Abstract
BACKGROUND
Global warming and extreme or adverse events induced by climatic fluctuations are an important threat for plants growth and agricultural production. Adaptability to environmental changes prevalently derives from a large set of genetic traits affecting physiological and agronomic parameters. Therefore, the identification of genotypes that are good yield performer at high temperatures is becoming increasingly necessary for future breeding programs. Here, we analyzed the performances of different tomato landraces grown under elevated temperatures in terms of yield and nutritional quality of the fruit. Finally, we evaluated the antioxidant and anti‐inflammatory activities of fruit extracts from the tomato landraces selected.
RESULTS
The tomato landraces analyzed here in a hot climate differed in terms of yield performance, physicochemical parameters of fruit (pH, titratable acidity, degrees Brix, firmness), bioactive compounds (ascorbic acid, carotenoids, and polyphenols), and anti‐inflammatory potential. Three of these landraces (named E30, E94, and PDVIT) showed higher fruit quality and nutritional value. An estimated evaluation index allowed identification of PDVIT as the best performer in terms of yield and fruit quality under high temperatures.
CONCLUSION
The analyses performed here highlight the possibility to identify new landraces that can combine good yield performances and fruit nutritional quality at high temperatures, information that is useful for future breeding programs. © 2020 The Authors. Journal of The Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: tomato, high temperature tolerance, yield performance, fruit quality, nutritional value
INTRODUCTION
One of the most important challenges facing us today is dealing with global warming, which can greatly impact on crop production and food accessibility. Together with water stress and high salinity, temperatures and light fluctuations during plant growth are the most important abiotic stresses that plants have to face.1
Tomato is one of the major cultivated crops worldwide and a model system to study plant response to the changing environmental conditions. The optimal range of temperatures for tomato cultivation is between 25 and 30 °C during the day and 20 °C during the night.2 However, tomato varieties or landraces can exhibit individual differences in terms of yield performances, depending on the genetic background and the adaptation to the environment in specific geographic areas. In this context, the exploration of the natural variation and the screening of genotypes and landraces that are good yield performers at high temperatures may help to understand the mechanisms underlying high‐temperature tolerance and can provide agronomic traits and genetic diversity useful for breeding.3
Increases in the average temperatures and UV radiation can have a significant impact on plant growth and, consequently, on crop yield and fruit quality. Indeed, different biochemical mechanisms, including plastid biogenesis and pigments/secondary metabolites synthesis, can be activated in plant cells in response to abiotic stresses.1, 4, 5, 6, 7 Since thermo‐tolerance requires the modulation of biochemical pathways involved in reactive oxygen species (ROS) detoxification, antioxidant compounds, including carotenoids, ascorbic acid (AsA) and polyphenols, are accumulated in response to heat stress.8, 9 Carotenoids can also contribute to membranes fluidity and permeability in response to temperature fluctuations.5, 6 Indeed, these molecules can exert a protective role in photosynthetic membranes and play important roles in structural stabilization, light harvesting, and photoprotection.5 The increase in the biosynthesis of polyphenolic compounds and flavonoids has also been reported following the exposure of many plant species to UV radiation, making their accumulation one of the most evolutionarily conserved responses to this type of abiotic stress.10, 11
Polyphenolic compounds, AsA, and carotenoids are also relevant for human health, since antioxidant and anti‐inflammatory properties have been described following their in vitro or in vivo administration.12, 13, 14 In particular, phenolic compounds may have therapeutic roles in inflammation‐based diseases and various type of cancers.15 Furthermore, ascorbic acid shows significant antioxidant and electron donor capability and is able to protect DNA from oxidation‐induced damage.15, 16 Finally, it has been demonstrated that carotenoids possess an apoptotic‐inducing effect in cancer cells and reduce oxidized low‐density lipoprotein cholesterol levels.17 The health‐promoting properties of these bioactive compounds contribute to the nutritional value of crops such as tomato and represent a parameter of preference in consumer choice of food products. Indeed, some of these bioactive compounds, such as AsA and carotenoids, cannot be synthesized by animals and thus have to be introduced with the diet.18 For these reasons, the daily consumption of plant‐derived food enriched in these compounds has been highly promoted in the last decade.
In this study, we analyzed the performances, in terms of final yield and fruit quality on a set of ten tomato landraces showing good performances when grown under elevated temperatures. Their productivity and field performances under restrictive environmental conditions were estimated. Moreover, the bioactive compounds content and antioxidants/anti‐inflammatory properties have been evaluated in extracts from ripe fruits from different tomato landraces, with the final aim of identifying the best ones in terms of both yield and nutritional fruit quality. Altogether, this study highlights the possibility to select tomato landraces combining desirable agronomic and nutritional traits, such as good yield performances and high nutritionally active phytonutrients content, for future applications in breeding programs.
MATERIALS AND METHODS
Plant materials
Plant material consisted of ten tomato indeterminate landraces (listed in Table 1) and the control variety ‘Moneymaker’. In 2017 they were grown in Apulia (Pulsano, 40° 23′ 03″ N latitude, 17° 21′ 17″ E longitude), a region of southern Italy greatly devoted to tomato cultivation and usually characterized by high temperatures during the growing season. Seeds were sown in plateau under a plastic‐house in April, and seedlings were then transplanted in open field in June. Plants were distributed following a complete randomized block design, with three replicate plots per landrace and ten plants per replicate. For fruit quality and nutritional traits analyses, ten fruits at the red ripe stage from at least three plants from each replicate plot were collected at the same time and pooled. Some traits were evaluated on fresh fruit, and others on fruit frozen in liquid nitrogen and stored at −80 °C until analysis. For yield determination, fruits at red ripe stage were collected on the same day from all the plants for each replicate plot. Total fruit number and fresh weight (FW) were measured to allow yield evaluation per plant.
Table 1.
List of the plant materials tested for production, quality, and nutritional traits under high temperature
| Genotype | Source | Accession no. | Common name | Country of origin | Collection site | Product destination | Fruit size | Fruit shape |
|---|---|---|---|---|---|---|---|---|
| E7 | CRA‐ORTa | Corbarino PC04 | Italy | Nocera (Salerno) | Processing | Small (25–30 g) | Ovate | |
| E8 | CRA‐ORTa | Corbarino PC05 | Italy | Sant'Antonio Abate (Salerno) | Processing | Small (20–25 g) | Elliptic | |
| E17 | CRA‐ORTa | Pantano Romanesco | Italy | Fondi (Latina) | Fresh market | Big (200–250 g) | Flattened | |
| E30 | CRA‐ORTa | Sel PC07 | Italy | Pagani (Salerno) | Processing | Small (15–20 g) | Ovate | |
| E32 | CRA‐ORTa | Sel PC16 | Italy | Nocera (Salerno) | Fresh market | Small (20–50 g) | Ovate | |
| E36 | Campania Regiona | Vesuvio Foglia Riccia | Italy | S. Vito (Naples) | Fresh market/processing | Small (25–30 g) | Ovate | |
| E53 | TGRC | LA0147 | — | Honduras | Tegucigalpa mercado | Fresh market | Medium (80–100 g) | Oblate |
| E76 | TGRC | LA4449 | Black plum | URSS | — | Processing | Small (20–25 g) | Ovate |
| E94 | NPGS | PI272890 | 1404 | Guatemala | Quetzaltenango, Guatemala | nd | Small (40–50 g) | Irregular |
| PDVIT | ARCA2010a | Caramella | Italy | Scafati (Salerno) | Fresh market/processing | Small (10–15 g) | Elliptic | |
| ‘Moneymaker’ | TGRC | LA2706 | Moneymaker | Great Britain | — | Fresh market | Medium (50–60 g) | Circular |
Germplasm collections maintained at Italian public institution.
Quality traits evaluation
Physicochemical traits were evaluated on fresh fruit. The determination of pH was carried out by using a pH meter (Mettler‐Toledo, Milan, Italy), and the total acidity was determined by titrating 10 mL of tomato juice with a solution of 0.1 mol L−1 sodium hydroxide. A few drops of tomato juice were also used to estimate the total soluble solids (degrees Brix) by adding them to the prism plate of a refractometer (Hanna Instruments). Finally, the firmness was measured by a penetrometer (PCE‐PTR200 penetrometer, Capannori, Italy) using an 8 mm tip. All extracts were from three biological replicates, and three technical assays were carried out on each biological repetition.
Extraction and detection of polyphenols
Whole tomato fruits were cut and frozen in liquid nitrogen, freeze‐dried, and finely ground. Samples of powder (200 mg) were extracted twice in methanol:water 80:20 (v/v). The extracts were centrifuged, and the supernatants were combined, filtered through a 0.22 μm filter, and stored at −20 °C until use. Polyphenols were detected at 320 nm by reversed‐phase high‐performance liquid chromatography with diode array detector (RP‐HPLC DAD) (Agilent 1100 HPLC system). Separation was performed on a C18 column (5 UltraSphere, 80 Å pore, 25 mm), with a linear gradient from 20% to 60% acetonitrile, in 55 min, with a flow of 1 mL min−1 at 25 °C. Concentrations were obtained by referring to calibration curves, and results were expressed in micrograms per gram or milligrams per gram (in the case of rutin and chlorogenic acid) of dried weight.
Carotenoids content
Freeze‐dried tomato powder (50 mg) was added to 2 mL of 60% potassium hydroxide, 2 mL of absolute ethanol, 1 mL of 1% sodium chloride (NaCl), 5 mL of 0.05% butylated hydroxytoluene in acetone. The mix was incubated at 60 °C for 30 min. A 1% solution of NaCl (15 mL) was added to the mix, and extractions were performed with 15 mL hexane:ethyl acetate 9:1 (v/v). Extracts were centrifuged, evaporated using a rotary evaporator, and collected in 1 mL of ethyl acetate. Analyses were performed using RP‐HPLC DAD (Agilent 1100 HPLC system) according to the method previously described.19
AsA determination
AsA determinations were carried out by a colorimetric method with modifications reported by Rigano et al.20 Briefly, 500 mg of frozen powder from tomato fruits was extracted with 300 μL of 6% trichloroacetic acid (TCA). The mixture was vortexed, incubated on ice for 15 min, and centrifuged at 15 700×g for 20 min. To 20 μL of supernatant were added 20 μL of 0.4 mol L−1 phosphate buffer (pH 7.4), 10 μL of double‐distilled water, and 80 μL of color reagent solution. This last solution was prepared by mixing solution A (31% (w/v) phosphoric acid, 4.6% (w/v) TCA, and 0.6% (w/v) ferric chloride) with solution B (4% (w/v) 2,2′‐dipyridyl). These mixtures were incubated at 37 °C for 40 min and measured at 525 nm using a UV–visible spectrophotometer (NanoPhotometer™; Implen). All extracts were from three biological replicates, and three technical assays were carried out on each biological repetition. The concentration was expressed in micrograms per gram FW.
Determination of antioxidant activity
2,2′‐Azino‐bis(3‐ethylbenzothiazoline‐6‐sulfonic acid) diammonium salt (ABTS; Sigma‐Aldrich) radical cations were prepared by mixing an aqueous solution of 2.45 mmol L−1 potassium persulfate (final concentration) with an aqueous solution of 7 mmol L−1 ABTS (final concentration) and allowed to stand in the dark at room temperature for 12–16 h before use. The ABTS radical cation solution was diluted in phosphate‐buffered saline (PBS; pH 7.4) to an absorbance of 0.40 at 734 nm. Trolox was used to prepare a standard calibration curve (0–16 μmol L−1). After the addition of 200 μL of diluted ABTS to 10 μL of Trolox standard or extracts diluted in PBS, in each well of a 96‐well plate (Costar), the absorbance was read at 734 nm 6 min after initial mixing using an Infinite200Pro plate reader (Tecan). All extracts were from three biological replicates, and three technical assays were carried out on each biological repetition. The percentage absorbance inhibition at 734 nm was calculated as a function of concentration of Trolox, and the Trolox equivalent antioxidant capacity (TEAC) value was expressed as Trolox equivalent (TE, μmol) using Magellan v7.2 software.
Culture of dendritic cells and enzyme‐linked immunosorbent assay
Bone‐marrow‐derived dendritic cells (BMDCs) were obtained from C57BL/6 mice, in agreement with national and international guidelines, approved by the authors' institutional review board (Organism for Animal Wellbeing – OPBA).
BMDCs were harvested as previously described19 and plated in Roswell Park Memorial Institute 1640 medium supplemented with fetal bovine serum, antibiotics, recombinant mouse granulocyte macrophage stimulating factor and recombinant mouse interleukin (IL)‐4 at 37° C in a humidified 5% carbon dioxide atmosphere. BMDCs were treated with tomato methanol extracts (100 mg lyophilized powder per milliliter, 1:25 final dilution), after administration of lipopolysaccharide (LPS; 1 μg mL−1) at day 8 for 24 h. BMDCs culture media were analyzed for IL‐6 and IL‐12 in triplicate using an enzyme‐linked immunosorbent assay (ELISA) kit as described by the manufacturer (R&D Systems).
Data analysis
Values are expressed as mean plus/minus standard deviation (SD). Group differences were analyzed and compared by paired two‐tailed Student's t‐tests. Yield differences among the genotypes analyzed were determined using SPSS Package 6, version 15.0. Significant different yields were determined by comparing mean values through a factorial analysis of variance with Duncan post hoc test at a significance level of 0.05. Spearman correlations were calculated to analyze co‐occurrence and associations among all traits measured. The P‐values obtained for multiple tests were corrected using the Benjamini and Hochberg false discovery rate (FDR). In order to identify the landraces with a desirable combination of traits, an evaluation index (EI) was estimated by assigning a score to each trait, which was a maximum of 11 to a minimum of 1 descending from the highest to the lowest value for all traits except for pH, IL‐6, and IL‐12, where the maximum score (11) was assigned to the lower value and the minimum score (1) to the highest value.
RESULTS AND DISCUSSION
Agronomic performances of tomato landraces under harsh temperature conditions
A group of tomato landraces was previously selected for yield performances under high temperatures in two regions of southern Italy, Campania and Apulia, in 2016.21 In the present study, we decided to test them again under high temperatures in an open field in the Apulia region in 2017. Plants were transplanted with a 1 month delay with respect to the usual agronomical practice of the area, exposing them to higher temperatures during the critical stages of flowering and fruit setting. Figure S1 reports the values of mean, maximum, and minimum temperatures recorded over the growing season, together with the average relative humidity of the whole day. As shown, more than 40 days (38.5% of the whole growing season, from the end of May to the beginning of September) reached temperatures over 32 °C, which is considered the critical temperature affecting the reproductive stage of tomato, as well as of other species.21 On 16 days (15.4%), a temperature higher than 35° C was even recorded. In addition, very high temperatures were also recorded in the night, and this is considered a critical point for pollen maturation.22
Based on the evaluation of production per plant (Table 2), four genotypes were classified as good (E8, E32, E36, ‘Moneymaker’), five as medium (E7, E17, E53, E76, PDVIT), and two as low producers (E30, E94) under high‐temperature conditions. Indeed, the first group exhibited a yield per plant higher than 4 kg, the second group yield ranged from 2 to 4 kg per plant, whereas the third group produced less than 2 kg per plant. As a whole, yield performances confirmed previous data and allowed a reliable classification of the genotypes in the aforementioned three groups.
Table 2.
Yield (kg) per plant measured on ten landraces and the control cv Moneymaker during 2017
| Genotype | Yield (kg)/plant | Production level |
|---|---|---|
| E7 | 3.43 ± 0.57 abc | Medium |
| E8 | 4.15 ± 1.12 c | High |
| E17 | 2.71 ± 0.29 ab | Medium |
| E30 | 1.70 ± 039 a | Low |
| E32 | 4.93 ± 0.83 c | High |
| E36 | 5.67 ± 0.58 d | High |
| E53 | 3.67 ± 0.25 bc | Medium |
| E76 | 2.59 ± 0.41 ab | Medium |
| E94 | 1.67 ± 0.71 a | Low |
| PDVIT | 2.98 ± 0.51 abc | Medium |
| ‘Moneymaker’ | 4.85 ± 0.60 c | High |
Following Duncan's post hoc test, the level of production under high temperature was also reported. Values followed by the same letters are not significantly different.
Assessment of main quality parameters
With the aim of identifying those landraces simultaneously exhibiting good productivity and good nutritional quality under high temperatures, and thus worth proposing as resilient varieties, we evaluated the main quality parameters of the ten landraces. As already reported in a previous study,23 we chose the indeterminate variety ‘Moneymaker’ as a control genotype, considering that it generally exhibits stable yields and fruit quality traits under different environmental conditions and in different years. Concerning the main fruit quality traits, the pH values ranged from 4.3 (landraces PDVIT and E76) to 4.6 (E17), with a mean value of 4.4, whereas the titratable acidity ranged from 0.38 g to 0.55 g of citric acid per 100 mL of tomato juice in E36 and E76 respectively (Table 3). The soluble solid content varied from 3.6 °Bx (E7) to 8.1 °Bx (E8). Finally, E94 showed the lowest level of firmness (8.7 kg cm−2) and PDVIT the maximum (18.9 kg cm−2). Noteworthy is that the three landraces E8, E30, and E76 showed high degrees Brix levels (>7), and two of these (E30 and E76) also had a titratable acidity value higher than 0.5. Altogether, these traits affect consumer taste, who generally prefer firm, sweet, and acid tomatoes,24 and consequently the commercial value of tomatoes.
Table 3.
Qualitative traits (mean and standard error) parameters measured on red ripe fruit. Statistical analysis was carried out by comparing values with those recorded in the cv Moneymaker (M)
| Genotype | pH | Soluble solid content (°Bx) | Titratable acidity (g citric acid/100 mL juice) | Firmness (kg cm−2) |
|---|---|---|---|---|
| E7 | 4.45 ± 0.05 | 3.62 + 0.26** | 0.43 + 0.01 | 14.31 + 1.11 |
| E8 | 4.41 ± 0.02 | 8.13 ± 0.01** | 0.46 + 0.01 | 15.16 + 0.12 |
| E17 | 4.65 + 0.01* | 4.29 + 0.17** | 0.49 + 0,02 | 14.02 + 2.10 |
| E30 | 4.42 + 0.04 | 7.88 + 0.21** | 0.53 + 0.04 | 13.21 + 1.34 |
| E32 | 4.49 + 0.02 | 6.58 + 0.24 | 0.39 + 0.01 | 15.03 + 0.18 |
| E36 | 4.40 + 0.05 | 3.60 + 0.45** | 0.38 + 0.03 | 14.05 + 2.51 |
| E53 | 4.40 + 0.04 | 5.92 + 0.20 | 0.47 + 0.02 | 11.17 + 1.45 |
| E76 | 4.32 + 0.02* | 7.74 + 0.26* | 0.55 + 0.03* | 10.91 + 0.98* |
| E94 | 4.36 + 0.04 | 6.78 + 0.12 | 0.48 + 0.03 | 8.68 + 0.01** |
| PDVIT | 4.31 + 0.06 | 5.92 + 0.01 | 0.50 + 0.02 | 18.90 + 1.50 |
| ‘Moneymaker’ | 4.47 + 0.04 | 6.17 + 0.27 | 0.43 + 0.02 | 15.18 + 1.05 |
*P < 0.05, **P < 0.01 (Student's t‐test).
In recent years, more attention has been paid to the nutritional value of food products, focusing on the beneficial effects of fresh fruit and vegetables. To verify if the selected landraces could also provide a good nutritional value when grown under high temperatures, the main tomato phytonutrients contents, such as AsA, polyphenols, and carotenoids, were determined. These compounds have been associated to health benefits and the reduction of inflammatory and aging‐related diseases.25, 26, 27 Therefore, the dietary intake of these compounds is highly recommended.
The AsA levels in the red ripe fruit of the ten tomato landraces and in the control cv Moneymaker are reported in Fig. 1. Most landraces exhibited values of approximately 350 μg g−1 FW, which was statistically different from the value recorded in ‘Moneymaker’ (232 μg g−1 FW), whereas two of them (E30 and E94) reached a mean value higher than 400 μg g−1 FW. The landrace PDVIT was the best performer, with a mean value over 500 μg g−1 FW. These values are in line with the values previously reported for tomato genotypes, where AsA ranged from 100 to 880 μg g−1 FW, even though commercial cultivars are generally characterized by lower contents (from 100 to 400 μg g−1 FW), probably due to the breeding process.28 However, the AsA content in fresh tomato fruits is also dependent on genotype, climatic conditions, fruit development, and maturation.16
Figure 1.
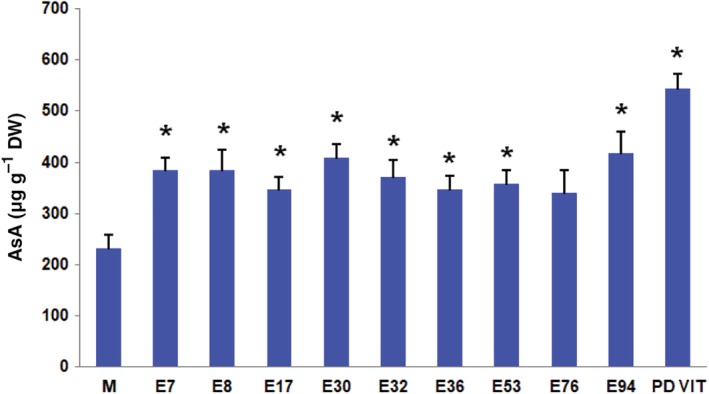
AsA content in the red ripe fruit of the ten tomato landraces and the control variety ‘Moneymaker’. Data are showed as mean ± SD (n = 3). *P < 0.05, **P < 0.01 (Student's t‐test).
Carotenoids accumulation has been reported following abiotic stresses, such as high or low temperature and high light, upon which the homeostasis of ROS metabolism is challenged. For these reasons, carotenoids are implicated in the thermo‐tolerance of different plant species, including tomato.2, 29 Therefore, herein, we analyzed the carotenoids content in the fruits of the tomato landraces considered here. Concerning the main carotenoids (i.e. lycopene, β‐carotene, and lutein), our data showed significantly higher levels for these compounds in E30, E36, and E94 compared with cv Moneymaker (Fig. 2). Noteworthy is that a higher β‐carotene content compared with the control cultivar was also evidenced in PDVIT. This compound is considered a provitamin, which can be converted into retinol, a phytochemical essential for vision. β‐Carotene is also known to act as a strong antioxidant and is the best quencher of singlet oxygen.16
Figure 2.
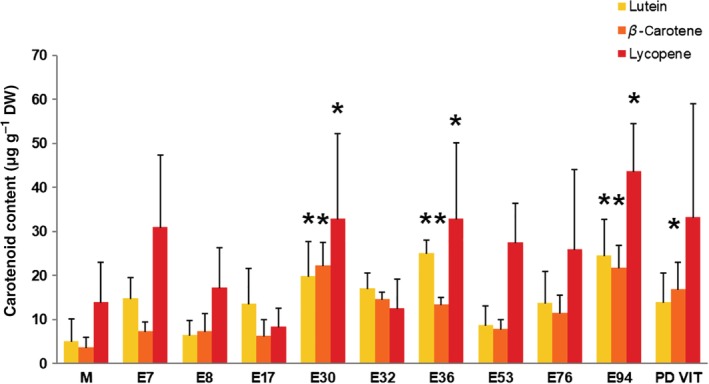
Content of the main carotenoids in tomato fruits of different landraces and the cv Moneymaker (M). Analyses were carried out by HPLC on fruit extracts. Statistical analysis was carried out by comparing the content of each compound with that recorded in the cv Moneymaker (M). Data are mean plus/minus SD (n = 3). *P < 0.05 (Student's t‐test).
The β‐carotene/lycopene ratio (Fig. S2) showed a different trend among the landraces tested, with higher levels recorded in E17, E30, and E32 (0.76, 0.68, and 1.16 respectively) compared with cv Moneymaker (0.27) and other landraces. The differences in the β‐carotene/lycopene ratio can be attributable to a possible different genetic background of the landraces tested, which might affect either the β‐carotene or lycopene biosynthesis. Regarding this point, further molecular analyses are needed to better elucidate possible genetic variations, which could explain the differences in the amounts of these phytochemicals. In addition, harsh environmental conditions, such as high temperature, can also impact lycopene accumulation in tomato fruits.30, 31, 32 Brandt et al.,30 for example, reported decreased lycopene biosynthesis under high temperatures in the tomato F1 variety ‘Lemance’.
Flavonoids, such as quercetin‐3‐rutinoside (rutin), and hydroxycinnamic acids (such as chlorogenic, ferulic, and caffeic acids) are the most representative phenolic compounds of tomatoes.33, 34 These compounds are characterized by the presence of phenolics rings and hydroxyl groups in their structure that can scavenge free radicals,35, 36 thus inhibiting the generation of ROS. In this study, no significant differences were detected in the amount of chlorogenic acid among the ten landraces and the commercial cv Moneymaker (Fig. 3). However, significantly higher rutin levels were detected in E36, E94, and PDVIT, compared with the control cultivar (Fig. 3). A lower rutin level was instead detected in E32. In the majority of the landraces analyzed in this study, higher levels of kaempferol‐O‐rutinoside, one of the most common derivatives of kaempferol in tomato fruit, were detected in E7, E17, E30, E32, E36, E94, and PDVIT in comparison with ‘Moneymaker’ (Fig. 4). A higher kaempferol‐O‐glucoside content was also detected in E94. Low levels of other phenolic compounds, such as naringenin, were overall detected in the majority of landraces (Fig. 4).
Figure 3.
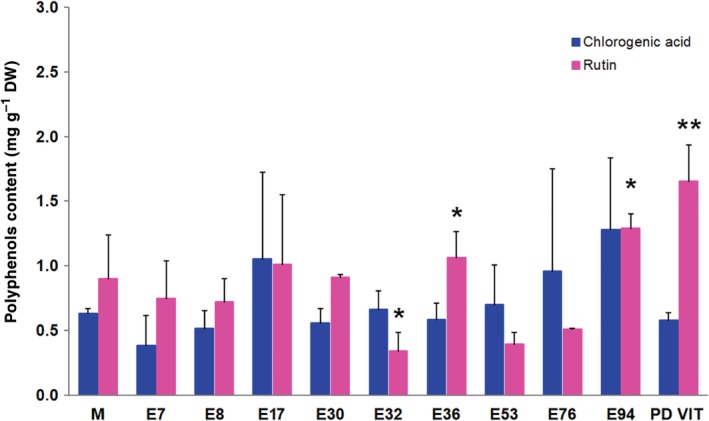
Chlorogenic acid and rutin content in fruits of different landraces and the cv Moneymaker (M). Quantification was carried out by HPLC using fruit methanolic extracts. Statistical analysis was carried out by comparing the content of each compound with that recorded in the cv Moneymaker (M). Data are mean plus/minus SD (n = 3). *P < 0.05, **P < 0.01 (Student's t‐test).
Figure 4.
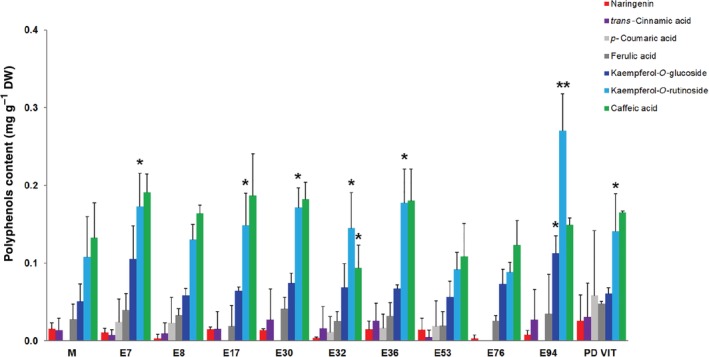
Polyphenols content in tomato fruits from different landraces and the cv Moneymaker (M). Quantification was carried out by HPLC using methanolic extracts of fruits. Statistical analysis was carried out by comparing the content of each compound with that recorded in the cv Moneymaker (M). Data are mean plus/minus SD (n = 3). *P < 0.05, **P < 0.01 (Student's t‐test).
The higher polyphenols levels detected in some of the tomato landraces analyzed could also be explained as a stronger response to abiotic stresses, including heat stress and exposure to UV radiation. Indeed, changes in the polyphenols content have already been reported in tomato following UV exposure.37 In fact, genes implicated in polyphenol biosynthesis are activated by light exposure, and a ‘sunscreen’ function has been proposed for these phytochemicals to protect the tissues from possible damage generated by UV radiation.10 Some authors reported that high temperatures and light exposure stimulate the production of phenolic acids and other flavonoids.38, 39 Indeed, heat stress positively modulates the activity of the enzyme phenylalanine ammonia‐lyase and affects the total phenols content by activating their biosynthesis and inhibiting their oxidation in tomato plants.38, 39
Antioxidant/anti‐inflammatory properties of tomato landraces
The antioxidant capacity of methanol extracts from fruits of the ten tomato landraces is reported in Fig. 5(a), using the TEAC assay. Previous studies reported that rutin and phenolic acids can contribute to the overall antioxidant capacity of tomato, neutralizing free radicals by acting as electron donors or inhibiting the activity of enzymes involved in the production of free radicals.39 Our results indicated a slight, not significant, higher antioxidant activity in E32, followed by E17 and E30. Conversely, a significant lower antioxidant activity was evidenced in E53 and E76, which also showed a lower polyphenols content (see Fig. 4). E76 also showed a lower AsA content than in the other landraces. Indeed, it is well known that the antioxidant property of a food matrix is dependent on the presence and levels of different compounds comprising phenolic species and AsA.38
Figure 5.
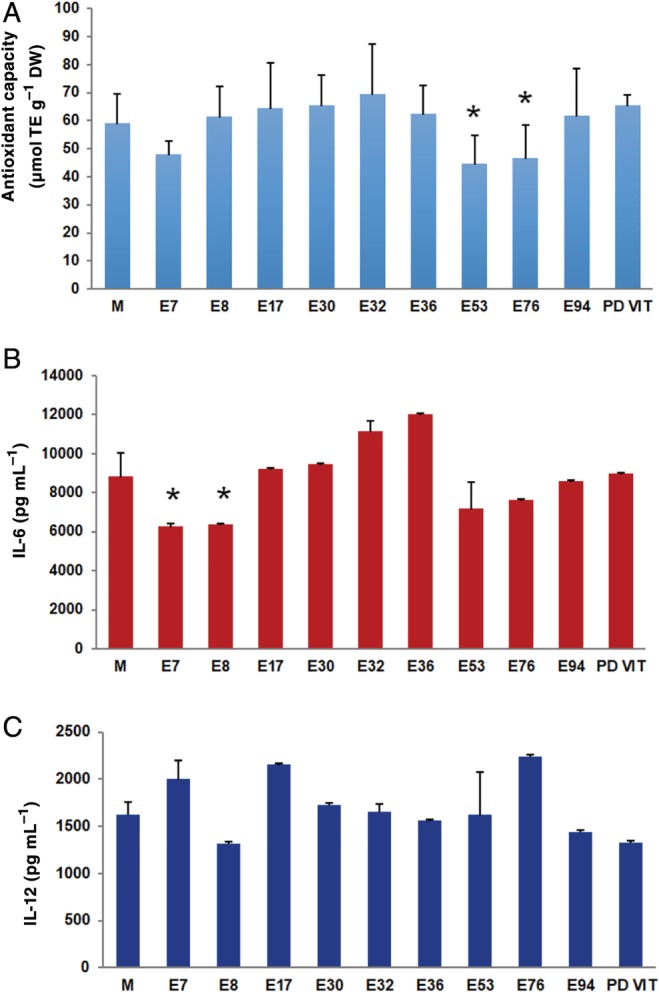
Antioxidant and anti‐inflammatory activities of tomato extracts from different landraces. (a) Antioxidant capacity profiles measured by TEAC. Data are mean μmol TE ± SD (n = 3), *P < 0.05 (Student's t‐test). Levels of pro‐inflammatory (b) IL‐6 and (c) IL‐12 in BMDCs stimulated with LPS and treated with methanolic tomato extracts from fruits of different tomato landraces. Concentrations of cytokines were determined by ELISA test. Data are expressed as mean plus/minus SD (n = 3); *P < 0.05 (Student's t‐test).
To assess the anti‐inflammatory potential and the ability to trigger an in vitro immune‐modulating response, tomato extracts were incubated with murine dendritic cells. LPS was used as an inflammatory stimulant. Figure 5(b) shows the effects of the administration of tomato extracts on the production of pro‐inflammatory IL‐6 and IL‐12. Significantly lower levels of IL‐6 were detected in the presence of E7 and E8 extracts. Both these landraces showed increased levels of AsA, which could be related to the decreased levels of the pro‐inflammatory activity of IL‐6. E8, together with E94 and PDVIT extracts, was also able to decrease the secretion of the pro‐inflammatory IL‐12 (Fig. 5(c)) even though the differences observed were not significant. Some studies have reported the reduction of pro‐inflammatory interleukins in dendritic cells mediated by the aglycone quercetin, thus demonstrating an anti‐inflammatory activity for this phytochemical.40, 41, 42 In this context, rutin, which is the most abundant phenolic compound in our quantification, could be responsible, at least in part, for the anti‐inflammatory activity detected in the tomato extracts. However, our data did not establish a clear correlation between the content in phenolic compounds and the anti‐inflammatory activity in all the landraces tested. Indeed, the role of flavonoids has been generally described using chemically pure standards, only partially reflecting the real anti‐inflammatory activity that can be exerted by a whole fruit or vegetable extracts.43
Combining yield and quality parameters
To examine the possible co‐occurrence of both yield and quality features in the landraces tested, we calculated Spearman correlations considering all the traits included in this study (Fig. 6(a)). Positive correlations, significant in some cases, were found among the secondary metabolites analyzed (AsA, carotenoids, phenolic acids, and flavonoids), indicating that the observed changes within the plant secondary metabolism involve the cross‐talk among different classes of phytochemicals and thus different biochemical pathways. Furthermore, some of these metabolites positively correlated to physicochemical parameters such as acidity, degrees Brix, and fruit firmness, indicating an association between secondary metabolites and traits influencing fruit organoleptic properties.
Figure 6.
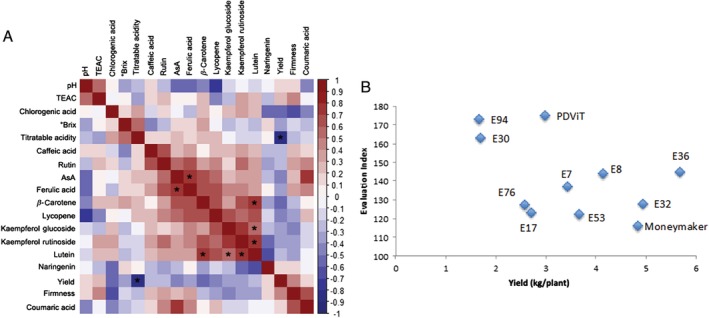
Correlation analysis and EI. (a) Spearman correlation analysis of the measured traits in the tested landraces. The correlation values range from (−1.00) (blue) to 1.00 (red); * FDR‐adjusted P‐values (q < 0.05). (b) Scatter diagram of the ten landraces and the control genotype ‘Moneymaker’ according to their EI and yield per plant production.
An estimated EI was also calculated considering the quality and nutritional traits analyzed all together. EI varied from a minimum of 123 for E17 to a maximum of 175 for PDVIT, with a mean value of 141.2. The distribution of the ten landraces according their EI value and yield is shown in Fig. 6(b). From this analysis, it is evident that three landraces (E30, E94, PDVIT) exhibit better performances in terms of fruit quality (EI > 160), though two of them (E30 and E94) showed low yield (less than 2 kg per plant) under high temperatures. All the better performing landraces in terms of yield (classified as high or medium producers), with the exception of PDVIT, exhibited lower EI levels, thus evidencing lower values of quality traits.
CONCLUSION
In this paper, one landrace (PDVIT) was selected as a good compromise between yield performances and good fruit quality and nutritional traits when growing under high temperature. Additional molecular and physiological analyses in other environments are in progress in order to further characterize this selected landrace. Indeed, this landrace shows a level of adaptability that can be useful in adverse conditions, making it a suitable candidate for breeding programs, since it can be considered as a ‘balanced landrace’44 in terms of stress resilience and fruit nutritional quality.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Figure S1. (a) Temperature and (b) relative humidity (RH) variation during the 2017 growing season in Apulia region. Each day maximum, mean and minimum temperatures were recorded (a). The mean relative humidity is also reported (b).
Figure S2. Ratio β‐carotene/lycopene in the tomato landraces analyzed in the study.
ACKNOWLEDGEMENTS
This work was in part founded by the European Union's Horizon 2020 research and innovation program through the TomGEM project under grant agreement no. 679796 and by the Apulia region through the SICURA project (KC3U5Y1). We acknowledge Mr Leone D'Amico for technical assistance.
REFERENCES
- 1. Bita CE and Gerats T, Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress‐tolerant crops. Front Plant Sci 4:273 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cameyo D, Rodriguez P, Angeles Morales M, Dell'Amico JM, Torrecillas A and Alarcon JJ, High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J Plant Physiol 162:281–289 (2005). [DOI] [PubMed] [Google Scholar]
- 3. Driedonks N, Wolters‐Arts M, Huber H, de Boer G‐J, Vriezen W, Mariani C et al, Exploring the natural variation for reproductive thermotolerance in wild tomato species. Euphytica 214:67 (2018). [Google Scholar]
- 4. Sun T, Yuan H, Cao H, Yazdani M, Tamdor Y and Li L, Carotenoid metabolism in plants: the role of plastids. Mol Plant 11:58–74 (2018). [DOI] [PubMed] [Google Scholar]
- 5. Spicher L, Glauser G and Kessler F, Lipid antioxidant and galactolipid remodeling under temperature stress in tomato plants. Front Plant Sci 7:167 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nisar N, Li L, Lu S, Khin NC and Pogson BJ, Carotenoid metabolism in plants. Mol Plant 8:68–82 (2015). [DOI] [PubMed] [Google Scholar]
- 7. Zhang R, Wise RR, Struck KR and Sharkey TD, Moderate heat stress of Arabidopsis thaliana leaves causes chloroplast swelling and plastoglobule formation. Photosynth Res 105:123–134 (2010). [DOI] [PubMed] [Google Scholar]
- 8. Mazzeo MF, Cacace G, Iovieno P, Massarelli I, Grillo S and Siciliano RA, Response mechanisms induced by exposure to high temperature in anthers from thermo‐tolerant and thermo‐sensitive tomato plants: a proteomic perspective. PLoS One 13:e0201027 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agati G, Azzarello E, Pollastri S and Tattini M, Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 196:67–76 (2012). [DOI] [PubMed] [Google Scholar]
- 10. Tohge T and Fernie AR, Leveraging natural variance towards enhanced understanding of phytochemical sunscreens. Trends Plant Sci 2:308–315 (2017). [DOI] [PubMed] [Google Scholar]
- 11. Mouradov A and Spangenberg G, Flavonoids: a metabolic network mediating plants adaptation to their real estate. Front Plant Sci 5:620 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cory H, Passarelli S, Szeto J, Tamez M and Mattei J, The role of polyphenols in human health and food systems: a mini‐review. Front Nutr 5:87 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carr AC. and Vissers MMC. eds, Vitamin C and Human Health, 1st ednNutrients, Special Issue. MDPI, Basel: (2014). [Google Scholar]
- 14. Yao LH, Jiang YM, Tomás‐Barberán FA, Datta N, Singanusong R and Chen SS, Flavonoids in food and their health benefits. Plant Foods Hum Nutr 59:113–122 (2004). [DOI] [PubMed] [Google Scholar]
- 15. Sacco A, Raiola A, Calafiore R, Barone A and Rigano MM, New insights in the control of antioxidants accumulation in tomato by transcriptomic analyses of genotypes exhibiting contrasting levels of fruit metabolites. BMC Genomics 20:43 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raiola A, Rigano MM, Calafiore R, Frusciante L and Barone A, Enhancing the health‐promoting effects of tomato fruit for biofortified food. Mediators Inflamm 2014:139873 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Del Giudice R, Raiola A, Tenore GC, Frusciante L, Barone A, Monti DM et al, Antioxidant bioactive compounds in tomato fruits at different ripening stages and their effects on normal and cancer cells. J Funct Foods 18:83–94 (2015). [Google Scholar]
- 18. Petruk G, Raiola A, Del Giudice R, Barone A, Frusciante L, Rigano MM et al, An ascorbic acid‐enriched tomato genotype to fight UVA‐induced oxidative stress in normal human keratinocytes. J Photochem Photobiol B 163:284–289 (2016). [DOI] [PubMed] [Google Scholar]
- 19. Scarano A, Butelli E, De Santis S, Cavalcanti E, Hill L, De Angelis M et al, Combined dietary anthocyanins, flavonols, and stilbenoids alleviate inflammatory bowel disease symptoms in mice. Front Nutr 4:75 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rigano MM, Lionetti V, Raiola A, Bellincampi D and Barone A, Pectic enzymes as potential enhancers of ascorbic acid production through the d‐galacturonate pathway in Solanaceae. Plant Sci 266:55–63 (2018). [DOI] [PubMed] [Google Scholar]
- 21. Ruggieri V, Calafiore R, Schettini C, Rigano MM, Olivieri F, Frusciante L et al, Exploiting genetic and genomic resources to enhance heat‐tolerance in tomatoes. Agronomy 9:22 (2019). [Google Scholar]
- 22. Huckstadt AB, Suthaparan A, Mortensen LM and Gislerod HR, The effect of low night and high day temperatures on photosynthesis in tomato. Am J Plant Sci 4:2323–2331 (2013). [Google Scholar]
- 23. Massaretto IL, Albaladejo I, Purgatto E, Flores FB, Plasencia F, Egea‐Fernandez JM et al, Recovering tomato landraces to simultaneously improve fruit yield and nutritional quality against salt stress. Front Plant Sci 9:1778 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Causse M, Friguet C, Coiret C, Lepicier M, Navez B, Lee M et al, Consumer preferences for fresh tomato at the European scale: a common segmentation on taste and firmness. J Food Sci 75:S531–S541 (2010). [DOI] [PubMed] [Google Scholar]
- 25. Rao AV and Rao LG, Carotenoids and human health. Pharmacol Res 55:207–216 (2007). [DOI] [PubMed] [Google Scholar]
- 26. Romier B, Schneider YJ, Larondelle Y and During A, Dietary polyphenols can modulate the intestinal inflammatory response. Nutr Rev 67:363–378 (2009). [DOI] [PubMed] [Google Scholar]
- 27. Scarano A, Chieppa M and Santino A, Looking at flavonoid biodiversity in horticultural crops: a colored mine with nutritional benefits. Plants 7:98 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruggieri V, Bostan H, Barone A, Frusciante L and Chiusano ML, Integrated bioinformatics to decipher the ascorbic acid metabolic network in tomato. Plant Mol Biol 91:397–412 (2016). [DOI] [PubMed] [Google Scholar]
- 29. Borghesi E, González‐Miret ML, Escudero‐Gilete ML, Malorgio F, Heredia FJ and Melendez‐Martinez AJ, Effects of salinity stress on carotenoids, anthocyanins, and color of diverse tomato genotypes. J Agric Food Chem 59:11676–11682 (2011). [DOI] [PubMed] [Google Scholar]
- 30. Brandt S, Zoltán P, Barna É, Lugasi A and Helyes L, Lycopene content and colour of ripening tomatoes as affected by environmental conditions. J Sci Food Agric 86:568–572 (2006). [Google Scholar]
- 31. Helyes L, Lugasi A and Pék Z, Effect of natural light on surface temperature and lycopene content of vine ripened tomato fruit. Can J Plant Sci 87:927–929 (2007). [Google Scholar]
- 32. Lokesha AN, Shivashankara KS, Laxman RH, Geetha GA and Shankar AG, Effect of high temperature on fruit quality parameters of contrasting tomato genotypes. Int J Curr Microbiol Appl Sci 8:1019–1029 (2019). [Google Scholar]
- 33. Slimestad R, Fossen T and Verheul MJ, The flavonoids of tomatoes. J Agric Food Chem 56:2436–2441 (2008). [DOI] [PubMed] [Google Scholar]
- 34. Luo J, Butelli E, Hill L, Parr A, Niggeweg R, Bailey P et al, AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: expression in fruit results in very high levels of both types of polyphenol. Plant J 56:316–326 (2008). [DOI] [PubMed] [Google Scholar]
- 35. Sroka Z and Cisowski W, Hydrogen peroxide scavenging, antioxidant and anti‐radical activity of some phenolic acids. Food Chem Toxicol 41:753–758 (2003). [DOI] [PubMed] [Google Scholar]
- 36. Scarano A and Santino A, The plant polyphenol metabolism as functional architecture and its nutritional exploitation. Nutr Food Sci Int J 8:26–30 (2019). [Google Scholar]
- 37. Luthria D, Mukhopadhyay S and Krizek DT, Content of total phenolics and phenolic acids in tomato (Lycopersicon esculentum Mill.) fruits as influenced by cultivar and solar UV radiation. J Food Compos Anal 19:771–777 (2006). [Google Scholar]
- 38. Toor RK, Geoffrey PS and Lister CE, Antioxidant activities of New Zealand‐grown tomatoes. Int J Food Sci Nutr 56:597–605 (2005). 10.1080/09637480500490400. [DOI] [PubMed] [Google Scholar]
- 39. Silva‐Beltrán NP, Ruiz‐Cruz S, Cira‐Chávez LA, Estrada‐Alvarado MI, Ornelas‐Paz JJ, Lopez‐Mata MA et al, Total phenolic, flavonoid, tomatine, and tomatidine contents and antioxidant and antimicrobial activities of extracts of tomato plant. Int J Anal Chem 2015:284071 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cavalcanti E, Vadrucci E, Delvecchio FR, Addabbo F, Bettini S, Liou R et al, Administration of reconstituted polyphenol oil bodies efficiently suppresses dendritic cell inflammatory pathways and acute intestinal inflammation. PLoS One 9:e88898 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Delvecchio FR, Vadrucci E, Cavalcanti E, De Santis S, Kunde D, Vacca M et al, Polyphenol administration impairs T‐cell proliferation by imprinting a distinct dendritic cell maturation profile. Eur J Immunol 45:2638–2649 (2015). [DOI] [PubMed] [Google Scholar]
- 42. Galleggiante V, De Santis S, Cavalcanti E, Scarano A, De Benedictis M, Serino G et al, Dendritic cells modulate iron homeostasis and inflammatory abilities following quercetin exposure. Curr Pharm Des 23:2139–2146 (2017). [DOI] [PubMed] [Google Scholar]
- 43. Liu RH, Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr 78:517S–520S (2003). [DOI] [PubMed] [Google Scholar]
- 44. Moreira‐Ascarrunz SD, Larsson H, Pietro‐Linde ML and Johansson E, Mineral nutritional yield and nutrient density of locally adapted wheat genotypes under organic production. Foods 5:E89 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (a) Temperature and (b) relative humidity (RH) variation during the 2017 growing season in Apulia region. Each day maximum, mean and minimum temperatures were recorded (a). The mean relative humidity is also reported (b).
Figure S2. Ratio β‐carotene/lycopene in the tomato landraces analyzed in the study.


