Abstract
Objectives
Autologous CD133+ bone marrow stem cells may improve cardiac function. This randomized, single‐blind clinical trial inquired whether a combined transepicardial and transseptal implantation of CD133+ stem cells during coronary artery bypass grafting (CABG) improve cardiac function with ejection fraction (EF) changes as a primary endpoint in patients with low EF.
Methods
Thirty patients with coronary heart disease and EF <35% were randomized to undergo CABG alone or CABG with transseptal and transepicardial implantation of CD133+. Cardiac function was evaluated using cardiac magnetic resonance imaging (MRI) before and 6 months after CABG.
Results
Preoperative EF was lower in the intervention group (25.88% ± 5.66%) than in the control group (30.18% ± 3.85%; P = .04). The adverse event incidence was similar between both groups. At 6 months, EF changes were significantly higher (8.69% ± 9.49; P = .04) in the CD133+ group than in the CABG‐only group. Compared to the control group, significant improvements were seen in the wall motion score index (P = .003) and scar size proportion (P = .047) in the CD133+ group. The quality of life (QOL), assessed by a 6‐minute walking test, showed considerable improvement in the CD133+ group compared to that in the control group (P = .03). The Minnesota Living with Heart Failure Questionnaire (MLHFQ) scale did not show improvement in the intervention group (P = .09, vs control).
Conclusion
Combined transepicardial and transseptal autologous CD133+ BMC implantation during bypass grafting improved cardiac function in low EF coronary artery disease patients.
Keywords: coronary artery bypass graft, coronary artery disease, heart failure, stem cell
1. INTRODUCTION
Coronary artery disease (CAD) is one of the leading causes of death in developing countries, including Indonesia. 1 It is estimated that by 2020, CAD will be the most common cause of death, accounting for approximately 36% of all deaths; the latter number is twice as high as the death rate due to malignancy. 2
The imbalance between demand and supply for myocardial oxygen leads to ischemia and myocardial infarction. A prolonged duration of ischemia results in the apoptosis of the myocardium, formation of fibrotic tissue, and subsequent heart failure. CAD is the primary cause of a high heart failure mortality rate worldwide, which can be as high as 14%. 3
Coronary artery bypass grafting (CABG) is one of the revascularization modalities used for the management of CAD. However, Haxhibeqiri‐Karabdic et al 4 have shown that postoperative improvement in patients with reduced global left ventricular ejection fraction (LVEF <35%) was not significant.
Even though multiple studies in the last decade have addressed the effects of stem cell therapy in improving cardiac function, the results, type of cells used, and implantation procedure varied considerably. CD133+ bone marrow cells have been shown to possess a high angiogenic capacity. 5 A study published by Nasseri et al revealed that transepicardial implantation of CD133+ cells during CABG in low LVEF patients showed no significant improvement. Nevertheless, they stated that this result might differ if the transseptal region was also included as a site of stem cell implantation. 6 Therefore, this study aimed to assess the role of CD133+ stem cells in improving cardiac function and included a transseptal implantation site along with the transepicardial site.
2. PATIENTS AND METHODS
2.1. Study protocol
This single‐blind randomized clinical trial was conducted at our facility, the Department of Cardiovascular Surgery of National Cardiovascular Center Harapan Kita, Jakarta, Indonesia, between March 2016 and June 2018 and was approved by the Institutional Review Board and the Ethics Committee of the institution (No. LB.02.01/VII/086/KEP.007/2016) and Universitas Indonesia (No: 999/UN2. F1/ETIK/XI/2016). The study was registered in http://ClinicalTrials.gov (NCT02870933). All patients enrolled in this study were informed about the procedures and provided the written informed consent form. Thirteen patients were randomly assigned to one of two groups, the intervention group that underwent elective on‐pump CABG+ dual‐site implantation of CD133+ or the control group that underwent only the CABG procedure.
Inclusion criteria for this study were as follows: (a) age <70 years, three or more vessels with CAD, and indications for CABG surgery; (b) an impaired resting global LVEF <35% with hypoperfused myocardium and abnormal septal wall motion, assessed by cardiac MRI; (c) LV hypoperfusion during resting state on MRI. Exclusion criteria were as follows: (a) a diagnosis of CAD with valvular disease requiring concomitant valve surgery; (b) acute myocardial infarction; (c) contraindications for MRI or bone marrow cell aspiration procedure; (d) a history of ventricular arrhythmia (≥Lown III); (e) coagulation disorders, including familial hemophilia, sign/symptoms of bleeding disorders, platelets <80 000 mL; (e) chronic obstructive pulmonary disease; (f) infectious diseases (HIV, HBV, and HCV); (g) abnormal liver and renal function tests; (h) ventricular conduction disorders; (i) electrolyte imbalance; (j) a history of immunosuppressant medications, cytotoxic agents, and radiotherapy, in the 4 months before surgery.
The sample size was calculated assuming a power (β) of 80% with a two‐sided α level of 0.05. A minimum of 13 patients per group was minimally required to show an intergroup difference in LVEF. We recruited 15 patients in each group to anticipate dropouts if any.
2.2. Bone marrow aspiration
Bone marrow (BM) aspiration was performed 24 hours before the scheduled CABG (Figure S2). Patients were administered local anesthesia, and marrow was aspirated from the posterior iliac crest. Approximately 190 to 210 mL of BM was harvested and diluted with 25 mL of normal saline containing 40 000 U heparin.
2.3. CD133+ separation process
The aspirate was then transferred to our stem cell facility. Phosphate buffer saline was added to obtain a total volume of 450 mL; the suspension was centrifuged to remove platelets, and 1.5 mL of anti‐CD133 labeled Magnetic MicroBeads® (Miltenyi Biotec) was added to the supernatant and incubated at room temperature for 30 minutes. CD133+ cells were separated on a CliniMACS® Magnetic Separation Device (Miltenyi Biotec) (Figure S3), and the final product was stored at 4 to 8°C until further use. The final volume for implantation was 20 mL, with a CD133+ count ranging from 5 to 10 million cells. Ten milliliters of the aspirate was drawn to assess cell numbers, proof of sterility, purity, and the viability of the CD133+ cells using flow‐cytometry on a FACS Aria III (BD Biosciences). The purity of the CD133+ end‐product was 84.4% to 90.1%, with viability of 95.2% to 94.4%.
2.4. Surgery
Anesthesia and CABG surgery were carried out according to the regular arrested heart surgical protocol. The CD133+ cells were prepared in 20 1‐mL syringes with 25G needles (0.5 × 25 mm) and implanted on transepicardial segments. After the operator released the aortic cross‐clamp, subsequently the CD133+ cells were implanted along the transseptal segment. This procedure was performed by going along the left anterior descending artery. Correct needle placement within the interventricular septum was ensured using transesophageal echocardiography (Figure 1). Each injection contained 0.5 mL volume of CD133+ cells and was injected at sites approximately 1.0‐cm apart. Ten injections were administered within the transseptal region (Figure S4), and the other 30 injections were administered along the border area of the hypokinetic/hypoperfused segment, which had been previously identified by MRI or was plainly visible due to scarring and epicardial muscle discoloration.
Figure 1.
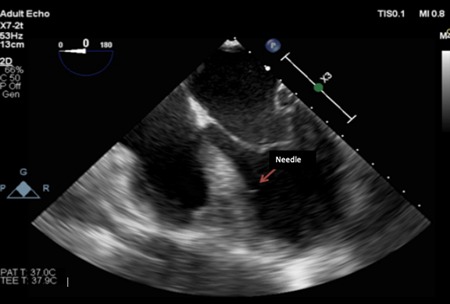
Echocardiography visualization of the transseptal injection
2.5. Cardiac magnetic resonance imaging and analysis
All patients underwent imaging by a Philips Achieva® 1.5T MRI (Netherland, 2005) at the same imaging facility in our hospital, before and 6 months after the surgery. All subjects underwent resting and stress (adenosine 140 μg/kg BW/min) MRI with contrast agent gadopentetate dimeglumine (0.05 mmol/kg) and the functional data evaluated under stress (Figures S8, S9). LVEF, scar size, and wall motion score index (WMSI) were analyzed using the software CVI42 (Canada, 2015).
2.6. Quality of life and aerobic capacity
MLHFQ and the 6‐minute walk test (6MWT) were used to evaluate the quality of life and aerobic capacity, respectively, before and 6 months after the surgery. Registered cardiologists evaluated the results of the 6MWT of all participants. The MLHFQ consisted of 21 questions that were used to assess the quality of life.
2.7. Statistical analysis
All statistical data were analyzed using the IBM SPSS Statistics version 21.0 (SPSS Inc, Chicago, IL). Continuous data are presented as mean ± standard deviation or median and interquartile range; categorical data are presented as number (n) and percentage. The Shapiro‐Wilk test was utilized to assess data distribution. Data were analyzed using the independent t‐test or the Mann–Whitney U‐test, as required. Scar size proportions are presented as categorical data, namely improved or nonimproved, and were compared by the Chi‐square test. In all cases, a P‐value of < .05 was considered statistically significant. All results were analyzed by comparing pre‐ and post‐intervention changes in both groups. Boxplot data represent median and minimum‐maximum.
3. RESULTS
3.1. Selection process and patient characteristics
Thirty patients were recruited for this trial and were randomly assigned to one of two groups. At the end of the trial, only 26 patients were evaluated, as four patients did not finish this study. The CONSORT flowchart (Figure S1) describes the process of group assignment.
Both groups demonstrated similar baseline characteristics in terms of age, gender, renal function, and other comorbidities, except for LVEF, which was significantly lower in the CD133+ group than in the control group. The selection of subjects was randomized and in compliance with the inclusion criteria. Other hemodynamic parameters were similar between the two groups. Most of the subjects were male (92.31%) and over the age of 50 years. A total of 20 subjects were active smokers, and 16 patients (61.54%) had hypertension and diabetes (Table 1). Thus, most patients in this trial had either modifiable or unmodifiable risk factors for CAD.
Table 1.
Baseline characteristic of trial subjects
| Baseline characteristic | Groups | P‐value | |
|---|---|---|---|
| CD133+ | Control | ||
| (n = 13) | (n = 13) | ||
| Age, y | 54.61 ± 8.07 | 57.46 ± 6.33 | .32 |
| Male population (f) | 12 | 12 | 1 |
| Systolic blood pressure (mm Hg) | 121.92 ± 14.37 | 126.53 ± 19.60 | .5 |
| Diastolic blood pressure (mm Hg) | 78.00 ± 12.03 | 72.46 ± 15.36 | .2 |
| Risk factors | |||
| Smoking (f) | 11 | 9 | .65 |
| Dyslipidaemia (f) | 6 | 11 | .09 |
| Hypertension (f) | 9 | 7 | .68 |
| Family history (f) | 7 | 9 | .69 |
| Menopause (f) | 1 | 1 | 1 |
| Diabetes mellitus (f) | 5 | 9 | .23 |
| History of previous infarction (f) | 11 | 11 | 1 |
| Blood glucose, mg/dL | 123.38 ± 35.74 | 129.00 ± 35.21 | .69 |
| NYHA Grade III‐IV | 4 | 4 | 1 |
| CCS Grade II‐III | 1 | 2 | .53 |
| 6‐min walk test (m) | 297.07 ± 72.56 | 308.92 ± 79.37 | .53 |
| LVEF (%) | 25.88 ± 5.66 | 30.18 ± 3.85 | .04 |
| Scar Size (%) | 27.76 ± 15.76 | 24.45 ± 13.73 | .47 |
| Wall motion score index | 2.32 ± 0.17 | 2.07 ± 0.31 | .08 |
| Minnesota living with heart failure score | 30.30 ± 13.73 | 21.46 ± 8.76 | .53 |
Abbreviations: LVEF, left ventricular ejection fraction; NYHA, New York Heart Association
3.2. Cardiac function
The primary endpoint of this study was an improvement in cardiac function, measured by changes in LVEF assessed by cardiac MRI. Changes in WMSI and scar size proportions were also measured to further support changes observed in LVEF.
We found a significant difference in the change in LVEF between the CD133+ and control groups when measured 6 months after surgery (respective means: Δ 8.69 ± 9.49 vs 1.43 ± 7.87, P = .04) (Figures 2, S5 and Table S1).
Figure 2.
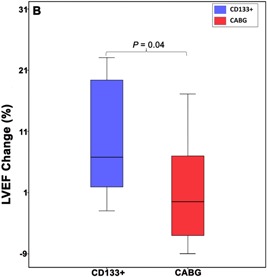
Boxplot diagram of left ventricular ejection fraction (LVEF) of patients treated with CABG plus CD133+ and CABG only. Comparison of changes (Δ) of LVEF. The improvement of LVEF in the CD133+ group after the intervention was significantly higher than CABG only group (P = .04). Data were presented as median and minimum‐maximum. CABG, coronary artery bypass grafting
In addition, improvements were seen in WMSI and scar size proportions. Specifically, at the six‐month follow‐up, WMSI in the CD133+ group decreased significantly by 0.51 ± 0.4, compared with that in the control group, in which WMSI increased; these changes were significantly different (P = .01) (Figures 3, S10, S11 and Table S1).
Figure 3.
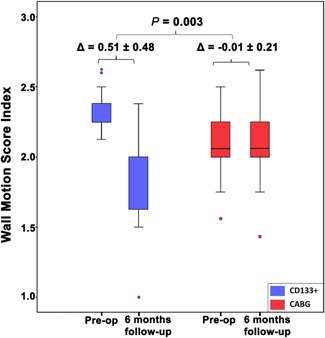
Comparison of wall motion score index (WMSI) changes between both groups. The decrement of the WMSI score (Δ) in the CD133+ group was significantly lower than the changes in the CABG group (P = .01). Box plot was presented in the median and minimum maximum. Δ score was presented in mean ± SD. CABG, coronary artery bypass grafting
Next, scar size proportion decreased significantly in the stem cell group (25.46% ± 12.91) compared to that in the control group (27.32% ± 12.92), and the improvement in scar size proportion was considerably higher in the stem cell group (decreased by 2.3%) than in the control group (increased by 3.01%). Categorical analysis using the Chi‐square test showed that greater improvements in scar size proportion were seen in the stem cell group than in the control group (P = .04) (Figure 4 and Table S2).
Figure 4.
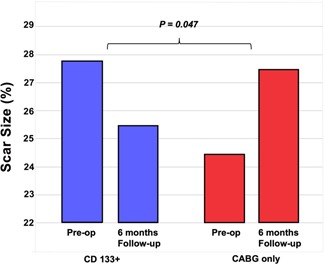
Comparison of scar size proportion improvement between both groups. There was a reduction in scar size proportion in the CD133+ group, on the contrary, there was an increment of scar size proportion in the CABG group (P = .04). Box plot is presented in median and minimum‐maximum. Δ score was presented in mean ± SD. CABG, coronary artery bypass grafting
3.3. Quality‐of‐Life assessment
The results of the MLHFQ and 6MWT are summarized in Table 2. Overall, MLHFQ showed an increase in QOL in both groups. MLHFQ score decreased significantly by 18.38 ± 17.89 points (P = .00) in the stem cell group and in the control group (9.00 ± 7.30 points; P = .00); however, the difference in QOL score reduction was not significantly different between the two groups (P = .09) (Figure S6)
Table 2.
Result of MLHFQ and 6MWT
| Variables | Stem cell group | Control group | P‐value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 months of follow‐up | Δ Score (Mean ± SD) | Baseline | 6 months of follow‐up | Δ Score (Mean ± SD) | ||
| MLHFQ | 27 | 12 | 18.38 ± 17.89 | 23 | 14 | 9.00 ± 7.30 | .09 |
| (Median IQR) | (20‐39.5) | (4.5‐16) | P = .00 | (15‐26) | (4.5‐15.5) | P = .00 | |
| 6MWT | 299 | 420 | 113.15 ± 97.47 | 298 | 378 | 58.84 ± 42.69 | .03 |
| (Median IQR) | (260.5‐350) | (381‐441) | P = .00 | (255‐371) | (335‐414.5) | P = .03 | |
Abbreviations: MLHFQ, Minnesota Living with Heart Failure Questionnaire; MWT, minute walk test.
The 6MWT showed significant improvement in patient performance as there was a significantly higher improvement in the score of the stem cell group than that in the score of the control group (Mean Δ score 113.15 ± 97.47 vs 58.84 ± 42.6; P = .03) (Figure S7). However, the absolute scores for the 6MWT were not significantly different between the two groups after 6 months (P = .10)
3.4. Adverse events
The incidence of subject dropout due to mortality was similar in both groups. In the CD133+ group, there were two deaths. The first patient died of sepsis, while the second patient died owing to hemodynamic instability during the post‐fasciotomy procedure due to compartment syndrome. In the control group, one patient decided to withdraw from the study, and another died of infection.
4. DISCUSSION
Many studies have reported the potential efficacy of stem cell therapy. Other preclinical studies revealed that autologous bone marrow–derived stem cells are the predominant cell type used for cardiac regeneration therapy in patients with heart failure due to myocardial infarction. The potential therapeutic effects of CD133+ stem cells, as immature endothelial progenitor cells, have been utilized in various trials, but they have yielded divergent clinical results.
Given this heterogeneity in published results, we hypothesized that combining transseptal with transepicardial in one procedure of CD133+ stem cell implantation would improve the effectiveness of the procedure. Clinically, our results confirmed the improvement of cardiac function by utilizing this combined approach. Thus, we have proposed a novel implantation site in the transseptal area. We directly injected the stem cells into this area. This way, the beneficial effects of stem cells could extend to the interventricular septum and enhance cardiac function improvement. Several studies, such as the Cardio133 Trial by Nasseri et al 6 and the trial by Ahmadi et al 7 have failed to show significant improvement in cardiac function. Nasseri et al have stated that the interventricular septum might not be accessible even with transepicardial injection. This could be the reason that led to the insignificant improvements of cardiac function in their trials. 6 The interventricular septum itself plays an important mechanical role in ensuring adequate left and right ventricular ejection function and heart contractility. 8 , 9 Ischemia at the septal area results in a loss of septal contractile ability, which then disrupts the regional intraventricular loading capability. The dyskinetic septum also disrupts the contractility of the remaining normokinetic segments. 9 Therefore, this novel implantation site could address issues arising due to a dyskinetic septum and thus enhance cardiac function improvement.
CD133+ stem cells improve cardiac function and repair myocardium by stimulating neovascularization and angiogenesis and also by inhibiting apoptosis and cardiomyogenesis. 5 , 10 Neovascularization and angiogenesis, in turn, increase tissue perfusion; hence, ischemic death of cells can be avoided. Eventually, this would result in a reduced scar tissue development. 11 The following mechanisms also play a role in decreasing scar size proportions. Previous studies have shown that bone marrow–derived endothelial progenitor cells could proliferate and differentiate into cardiomyocytes and endothelial cells and lead to new tissue formations, and this process could directly repair both cardiac function and contractility. 12 , 13 Neovascularization also releases various factors that mobilize progenitor cells from the bone marrow and local cardiac stem cells via a paracrine mechanism. 5 , 10 These stem cells then undergo differentiation and proliferation to augment cardiac regeneration. Our results on the improvement in scar size proportion and WMSI support the validity of such a mechanism of action of the CD133+ stem cells. Ahmadi et al 7 have previously commented that akinetic segments could only regain perfusion but not their contractile functions, whereas hypokinetic segments showed improved contractility after reperfusion. Our observations on the reduction in scar size proportion, along with improvement in contractility, may indicate that cardiac regeneration can indeed occur. The decrease in scar size proportion was objectively proven by an increase in the 6MWT of the CD133+ intervention group. In contrast, the cause of the increase of scar size proportion in the control group remains unknown.
The WMSI test showed that while some of the akinetic segments reverted to becoming hypokinetic, some of the hypokinetic segments regained normal movement. These phenomena suggest that stem cells can improve cardiac regeneration and also increase the score index of contractile elements in viable cells within the hypokinetic segment. It can be said that these processes contribute to cardiac repair, which is reflected as an increase in ejection fraction.
Although the increase in LVEF changes was merely 8%, these changes were followed by an increase in the aerobic capacity. The 6MWT and MLHFQ were used to assess the patients' quality of life after the procedure. However, while MLHFQ scores showed insignificant results, the 6MWT showed a significant improvement in walking distance of the CD133+ patients. MLHFQ is a subjective test and can be influenced by many factors, including patient interpretation, which may have affected the result. In contrast, the 6MWT relies on objective measurements that might better capture any improvement in the patient's condition.
During the trial, none of the adverse events were related to the CD133+ stem cell implantation. The participants could complete the trial without any significant events or prolonged hospitalization. Deaths in the stem cell group were also due to infections unrelated to stem cell therapy.
We acknowledge that there are a few limitations of this study, namely the difference in preoperative EF between the two groups. In addition, there were four dropouts; two patients died due to noncardiac causes (eg, septicemia) in the intervention group, while one patient in the control group also died of noncardiac events (eg, septicemia), and another patient withdrew.
5. CONCLUSION
Our findings showed that combined transseptal and transepicardial implantation of CD133+ stem cells can have a potentially beneficial effect on cardiac function. They can help improve the recovery of the ischemic myocardium, increasing cardiac regeneration, and viability, of cells that improve wall motion and by reducing scar size proportion. All of these factors lead to an improvement in the quality of life of patients.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Supporting information
Supporting information
ACKNOWLEDGMENTS
We extend our gratitude to Dr. Elen, Dr. Manoefris Kasim, and Dr. Habibie Arifianto for their contributions to interpreting the cardiac imaging. We thank Dr. Dicky Aligheri Wartono and Dr. Amin Tjubandi for their role in the surgical procedure. We also thank Dr. Christian, Dr. Erik Hoetama, Dr. Beta Canina Harlyjoy, Dr. Geswin Aditya Hermawan, Dr. Natasha Marianne Setiabakti, Dr. Maria Felinsianita, Dr. Stella Aprilia, and Dr. Florence Low for their assistance during this whole study. A professional native proofreader has reviewed this manuscript. This study was supported by a grant from National Cardiovascular Center Harapan Kita Hospital (Grant Number: LB.0101/VII/32/2016).
Soetisna TW, Sukmawan R, Setianto B, et al. Combined transepicardial and transseptal implantation of autologous CD 133+ bone marrow cells during bypass grafting improves cardiac function in patients with low ejection fraction. J Card Surg. 2020;35:740–746. 10.1111/jocs.14454
Contributor Information
Tri Wisesa Soetisna, Email: tricts2000@yahoo.com.
Anwar Santoso, Email: anwarsantoso@inaheart.org.
REFERENCES
- 1. Indonesian Primary Health Survey Report 2013. Ministry of health republic of Indonesia, lembaga penerbitan balitbangkes kementrian kesehatan republik Indonesia, Jakarta, 2013. [Google Scholar]
- 2. World Health Organization . WHO Annual Report ; 2012.
- 3. Gheorghiade M, Sopko G, De Luca L, et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation. 2006;114:1202‐1213. [DOI] [PubMed] [Google Scholar]
- 4. Haxhibeqiri‐Karabdic I, Hasanovic A, Kabil E, Straus S. Improvement of ejection fraction after coronary artery bypass grafting surgery in patients with impaired left ventricular function. Med Arch. 2014;68:332‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343‐353. [DOI] [PubMed] [Google Scholar]
- 6. Nasseri BA, Ebell W, Dandel M, et al. Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 trial. Eur Heart J. 2014;35:1263‐1274. [DOI] [PubMed] [Google Scholar]
- 7. Ahmadi H, Baharvand H, Ashtiani SK, et al. Safety analysis and improved cardiac function following local autologous transplantation of CD133(+) enriched bone marrow cells after myocardial infarction. Curr Neurovasc Res. 2007;4:153‐160. [DOI] [PubMed] [Google Scholar]
- 8. Fomina IG, Georgadze ZO, Galanina NA, Gaidamakina NE, Kiniasheva NB. The role of interventricular septum in kinetics of left and right ventricular contraction in patients with ischemic heart disease and chronic cardiac failure. Ter Arkh. 2006;78:19‐24. [PubMed] [Google Scholar]
- 9. Goldstein JA, Tweddell JS, Barzilai B, Yagi Y, Jaffe AS, Cox JL. Importance of left ventricular function and systolic ventricular interaction to right ventricular performance during acute right heart ischemia. J Am Coll Cardiol. 1992;19:704‐711. [DOI] [PubMed] [Google Scholar]
- 10. Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steinhoff G, Choi Y‐H, Stamm C. Intramyocardial bone marrow stem cell treatment for myocardial regeneration. Eur Heart J. 2006;8(suppl):H32‐H39. [Google Scholar]
- 12. Dawn B, Abdel‐Latif A, Sanganalmath SK, Flaherty MP, Zuba‐Surma EK. Cardiac repair with adult bone marrow‐derived cells: the clinical evidence. Antioxid Redox Signal. 2009;11:1865‐1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Strauer BE, Brehm M, Zeus T, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913‐1918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


