Abstract
NGM282, an engineered fibroblast growth factor 19 analogue, rapidly and significantly reduced liver fat content in a multicenter, randomized, double‐blind, placebo‐controlled study in patients with biopsy‐confirmed nonalcoholic steatohepatitis (NASH). However, it is unclear whether these changes would be accompanied by histological improvement. In this open‐label study, we assessed the histological efficacy of NGM282 in patients with biopsy‐confirmed nonalcoholic steatohepatitis. Paired liver biopsies from 43 patients who received subcutaneous NGM282 (1 mg, n = 24; 3 mg, n = 19) once daily for 12 weeks were evaluated blinded to time point, subject, and clinical information. At week 12, NGM282 significantly reduced nonalcoholic fatty liver disease activity score (NAS; −1.9; 95% confidence interval, −2.6 to −1.2; P < 0.001 in the 1 mg group; −2.2, −3.1 to −1.3; P < 0.001 in the 3 mg group) and fibrosis (−0.5; −0.9 to 0; P = 0.035 in the 3 mg group) scores. Overall, 50% and 63% of the patients receiving NGM282 1 mg or 3 mg, respectively, improved NAS by 2 or more points without fibrosis worsening. Of the patients receiving NGM282 1 mg or 3 mg, 25% and 42%, respectively, improved liver fibrosis by one stage or more without worsening of steatohepatitis. Treatment with NGM282 led to relative reductions in liver fat content (−58% and −67% in the 1 mg and 3 mg groups, respectively), corrected T1 (cT1; −8% and −9%), alanine aminotransferase (ALT) (−67% and −60%), aspartate aminotransferase (−57% and −52%), and fibrogenesis biomarkers neoepitope‐specific N‐terminal propeptide of type III collagen (Pro‐C3; −22% and −33%) and enhanced liver fibrosis score (ELF; −3% and −6%) at week 12. Greater reductions in Pro‐C3, ELF, and cT1, but not in liver fat content, 7alpha‐hydroxy‐4‐cholesten‐3‐one, or ALT, were observed in histological responders than in nonresponders. Conclusion: In this open‐label study, NGM282 improved the histological features of NASH in 12 weeks with significant reductions in NAS and fibrosis scores, accompanied by improvements in noninvasive imaging and serum markers.
Abbreviations
- AE
adverse event
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- C4
7alpha‐hydroxy‐4‐cholesten‐3‐one
- cT1
corrected T1
- CYP7A1
cholesterol 7alpha‐hydroxylase
- ELF
enhanced liver fibrosis
- EOT
end of treatment
- FGF19
fibroblast growth factor 19
- LDL‐C
low‐density lipoprotein cholesterol
- mITT
modified intention‐to‐treat
- MRI‐PDFF
magnetic resonance imaging‐proton density fat fraction
- NAS
nonalcoholic fatty liver disease activity score
- NASH
nonalcoholic steatohepatitis
- NASH CRN
NASH Clinical Research Network
- Pro‐C3
neoepitope‐specific N‐terminal propeptide of type III collagen
- SD
standard deviation
Nonalcoholic steatohepatitis (NASH), a progressive form of nonalcoholic fatty liver disease (NAFLD), is a growing clinical concern associated with the increasing prevalence of obesity, type 2 diabetes, and metabolic syndrome.1 NASH is characterized by the presence of hepatic steatosis, inflammation, and hepatocellular injury and is predicted to be the leading indication for liver transplantation by 2020.1, 2 Patients with NASH have an increased risk of developing cirrhosis and its complications, such as ascites, variceal hemorrhage, hepatic encephalopathy, hepatocellular carcinoma, and liver failure. Weight loss and lifestyle modifications are the recommended treatments for NASH, yet most patients do not achieve or maintain dietary goals and weight loss. Currently, there is no approved medicine for NASH, and therapies to arrest or reverse disease progression are urgently needed.
Liver fibrosis is the most important determinant of clinical disease progression and mortality in patients with NASH.3 Although the mechanism underlying the development and progression of fibrosis in NASH is poorly understood, emerging evidence supports a role for bile acids in the pathogenesis of liver fibrosis and inflammation.4 Fibroblast growth factor 19 (FGF19), an endocrine gastrointestinal hormone, controls bile acid metabolism through actions on cholesterol 7alpha‐hydroxylase (CYP7A1), the first and rate‐limiting enzyme in the classic pathway of bile acid synthesis from cholesterol.5 Multiple independent groups have reported reduced circulating FGF19 concentrations and elevated bile acid levels in patients with NASH.6, 7, 8 Although the mechanistic and clinical significance of these observations is incompletely understood, accumulating evidence suggests that the supraphysiological bile acid concentration may contribute to NASH‐related liver injury and progressive fibrosis.
NGM282 is an engineered analogue of FGF19 that potently inhibits de novo bile acid synthesis while eliminating FGF19‐associated tumorigenicity.9, 10 Preclinical studies have demonstrated that NGM282 impacts multiple pathways relevant to NASH pathogenesis by inhibiting de novo lipogenesis, improving insulin sensitivity, correcting mitochondrial dysfunction, and reducing hepatic inflammation and fibrosis.11 In a randomized, double‐blind, placebo‐controlled trial in patients with NASH, treatment with NGM282 for 12 weeks produced rapid and significant reductions in liver fat content, levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and serum markers of fibrogenesis.12 However, the histological features of NASH were not assessed at the end of that study.12 We therefore designed and conducted an open‐label trial of NGM282 to test its effect on histological endpoints in patients with NASH.
Patients and Methods
Trial Design and Oversight
This open‐label, multicenter trial was conducted at 3 investigative sites: Pinnacle Clinical Research (San Antonio, TX), Brooke Army Medical Center (San Antonio, TX), and Liver Consultants of Texas (Dallas, TX). The protocol was approved by the local ethics committee prior to study initiation. The trial was conducted in compliance with the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonization. All patients provided written informed consent. The NGM282 dose was selected on the basis of previous data that showed similar efficacy but better tolerability of 3 mg than 6 mg.12 Data were collected by investigators and managed by INC Research.
Patients
Patients were eligible if they met the following inclusion criteria: 18‐75 years of age at the time of screening; histological diagnosis of NASH on the basis of liver biopsy performed within 3 months of screening; NAFLD activity score (NAS, defined by the NASH Clinical Research Network [NASH CRN] Histologic Scoring System) ≥4 (with ≥1 point in each component of steatosis, lobular inflammation, and hepatocellular ballooning); stage 1, 2, or 3 fibrosis; liver fat content ≥8% as assessed by magnetic resonance imaging‐proton density fat fraction (MRI‐PDFF); and elevated ALT (≥19 IU/L in women; ≥30 IU/L in men). Detailed inclusion and exclusion criteria are included in the Supporting Information.
Procedures
During the screening period, patients underwent a liver biopsy or provided a liver biopsy tissue specimen obtained within the previous 3 months. At the end of treatment (EOT) at week 12, patients underwent a second liver biopsy (Fig. 1). Tissue samples were prepared and read by a qualified local pathologist to verify NASH and determine the NAS at enrollment. Patients underwent MRI for assessment of MRI‐PDFF and LiverMultiScan (Perspectum Diagnostics, Oxford, UK) during screening, at week 6, and at EOT/week 12 or early withdrawal (Fig. 1).
Figure 1.
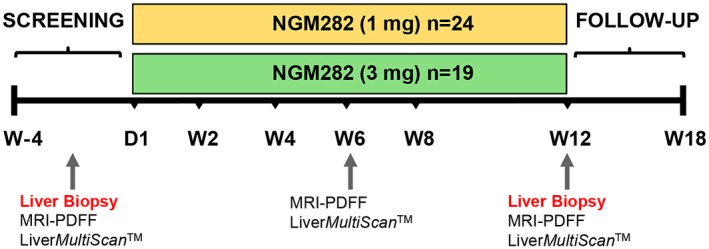
Study schema. Patients received NGM282 at a dose of 1 mg or 3 mg per day subcutaneously for 12 weeks. Liver biopsies were obtained at baseline and at week 12 (EOT). Liver fat content was measured by MRI‐PDFF at baseline, week 6, and week 12. Assessment of cT1 was performed at baseline, week 6, and week 12 by multimetric MRI LiverMultiScan. Serum markers of liver injury and fibrosis were measured by standard laboratory methods. Abbreviations: D, day; W, week.
Blood samples were obtained for measurements of 7alpha‐hydroxy‐4‐cholesten‐3‐one (C4, a serum marker of hepatic CYP7A1 activity), bile acids, chemistry, and fibrosis biomarkers at day 1 and week 12. Because serum cholesterol concentrations increased more in the NGM282‐treated patients than in the placebo‐treated patients in the previously reported randomized, double‐blind study,12 a lipid management approach was adopted by adding rosuvastatin if low‐density lipoprotein cholesterol (LDL‐C) levels elevated by >10 mg/dL from baseline.13 Adverse events (AEs) were evaluated at each study visit.
Assessments
We used a modified intention‐to‐treat (mITT) analysis that included all of the patients who enrolled and had both baseline and week 12 liver biopsies. Histological assessment of paired liver biopsies was performed by a pathologist (C.D.G.), blinded to time point, subject, and clinical and imaging information. Grading and staging of biopsies for the purpose of enrollment were done by the local pathologists at the site of enrollment but centrally read at the end of the study. Biopsies were graded with the use of NASH CRN criteria. The histological outcome measure was change in NAS or fibrosis scores from baseline to week 12. Responders were defined as those who achieved a decrease in NAS by at least 2 points without worsening of fibrosis (defined as any numerical increase in fibrosis stage) or a decrease in fibrosis by at least one stage without worsening of NASH. Resolution of NASH is defined as a score of 0‐1 for inflammation, 0 for ballooning, and any value for steatosis. Improvement in fibrosis was defined as any numerical decrease in fibrosis stage.
Noninvasive imaging outcome measures, including liver fat content as measured by MRI‐PDFF and corrected T1 (cT1, a standardized, vendor‐neutral imaging biomarker of hepatic fibroinflammatory disease) by LiverMultiScan, were assessed independently of each other at the central radiology core facilities (MRI‐PDFF at Duke University, cT1 at Perspectum Diagnostics) by central readers who were unaware of the subject, laboratory, clinical and histological information. Noninvasive serum outcome measures included change from baseline to week 12 in C4, bile acids, ALT and AST. Serum fibrosis biomarkers, including neoepitope‐specific N‐terminal propeptide of type III collagen (Pro‐C3; Nordic Bioscience) and enhanced liver fibrosis (ELF) score (with three components: the N‐terminal propeptide of type III collagen, the tissue inhibitor of metalloproteinase 1, and hyaluronic acid) were also determined. AEs were assessed using the Common Terminology Criteria for Adverse Events, v4.03.
Statistical Analysis
Sample size was empirically chosen on the basis of our previous data from a double‐blind, placebo‐controlled trial in patients with NASH.12 All patients with paired liver biopsies at baseline and week 12 were included in the mITT analysis. Change from baseline at week 12 (EOT) for all outcome measures was analyzed using a two‐sided, one‐sample t test at the 5% level of significance. To compare across treatment groups in changes from baseline to week 12, we used analysis of covariance with treatment group and baseline value as covariates at the 5% level of significance. Means with standard deviations (SDs) and the corresponding P values were presented. We also performed sensitivity analysis using the Wilcoxon matched‐pairs signed rank test. SAS version 9.4 (SAS Institute, Cary, NC) was used to conduct the analyses. The clinicaltrials.gov trial number is NCT02443116.
Results
Patient Characteristics
From April 2017 through June 2018, a total of 50 eligible patients with histologically proven NASH received once‐daily NGM282 1 mg or 3 mg treatment (Supporting Fig. S1). Seven patients did not have the week 12 liver biopsies and were not included in the mITT analysis (reasons are listed in Supporting Table S1). Baseline demographics and disease characteristics of patients in this study are summarized in Table 1. The mean total NASs were 5.4 (SD, 1.5) and 5.7 (SD, 1.5), and the mean fibrosis scores were 2.3 (SD, 0.8) and 2.5 (SD, 0.8), respectively, in the NGM282 1 mg (n = 24) and 3 mg (n = 19) groups. On central consensus review, stage 3 fibrosis was present in 38% and 53% patients in the NGM282 1 mg and 3 mg groups, respectively. Fifty patients were included in the safety analysis.
Table 1.
Baseline Demographics and Characteristics
| NGM282 1 mg (n = 24) | NGM282 3 mg (n = 19) | |
|---|---|---|
| Age (years) | 49.5 (10.7) | 51.4 (12.6) |
| Sex | ||
| Female | 19 (79%) | 15 (79%) |
| Male | 5 (21) | 4 (21%) |
| Ethnicity | ||
| Hispanic/Latino | 20 (83%) | 13 (68%) |
| Non‐Hispanic/Latino | 4 (17) | 6 (32%) |
| Type 2 diabetes | ||
| Yes | 9 (38%) | 7 (37%) |
| No | 15 (62%) | 12 (63%) |
| Statin status | ||
| Naive | 20 (83%) | 13 (68%) |
| Experienced | 4 (17%) | 6 (32%) |
| Histology | ||
| Fibrosis score | 2.3 (0.8) | 2.5 (0.8) |
| Fibrosis stage | ||
| 1 | 4 (17%) | 3 (16%) |
| 2 | 10 (42%) | 5 (26%) |
| 3 | 9 (38%) | 10 (53%) |
| 4 | 1 (4%) | 1 (5%) |
| NAS | 5.4 (1.5) | 5.7 (1.5) |
| Ballooning | ||
| 0 (none) | 4 (17%) | 1 (5%) |
| 1 (few ballooned cells) | 6 (25%) | 2 (10%) |
| 2 (many ballooned cells) | 14 (58%) | 16 (84%) |
| Steatosis | ||
| 0 (<5%) | 0 | 0 |
| 1 (5%‐33%) | 11 (46%) | 11 (58%) |
| 2 (34%‐66%) | 4 (17%) | 4 (21%) |
| 3 (>66%) | 9 (38%) | 4 (21%) |
| Inflammation | ||
| 0 (none) | 0 | 0 |
| 1 (<2) | 3 (12%) | 2 (10%) |
| 2 (2‐4) | 18 (75%) | 10 (53%) |
| 3 (>4) | 3 (12%) | 7 (37%) |
Shown are mean (SD) or n (%).
Histological Outcomes
Forty‐three patients had paired liver biopsies both at baseline and at 12 weeks, received treatment, and were included in the mITT analysis of the histological outcome measure. At week 12, the mean NAS improved by 1.9 and 2.2 points in patients treated with NGM282 1 mg or 3 mg, respectively (P < 0.001 versus baseline for both doses) (Fig. 2A and Table 2). Significant reduction in components of NAS was also noted. Distributions of NAS scores at baseline and week 12 are provided in Supporting Fig. S2. At week 12, the mean fibrosis score fell 0.1 and 0.5 points in patients receiving NGM282 1 mg or 3 mg, respectively (P = 0.035 for NGM282 3 mg) (Fig. 2B,C and Table 2).
Figure 2.
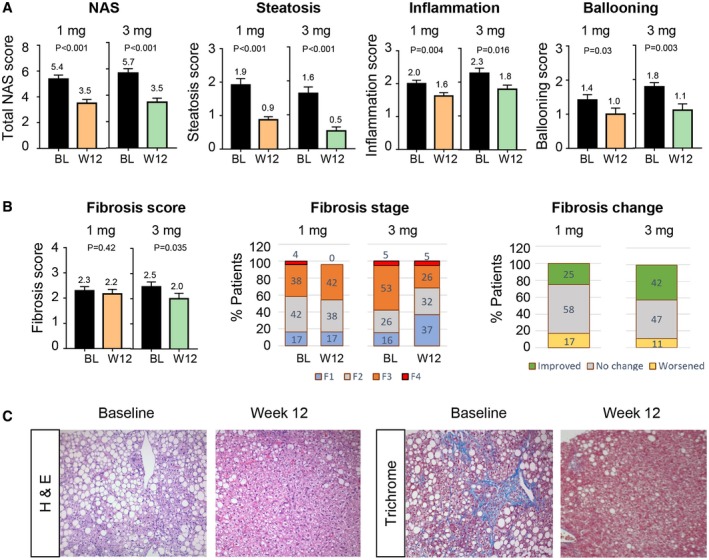
Histological outcome measures. (A) NAS and components at baseline and week 12. Details regarding the scoring systems are provided in Patients and Methods. (B) Fibrosis scores at baseline and week 12. Shown is the distribution of patients according to the NASH CRN fibrosis score (scores range from 0 to 4, with higher scores indicating more severe fibrosis) at baseline and week 12. A value of 100% represents all participants who had paired liver biopsies. Percentages may not total 100 because of rounding. (C) Representative hematoxylin and eosin and trichrome images of liver biopsies at baseline and week 12 in a patient treated with NGM282 3 mg. Liver fat droplets appear as white vacuoles in hematoxylin and eosin and trichrome images. Blue stains on trichrome staining (measuring collagen content) reveal fibrosis in the liver. Shown are mean ± SEM; P values by one‐sample t test. Abbreviations: BL, baseline; H&E, hematoxylin and eosin; W, week.
Table 2.
Change from Baseline to Week 12 in Key Outcomes
| NGM282 1 mg (n = 24) | NGM282 3 mg (n = 19) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | Change From Baseline to Week 12 | P | Baseline | Week 12 | Change From Baseline to Week 12 | P | |
| Histology | ||||||||
| Total NAS score | 5.4 (1.5) | 3.5 (1.4) | −1.9 (1.6) | <0.001 | 5.7 (1.5) | 3.5 (1.3) | −2.2 (1.8) | <0.001 |
| Baseline NAS 3‐6 | 4.6 (1.2) | 3.3 (1.6) | −1.2 (1.5) | 0.005 | 5.0 (1.0) | 3.5 (1.3) | −1.5 (1.3) | 0.001 |
| Baseline NAS 7‐8 | 7.0 (0) | 3.9 (1.1) | −3.1 (1.1) | <0.001 | 7.6 (0.5) | 3.4 (1.3) | −4.2 (1.5) | 0.003 |
| Steatosis | 1.9 (0.9) | 0.9 (0.4) | −1.0 (0.8) | <0.001 | 1.6 (0.8) | 0.5 (0.5) | −1.1 (0.9) | <0.001 |
| Ballooning | 1.4 (0.8) | 1.0 (0.9) | −0.4 (0.9) | 0.030 | 1.8 (0.5) | 1.1 (0.8) | −0.7 (0.9) | 0.003 |
| Inflammation | 2.0 (0.5) | 1.6 (0.5) | −0.4 (0.6) | 0.004 | 2.3 (0.6) | 1.8 (0.5) | −0.5 (0.8) | 0.016 |
| Total fibrosis score | 2.3 (0.8) | 2.2 (0.9) | −0.1 (0.7) | 0.42 | 2.5 (0.8) | 2.0 (0.9) | −0.5 (0.9) | 0.035 |
| Baseline F1 | 1.0 (0) | 1.3 (0.6) | 0.3 (0.6) | 1.0 (0) | 1.3 (0.6) | 0.3 (0.6) | ||
| Baseline F2 | 2.0 (0) | 1.8 (1.0) | −0.2 (1.0) | 2.0 (0) | 1.6 (0.5) | −0.4 (0.5) | ||
| Baseline F3 | 3.0 (0) | 2.9 (0.4) | −0.1 (0.4) | 3.0 (0) | 2.3 (1.0) | −0.7 (1.0) | ||
| Baseline F4 | 4.0 (0) | 3.0 (0) | −1.0 (0) | 4.0 (0) | 3.0 (0) | −1.0 (0) | ||
| Histological response | 12 (50%) | 13 (68%) | ||||||
| NAS ≥2 reduction without fibrosis worsening | 12 (50%) | 12 (63%) | ||||||
| Fibrosis ≥1 reduction without NASH worsening | 6 (25%) | 8 (42%) | ||||||
| NASH resolution without fibrosis worsening | 3 (12%) | 2 (10%) | ||||||
| Imaging | ||||||||
| Liver fat content by MRI‐PDFF (%) | 19.0 (7.2) | 8.0 (5.2) | −11.0 (5.0) | <0.001 | 17.1 (5.6) | 5.9 (4.4) | −11.2 (4.2) | <0.001 |
| cT1 by LiverMultiScan | 918.8 (80.7) | 848.8 (73.8) | −78.2 (53.0) | <0.001 | 891.4 (56.2) | 812.2 (57.6) | −81.6 (57.4) | <0.001 |
| Serum markers of target engagement | ||||||||
| C4 (ng/mL) | 37.7 (27.1) | 6.8 (9.9) | −30.9 (29.3) | <0.001 | 35.0 (24.2) | 1.8 (2.3) | −33.2 (24.4) | <0.001 |
| Total bile acids (μmol/L) | 5.3 (5.6) | 1.7 (1.5) | −3.6 (5.0) | 0.002 | 5.3 (3.1) | 1.4 (1.1) | −4.0 (3.2) | <0.001 |
| Liver enzymes | ||||||||
| ALT (U/L) | 92.2 (53.0) | 28.4 (19.9) | −63.8 (41.9) | <0.001 | 81.7 (39.3) | 28.8 (14.4) | −52.9 (35.8) | <0.001 |
| AST (U/L) | 69.8 (39.0) | 27.4 (17.4) | −42.5 (33.7) | <0.001 | 64.5 (31.9) | 27.2 (9.3) | −37.3 (26.2) | <0.001 |
| Serum fibrosis markers | ||||||||
| Pro‐C3 (ng/mL) | 16.3 (10.1) | 11.8 (5.2) | −4.5 (7.1) | 0.005 | 25.0 (21.6) | 13.9 (8.4) | −11.1 (18.4) | 0.017 |
| ELF score | 9.8 (0.8) | 9.5 (0.8) | −0.3 (0.4) | <0.001 | 10.1 (1.0) | 9.5 (1.0) | −0.6 (0.6) | <0.001 |
| Hyaluronic acid (μg/L) | 61.1 (49.4) | 61.8 (53.0) | 0.7 (27.7) | 0.90 | 91.0 (79.9) | 73.0 (90.7) | −18.1 (50.3) | 0.13 |
| PIIINP (μg/L) | 12.8 (5.9) | 9.7 (3.3) | −3.1 (3.7) | <0.001 | 13.6 (4.7) | 10.3 (3.4) | −3.3 (3.5) | <0.001 |
| TIMP‐1 (μg/L) | 283.0 (64.1) | 243.4 (51.0) | −39.6 (28.2) | <0.001 | 270.5 (68.0) | 227.7 (54.6) | −42.7 (44.6) | <0.001 |
Shown are mean (SD) or n (%). P values by one‐sample t test. Histological response is defined as a 2‐point or greater improvement in NAS without worsening of fibrosis or improvement in fibrosis of one stage or more without worsening of NASH (defined as no increase in NAS for ballooning, inflammation, or steatosis). Resolution of NASH is defined as a score of 0‐1 for inflammation, 0 for ballooning, and any value for steatosis.
Abbreviations: PIIINP, N‐terminal pro‐peptide of collagen III; TIMP‐1, tissue inhibitor of metalloproteinase 1.
Of the patients treated with NGM282 1 mg or 3 mg, 50% (12/24) and 68% (13/19), respectively, had histological response (2‐point or greater improvement in NAS without worsening of fibrosis or improvement in fibrosis without worsening of NASH). When compared with those at baseline, the NAS decreased in 75% and 84% of the patients and the fibrosis score decreased in 25% and 42% of the patients in the NGM282 1 mg and 3 mg groups, respectively. Of patients with F3 fibrosis at baseline in the NGM282 3 mg group, 30% (3/10) achieved a two‐stage improvement in fibrosis. Of the patients in the NGM282 1 mg and 3 mg groups, 12% and 10%, respectively, achieved NASH resolution without fibrosis worsening at week 12.
A sensitivity analysis of the histological outcome measure, in which patients with a missing week 12 liver biopsy were defined as nonresponders, showed that 43% (1 mg) and 59% (3 mg) of patients receiving NGM282 achieved histological improvement. Similar results were obtained when the Wilcoxon matched‐pairs signed rank test was used (Supporting Table S2).
The pragmatic design in the current trial about biopsies read by local pathologists to assess enrollment eligibility led to unavoidable discordant interpretations due to interobserver variability, as it did in other NASH trials.14, 15 Six patients from the NGM282 1 mg group and 3 patients from the 3 mg group no longer met inclusion criteria (NAS ≥4, with at least 1 point each in steatosis, ballooning, and inflammation) at central pathology review. Further sensitivity analysis of histological outcome measures excluding these patients did not alter the principal conclusions regarding histological improvements (Supporting Tables S3 and S4).
Imaging Outcomes
At week 12, absolute liver fat content was significantly reduced with NGM282 versus baseline (Fig. 3A and Table 2). Most of the beneficial effect of NGM282 related to the reduction in liver fat content was achieved as early as week 6 (Fig. 3B). At week 12, 92% and 100% of patients receiving NGM282 1 mg or 3 mg, respectively, achieved a clinically meaningful ≥5% reduction in absolute liver fat content; 92% (1 mg) and 100% (3 mg) of patients also achieved a ≥30% relative reduction of liver fat content. Liver fat content completely normalized in 33% (1 mg) and 63% (3 mg) of patients treated with NGM282.
Figure 3.
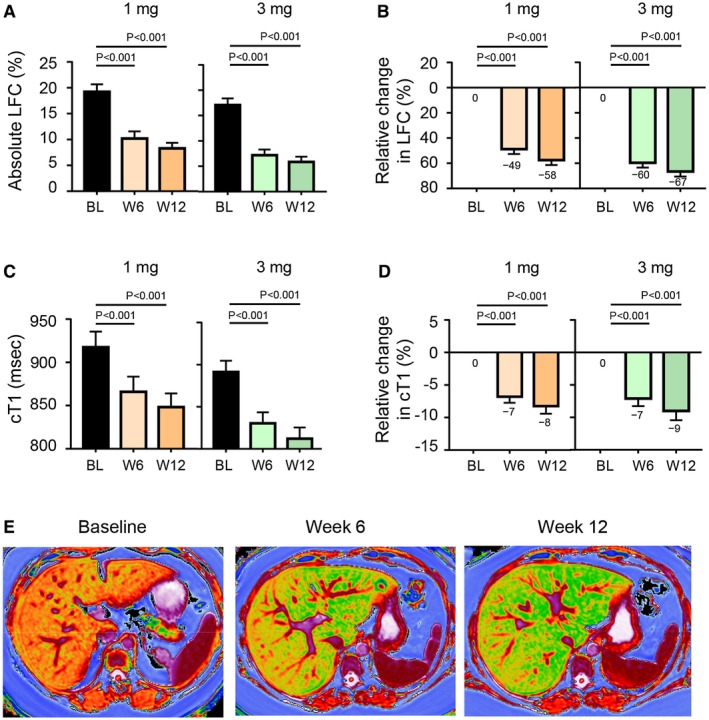
Imaging outcome measures. (A) Absolute liver fat content as measured by MRI‐PDFF at baseline, week 6, and week 12. (B) Relative change in liver fat content from baseline at week 6 and week 12. (C) cT1 values as measured by LiverMultiScan. (D) Relative change in cT1. (E) Representative LiverMultiScan images showing cT1 change over 12 weeks. Shown are mean ± SEM; P values by one‐sample t test. Abbreviations: BL, baseline; LFC, liver fat content; W, week.
Assessments by LiverMultiScan revealed that NGM282 treatment significantly lowered cT1 values, a standardized imaging biomarker of hepatic fibroinflammation16 (–78.2 milliseconds and –81.6 milliseconds in the 1 mg and 3 mg groups, respectively, at week 12; P < 0.001 versus baseline for both doses; Fig. 3C‐E).
Serum Parameters
Circulating levels of C4, a marker of hepatic CYP7A1 activity indicative of target engagement, were reduced by 76% and 93% in patients receiving NGM282 1 mg or 3 mg, respectively, at week 12 (Fig. 4A). Greater reductions in C4 were achieved in the NGM282 3 mg group than the 1 mg group (Supporting Table S5). Serum concentrations of total bile acids also decreased significantly from baseline (Fig. 4B).
Figure 4.
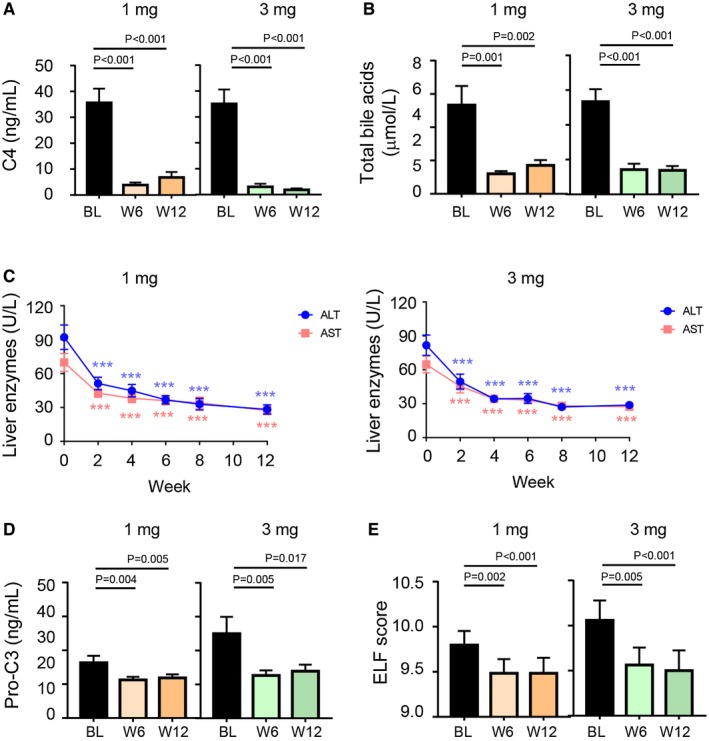
Serum outcome measures. (A) Concentrations of C4 at baseline, week 6, and week 12. (B) Levels of total bile acids at baseline, week 6, and week 12. (C) ALT and AST over time. (D) Concentrations of Pro‐C3 at baseline, week 6, and week 12. (E) ELF scores at baseline, week 6, and week 12. Shown are mean ± SEM; *** P < 0.001 versus baseline; P values by one‐sample t test. Abbreviations: BL, baseline; W, week.
Treatment with NGM282 resulted in significant reductions in ALT and AST from baseline to week 12 (Fig. 4C and Table 2). Most of the beneficial effects of NGM282 related to ALT and AST were achieved by 2 weeks of treatment and were sustained throughout treatment. No increases in gamma‐glutamyltransferase or alkaline phosphatase were observed (Supporting Fig. S3).
Significant reductions in noninvasive serum fibrosis biomarkers were observed as early as 6 weeks of treatment with NGM282 and were sustained for the duration of treatment (Fig. 4D,E). Reductions in concentrations of Pro‐C3, which directly reflects collagen formation during fibrogenesis,17 were significant at both week 6 and week 12 compared with baseline (Fig. 4D). Significant reductions in ELF, as well as components of ELF, from baseline were observed in the NGM282‐treated patients (Fig. 4E).
Consistent with the mechanism by which NGM282 inhibits the conversion of cholesterol to bile acids, serum levels of LDL‐C were elevated at week 2 in both the NGM282 1 mg (Fig. 5A) and 3 mg (Fig. 5B) groups. Rosuvastatin was added at week 2, with biweekly incremental doses to a maximum of 40 mg daily.13 Both drugs were continued until EOT at week 12. NGM282‐associated elevation of LDL‐C was effectively managed with rosuvastatin, reaching levels below baseline at week 12 (Fig. 5). Serum concentrations of triglyceride steadily declined over time in both NGM282 dose groups. Proportions of patients taking rosuvastatin 20 mg or 40 mg at weeks 2, 4, 8, and 12 are shown in Supporting Fig. S4.
Figure 5.
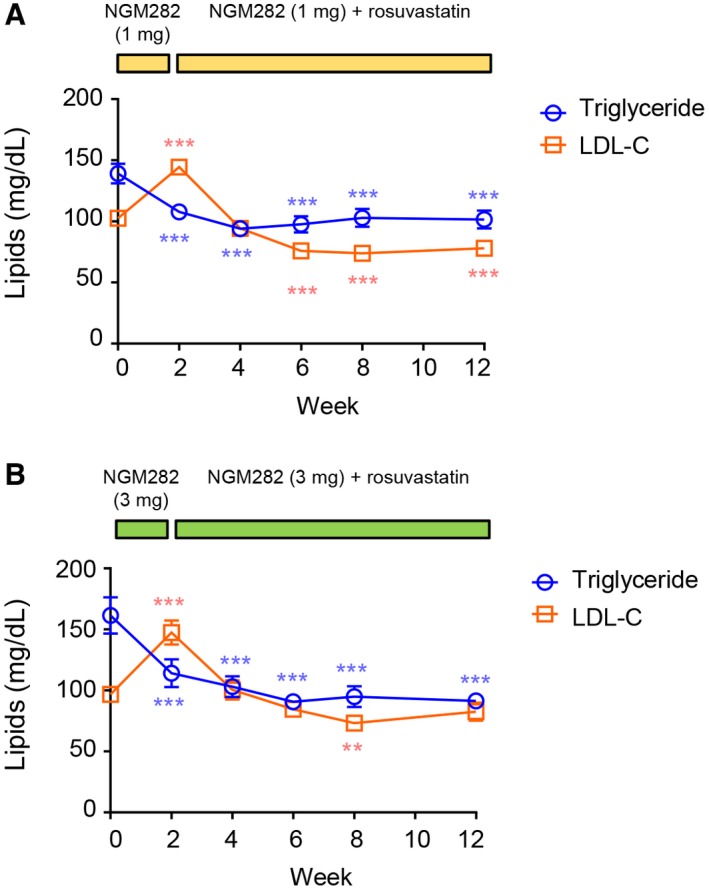
Change in lipids over time. (A) Serum LDL‐C and triglyceride levels over time in the NGM282 1 mg group. (B) Serum LDL‐C and triglyceride levels over time in the NGM282 3 mg group. Concentrations of LDL‐C and triglycerides were measured at baseline (week 0) and weeks 2, 4, 6, 8, and 12 in patients treated with NGM282 1 mg or 3 mg. Shown are mean ± SEM; *** P < 0.001, ** P < 0.01, * P < 0.05 versus baseline by one‐sample t test.
Features Associated with Histological Response in the NGM282 3 mg Group
To investigate factors associated with histological outcome, we categorized patients according to whether they had a histological response or did not have a histological response. Among the clinical or laboratory test changes that occurred in patients who had histological improvement with NGM282 treatment compared with patients who did not have histological improvement, greater reductions from baseline in Pro‐C3 (−56% versus −9% for responders and nonresponders, respectively; Fig. 6A) and ELF (−7% versus −3% for responders and nonresponders, respectively; Fig. 6B) levels were observed for histological responders.
Figure 6.
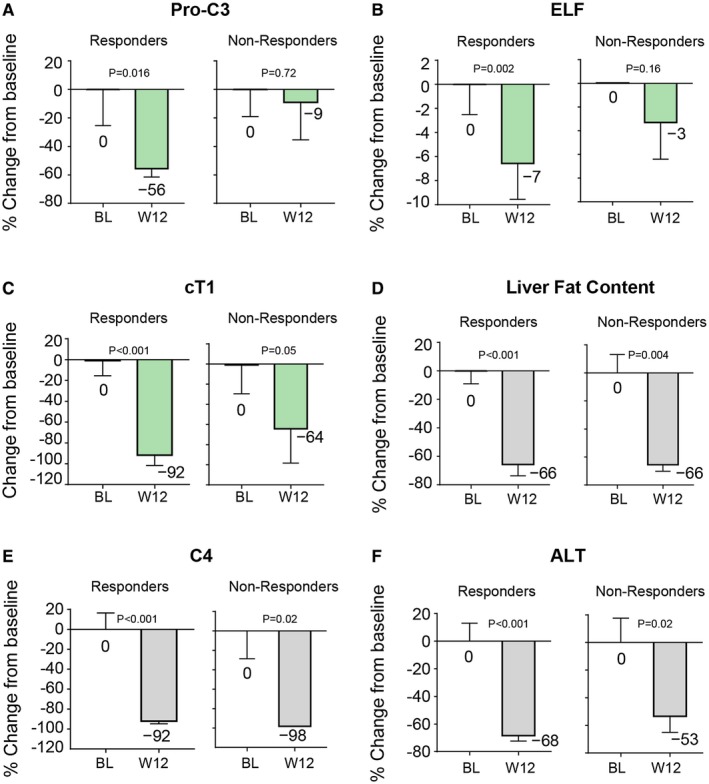
Change in key parameters in histological responders and nonresponders in the NGM282 3 mg group. (A) Pro‐C3. (B) ELF score. (C) cT1. (D) Liver fat content by MRI‐PDFF. (E) C4. (F) ALT. Histological response is defined as 2‐point or greater improvement in NAS without worsening of fibrosis or improvement in fibrosis of one stage or more without worsening of NASH (defined as no increase in NAS for ballooning, inflammation, or steatosis). Shown are mean ± SEM; P values by one‐sample t test. Abbreviations: BL, baseline; W, week.
cT1 reduction was more marked in histological responders (−92 milliseconds) than nonresponders (−64 milliseconds) (Fig. 6C), in contrast to the reductions in liver fat content being similar in both groups (−66% and −66% relative change from baseline for responders and nonresponders, respectively; Fig. 6D). Similar reductions in C4 were observed in histological responders (−92%) and nonresponders (−98%) (Fig. 6E). Changes in ALT were not significantly different in the comparison of responders and nonresponders (Fig. 6F). No associations were apparent between histological response and age or gender.
Safety
AEs that occurred during treatment were mild or moderate in severity, consistent with the previously reported safety profile of NGM282.12 The most common AEs were gastrointestinal (diarrhea, nausea, frequent bowel movements, and abdominal pain). Diarrhea was typically transient and was managed by separating the timing of injection around meals and decreasing meal size. Serious AEs were recorded in 4 patients, all deemed unrelated to study drug by the investigators, and were resolved without sequelae: 1 patient (from the 1 mg group) had a kidney mass detected by MRI during follow‐up; 1 patient (3 mg) had a grade 2 chest pain during follow‐up and was briefly hospitalized and discharged the same day; 1 patient (3 mg) had pleurisy; 1 patient (3 mg) with a medical history of congestive heart failure, type 2 diabetes, hypertension, and obesity had cardiac arrest. No deaths or cases of pancreatitis were reported during the trial.
Discussion
In this open‐label study, 12‐week treatment with NGM282, an engineered FGF19 analogue, produced marked antifibrotic, antisteatotic, and anti‐inflammatory activities in patients with NASH. High response rates were observed in patients with biopsy‐confirmed NASH, 50% (NGM282 1 mg) to 68% (NGM282 3 mg) of whom achieved significant histological improvement (reduction in NAS of 2 or more points with no worsening of fibrosis or improvement in fibrosis with no worsening of NASH). Importantly, 42% of the patients treated with NGM282 3 mg had a decrease in fibrosis score from baseline of at least one stage in 12 weeks. Moreover, all components of NAS (steatosis, hepatocellular ballooning, and lobular inflammation) improved significantly from baseline upon NGM282 treatment. NGM282 was generally well tolerated, with most treatment‐related AEs being mild in severity.
Previous NASH trials have traditionally evaluated histological outcome measures after a treatment duration of 48‐96 weeks. The current study reports an effective longitudinal histological assessment of a 12‐week therapy. To reduce the risk of unnecessary exposure of patients in the placebo group to two biopsy procedures in short succession, we conducted open‐label enrollment with a central read on slides blinded to time points and compared our results to historical placebo responses from a meta‐analysis of 39 randomized controlled trials comprising 1,463 patients with NASH.18 A greater proportion of patients treated with NGM282 3 mg compared with historical placebo control had improvement in fibrosis (42% versus 21%), steatosis (74% versus 33%), hepatocellular ballooning (53% versus 30%), and lobular inflammation (42% versus 32%). The mean change in these parameters was also greater in patients treated with NGM282 3 mg than historical placebo control (−0.5 versus −0.04, −2.3 versus −0.7, −1.1 versus −0.4, −0.7 versus −0.2, and −0.5 versus −0.2 for fibrosis, NAS, steatosis, ballooning, and inflammation scores, respectively).
Furthermore, we show that NGM282 produced rapid and sustained improvement in liver imaging parameters, potent target engagement as demonstrated by decreases in C4 and bile acids, and significant reductions in ALT, AST, and noninvasive serum fibrosis biomarkers (Pro‐C3 and ELF score). At present, liver biopsy, however imperfect due to its invasive and heterogeneous nature, remains a valuable endpoint in NASH trial design and approval. Although the short‐term (12‐week) repeat liver biopsy was well tolerated with no complications related to liver biopsy in experienced centers in the current study, noninvasive imaging and biochemical and molecular parameters, such as Pro‐C3, ELF, and cT1, are in urgent need of identification and validation in order to replace histological procedures in the future.
A lipid management approach was adopted in the current trial by coadministration of rosuvastatin,13 given that serum cholesterol concentrations increased more in the NGM282‐treated patients. Although we cannot discount any effect of statin on the outcome of NASH, data suggest that statin has a small effect on histological endpoints including fibrosis stage and NAS. In a randomized, double‐blind, placebo‐controlled trial, treatment of patients with biopsy‐proven NASH with simvastatin for 12 months resulted in no histological improvement in stage of fibrosis, steatosis, or necroinflammatory activity, although a 26% reduction in LDL‐C was seen in the simvastatin group compared with placebo.19 Another study showed that treatment with rosuvastatin for 24 months had no effect on fibrosis stage or NAS despite significantly improving the lipid profile.20 Overall, statins appear to be a safe and effective approach for managing NGM282‐associated cholesterol changes.
The magnitude of benefit from NGM282 treatment, as measured by reduction in NAS and fibrosis scores from baseline to week 12, compares favorably with those in other trials of much longer duration. The mean changes in NAS was −2.2 (SD, 1.8) after 12 weeks of NGM282 3 mg treatment, compared with −1.7 (SD, 1.8) for obeticholic acid (72 weeks),14 −1.3 (SD, 1.6) for liraglutide (48 weeks),15 −1.9 (SD, 2.1) for vitamin E (96 weeks),21 and −1.9 (SD, 1.8) for pioglitazone (96 weeks).21 A similar or greater proportion of patients receiving NGM282 3 mg achieved fibrosis improvement (42% in 12 weeks of treatment duration) compared to those previously reported with obeticholic acid (35% in 72 weeks),14 liraglutide (26% in 48 weeks),15 selonsertib (30%‐40% in 24 weeks),22 cenicriviroc (20% in 52 weeks),23 simtuzumab (18%‐19% in 96 weeks),24 and MGL‐3196 (29% in 36 weeks).25 Our data strengthen the notion that the farnesoid X receptor (FXR) mechanism may be largely dependent on the induction of its target gene FGF19.
The pathogenesis of NASH is believed to involve a multitude of mechanisms, including insulin resistance underlying the metabolic syndrome, lipotoxicity attributable to the accumulation of lipids and lipid metabolites, and the infiltration of proinflammatory cells causing hepatic injury.26 Emerging studies have identified bile acids as an important risk factor for chronic liver diseases including NASH.4 When accumulated within hepatocytes, bile acids can cause hepatic stellate cell activation, mitochondrial dysfunction, and endoplasmic reticulum stress, leading to cell death, inflammation, and liver injury.4 NGM282 targets the FGF receptor 4 (FGFR4)‐KLB receptor to inhibit CYP7A1, the key enzyme catalyzing the classical pathway of bile acid synthesis. Given this mechanism of action, our results suggest that the reduced bile acid pool may contribute to additional anti‐inflammatory, antifibrotic benefits beyond those associated with liver fat reduction. Of note, levels of ALT and AST decline very rapidly, as early as the first 2 weeks on NGM282 therapy (ALT, −41% and −37% in the NGM282 1 mg and 3 mg groups, respectively; AST, −33% and −26% in the NGM282 1 mg and 3 mg groups, at week 2), likely before the disappearance of the liver fat. Despite the relatively short duration of this trial, 42% patients in the NGM282 3 mg group had histological improvement in fibrosis. The regression of fibrosis was more substantial among patients with advanced fibrosis, with 3 of the 10 (30%) patients who were F3 at baseline achieving a two‐stage fibrosis reduction. The benefit of NGM282 therapy on the biochemical, imaging, and histological outcomes, with significant improvement in fibrosis in addition to NAS, adds to the weight of evidence supporting that bile acids may indeed be a driver of inflammation, injury, and fibrosis in patients with NASH. In this context, bile acids may be a direct contributor to the pathophysiology of NASH rather than merely a marker of a diseased liver. Overall, the observed histological benefit of NGM282 is probably a consequence of its cumulative hepatic and systemic actions in modulating bile acid metabolism, decreasing fatty acid synthesis and de novo lipogenesis, and improving insulin sensitivity.11
Levels of Pro‐C3, ELF, and cT1 had greater reductions in patients with histological response compared with nonresponders. However, the effect of NGM282 on certain biochemical and imaging outcome measures (ALT, AST, liver fat content) in histological responders was similar in magnitude and direction to that observed in nonresponders. Although C4 was suppressed to a similar degree in histological responders and nonresponders, a greater reduction in C4 was observed in the NGM282 3 mg group than the 1 mg group, which had a less profound fibrosis regression. Furthermore, the 12‐week treatment duration may not yet capture the full benefit of the intervention and that nonresponders from a 12‐week study may become responders after 24‐48 weeks of treatment. Future investigations on the genetic status of patatin‐like phospholipase domain containing 3 p.I148M,27 HSB17B13,28 and FGFR4 p.G488R29 may shed light on patient response, given that single‐nucleotide polymorphism variants in these genes are associated with risk of developing chronic liver disease including NASH and increased hepatic de novo lipogenesis in humans.
Despite strong anti‐inflammatory activity as demonstrated by the rapid decline in ALT and AST levels, the rate of NASH resolution with the 12‐week NGM282 therapy is low (12% and 10% in the 1 mg and 3 mg groups, respectively). Seven patients (3 and 4 in the 1 mg and 3 mg groups, respectively) reached a ballooning score of 0 at week 12 from a score of 2 at baseline; however, 4 of the 7 patients (57%) still had an inflammation score of >1 and therefore did not meet the criteria for NASH resolution. Explaining this phenomenon may require a more granular understanding of the mechanism of fibrosis resolution. Immune cells represent an important component of the liver microenvironment, providing a defense against pathogens, a mechanism for tissue repair and regeneration, as well as a clearance process during fibrosis regression. Ramachandran and colleagues reported that the presence of “restorative macrophage,” phagocytic Ly6Clow cells that do not conform to the traditional M1‐M2 paradigm is a feature of fibrosis regression30; and there is evidence for intrahepatic aggregates of T and B cells in response to antiviral treatment.31 It is possible that in NGM282‐treated liver, histological improvement in inflammation may not yet be visible beneath the background of immunoreparative activities during fibrosis regression. Future studies should examine distinct subsets of intrahepatic immune cells by immunohistochemistry or immunofluorescence staining and whether longer duration of NGM282 treatment would improve NASH resolution.
This study has a number of strengths. First, it reports the longitudinal change in NASH histology in response to a therapeutic agent in 12 weeks. The study design addressed the most relevant clinical question in NASH, according to the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver guidelines—namely, the histological changes in NAS and fibrosis that are used for the diagnosis of NASH. Second, it reports the effect of an FGF19 analogue on liver histology in patients with NASH. Given the continued interest in bile acid and FGF19 biology, our results affirm that modulating the FGF19‐FXR axis has great potential as therapy for liver diseases. Third, given the intravariability and intervariability in the assessment of liver biopsies, we had a NASH CRN liver pathologist, blinded to treatment time sequences and subject information, perform rigorous central assessment and confirmation of randomized liver biopsies at final reading to reduce bias that might have resulted from knowledge of time points. Fourth, we included the use of innovative, noninvasive imaging methods such as MRI‐PDFF and cT1 to evaluate changes in liver fat content, inflammation, and fibrosis, in addition to histological evaluation. We simultaneously evaluated a range of histological, imaging, biochemical, and serum fibrosis biomarkers, all in 12 weeks. This represents a continued evolution in the design of NASH trials, incorporating modern clinical science alongside regulatory approvable endpoints. The state‐of‐the‐art imaging systems (MRI‐PDFF and LiverMultiScan) used in this trial evaluate areas of the liver much larger than biopsies, providing an accurate and holistic assessment of liver health. All centers were required to demonstrate proficiency in image acquisition according to standardized protocol on qualified instruments. The central evaluation of the images of MRI‐PDFF and LiverMultiScan blinded to subject, clinical, laboratory, and histological information allow a more objective and complete characterization of NASH. The robustness of reductions in liver fat content and cT1 values, coupled with consistent decreases in laboratory parameters (ALT, AST) and improvements in fibrosis biomarkers (ELF, Pro‐C3), supports the validity of the principal findings. Lastly, 49% of patients with paired biopsies in this trial had advanced fibrosis (F3/F4) at baseline, a population that contributes disproportionately to the disease burden and cost of care and that is in urgent need of therapy.
An important limitation of the present study is the lack of a placebo group. While the results from this study are encouraging, it should be noted that in short‐term trials such as ours with a 12‐week treatment period, patients are more susceptible to the Hawthorne (placebo) effect due to frequent office visits, regular monitoring, and associated behavior change.32 The lack of a placebo‐controlled arm was due to ethical concern to perform serial liver biopsy after only 12 weeks in placebo‐treated patients. Although the effects of NGM282 on histological outcome measures compare favorably to other studies, we should note that comparison with historical placebo rates or other trials is of limited value due to heterogeneity between studies, including patient population, study design, study duration, and the inherent sampling variability associated with the liver biopsy procedure. The open‐label design of the study and small sample size require that a larger group of patients be evaluated in a placebo‐controlled trial, given that durable efficacy of therapeutics is of particular importance for the treatment of a chronic disease such as NASH. The pragmatic design in the current trial about biopsy read by local pathologists to assess enrollment eligibility led to unavoidable discordant interpretations due to interobserver variability, as it did in other NASH trials. Some patients were actually F4 or NAS = 3 at baseline despite inclusion criterial specifying F1‐F3 and NAS ≥4 for enrollment. Further sensitivity analysis of histological outcome measures excluding these patients did not alter the principal conclusions regarding histological improvements. Lastly, rosuvastatin was administered to manage NGM282‐associated cholesterol elevation. Although statins have been shown not to affect fibrosis stages in patients with NASH, we cannot exclude the possibility that statins might have some influence on the trial results. Despite these limitations, this trial builds on and adds to the accumulating evidence that the use of an FGF19 analogue in patients with NASH not only results in lower liver fat content and transaminases but also leads to reverse matrix remodeling, as indicated by improvements in histological endpoints.
NASH now afflicts approximately 6% of the global population. This high burden represents one of our most urgent and most neglected global health crises, with broad impact on public health. Developing therapeutics for NASH poses a vexing challenge for clinicians and researchers, which requires invasive procedures and lengthy trials. Fibrosis regression is generally presumed to take a long time; however, our results demonstrate that it is possible to shift the needle on fibrosis in a very short period (12 weeks). Longer‐term intervention may further enhance the histological improvements observed in the current study. Further studies are needed to determine whether histological improvement observed in this study can translate into benefits in clinical outcome, such as prevention of progression to cirrhosis and its complications. A phase 2b, randomized, double‐blind, placebo‐controlled, 24‐week paired biopsy study in patients with NASH is enrolling patients.
In conclusion, 12‐week treatment with NGM282 resulted in improvements in histological outcomes, as evidenced by reductions in NAS and fibrosis scores, in patients with NASH.
Supporting information
Acknowledgment
We express our appreciation to all of the patients who participated in this study and the study coordinators and staff of the participating clinical centers for their support and assistance. We thank Dr. Elizabeth Brunt for blinded, confirmatory reviewing of liver biopsies.
Supported by NGM Biopharmaceuticals.
Potential conflict of interest: Dr. Ling is employed by and owns stock in NGM Bio. Dr. Rossi is employed by and owns stock in NGM Bio. Dr. DePaoli is employed by and owns stock in NGM Bio. Dr. Baxter is employed by and owns stock in NGM Bio. Dr. Jaros consults for NGM Bio. Dr. Bashir consults for RadMD. He received grants from NGM Bio, Madrigal, Metacrine, Pinnacle, and Prosciento. Dr. Banerjee is employed by, received grants from, and owns stock in Perspectum. Dr. Guy consults for Madrigal, NGM Bio, and Cymabay. Dr. Harrison consults for, advises, received grants from, and owns stock in Madrigal, Genfit, and Galectin. He consults for, advises, and received grants from Gilead, Intercept, Cirius, NGM Bio, Novo Nordisk, Novartis, Pfizer, CymaBay, Hightide, Second Genome, and Axcella. He consults for, advises, and owns stock in Metacrine and Akero. He consults for and advises Echosens, Perspectum, Prometheus, Civi, Corcept, Bristol‐Myers Squibb, HistoIndex, Medpace, PPD, IQVIA, CLDF, Innovate, Albireo, Terns, Consynance, Prometic, and Lipocine. He is on the speakers’ bureau for AbbVie and Alexion. He received grants from Galmed, Immuron, Conatus, and Tobira/Allergan.
See Editorial on page https://doi.org/10.1002/hep.31213
References
Author names in bold designate shared co‐first authorship.
- 1. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313:2263‐2273. [DOI] [PubMed] [Google Scholar]
- 2. Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547‐555. [DOI] [PubMed] [Google Scholar]
- 3. Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta‐analysis. Hepatology 2017;65:1557‐1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology 2017;65:350‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Degirolamo C, Sabba C, Moschetta A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov 2016;15:51‐69. [DOI] [PubMed] [Google Scholar]
- 6. Wojcik M, Janus D, Dolezal‐Oltarzewska K, Kalicka‐Kasperczyk A, Poplawska K, Drozdz D, et al. A decrease in fasting FGF19 levels is associated with the development of non‐alcoholic fatty liver disease in obese adolescents. J Pediatr Endocrinol Metab 2012;25:1089‐1093. [DOI] [PubMed] [Google Scholar]
- 7. Caussy C, Hsu C, Singh S, Bassirian S, Kolar J, Faulkner C, et al. Serum bile acid patterns are associated with the presence of NAFLD in twins, and dose‐dependent changes with increase in fibrosis stage in patients with biopsy‐proven NAFLD. Aliment Pharmacol Ther 2019;49:183‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Puri P, Daita K, Joyce A, Mirshahi F, Santhekadur PK, Cazanave S, et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology 2018;67:534‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou M, Wang X, Phung V, Lindhout DA, Mondal K, Hsu JY, et al. Separating tumorigenicity from bile acid regulatory activity for endocrine hormone FGF19. Cancer Res 2014;74:3306‐3316. [DOI] [PubMed] [Google Scholar]
- 10. Zhou M, Yang H, Learned RM, Tian H, Ling L. Non‐cell‐autonomous activation of IL‐6/STAT3 signaling mediates FGF19‐driven hepatocarcinogenesis. Nat Commun 2017;8:15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou M, Learned M, Rossi SJ, DePaoli AM, Tian H, Ling L. Engineered FGF19 eliminates bile acid toxicity and lipotoxicity leading to resolution of steatohepatitis and fibrosis in mice. Hepatol Commun 2017;1:1024‐1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harrison SA, Rinella ME, Abdelmalek MF, Trotter JF, Paredes AH, Arnold HL, et al. NGM282 for treatment of non‐alcoholic steatohepatitis: a multicentre, randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet 2018;391:1174‐1185. [DOI] [PubMed] [Google Scholar]
- 13. Rinella ME, Trotter JF, Abdelmalek MF, Paredes AH, Connelly MA, Jaros MJ, et al. Rosuvastatin improves the FGF19 analogue NGM282‐associated lipid changes in patients with nonalcoholic steatohepatitis. J Hepatol 2018; 10.1016/jhep.2018.11.032. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14. Neuschwander‐Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non‐cirrhotic, non‐alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo‐controlled trial. Lancet 2015;385:956‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non‐alcoholic steatohepatitis (LEAN): a multicentre, double‐blind, randomised, placebo‐controlled phase 2 study. Lancet 2016;387:679‐690. [DOI] [PubMed] [Google Scholar]
- 16. Banerjee R, Pavlides M, Tunnicliffe EM, Piechnik SK, Sarania N, Philips R, et al. Multiparametric magnetic resonance for the non‐invasive diagnosis of liver disease. J Hepatol 2014;60:69‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nielsen MJ, Nedergaard AF, Sun S, Veidal SS, Larsen L, Zheng Q, et al. The neo‐epitope specific PRO‐C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am J Transl Res 2013;5:303‐315. [PMC free article] [PubMed] [Google Scholar]
- 18. Han MAT, Altayar O, Hamdeh S, Takyar V, Rotman Y, Etzion O, et al. Rates of and factors associated with placebo response in trials of pharmacotherapies for nonalcoholic steatohepatitis: systematic review and meta‐analysis. Clin Gastroenterol Hepatol 2019;17:616‐629. [DOI] [PubMed] [Google Scholar]
- 19. Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: a randomized placebo‐controlled trial. J Clin Gastroenterol 2009;43:990‐994. [DOI] [PubMed] [Google Scholar]
- 20. Nakahara T, Hyogo H, Kimura Y, Ishitobi T, Arihiro K, Aikata H, et al. Efficacy of rosuvastatin for the treatment of non‐alcoholic steatohepatitis with dyslipidemia: an open‐label, pilot study. Hepatol Res 2012;42:1065‐1072. [DOI] [PubMed] [Google Scholar]
- 21. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology 2018;67:549‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, et al. A randomized, placebo‐controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018;67:1754‐1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harrison SA, Abdelmalek MF, Caldwell S, Shiffman ML, Diehl AM, Ghalib R, et al. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology 2018;155:1140‐1153. [DOI] [PubMed] [Google Scholar]
- 25. Harrison SA, Guy CD, Bashir M, Frias JP, Alkhouri N, Baum S, et al. In a placebo‐controlled 36‐week phase 2 trial, treatment with Mgl‐3196 compared to placebo results in significant reductions in hepatic fat (MRI‐PDFF), liver enzymes, fibrosis biomarkers, atherogenic lipids, and improvement in NASH on serial liver biopsy. Hepatology 2018;68:9A. [Google Scholar]
- 26. Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med 2017;377:2063‐2072. [DOI] [PubMed] [Google Scholar]
- 27. Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461‐1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abul‐Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P, et al. A protein‐truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med 2018;378:1096‐1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lutz SZ, Hennige AM, Peter A, Kovarova M, Totsikas C, Machann J, et al. The Gly 385(388)Arg polymorphism of the FGFR4 receptor regulates hepatic lipogenesis under healthy diet. J Clin Endocrinol Metab 2018; 10.1210/jc.2018-01573. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30. Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, et al. Differential Ly‐6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA 2012;109:E3186‐E3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li L, Barry V, Daffis S, Niu C, Huntzicker E, French DM, et al. Anti‐HBV response to toll‐like receptor 7 agonist GS‐9620 is associated with intrahepatic aggregates of T cells and B cells. J Hepatol 2018;68:912‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne effect: a randomised, controlled trial. BMC Med Res Methodol 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


