Abstract
Background
The AdaptivCRT (aCRT) algorithm continuously adjusts cardiac resynchronization therapy (CRT) according to intrinsic atrioventricular conduction, providing synchronized left ventricular pacing in patients with normal PR interval and adaptive BiV pacing in patients with prolonged PR interval. Previous analyses demonstrated an association between aCRT and clinical benefit. We evaluated the incidence of patient mortality and atrial fibrillation (AF) with aCRT compared with standard CRT in a real‐world population.
Methods and Results
Patients enrolled in the Medtronic Personalized CRT Registry and implanted with a CRT from 2013‐2018 were divided into aCRT ON or standard CRT groups based upon device‐stored data. A Frailty survival model was used to evaluate the potential survival benefit of aCRT, accounting for patient heterogeneity and center variability. Daily AF burden and first device‐detected AF episodes of various durations were recorded by the device during follow‐up.
A total of 1814 CRT patients with no reported long‐standing AF history at implant were included. Mean follow‐up time was 26.1 ± 16.5 months and 1162 patients (64.1%) had aCRT ON. Patient survival probability at 36 months was 88.3% for aCRT ON and 83.7% for standard CRT (covariate‐adjusted hazard ratio [HR] = 0.71, 95% CI: 0.53‐0.96, P = .028). Mean AF burden during follow‐up was consistently lower in aCRT ON patients compared with standard CRT. At 36 months, the probability of AF was lower in patients with aCRT ON, regardless of which AF definition threshold was applied (6 minutes‐30 days, all P < .001).
Conclusion
Use of the AdaptivCRT algorithm was associated with improved patient survival and lower incidence of AF in a real‐world, prospective, nonrandomized registry.
Keywords: atrial fibrillation, AV conduction, cardiac resynchronization therapy, heart failure, optimized pacing, synchronized pacing
1. INTRODUCTION
Cardiac resynchronization therapy (CRT) is an established therapy for patients with symptomatic heart failure (HF) and reduced ejection fraction. 1 , 2 Randomized clinical trials have consistently demonstrated a benefit in reducing mortality and heart failure hospitalizations (HFH) and improving symptoms. 3 , 4 Two individual patient data meta‐analyses have confirmed the mortality and HFH findings. 3 , 5 The effect of CRT on atrial fibrillation (AF), a common comorbidity in HF patients that has been linked to increased risk of mortality, stroke, and hospitalizations, is less certain. 6 , 7 , 8 , 9 Despite the benefits of CRT, not all patients improve with therapy, with nearly one‐third of the patients having persistent HF symptoms and/or lack of left ventricular reverse remodeling. Potential reasons for suboptimal response include both patient factors, such as arrhythmias and device factors, such as suboptimal atrioventricular (AV) timing. 10
The AdaptivCRT (aCRT) algorithm was developed to continuously measure intrinsic conduction and dynamically adjust CRT pacing parameters. 11 When AV conduction time is normal, the algorithm provides synchronized left ventricular (LV) pacing, whereas when AV conduction time is prolonged, the algorithm continuously optimizes AV and ventriculoventricular (VV) intervals to provide BiV pacing. Initial clinical results from the Investigational Device Exemption (IDE) study demonstrated that the aCRT algorithm was noninferior to echo‐optimized pacing. 12 Additionally, the aCRT algorithm was associated with a significantly reduced risk of both HFH readmissions and all‐cause 30‐day admissions compared with echo‐optimized CRT. 13 Subsequent subgroup analysis showed a significant increase in the proportion of patients with an improved clinical composite score (CCS) with the aCRT algorithm in patients with a high percentage of synchronized LV pacing and in patients with normal AV conduction. 14 In this same subset of patients, there was a lower risk of death or HF hospitalization with aCRT. More recently, an analysis of the Adaptive CRT randomized clinical trial reported a 46% reduced risk of developing an AF event longer than 48 hours with aCRT vs echo‐optimized CRT. 15 These findings were also observed within a large de‐identified device database (CareLink) of over 37 000 patients where a 54% risk reduction was found. 16 These positive outcomes, however, have not been replicated in the real‐world population within a prospective registry. We sought to evaluate the potential association of the AdaptivCRT algorithm with mortality and the incidence/burden of AF in the real world in follow‐up extending up to 36 months.
2. METHODS
2.1. Study design
The Personalized CRT Study, part of the Medtronic Product Surveillance Registry (PSR) (http://ClinicalTrials.gov ID: NCT01524276), is a prospective, observational, real‐world clinical trial evaluating CRT response under the standard of care practices to optimize treatment of patients with HF. Continually enrolling, the PSR includes data from cardiac resynchronization therapy patients at 300 centers in 16 countries. The protocol was approved by the ethics committee at each of the participating centers. Adverse events were adjudicated by a Clinical Events Committee comprised of independent physicians.
2.2. Patients and procedures
All patients provided written informed consent to participate in the study before implant.
Device performance and mortality status were submitted via electronic data capture. In‐person follow‐up of enrolled patients is conducted as per the care provider's routine practice providing “real‐world” representation of device performance. Patients and their products are followed in personalized CRT over the continuum of the product lifecycle, until patient death or withdrawal of consent.
The cohort for this retrospective analysis included patients who were at least 18‐years old, were implanted with a CRT in 2013 or later (after US market release of the AdaptivCRT algorithm), and had device transmission and baseline characteristic data available. Patients with no HF, QRS duration less than 120 ms, or left ventricular ejection fraction (LVEF) greater than 50% were excluded. Additionally, to investigate the impact of aCRT on developing AF, patients with a reported history of permanent/persistent or long‐standing AF were excluded. AdaptivCRT is a programmable parameter and its configuration is stored in the device data. Device files collected through either on‐site interrogation or remote CareLink transmission were used to identify the CRT system configurations for all enrolled patients. The AdaptivCRT group included patients who had AdaptivCRT enabled at any time after implant (ie, AdaptivCRT ever ON). The “control” group consisted of patients who did not have aCRT enabled at any time postimplant (ie, AdaptivCRT never ON).
2.3. AdaptivCRT algorithm
The aCRT algorithm provides fusion pacing by evaluating intrinsic conduction every minute. When the device is programmed to Adaptive BiV+LV mode, during normal AV conduction (defined as PR interval, ≤200 ms), the algorithm provides synchronized LV‐only pacing by pre‐empting the atrial to RV sense interval by 40 ms or longer. During prolonged AV conduction (PR interval, >200 ms), BiV pacing is provided with automatic adjustment to the AV and VV timing based on intervals of atrial to RV sense, atrial to P‐wave end, and RV sense to QRS end. BiV pacing is also provided when the heart rate is greater than 100 beats per minute or if the aCRT algorithm is programmed to BiV only (referred to as Adaptive BiV mode). A detailed description of the algorithm has been previously published. 11
2.4. Endpoints
The first endpoint of interest for the analysis was patient survival. Patient survival status was reported by clinical sites through electronic case report forms. The second endpoint of interest was the incidence of atrial fibrillation, which was examined using two approaches. Atrial arrhythmias (referred to hereafter as AF) were detected and recorded by the device system. The first approach calculated mean AF burden, defined as hours of AF per day recorded by the device during follow‐up. Specifically, time to the first incidence of AF burden of 5.5 hours or longer was used, as arrhythmia burden of 5.5 hours or longer has been shown to double the risk of thromboembolic events compared with no burden. 17 The second approach was to evaluate time to the first occurrence of an atrial fibrillation episode of 48‐hours duration or longer. Additionally, using this method, differing durations of AF episode duration (ie, 6 minutes, 6 hours, 24 hours) were also examined.
2.5. Statistical analysis
Summary statistics are reported for patient baseline characteristics for the two groups. To account for center effect and heterogeneity of patients, the Frailty survival regression models 18 were used to evaluate the potential survival benefit, as well as a reduction in the incidence of AF and AF burden, of the aCRT algorithm. Site‐reported baseline factors (patient age, sex, LVEF, NYHA [New York Heart Association], diabetes, renal disease [with and without dialysis], LBBB [left bundle branch block], AV block, CAD [coronary artery disease], and hypertension) were candidate variables for stepwise model selection for both the mortality and AF analysis. Within center correlation was considered as a “random effect” in the statistical model. The marginal log‐likelihood statistics were examined for model selection to balance goodness‐of‐fit and simplicity. Candidate variables significantly (at the α = .10 level) associated with the outcomes of interest were included in the final model for risk reduction estimate. For patients who did not reach the endpoints, the last follow‐up date (mortality) or device transmission date (atrial arrhythmias) was used as the censoring date for the survival analysis. The statistical analyses were completed using SAS 9.4 (Cary, NC) and R (Vienna, Austria) statistical software packages.
3. RESULTS
3.1. Patients
A total of 1814 patients were implanted with a CRT device between 2013 and 2018 and met the criteria for inclusion in the analysis (Figure S1). Of these, 1162 (64.1%) had aCRT programmed ON, based upon device data. Mean follow‐up was 23.2 ± 14.5 months in the aCRT group (range, 0.0‐58.9) and 32.1 ± 18.6 months in the standard CRT group (range, 0.0‐67.0). There were 17 (1.5%) patients lost to follow‐up in the ON group and 23 (3.5%) in the OFF group. On average, patients in the aCRT group were younger, more often female, less frequently had AV block, and more frequently had LBBB compared with patients in the standard CRT group (Table 1). The average %CRT pacing delivered per patient during follow‐up was 95.3% in the aCRT group and 92.5% in the standard CRT group. Among patients in the aCRT group, 16.4% were programmed to Adaptive BiV and 83.6% were programmed to Adaptive BiV+LV. Mean and median %LV‐only pacing in the Adaptive BiV+LV subgroup were 55.4% and 71.6%, respectively.
Table 1.
Patient characteristics
| Patient characteristic | AdaptivCRT (n = 1162) | Standard CRT (n = 652) | P value |
|---|---|---|---|
| Age (±SD), y | 67.0 ± 10.9 | 68.7 ± 11.7 | .0019 |
| Sex (Female), n (%) | 400 (34.4) | 188 (28.8) | .0147 |
| NYHA class, n (%) | |||
| I | 33 (2.8) | 27 (4.1) | .0560 |
| II | 452 (38.9) | 216 (33.1) | |
| III | 648 (55.8) | 394 (60.4) | |
| IV | 29 (2.5) | 15 (2.3) | |
| QRS, ms | 158.9 ± 21.2 | 160.6 ± 23.1 | .1168 |
| LVEF, % | 26.6 ± 7.8 | 27.1 ± 8.2 | .2110 |
| AV block, a n (%) | 244 (21.0) | 205 (31.4) | <.0001 |
| LBBB, n (%) | 901 (77.5) | 406 (62.3) | <.0001 |
| CAD, n (%) | 607 (52.2) | 345 (52.9) | .7819 |
| Hypertension, n (%) | 808 (69.5) | 468 (71.8) | .3154 |
| Renal dysfunction, b n (%) | 212 (18.2) | 101 (15.5) | .1364 |
| Diabetes, n (%) | 368 (31.7) | 220 (33.7) | .3654 |
Abbreviations: AV, atrioventricular; CAD, coronary artery disease; CRT, cardiac resynchronization therapy; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
AV block includes 1st, 2nd, or 3rd degree AV block.
Renal dysfunction including with and without dialysis.
3.2. Mortality
There were a total of 84 deaths for any cause in the aCRT group, of which 13 were due to sudden cardiac death, 34 were due to non‐sudden cardiac death, 31 were due to noncardiac death, and six were for unknown reasons. In comparison, there were 97 deaths for any cause in the standard CRT group: 15 sudden cardiac deaths, 32 non‐sudden cardiac deaths, 34 noncardiac deaths, and 16 deaths of unknown cause. Through 36 months postimplant, the survival rate was significantly higher in the aCRT ON group (88.3%) compared with the standard CRT group (83.7%) (HR: 0.68, 95% CI: 0.50‐0.91, P = .01) (Figure 1A). To take into account other potential risk factors that may also have an impact on survival, patient baseline characteristics collected in the study (variables from Table 1) were included in statistical models. Potential effect modifiers were also examined using bivariate models, in which each potential risk factor was tested for its main effect as well as its interaction with aCRT. Across patient subgroups of age, sex, and comorbidities, aCRT was consistently associated with a survival benefit compared with standard CRT (Figure 2). Results from univariate modeling, full modeling, and a final statistical model are shown in Table S1. The final model for the mortality analysis included patient age, LVEF, NYHA, and patient comorbidity status of diabetes and renal disease. After adjusting for these variables, the probability of survival remained significantly higher in the aCRT ON group (adjusted HR: 0.71, 95% CI: 0.53‐0.96, P = .028) (Table S1), representing a 29% relative risk reduction in mortality with aCRT.
Figure 1.
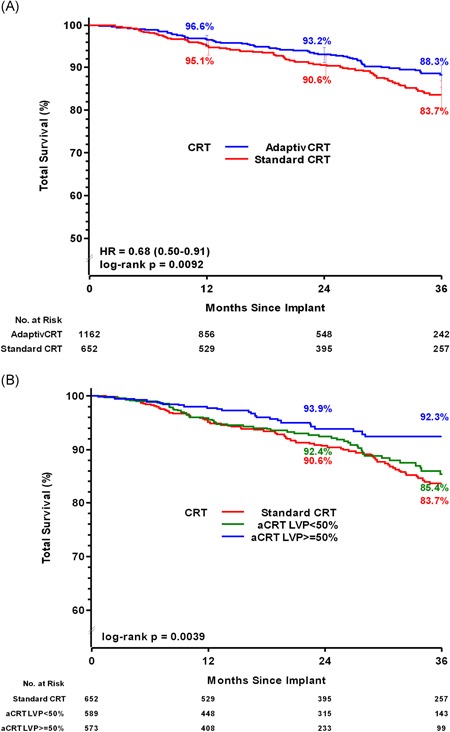
A, Patient Survival for aCRT vs standard CRT. Curves reflect unadjusted Kaplan‐Meier estimates of cumulative survival probability in patients programmed to aCRT (blue line) and standard CRT (red line) through 36 months follow‐up. Unadjusted hazard ratio derived from data through 36 months for each cohort using Frailty survival regression models. B, Patient survival stratified by percent LV pacing (LVP). Curves reflect unadjusted Kaplan‐Meier estimates of cumulative survival probability among aCRT patients and standard CRT patients (red line). aCRT patients were stratified by percent LV pacing (≥50% [blue line] and less than 50% [green line]). CRT, cardiac resynchronization therapy; HR, hazard ratio; LV, left ventricular
Figure 2.
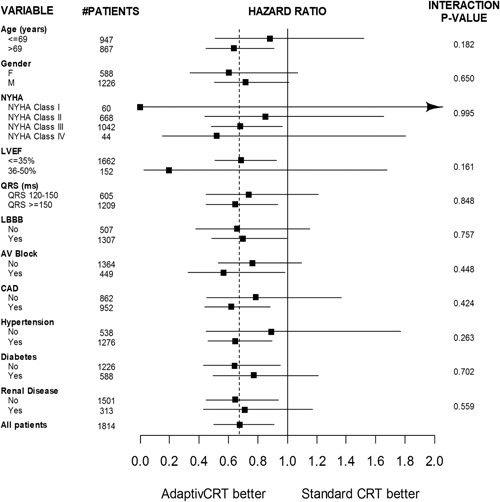
Mortality subgroup analysis hazard ratio forest plot. Risk of mortality through 36 months postimplant comparing aCRT and standard CRT. The vertical solid line corresponds to equal risk. The vertical dashed line represents the hazard ratio from the full comparison. The horizontal solid lines represent the 95% confidence intervals for the hazard ratios. AV, atrioventricular; CAD, coronary artery disease; CRT, cardiac resynchronization therapy; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; LVP, left ventricular pacing; NYHA, New York Heart Association
Patients in the aCRT ON group were further stratified into those receiving 50% or greater LV pacing (N = 573, 49%) and those with less than 50% LV pacing (N = 589, 51%). Through 36 months, the survival rate was significantly higher in those patients with 50% or greater LV pacing compared with patients who had less than 50% LV pacing (92.3% vs 85.2%, respectively; Figure 1B), representing a 45% reduced risk of mortality (HR: 0.55, 95% CI: 0.34–0.89, P = .014).
3.3. Atrial fibrillation
The mean AF burden was consistently lower in the aCRT ON group compared with the standard CRT group at all follow‐up durations examined (Figure 3A). The mean daily AF burden at 30 to 36 months postimplant was 6.0 ± 21.5 hours for the aCRT ON group and 14.2 ± 31.3 hours for the standard CRT group. Through 36 months, the probability of experiencing at least one daily AF burden of 5.5 hours or longer was significantly lower in the aCRT ON group compared with the standard CRT group (24.4% vs 35.8%, P < .0001; Figure 3B). Similar to the mortality analysis, to adjust for differences in patient baseline characteristics, variables from Table 1 were considered for statistical model building. After the model selection process, the final model included patient age and sex. The reduction in AF burden remained significant after adjusting for both covariates (HR: 0.72; 95% CI: 0.59‐0.87, P = .0007).
Figure 3.
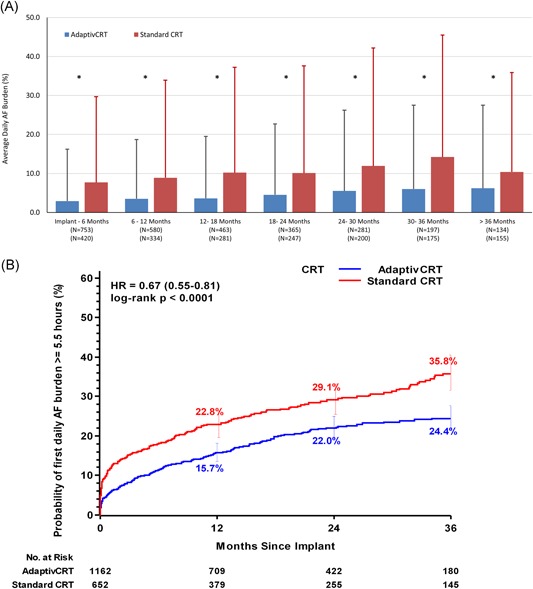
A, Daily AF burden summary. Average daily AF burden summary in 6‐month time periods comparing aCRT (blue bars) with standard CRT (red bars). Error bars reflect the standard deviation. B, Incidence of AF burden of 5.5 hours or longer. Curves reflect unadjusted Kaplan‐Meier estimates of cumulative incidence of developing daily AF burden of 5.5 hours or longer in patients programmed to aCRT (blue line) and standard CRT (red line) through 36‐month follow‐up. Unadjusted hazard ratio derived from data through 36 months for each cohort using Frailty survival regression models. AF, atrial fibrillation; CRT, cardiac resynchronization therapy; HR, hazard ratio. *P < .05, t test
Through the follow‐up period of 36 months, 11.2% of patients in the aCRT group and 21.5% of patients in the standard CRT group experienced an AF event of 48 hours or longer (HR: 0.53, 95% CI: 0.40‐0.70, P < .0001). After adjusting for age and sex, the risk for greater than or equal to 48 hours of AF remained significantly reduced (adjusted HR: 0.57, 95% CI: 0.43‐0.75, P < .0001), representing a 43% reduced risk with aCRT. Similar findings were observed when additionally excluding patients (N = 37) that had greater than or equal to 48 hours of AF in the first 30 days postimplant (HR: 0.54, 95% CI: 0.40‐0.74; P < .0001; Figure S2). In further analyses examining different AF duration cut‐points to define the incidence of AF, patients in the aCRT group consistently experienced a lower risk of developing AF, from 6 minutes to 30 days (P < .01 for all durations) (Table 2).
Table 2.
Occurrence of AF episodes of various duration: AdaptivCRT vs Standard CRT
| AF episode duration | Log‐rank P value | Univariate Cox regression model | Multivariate Cox regression model | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| 6 min | <.0001 | 0.71 (0.60‐0.85) | <.0001 | 0.75 (0.63‐0.89) | .0009 |
| 6 hr | .0008 | 0.70 (0.57‐0.86) | .0008 | 0.74 (0.60‐0.92) | .0054 |
| 24 hr | <.0001 | 0.57 (0.44‐0.74) | <.0001 | 0.62 (0.48‐0.81) | .0004 |
| 48 hr | <.0001 | 0.53 (0.40‐0.70) | <.0001 | 0.57 (0.43‐0.75) | <.0001 |
| 30 d | <.0001 | 0.32 (0.20‐0.50) | <.001 | 0.34 (0.22‐0.55) | <.001 |
Abbreviations: AF, atrial fibrillation; CI, confidence interval; CRT, cardiac resynchronization therapy.
4. DISCUSSION
The Personalized CRT study is a prospective, observational, real‐world registry evaluating CRT response. In this analysis, involving a cohort of 1814 patients, the data have shown a significant reduction in mortality in patients with aCRT programmed ON. This mortality reduction persisted after adjustment for demographic and clinical variables and the mortality benefit was observed across most subgroups. In addition, the occurrence and burden of AF were significantly reduced, regardless of the definition of AF used (Figure 4).
Figure 4.
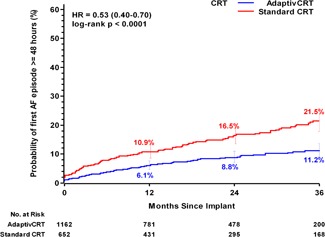
Incidence of AF episode of 48 hours or longer. Curves reflect unadjusted Kaplan‐Meier estimates of cumulative incidence of developing 48 hours of AF in patients programmed to aCRT (blue line) and standard CRT (red line) through 36‐month follow‐up. Unadjusted hazard ratio derived from data through 36 months for each cohort using Frailty survival regression models. AF, atrial fibrillation; CRT, cardiac resynchronization therapy; HR, hazard ratio
The aCRT algorithm aims to optimize CRT by allowing intrinsic conduction in patients with normal right ventricular activation by avoiding RV pacing and concomitant dynamic AV and VV adjustment based on electrical conduction timing measurements. The previous randomized controlled trial comparing aCRT and conventional echo‐optimized BiV pacing demonstrated the clinical benefits of the aCRT algorithm; aCRT was demonstrated to reduce the occurrence of AF and a greater %LVP (left ventricular pacing) was independently associated with a reduced risk of death, HF hospitalizations, and an improvement in Packer's CCS. 12 , 15 The findings of this analysis are consistent with the previous studies.
4.1. Mortality
Notably, when aCRT was programmed on there was a 29% relative risk reduction in mortality. The mortality benefit was seen across multiple subgroups and was greatest in patients with a higher percent of LV pacing (defined as ≥50% LV pacing in this analysis). This is consistent with the IDE study, which demonstrated a relative risk reduction of nearly 50% for mortality or HF hospitalizations in favor of aCRT in patients with %LV pacing greater than 50% compared with %LV pacing less than 50%. 14 To fully understand our initial observation of a clear separation in the survival curves between aCRT ON patients and patients with standard CRT, we examined patient demographics and available medical history data in a proportional hazard regression model. The results agreed with the initial Kaplan‐Meier analysis that patient survival probability was higher with AdaptivCRT enabled after adjusting for statistically significant risk factors in the model.
The mortality benefits of aCRT have several potential mechanisms. In patients with LBBB, the right bundle typically functions normally preserving the intrinsic activation of the septum and the RV. An increasing volume of literature supports the importance of optimally timed LV stimulation. 19 , 20 Improved dP/dt max, narrower QRSd, and even better clinical outcomes are found by fusing the LV pacing stimulus with intrinsic activation. 21 This more physiological activation may also reduce RV pacing‐induced dyssynchrony. Previous small analyses have supported the benefits of LV pacing compared with RV pacing and BiV pacing in reducing RV activation times, improving RV dP/dt max, and RV stroke volume. 22 , 23
The second major component of the aCRT algorithm is the dynamic adjustment of the AV and VV intervals. The algorithm works to fuse intrinsic conduction with sensed delayed LV conduction. CRT optimization has always been limited by the variable results with changes over time and clinical situations. While single‐center studies have demonstrated the benefits of device optimization, the SmartAV randomized clinical trial using static algorithms failed to show a benefit of device‐based algorithms over echocardiographic optimization. 24 However, the Respond CRT randomized study of ambulatory optimization using an accelerometer sensor showed an improvement in clinical response compared with empirical programming, which was consistent across patient subgroups. 25 , 26 A dynamic algorithm such as aCRT will potentially maintain the benefits of optimal resynchronization during postural changes and exercise. In addition, dynamic adjustment is also advantageous in the setting of intermittent AV block, where there may be marked variability in conduction throughout the day, thereby requiring changes in CRT settings throughout the day.
4.2. Atrial fibrillation
The effects of CRT on the burden of AF have raised some conflicting results. Many observational studies have suggested that CRT reduced AF, whereas some of the large CRT clinical trials (CARE HF and MADIT CRT) failed to demonstrate a significant reduction in the burden of AF. 7 , 27 RAFT even suggested an increase in AF in the CRT arm, although CARE HF and RAFT used clinical AF rather than device‐detected AF. 9 This study has demonstrated significant reductions in AF in patients with aCRT programmed ON. The reduction in AF was present irrespective of whether the definition of AF was based on time to AF burden (≥5.5 hours) or time to first AF occurrence at various episode durations (6 minutes to 30 days).
The mechanism of the reduction in AF is uncertain. The benefits in AF reduction in the aCRT IDE trial were greater in patients with longer baseline AV conduction and did not appear to be related to a reduction in RV pacing. 15 The importance of the AV interval in response to CRT and the development of AF have been postulated. Perhaps the more physiological AV intervals may explain the benefit of the algorithm. The RAFT investigators postulated that overly short programming of AV intervals may explain their observation of increased AF with CRT. 9
Looking beyond the individual components of the aCRT algorithm to the reported clinical benefits associated with aCRT may shed more light upon potential mechanisms of benefit. As previously reported, and replicated in the present analysis, aCRT is associated with a reduction in both AF incidence and daily AF burden. 15 , 16 Population‐based studies have consistently shown that AF is associated with increased mortality risk. 6 Additionally, a recent single‐center study found that CRT patients with intermittent AF are at a significantly increased risk of developing acute decompensated HF or death. 28 Thus, it is plausible that the reduced incidence of AF associated with the aCRT algorithm may contribute to the reduction in mortality observed.
Our findings provide a signal for aCRT benefit in reducing mortality and AF burden, in a real‐world population. Ongoing studies in the randomized setting will be important in confirming this benefit in a controlled setting. The AdaptResponse trial (NCT02205359) is a prospective, randomized, parallel, controlled, single‐blinded global trial, which plans to enroll 3800 patients with LBBB, PR interval less than or equal to 200 ms, and QRS duration of 140 ms or longer for males or 130 ms or longer for females. Risk of HF event or all‐cause mortality and risk of AF will be compared between aCRT and standard BiV pacing.
5. LIMITATIONS
This is a prospective nonrandomized registry and has inherent weaknesses related to this design. Nonetheless, real‐world observational studies have become increasingly important to describe clinical practice particularly in terms of clinical outcomes, safety, and economic value.
Patient medical history and comorbidities are self‐reported and could be underreported. Our analysis was retrospective in nature and programming requirements were not prespecified. There were also no requirements for device interrogation frequency. We observed patient demographics difference at baseline. These differences may be due to physician practices preferences or specific patient populations at the site. Information on why aCRT was not utilized in available devices was not collected, nor was information on social class or access to health care. Despite these limitations, the large, real‐world cohort and use of mortality as an endpoint contribute to the robustness of the analysis.
6. CONCLUSIONS
The use of the AdaptivCRT algorithm was associated with improved patient survival compared with standard CRT in a large real‐world, prospective, nonrandomized registry. The aCRT algorithm was also associated with a reduced risk of developing AF, as well as a reduction in daily AF burden.
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors thank Shufeng Liu of Medtronic for statistical analysis support and Dedra Fagan also of Medtronic for assistance in the preparation of this manuscript. The Personalized CRT Study, part of the Medtronic Product Surveillance Registry (PSR) (http://ClinicalTrials.gov ID: NCT01524276), is funded by Medtronic, Inc.
Singh JP, Cha Y‐M, Lunati M, et al. Real‐world behavior of CRT pacing using the AdaptivCRT algorithm on patient outcomes: Effect on mortality and atrial fibrillation incidence. J Cardiovasc Electrophysiol. 2020;31:825–833. 10.1111/jce.14376
Disclosures: JPS: Compensation for Services; Medtronic, Boston Scientific, Abbott Laboratories, LivaNova, Impulse Dynamics, Biotronik; YMC: Compensation for Services; Medtronic; SL: Employee/Shareholder: Medtronic; PS: Employee/Shareholder: Medtronic; DO: Compensation for Services; Medtronic; Fellowship Support; Medtronic, Abbott. Other author: No disclosure.
REFERENCES
- 1. Brignole M, Auricchio A, Baron‐Esquivias G, et al. ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34:2281‐2329. [DOI] [PubMed] [Google Scholar]
- 2. Zipes DP, Camm AJ, Borggrefe M, et al. American College of Cardiology/American Heart Association Task Force, European Society of Cardiology Committee for Practice Guidelines, European Heart Rhythm Association, Heart Rhythm Society: ACC/AHA/ESC 2006 Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention Of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385‐e484. [DOI] [PubMed] [Google Scholar]
- 3. Cleland JG, Abraham WT, Linde C, et al. An individual patient meta‐analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J. 2013;34:3547‐3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Linde C, Abraham WT, Gold MR, et al. Predictors of short‐term clinical response to cardiac resynchronization therapy. Eur J Heart Fail. 2017;19:1056‐1063. [DOI] [PubMed] [Google Scholar]
- 5. Woods B, Hawkins N, Mealing S, et al. Individual patient data network meta‐analysis of mortality effects of implantable cardiac devices. Heart. 2015;101:1800‐1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946‐952. [DOI] [PubMed] [Google Scholar]
- 7. Hoppe UC, Casares JM, Eiskjaer H, et al. Effect of cardiac resynchronization on the incidence of atrial fibrillation in patients with severe heart failure. Circulation. 2006;114:18‐25. [DOI] [PubMed] [Google Scholar]
- 8. Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920‐2925. [DOI] [PubMed] [Google Scholar]
- 9. Wilton SB, Exner DV, Wyse DG, et al. Frequency and outcomes of postrandomization atrial tachyarrhythmias in the Resynchronization/Defibrillation in Ambulatory Heart Failure Trial. Circ Arrhythm Electrophysiol. 2016;9(5):e003807. [DOI] [PubMed] [Google Scholar]
- 10. Mullens W, Grimm RA, Verga T, et al. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009;53:765‐773. [DOI] [PubMed] [Google Scholar]
- 11. Krum H, Lemke B, Birnie D, et al. A novel algorithm for individualized cardiac resynchronization therapy: rationale and design of the adaptive cardiac resynchronization therapy trial. Am Heart J. 2012;163:747‐752. [DOI] [PubMed] [Google Scholar]
- 12. Martin DO, Lemke B, Birnie D, et al. Study Investigators: Investigation of a novel algorithm for synchronized left‐ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: results of the adaptive CRT trial. Heart Rhythm. 2012;9:1807‐1814. [DOI] [PubMed] [Google Scholar]
- 13. Starling RC, Krum H, Bril S, et al. Impact of a novel adaptive optimization algorithm on 30‐day readmissions: evidence from the Adaptive CRT Trial. JACC Heart Fail. 2015;3:565‐572. [DOI] [PubMed] [Google Scholar]
- 14. Birnie D, Lemke B, Aonuma K, et al. Clinical outcomes with synchronized left ventricular pacing: analysis of the adaptive CRT trial. Heart Rhythm. 2013;10:1368‐1374. [DOI] [PubMed] [Google Scholar]
- 15. Birnie D, Hudnall H, Lemke B, et al. Continuous optimization of cardiac resynchronization therapy reduces atrial fibrillation in heart failure patients: results of the Adaptive Cardiac Resynchronization Therapy Trial. Heart Rhythm. 2017;14:1820‐1825. [DOI] [PubMed] [Google Scholar]
- 16. Hsu JC, Birnie D, Stadler RW, Cerkvenik J, Feld GK, Birgersdotter‐Green U. Adaptive cardiac resynchronization therapy is associated with decreased risk of incident atrial fibrillation compared to standard biventricular pacing: A real‐world analysis of 37,450 patients followed by remote monitoring. Heart Rhythm. 2019;16:983‐989. [DOI] [PubMed] [Google Scholar]
- 17. Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474‐480. [DOI] [PubMed] [Google Scholar]
- 18. Hougaard P. Frailty models for survival data. Lifetime Data Anal. 1995;1:255‐273. [DOI] [PubMed] [Google Scholar]
- 19. Kass DA, Chen CH, Curry C, et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99:1567‐1573. [DOI] [PubMed] [Google Scholar]
- 20. Leclercq C, Kass DA. Retiming the failing heart: principles and current clinical status of cardiac resynchronization. J Am Coll Cardiol. 2002;39:194‐201. [DOI] [PubMed] [Google Scholar]
- 21. van Gelder BM, Bracke FA, Meijer A, Pijls NH. The hemodynamic effect of intrinsic conduction during left ventricular pacing as compared to biventricular pacing. J Am Coll Cardiol. 2005;46:2305‐2310. [DOI] [PubMed] [Google Scholar]
- 22. Lee KL, Burnes JE, Mullen TJ, Hettrick DA, Tse HF, Lau CP. Avoidance of right ventricular pacing in cardiac resynchronization therapy improves right ventricular hemodynamics in heart failure patients. J Cardiovasc Electrophysiol. 2007;18:497‐504. [DOI] [PubMed] [Google Scholar]
- 23. Varma N, Jia P, Ramanathan C, Rudy Y. RV electrical activation in heart failure during right, left, and biventricular pacing. JACC Cardiovasc Imaging. 2010;3:567‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ellenbogen KA, Gold MR, Meyer TE, et al. Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART‐AV) trial: a randomized trial comparing empirical, echocardiography‐guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation. 2010;122:2660‐2668. [DOI] [PubMed] [Google Scholar]
- 25. Brugada J, Delnoy PP, Brachmann J, et al. Investigators: contractility sensor‐guided optimization of cardiac resynchronization therapy: results from the RESPOND‐CRT trial. Eur Heart J. 2017;38:730‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh JP, Aydin A, Murgatroyd F, et al. Automatic contractility sensor‐guided optimization is associated with improved outcomes in CRT subgroups at high risk of non‐response. Heart Rhythm. 2017;38:14‐738. Abstract. [Google Scholar]
- 27. Brenyo A, Link MS, Barsheshet A, et al. Cardiac resynchronization therapy reduces left atrial volume and the risk of atrial tachyarrhythmias in MADIT‐CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy). J Am Coll Cardiol. 2011;58:1682‐1689. [DOI] [PubMed] [Google Scholar]
- 28. Nakajima I, Noda T, Kanzaski H, et al. Development of heart failure from transient atrial fibrillation attacks in responders to cardiac resynchronization therapy. JACC Clin Electrophysiol. 2018;4:1227‐1234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


