recovery of 113% ± 2%, which most likely is caused by the uptake of tryptone for biomass production. Taking into account that tryptone uptake is equivalent to the nitrogen requirements for biomass production, the carbon recovery would be 100% ± 2%.
The mineral medium enrichment showed a 25% higher biomass yield on glucose than the complex medium enrichment culture. The µ max values for the cultures were derived with a cut‐off at 20 SRTs (Figure S2). The µ max in the complex medium enrichment was 58% higher than the mineral medium enrichment, while the maximal biomass specific substrate uptake rate (q s max) was even 94% higher (Table 1).
Table 1.
Key kinetic, stoichiometric and bioenergetic parameters of the glucose fermenting SBR enrichment cultures
| Enrichment medium | µ max (hr−1) | qs max (C‐molS C‐molX −1 hr−1) | Yx,s (C‐molX C‐molS ‐1) | YATP,s (molATP molS −1) | Yx,ATP (gX molATP −1) |
|---|---|---|---|---|---|
| Mineral | 0.17 ± 0.02 | 1.17 ± 0.30 | 0.15 ± 0.04 | 3.04 [3.02, 3.05] | 7.0 [5.4, 8.7] |
| Complex | 0.27 ± 0.01 | 2.27 ± 0.11 | 0.12 ± 0.00 | 2.33 [2.29, 2.37] | 7.9 [7.7, 8.0] |
Note: Observed µ max obtained through processing of online base‐dosage data after 20 SRTs (1 SRT = 12 hr), estimated q s max and biomass yield on glucose (Y x,s). Calculated Y x,ATP using the observed Y x,s and the Y ATP,s obtained from the best fitting catabolic product distribution profile with the 95% confidence interval values given in brackets.
3.2. Cycle analysis reveals potential storage of glucose when using a mineral medium and an intermediate lactate peak when using a complex medium
The product and substrate concentrations during a representative cycle in the SBR are shown in Figure 2 for both enrichments. In the enrichment culture on mineral medium, glucose was converted to mainly acetate and butyrate with minor amounts of ethanol and lactate (Figure 2a). The formation of fermentation products proceeds after glucose depletion. The COD recovery during the cycle showed that during the glucose consumption phase the COD of consumed glucose is not fully recovered in the measured products (Figure S7A), while in the subsequent period in the cycle the product concentration increased and finally a full recovery of consumed COD is observed. This indicates formation of an intermediate product which is probably a storage product, most likely a polymer of glucose.
Figure 2.
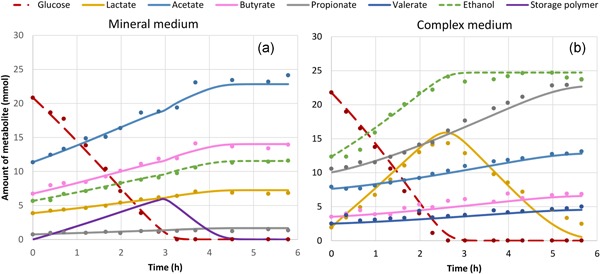
Observed (points) and modelled (lines) amount of substrate and product in the mineral (a) and complex (b) medium and enrichment at Day 33 (40 SRTs) and Day 29 (49 SRTs) respectively. SRT, solid retention time [Color figure can be viewed at http://wileyonlinelibrary.com]
In the complex medium enrichment, lactate and ethanol were formed in the glucose consumption phase (Figure 2b). When glucose was depleted, ethanol formation stopped, while lactate was consumed. In this secondary fermentation, acetate, propionate, butyrate, valerate and CO2 were formed. This secondary fermentation of lactate was confirmed by a pulse experiment with lactate, peptides and B vitamins (Figure S5). The COD recovery showed no indications of an intermediate product or storage of glucose, since all COD consumed could be accounted for in the dissolved or gaseous products formed when the glucose was depleted (Figure S7B). No homoacetogenesis was observed when H2 and CO2 were used as a substrate in a batch experiment (Figure S6) and no methane was measured during the cycles in the complex medium enrichment (data not shown).
3.3. Supplementing peptides and B vitamins led to significant presence of lactic acid bacteria and more microbial diversity
Clostridium and Ethanoligenens were the dominant OTUs in the mineral medium enrichment culture (Figure 3). FISH analysis showed a very different result and demonstrated dominance in biovolume of Ruminococcaceae using the Rums278 probe, to which the genus of Ethanoligenens belongs. Only a minor biovolume of Clostridium was detected using the Chis150 probe. Lactobacillus was present as a very minor population (Figure S4) in the mineral medium enrichment. The discrepancy between sequencing results and FISH evaluation shows that complementary observations of the microbial community structure are needed when analysing a microbial community, referred to as the “full cycle rRNA analysis” by Schleifer, Amann, and Ludwig (1995).
Figure 3.
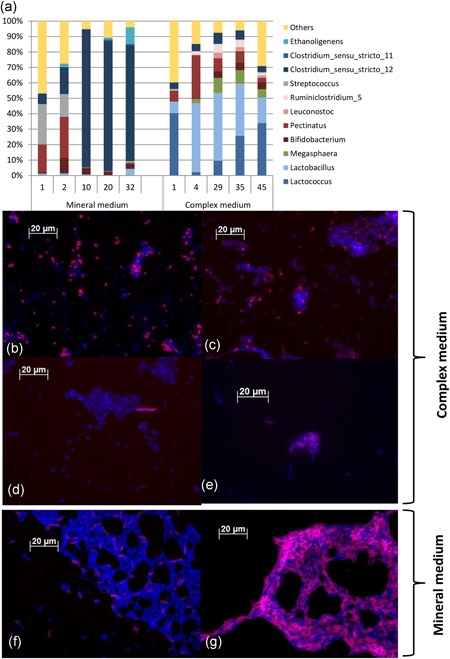
Result of the FISH and V3‐V4 16S rRNA gene analysis with OTUs grouped at the genus level. 16S rRNA gene identification of the microbial community composition in time for both enrichments (as SRTs) at genus level (a). Less than 3% genera are grouped as others. FISH is shown with target‐probe in red using Cy3 as fluorescence marker and EUB338 targeting all eubacterial biomass in blue using Cy5. The pictures show complex medium enrichment probed with Lactococcus4 (b), Lacto722 (c), Chis150 (d) and Mega‐X (e) and the mineral medium enrichment probed with Chis150 (f) and rums278 (g). FISH, fluorescence in situ hybridisation; OTU, operational taxonomic unit; rRNA, ribosomal RNA; SRT, solid retention time [Color figure can be viewed at http://wileyonlinelibrary.com]
Lactococcus and Lactobacillus were dominant OTUs in the complex medium enrichment (Figure 3). Their dominance was confirmed by FISH analysis using the Lactococcus4 (Lactococcus) and Lacto722 (Lactobacillus) probes. The presence of Megasphaera and minor presence of Clostridium was also confirmed using the Mega‐X (Megasphaera) and the Chis150 (Clostridium) probes.
The diversity of the obtained microbial community structures using 16S rRNA amplicon sequencing can be calculated using the Shannon index for the observed genera with a presence >3% of the total OTUs. For the mineral medium the Shannon index was 0.94 (sample at 32 SRTs) and for the complex medium, this was 1.34 (sample taken at 41 SRTs). Thus, more microbial diversity was observed in the complex medium enrichment compared to the mineral medium enrichment on the basis of the number of genera found (Figure 3).
4. DISCUSSION
4.1. Supplementation of peptides and B vitamins leads to a dominance of lactic acid bacteria and high q s max through resource allocation
In this study we demonstrate that lactic acid bacteria outcompete prototrophic type fermenters (e.g., Clostridium species) when nutritive conditions were favourable, that is, with sufficient amount of amino acids and B vitamins in an SBR cultivation mode. Kim et al. (2016) have shown that lactic acid bacteria can be enriched in a continuous‐flow stirred tank reactor (CSTR) process. They operated the CSTR anaerobically, at pH 5.0 and thermophilic (50°C) conditions with an SRT of 12 hr, with glucose and yeast extract as fermentable organic substrates. Yeast extract is a well‐known source of peptides, amino acids, B vitamins and carbohydrates. In cabbage fermentation lactic acid bacteria are known to be the dominant organism (Plengvidhya, Breidt, Lu, & Fleming, 2007), while fermentable substrates with low protein content, such as starch, Clostridium species are the dominant organism (Lin, Chang, & Hung, 2008).
Lactic acid bacteria are well known to be auxotrophic for amino acids (Kitay & Snell, 1950), while their auxotrophy for B vitamins is more ambiguous. Some lactic acid bacteria might actually be producers of B vitamins (LeBlanc et al., 2011). Studies with lactic acid bacteria on synthetic medium have demonstrated the specific compounds needed for growth (Novak, Cocaign‐Bousquet, Lindley, & Loubiere, 1997), up to individual amino acids (Cocaign‐Bousquet et al., 1995). The effect of decreasing medium complexity has been illustrated by Olmos‐Dichara, Ampe, Uribelarrea, Pareilleux, and Goma (1997). When the “richness” of the growth medium was decreased, the q s max remained stable, while the growth yield decreased. This shows that the medium complexity directly influences the bioenergetics of Lactobacillus casei, resulting in a lower biomass production when peptides and/or B vitamins are insufficiently supplied in the medium.
Lactic acid bacteria have a kinetic advantage leading to their dominance in enrichments at high growth rates and complex media. The biomass yield of the complex medium enrichment culture was 20% lower than for the enrichment culture on a mineral medium. The maximal substrate uptake rate was almost double for the community enriched on a complex medium versus mineral medium (Table 1). Lactic acid production is clearly a metabolic strategy of high flux but low efficiency. This is supported by the observation that lactic acid bacteria switch to acetate and ethanol production when substrate conversion rates decrease, that is, lower growth rates (De Vries, Kapteijn, Van Der Beek, & Stouthamer, 1970). Acetate/ethanol production generates 3 mol instead of 2 mol ATP for lactate fermentation on glucose. This can be placed well in the context of resource allocation theories, given a certain protein budget (Bachmann, Molenaar, Branco dos Santos, & Teusink, 2017). Less biosynthetic enzymes needed for amino acids and B vitamin synthesis lead to a smaller anabolic proteome. A smaller anabolic proteome can imply a bigger catabolic proteome, as demonstrated when comparing the proteome from E. coli grown in a mineral and complex medium (Li, Nimtz, & Rinas, 2014). Lactate catabolism requires one enzyme from pyruvate, while acetate/ethanol production requires at least five enzymes. Furthermore, at increasing growth rates, ribosome and RNA polymerase content is higher (Bosdriesz, Molenaar, Teusink, & Bruggeman, 2015). Lactic acid bacteria are assumed to have optimally distributed their metabolic enzyme levels (Teusink, Bachmann, & Molenaar, 2011), enabling a high overall metabolic flux.
4.2. Mineral medium enriched for an acetate‐butyrate type fermentation, potential glucose storage and the class of Clostridia
The product formation spectrum from the mineral medium enrichment culture was evaluated to identify the most dominant catabolic route. It was found that our flux‐based model fitted best when 42% of the glucose was converted through the acetate–butyrate pathway involving electron bifurcation (Table S5). The microbial community was populated by two genera from the class of Clostridia: an Ethanoligenens population and Clostridium population. The Ethanoligenens population showed dominance, as Ruminococcaceae were dominant while Clostridium was a minority which was shown with fluorescent in situ hybridisation (Figure 3f,g). Ethanoligenens harbinense is a strictly anaerobic species known to produce ethanol, acetate and butyrate from carbohydrates (Tang, Yuan, Guo, & Ren, 2012; Xing et al., 2006). Cluster 12 (sensu strcito XII) of Clostridium was identified (Figure 3a,f). Clostridium pasteurianum is a well‐studied species in this cluster (SILVA release 138), and is known for acetate‐butyrate production involving electron bifurcation (Buckel & Thauer, 2013). This organism has also been found in a fermentative granular enrichment culture which stored poly‐glucose (Tamis, Joosse, Loosdrecht, & Kleerebezem, 2015). In a glucose‐fermenting methanogenic community, storage of glucose has been characterised as the storage of mainly trehalose (Shimada, Zilles, Raskin, & Morgenroth, 2007). In the mineral medium enrichment, effectively 20% of the glucose was potentially metabolised via a carbon storage pool (Figure S6A). As the community is dominated by Ethanoligenens and the storage of poly‐glucose is significant it is likely that both Clostridium and Ethanoligenens species were performing the storage response. The storage response in the mineral medium enrichment causes uncoupling of substrate uptake and growth. The community thereby maximises its substrate uptake rate (competitive advantage) while growing at a more balanced growth rate over the SBR cycle.
4.3. Complex medium enriched for production of VFAs through lactic acid formation and consumption and is linked with lactic acid bacteria and megasphaera
Evaluating the pathways for the complex medium enrichment showed the best fit when glucose was catabolised through the heterofermentative (69%) and homofermentative (31%) pathway (Table S5). Lactate was subsequently fermented into propionate, butyrate, valerate, H2 and CO2. The secondary lactate fermentation was confirmed in a batch experiment with the enrichment culture and replacing glucose with lactate (Figure S5). The microbial community analysis revealed a dominance of Lactobacillus, Lactococcus and Megasphaera (Figure 3b,c,e).
Lactococcus species are known homofermentative lactic acid bacteria, while Lactobacillus and Leuconostoc species can also be heterofermentative (Madigan & Martinko, 2006). Megasphaera elsdenii is known to produce acetate, propionate, butyrate and valerate from lactate (Prabhu, Altman, & Eiteman, 2012). Megasphaera is known to convert lactate in the intestinal tract of cows, pigs and humans (Duncan, Louis, & Flint, 2004) and is linked to lactate‐mediated medium‐chain length carboxylate production microbiomes (Spirito, Richter, Stams, & Angenent, 2014). Megasphaera elsdenii interestingly prefers lactate uptake over glucose uptake, taking up limited amounts of glucose when lactate is present (Marounek, Fliegrova, & Bartos, 1989), which argues for the Megasphaera species having a mainly lactate consuming role in the community.
4.4. Bioenergetics of complex and mineral‐type fermentation: supplementation might lead to a more efficient metabolism
The lower growth yield in the complex medium (Table 1) is counterintuitive since these bacteria grow on a complex medium and do not need to produce the amino acids themselves. To compare the impact of the supplementation of peptides and B vitamins on the metabolism, we calculated the Y x,ATP for both enrichments from the catabolic ATP yields estimated (Table 1). For the mineral enrichment a 11% lower Y x,ATP value was estimated, but is not statistically significant (one‐tailed t test gives p = 0.45). Amino acid degradation (Stickland fermentation) was omitted from this bioenergetic evaluation, as the metabolic evaluation of 20 different amino acid utilising pathways would seriously complicate the bioenergetic evaluation. Incorporating amino acid degradation might lower the Y X,ATP for the complex medium enrichment, bringing it closer to the value of the mineral medium enrichment.
Prototrophic fermenters such as Escherichia coli and auxotrophic fermenters such as L. lactis have similar protein and RNA content (Table S6). Stouthamer estimated that the supplementation of amino acids induces a 0.7% decrease in Y x,ATP (Stouthamer, 1973). The biomass yield of E. coli fermenting glucose in complex medium is 13% higher than when fermenting in mineral medium (Lawford & Rousseau, 1995). According to Stouthamer (1973, Table 5) the synthesis of amino acids consumes a relatively low amount of ATP, while uptake of amino acids or ammonium consumes ATP, making both environment bioenergetically equivalent. The polymerisation process for proteins consumes most ATP, about 55% of the available ATP (Stouthamer, 1973). We expect that the biosynthesis of B vitamins requires a relatively small ATP‐flux, as B vitamins are present in trace amounts in bacterial biomass (<10−5 in g g−1; Waller & Lichstein, 1965).
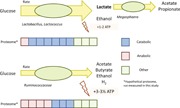
The difference in the estimated anabolic efficiency is much less than the difference observed in µ max, indicating that the complex medium promotes high substrate uptake rates rather than high biomass yields. Functional protein is a valuable resource for a cell. Not only minimising the fermentative pathway length, but also minimising biosynthetic enzymes for amino acid and B vitamin production makes that more cellular protein can be allocated to increase the growth rate of the cell. This is in essence the “trick” that lactic acid bacteria use to dominate anaerobic environments where carbohydrates and peptides are available (Figure 4).
Figure 4.
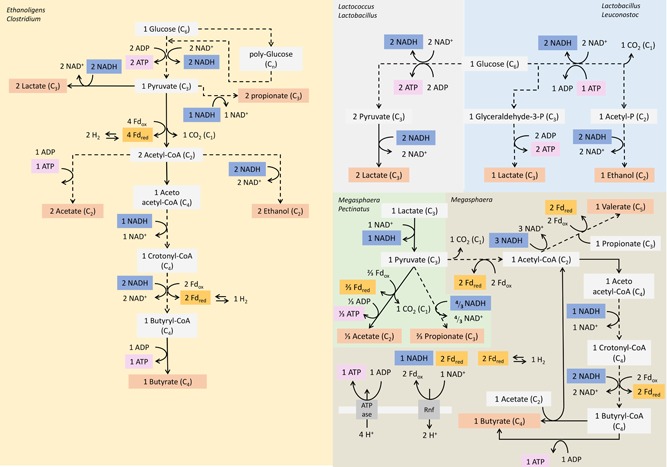
Dominant metabolic pathways and associated taxa in the mineral medium enrichment culture (left) and the complex medium enrichment culture (right). Dotted lines denote lumped reactions. A detailed explanation on the basis of these metabolic networks is given in Appendix C [Color figure can be viewed at http://wileyonlinelibrary.com]
4.5. The impact of feedstock protein content on fermentative product spectrum
The consequence of these different ecological types of fermentations are important for understanding applications of mixed‐culture fermentation, especially for non‐aseptic or “open” bioprocesses aimed to produce economically interesting compounds. The difference in hydrogen production we observed here (Figure 1) has been reflected in a meta‐study comparing different feedstocks for the production of hydrogen: food and municipal waste streams generate 32–42% less hydrogen than industrial and (pretreated) agricultural residue waste streams (Moscoviz, Trably, Bernet, & Carrère, 2018). Food waste typically contains more than 10% (w:w) of protein (Paritosh et al., 2017), while agricultural residues contain low amounts of protein, for example, wheat straw contains 0.6% (w:v; Kaparaju, Serrano, Thomsen, Kongjan, & Angelidaki, 2009). This leads to food waste fermentations being dominated by lactic acid bacteria and the secondary lactate fermentation producing no or small amounts of hydrogen gas. In contrast, fermentations of (pretreated) agricultural residues are dominated by acetate/butyrate producing bacteria, such as Clostridium species, resulting in significant amounts of hydrogen produced. This difference in performance is a direct consequence of the ecology of these two different fermentative microbial groups. Lactic acid bacteria seem to dominate environments abundant in carbohydrates and peptides where selection occurs on a maximal growth rate or maximal substrate uptake rate. The consequence of striving for a high growth rate is that the organisms have to optimise their proteome in preference for high growth rate enabling proteins, making them auxotrophic, for example, amino acids and vitamins.
Using this ecological concept, feedstocks with readily fermentable carbohydrates and a sufficient protein content are a good target to directly produce lactic acid. Protein‐poor feedstocks on the other hand are a good target to produce VFAs and hydrogen (Figure 5). In this study, we have obtained an enrichment producing only 0.11 Cmol per Cmol of lactate at the end of the batch (Figure 1). If lactic acid production from low‐value feedstocks is the desired bioprocess, lactate consumption has to be managed effectively. Lactate consumption can be managed by creating a selective environment which does not select for lactate consuming organisms such as Megasphaera, by using a different pH or solid retention time for example.
Figure 5.
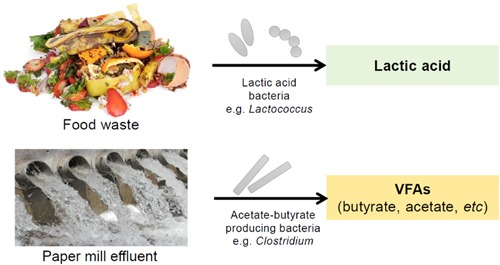
Ecology‐based design of fermentative bioprocesses utilising low‐value streams such as protein‐rich food waste and protein‐poor paper mill effluent to produce economically interesting products [Color figure can be viewed at http://wileyonlinelibrary.com]
Here we used enrichment culture to better understand the ecological niche of lactic acid‐producing bacteria which showed:
-
1.
Lactic acid bacteria outcompete prototrophic type fermentative bacteria on high biomass‐specific substrate uptake rate and growth rate.
-
2.
This behaviour can be explained in line with the resource allocation hypothesis for protein allocation: LAB can dedicate a higher share of their proteome to catabolism, ribosomes and RNA polymerases and therefore are able to attain a significantly higher substrate uptake rate and growth rate.
-
3.
The anabolic efficiency of the microbial community enriched on complex medium is higher but not significantly, and only accounts for a minor possible increase in µ max.
-
4.
Intermediately formed lactic acid is fermented to acetate, propionate, butyrate, valerate, H2 and CO2, resulting in a different fermentation product spectrum when lactate is an intermediate fermentation product.
-
5.
A relatively high protein content of a feedstock can enhance the competitiveness of lactic acid bacteria, leading to lower hydrogen yield and the possibility of producing lactic acid by enrichment cultures.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors wish to thank Cor Ras, Max Zomerdijk and Dirk Geerts at Delft University of Technology for their assistance in obtaining accurate stoichiometric data and designing and setting up the reactor equipment. Furthermore, we acknowledge the contribution of Udo van Dongen in performing the methane‐targeted GC measurement and improving the FISH analysis, Ben Abbas for assisting in the sequencing, the waste water treatment plant at Harnaschpolder, the Netherlands for providing the inoculum and Miguel Mauricio‐Iglesias for assistance in parameter estimation. A. Regueira would like to thank the support of the Spanish Ministry of Education (FPU14/05457) and of the CRETUS Strategic Partnership for a research stay grant. A. Regueira belongs to the Galician Competitive Research Group ED431C2017/029 and to the CRETUS Strategic Partnership (ED431E 2018/01), both programs are co‐funded by FEDER (EU). This study was supported by the Soenghen Institute for Anaerobic Microbiology (SIAM), SIAM gravitation grant, the Netherlands Organization for Scientific Research (024.002.002) and Spanish Ministry of Education (FPU14/05457).
APPENDIX A. USE OF REFRACTION INDEX AND UC DETECTORS IN THE HPLC ANALYSIS
In the HPLC analysis, the refraction index (RI) spectrum was always used to check for the presence of peaks in the UV spectrum, as all compounds cause a change in refractive index, while only carboxylic acids absorb in the UV spectrum. In the mineral medium, glucose was quantified from the RI spectrum, and organic acids from the UV spectrum. A significant peak of succinate in the UV was not present in the RI spectrum, thus is not quantified from UV.
In the complex medium, acetate and succinate were quantified from the RI spectrum. At the retention time of these two compounds, a peak was present in the UV spectrum when running a sample of the combined medium A and B, indicating the presence of UV absorbing compounds in the influent.
For the cycle analysis, samples of the complex medium, propionate and butyrate were quantified using RI detection, since absorbance on the UV channel was poor for these peaks. For the cycle analysis, measurements of samples of mineral medium, acetate and butyrate concentrations were obtained from the RI channel.
For the lactate pulse experiment in the complex medium enrichment, acetate and lactate were quantified from the RI spectrum.
APPENDIX B. BOOTSTRAP METHOD
To estimate robust parameters and to avoid being stuck at local minima, the bootstrap methodology was followed (Frutiger, Marcarie, Abildskov, & Sin, 2016). With it the expected parameter values and their confidence intervals were determined.
The model minimises the normalised root squared mean deviation (Equation B1) between the experimental data and the simulated data of the model.
| (B1) |
where m is the number of measurement times, n the number of measured compounds (n = 9 in the mineral medium enrichment and n = 10 in the complex medium enrichment), y is the simulated concentration value, is the experimental concentration value, represent the calibration parameters and σ is the experimental standard deviation of the concentration values of a compound. The subscript i refers to the different measurements over time and the subscript j refers to the different compounds.
After the first parameter estimation, the reference residuals (i.e. the difference between the experimental and simulated concentration) are calculated. These residuals are used to generate new synthetic experimental data, which is then used to estimate a new set of parameters. A population of parameters is generated by iterating until convergence and can be used to determine robust estimates and uncertainty quantifications.
A Monte Carlo procedure was used to propagate the uncertainty of the estimated parameters. Samples of the parameter population are chosen using Latin Hypercube Sampling to ensure a maximal coverage of the parameter space (Helton & Davis, 2003). The Monte Carlo procedure can be briefly summarised in three steps: (i) select a random sample of the estimated parameter population; (ii) run the model and store the solution; (iii) iterating 500 times steps (i) and (ii) until the distribution of model solutions converges.
A reference set of parameters is estimated using the Matlab command lsqnonlin (Equation B2).
| (B2) |
where is the set of parameters to estimate, y is the simulated concentration value, is the experimental concentration value and σ is the experimental standard deviation of the concentration values of a compound. The subscript i refers to the different measurements over time and the subscript j refers to the different compounds.
The vector of reference residuals are determined (Equation B3)
| (B3) |
where e is the reference residuals.
Synthetic data is generated by randomly adding the reference residuals to the experimental data (Equation B4).
| (B4) |
where is the new synthetic experimental data and ek is the element randomly sampled from e.
A new set of parameters is determined with the new synthetic data (Equation B5).
| (B5) |
where is the set of estimated parameters corresponding to iteration j.
Iterate through steps 3 and 4 (). The mean and standard variation of the population of estimated parameters are used as convergence indicators.
For a high number of iterations, the expected value of the parameter set can be approximated to the mean value of the distribution of parameters (Equation B6). The confidence intervals for an α = 0.05 significance are determined by the quantile function (Equation B7)
| (B6) |
where is the parameter set expected values.
| (B7) |
APPENDIX C. SELECTION OF RELEVANT CATABOLIC PATHWAYS
C.1 | Mineral medium
Ethanoligenens and Clostridium populated the mineral medium enrichment, with Lactobacillus confirmed to be absent (Figures 3 and S3B). Ethanoligenens species are known to produce acetate, ethanol and butyrate (Tang et al., 2012; Xing et al., 2006), though electron bifurcation is not investigated in this genus. Clostridium species belonging to cluster 12 are known to produce, acetate, ethanol, butyrate and are confirmed to use electron bifurcation (Buckel & Thauer, 2013). Lactate utilisation was not included, as lactate was not consumed during the cycle (Figure 2). Homoacetogenesis and methanogenesis were not considered relevant in this environment. Lactate production from glucose has been measured for Clostridium pasteurianum (Dabrock, Bahl, & Gottschalk, 1992). Propionate production was assumed to be performed through the acrylate pathway (Gonzalez‐Garcia et al., 2017), using pyruvate directly from glucose. This is a pathway that generates less ATP but attains higher growth rates (Seeliger, Janssen, & Schink, 2002) and propionate is formed directly from glucose, as no lactate consumption is observed. This catabolism is not matched with the microbial community.
C.2 | Complex medium
Lactic acid bacteria and Megasphaera were observed to be the dominant populations in the complex medium enrichment. Lactic acid bacteria are known to be homo or heterofermentative (reaction 1 and 2, Table S5; Madigan & Martinko, 2006). They are also known to produce acetate and ethanol coupled to formate and hydrogen (Madigan & Martinko, 2006). When including this reaction in the modelling it was not selected, thus it is not used here. Megasphaera species are known to utilise all three lactate utilising pathways (reaction 7, 8 and 9). Homoacetogenesis and methanogenesis were not considered in the modelling of the carbon fluxes in as both reactions were measured not to be active in this enrichment (Figure S6 and data not shown). Megasphaera are species are also known to posses and utilise the Rnf complex (Buckel & Thauer, 2018), thereby enabling them to obtain energy from the conversion of reduced ferredoxin to NADH and driving a sodium motive force.
Rombouts JL, Kranendonk EMM, Regueira A, Weissbrodt DG, Kleerebezem R, van Loosdrecht MCM. Selecting for lactic acid producing and utilising bacteria in anaerobic enrichment cultures. Biotechnology and Bioengineering. 2020;117:1281–1293. 10.1002/bit.27301
Contributor Information
Julius Laurens Rombouts, Email: julesrombouts@gmail.com.
Mark Cornelis Maria van Loosdrecht, Email: m.c.m.vanloosdrecht@tudelft.nl.
REFERENCES
- APHA . (1998). Standard methods for the examination of water and wastewater (20th ed.). Washington DC: American Public Health Association. [Google Scholar]
- Axelsson, L. , & Ahrné, S. (2000). Lactic acid bacteria, Applied microbial systematics (pp. 367–388). Berlin: Springer. [Google Scholar]
- Bachmann, H. , Molenaar, D. , Branco dos Santos, F. , & Teusink, B. (2017). Experimental evolution and the adjustment of metabolic strategies in lactic acid bacteria. FEMS Microbiology Reviews, 41, S201–S219. [DOI] [PubMed] [Google Scholar]
- Bosdriesz, E. , Molenaar, D. , Teusink, B. , & Bruggeman, F. J. (2015). How fast‐growing bacteria robustly tune their ribosome concentration to approximate growth‐rate maximization. FEBS Journal, 282, 2029–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckel, W. , & Thauer, R. K. (2013). Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation. Biochimica et Biophysica Acta—Bioenergetics, 1827, 94–113. [DOI] [PubMed] [Google Scholar]
- Buckel, W. , & Thauer, R. K. (2018). Flavin‐based electron bifurcation, ferredoxin, flavodoxin, and anaerobic respiration with protons (Ech) or NAD+ (Rnf) as electron acceptors: A historical review. Frontiers in Microbiology, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce, J. T. , Ndam, E. , Ofiteru, I. D. , Moore, A. , & Graham, D. W. (2018). A review of phosphorus removal technologies and their applicability to small‐scale domestic wastewater treatment systems. Frontiers in Environmental Science, 6, 1–15. [Google Scholar]
- Caporaso, J. G. , Kuczynski, J. , Stombaugh, J. , Bittinger, K. , Bushman, F. D. , Costello, E. K. , … Knight, R. (2010). QIIME allows analysis of high‐throughput community sequencing data. Nature Methods, 7, 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso, J. G. , Lauber, C. L. , Walters, W. A. , Berg‐Lyons, D. , Lozupone, C. A. , Turnbaugh, P. J. , … Knight, R. (2011). Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the United States of America, 108(Suppl.), 4516–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocaign‐Bousquet, M. , Garrigues, C. , Novak, L. , Lindley, N. D. , & Loublere, P. (1995). Rational development of a simple synthetic medium for the sustained growth of Lactococcus lactis . Journal of Applied Microbiology, 79, 108–116. [Google Scholar]
- Dabrock, B. , Bahl, H. , & Gottschalk, G. (1992). Parameters affecting solvent production by Clostridium pasteurianum . Applied and Environmental Microbiology, 58, 1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, S. H. , Louis, P. , & Flint, H. J. (2004). Lactate‐utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Applied and Environmental Microbiology, 70, 5810–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutiger, J. , Marcarie, C. , Abildskov, J. , & Sin, G. (2016). A comprehensive methodology for development, parameter estimation, and uncertainty analysis of group contribution based property models—An application to the heat of combustion. Journal of Chemical and Engineering Data, 61, 602–613. [Google Scholar]
- Gonzalez‐Garcia, R. , McCubbin, T. , Navone, L. , Stowers, C. , Nielsen, L. , & Marcellin, E. (2017). Microbial propionic acid production. Fermentation, 3, 21. [Google Scholar]
- Gonzalez‐Gil, L. , Mauricio‐iglesias, M. , Carballa, M. , & Lema, J. M. (2018). Why are organic micropollutants not fully biotransformed? A mechanistic modelling approach to anaerobic systems. Water Research, 142, 115–128. [DOI] [PubMed] [Google Scholar]
- de Groot, DH , van Boxtel, C , Planqué, R , Bruggeman, FJ , & Teusink, B. (2018). The number of active metabolic pathways is bounded by the number of cellular constraints at maximal metabolic rates. bioRxiv:167171. [DOI] [PMC free article] [PubMed]
- Hasona, A. , Kim, Y. , Healy, F. G. , Ingram, L. O. , & Shanmugam, K. T. (2004). Pyruvate formate lyase and acetate kinase are essential for anaerobic growth of Escherichia coli on xylose. Journal of Bacteriology, 186, 7593–7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton, J. C. , & Davis, F. J. (2003). Latin hypercube sampling and the propagation of uncertainty in analyses of complex systems. Reliability Engineering and System Safety, 81, 23–69. [Google Scholar]
- Jia, H.‐R. , Geng, L.‐L. , Li, Y.‐H. , Wang, Q. , Diao, Q.‐Y. , Zhou, T. , & Dai, P.‐L. (2016). The effects of Bt Cry1Ie toxin on bacterial diversity in the midgut of Apis mellifera ligustica (Hymenoptera: Apidae). Scientific Reports, 6, 24664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, K. , Jiang, Y. , Kleerebezem, R. , Muyzer, G. , & Loosdrecht, M. C. M. Van (2009). Enrichment of a mixed bacterial culture with a high polyhydroxyalkanoate storage capacity. Biomacromolecules, 10, 670–676. [DOI] [PubMed] [Google Scholar]
- Kaparaju, P. , Serrano, M. , Thomsen, A. B. , Kongjan, P. , & Angelidaki, I. (2009). Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresource Technology, 100, 2562–2568. [DOI] [PubMed] [Google Scholar]
- Kim, D.‐H. , Lee, M.‐K. , Hwang, Y. , Im, W.‐T. , Yun, Y.‐M. , Park, C. , & Kim, M.‐S. (2016). Microbial granulation for lactic acid production. Biotechnology and Bioengineering, 113, 101–111. [DOI] [PubMed] [Google Scholar]
- Kim, Y. , Ingram, L. O. , & Shanmugam, K. T. (2007). Construction of an Escherichia coli K‐12 mutant for homoethanologenic fermentation of glucose or xylose without foreign genes. Applied and Environmental Microbiology, 73, 1766–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitay, E. , & Snell, E. E. (1950). Some additional nutritional requirements of certain lactic acid bacteria. Journal of Bacteriology, 60, 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleerebezem, R. , & van Loosdrecht, M. C. (2007). Mixed culture biotechnology for bioenergy production. Current Opinion in Biotechnology, 18, 207–212. [DOI] [PubMed] [Google Scholar]
- Lawford, H. G. , & Rousseau, J. D. (1995). Comparative energetics of glucose and xylose metabolism in ethanologenic recombinant Escherichia coil B. Applied Biochemistry and Biotechnology, 51, 179–195. [DOI] [PubMed] [Google Scholar]
- LeBlanc, J. G. , Laiño, J. E. , del Valle, M. J. , Vannini, V. , van Sinderen, D. , Taranto, M. P. , … Sesma, F. (2011). B‐Group vitamin production by lactic acid bacteria—Current knowledge and potential applications. Journal of Applied Microbiology, 111, 1297–1309. [DOI] [PubMed] [Google Scholar]
- Leroy, F. , & De Vuyst, L. (2004). Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends in Food Science and Technology, 15, 67–78. [Google Scholar]
- Li, Z. , Nimtz, M. , & Rinas, U. (2014). The metabolic potential of Escherichia coli BL21 in defined and rich medium. Microbial Cell Factories, 13, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C. , Chang, C. , & Hung, C. (2008). Fermentative hydrogen production from starch using natural mixed cultures. International Journal of Hydrogen Energy, 33, 2445–2453. [Google Scholar]
- Madigan, M. T. , & Martinko, J. M. (2006). Brock biology of microorganisms (11th ed., p. 992). Pearson Prentice Hall. [Google Scholar]
- Magnúsdóttir, S. , Ravcheev, D. , de Crécy‐Lagard, V. , & Thiele, I. (2015). Systematic genome assessment of B‐vitamin biosynthesis suggests co‐operation among gut microbes. Frontiers in Genetics, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marounek, M. , Fliegrova, K. , & Bartos, S. (1989). Metabolism and some characteristics of ruminal strains of Megasphaera elsdenii. Applied and Environmental Microbiology, 55, 1570–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar, D. , van Berlo, R. , de Ridder, D. , & Teusink, B. (2009). Shifts in growth strategies reflect tradeoffs in cellular economics. Molecular Systems Biology, 5, 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscoviz, R. , Trably, E. , Bernet, N. , & Carrère, H. (2018). The environmental biorefinery: State‐of‐the‐art on the production of hydrogen and value‐added biomolecules in mixed‐culture fermentation. Green Chemistry, 20, 3159–3179. [Google Scholar]
- Muyzer, G. , de Waal, E. C. , & Uitterlinden, A. G. (1993). Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction‐amplified genes coding for 16S rRNA. Applied and Environmental Microbiology, 59, 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak, L. , Cocaign‐Bousquet, M. , Lindley, N. D. , & Loubiere, P. (1997). Metabolism and energetics of Lactococcus lactis during growth in complex or synthetic media. Applied and Environmental Microbiology, 63, 2665–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos‐Dichara, A. , Ampe, F. , Uribelarrea, J.‐L. , Pareilleux, A. , & Goma, G. (1997). Growth and lactic acid production by Lactobacillus casei ssp. rhamnosus in batch and membrane bioreactor: Influence of yeast extract and Tryptone enrichment. Biotechnology Letters, 19, 709–714. [Google Scholar]
- Paritosh, K. , Kushwaha, S. K. , Yadav, M. , Pareek, N. , Chawade, A. , & Vivekanand, V. (2017). Food waste to energy: An overview of sustainable approaches for food waste management and nutrient recycling. BioMed Research International, 2017. 2370927‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirt, S. J. (1965). The maintenance energy of bacteria in growing cultures. Proceedings of the Royal Society of London. Series B. 163, 224–231. LP – 231. [DOI] [PubMed] [Google Scholar]
- Plengvidhya, V. , Breidt, F., Jr. , Lu, Z. , & Fleming, H. P. (2007). DNA fingerprinting of lactic acid bacteria in sauerkraut fermentations. Applied and Environmental Microbiology, 73, 7697–7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu, R. , Altman, E. , & Eiteman, M. A. (2012). Lactate and acrylate metabolism by Megasphaera elsdenii under batch and steady‐state conditions. Applied and Environmental Microbiology, 78, 8564–8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels, J. A. (1983). Energetics and kinetics in biotechnology. Amsterdam: Elsevier B.V. [Google Scholar]
- Roger, P. , Delettre, J. , Bouix, M. , & Béal, C. (2011). Characterization of Streptococcus salivarius growth and maintenance in artificial saliva. Journal of Applied Microbiology, 111, 631–641. [DOI] [PubMed] [Google Scholar]
- Rombouts, J. L. , Mos, G. , Weissbrodt, D. G. , Kleerebezem, R. , & van Loosdrecht, M. C. M. (2019). Diversity and metabolism of xylose and glucose fermenting microbial communities in sequencing batch or continuous culturing. FEMS Microbiology Ecology, 95. [DOI] [PubMed] [Google Scholar]
- Schleifer, K. H. , Amann, R. I. , & Ludwig, W. (1995). Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Phylogenetic Identification and In Situ Detection of Individual Microbial Cells without Cultivation, 59, 143–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeliger, S. , Janssen, P. H. , & Schink, B. (2002). Energetics and kinetics of lactate fermentation to acetate and propionate via methylmalonyl‐CoA or acrylyl‐CoA. FEMS Microbiology Letters, 211, 65–70. [DOI] [PubMed] [Google Scholar]
- Shimada, T. , Zilles, J. , Raskin, L. , & Morgenroth, E. (2007). Carbohydrate storage in anaerobic sequencing batch reactors. Water Research, 41, 4721–4729. [DOI] [PubMed] [Google Scholar]
- Solís, G. , de los Reyes‐Gavilan, C. G. , Fernández, N. , Margolles, A. , & Gueimonde, M. (2010). Establishment and development of lactic acid bacteria and bifidobacteria microbiota in breast‐milk and the infant gut. Anaerobe, 16, 307–310. [DOI] [PubMed] [Google Scholar]
- Spirito, C. M. , Richter, H. , Stams, A. J. , & Angenent, L. T. (2014). Chain elongation in anaerobic reactor microbiomes to recover resources from waste. Current Opinion in Biotechnology, 27, 115–122. [DOI] [PubMed] [Google Scholar]
- Stouthamer, A. H. (1973). A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antonie Van Leeuwenhoek, 39, 545–565. [DOI] [PubMed] [Google Scholar]
- Straathof, A. J. J. (2014). Transformation of Biomass into Commodity Chemicals Using Enzymes or Cells. Chemical Reviews, 114, 1871–1908. [DOI] [PubMed] [Google Scholar]
- Tamis, J. , Joosse, B. M. , Loosdrecht, M. C. M. , & Kleerebezem, R. (2015). High‐rate volatile fatty acid (VFA) production by a granular sludge process at low pH. Biotechnology and Bioengineering, 112, 2248–2255. [DOI] [PubMed] [Google Scholar]
- Tang, J. , Yuan, Y. , Guo, W. Q. , & Ren, N. Q. (2012). Inhibitory effects of acetate and ethanol on biohydrogen production of Ethanoligenens harbinese B49. International Journal of Hydrogen Energy, 37, 741–747. [Google Scholar]
- Temudo, M. F. (2008). Directing product formation by mixed culture fermentation, Delft, the Netherlands. [Google Scholar]
- Temudo, M. F. , Kleerebezem, R. , & van Loosdrecht, M. (2007). Influence of the pH on (open) mixed culture fermentation of glucose: A chemostat study. Biotechnology and Bioengineering, 98, 69–79. [DOI] [PubMed] [Google Scholar]
- Teusink, B. , Bachmann, H. , & Molenaar, D. (2011). Systems biology of lactic acid bacteria: A critical review. Microbial Cell Factories, 10, S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, T. D. , Ellwood, D. C. , & Longyear, V. M. C. (1979). Change from homo‐ to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. Journal of Bacteriology, 138, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries, W. , Kapteijn, W. M. C. , Van Der Beek, E. G. , & Stouthamer, A. H. (1970). Molar growth yields and fermentation balances of Lactobacillus casei L3 in Batch cultures and in continuous cultures. Microbiology, 63, 333–345. [DOI] [PubMed] [Google Scholar]
- Waller, J. R. , & Lichstein, H. C. (1965). Biotin transport and accumulation by cells of Lactobacillus plantarum. II. Kinetics of the system. Journal of Bacteriology, 90, 853–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Garrity, G. M. , Tiedje, J. M. , & Cole, J. R. (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology, 73, 5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, D. , Ren, N. , Li, Q. , Lin, M. , Wang, A. , & Zhao, L. (2006). Ethanoligenens harbinense gen. nov., sp. nov., isolated from molasses wastewater. International Journal of Systematic and Evolutionary Microbiology, 56, 755–760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


