Summary
This study was conducted to evaluate the expression of fibrinogen receptors on platelets of Philadelphia‐negative chronic myeloproliferative neoplasm (MPN) patients. We collected blood samples from 40 consecutive MPN patients and healthy volunteers. We performed flow cytometry analysis of P‐selectin expression and integrin beta‐3, activation of glycoprotein (GP) IIb/IIIa and fibrinogen receptor exposure (PAC‐1 binding). Surprisingly, we found a very low PAC‐1 binding capacity in MPN patients; however, the expression of PAC‐1 was almost completely recovered with aspirin intake. We hypothesize that the hypercoagulable states observed in MPN patients could depend on a primarily plasma‐driven impairment of fibrin turnover and thrombin generation.
Keywords: platelet fibrinogen receptor, myeloproliferative neoplasms, PAC‐1, aspirin
Patients affected by Philadelphia‐negative chronic myeloproliferative neoplasms (MPNs) are considered at high risk of thrombo‐haemorrhagic events, but the role of the platelet count in the assessment of the risk of vascular events is still controversial (Bucalossi et al., 1996; Finazzi et al., 1996).
A tight correlation was found between the platelet count and plasma sCD40L (Viallard et al., 2002), which appears to be required for thrombus formation in vivo (André et al., 2002). However, sCD40L is increased both in MPNs and reactive thrombocytosis (Viallard et al., 2002). Intravascular aggregates of platelets and leukocytes, mediated by P‐selectin on the former and CD11b on the latter, have been observed (Cervantes et al., 2009). The expression of CD11b is even more evident in patients with mutation of the JAK2 gene Val617Phe (Coucelo et al., 2014). Both of these processes should imply a perpetual — and measurable — platelet activation. Several studies on platelet function have been already proposed. Nevertheless, the mechanisms through which platelets are able to trigger vascular events are not yet adequately clarified. A refined method for the determination of platelet activation appears to be the use of platelet PAC‐1 antibody, able to identify the expression of the fibrinogen receptor of platelet glycoprotein IIb/IIIa (Lu & Malinauskas, 2011). This expression is indeed unique in the process of platelet activation, and yet rarely analyzed. One of the few reports demonstrating platelet fibrinogen receptor expression in MPN patients documented a decrease in PAC‐1 binding, despite a normal expression of GP IIb/IIIa. This finding was more marked in myelofibrosis (MF) than in essential thrombocythaemia (ET) or polycythaemia vera (PV) (Jensen et al., 2000). Moreover, since the exposure of platelet fibrinogen receptor seems to be influenced by turbulence in blood flow, we estimated that its evaluation could provide important biological evidence to explain some clinical manifestations, such as microvascular disturbances.
Patients and Methods
After obtaining informed consent from patients and the approval of the local Ethics Committee, blood samples from 40 consecutive MPNs patients who never received cytoreductive agents were obtained. Clinical characteristics of patients are reported in Table 1. In all, 28/40 patients were receiving a continuative antiplatelet prophylaxis with low‐dose aspirin (ASA, 75–100 mg) at the time of collection, while 12/40 of them were not on such therapy.
Table 1.
Patients and characteristics.
| MPNs | |||
|---|---|---|---|
| No. patients | 40 | ||
| Gender | |||
| Male | 17 (42·5) | ||
| Female | 23 (57·5) | ||
| Median age, years (range) | 64 (32–86) | ||
| Pathology | |||
| (A) Essential thrombocythaemia | 22/40 | ||
| Platelet count 109/l (mean ± SD) | 691·18 ± 264·40 | ||
| Haematocrit (mean ± SD) | 41·00 ± 5·50 | ||
| JAK2 allele burden (mean ± SD) | 17·67 ± 15·46 | ||
| CALR mut exon 9 – Type 2 | 2/22 (9%) | ||
| (B) Polycythaemia vera | 13/40 | ||
| Platelet count 109/l (mean ± SD) | 415·08 ± 176·96 | ||
| Haematocrit (mean ± SD) | 48·93 ± 3·06 | ||
| JAK2 allele burden (mean ± SD) | 47·85 ± 34·18 | ||
| CALR mut exon 9 – Type 2 | 0/13 (0%) | ||
| (C) Myelofibrosis | 5/40 | ||
| Platelet count 109/l (mean ± SD) | 461·60 ± 32·18 | ||
| Haematocrit (mean ± SD) | 43·64 ± 7·73 | ||
| JAK2 allele burden (mean ± SD) | 12·63 ± 11·74 | ||
| CALR mut exon 9 – Type 2 | 1/5 (20%) | ||
Flow cytometric analyses were performed using a FACSCanto flow cytometer (Becton–Dickinson, Franklin Lakes, NJ, USA) and 50 000 events were recorded for each sample. Our aim was to verify the expression of platelet fibrinogen receptors (PFRs) in the two different groups of patients compared to healthy volunteers, using whole blood flow cytometry. In each experiment sodium citrate and heparin tubes were collected from the same patient (positive control of platelet activation). Within 10 min from blood sampling, 5 μl of whole blood from each tube was incubated for 20 min at room temperature in the dark with a saturated concentration of CD61 peridinin‐chlorophyll proteins (PerCP), CD62P phycoerythrin (PE) and PAC‐1 fluorescein isothiocyanate (FITC). Positive control was also incubated with PAC‐1 in the presence of Arg–Gly–Asp–Ser (RGDS) in order to test the specific antibody binding. Samples were fixed with paraformaldehyde 1% for 30 min at 4°C in the dark and analyzed on a flow cytometer. Prism 8.0.1 (GraphPad, San Diego, CA, USA) was used for statistical analysis. To compare continuous response variables between two groups a Mann–Whitney U‐test was performed. P < 0·05 was considered as statistically significant. To measure the linear correlation between two variables the Pearson correlation coefficient was used.
Results
Surprisingly, we were able to verify a very low binding of PAC‐1 to platelets in patients with MPNs not receiving cytoreduction nor antiplatelet agents if compared to that observed in healthy subjects (35·3 ± 12·9 vs 65·3 ± 24·2 respectively, P = 0·008). Conversely, the use of aspirin seems to restore the expression of platelet fibrinogen receptor, as PAC‐1’s binding capacity is comparable to that of healthy volunteers (56·7 ± 18·7) (Fig 1). No difference was found with respect to the JAK2 Val617Phe mutation and its allele burden (data not shown). Interestingly, by focusing on the group of patients under antiplatelet prophylaxis and with no history of thrombosis, it was found that subjects with persistent microcirculatory disorders (MD) show a higher PAC‐1 binding capacity if compared to the asymptomatic ones (67·7 ± 17·8 vs 51·7 ± 14·0 respectively, P = 0·030) (Fig 2).
Figure 1.
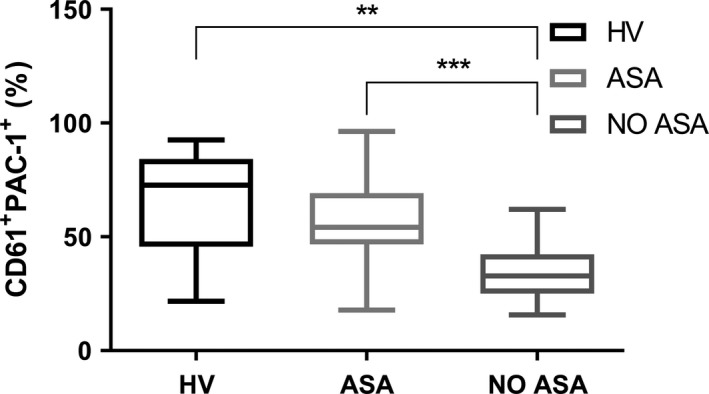
PAC‐1 frequency in whole blood obtained from healthy volunteers (HV, n = 10) and myeloproliferative neoplasm (MPN) patients treated or not with low‐dose aspirin (ASA, n = 28 and NO ASA, n = 12). **P = 0·008, ***P < 0·001.
Figure 2.
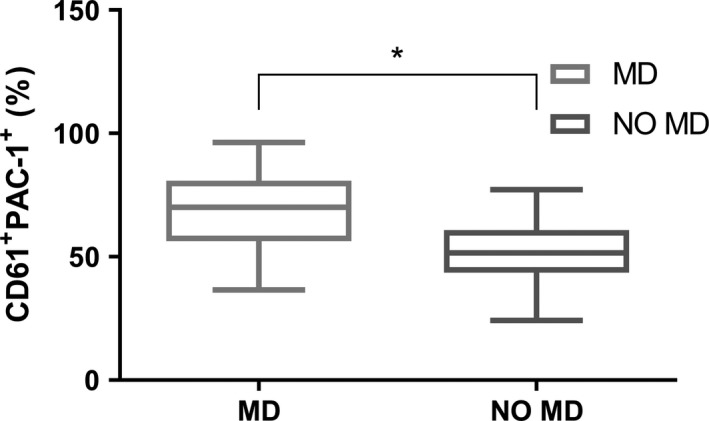
PAC‐1 frequency in whole blood obtained from MPN patients stratified according to the presence of microcirculatory disorders (MD, n = 13 and NO MD, n = 9). *P < 0·05.
Discussion
The expression of fibrinogen receptors has already been previously evaluated in patients with microcirculation disorders similar to those observed in MPNs (Raynaud's phenomenon). Interestingly, in the paper by Polidoro et al. (2012), particularly high values of PAC‐1 expression were reported, but with an inverse relation to the circulating levels of thromboxane B2. The authors hypothesized a possible downregulation of GP IIb/IIIa as a response to chronic exposure to thromboxanes. In MPNs, platelet counts are usually high, as well as the rate of vascular events. Moreover, since MPNs are characterized by high turbulence of blood flow — and consequently by an increase in endothelial shear stress — the PAC‐1‐binding capacity was expect likely to be high, by analogy to Raynaud's phenomenon. As a matter of fact, there is already evidence of how the binding between fibrinogen and GP IIb/IIIa is important in shear stress‐induced platelet aggregation (SIPA). Inhibitors of thromboxane synthesis seem able to counteract SIPA (Ikeda et al., 1988; Floyd & Ferro, 1988). Surprisingly, we observed that in untreated MPNs large amounts of platelets are resting in a conformation that is unable to bind fibrinogen, as demonstrated by the low PAC‐1 expression in cytofluorometry. This lack of activity can be reversed by administering ASA, which is also known for its fibrinolytic and hypoprothrombinaemic effects. We hypothesize that the hypercoagulable states observed in these patients could depend on a primarily plasma‐driven impairment of fibrin turnover and thrombin generation. A study by Moore et al. (2013) found an impaired fibrinogen binding in ET patients, despite normal levels of GP IIb/IIIa receptors. In the same study, interestingly, the authors describe enhanced Protease‐Activated Receptor‐1 (PAR1)‐mediated expression of GP IIb/IIIa after thrombopoietin stimulation, followed by the disappearance of fibrinogen binding sites. Starting from these observations, we can speculate that an increased generation of thrombin in MPN patients could secondarily lead to PAR1 activation, determining both a major conversion of fibrinogen into fibrin and the disappearance of fibrinogen binding sites on platelets with a reduced PAC‐1 expression. As is well known, PAR receptors are expressed in platelets, endothelium, and smooth muscle, contributing to both normal and pathological haemostasis (Leger et al., 2006). PAR1 activation could thus be a key element for the pathogenesis of thrombosis in MPNs. The results of our experiment are also similar to those observed in patients with pre‐infarct angina, where a reduced expression of PAC‐1 is described (Scalone et al., 2013), and to what occurs months after an acute ST segment elevation myocardial infarction, with a progressive increase in the expression of the platelet fibrinogen receptor despite dual antiplatelet therapy (Scalone et al., 2011). Indeed, in acute cardiovascular diseases the role of plasma procoagulants such as tissue factor was considered fundamental (Steffel et al., 2006). Finally, PAC‐1 could at the same time be a good marker of aspirin resistance in patients experiencing microcirculatory disorders. Further investigations are continuing in our group.
Conflict of Interest
The authors declare no potential conflicts of interest.
Author contributions
AL devised and directed the research project, and wrote the paper. SC and RN designed and performed experiments, analysed data and co‐wrote the paper. SDM and MG performed experiments and contributed to improve the methodology. AL, G Musuraca, MP, AFA and PPF worked on patient enrollment and provided clinical data. GG and G Martinelli contributed to the interpretation of the results. All authors provided critical feedback and approved the final version of the manuscript.
Supporting information
Table SI. Patients and characteristics.
References
- André, P. , Prasad, K.S. , Denis, C.V. , He, M. , Papalia, J.M. , Hynes, R.O. , Phillips, D.R. & Wagner, D.D. (2002) CD40L stabilizes arterial thrombi by a beta3 integrin–dependent mechanism. Nature Medicine, 8, 247–252. [DOI] [PubMed] [Google Scholar]
- Bucalossi, A. , Marotta, G. , Bigazzi, C. , Galieni, P. & Dispensa, E. (1996) Reduction of antithrombin III, protein C, and protein S levels and activated protein C resistance in polycythemia vera and essential thrombocythemia patients with thrombosis. American Journal of Hematology, 52, 14–20. [DOI] [PubMed] [Google Scholar]
- Cervantes, F. , Arellano‐Rodrigo, E. & Alvarez‐Larrán, A. (2009) Blood cell activation in myeloproliferative neoplasms. Haematologica, 94, 1484–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coucelo, M. , Caetano, G. , Sevivas, T. , Almeida Santos, S. , Fidalgo, T. , Bento, C. , Fortuna, M. , Duarte, M. , Menezes, C. & Ribeiro, M.L. (2014) JAK2V617F allele burden is associated with thrombotic mechanisms activation in polycythemia vera and essential thrombocythemia patients. International Journal of Hematology, 99, 32–40. [DOI] [PubMed] [Google Scholar]
- Finazzi, G. , Budde, U. & Michiels, J.J. (1996) Bleeding time and platelet function in essential thrombocythemia and other myeloproliferative syndromes. Leukemia & Lymphoma, 22, 71–78. [DOI] [PubMed] [Google Scholar]
- Floyd, C.N. & Ferro, A. (2012) The platelet fibrinogen receptor: from megakaryocyte to the mortuary. JRSM Cardiovascular Disease, 1, pii: cvd.2012.012007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, Y. , Murata, M. , Araki, Y. , Watanabe, K. , Ando, Y. , Itagaki, I. , Mori, Y. , Ichitani, M. & Sakai, K. (1988) Importance of fibrinogen and platelet membrane glycoprotein IIb/IIIa in shear‐induced platelet aggregation. Thrombosis Research, 51, 157–163. [DOI] [PubMed] [Google Scholar]
- Jensen, M.K. , de Nully Brown, P. , Lund, B.V. , Nielsen, O.J. & Hasselbalch, H.C. (2000) Increased platelet activation and abnormal membrane glycoprotein content and redistribution in myeloproliferative disorders. British Journal of Haematology, 110, 116–124. [DOI] [PubMed] [Google Scholar]
- Leger, A.J. , Covic, L. & Kuliopulos, A. (2006) Protease‐activated receptors in cardiovascular diseases. Circulation, 114, 1070–1077. [DOI] [PubMed] [Google Scholar]
- Lu, Q. & Malinauskas, R.A. (2011) Comparison of two platelet activation markers using flow cytometry after in vitro shear stress exposure of whole human blood. Artificial Organs, 35, 137–144. [DOI] [PubMed] [Google Scholar]
- Moore, S.F. , Hunter, R.W. , Harper, M.T. , Savage, J.S. , Siddiq, S. , Westbury, S.K. , Poole, A.W. , Mumford, A.D. & Hers, I. (2013) Dysfunction of the PI3 kinase/Rap1/integrin α(IIb)β(3) pathway underlies ex vivo platelet hypoactivity in essential thrombocythemia. Blood, 121, 1209–1219. [DOI] [PubMed] [Google Scholar]
- Polidoro, L. , Barnabei, R. , Giorgini, P. , Petrazzi, L. , Ferri, C. & Properzi, G. (2012) Platelet activation in patients with the Raynaud phenomenon. Internal Medicine Journal, 42, 531–535. [DOI] [PubMed] [Google Scholar]
- Scalone, G. , Coviello, I. , Barone, L. , Battipaglia, I. , Aurigemma, C. , Careri, G. , Pinnacchio, G. , Tarzia, P. , Lanza, G.A. & Crea, F. (2011) Evidence of increased platelet reactivity in the first six months after acute ST segment elevation myocardial infarction. Thrombosis Research, 128, 174–178. [DOI] [PubMed] [Google Scholar]
- Scalone, G. , Aurigemma, C. , Tomai, F. , Corvo, P. , Battipaglia, I. , Lanza, G.A. & Crea, F. (2013) Effect of pre‐infarction angina on platelet reactivity in acute myocardial infarction. International Journal of Cardiology, 167, 51–56. [DOI] [PubMed] [Google Scholar]
- Steffel, J. , Lüscher, T.F. & Tanner, F.C. (2006) Tissue factor in cardiovascular diseases: molecular mechanisms and clinical implications. Circulation, 113, 722–731. [DOI] [PubMed] [Google Scholar]
- Viallard, J.F. , Solanilla, A. , Gauthier, B. , Contin, C. , Déchanet, J. , Grosset, C. , Moreau, J.F. , Praloran, V. , Nurden, P. , Pellegrin, J.L. , Nurden, A.T. & Ripoche, J. (2002) Increased soluble and platelet‐associated CD40 ligand in essential thrombocythemia and reactive thrombocytosis. Blood, 99, 2612–2614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Patients and characteristics.


