Abstract
The term gut-liver axis is used to highlight the close anatomical and functional relationship between the intestine and the liver. It has been increasingly recognized that the gut-liver axis plays an essential role in the development and progression of liver disease. In particular, in non-alcoholic fatty liver disease (NAFLD) and alcohol-related liver disease (ALD), the two most common causes of chronic liver disease, a dysbiotic gut microbiota can influence intestinal permeability allowing some pathogens or bacteria-derived factors from the gut reaching the liver via the enterohepatic circulation contributing to liver injury, steatohepatitis and fibrosis progression. Pathways involved are multiple, including changes in bile acid metabolism, intestinal ethanol production, generation of short-chain fatty acids, and other by-products. Bile acids act through dedicated bile acid receptors farnesoid X receptor and TGR5 in both the ileum and the liver, influencing lipid metabolism, inflammation, and fibrogenesis. Currently, both NAFLD and ALD lack of effective therapies and therapeutic targeting of gut microbiota and bile acids enterohepatic circulation hold promise. In this review, we summarize current knowledge about the role of gut microbiota in the pathogenesis of NAFLD and ALD, as well as the relevance of microbiota or bile acid-based approaches in the management of those liver diseases.
Keywords: gut-liver axis, cirrhosis, NAFLD, ALD, alcohol, fatty liver, steatosis, microbiota, translocation, bile acids
INTRODUCTION
The term gut-liver axis highlights the increasingly recognized crosstalk between both organs that are strictly anatomically and functionally related (1-3). The gut and the liver communicate through the biliary tract, the portal vein, and systemic circulation exchanging a myriad of signaling compounds. On the one hand, the liver secretes bile acids and other bioactive mediators and releases them into the biliary tract reaching the intestine, and on the other hand, the intestine signals back to the liver secreting enterokines from the terminal ileum. In addition, in the distal gut, microbiota, which is mainly composed of bacteria and other microbial components such as fungi, have many functional roles in health and disease (such as digestion, vitamins production, resistance to colonization by pathogenic bacteria, and stimulation of the immune system) (4). Gut microbiota composition is modified by diet, alcohol consumption, and medications (antibiotics, probiotics, proton pump inhibitors, etc.) (5, 6). Also, microbiota metabolizes bile acids and amino acids, which are transported to the splanchnic blood vessels to reach the liver. The liver receives 75% of its blood supply through the portal vein, which it comes from the gut carrying both nutrients and microbial products exposing the liver to a multiple types of antigens. Despite the highly specialized intestinal epithelial barrier, some bacteria-derived molecules will enter the enterohepatic circulation reaching the liver and acting on both parenchymal and non-parenchymal cells (7).
It has been increasingly recognized that the gut-liver axis plays critical roles in the pathogenesis and progression of the most common causes of liver disease worldwide, non-alcoholic fatty liver disease (NAFLD), and alcohol-related liver disease (ALD). Gut-liver axis-related events facilitating development and progression of liver disease in both NAFLD and ALD include mainly the occurrence of intestinal dysbiosis, defined as the imbalance between microbial communities leading to disruption of the symbiotic relationship between gut resident microbes and the host, and increased intestinal permeability leading to a pro-inflammatory state (3). The molecular underpinnings of how these phenomena modulate liver disease are still incompletely understood, but significant advances have been made in recent years (8, 9). Dysbiosis and the alteration of the intestinal barrier have been described to act as a disease-drivers in NAFLD and ALD by influencing liver injury (i.e. promoting steatosis, inflammation, and fibrosis) through the modulation of the immune system by multiple mechanisms (10).
The hepatic immune system must balance its responses differentiating between harmless stimuli and dangerous bacterial pathogens, preventing them from reaching the systemic circulation (7, 11). If the latter fails, the subsequent proinflammatory response from pathogen-derived substances may promote the development and progression of chronic liver disease (12). Hence, in order to maintain the homeostasis, a complex interaction must be established between the gut epithelia, the microbiota, the immune system, and the liver. When an imbalance occurs, microbial products translocation drives disease progression. The present review aims to summarize the current knowledge about the role that gut microbiota plays in liver disease, especially in NAFLD and ALD.
Intestinal permeability and microbiota
The highly specialized intestinal epithelial barrier allows the transport of nutrients, but, at the same time, protects against microbial-derived products and pathogens (13). This barrier is composed of a mucus layer that capture bacteria and large molecules avoiding them to reach the epithelium (14); a monolayer of epithelial cells that actively limit the transit of hydrophilic molecules; and finally, the intercellular tight junctions (claudins, occludins, and zonula occludens) that maintain closed the space between cells controlling the passage across the intestinal mucosa. It is known that alcohol, in particular acetaldehyde (a byproduct of the intestinal metabolism of alcohol), can disrupt the intestinal barrier by impairing the integrity and expression of the intercellular tight junctions leading to translocation and endotoxemia (15-18). Alcohol also induces changes in the expression of zonula occludens-1 and claudin-1, impairing the epithelial barrier function (19, 20), similar to the effect produced by pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and interferon-gamma (21, 22).
Dysbiosis and bacterial translocation due to disruption of the intestinal epithelial barrier in patients with advanced chronic liver disease is detrimental in natural history and can lead to serious infections (23). This chronic activation of the immune system by bacterial products perpetuates liver injury and inflammation (24). The immune system identifies bacterial products through recognition of specific pathogen-associated molecular patterns (PAMPs), which are a limited and defined set of conserved molecular patterns carried by all microorganisms of a given class (25), such as lipopolysaccharide (LPS) from gram-negative bacteria. Intraperitoneal LPS administration has shown to increase portal pressure (26-28) and influence intestinal permeability (29, 30). The liver has anti-inflammatory mechanisms to maintain homeostasis and immunotolerance, such as the hepatic antigen-presenting cells that drives the tolerogenic adaptative response (11).
When microbial products reach the liver through the portal vein, activation of membrane-bound Toll-like receptors (TLRs) and the cytoplasmic nucleotide-binding oligomerization domain-like receptors (NLRs) present in both parenchymal and non-parenchymal cells occur. TLRs recognize PAMPs and DAMPs (damage-associated molecular patterns) and trigger the innate immune system activation (i.e. macrophages and dendritic cells) (31, 32), but also activation of hepatic stellate cells and endothelial cells that will amplify the inflammatory and fibrotic response (24, 33). Downstream TLRs activate NF-κB (34), which is constitutively expressed in all cell types and has a pivotal role in the regulation of the inflammatory response in the liver (inducing the release of pro-inflammatory cytokines such as TNFα, IL-6, and IL-1β) and it is known to drive the pathogenetic process in many liver diseases (35-37). Activation of TLRs leads to sterile inflammation and plays a role in the pathogenesis of the non-alcoholic and alcoholic liver disease (31, 38-42).
In patients with cirrhosis, impaired intestinal barrier function leads to microbial products to reach the liver triggering a pro-inflammatory response (43, 44). This has been particularly described in the pathogenesis of alcohol-related liver disease (45, 46), end-stage liver disease, and acute-on-chronic liver failure (ACLF) (47). In advanced cirrhosis, with worsening portal hypertension, there is a dysfunction of the intestinal tight junctions, as well as, intestinal bacterial overgrowth, and changes in microbiota, favoring bacterial translocation (48). Small intestine bacterial overgrowth (SIBO) also occurs in cirrhotic patients (49) by multiple mechanisms including decreased bile acid secretion and impaired intestinal motility (autonomic neuropathy, inflammatory mediators, dysbiosis, and neuropeptides) (50, 51). Of note, SIBO is commonly seen in patients with cirrhosis with or without intestinal motility dysfunction (52). Cirrhotic patients have a decrease in the beneficial normal microbiota (Lactobacillus, Bifidobacterium, and Bacteroides species), which it can exacerbate liver disease (53). Additionally, there is an increase in Proteobacteria (particularly Enterobacteriaceae), Fusobacterium spp., Veillonellaceae, and Streptococcaceae, which are potentially pathogenic agents, responsible of most cases of spontaneous bacterial peritonitis (54-57).
In order to target gut microbiota in liver disease, non-absorbable disaccharides, such as lactulose and lactitol have been used. However, despite its widespread use, no studies have clearly shown that lactulose leads to significant changes to microbiota composition or function (58). The proposed mechanisms of action of lactulose are: laxative, prebiotic, acidifying and modifying the colonic flora (59, 60). A recent trial assessed the effects of single-dose lactulose ingestion on the growth of intrinsic Escherichia coli. The authors concluded that the ingestion of a single dose of 50 g lactulose does not significantly alter E. coli density in stool samples of healthy volunteers, however, this dose seems unlikely to be sufficient to alter alter gut microbiota (61). Gut microbiota changes after the use of rifaximin has been also evaluated (62, 63). An elegant study by Bajaj et al. (64) showed that cirrhotic patients under rifaximin treatment despite having a slight change in microbiota composition, have less endotoxemia and an improvement in cognition. In the same study, rifaximin changed bile acid composition. Recently, a randomized, double-blind, placebo-controlled trial in 54 patients with cirrhosis and ascites showed no effect on hemodynamics (hepatic venous pressure gradient or systemic hemodynamics) (65).
Bile acids and enterohepatic circulation
Bile acids (BAs) are amphipathic steroid molecules synthesized in the liver from cholesterol and excreted into bile as one of its main components. BAs (amino-acyl-conjugates of the primary BAs, cholic acid [CA] and chenodeoxycholic acid [CDCA], and their secondary metabolites) are actively secreted by the hepatocyte into the canaliculus where they serve as the main driving force for bile production by specific transporters (i.e., bile salt export pump, BSEP)(66). Once in the small intestine, BAs function aiding in the emulsification and absorption of dietary fat, cholesterol, and fat-soluble vitamins. After reaching the terminal ileum, BAs are efficiently absorbed (95% recapture) by an active uptake mechanism mediated by the apical sodium bile acid transporter (Asbt). BAs loss in feces are approximately 0.2-0.6 g/day, which is balanced by the daily hepatic synthesis of BAs. In the gut, the primary BAs, CA and CDCA, undergo deconjugation and dehydroxylation by microbiota, resulting in the formation of secondary BAs (i.e., deoxycholic acid [DCA] and lithocholic acid [LCA]) (67). These secondary BAs can be reabsorbed passively and constitute a portion of the total BA pool that cycles in the enterohepatic circulation, a system of exchange between the gut and the liver. (68). As a result of their efficient hepatic extraction, the concentration of BAs in the systemic circulation and peripheral tissues is extremely low, with only small incremental rises in postprandial periods (69). For decades BA were considered just detergents helping digestion of ingested food, but in last decades BA have emerged as relevant signaling molecules that may act at both hepatic and extrahepatic tissues to regulate both lipid and carbohydrate metabolism as well as energy homeostasis (67, 70). These actions are exercised through activation or modulation of BA receptors, such as the farnesoid X receptor (FXR; also known as NR1H4) and G protein-coupled bile acid receptor 1 (GPBAR1; also known as TGR5), and may be influenced by changes in abundance or activity of BA transporters, such as the Asbt, the sodium-dependent taurocholate polypeptide (NTCP) or the export pump BSEP (66, 67, 71). Bile acids activate FXR in the ileum and liver, leading to the production of fibroblast growth factor 19 (FGF19; FGF15 in mouse). FGF19 is an endocrine, gastrointestinal hormone that suppresses the hepatocyte expression of CYP7A1, a rate-limiting enzyme in the synthesis of BAs, thereby creating a negative feedback loop. Activation of FXR and TGR5 may affect both steatotic and inflammatory responses and therefore influence NAFLD and ALD pathogenesis at multiple levels (72, 73). FGF19 has shown to regulate glucose homeostasis, body weight and alcohol consumption at central nervous system level (74, 75). Of note, dysregulated BA levels have also been found in patients with severe AH (76). Additionally, BAs bind to TGR5 on the plasma membrane and act on tissues beyond enterohepatic circulation. This binding mediates host energy expenditure (77, 78), glucose homeostasis (79), and anti-inflammatory immune responses (80, 81).
There is a close, and the bidirectional interplay between BA metabolism and the gut microbiota and cholestasis may alter intestinal bacterial populations (3, 82). Changes in BA pool composition have been found in ALD patients suggesting that FXR activation may be decreased (83). The role of gut microbiota in controlling BA pool composition has also been recognized as BAs, and gut microbiota have a reciprocal relationship (84-86). Indeed, on the one hand, BAs shape the intestinal microbiome through direct antimicrobial effects and FXR-induced production of antimicrobial peptides and in the other hand gut microbiota modify the BA pool composition through defined enzymatic activities (such as deconjugation, dihydroxylation, oxidation, and epimerization, among others) (87, 88). Additionally, FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation (89). In the setting of NAFLD and ALD, both altered BA metabolism and changes in microbiota composition have been found, which potentially promotes disease development (83, 90-94). Recent studies have explored the effects of ursodeoxycholic acid (UDCA) on gut microbiome composition in healthy subjects and also in individuals with liver dysfunction (95, 96). Interestingly, UDCA influenced bacterial populations inducing a marked decrease in abundance of Bifidobacterium, Lactobacillus, and Lactobacillaceae (95). If these effects have any relevance for the therapeutic action of UDCA, remain to be determined. One interesting recent study showed that the absence of the intestinal microbiota results in exacerbation of liver injury in a murine model of primary sclerosing cholangitis (PSC), the mdr2−/− mice (97). This genetically engineered mouse is deficient in the canalicular transporter of phospholipid and has very low levels of biliary phosphatidylcholine, which results in biliary injury. The biliary alterations of this experimental model are similar to those observed in PSC (98). In the study by Tabibian et al. (97), germ-free mdr2−/− mice exhibited significantly worse liver chemistry and histological lesions than conventionally housed mice underscoring the importance of commensal microbiota in protecting against biliary damage. Furthermore, few studies have analyzed the gut microbiome in cholestatic diseases (99, 100). Of note, a significant reduction of within-individual microbial diversity has been found in primary biliary cholangitis (PBC) (101), which is partially relieved by UDCA administration. Similarly, reduced diversity and significant shifts in the microbiome composition have been found in stool samples from PSC patients (102) but is unclear the relationship to the bile secretory failure present in cholestatic diseases. Furthermore, oral microbiota correlates with gut microbiota, and oral dysbiosis influences liver disease (103-108). Collectively, these findings suggest that an imbalance in BAs and gut microbiota elicits a cascade of host immune responses relevant to the progression of liver diseases.
Microbiota and ALD
Gut microbiota modulates ALD, however, the exact mechanisms are not fully understood (67, 109-111). Ethanol is absorbed in the stomach (20%) and small intestine (70%) by simple diffusion (112, 113). The largest portion of ethanol in the intestine comes from the systemic circulation, although microbial fermentation also contributes to luminal ethanol concentration (114).
ALD is characterized by increased levels (both luminal and circulating) of ethanol and its metabolites (115, 116). These high levels promote leaky gut with translocation of bacterial products, triggering inflammatory and adaptative host immune responses. Gut microbiota and enterocytes metabolize alcohol through enzymes such as alcohol dehydrogenase into byproducts like acetaldehyde (117, 118). Once alcohol reaches the liver is also metabolized, and the liver can upregulate its metabolic pathways to adapt to higher concentrations (118, 119).
When there is chronic and high alcohol consumption, ALD can develop. ALD is a consequence of multiple environmental (diet, viral hepatitis, etc.), genetic/epigenetic, immune, and microbiome factors interaction (120-122). Similar to what occurs in NAFLD, the early stage of ALD is characterized by the accumulation of fat within the liver (steatosis), and it can progress to more advanced forms of liver disease with inflammation and liver injury (alcoholic steatohepatitis [ASH]).
During recent years, many studies at the preclinical and experimental level have shed light on the relationship between ALD and gut microbiota. Dysbiosis and SIBO have been demonstrated as relevant disease factors in both human (123, 124) and mouse models (122, 125). Microbiota in subjects with ALD is characterized by marked enrichment of Enterobacteriaceae and reduction of Bacteroidetes and Lactobacillus (56, 125, 126). This phenomenon of dysbiosis is only partially reversible by alcohol withdrawal or probiotic therapy (56, 127). The presence of SIBO has been shown to significantly correlate with a higher prevalence of spontaneous bacterial peritonitis and with the severity of alcohol-related cirrhosis (128). These changes in the gut microbiota of ALD patients seem to be accompanied by changes in colonic pH and liver steatosis (129). It also correlates with a higher level of serum endotoxin and increased intestinal TNF-α levels, as well as increased levels of nitric oxide, IL-6, and IL-8 (2). Another recent discovery is that patients with ALD not only have bacterial dysbiosis but also display reduced fungal diversity as well as Candida overgrowth (130-133). Indeed, using antifungal agents in mouse models have shown to decrease β-glucan translocation and ameliorate alcohol-induced liver injury produced via the C--type lectin domain family 7 member A receptor on hepatic Kupffer cells (130). In the same line, since microbiota has been shown to be a relevant disease driver in ALD, fecal microbiota transplantation (FMT) has been explored as a therapeutic option for ALD (122, 134). Philips et al. demonstrated an improvement in 1-year survival rate in FMT-treated patients compared to historical controls (87.5% vs. 33.3%). The FMT was given daily for 7 consecutive days in 8 patients (134). However, larger and carefully designed trials are needed before FMT can be considered safe in routine clinical practice for managing ALD. Careful donor selection is recommended considering the risk of transmission of drug-resistant bacteria (135).
Preclinical studies using animal models of ALD have advanced our knowledge regarding the role of microbiota in the pathogenesis and progression of the disease. Using TLR4 chimeric mice, it has been shown that endotoxin--induced release of TGFβ is mediated by an MYD88–NF-κB-dependent pathway, providing an explanatory mechanism for endotoxin-induced liver inflammation (136). Other studies have used Reg3b/g KO or Muc2-deficient mice to show that REG3 lectins protect against alcohol-induced liver injury by reducing mucosa-associated microbiota, thereby preventing translocation of viable bacteria (137, 138). Moreover, IgA KO mice led to increased levels of IgM and overall protection against alcohol-induced liver injury (139). Recently, Duan Y et al. described that the presence of cytolysin-positive E. faecalis correlated with the severity of liver disease and with mortality in patients with AH. Furthermore, using humanized mice that were colonized with bacteria obtained from feces of patients with AH, they investigated the therapeutic effects of bacteriophages that target cytolytic E. faecalis. The authors found that bacteriophages decreased cytolysin in the liver and abolish ethanol-induced liver disease in humanized mice. These findings link cytolytic E. faecalis with more severe clinical outcomes and increased mortality in patients with AH, and it can specifically be targeted by bacteriophage againts cytolytic E. faecalis (140).
Besides bacterial product translocation and immunological responses, it has been recognized the role of bile acids as signaling compounds. Alcohol leads to an increase in bile acid biosynthesis in both humans and mice (141, 142). Of note, clinically, as in other chronic liver diseases, mild cholestasis is common in patients with ALD (143). Bile acids activate FXR in the ileum; impaired FXR activation has been associated with more alcohol-induced liver injury (144). Currently, multiple FXR agonists are being tested, and initial results have shown a protective effect against alcoholic steatohepatitis (145). A recent preclinical study showed that obeticholic acid (OCA), INT-767, or INT-777 (BA derivatives with selective agonist properties for FXR, TGR5, or both, respectively) administration are effective in reducing acute and chronic ethanol-induced steatosis and inflammation in mice, with varying degrees of efficacy depending on the duration of ethanol administration, indicating that both FXR and TGR5 activation can protect from liver injury in ALD models (146). Additionally, it has been shown that the modulation of the intestinal BA/FXR/FGF15 axis improves ALD in mice by modulation of hepatic Cyp7a1 and lipid metabolism (92). Concordantly, Lactobacillus rhamnosus GG showed to prevent liver fibrosis through inhibiting hepatic bile acid synthesis and enhancing bile acid excretion in mice (92). Lactobacillus rhamnosus GG supplementation decreased hepatic BA by increasing intestinal FXR/FGF15 signaling pathway-mediated suppression of BA de novo synthesis and enhanced BA excretion, which prevents excessive BA-induced liver injury and fibrosis in mice (92).
Microbiota and NAFLD
Non-alcoholic fatty liver disease (NAFLD) is an umbrella term used to describe a clinicopathological entity defined by the presence of a spectrum of hepatic histological changes that range from simple steatosis, steatohepatitis to cirrhosis (147). The hepatic histological findings in NAFLD are similar to those observed in heavy-drinkers but detected in patients that deny significant alcohol consumption and in whom other known causes of chronic liver disease (i.e., viral hepatitis, autoimmune liver disease or exposure to hepatotoxic drugs) are excluded (148). The histological hallmark of NAFLD is steatosis, which refers to the pathological accumulation of fat in the liver predominantly in the form of triglycerides, although several additional lipid species accumulate inside hepatocytes in this setting. Hepatic steatosis may or may not be accompanied by the presence of necro-inflammatory changes (i.e., cellular ballooning) and various degrees of hepatic fibrosis. When the latter features are present, the term non-alcoholic steatohepatitis (NASH) is used (149). NAFLD is commonly associated with overweight or obesity as well as impaired glucose tolerance, type 2 diabetes mellitus, arterial hypertension, and hypertriglyceridemia (148). For this reason, NAFLD is widely considered as the hepatic manifestation of the metabolic syndrome and is thought to be mainly driven by the occurrence of insulin resistance (148, 150).
In recent years, it has become evident that NAFLD pathophysiology is complex and involves diverse immunological and metabolic pathways. Importantly, these pathways can influence disease phenotype in diverse fashion, thus determining disease heterogeneity (151, 152). Several studies have highlighted the role of the gut microbiota in NAFLD (153-155). Pre-clinical studies have shown that germ-free mice are protected against obesity and hepatic steatosis (156). Despite the large number of preclinical data investigating and demonstrating a relationship between microbiota and NAFLD, only a limited number of human studies, mostly cross-sectional, are available with variable results. NAFLD patients have a higher prevalence of dysbiosis with increased Bacteroides, Escherichia, and Ruminococcus and decreased Prevotella bacteria (115, 157) in those with advanced forms of the disease indicating an association between Gram-negative bacteria and progression of liver fibrosis (108). Additionally, fecal-microbiome-derived signatures associated with fibrotic NASH and NAFLD-related cirrhosis has been described (158, 159). Also, a significant association between NAFLD and SIBO has been established (111, 160, 161), Studies using mouse models have shown that impairment of intestinal permeability, achieved by using junctional adhesion molecule A protein (Jam1)-knockout mice or mice deficient in Muc2, leads to increased liver inflammation when the high-fat diet is administrated (138, 162). The role of translocated bacterial products has also been assessed by using inflammasome-deficient mouse models (Nlrp3 KO or Nlrp6 KO). NLRP3 inflammasome pathway is important in the modulation of microbiota in the intestine. Defective NLRP3 inflammasome pathway results in dysbiosis with an increased translocation of endotoxins, accumulation of PAMPs in the portal circulation, and, thereby, promoting liver inflammation and NASH progression (10).
A growing number of studies confirm the association between NAFLD and microbiota at both the observational and mechanistic levels. Microbiota from adult subjects with NAFLD exhibits differences in carbon and amino acid metabolism (157). Also, they have increased serum TMAO and hepatic bile acid synthesis (123) and decreased the production of phosphatidylcholine (163). Interestingly, a recent elegant paper by Yuan J. et al. showed that high-alcohol-producing Klebsiella pneumoniae was associated with up to 60% of individuals with NAFLD in a Chinese cohort. (164). These results suggest that, at least in some cases of NAFLD, an alteration in the gut microbiome drives the condition due to excess endogenous alcohol production, highlighting the link between NAFLD and ALD, and the pivotal role of microbiota (133, 165). Gut microbiota is also altered in subjects with hepatocellular carcinoma-associated NASH. Clostridium, Escherichia, and Helicobacter species have been found to be enriched in mouse models of NASH-related hepatocellular carcinoma as well as in humans with this neoplasia (166-171).
The role various bacterial metabolites and microbiota-generated secondary BA in NAFLD pathophysiology has been unveiled in recent years (165). These substances may affect a myriad of signaling pathways that may directly influence metabolic dysfunction and contribute to NAFLD development and progression. Importantly, gut microbiota can modulate BA metabolism contributing to diversification of host BA, thus regulating BA-dependent pathways mediated by dedicated BA receptors such as FXR and TGR5 (67, 172). In addition, similar to ALD, early in the disease, subjects with NAFLD may exhibit impaired BA secretion resulting in increased intracellular BA concentrations (173). Thus, BA retention and changes in BA metabolism might have a role as mediators of liver injury and triggers of inflammation, promoting disease progression (67, 174) including HCC development (90). On these grounds, therapeutic approaches based in the activation or modulation of FXR and TGR5, as well as of specific BA transporters, such as the ileal apical sodium-dependent bile acid transporter, has been explored in NAFLD/NASH and hold promise (67). Also, modulation of gut microbiota using probiotics, prebiotics, symbiotics of FMT could have impact on NAFLD/NASH through their effects in BA metabolism among other mechanisms (175). Given the shared histological features of AH and NASH, BA dependent targets investigated in NASH could be tested in AH (176), for example, the already available NGM282 (a non-tumorigenic variant of FGF19 analogue) and tropifexor (non-steroidal FXR agonist). Currently, an FXR agonist is already available and under study as a therapeutic agent for severe AH (TREAT, NCT02039219 on ClinicalTrials.gov). Further preclinical and clinical studies are needed to advance our knowledge about the relationship between gut microbiota and NAFLD.
Conclusions
It has been increasingly recognized that the gut-liver axis plays an important role in the development and progression of liver disease, where bacterial products and bile acids reach the liver through the portal circulation and modulate liver injury (Figure 1). NAFLD and ALD are the two most common causes of liver disease, and in both effective therapies are urgently needed. Gut-liver axis signaling pathways such as BA-related pathways and microbiota-related mechanisms (i.e., dysbiosis and endogenous ethanol production are attractive candidates for new targeted therapies.
Figure 1:
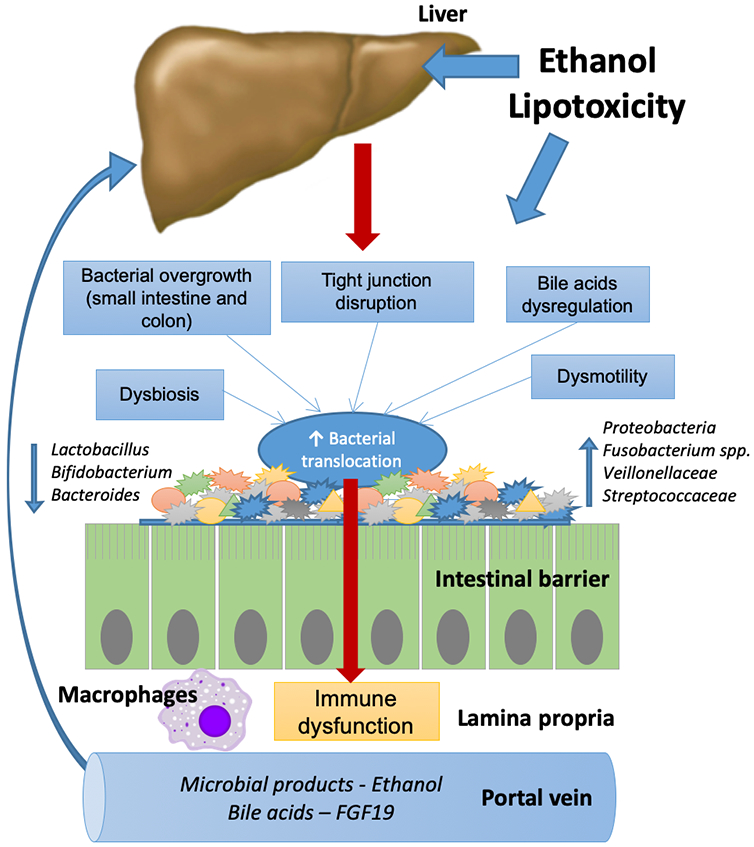
Gut-liver axis in NAFLD and ALD: In response to ethanol or diet, bacterial overgrowth, dysbiosis, impaired intestinal permeability, bile acids dysregulation, and dysmotility promote bacterial translocation from the intestinal lumen to the portal vein. Microbial products and ethanol can reach the liver contributing to liver disease.
Acknowledgments:
This work was supported by grant(s) NIH DK59615 and AA021171 (VHS), the Clinical Core of the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30DK084567). This article was partially supported by the Chilean government through the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT 1200227 to JPA and 1119145 to MA) and the Comisión Nacional de Investigación Científica y Tecnológica (grant CONICYT PIA/Basal PFB12, Basal Centre for Excellence in Science and Technology to MA).
Abbreviations used in this paper:
- ACLF
acute-on-chronic liver failure
- ALD
alcohol-related liver disease
- Asbt
apical sodium bile acid transporter
- ASH
alcoholic steatohepatitis
- BAs
bile acids
- BSEP
bile salt export pump
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- DAMPs
damage-associated molecular patterns
- DCA
deoxycholic acid
- FGF19
fibroblast growth factor 19
- FMT
fecal microbiota transplantation
- FXR
farnesoid X receptor
- NAFLD
non-alcoholic liver disease
- NASH
non-alcoholic steatohepatitis
- NLR
nucleotide-binding oligomerization domain-like receptors
- NTCP
sodium-dependent taurocholate polypeptide
- LCA
lithocholic acid
- LPS
lipopolysaccharide
- OCA
obeticholic acid
- PAMPs
pathogen-associated molecular patterns
- PBC
primary biliary cholangitis
- PSC
primary sclerosing cholangitis
- SIBO
small intestine bacterial overgrowth
- TNF-α
tumor necrosis factor-α
- TLRs
toll-like receptors
- UDCA
ursodeoxycholic acid
Footnotes
Conflict of Interest: The authors have nothing to disclose.
REFERENCES
- 1.Albillos A, Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2019. [DOI] [PubMed] [Google Scholar]
- 2.Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15(7):397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arab JP, Martin-Mateos RM, Shah VH. Gut-liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatol Int. 2018;12(Suppl 1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biedermann L, Rogler G. The intestinal microbiota: its role in health and disease. European journal of pediatrics. 2015;174(2):151–67. [DOI] [PubMed] [Google Scholar]
- 5.Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vassallo G, Mirijello A, Ferrulli A, Antonelli M, Landolfi R, Gasbarrini A, et al. Review article: Alcohol and gut microbiota - the possible role of gut microbiota modulation in the treatment of alcoholic liver disease. Alimentary pharmacology & therapeutics. 2015;41(10):917–27. [DOI] [PubMed] [Google Scholar]
- 7.Koch M Gut Microbiota and the Liver: A Tale of 2 Cities: A Narrative View in 2 Acts. Journal of clinical gastroenterology. 2016;50 Suppl 2, Proceedings from the 8th Probiotics, Prebiotics & New Foods for Microbiota and Human Health meeting held in Rome, Italy on September 13-15, 2015:S183–S7. [DOI] [PubMed] [Google Scholar]
- 8.Tilg H, Zmora N, Adolph TE, Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol. 2019. [DOI] [PubMed] [Google Scholar]
- 9.Bajaj JS. Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16(4):235–46. [DOI] [PubMed] [Google Scholar]
- 10.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crispe IN. Immune tolerance in liver disease. Hepatology. 2014;60(6):2109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pradere JP, Troeger JS, Dapito DH, Mencin AA, Schwabe RF. Toll-like receptor 4 and hepatic fibrogenesis. Seminars in liver disease. 2010;30(3):232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annual review of pathology. 2010;5:119–44. [DOI] [PubMed] [Google Scholar]
- 14.Johansson ME, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L, Xu H, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63(2):281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1(8370):179–82. [DOI] [PubMed] [Google Scholar]
- 16.Draper LR, Gyure LA, Hall JG, Robertson D. Effect of alcohol on the integrity of the intestinal epithelium. Gut. 1983;24(5):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. Journal of hepatology. 2000;32(5):742–7. [DOI] [PubMed] [Google Scholar]
- 18.Dunagan M, Chaudhry K, Samak G, Rao RK. Acetaldehyde disrupts tight junctions in Caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2012;303(12):G1356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Tong J, Chang B, Wang B, Zhang D, Wang B. Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction-associated proteins. Molecular medicine reports. 2014;9(6):2352–6. [DOI] [PubMed] [Google Scholar]
- 20.Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, et al. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcoholism, clinical and experimental research. 2008;32(2):355–64. [DOI] [PubMed] [Google Scholar]
- 21.Al-Sadi R, Ye D, Boivin M, Guo S, Hashimi M, Ereifej L, et al. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PloS one. 2014;9(3):e85345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen P, Starkel P, Turner JR, Ho SB, Schnabl B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology. 2015;61(3):883–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. Journal of hepatology. 2014;60(6):1310–24. [DOI] [PubMed] [Google Scholar]
- 24.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48(1):322–35. [DOI] [PubMed] [Google Scholar]
- 25.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. International reviews of immunology. 2011;30(1):16–34. [DOI] [PubMed] [Google Scholar]
- 26.Steib CJ, Hartmann AC, v Hesler C, Benesic A, Hennenberg M, Bilzer M, et al. Intraperitoneal LPS amplifies portal hypertension in rat liver fibrosis. Laboratory investigation; a journal of technical methods and pathology. 2010;90(7):1024–32. [DOI] [PubMed] [Google Scholar]
- 27.Tazi KA, Moreau R, Herve P, Dauvergne A, Cazals-Hatem D, Bert F, et al. Norfloxacin reduces aortic NO synthases and proinflammatory cytokine up-regulation in cirrhotic rats: role of Akt signaling. Gastroenterology. 2005;129(1):303–14. [DOI] [PubMed] [Google Scholar]
- 28.Wiest R, Das S, Cadelina G, Garcia-Tsao G, Milstien S, Groszmann RJ. Bacterial translocation in cirrhotic rats stimulates eNOS-derived NO production and impairs mesenteric vascular contractility. The Journal of clinical investigation. 1999;104(9):1223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiva M, Guarner C, Peralta C, Llovet T, Gomez G, Soriano G, et al. Intestinal mucosal oxidative damage and bacterial translocation in cirrhotic rats. European journal of gastroenterology & hepatology. 2003;15(2):145–50. [DOI] [PubMed] [Google Scholar]
- 30.Clements WD, Erwin P, McCaigue MD, Halliday I, Barclay GR, Rowlands BJ. Conclusive evidence of endotoxaemia in biliary obstruction. Gut. 1998;42(2):293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo J, Friedman SL. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis & tissue repair. 2010;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrasek J, Mandrekar P, Szabo G. Toll-like receptors in the pathogenesis of alcoholic liver disease. Gastroenterology research and practice. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crispe IN. The liver as a lymphoid organ. Annual review of immunology. 2009;27:147–63. [DOI] [PubMed] [Google Scholar]
- 34.Verstak B, Nagpal K, Bottomley SP, Golenbock DT, Hertzog PJ, Mansell A. MyD88 adapter-like (Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and TLR4-mediated NF-kappaB proinflammatory responses. The Journal of biological chemistry. 2009;284(36):24192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakraborty JB, Mann DA. NF-kappaB signalling: embracing complexity to achieve translation. J Hepatol. 2010;52(2):285–91. [DOI] [PubMed] [Google Scholar]
- 36.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. [DOI] [PubMed] [Google Scholar]
- 37.Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate Immunity and Inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61(5):1294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nature reviews Immunology. 2010;10(12):826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutterwala FS, Ogura Y, Flavell RA. The inflammasome in pathogen recognition and inflammation. J Leukoc Biol. 2007;82(2):259–64. [DOI] [PubMed] [Google Scholar]
- 40.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–65. [DOI] [PubMed] [Google Scholar]
- 41.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–26. [DOI] [PubMed] [Google Scholar]
- 42.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20(3):319–25. [DOI] [PubMed] [Google Scholar]
- 43.Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. Journal of immunology. 2009;183(2):1320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Seminars in liver disease. 2009;29(2):141–54. [DOI] [PubMed] [Google Scholar]
- 45.Szabo G, Mandrekar P, Petrasek J, Catalano D. The unfolding web of innate immune dysregulation in alcoholic liver injury. Alcoholism, clinical and experimental research. 2011;35(5):782–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szabo G Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148(1):30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. Journal of hepatology. 2015;63(5):1272–84. [DOI] [PubMed] [Google Scholar]
- 48.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41(3):422–33. [DOI] [PubMed] [Google Scholar]
- 49.Bauer TM, Schwacha H, Steinbruckner B, Brinkmann FE, Ditzen AK, Aponte JJ, et al. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. The American journal of gastroenterology. 2002;97(9):2364–70. [DOI] [PubMed] [Google Scholar]
- 50.Raedsch R, Stiehl A, Gundert-Remy U, Walker S, Sieg A, Czygan P, et al. Hepatic secretion of bilirubin and biliary lipids in patients with alcoholic cirrhosis of the liver. Digestion. 1983;26(2):80–8. [DOI] [PubMed] [Google Scholar]
- 51.Gunnarsdottir SA, Sadik R, Shev S, Simren M, Sjovall H, Stotzer PO, et al. Small intestinal motility disturbances and bacterial overgrowth in patients with liver cirrhosis and portal hypertension. The American journal of gastroenterology. 2003;98(6):1362–70. [DOI] [PubMed] [Google Scholar]
- 52.Chesta J, Defilippi C, Defilippi C. Abnormalities in proximal small bowel motility in patients with cirrhosis. Hepatology. 1993;17(5):828–32. [PubMed] [Google Scholar]
- 53.Rayes N, Pilarski T, Stockmann M, Bengmark S, Neuhaus P, Seehofer D. Effect of pre- and probiotics on liver regeneration after resection: a randomised, double-blind pilot study. Beneficial microbes. 2012;3(3):237–44. [DOI] [PubMed] [Google Scholar]
- 54.Betrapally NS, Gillevet PM, Bajaj JS. Gut microbiome and liver disease. Translational research : the journal of laboratory and clinical medicine. 2017;179:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. Journal of hepatology. 2014;60(5):940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54(2):562–72. [DOI] [PubMed] [Google Scholar]
- 57.Chen P, Schnabl B. Host-microbiome interactions in alcoholic liver disease. Gut and liver. 2014;8(3):237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bajaj JS, Gillevet PM, Patel NR, Ahluwalia V, Ridlon JM, Kettenmann B, et al. A longitudinal systems biology analysis of lactulose withdrawal in hepatic encephalopathy. Metabolic brain disease. 2012;27(2):205–15. [DOI] [PubMed] [Google Scholar]
- 59.Riggio O, Varriale M, Testore GP, Di Rosa R, Di Rosa E, Merli M, et al. Effect of lactitol and lactulose administration on the fecal flora in cirrhotic patients. Journal of clinical gastroenterology. 1990;12(4):433–6. [DOI] [PubMed] [Google Scholar]
- 60.Mortensen PB, Holtug K, Bonnen H, Clausen MR. The degradation of amino acids, proteins, and blood to short-chain fatty acids in colon is prevented by lactulose. Gastroenterology. 1990;98(2):353–60. [DOI] [PubMed] [Google Scholar]
- 61.Wotzka SY, Kreuzer M, Maier L, Zund M, Schlumberger M, Nguyen B, et al. Microbiota stability in healthy individuals after single-dose lactulose challenge-A randomized controlled study. PLoS One. 2018;13(10):e0206214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. American journal of physiology Gastrointestinal and liver physiology. 2012;302(1):G168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. American journal of physiology Gastrointestinal and liver physiology. 2012;303(6):G675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PloS one. 2013;8(4):e60042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kimer N, Pedersen JS, Busk TM, Gluud LL, Hobolth L, Krag A, et al. Rifaximin has no effect on hemodynamics in decompensated cirrhosis: A randomized, double-blind, placebo-controlled trial. Hepatology. 2017;65(2):592–603. [DOI] [PubMed] [Google Scholar]
- 66.Chiang JYL, Ferrell JM. Bile Acid Metabolism in Liver Pathobiology. Gene Expr. 2018;18(2):71–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology. 2017;65(1):350–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arab JP, Cabrera D, Arrese M. Bile Acids in Cholestasis and its Treatment. Ann Hepatol. 2017;16(Suppl. 1: s3-105.):s53–s7. [DOI] [PubMed] [Google Scholar]
- 69.Dawson PA, Karpen SJ. Intestinal transport and metabolism of bile acids. J Lipid Res. 2015;56(6):1085–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chiang JYL, Ferrell JM. Bile Acids as Metabolic Regulators and Nutrient Sensors. Annu Rev Nutr. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Masyuk AI, Huang BQ, Radtke BN, Gajdos GB, Splinter PL, Masyuk TV, et al. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am J Physiol Gastrointest Liver Physiol. 2013;304(11):G1013–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Copple BL, Li T. Pharmacology of bile acid receptors: Evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol Res. 2016;104:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102(6):731–44. [DOI] [PubMed] [Google Scholar]
- 74.Song P, Zechner C, Hernandez G, Canovas J, Xie Y, Sondhi V, et al. The Hormone FGF21 Stimulates Water Drinking in Response to Ketogenic Diet and Alcohol. Cell Metab. 2018;27(6):1338–47 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lan T, Morgan DA, Rahmouni K, Sonoda J, Fu X, Burgess SC, et al. FGF19, FGF21, and an FGFR1/beta-Klotho-Activating Antibody Act on the Nervous System to Regulate Body Weight and Glycemia. Cell Metab. 2017;26(5):709–18 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brandl K, Hartmann P, Jih LJ, Pizzo DP, Argemi J, Ventura-Cots M, et al. Dysregulation of serum bile acids and FGF19 in alcoholic hepatitis. Journal of hepatology. 2018;69(2):396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. Journal of hepatology. 2011;54(6):1263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Broeders EP, Nascimento EB, Havekes B, Brans B, Roumans KH, Tailleux A, et al. The Bile Acid Chenodeoxycholic Acid Increases Human Brown Adipose Tissue Activity. Cell Metab. 2015;22(3):418–26. [DOI] [PubMed] [Google Scholar]
- 79.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perino A, Schoonjans K. TGR5 and Immunometabolism: Insights from Physiology and Pharmacology. Trends Pharmacol Sci. 2015;36(12):847–57. [DOI] [PubMed] [Google Scholar]
- 81.Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol. 2014;11(1):55–67. [DOI] [PubMed] [Google Scholar]
- 82.Kang DJ, Hylemon PB, Gillevet PM, Sartor RB, Betrapally NS, Kakiyama G, et al. Gut microbial composition can differentially regulate bile acid synthesis in humanized mice. Hepatol Commun. 2017;1(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ciocan D, Voican CS, Wrzosek L, Hugot C, Rainteau D, Humbert L, et al. Bile acid homeostasis and intestinal dysbiosis in alcoholic hepatitis. Aliment Pharmacol Ther. 2018;48(9):961–74. [DOI] [PubMed] [Google Scholar]
- 84.Staley C, Weingarden AR, Khoruts A, Sadowsky MJ. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl Microbiol Biotechnol. 2017;101(1):47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leclercq S, Starkel P, Delzenne NM, de Timary P. The gut microbiota: A new target in the management of alcohol dependence? Alcohol. 2019;74:105–11. [DOI] [PubMed] [Google Scholar]
- 86.Jacobs JP, Dong TS, Agopian V, Lagishetty V, Sundaram V, Noureddin M, et al. Microbiome and bile acid profiles in duodenal aspirates from patients with liver cirrhosis: The Microbiome, Microbial Markers and Liver Disease Study. Hepatol Res. 2018;48(13):1108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Best N, Jansen PL, Rensen SS. The gut microbiota of nonalcoholic fatty liver disease: current methods and their interpretation. Hepatol Int. 2015;9(3):406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han R, Ma J, Li H. Mechanistic and therapeutic advances in non-alcoholic fatty liver disease by targeting the gut microbiota. Front Med. 2018;12(6):645–57. [DOI] [PubMed] [Google Scholar]
- 89.Sorribas M, Jakob MO, Yilmaz B, Li H, Stutz D, Noser Y, et al. FXR-modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. Journal of hepatology. 2019. [DOI] [PubMed] [Google Scholar]
- 90.Yamada S, Takashina Y, Watanabe M, Nagamine R, Saito Y, Kamada N, et al. Bile acid metabolism regulated by the gut microbiota promotes non-alcoholic steatohepatitis-associated hepatocellular carcinoma in mice. Oncotarget. 2018;9(11):9925–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cassard AM, Ciocan D. Microbiota, a key player in alcoholic liver disease. Clin Mol Hepatol. 2018;24(2):100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hartmann P, Hochrath K, Horvath A, Chen P, Seebauer CT, Llorente C, et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology. 2018;67(6):2150–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bajaj JS, Kakiyama G, Zhao D, Takei H, Fagan A, Hylemon P, et al. Continued Alcohol Misuse in Human Cirrhosis is Associated with an Impaired Gut-Liver Axis. Alcohol Clin Exp Res. 2017;41(11):1857–65. [DOI] [PubMed] [Google Scholar]
- 94.Cassard AM, Gerard P, Perlemuter G. Microbiota, Liver Diseases, and Alcohol. Microbiol Spectr. 2017;5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim DJ, Yoon S, Ji SC, Yang J, Kim YK, Lee S, et al. Ursodeoxycholic acid improves liver function via phenylalanine/tyrosine pathway and microbiome remodelling in patients with liver dysfunction. Sci Rep. 2018;8(1):11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pearson T, Caporaso JG, Yellowhair M, Bokulich NA, Padi M, Roe DJ, et al. Effects of ursodeoxycholic acid on the gut microbiome and colorectal adenoma development. Cancer Med. 2019;8(2):617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tabibian JH, O'Hara SP, Trussoni CE, Tietz PS, Splinter PL, Mounajjed T, et al. Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis. Hepatology. 2016;63(1):185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mariotti V, Cadamuro M, Spirli C, Fiorotto R, Strazzabosco M, Fabris L. Animal models of cholestasis: An update on inflammatory cholangiopathies. Biochim Biophys Acta Mol Basis Dis. 2019;1865(5):954–64. [DOI] [PubMed] [Google Scholar]
- 99.Quigley EM. Primary Biliary Cirrhosis and the Microbiome. Semin Liver Dis. 2016;36(4):349–53. [DOI] [PubMed] [Google Scholar]
- 100.Li Y, Tang R, Leung PSC, Gershwin ME, Ma X. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun Rev. 2017;16(9):885–96. [DOI] [PubMed] [Google Scholar]
- 101.Tang R, Wei Y, Li Y, Chen W, Chen H, Wang Q, et al. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut. 2018;67(3):534–41. [DOI] [PubMed] [Google Scholar]
- 102.Kummen M, Holm K, Anmarkrud JA, Nygard S, Vesterhus M, Hoivik ML, et al. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut. 2017;66(4):611–9. [DOI] [PubMed] [Google Scholar]
- 103.Olsen I, Yamazaki K. Can oral bacteria affect the microbiome of the gut? J Oral Microbiol. 2019;11(1):1586422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xun Z, Zhang Q, Xu T, Chen N, Chen F. Dysbiosis and Ecotypes of the Salivary Microbiome Associated With Inflammatory Bowel Diseases and the Assistance in Diagnosis of Diseases Using Oral Bacterial Profiles. Front Microbiol. 2018;9:1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Acharya C, Bajaj JS. Gut Microbiota and Complications of Liver Disease. Gastroenterol Clin North Am. 2017;46(1):155–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hartmann P, Seebauer CT, Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin Exp Res. 2015;39(5):763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Acharya C, Sahingur SE, Bajaj JS. Microbiota, cirrhosis, and the emerging oral-gut-liver axis. JCI Insight. 2017;2(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bajaj JS, Betrapally NS, Hylemon PB, Heuman DM, Daita K, White MB, et al. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology. 2015;62(4):1260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boursier J, Diehl AM. Nonalcoholic Fatty Liver Disease and the Gut Microbiome. Clin Liver Dis. 2016;20(2):263–75. [DOI] [PubMed] [Google Scholar]
- 110.Quigley EM, Monsour HP. The Gut Microbiota and Nonalcoholic Fatty Liver Disease. Semin Liver Dis. 2015;35(3):262–9. [DOI] [PubMed] [Google Scholar]
- 111.Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63(3):764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Levitt MD, Li R, DeMaster EG, Elson M, Furne J, Levitt DG. Use of measurements of ethanol absorption from stomach and intestine to assess human ethanol metabolism. Am J Physiol. 1997;273(4):G951–7. [DOI] [PubMed] [Google Scholar]
- 113.Norberg A, Jones AW, Hahn RG, Gabrielsson JL. Role of variability in explaining ethanol pharmacokinetics: research and forensic applications. Clin Pharmacokinet. 2003;42(1):1–31. [DOI] [PubMed] [Google Scholar]
- 114.Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol. 2012;590(3):447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–9. [DOI] [PubMed] [Google Scholar]
- 116.Setshedi M, Wands JR, Monte SM. Acetaldehyde adducts in alcoholic liver disease. Oxid Med Cell Longev. 2010;3(3):178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hamarneh SR, Kim BM, Kaliannan K, Morrison SA, Tantillo TJ, Tao Q, et al. Intestinal Alkaline Phosphatase Attenuates Alcohol-Induced Hepatosteatosis in Mice. Dig Dis Sci. 2017;62(8):2021–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen P, Miyamoto Y, Mazagova M, Lee KC, Eckmann L, Schnabl B. Microbiota Protects Mice Against Acute Alcohol-Induced Liver Injury. Alcohol Clin Exp Res. 2015;39(12):2313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ansari RA, Husain K, Rizvi SA. Role of Transcription Factors in Steatohepatitis and Hypertension after Ethanol: The Epicenter of Metabolism. Biomolecules. 2016;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rao RK. Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer. Methods Mol Biol. 2008;447:171–83. [DOI] [PubMed] [Google Scholar]
- 121.Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286(6):G881–4. [DOI] [PubMed] [Google Scholar]
- 122.Ferrere G, Wrzosek L, Cailleux F, Turpin W, Puchois V, Spatz M, et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. Journal of hepatology. 2017;66(4):806–15. [DOI] [PubMed] [Google Scholar]
- 123.Mouzaki M, Wang AY, Bandsma R, Comelli EM, Arendt BM, Zhang L, et al. Bile Acids and Dysbiosis in Non-Alcoholic Fatty Liver Disease. PLoS One. 2016;11(5):e0151829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hartmann P, Chu H, Duan Y, Schnabl B. Gut microbiota in liver disease: too much is harmful, nothing at all is not helpful either. Am J Physiol Gastrointest Liver Physiol. 2019;316(5):G563–G73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, et al. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302(9):G966–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Starkel P, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111(42):E4485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Casafont Morencos F, de las Heras Castano G, Martin Ramos L, Lopez Arias MJ, Ledesma F, Pons Romero F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci. 1996;41(3):552–6. [DOI] [PubMed] [Google Scholar]
- 129.Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. 2013;8(1):e53028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang AM, Inamine T, Hochrath K, Chen P, Wang L, Llorente C, et al. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest. 2017;127(7):2829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lang S, Duan Y, Liu J, Torralba MG, Kuelbs C, Ventura-Cots M, et al. Intestinal Fungal Dysbiosis and Systemic Immune Response to Fungi in Patients With Alcoholic Hepatitis. Hepatology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chu H, Duan Y, Lang S, Jiang L, Wang Y, Llorente C, et al. The Candida albicans exotoxin Candidalysin promotes alcohol-associated liver disease. Journal of hepatology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sarin SK, Pande A, Schnabl B. Microbiome as a therapeutic target in alcohol-related liver disease. Journal of hepatology. 2019;70(2):260–72. [DOI] [PubMed] [Google Scholar]
- 134.Philips CA, Pande A, Shasthry SM, Jamwal KD, Khillan V, Chandel SS, et al. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin Gastroenterol Hepatol. 2017;15(4):600–2. [DOI] [PubMed] [Google Scholar]
- 135.DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, et al. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl J Med. 2019;381(21):2043–50. [DOI] [PubMed] [Google Scholar]
- 136.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13(11):1324–32. [DOI] [PubMed] [Google Scholar]
- 137.Wang L, Fouts DE, Starkel P, Hartmann P, Chen P, Llorente C, et al. Intestinal REG3 Lectins Protect against Alcoholic Steatohepatitis by Reducing Mucosa-Associated Microbiota and Preventing Bacterial Translocation. Cell Host Microbe. 2016;19(2):227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hartmann P, Chen P, Wang HJ, Wang L, McCole DF, Brandl K, et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58(1):108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Inamine T, Yang AM, Wang L, Lee KC, Llorente C, Schnabl B. Genetic Loss of Immunoglobulin A Does Not Influence Development of Alcoholic Steatohepatitis in Mice. Alcohol Clin Exp Res. 2016;40(12):2604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Duan Y, Llorente C, Lang S, Brandl K, Chu H, Jiang L, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575(7783):505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Axelson M, Mork B, Sjovall J. Ethanol has an acute effect on bile acid biosynthesis in man. FEBS Lett. 1991;281(1-2):155–9. [DOI] [PubMed] [Google Scholar]
- 142.Xie G, Zhong W, Li H, Li Q, Qiu Y, Zheng X, et al. Alteration of bile acid metabolism in the rat induced by chronic ethanol consumption. FASEB J. 2013;27(9):3583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jungst C, Berg T, Cheng J, Green RM, Jia J, Mason AL, et al. Intrahepatic cholestasis in common chronic liver diseases. Eur J Clin Invest. 2013;43(10):1069–83. [DOI] [PubMed] [Google Scholar]
- 144.Wu WB, Chen YY, Zhu B, Peng XM, Zhang SW, Zhou ML. Excessive bile acid activated NF-kappa B and promoted the development of alcoholic steatohepatitis in farnesoid X receptor deficient mice. Biochimie. 2015;115:86–92. [DOI] [PubMed] [Google Scholar]
- 145.Wu WB, Xu YY, Cheng WW, Wang YX, Liu Y, Huang D, et al. Agonist of farnesoid X receptor protects against bile acid induced damage and oxidative stress in mouse placenta--a study on maternal cholestasis model. Placenta. 2015;36(5):545–51. [DOI] [PubMed] [Google Scholar]
- 146.Iracheta-Vellve A, Calenda CD, Petrasek J, Ambade A, Kodys K, Adorini L, et al. FXR and TGR5 Agonists Ameliorate Liver Injury, Steatosis, and Inflammation After Binge or Prolonged Alcohol Feeding in Mice. Hepatol Commun. 2018;2(11):1379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57. [DOI] [PubMed] [Google Scholar]
- 148.Younossi ZM, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology. 2018;68(1):349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bedossa P Diagnosis of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: Why liver biopsy is essential. Liver international : official journal of the International Association for the Study of the Liver. 2018;38 Suppl 1:64–6. [DOI] [PubMed] [Google Scholar]
- 150.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nature medicine. 2018;24(7):908–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Noureddin M, Sanyal AJ. Pathogenesis of NASH: The Impact of Multiple Pathways. Curr Hepatol Rep. 2018;17(4):350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15(6):349–64. [DOI] [PubMed] [Google Scholar]
- 153.Wieland A, Frank DN, Harnke B, Bambha K. Systematic review: microbial dysbiosis and nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2015;42(9):1051–63. [DOI] [PubMed] [Google Scholar]
- 154.Jayakumar S, Loomba R. Review article: emerging role of the gut microbiome in the progression of nonalcoholic fatty liver disease and potential therapeutic implications. Aliment Pharmacol Ther. 2019;50(2):144–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sharpton SR, Ajmera V, Loomba R. Emerging Role of the Gut Microbiome in Nonalcoholic Fatty Liver Disease: From Composition to Function. Clin Gastroenterol Hepatol. 2019;17(2):296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bajzer M, Seeley RJ. Physiology: obesity and gut flora. Nature. 2006;444(7122):1009–10. [DOI] [PubMed] [Google Scholar]
- 157.Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, et al. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2019;30(3):607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Caussy C, Tripathi A, Humphrey G, Bassirian S, Singh S, Faulkner C, et al. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat Commun. 2019;10(1):1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, et al. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017;25(5):1054–62 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Soza A, Riquelme A, Gonzalez R, Alvarez M, Perez-Ayuso RM, Glasinovic JC, et al. Increased orocecal transit time in patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2005;50(6):1136–40. [DOI] [PubMed] [Google Scholar]
- 161.Wijarnpreecha K, Lou S, Watthanasuntorn K, Kroner PT, Cheungpasitporn W, Lukens FJ, et al. Small intestinal bacterial overgrowth and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2019. [DOI] [PubMed] [Google Scholar]
- 162.Rahman K, Desai C, Iyer SS, Thorn NE, Kumar P, Liu Y, et al. Loss of Junctional Adhesion Molecule A Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol. Gastroenterology. 2016;151(4):733–46 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Arendt BM, Ma DW, Simons B, Noureldin SA, Therapondos G, Guindi M, et al. Nonalcoholic fatty liver disease is associated with lower hepatic and erythrocyte ratios of phosphatidylcholine to phosphatidylethanolamine. Appl Physiol Nutr Metab. 2013;38(3):334–40. [DOI] [PubMed] [Google Scholar]
- 164.Yuan J, Chen C, Cui J, Lu J, Yan C, Wei X, et al. Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab. 2019;30(4):675–88 e7. [DOI] [PubMed] [Google Scholar]
- 165.Chu H, Duan Y, Yang L, Schnabl B. Small metabolites, possible big changes: a microbiota-centered view of non-alcoholic fatty liver disease. Gut. 2019;68(2):359–70. [DOI] [PubMed] [Google Scholar]
- 166.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. [DOI] [PubMed] [Google Scholar]
- 167.Xie G, Wang X, Liu P, Wei R, Chen W, Rajani C, et al. Distinctly altered gut microbiota in the progression of liver disease. Oncotarget. 2016;7(15):19355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Grat M, Krasnodebski M, Patkowski W, Wronka KM, Masior L, Stypulkowski J, et al. Relevance of Pre-Transplant alpha-fetoprotein Dynamics in Liver Transplantation for Hepatocellular Cancer. Ann Transplant. 2016;21:115–24. [DOI] [PubMed] [Google Scholar]
- 169.Fox JG, Feng Y, Theve EJ, Raczynski AR, Fiala JL, Doernte AL, et al. Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut. 2010;59(1):88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huang Y, Fan XG, Wang ZM, Zhou JH, Tian XF, Li N. Identification of helicobacter species in human liver samples from patients with primary hepatocellular carcinoma. J Clin Pathol. 2004;57(12):1273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kruttgen A, Horz HP, Weber-Heynemann J, Vucur M, Trautwein C, Haase G, et al. Study on the association of Helicobacter species with viral hepatitis-induced hepatocellular carcinoma. Gut Microbes. 2012;3(3):228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2019:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pizarro M, Balasubramaniyan N, Solis N, Solar A, Duarte I, Miquel JF, et al. Bile secretory function in the obese Zucker rat: evidence of cholestasis and altered canalicular transport function. Gut. 2004;53(12):1837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Arab JP, Barrera F, Arrese M. Bile Acids and Portal Hypertension. Ann Hepatol. 2017;16(Suppl. 1: s3-105.):s83–s6. [DOI] [PubMed] [Google Scholar]
- 175.Knudsen C, Neyrinck AM, Lanthier N, Delzenne NM. Microbiota and nonalcoholic fatty liver disease: promising prospects for clinical interventions? Curr Opin Clin Nutr Metab Care. 2019;22(5):393–400. [DOI] [PubMed] [Google Scholar]
- 176.Hameed B, Terrault NA, Gill RM, Loomba R, Chalasani N, Hoofnagle JH, et al. Clinical and metabolic effects associated with weight changes and obeticholic acid in non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2018;47(5):645–56. [DOI] [PMC free article] [PubMed] [Google Scholar]


