Abstract
Childhood asthma is a huge global health burden. The spectrum of disease, diagnosis, and management vary depending on where children live in the world and how their community can care for them. Global improvement in diagnosis and management has been unsatisfactory, despite ever more evidence‐based guidelines. Guidelines alone are insufficient and need supplementing by government support, changes in policy, access to diagnosis and effective therapy for all children, with research to improve implementation. We propose a worldwide charter for all children with asthma, a roadmap to better education and training which can be adapted for local use. It includes access to effective basic asthma medications. It is not about new expensive medications and biologics as much can be achieved without these. If implemented carefully, the overall cost of care is likely to fall and the global future health and life chance of children with asthma will greatly improve. The key to success will be community involvement together with the local and national development of asthma champions. We call on governments, institutions, and healthcare services to support its implementation.
Keywords: asthma, charter, childhood asthma, children, global pediatric asthma
1. INTRODUCTION
1.1. Why we need a charter
Asthma is the commonest chronic childhood disease and encompasses a spectrum of airway diseases with similar symptoms. 1 , 2 Inaccurate diagnosis remains common, especially in younger children, with failure to characterize the different “asthmas.” 3 , 4 Children worldwide repeatedly suffer symptoms which severely affect their everyday lives. 5 Children die from asthma, especially in low and middle‐income countries (LMICs). 6 , 7 In many countries, asthma prevalence is rising. Access to effective care and changing environments are hugely variable and may explain the higher morbidity in inner‐city children, in LMICs, and in deprived populations in high‐income countries. 8 , 9 Despite the disease being eminently controllable, morbidity and mortality persist. 10 , 11
We previously described the barriers to better management of paediatric asthma, 12 and proposed a global Bill of Rights for children with asthma as guiding principles. 13 Global health organizations, governments, policymakers, community leaders, local authority workers, schools, and teachers all need to play their part with the children, their families, communities, and their healthcare workers. Guidelines set standards, but with suboptimal implementation they are insufficient. Community‐led projects using inexpensive medications, supported by effective policies, have greatly improved outcomes in countries such as Finland, Nigeria, Costa Rica, and Brazil. 14 , 15 , 16 , 17 Important principles include (a) asthma in most children can be diagnosed by a few specific clinical questions asked by healthcare workers; (b) most children with asthma respond to inhaled corticosteroids (ICS) and a short‐acting beta‐agonist (SABA) using a spacer; (c) repeated, simple education can deliver better, safer care that addresses individual and community beliefs, and; (d) asthma action plans effectively reduce risk.
We aim to highlight that:
-
1.
Children worldwide are suffering and dying from a treatable disease through misdiagnosis and failure to implement effective treatments.
-
2.
Universal health coverage must be extended to include diagnosis and treatment of childhood asthma
-
3.
The Children's Asthma Charter is a way to improve care globally.
-
4.
Solutions exist for the current barriers to effective asthma care.
-
5.
A globally adaptable educational and training program tailored to local needs is available.
2. PREVALENCE OF ASTHMA IN CHILDREN
2.1. The global view
In 2007 the estimated global prevalence of asthma was 11% in children aged 6 to 7 years and 14% in those aged 13 to 14 years 18 but is increasing, particularly in LMICs. Asthma in children is a low‐ranked global noncommunicable disease (NCD), although it causes more loss of disability‐adjusted life‐years, than many other conditions. 19 Also, NCD risk factors including lack of immunizations, poor diet, physical inactivity, obesity, smoking, cigarettes, and pollution exposure apply equally to asthma as to diabetes and cancer. Yet asthma can be well managed at low cost if there is universal access to primary care services and the medicines recommended by the 2017 World Health Organization (WHO) Essential Medicines List including ICS, inhaled SABAs, and oral corticosteroids.
Most national formularies include essential asthma medicines but access remains inequitable and spacers are rarely provided. 20 , 21 Reducing the burden of asthma in children requires seven achievable goals (Table 1).
Table 1.
The seven achievable goals to reduce the asthma burden
|
2.2. Issues specific to LMICs
Asthma is the commonest NCD in children in LMICs and is often more severe than in high‐income countries which may be due to factors including wrong or missed diagnosis, inadequate access to care and health systems in which asthma is low priority. Diagnosis is especially challenging due to the high incidence of respiratory tract infections and poor access to care, 22 with under‐diagnosis of even severe asthma common. 23 In addition, treatment guidelines, appropriate medicines, and spacers are frequently unaffordable or inaccessible. 20
Risk factors including tobacco and indoor biomass smoke, outdoor air pollution, and viral infection are particularly common in LMICs 24 , 25 , 26 , 27 , 28 and in deprived communities in high‐income countries. Environmental disasters, such as wildfires, hailstorms or floods, also worsen asthma control. Smoking compounds the pernicious effects of environmental tobacco smoke exposure, family psychosocial stresses and cultural factors which affect disease severity, the caregiver and the child's perception of their asthma and its treatment.
3. CLINICAL AND RESEARCH BARRIERS TO ASTHMA IN CHILDREN
3.1. Unmet needs: diagnosis, risk assessment, and mitigation
Fixed or variable airflow obstruction, inflammation, and infection may all result from multiple endotypes, such that “asthma is solely a clinical description of wheeze, chest tightness, breathlessness, +/− cough which makes no assumptions about its underlying pathology.” 2 We need to know what sort of asthma the child has, such as eosinophilic inflammation, to define a treatment strategy. Given different environments, infective agents, and genetic/epigenetic factors, asthma in Brazil, New York, and Africa is likely to have different causations, different attack risks, 29 and may need different management strategies. The start of successful management is making the correct diagnosis or perhaps better determining which treatable traits are present.
Possible biomarkers need further research to assess utility and affordability in LMICs. Elevated levels of exhaled nitric oxide (FeNO) or raised blood eosinophil counts likely reflect TH2 inflammation worldwide, but, where eosinophil levels are commonly elevated by parasitic infections or other causes, their value may be less clear.
Healthcare workers caring for children suspected of having asthma should undertake a full medical history including smoking history and clinical examination. They should also consider organizing spirometry with bronchodilator reversibility in children older than 5 years, FeNO, a blood eosinophil count, allergen skin prick tests, immunoglobulin E, and cough swab/sputum culture if there is poor response to ICS. These tests are frequently omitted leading to over‐diagnosis. 30 , 31 In LMICs, under‐diagnosis is more likely. 32
ICS should be prescribed to children with eosinophilic airway inflammation. In the INFANT study of children aged 12 to 59 months, 33 a blood eosinophil count more than 300/μL and aeroallergen sensitization predicted response to regular ICS (Figure 1). Because the analysis was post hoc, the results need confirmation, including in populations with eosinophil levels elevated for other reasons. This study is proof of the concept that ICS are more likely to be effective with eosinophilic airway inflammation. Without eosinophilic inflammation, alternative treatment strategies need consideration. Clearly better biomarkers for both high and low type 2 inflammation are needed. 34
Figure 1.
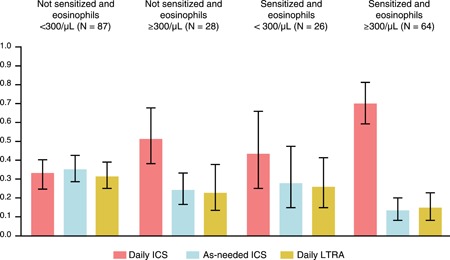
The probability of best response based on combinations of sensitization and peripheral blood eosinophil count. P values correspond to the test of interaction between the predictor and treatment and indicate whether the pattern of treatment response differs according to subgroup. Sample sizes correspond to participants with evaluable data (N = 230). Reproduced with permission from Fitzpatrick et al 33 [Color figure can be viewed at http://wileyonlinelibrary.com]
Objective biomarkers can also monitor risk. Day‐to‐day asthma control may be good, but the child remains at risk from an asthma attack and/or side effects of medical treatment, and impaired lung growth or fixed airway obstruction later in life. An asthma attack is the strongest predictor of risk for a future attack, but, all too often, is treated as an isolated event. 35 , 36 Risk is also increased in uncontrolled type 2 inflammation. 37 , 38 Measuring FeNO levels and titrating ICS dose may reduce future attacks, but successful asthma care really centers on careful history taking, good rapport with the family, ensuring treatment adherence, education, asthma management plans, and regular follow‐up.
Longitudinal lung function measurements monitor lung growth. Acute asthma attacks may be associated with reduced airway growth 39 and a persisting low lung function trajectory (Figure 2) which is attenuated by the use of regular, low dose ICS.
Figure 2.
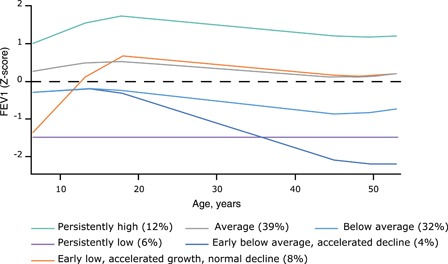
Trajectories of lung function (forced expiratory volume in 1 second z‐score) from 7 to 53 years of age. The six trajectories represent the latest growth patterns of lung function. The group prevalences do not add up to 100% because of rounding. Reproduced with permission from Bui et al 40 [Color figure can be viewed at http://wileyonlinelibrary.com]
Poor lung growth risks adulthood fixed airway obstruction. 40 , 41 Unfortunately, currently we have no specific strategies to correct an adverse trajectory. Preventing LRTIs and exposure to indoor and outdoor pollution (antenatally and postnatally) may improve lung growth. 42 , 43 In California, improvements in air quality resulted in better lung growth. 43 Trajectories may also improve in those with later onset puberty. 44 The mechanism is unknown, but when discovered might reveal ways to normalize a low lung function trajectory. 45
4. A CHARTER FOR ALL CHILDREN WITH ASTHMA
Patient advocacy and community organizations are critical in improving asthma care and holding health services to account. 46 We call on all governments and communities to support the Rights of Children with asthma by actions using a charter modified for global use in children (Table 2), which has been developed from principles published for the care of adults with severe asthma. 46 We recommend the development and recruitment of national champions together with additional professional support to improve global asthma care (Table 3).
Table 2.
The rights of children with asthma: a charter modified for global use in children, based on principles for the care of adults with severe asthma 18
|
Table 3.
An asthma champion plan to universally improve care in children
|
5. SETTING STANDARDS AND IMPROVING GUIDELINES FOR PRIMARY CARE
5.1. Where we need to go
Most paediatric asthma management takes place within primary care/the community where the understanding of the wider context and risk factors for local families is implicit. We need a practical, implementable package that has been codeveloped and tested in a primary care context and found to be feasible, effective, and affordable. The WHO guidelines on the management of pneumonia are an example of an effective primary care guideline, which has substantially reduced childhood mortality. Guidelines for a chronic disease like asthma are more complex, and it is possible that more equipment will be needed, such as exhaled FeNO measures, to improve the care of asthma. 47
Simple transfer of information fails to achieve good asthma management in adults, 48 and the same is probably true in children. Asthma care requires accurate and practical educational tools which can be used by the child, family, caregivers, healthcare workers, school teachers, and community health workers. The information needs to be adapted to local needs, culturally sensitive, freely available in many languages, and in narrative formats with simple illustrations to overcome poor literacy.
For asthma management, community engagement is essential. 12 , 49 Guidelines need to focus on implementation, prioritization of health policy and training of healthcare staff to ensure patients understand their asthma and use what is prescribed. If local guidelines are to work, their development should include users in the wider community.
Changing perceptions of “what asthma really is,” especially in young children, have made the diagnosis, management, and labeling of wheezing disorders controversial, resulting in inconsistent guidelines. 50 , 51 Management plans need to be clear, consistent, simple, and well‐communicated to be useful. The healthcare workers and families need to approach asthma in children as a life‐course respiratory disease. Healthcare providers should refer to secondary care if treatment is not working.
It is easy to agree to a set of standards from the evidence, as typified by the National Institute for Health and Care Excellence Asthma Quality Standard. 52 What is more important to improve outcomes, however, is the process by which they are agreed, to ensure ownership from all stakeholders, and the incentive system to encourage implementation. The London Children and Young People Asthma Standards is a good example of the codevelopment process that involved local government, health organizations, professional societies, and civil society responsible for the health of children in London, a city of 8.9 million people. 53 These standards have then been adapted and integrated into a comprehensive plan at a more local level for a population of about 92 000 young people. 54 National health guidelines for asthma management improve outcomes across different socioeconomic settings, for example in Finland, Brazil, and Poland. 55
5.2. Changes needed in global clinical asthma care
All countries must have a trained, available workforce to deliver appropriate care for each child with asthma. We need quality standards applicable worldwide, adapted for individual countries to account for local factors. 13
Asthma education in primary care saves lives and reduces health costs. 56 Improving diagnostic accuracy must first be addressed, using a clear educational strategy. Guidelines need simplification for primary care with recommendations on how to monitor asthma control and future risk. Asthma outcome data must be used to assess care, focusing on improving asthma control, and reducing future attacks and asthma deaths, 57 , 58 , 59 , 60 as was done to assess progress against the Millennium Development Goals. 61 Most children can achieve asthma control simply by the use of inhaled low dose ICS and SABAs via a metered‐dose inhaler (MDI) and spacer. 62
All children should have access to care that meets their needs, including clean air and a smoke‐free environment. Clean air encompasses not merely industrial and other environmental pollutants, but also freedom from exposure to second‐hand tobacco smoke and the effluvium from e‐cigarettes, especially if these have been “cut” with substances of abuse or other illicit contaminants.
There must be access to local guidance‐recommended, affordable treatment, developed and supported by local children's asthma champions. The International Primary Care Respiratory Group has developed a “Teach the Teacher” program, to build capacity for evidence‐based practice and teaching/learning strategies appropriate to primary care. The model has been tested in eight countries for use in adults with asthma. 63 The group has now developed a three‐tier program with a clinical focus on children's asthma. Key primary care health professionals have attended inter‐disciplinary international workshops to gain the skills and knowledge required to adapt the curriculum and materials, and develop and lead in‐country appropriate programs. These are cascaded to healthcare educators in the community and in and across primary care. These educators deliver relevant modules within their communities, assessing progress by collecting and sharing data (http://www.theipcrg.org).
5.3. Changes needed in policy
Policies must ensure all children have access to affordable basic asthma care within their local communities. 64 , 65 All asthma outcomes must be monitored centrally.
Communication between health care teams can be greatly improved. Primary care visits may, of necessity, be short. Information from asthma specialists may not automatically be shared with the primary care team. A school nurse (if such a person exists, and if the child actually attends school) may recognize clinical problems but be unable to contact other health care workers. Asthma management plans should be copied to schools to coordinate care. Poor communication can result in different treatment plans from different teams, confusion, unnecessary costs, and bad outcomes. 58 , 66 New technologies can improve asthma self‐management with families having direct access, through their electronic health record, to their community healthcare professionals. They could help enable prompt primary care review after hospital discharge. We need research into better asthma communication between patients, primary care physicians, asthma specialists, teachers, and community health care professionals.
5.4. Schools
Self‐management educational interventions improve lung function and decrease some measures of morbidity and healthcare use in children and adolescents with asthma. 67 Schools are important settings to offer asthma teaching and training to children. School nurses can recognize the need for referral to a primary care doctor or asthma specialist, uncover the social or psychological issues which are affecting asthma control and provide an important support system by contributing to the medical education of the child and the family. They can also educate the whole school community.
Teachers need to know how to respond to an asthma attack and help counteract stigma. They need up to date asthma information and knowledge of available resources. Many countries do not have school nurses, but professionals other than nurses can be identified and trained to undertake a similar role. Directly‐observed therapy using community workers functions well in tuberculosis management and could be extended to asthma. Many community pharmacists want involvement in this process. We need to develop asthma champions from varied backgrounds depending on the locality.
School‐centered programs can identify those with uncontrolled asthma, help them understand and monitor their asthma better, relate with healthcare providers, 62 , 63 and reduce attacks and school absences. 68 , 69 , 70 , 71 Trained community health workers can assist school nurses. 72 However, in many countries, schooling is far from universal, and this is where the local community “asthma champion” has a role, identifying children who are not at school and advocating for them.
An asthma management program, SAMPRO, 73 has developed a tool kit to support schoolchildren with asthma in the USA. It provides a school asthma management plan, addresses home environmental trigger factors and monitors asthma school absences. Other countries have similar programs.
5.5. Poverty
Poverty, neighborhood violence, maternal anxiety, depression in pregnancy, and childhood stress all significantly affect asthma outcomes. 74 , 75 , 76 , 77 Community programs must address such issues. Once started, such programs often find innovative ways to develop and progress. 78 , 79 It is possible to improve asthma control using different methods. An individual tailored, multifaceted intervention by social workers reduced asthma symptoms amongst inner‐city children, 80 and a detailed review of seven successful interventions have also been reported. 81 Other detailed summaries addressing asthma disparities have demonstrated multiple successful interventions. 64 , 82
Ambitious implementation studies are needed to test whole systems approaches to “at‐risk” families. Important lessons are available from the Seattle Healthy Homes Project where greater improvements were noted with a high intensity as compared to a low‐intensity program. 83
5.6. Environment
A recent systematic review identified 35 well‐designed studies from North America, Europe, and China assessing the impact of smoke‐free legislation on child health with clear benefits on hospital admissions and improved asthma control. 84 A Cochrane review of interventions to reduce childhood ETS exposure (including 11 from LMICs) found that a few interventions reduced children's exposure to ETS and improved children's health. Features of effective interventions were not clearly definable. 85 Provision of better housing improved asthma symptoms and severity of disease in children in Massachusetts. 86
5.7. Improved health care communication
Applying real‐world primary care data can help predict asthma attacks and can help to improve asthma control in children. 87 The British Lung Foundation asthma management program involving 2800 children with asthma demonstrated significant improvement in asthma control after educational intervention. 88
There are already examples of how data can create maps of areas of deprivation in growth failure and education, and also how to reduce the burden of respiratory infection, all highly relevant to asthma. 89 , 90 , 91 , 92 Although important themes such as increasing immunization cover and reducing indoor pollution emerged, it was clear that preventive measures needed to be individualized; one size certainly did not fit all.
There is strong evidence that better communication following integrated, multifaceted practice‐based approaches for patient management, improves outcomes and reduces the need for referral to secondary care. Coordinated practice systems combining several interventions (such as decision support tools, flagging of electronic records, use of care pathways, staff training and structured approaches to patient education) afford the greatest benefits. 1 , 2 , 93 , 94
Matrix‐support collaborative care (which includes training and support for primary care physicians/nurses from specialists including joint consultations, case discussions, and tailored education), is well‐accepted by primary care professionals and reduces respiratory secondary care referrals. 94 One such approach was in a deprived area of inner‐city London, called “Connecting Care for Children” (https://www.cc4c.imperial.nhs.uk/). This model of outreach by hospital paediatric specialists to primary care/hubs to build competence and confidence through case discussion and also offer individual consultations has been shown to yield a very positive impact on walk‐in emergency attendances. 95 Such an approach may reduce subsequent all‐cause emergency room attendances by half. 96
A Cochrane review in both HICs and LMICs showed the use of community health care workers was a cost‐effective intervention for childhood asthma. 97 This may be a good strategy to strengthen asthma care in LMICs with limited medical or nursing personnel.
As children from deprived areas may make greater use of unplanned emergency care, it is important to provide proactive and protocol‐driven emergency department care. A Cochrane review showed fewer admissions and hospital attendances following the education of the carer and/or child in the emergency department. 98 Given the association between deprivation, asthma triggers, and hospital attendance, guidance on how to avoid asthma triggers at home is recommended as part of the intervention (eg, smoking cessation advice).
Continuity of care is likely to be important to reduce the frequency of hospital admissions. 99 Primary care offers this opportunity and can be underpinned by an electronic health record, or patient‐held record to facilitate continuity of decision‐making. Lower continuity of primary care has been associated with more hospital admissions in children, 100 and in children and adults enrolled in a Medicaid program. 101
Even in areas of conflict, it is possible to improve systems of care. In Syria, war shelters are now integrated into the system of care to ensure essential anti‐inflammatory inhaled medicines are available. 102 Also, in a systematic review, quality improvement interventions have been shown to improve outcomes in children with asthma. 103
5.8. Research priorities
Research is needed to persuade governments that there are better ways to diagnose and manage asthma in children. We need targeted research into better diagnosis, particularly in younger children, with a review of new and existing biomarkers to identify different endotypes. Exacerbation risk research is needed with the recognition that factors driving it will be different between and within high‐income countries and LMICs. We should start by a meeting of policymakers to determine data requirements that are needed to facilitate policy changes which improve important outcomes.
How do we move from a global philosophy where management of acute attacks is the priority to one where longitudinal care or preferably prevention of symptom development is the rule? We now have measures of asthma burden such as the Composite Asthma Severity Index and Asthma Severity Scoring System 104 , 105 , 106 and tools to predict who is at risk for seasonal exacerbations. 36 , 37 These tools need studying and probably modifying in LMICs. Ultimately, we need to progress toward a primary prevention strategy for childhood asthma, which should be part of the research plan.
Asthma data are inadequately collected at a national level with no global international vision. In contrast, the success of the cystic fibrosis registries and their quality improvement programs is enviable. 107 , 108 Setting up local, national, and global severe children's asthma registries will help, progressing then to other asthma categories. These registries should contain longitudinal information on lung trajectories, the frequency of asthma attacks, medication adherence patterns, patient behaviors, and environmental details.
Further research into the feasibility of an international standard for asthma care is needed with transparent benchmarks for diagnosis, management outcomes and medications prescribed, organized and funded at a national level and data shared with international registries, similar to that developed for adults with severe asthma. 109 It will improve asthma care 11 and inform policymakers. Financial support is required for the implementation of minimal standards together with real world‐research into its outcomes.
Technology advances will enable electronic medication monitoring, as well as the organization of medical records to identify those children with frequent hospital admissions. Such technology could be used to identify children receiving suboptimal care. While these potential advances may not be available in LMICs, they are feasible in high‐income countries containing deprived communities. The asthma community needs to encourage research into these techniques. If benefits are shown, such advances will enter into asthma guidelines with subsequent adaptation in both high income and LMICs. Table 4 summarises the key elements of research policy and public expectation needed to really make a difference.
Table 4.
The future clinical, research, and policy direction for asthma in children
|
6. CONCLUSIONS
Our recommendations enable us to examine the understanding of global asthma in children in a comprehensive way. Table 2 sets out measurable next steps to improve asthma diagnosis and management in a way which is adaptable to each individual country.
The current approach requires supplementation with new, collaborative, community‐based approaches that work toward more acceptable standards of asthma care for all. By directing attention to the risk domain, future asthma attacks can be reduced together with the consequent risk of disease progression and the associated irrecoverable loss of pulmonary function.
Asthma clinicians in all countries need a better understanding of their local “asthmas” to facilitate local network strategies to increase access to basic asthma care. This will influence governments to recognize the benefits. Academic centers should develop higher national standards of care alongside the Global Asthma Network and the WHO. All should work with national governments to convince them care and outcomes can improve if training and education in childhood asthma are optimized. If the local, national, and international building blocks in Table 5 (A, B, and C) are put in place, it is likely that the remarkable outcomes of reduction in asthma morbidity and mortality seen in Finland can also be realized globally.
Table 5.
Building blocks to reduce worldwide morbidity and mortality in children with asthma
| A: Local requirement |
|
| B: National requirement |
|
| C: International requirement |
|
Note: The Tables set out broad general principles, but the detailed practical implementation will depend on the setting, and should be determined in partnership between local communities and health care professionals.
CONFLICT OF INTERESTS
SJS reports acting as a consultant for and research with Boehringer Ingelheim, GlaxoSmithKline plc, AstraZeneca, Novartis, Regeneron, and Sanofi and grants from GlaxoSmithKline plc and Propeller Health. DAF has consulted for GlaxoSmithKline plc and Merck, Sharp & Dohme. YA reports grants from Astellas, GlaxoSmithKline plc, and Pfizer and personal fees from Merck Sharp & Dohme, GlaxoSmithKline plc, and Kyorin Pharmaceuticals. MF and WL are former GlaxoSmithKline plc employees and hold GlaxoSmithKline plc shares. SP reports personal fees from AstraZeneca, ALK, Thermo Fisher Scientific, and Phadia. AØ has consulted for GlaxoSmithKline plc and Boehringer Ingelheim. HJZ reports grants from The Allergy Society of South Africa, South Africa Medical Research Council and acting as a consultant to GSK. All other authors have no competing interests to disclose.
AUTHOR CONTRIBUTIONS
All authors were involved in the development of this manuscript and approved the submitted version.
ON BEHALF OF THE CHILDREN'S ASTHMA GROUP
With thanks to the expert attendees at the Washington Children's Asthma Forum, 31st October to 1st November 2018: Yuichi Adachi, Lara Akinbami, Kathryn Blake, Andrew Bush, Michael Cabana, Lisa Cicutto, Adnan Custovic, Iolo Doull, Gilberto Fischer, Dominic Fitzgerald, Monica Fletcher, Jonathan Grigg, Jianguo Hong, Rod Hughes, Christina Keen, David Leather, Rob Lemanske, Warren Lenney, Luis García‐Marcos, Donna Mazyck, Anders Østrem, Søren Pedersen, Bruce Rubin, Aziz Sheikh, Kunling Shen, Peter Sly, Renato Stein, Jim Stout, Padmaja Subbarao, Stanley Szefler, Raj Tilak, Tonya Winders, Siân Williams, Heather Zar.
ACKNOWLEDGMENT
This work is supported by the GlaxoSmithKline plc.
Szefler SJ, Fitzgerald DA, Adachi Y, et al. A worldwide charter for all children with asthma. Pediatric Pulmonology. 2020;55:1282–1292. 10.1002/ppul.24713
Monica Fletcher and Warren Lenney were employees of GlaxoSmithKline plc Respiratory Therapy Area, Brentford, London, UK until January 28, 2020.
REFERENCES
- 1. The Global Asthma Network Report 2018. Auckland, New Zealand, Global Asthma Network 2018. [Google Scholar]
- 2. Pavord ID, Beasley R, Agusti A, et al. After asthma‐redefining airways diseases A Lancet Commission. Lancet. 2018;391:350‐400. [DOI] [PubMed] [Google Scholar]
- 3. Looijmans‐Van den akker I, van Luijn K, Verheij T. Overdiagnosis of asthma in children in primary care: a retrospective analysis. Br J Gen Pract. 2016;66:e152‐e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pedersen SE, Hurd SS, Lemanske RF, et al. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr Pulmonol. 2011;46:1‐17. [DOI] [PubMed] [Google Scholar]
- 5. Carroll WD, Wildhaber J, Brand PLP. Parent misperception of control in childhood/adolescent asthma: the Room to Breathe survey. ERJ. 2012;39:90‐96. [DOI] [PubMed] [Google Scholar]
- 6. Cruz ÁA, Stelmach R, Ponte EV. Asthma prevalence and severity in low‐resource communities. Curr Opin Allergy Clin Immunol. 2017;17(3):188‐193. 10.1097/ACI.0000000000000360 [DOI] [PubMed] [Google Scholar]
- 7. Ferrante G, Grutta La. The burden of pediatric asthma. Front Pediatr. 2018;6:186 10.3389/fped.2018.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Szefler SJ. Asthma across the lifespan: time for a paradigm shift. J Allergy Clin Immunol. 2018;142(3):773‐780. [DOI] [PubMed] [Google Scholar]
- 9. Asher I, Bissell K, Chiang CY, et al. Calling time on asthma deaths in tropical regions—how much longer must people wait for essential medicines? Lancet Respi Med. 2019;7(1):13‐15. [DOI] [PubMed] [Google Scholar]
- 10. NRAD 2014. Why asthma still kills. RCP Publications London: Available at: https://www.rcplondon.ac.uk/projects/outputs/why-asthma-still-kills. Accessed July 4, 2019. [Google Scholar]
- 11. Nuffield Trust Report 2019. Available at: https://www.nuffieldtrust.org.uk/research/international-comparisons-of-health-and-wellbeing-in-adolescence-and-early-adulthood. Accessed July 4, 2019.
- 12. Lenney W, Bush A, Fitzgerald DA, et al. Improving the global diagnosis and management of asthma in children. Thorax. 2018;73:662‐669. [Google Scholar]
- 13. Lenney W, Adachi Y, Bush A, et al. Asthma: moving toward a global children's charter. The Lancet Respi Med. 2019;7(4):299‐300. [DOI] [PubMed] [Google Scholar]
- 14. Haahtela T, Tuomisto LE, Pietinalho A, et al. A 10 year asthma programme in Finland: major change for the better, A 10 year asthma programme in Finland: major change for the better. Thorax. 2006;61:663‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ade G, Gninafon M, Tawo L, Aït‐Khaled N, Enarson DA, Chiang CY. Management of asthma in Benin: the challenge of loss to follow‐up. Public Health Action. 2013;3(1):76‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soto‐Martinez M, Avila L. Trends in hospitalizations and mortality from asthma in Costa Rica over a 12‐15 year period. JACI in Pract. 2014;2:85‐90. [DOI] [PubMed] [Google Scholar]
- 17. Souza‐Machado C, Souza‐Machado A, Franco R, et al. Rapid reduction in hospitalisations after an intervention to manage severe asthma. Eur Respir J. 2010;35:515‐521. [DOI] [PubMed] [Google Scholar]
- 18. Pearce N, Ait‐Khaled N, Beasley R, et al. Worldwide trends in the prevalence of asthma symptoms: phase 111 of the ISAAC studies. Thorax. 2007;62:758‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. GBD 2015 . Disease and injury incidence and prevalence collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries,1990‐2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respi Med. 2016;388(10053):P1545‐P1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beran D, Zar HJ, Perrin C, Menezes AM, Burney P. Forum of international respiratory societies working group c. Burden of asthma and chronic obstructive pulmonary disease and access to essential medicines in low‐income and middle‐income countries. The Lancet Respi med. 2015;3(2):159‐170. [DOI] [PubMed] [Google Scholar]
- 21. Zar HJ, Levin ME. Challenges in treating pediatric asthma in developing countries. Paediatr Drugs. 2012;14(6):353‐359. [DOI] [PubMed] [Google Scholar]
- 22. Zar HJ, Ferkol TW. The global burden of respiratory disease—Impact on child health. Ped Pulmonol. 2014;49:430‐434. [DOI] [PubMed] [Google Scholar]
- 23. Herrera AM, Brand P, Cavada G, et al. Hospitalizations for asthma exacerbation in Chilean children: a multicenter observational study. Allergol Immunopathol (Madr). 2018;46(6):533‐538. [DOI] [PubMed] [Google Scholar]
- 24. Ayuk AC, Ramjith J, Zar HJ. Environmental risk factors for asthma in 13‐14 year old African children. Pediatr Pulmonol. 2018;53(11):1475‐1484. [DOI] [PubMed] [Google Scholar]
- 25. Britton J. Smoke‐free policy and child health. Lancet Resp Med. 2017;2(9):e392‐e393. [DOI] [PubMed] [Google Scholar]
- 26. Ellwood P, Asher MI, García‐Marcos L, et al. Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the International Study of Asthma and Allergies in Childhood (ISAAC) phase three. Thorax. 2013;68(4):351‐360. [DOI] [PubMed] [Google Scholar]
- 27. Beasley R, Clayton T, Crane J, et al. Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6‐7 years: analysis from phase three of the ISAAC programme. Lancet. 2008;372(9643):1039‐1048. [DOI] [PubMed] [Google Scholar]
- 28. Wilson KM, Torok MR, Wei B, Wang L, Lowary M, Blount BC. Marijuana and tobacco coexposure in hospitalized children. Pediatrics. 2018;142(6):e20180820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pereira MU, Sly PD, Pitrez PM, et al. Non‐atopic asthma is associated with helminthic infections and bronchiolitis in poor children. ERJ. 2007;29:1154‐1160. [DOI] [PubMed] [Google Scholar]
- 30. The Tobacco Atlas, Youth Smoking, December 19, 2018, https://tobaccoatlas.org/2018/12/19/youth-smoking/. Accessed January 25, 2020.
- 31. Aaron SD, Vandemheen KL, Fitzgerald JM, et al. Canadian research network, reevaluation of diagnosis in adults with physician‐diagnosed asthma. JAMA. 2017;317:269‐279. [DOI] [PubMed] [Google Scholar]
- 32. Oluwole O, Arinola GO, Huo D, Olopade CO. Household biomass fuel use, asthma symptoms severity, and asthma underdiagnosis in rural schoolchildren in Nigeria: a cross‐sectional observational study. BMC Pulm Med. 2017;17:3 10.1186/s12890-016-0352-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fitzpatrick AM, Jackson DJ, Mauger DT, et al. Individualized therapy for persistent asthma in young children. J Allergy Clin Immunol. 2016;138:1608‐1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fleming L, Murray C, Bansal AT, et al. The burden of severe asthma in childhood and adolescence: results from the paediatric U‐BIOPRED cohorts. ERJ. 2015;46:1322‐1333. [DOI] [PubMed] [Google Scholar]
- 35. Nantanda R, Ostergaard MS, Ndeesi G, et al. Clinical Outcomes of Children with Acute Asthma and Pneumonia in Mulago hospital 14 Uganda: a prospective study BMC Pediatr; 2014:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buelo A, McLean S, Julious S, et al. the ARC Group . At‐risk children with asthma (ARC): a systematic review. Thorax. 2018;73:813‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Teach SJ, Gergen PJ, Szefler SJ, et al. Seasonal risk factors for asthma exacerbations among inner city children. J Allergy Clinical Immunol. 2015;135:1465‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoch HE, Calatroni A, West JB, et al. Can we predict fall asthma exacerbations? Validation of the seasonal asthma exacerbation index. J Allergy Clin Immunol. 2017;140:1130‐1137.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murray CS, Woodcock A, Langley SJ, Morris J, Custovic A. Secondary prevention of asthma by the use of inhaled fluticasone propionate in Wheezy INfants (IFWIN): double‐blind, randomised, controlled study. Lancet. 2006;368:708‐710. [DOI] [PubMed] [Google Scholar]
- 40. Bui DS, Lodge CJ, Burgess JA, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018;6:535‐544. [DOI] [PubMed] [Google Scholar]
- 41. Tai A, Tran H, Roberts M, et al. Outcomes of childhood asthma to the age of 50 years. JACI. 2014;133:1572‐1578. [DOI] [PubMed] [Google Scholar]
- 42. Gray DM, Turkovic L, Willemse L, et al. Lung function in African infants in the Drakenstein Child Health Study. Impact of lower respiratory tract illness. AmJ Respir Crit Care Med. 2017;195:212‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gauderman WJ, Urman R, Avol E, et al. Association of improved air quality with lung development in children. NEJM. 2015;372:905‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suresh S, O'Callaghan M, Sly PD, Mamun AA. Impact of childhood anthropometry trends on adult lung function. Chest. 2015;147:1118‐1126. [DOI] [PubMed] [Google Scholar]
- 45. O'Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW. Severe exacerbations and the decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179(1):19‐24. [DOI] [PubMed] [Google Scholar]
- 46. Menzies‐Gow A, Canonica GW, Winders TA, Correia de Sousa J, Upham JW, Fink‐Wagner AH. A charter to Improve patient care in severe asthma. Adv Ther. 2018;35(10):1485‐1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Jong CCM, Pedersen ESL, Mozun R, et al. Diagnosis of asthma in children: the contribution of a detailed history and test results. Eur Respir J. Dec 2019;54(6):1901326 10.1183/13993003.01326-2019 [DOI] [PubMed] [Google Scholar]
- 48. Gibson PG, Powell H, Wilson A, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database Syst Rev. 2002;(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shelef DQ, Rand C, Streisand R. Using stakeholder engagement to develop a patient‐centered pediatric asthma intervention. JACI. 2016;138:1512‐1517. [DOI] [PubMed] [Google Scholar]
- 50. Keeley D, Baxter N. Conflicting asthma guidelines cause confusion in primary care. BMJ. 2018;360:k29 10.1136/bmj.k29 [DOI] [PubMed] [Google Scholar]
- 51. Reddel HK, Bateman ED, Becker A, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J. 2015;46(3):622‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. National Institute for Health and Care Excellence Asthma Quality Standard . September 2018. https://www.nice.org.uk/guidance/qs25/chapter/Quality-statements. Accessed January 19, 2020.
- 53. London children and young people strategic clinical network, London asthma standards for children and young people, June 2015, http://www.londonscn.nhs.uk/wp-content/uploads/2015/07/cyp-asthma-stds-062015.pdf. Accessed January 19, 2020.
- 54. Whole System Paediatric Asthma Plan: Improving outcomes for children andyoung people across Barnet and North Central London, North London Partner in Health and Care, March 2019, https://barnet.moderngov.co.uk/documents/s51811/Appendix%20A%20-%20Developing%20the%20CYP%20Asthma%20Plan.pdf. Accessed January 19, 2020.
- 55. Kupczyk M, Haahtela T, Cruz AA, Kuna P. Reduction of asthma burden is possible through National Asthma Plans. Allergy. 2010;65:415‐419. [DOI] [PubMed] [Google Scholar]
- 56. Haahtela T, Herse F, Karjalainen J, et al. The Finnish experience to save asthma costs by improving care in 1987‐2013. J Allergy Clin Immunol. 2017;139:408‐414. [DOI] [PubMed] [Google Scholar]
- 57. Anderson WC, Szefler SJ. New and future strategies to improve asthma control in children. J Allergy Clin Immunol. 2015;136:848‐859. [DOI] [PubMed] [Google Scholar]
- 58. Fitzgerald DA, Gillis J. Asthma deaths in children in New South Wales 2004‐2013: could we have done more? J Paediatr Child Health. 2015;51(11):1127‐1133. [DOI] [PubMed] [Google Scholar]
- 59. Puranik S, Forno E, Bush A, Celedón JC. Predicting severe asthma exacerbations in children. Am J Respir Crit Care Med. 2017;195(7):854‐859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kivistö JE, Karjalainen J, Kivelä L, Huhtala H, Protudjer JLP. Very low asthma death incidence among Finnish children from 1999 to 2015. Pediatr Pulmonol. 2018;53(8):1009‐1013. [DOI] [PubMed] [Google Scholar]
- 61. United Nations report 2010. Keeping the promise: a forward‐looking review to promote an agreed action agenda to achieve the Millennium Development Goals by 2015. Available at: https://undocs.org/A/64/665. Accessed July 4, 2019.
- 62. O'Byrne PM, Pedersen S, Schatz M, et al. The poorly explored impact of uncontrolled asthma. Chest. 2013;143(2):511‐523. [DOI] [PubMed] [Google Scholar]
- 63. McDonnell J, Correia de Sousa J, Baxter N, et al. Building capacity to improve respiratory care: the education strategy of the International Primary Care Respiratory Group. NPJ Prim Care Respir Med. 2014;24:14072 10.1038/npjpcrm.2014.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Volerman A, Chin MH, Press VG. Solutions for asthma disparities. Pediatrics. 2017;139:e20162546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lynn J, Oppenheimer S, Zimmer L. Using public policy to improve outcomes for asthmatic children in schools. J Allergy Clin Immunol. 2014;134:1238‐1244. [DOI] [PubMed] [Google Scholar]
- 66. Stille CJ, Honigfeld L, Heitlinger LA, Kuo DZ, Werner EJ. The pediatric primary care‐specialist interface: a call for action. J Pediatr. 2017;187:303‐308. [DOI] [PubMed] [Google Scholar]
- 67. Wolf FM, Guevara JP, Grum CM, Clark NM, Cates CJ. Educational interventions for asthma in children. Cochrane Database Syst Rev. 2003;326(1):CD000326 http://ebn.bmj.com/. Accessed January 10, 2020. [DOI] [PubMed] [Google Scholar]
- 68. Harris K, Kneale D, Lasserson TJ, McDonald VM, Grigg J, Thomas J. School‐based self‐management interventions for asthma in children and adolescents: a mixed methods systematic review. Cochrane Database Syst Rev. 2019;1:1CD011651 10.1002/14651858.CD011651.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kneale D, Harris K, McDonald VM, Thomas J, Grigg J. Effectiveness of school‐based self‐management interventions for asthma among children and adolescents: findings from a Cochrane review and metanalysis. Thorax. 2019;74(5):432‐438. [DOI] [PubMed] [Google Scholar]
- 70. Cicutto L, Gleason M, Haas‐Howard C, et al. Building bridges for asthma care program: a school‐centered program connecting schools, families, and community health‐care providers. J Sch Nurs. 2018:1059840518805824 10.1177/1059840518805824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Szefler SJ, Cloutier MM, Villarreal M, et al. Building bridges for asthma care: reducing school absence for inner city children with health disparities. J Allergy Clin Immunol. 2019;143(2):746‐754.e2. [DOI] [PubMed] [Google Scholar]
- 72. Liptzin DR, Gleason MC, Cicutto LC, et al. Developing, implementing, and evaluating a school‐centered asthma program: step up asthma program. J Allergy Clin Immunol: In Practice. 2016;4:972‐979. [DOI] [PubMed] [Google Scholar]
- 73. Lemanske RF, Kakumanu S, Shanovich K, et al. Creation and implementation of SAMPRO™: a school‐based asthma management program. J Allergy Clin Immunol. 2016;138:711‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tobin ET, Zilioli S, Imami L, Saleh DJ, Kane HS, Slatcher RB. Neighborhood stress, depressive symptoms and asthma morbidity in youth. J Ped Psychol. 2016;41:952‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ramratnam SK, Visness CM, Jaffee KF, et al. Relationships among maternal stress and depression, type 2 responses, and recurrent wheezing at age 3 years in low‐income urban families. AJRCCM. 2017;195:674‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wright RJ, Visness CM, Calatroni A. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner‐city cohort. AJRCCM. 2010;182:25‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ramratnam SK, Han YY, Rosas‐Salazar C. Exposure to gun violence and asthma among children in Puerto Rico. Respir Med. 2015;109:975‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Creating an asthma friendly school . Available at: https://www.cdc.gov/healthyschools/asthma/creatingafs/index.htm. Accessed July 4, 2019.
- 79. Stempel H, Federico MJ, Szefler SJ. Applying a biopsychosocial model to inner city asthma: recent approaches to address paediatric asthma health disparities. Paediatr Respir Rev. 2019;32:10‐15. [DOI] [PubMed] [Google Scholar]
- 80. Evans R, Gergen PJ, Mitchell H, et al. A randomized clinical trial to reduce asthma morbidity among inner‐city children: results of the national cooperative asthma study. J Pediatr. 1999;135:332‐338. [DOI] [PubMed] [Google Scholar]
- 81. Kopel LS, Phipatanakul W, Gaffin JM. Social disadvantage and asthma control in. Children Paed Resp Rev. 2014;15:256‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of home‐based environmental interventions among urban children with asthma. N Engl J Med. 2004;351:1068‐1080. [DOI] [PubMed] [Google Scholar]
- 83. Krieger JW, Takaro TK, Song L, Weaver M. The Seattle‐King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health. 2005;95(4):652‐659. 10.2105/AJPH.2004.042994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bean JV, Sheik A. Tobacco control policies in relation to child health and perinatal health outcomes. Arch Dis Child. 2018;103:817‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Behbod B, Sharma M, Baxi R, et al. Family and carer smoking control programmes for reducing children's exposure to environmental tobacco smoke. Cochrane Database Syst Rev. 2018;1:CD001746 10.1002/14651858.CD001746.pub4, Review. PubMed PMID: 29383710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Turcotte DA, Chaves E, Gore R, Adejumo KL, Woskie S. The impact of housing type on low‐income asthmatic children receiving multifaceted home interventions. Public Health. 2018;164:107‐114. [DOI] [PubMed] [Google Scholar]
- 87. Turner SW, Murray C, Thomas M. Applying UK real world primary care data to predict asthma attacks in 3776 well‐characterised children: a retrospective cohort study. NJP Prim Care Resp Med. 2018;28:28 10.1038/s41533-018-0095-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Carroll W, Gilchrist FJ, Lenney W, et al. The british lung foundation asthma management programme. Eur Respir J. 2017;50:PA592. [Google Scholar]
- 89. Smith JR, Musgrave S, Payerne E, et al. At‐risk registers integrated into primary care to stop asthma crises in the UK (ARRISA‐UK): study protocol for a pragmatic, cluster randomised trial with nested health economic and process evaluations. Trials. 2018;19:466 10.1186/s13063-018-2816-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Local Burden of Disease Child Growth Failure Collaborators. Mapping child growth failure across low‐ and middle‐income countries. Nature. 2020; 577: 231‐234. [DOI] [PMC free article] [PubMed]
- 91. Local Burden of Disease Educational Attainment Collaborators. Mapping disparities in education across low‐ and middle‐income countries. Nature. 2020; 577: 235‐238. [DOI] [PMC free article] [PubMed]
- 92. GBD . Lower respiratory infections collaborators. Quantifying risks and interventions that have affected the burden of lower respiratory infections among children younger than 5 years: an analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2017;2020(20):60‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ryan D, Murphy A, Ställberg B, Baxter N, Heaney LG. ‘SIMPLES’: a structured primary care approach to adults with difficult asthma. Prim Care Respir J. 2013;22:365‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Martins SM, Salibe‐Filho W, Tonioli LP, et al. Implementation of 'matrix support' (collaborative care) to reduce asthma and COPD referrals and improve primary care management in Brazil: a pilot observational study. NPJ Prim Care Respir Med. 2016;26:16047 10.1038/npjpcrm.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Montgomery‐Taylor S, Watson M, Klaber R. Child health general practice hubs: a service evaluation. Arch Dis Child. 2016;101:333‐337. [DOI] [PubMed] [Google Scholar]
- 96. Steele L, Coote N, Klaber R, Watson M, Coren M. Understanding case mix across three paediatric services: could integration of primary and secondary general paediatrics alter walk‐in emergency attendances? Arch Dis Child. 2019;104:432‐436. [DOI] [PubMed] [Google Scholar]
- 97. Nkonki L, Tugendhaft A, Hofman K. A systematic review of economic evaluations of CHW interventions aimed at improving child health outcomes. Hum Resour Health. 2017;15(1):19 10.1186/s12960-017-0192-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Boyd M, Lasserson T, McKean M, Gibson P, Ducharme F, Haby M (2009). ‘Interventions for educating children who are at risk of asthma‐related emergency department attendance (Cochrane Review)’. Cochrane Database of Systematic Reviews, issue 2, article CD001290 10.1002/14651858.CD001290.pub2 [DOI] [PMC free article] [PubMed]
- 99. Purdy S Avoiding hospital admissions: What does the research evidence say? The Kings Fund, December 2010, https://www.kingsfund.org.uk/sites/default/files/Avoiding-Hospital-Admissions-Sarah-Purdy-December2010.pdf. Accessed January 19, 2020.
- 100. Christakis DA, Mell L, Koepsell TD, Zimmerman FJ, Connell FA. Association of lower continuity of care with greater risk of emergency department use and hospitalization in children. Pediatrics. 2001;107:524‐529. [DOI] [PubMed] [Google Scholar]
- 101. Gill J, Mainous A. The role of provider continuity in preventing hospitalizations. Arch Fam Med. 1998;7:352‐357. [DOI] [PubMed] [Google Scholar]
- 102. Mohammad Y, Rafea S, Latifeh Y, et al. Uncontrolled and under‐diagnosed asthma in a Damascus shelter during the Syrian crisis. J Thorac Dis. 2017;9:3415‐3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bravata DM, Gienger AL, Holty J‐EC, et al. Quality improvement strategies for children with asthma. Arch Pediatr Adolesc Med. 2009;163:572‐581. [DOI] [PubMed] [Google Scholar]
- 104. Wildfire JJ, Gergen PJ, Sorkness CA, et al. Development and validation of the composite asthma severity index—an outcome measure for use in children and adolescents. J Allergy Clin Immunol. 2012;129:694‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Krouse RZ, Sorkness CA, Wildfire JJ, et al. Minimally important differences and risk levels for the composite asthma severity index. J Allergy Clin Immunol. 2017;139:1052‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fitzpatrick AM, Szefler SJ, Mauger DT, et al. Development and initial validation of the Asthma Severity Scoring System (ASSESS). J Allergy Clin Immunol. 2020;145:127‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Quon BS, Goss C. A story of success: continuous quality improvement in cystic fibrosis care in the USA. Thorax. 2011;66(12):1106‐1108. [DOI] [PubMed] [Google Scholar]
- 108. James BC. The cystic fibrosis improvement story: we count our successes in lives. BMJ Qual Saf. 2014;23:268‐271. [DOI] [PubMed] [Google Scholar]
- 109. Eileen W, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry (ISAR). Chest. 2019. 10.1016/j.chest.2019.10.053 [DOI] [PubMed] [Google Scholar]


