Abstract
Introduction
Respiratory syncytial virus infection in early childhood has been linked to longer‐term respiratory morbidity; however, debate persists around its impact on asthma. The objective was to assess the association between respiratory syncytial virus hospitalization and childhood asthma.
Methods
Asthma hospital admissions and medication use through 18 years were compared in children with (cases) and without (controls) respiratory syncytial virus hospitalization in the first 2 years of life. All children born in National Health Service Scotland between 1996 and 2011 were included.
Results
Of 740 418 children (median follow‐up: 10.6 years), 15 795 (2.1%) had a respiratory syncytial virus hospitalization at ≤2 years (median age: 143 days). Asthma hospitalizations were three‐fold higher in cases than controls (8.4% vs 2.4%; relative risk: 3.3, 95% confidence interval [CI]: 3.1‐3.5; P < .0001) and admission rates were four‐fold higher (193.2 vs 46.0/1000). Cases had two‐fold higher asthma medication usage (25.5% vs 14.7%; relative risk: 1.7, 95% CI: 1.7‐1.8; P < .0001) and a three‐fold higher rate of having both an asthma admission and medication (4.8% vs 1.5%; relative risk 3.1, 95% CI: 2.9‐3.3; P < .0001). Admission rates and medication use remained significantly (P < .001) higher for cases than controls throughout childhood (admissions: ≥2‐fold higher; medication: ≥1.5‐fold higher). Respiratory syncytial virus hospitalization was the most significant risk factor for asthma hospitalizations±medication use (odds ratio: 1.9‐2.8; P < .001).
Conclusions
Respiratory syncytial virus hospitalization was associated with significantly increased rates and severity of asthma throughout childhood, which has important implications for preventive strategies.
Keywords: bronchiolitis, long‐term, LRTI, respiratory morbidity, wheezing
1. INTRODUCTION
Respiratory syncytial virus (RSV) lower respiratory tract infection (LRTI) causes a substantial burden to pediatric services during the winter months, with an estimated 383 000 RSV hospitalizations (RSVHs) and 3300 deaths in children aged less than 5 years in high‐income countries during 2015. 1 In addition to this acute burden, there is increasing evidence that RSV LRTI in young children (<3 years) is associated with longer‐term respiratory morbidity, namely recurrent wheezing and asthma. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 Studies investigating recurrent wheezing following RSV LRTI suggest that this might be transient, persisting for several years, but normalizing by around 6 to 11 years. 3 , 4 , 9 The evidence for asthma is more equivocal, with some studies suggesting no clear evidence of a definitive association, 5 , 10 while others report a significantly increased risk throughout childhood. 6 , 7 , 8 Demonstrating an association with asthma is challenging, particularly due to the difficulties in confirming an asthma diagnosis in young children, establishing a causal link with RSV LRTI, ratifying how this relates to severity of infection, and the need for long‐term (throughout childhood) follow‐up. 2 , 11 How asthma may be defined is also a key consideration, including whether it is current/active. 2
The objective of this study was to use national population data from Scotland to determine whether severe RSV LRTI in young children (≤2 years) is associated with increased rates of asthma development and to quantify this association throughout childhood (up to 18 years of age). Our study focused on severe RSV LRTI, as defined by RSVH, and asthma defined by diagnostic hospital codes and medication use to provide more in‐depth appreciation of the true link.
2. MATERIALS AND METHODS
2.1. Study population
The Information Services Division (ISD) datasets (Scottish Maternity and Birth Record [SMR02], Scottish Birth Record [SBR], General/Acute Inpatient and Day Case Record [SMR01], and Outpatient Attendance Record [SMR00]) provide linked data on more than 5 million people receiving care in NHS Scotland hospitals. 12 Data on all live births from 1996 to 2011 were assessed, with children followed until 18 years or until the study end in 2014, whichever came sooner. Those who died or left Scotland during the study period or whose records were unable to be linked between datasets were excluded.
The study population was split into two cohorts: children with a recorded RSVH during their first 2 years of life (cases) and a comparator group containing all other children (controls). RSVHs were identified using three RSV‐specific, International Classification of Diseases 10th Revision (ICD‐10) codes (Table 1). Age at first RSVH, length of stay (LOS), and intensive care unit (ICU) admission were assessed.
Table 1.
Diagnostic codes for RSV, asthma, and comorbidities
| Condition | ICD‐10 code | ICD‐10 code definition |
|---|---|---|
| RSV | J12.1 | RSV pneumonia |
| J20.5 | Acute bronchitis due to RSV | |
| J21.0 | Acute bronchiolitis due to RSV | |
| Asthma | J45 | Asthma |
| J46 | Status asthmaticus | |
| Comorbidities | Q20‐Q26 | Congenital heart disease or pulmonary hypertension |
| P271, Q30‐Q34 | Congenital lung disease or bronchopulmonary dysplasia | |
| Q90 | Down syndrome | |
| E84 | Cystic fibrosis | |
| G80 | Cerebral palsy | |
| G71 | Neuromuscular disorders |
Abbreviations: ICD‐10, International Classification of Diseases 10th revision; RSV, respiratory syncytial virus.
2.2. Baseline and demographic factors
Sex, gestational age at birth, birth weight, and diagnosis with a comorbidity associated with increased RSVH risk (identified using ICD‐10 codes; Table 1) were assessed for all children. Need for emergency cesarean, multiple births, mother's age at birth, mother's smoking history at predelivery assessment (self‐declared), mother's previous pregnancies, and mother's socioeconomic status, using the Scottish Index of Multiple Deprivation (SIMD; based on post code/Zip code, where 1 = most deprived and 5 = least deprived), 13 were also assessed.
2.3. Asthma outcomes
There were two primary outcomes: asthma admissions (identified using ICD‐10 codes; Table 1) and asthma medication use (from the Prescribing Information System for Scotland) through 18 years. 14 The number of children hospitalized for asthma and the total number of asthma admissions (to assess multiple admissions for a child) were captured. Prescribing data were available for the final 3 years of the study (2011‐2014) and collected on the following asthma medications: bronchodilators (short‐ and long‐acting), corticosteroids (inhaled), cromoglycate and related therapy, leukotriene receptor antagonists, and phosphodiesterase type‐4 inhibitors. A composite outcome of “confirmed asthma” was defined as a child with an asthma admission and requirement for (any) asthma medication. Incidence and rates per 1000 children, overall and over time, were calculated. An analysis of asthma admissions from 2 years of age onwards was undertaken to assess the impact of early asthma diagnosis during the time period of RSVH.
2.4. Statistical analysis
χ 2 tests and t‐tests were used to compare baseline and demographic characteristics between cohorts. Differences in incidence and rates between cohorts were expressed as a weighted relative risk (RR) with 95% confidence intervals (CIs). Age at first asthma admission for cases and controls was compared by Kaplan–Meier analysis and Mantel–Cox log rank test. Logistic regression was used to test the association of asthma with RSVH and all baseline and demographic factors, with adjusted odds ratios (ORs) calculated.
All analyses were performed using SPSS for Windows version 15.0 and Microsoft Excel.
2.5. Ethical considerations
This study was conducted in accordance with applicable local regulations and the Declaration of Helsinki principles. The National Research Ethics Service confirmed formal ethical approval was not required and the ISD Privacy Advisory Committee gave their approval. All data were accessed and analyzed using the National Services Scotland “Safe Haven” system to ensure security, anonymity, and confidentiality.
3. RESULTS
3.1. Study population
A total of 791 684 children were identified and, after exclusion of émigrés (47 345) and those that died during the study period (3921), 740 418 were included in the study for a median follow‐up of 10.6 (interquartile range [IQR]: 6.9‐14.6) years. Of the 740 418 children, 15 795 (2.1%) had a RSVH in the first 2 years of life (17 271 total admissions). The median age at first RSVH was 143 days (IQR: 64‐274), with 85.8% (13 472/17 271) occurring within the 1st year of life (59.3% [10 237] in first 6 months). Median LOS in hospital was 3 days (IQR: 1‐5). ICU admission was required by 3.8% (657) of RSV admissions for a median of 5 (IQR: 2‐8) days. The remaining 724 623 (97.9%) children were controls. Median follow‐up was 10.2 (IQR: 6.6‐14.4) years for cases and 10.6 (IQR: 6.9‐14.6) years for controls.
Cases were significantly (P < .0001) more likely than controls to be male (56.6% vs 51.0%, respectively), part of a multiple birth (5.4% vs 2.8%), delivered by emergency cesarean (16.0% vs 14.3%), born at a lower gestational age (median 39 vs 40 weeks), have lower birth weight (median 3270 vs 3410 g), or have a comorbidity that placed them at increased risk of RSVH (8.0% vs 1.5%; Table 2). Mothers of RSV‐hospitalized children were significantly (P < .0001) younger at the time of birth (median 28 vs 29 years), were more likely to be current smokers at predelivery assessment (36.7% vs 25.9%), to have had ≥1 prior pregnancy (75.0% vs 63.8%), or to be resident in the most socially deprived areas (SIMD 1: 31.7% vs 25.7%) compared with their counterparts.
Table 2.
Baseline characteristics and demographics
| Cases | Controls | ||
|---|---|---|---|
| Number of children | 15 795 | 724 623 | |
| Mothers | |||
| Age at giving birth, y | Mean (SD) | 28 (1.5) a | 29 (1.5) |
| Median (IQR) | 28 (23‐32) | 29 (24‐33) | |
| Mother's SIMD quintile, n/N (%) | 1 – most deprived | 4992/15 758 (31.7) a | 185 494/722 908 (25.7) |
| 2 | 3404/15 758 (21.6) b | 148 955/722 908 (20.6) | |
| 3 | 2684/15 758 (17.0) a | 133 638/722 908 (18.5) | |
| 4 | 2409/15 758 (15.3) a | 129 472/722 908 (17.9) | |
| 5 – least deprived | 2269/15 758 (14.4) a | 125 349/722 908 (17.3) | |
| Mother's smoking history at predelivery assessment, n/N (%) | Current | 5234/14 271 (36.7) a | 169 931/656 611 (25.9) |
| Former | 1248/14 271 (8.7) a | 66 310/656 611 (10.1) | |
| Never | 7789/14 271 (54.6) a | 420 370/656 611 (64.0) | |
| Mother's previous pregnancies, n/N (%) | 0 | 3948/15 795 (25.0) a | 262 133/724 621 (36.2) |
| 1 | 5155/15 795 (32.6) c | 225 966/724 621 (31.2) | |
| 2+ | 6692/15 795 (42.4) c | 236 522/724 621 (32.6) | |
| Children and births | |||
| Sex, n/N (%) | Male | 8934/15 793 (56.6) a | 369 792/724 596 (51.0) |
| Multiple births, n/N (%) | Multiple | 857/15 793 (5.4) a | 20 271/724 599 (2.8) |
| Emergency cesarean | |||
| n/N (%) | Yes | 2527/15 795 (16.0) a | 103 637/724 623 (14.3) |
| Gestational age at birth, wk | Mean (SD) | 38 (0.3) a | 39 (0.3) |
| Median (IQR) | 39 (37‐40) | 40 (39‐41) | |
| Birth weight, g | Mean (SD) | 3162 (143) a | 3381 (117) |
| Median (IQR) | 3270 (2790‐3660) | 3410 (3050‐3760) | |
| Comorbidities, n/N (%) | Any comorbidity d | 1269/15 795 (8.0) a | 10 566/724 623 (1.5) |
Abbreviations: IQR, interquartile range; RSV, respiratory syncytial virus; SD, standard deviation; SIMD, Scottish Index of Multiple Deprivation.
P < .0001 vs control group.
P < .01 vs control group.
P < .0001 for combined ≥1 pregnancies vs control group.
Diagnosis with any predefined comorbidity as detailed in Table 1.
3.2. Asthma hospitalizations
Significantly more cases than controls were hospitalized for asthma during childhood (8.4% vs 2.4%, respectively; RR: 3.3, 95% CI: 3.1‐3.5, P < .0001; Mantel–Cox log rank test: P < .001; Table 3). Nearly half (43.7%) of cases required more than one asthma admission compared with approximately a third (35.2%) of controls (RR: 4.0, 95% CI: 3.7‐4.3, P < .0001). The relative difference between cases and controls was greater for children who had more than two asthma admissions (23.2% vs 17.1%, respectively; RR: 4.3, 95% CI: 3.9‐4.8, P < .0001). The mean number of asthma admissions per hospitalized child was 2.3 for cases and 1.9 for controls (P < .0001). Despite cases representing only 2.1% of the total study population, this cohort accounted for 7.0% (1326/18 967) of all children with an asthma hospitalization and 8.4% (3052/36 403) of all recorded asthma admissions. Overall, the asthma admission rate was 193.2/1000 for cases compared with 46.0/1000 for controls.
Table 3.
Asthma admissions and asthma medication usage
| Cases (n = 15 795) | Controls (n = 724 623) | RR (95% CI) | |
|---|---|---|---|
| Children admitted for asthma, n (%) | 1326 (8.4) | 17 641 (2.4) | 3.3 (3.1‐3.5) a |
| Total asthma admissions, n | 3052 | 33 351 | … |
| Admission rate per 1000 children | 193.2 | 46.0 | … |
| Asthma admissions in first year of life, n/N (%) | 46/3025 (1.5) | 194/33 351 (0.6) | … |
| Children with 1 asthma admission, n/N (%) | 747/1326 (56.3) | 11 423/17 641 (64.8) | 2.9 (2.7‐3.1) a |
| Children with >1 asthma admission, n/N (%) | 579/1326 (43.7) | 6218/17 641 (35.2) | 4.0 (3.7‐4.3) a |
| Children with >2 asthma admissions, n/N (%) | 308/1326 (23.2) | 3018/17 641 (17.1) | 4.3 (3.9‐4.8) a |
| Mean asthma admissions per child admitted, n | 2.3 b | 1.9 | … |
| Median age at first asthma admission, days | 893 c | 944 | … |
| Children using asthma medication, d n (%) | 4023 (25.5) | 106 828 (14.7) | 1.7 (1.7‐1.8) a |
| Total children with “confirmed asthma”, n (%) | 756 (4.8) | 10 655 (1.5) | 3.1 (2.9‐3.3) a |
| Total asthma admissions for children with “confirmed asthma”, n | 1857 | 21 363 | … |
| Admission rate per 1000 children for “confirmed asthma” | 117.6 | 29.5 | … |
| Mean “confirmed asthma” admissions per child admitted, n | 2.5 b | 2.0 | … |
Note: Blank cells are included where relative risks are not relevant and could not be calculated.
Abbreviations: CI, confidence interval; RR, relative risk.
P < .0001.
P < .0001 vs control group.
P < .001 vs control group.
Short‐ and long‐acting bronchodilators, inhaled corticosteroids, cromoglycate and related therapy, leukotriene receptor antagonists, and phosphodiesterase type‐4 inhibitors.
Admission rates were at least two‐fold higher in cases than controls at all ages throughout childhood, with the difference being most pronounced (≥3.5‐fold) below the age of 7 years (Figure 1). The asthma admission rate peaked around 2 to 4 years of age in both cohorts, with cases having their first asthma hospitalization at a median age of 893 days compared to 944 days for controls (hazard ratio: 3.7, 95% CI: 3.5‐3.9; Mantel–Cox log rank test: P < .001). At 17 to 18 years, the asthma admission rate was 9.6/1000 for cases and 2.8/1000 for controls (RR: 3.3, 95% CI: 2.0‐5.4, P < .0001).
Figure 1.
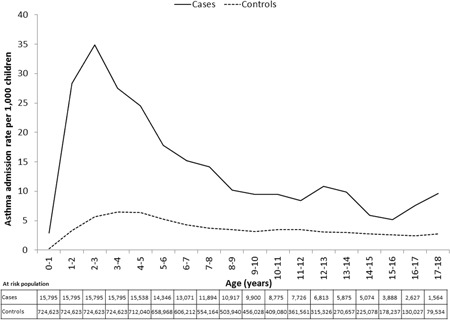
Asthma admission rate through childhood. Figure plots asthma admission rate (per 1000 children) against age (years) for cases and controls. Median follow‐up time was 10.2 (interquartile range: 6.6‐14.4) years for cases and 10.6 (6.9‐14.6) years for controls
The incidence of asthma admissions remained significantly higher for cases (5.2%; 797/15 246) than controls (2.0%; 14 480/721 367) when restricted to those occurring from year 2 onwards (RR: 2.5, 95% CI: 2.3‐2.7, P < .0001; Mantel–Cox log rank test; P < .001).
3.3. Asthma medication use
Almost twice as many cases as controls received one or more asthma medications during childhood (25.5% vs 14.7%, respectively; RR: 1.7, 95% CI: 1.7‐1.8, P < .0001, Table 3). Cases (2.1% of total study population) accounted for 3.6% (4023/110 851) of all children on asthma medication. At all ages through childhood, a significantly (P < .001) higher proportion of cases than controls were on asthma medication (Figure 2). Between the ages of 14 and 18 years, approximately a fifth (18.0%) of cases had taken asthma medication, a rate approximately 40% higher than for controls (13.0%, P < .0001).
Figure 2.
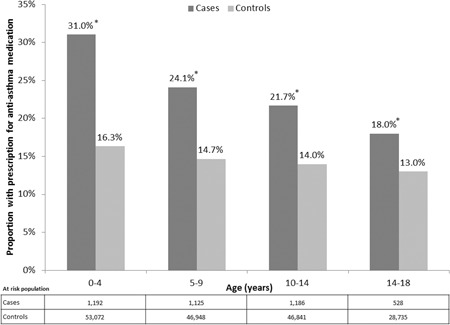
Asthma medication usage by age. *P < .0001 vs control group
3.4. Confirmed asthma
The incidence of “confirmed asthma” was significantly higher in cases than controls (4.8% vs 1.5%; RR: 3.1, 95% CI: 2.9‐3.3, P < .0001, Table 3). Cases accounted for 6.6% (756/11 411) of children with “confirmed asthma” and 8.0% (1857/23 220) of all admissions in these children. The mean number of admissions per child (2.5 vs 2.0, P < .0001) and the overall admission rate (117.6/1000 vs 29.5/1000) were both higher in cases than controls.
3.5. Risk factors for asthma
Logistic regression analysis showed that prior RSVH was the most salient risk factor for asthma hospitalizations (OR: 2.80, 95% CI: 2.60‐3.02, P < .001) and “confirmed asthma” (OR: 1.85, 95% CI: 1.78‐1.92, P < .001; Supporting Information eFigures 1 and 2). Other significant (P < .001) risk factors had considerably lower ORs (asthma hospitalizations: <1.8; “confirmed asthma”: ≤1.3), with the next three highest being for male sex (1.71, 95% CI: 1.65‐1.78; 1.29, 95% CI: 1.27‐1.30), presence of a predefined comorbidity (1.77, 95% CI: 1.59‐1.97; 1.26, 95% CI: 1.20‐1.32), and multiple births (1.36, 95% CI: 1.21‐1.52; 1.30, 95% CI: 1.24‐1.35).
4. DISCUSSION
This population‐level study of more than 740 000 children demonstrated that RSVH in early childhood (≤2 years) was an independent risk factor for asthma through 18 years of age (OR: 1.9‐2.8). In children with a history of RSVH, there was an approximately three‐fold higher (RR: 3.3) incidence of asthma hospitalizations, two‐fold higher rate of asthma medication use (RR: 1.7), and over three‐fold higher incidence of “confirmed asthma” (RR: 3.1), compared to controls. Asthma within the RSVH cohort appeared to be of greater severity, with increased rates of multiple admissions for both asthma and “confirmed asthma” compared to controls, with cases accounting for approximately 10% of all childhood asthma hospitalizations despite representing less than 3% of the total study population. Importantly, the significantly increased rates of asthma hospitalizations and asthma medication use in the RSVH cohort persisted throughout childhood, admission rates being more than double those seen in controls at any time point.
A number of previous studies have reported an association between RSV LRTI in early childhood (typically <3 years) and an increased rate of subsequent asthma. 6 , 7 , 8 , 15 , 16 , 17 , 18 , 19 Four of these studies included assessment that covered most or all of childhood (14‐20 years) 6 , 7 , 10 , 18 ; with the rest typically having a follow‐up of less than 8 years. 8 , 15 , 16 , 17 , 19 In the four studies covering most of childhood, the asthma rate was approximately 1.5 to 4.5 times higher in the RSVH group than control group at final follow‐up, 6 , 7 , 10 , 18 which is in line with the 1.5‐fold difference found in our study for asthma hospitalizations at 17 to 18 years. In contrast to our study, however, a Finnish study by Korppi et al 10 found RSVH at less than 2 years not to be an independent risk factor for current doctor‐diagnosed asthma at 18 to 20 years. These contradictory findings may be due to several reasons, including how asthma was defined (on‐going asthma medication or doctor‐diagnosed vs hospitalizations and/or medication use in our study), substantially different patient numbers (N = 81 vs N = 740 418), and, perhaps most importantly, that we assessed asthma through childhood (up to 18 years) rather than at one age band (18‐20 years). It is also interesting to note that a recent study by Homaira et al 20 demonstrated that the incidence of asthma was highest in children with an RSVH at ≥6 months compared to those who had RSVH at <6 months; such an association was not investigated within our study.
An important finding from our study was that RSVH appeared to be not only associated with an increased incidence of asthma, but also with an increased severity, as indicated by number of admissions per child. Somewhat similar results were reported in a Canadian study by Szabo et al 15 involving a cohort of 145 430 children followed up for up to 10 years. 15 While a diagnosis of chronic respiratory morbidity (>98% asthma) was reported to be approximately three‐fold higher in those with a history of RSVH at <2 years vs nonhospitalized controls, the incidence of hospitalizations for respiratory morbidity was 4.5‐fold higher; however, it is unclear whether this difference might relate to increased non‐asthma, respiratory admissions. 15 Our study, therefore, appears to be the first to clearly indicate an increased severity as well as incidence of asthma following RSVH in early childhood. It may be that the previously observed long‐term alterations to respiratory tissues linked to RSV infection, such as to the lung macrophage population and phenotype, as well as changes to cell‐mediated immunity (eg, TH1/TH2 balance), 21 , 22 lead to more severe asthma exacerbations than are found in those children without such a history of infection. The severity of RSV infection might also be an important factor, as it has been reported in a US study (N = 72 602) that this was positively correlated with the incidence of wheezing/asthma. 23 There is also the potential that underlying risk factors may lead to an increased predisposition to both severe RSV and asthma, with a number of polymorphisms in immune system genes associated with both conditions. 24 Further studies are required to support these findings and to elucidate the potential mechanisms behind it.
The main limitations of this retrospective study were a reliance on the available datasets and the accuracy of coding within them. As RSV was routinely tested at virtually all pediatric inpatients unit in Scotland during the study period (email communication between Scottish units and Jonathan Coutts, MBChB, December 2018); the coding can be assumed to be generally reliable. In terms of the ISD datasets, these have been proven to be comprehensive and robust, with analysis finding that during the study period, 98.2% of all births in Scotland were recorded, 25 almost complete population‐level coverage. Other limitations of the available data include that only self‐reported smoking data was available (smoke exposure is a key known risk factor for RSVH), prescribing data were available only at the end of the study period, and sufficient detail was not available to be able to account for all potential confounding factors, such as atopic comorbidities (eg, atopic dermatitis) and food allergies. However, none of these data limitations would be expected to have a significant impact on the main result—a significant association between RSVH and subsequent asthma. Whether the presence of atopic comorbidities, familial atopy, and food allergies, etc. influences the incidence or severity of asthma following RSVH warrants further exploration.
A prominent issue for studies such as ours is the recognized difficulties in confirming an asthma diagnosis, particularly in younger children, which has led to a variety of definitions being used. A recent expert review recommended that to provide clarity on the relationship between RSV LRTI and asthma, future studies should use a definition of physician‐confirmed asthma combined with usage of asthma medication. 2 Our study followed this recommendation with the combined “confirmed asthma” outcome of asthma hospitalization and medication use. It is acknowledged that asthma hospitalizations are somewhat limited in excluding recurrent wheeze in younger children by being defined by ICD‐10 codes (only method available); however, asthma specific codes were used and the data from older children, where a more robust diagnosis of asthma is possible, support the reported association.
This population‐level study provides strong evidence for a significant, independent link between severe RSV LRTI in young children and asthma persisting throughout childhood. This association was demonstrated by an increased rate of asthma hospitalizations and increased prescribing of asthma medications in children who had RSVH. RSVH was also found to increase the frequency of asthma admissions, suggesting increased severity of disease. The clarification of this relationship has important implications for RSV prevention and treatment, particularly surrounding the potential benefits of RSV prophylaxis in reducing long‐term asthma morbidity.
CONFLICT OF INTERESTS
JC and RT have received research funding and/or compensation as advisor/lecturer from AbbVie. BRG, SB and JF are employees of Strategen. Strategen has received consultancy and research fees from AbbVie for work on this and other projects. CM is an employee of ISD Scotland. ISD Scotland has received research fees from AbbVie for data analysis work on this project. EG is an employee of AbbVie and holds stock in AbbVie.
AUTHOR CONTRIBUTION
AbbVie participated in the study design, research, data collection, analysis and interpretation of data, writing, reviewing, and approving the publication. All authors contributed to the development of the publication and maintained control over the final content.
Supporting information
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors wish to thank Suhail Iqbal of ISD Scotland for his work as a data analyst on this study and Matthew Freddi of Strategen for his medical writing and editing services in the development of this publication. AbbVie provided funding for these services.
Coutts J, Fullarton J, Morris C, et al. Association between respiratory syncytial virus hospitalization in infancy and childhood asthma. Pediatric Pulmonology. 2020;55:1104–1110. 10.1002/ppul.24676
REFERENCES
- 1. Shi T, McAllister DA, O'Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet. 2017;390(10098):946‐958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fauroux B, Simões EAF, Checchia PA, et al. The burden and long‐term respiratory morbidity associated with respiratory syncytial virus infection in early childhood. Infect Dis Ther. 2017;6(2):173‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carbonell‐Estrany X, Pérez‐Yarza EG, García LS, Guzmán Cabañas JM, Bòria EV, Atienza BB IRIS (Infección Respiratoria Infantil por Virus Respiratorio Sincitial) Study Group . Long‐term burden and respiratory effects of respiratory syncytial virus hospitalization in preterm infants‐the SPRING study. PLoS One. 2015;10(5):e0125422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354(9178):541‐545. [DOI] [PubMed] [Google Scholar]
- 5. Scheltema NM, Nibbelke EE, Pouw J, et al. Respiratory syncytial virus prevention and asthma in healthy preterm infants: A randomised controlled trial. Lancet Respir Med. 2018;6(4):257‐264. [DOI] [PubMed] [Google Scholar]
- 6. Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 year after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65(12):1045‐1052. [DOI] [PubMed] [Google Scholar]
- 7. Green CA, Yeates D, Goldacre A, et al. Admission to hospital for bronchiolitis in England: trends over five decades, geographical variation and association with perinatal characteristics and subsequent asthma. Arch Dis Child. 2016;101(2):140‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: A longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16(5):386‐392. [DOI] [PubMed] [Google Scholar]
- 9. Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171(2):137‐141. [DOI] [PubMed] [Google Scholar]
- 10. Korppi M, Piippo‐Savolainen E, Korhonen K, Remes S. Respiratory morbidity 20 years after RSV infection in infancy. Pediatr Pulmonol. 2004;38(2):155‐160. [DOI] [PubMed] [Google Scholar]
- 11. Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention, 2018. https://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/. Accessed June 20 2018.
- 12. Information Services Division (ISD) Scotland . About ISD. http://www.isdscotland.org/. Accessed August 30 2018.
- 13. Scottish Government . Scottish Index of Multiple Deprivation: Background and methodology. http://www.gov.scot/Topics/Statistics/SIMD/BackgroundMethodology. Accessed September 09 2019.
- 14. Alvarez‐Madrazo S, McTaggart S, Nangle C, Nicholson E, Bennie M. Data resource profile: The Scottish national Prescribing Information System (PIS). Int J Epidemiol. 2016;45(3):714‐715f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Szabo SM, Gooch KL, Korol EE, et al. A population‐based study of childhood respiratory morbidity after severe lower respiratory tract infections in early childhood. J Pediatr. 2014;165(1):123‐128. [DOI] [PubMed] [Google Scholar]
- 16. James KM, Gebretsadik T, Escobar GJ, et al. Risk of childhood asthma following infant bronchiolitis during the respiratory syncytial virus season. J Allergy Clin Immunol. 2013;132(1):227‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Homaira N, Briggs N, Pardy C, et al. Association between respiratory syncytial viral disease and the subsequent risk of the first episode of severe asthma in different subgroups of high‐risk Australian children: A whole‐of‐population‐based cohort study. BMJ Open. 2017;7(11):e017936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruotsalainen M, Hyvärinen MK, Piippo‐Savolainen E, Korppi M. Adolescent asthma after rhinovirus and respiratory syncytial virus bronchiolitis. Pediatr Pulmonol. 2013;48(7):633‐639. [DOI] [PubMed] [Google Scholar]
- 19. Zomer‐Kooijker K, van der Ent CK, Ermers MJ, Uiterwaal CS, Rovers MM, Bont LJ RSV Corticosteroid Study Group . Increased risk of wheeze and decreased lung function after respiratory syncytial virus infection. PLoS One. 2014;9(1):e87162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Homaira N, Briggs N, Oei JL, et al. Association of age at first severe RSV disease with subsequent risk of severe asthma: A population‐based cohort study. J Infect Dis. 2019;220:550‐556. [DOI] [PubMed] [Google Scholar]
- 21. Restori KH, Srinivasa BT, Ward BJ, Fixman ED. Neonatal immunity, respiratory virus infections, and the development of asthma. Front Immunol. 2018;9:1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Korppi M, Nuolivirta K, Lauhkonen E, et al. IL‐10 gene polymorphism is associated with preschool atopy and early‐life recurrent wheezing after bronchiolitis in infancy. Pediatr Pulmonol. 2017;52(1):14‐20. [DOI] [PubMed] [Google Scholar]
- 23. Escobar GJ, Masaquel AS, Li SX, Walsh EM, Kipnis P. Persistent recurring wheezing in the fifth year of life after laboratory‐confirmed, medically attended respiratory syncytial virus infection in infancy. BMC Pediatr. 2013;13:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feldman AS, He Y, Moore ML, Hershenson MB, Hartert TV. Toward primary prevention of asthma. Reviewing the evidence for early‐life respiratory viral infections as modifiable risk factors to prevent childhood asthma. Am J Respir Crit Care Med. 2015;191:34‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Information Services Division . Births in Scottish Hospitals. http://www.isdscotland.org/Health-Topics/Maternity-and-Births/Births/. Accessed September 09 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Supporting information


