Abstract
Background and Aim
The CHORUS study (Chronic venous and HemORrhoidal diseases evalUation and Scientific research) was conducted to provide data on patients presenting with hemorrhoidal disease (HD) in clinical practice and to explore the frequency with which it coexists with chronic venous disease (CVD) and shared risk factors.
Methods
This international, noninterventional study enrolled adult patients attending a consultation for hemorrhoidal complaints. The questionnaire completed by physicians established the subjects' demographic and lifestyle characteristics and collected information on HD grade and symptoms and signs of CVD.
Results
A total of 5617 patients were analyzed. Symptoms commonly reported were bleeding (71.8%), pain (67.4%), swelling (55.0%), itching (44.1%), and prolapse (36.2%). Multivariate analysis revealed the variables with the strongest association with HD severity were older age, higher CVD CEAP (Clinical manifestations, Etiologic factors, Anatomic distribution of disease, and underlying Pathophysiology) class, constipation, and male gender (all P < 0.0001). Elevated BMI was a risk factor for HD recurrence. Among women, number of births had a significant association with both HD grade and recurrence. The presence of CVD, reported in approximately half the patients (51.2%), was strongly associated with advanced grade of HD (P < 0.0001). Treatments most commonly prescribed were venoactive drugs (94.3%), dietary fiber (71.4%), topical treatment (70.3%), analgesics (26.3%), and surgery (23.5%).
Conclusions
CHORUS provides a snap shot of current profiles, risk factors, and treatments of patients with HD across the globe. The coexistence of HD and CVD in more than half the study population highlights the importance of examining for CVD among patients with a hemorrhoid diagnosis, particularly when shared risk factors are present.
Keywords: chronic venous disease, hemorrhoidal disease, risk factors
Introduction
Hemorrhoidal disease (HD) is a common cause of anal pathology,but its exact prevalence is difficult to determine as patients are often reluctant to seek medical attention.1, 2 Levels of spontaneous consultation for anal symptoms are only around 2%, increasing to around 14% when patients presenting for an unrelated condition are subject to targeted questioning.1, 2
Most population‐based information on anal symptoms is collected via patient surveys alone.3 However, many HD symptoms are nonspecific, and without any confirmatory anal examination prevalence results can vary widely.1, 3, 4, 5
Data on the coexistence of hemorrhoids with other conditions are also sparse. Some data are consistent with a common pathophysiological link between straining at stool, constipation, and obstetrical events such as pregnancy and delivery.6 These factors are also involved in the development of chronic venous disease (CVD). Interference with venous return in the internal hemorrhoidal plexuses and saphenous veins may be a common anatomical mechanism.7
Given the paucity of information on the profiles of patients with HD, the aim of the CHORUS study (Chronic venous and HemORrhoidal diseases evalUation and Scientific research) was to provide current, global data on patients presenting with HD in clinical practice and to explore the frequency of its coexistence with CVD and their shared risk factors.
Methods
This was an international, cross‐sectional, observational study, consisting of a one‐time survey conducted between January 2015 and December 2016. Physicians (general practitioners, proctologists, gastroenterologists, and other types of physician) from seven countries enrolled subjects aged 18 and over attending a consultation for hemorrhoidal complaints. The only patients excluded were those attending for emergency visits. Informed consent was obtained.
The CHORUS study assessed two pathologies managed by different specialties with a diagnosis based on patient interview with or without a physical examination. As not all physicians may have been familiar with the requirements for a CVD or hemorrhoid diagnosis, they were provided with simple classifications based on clinical criteria in the form of the Goligher's classification8 (hemorrhoidal disease) and CEAP classification9 (CVD), both of which are designed to allow easy use and reproducibility, regardless of the practitioner. Demographic data were collected and subjects asked to describe their anal complaints including symptoms over the last 15 days, presence and duration of constipation, use of laxatives, time required for stool evacuation, stool consistency based on the seven‐category Bristol stool scale,10 and any previous consultations for hemorrhoids. The patient examination, if performed, was described (digital and/or anoscopy), and the type (internal, external, or mixed) and grade of hemorrhoids were noted. Internal hemorrhoids were graded from I to IV according to the classification of Goligher et al's.8 CVD of the lower extremities was graded according to the revised CEAP (Clinical manifestations, Etiologic factors, Anatomic distribution of disease, and underlying Pathophysiology) classification (Table S1).9 Physicians recorded details of any hemorrhoidal treatment prescribed including dietary fiber, topical treatment, venoactive drugs, pain killers, and surgery (including minimally invasive procedures).
For the statistical analysis, continuous variables were expressed as mean ± SD and categorical variables as number and frequency. Between‐group differences were evaluated by the chi‐squared analysis, Fisher's exact test, independent Student t‐test, or the Wilcoxon rank‐sum test as appropriate depending on whether variables were categorical or continuous.
Between‐group differences according to CVD CEAP class were evaluated by the Cochran–Mantel–Haenszel statistic. Univariate and multivariate logistic regression analyses were used to identify potential risk factors for HD and its recurrence. All variables testing significant in univariate analysis were included in the multivariate models (stepwise algorithm with a cumulative logit model). All P values were based on two‐tailed tests and P < 0.05 was considered significant. Data were analyzed using SAS® (SAS Institute, Cary, North Carolina, USA) software 9.4 TS1M4 for Windows Professional 64 bits.
Results
Patient characteristics
CHORUS enrolled 9381 subjects. The analysis set comprised 5617 (59.9%) subjects with available data for the main variables and no major deviations from protocol. Subjects were recruited from the following countries: Russia (n = 2362), India (n = 1510), Pakistan (n = 628), Mexico (n = 465), Belgium (n = 424), Slovenia (n = 129), and Thailand (n = 99). Comparison of the analysis set with the total population showed both were similar for demographic variables and baseline characteristics (Table 1).
Table 1.
Patient demographics and baseline characteristics for total enrolled population and analysis population
| Number (%) | ||
| Analysis population (N = 5617) | Enrolled population (N = 9381) | |
| Gender | ||
| Male/female | 3020 (53.7) /2597 (46.2) | 4768 (51.2) /4548 (48.8) |
| Missing data | 0 (0.0) | 65 (0.7) |
| Age (years) (mean ± SD) | 45.3 ± 13.8 | 45.6 ± 14.1 |
| Age groups (years): | ||
| 18–34 | 1416 (25.2) | 2199 (24.6) |
| 35–50 | 2199 (39.2) | 3495 (39.1) |
| 51–64 | 1465 (26.1) | 2310 (25.9) |
| ≥65 | 537 (9.6) | 924 (10.3) |
| Missing data | 0 (0.0) | 453 (4.8) |
| BMI (kg/m2) (mean ± SD) | 26.7 ± 4.8 | 27.2 ± 5.3 |
| BMI groups (kg/m2): | ||
| ≤18 | 112 (2.0) | 157 (1.8) |
| 19–24 | 1825 (32.5) | 2582 (29.6) |
| 25–30 | 2696 (48.0) | 4219 (48.4) |
| ≥31 | 984 (17.5) | 1761 (20.2) |
| Missing data | 0 (0.0) | 662 (7.1) |
| Hemorrhoid grade (Goligher et al9): | ||
| I | 1631 (29.0) | 2166 (29.5) |
| II | 2398 (42.7) | 3092 (42.1) |
| III | 1301 (23.2) | 1693 (23.0) |
| IV | 287 (5.1) | 398 (5.4) |
| Missing data | 0 (0.0) | 2032 (21.7) |
| Constipation | ||
| Yes | 2685 (47.8) | 4474 (51.1) |
| No | 2759 (49.1) | 4273 (48.9) |
| Missing data | 173 (3.1) | 634 (6.8) |
| Current venous leg problems | ||
| Yes | 2878 (51.2) | 4493 (51.9) |
| No | 2739 (48.8) | 4162 (48.1) |
| Missing data | 0 (0.0) | 726 (7.7) |
| Time spent standing | ||
| ≤2 h | 1727 (30.8) | 2801 (30.3) |
| 3–6 h | 2780 (49.5) | 4438 (48.0) |
| ≥7 h | 1110 (19.8) | 2005 (21.7) |
| Missing data | 0 (0.0) | 137 (1.5) |
| Smoking status: | ||
| Never smoked | 3222 (57.4) | 5496 (59.6) |
| Current smoker | 1468 (26.1) | 2212 (24.0) |
| Previous smoker | 927 (16.5) | 1511 (16.4) |
| Missing data | 0 (0.0) | 162 (1.7) |
| Previous consultation for hemorrhoids | 3176 (56.5) | 4759 (50.7) |
| Number of previous consultations: | ||
| One | 1210 (38.1) | 1737 (37.3) |
| Two | 1151 (36.2) | 1640 (35.2) |
| 3–5 times | 667 (21.0) | 1022 (22.0) |
| ≥6 times | 148 (4.7) | 257 (5.5) |
| Missing data | 0 (0.0) | 103 (2.2) |
| Among women participants: | N = 2597 | N = 4548 |
| Use of hormonal contraception: | ||
| Yes | 464 (17.9) | 765 (18.4) |
| No | 1998 (76.9) | 3387 (92.4) |
| Missing data | 135 (5.2) | 396 (8.7) |
| Use of hormone replacement therapy: | ||
| Yes | 157 (6.1) | 294 (7.6) |
| No | 2177 (83.8) | 3593 (92.4) |
| Missing data | 263 (10.1) | 661 (14.5) |
| Given birth: | ||
| Yes | 2101 (80.9) | 3569 (83.1) |
| No | 496 (19.1) | 724 (16.9) |
| Missing data | 0 (0.0) | 255 (5.6) |
| Number of births (among women with history of pregnancy)† (mean ± SD) | 2.18 ± 1.34 | 2.51 ± 1.62 |
Among the 2597 women in the study, 2101 had given birth of whom 2080 had the number of births recorded and 21 did not.
BMI, body mass index.
Hemorrhoidal symptoms and diagnosis
Most subjects with HD had suffered anal symptoms in the last 15 days (97.4%). The mean number reported was 2.9 ± 1.3 most commonly bleeding (71.8%), pain (67.4%), swelling (55.0%), itching (44.1%), prolapse (36.2%), soiling (9.2%), and fecal incontinence (2.9%). The frequency of symptoms increased with disease severity but occurred in a high proportion of subjects from as early as Grade I disease (Fig. 1).
Figure 1.
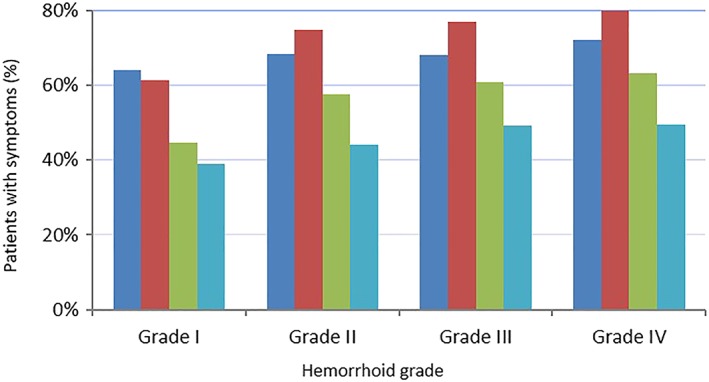
Frequencies of declared hemorrhoidal symptoms according to hemorrhoid grade.  , Pain;
, Pain;  , Bleeding;
, Bleeding;  , Swelling;
, Swelling;  , Itching. [Color figure can be viewed at http://wileyonlinelibrary.com]
, Itching. [Color figure can be viewed at http://wileyonlinelibrary.com]
A physical examination was performed in 5572 (99.2%) subjects: digital (90.0%) and/or anoscopic (71.8%). General practitioners were more likely to perform a digital examination (78.7%) than anoscopic (25.9%). Hemorrhoids were diagnosed in 98.5% of respondents and a previous history of hemorrhoids in 56.5% of respondents.
Influence of subject characteristics on severity of hemorrhoidal disease
Variables shown by univariate analysis to be significantly associated with grade of HD were male gender, older age, elevated BMI, previous pregnancies and number of births, constipation, and CVD (Table 2). In the multivariate analysis, the variables with the strongest association with hemorrhoid grade were in descending order: age group, CVD CEAP class, constipation, and male gender (all P values < 0.0001). In women, constipation and number of births were stronger risk factors than CEAP class, while in men, CEAP class was a stronger risk factor than constipation.
Table 2.
Patients' characteristics at baseline according to hemorrhoid grade
| Grade of hemorrhoid: | Patients' characteristics (%) | ||||||||||||
| Gender* | Age group** | BMI** | Given birth** | Presence of constipation** | Presence of CVD** | ||||||||
| Male (N = 3020) | Female (N = 2597) | 18–34 (N = 1416) | 35–50 (N = 2199) | 51–64(N = 1465) | ≥65 (N = 537) | 12–18 (N = 112) | 19–24 (N = 1825) | 25–30 (N = 2696) | ≥31 (N = 984) | (N = 2101) | (N = 2685) | (N = 2878) | |
| I (N = 1631) | 51.93 | 48.07 | 38.20 | 35.25 | 18.88 | 7.66 | 2.94 | 38.07 | 45.25 | 13.73 | 70.92 | 40.53 | 43.10 |
| II (N = 2398) | 52.63 | 47.37 | 23.31 | 41.49 | 28.86 | 8.34 | 1.79 | 32.65 | 47.71 | 17.85 | 83.18 | 48.50 | 54.00 |
| III (N = 1301) | 58.11 | 41.89 | 15.30 | 40.58 | 32.13 | 11.99 | 1.31 | 26.21 | 51.58 | 20.91 | 89.72 | 54.50 | 54.80 |
| IV (N = 287) | 54.01 | 45.99 | 12.20 | 35.19 | 33.10 | 19.51 | 1.39 | 27.87 | 49.83 | 20.91 | 84.09 | 52.96 | 58.19 |
Univariate analysis:
P < 0.004.
P < 0.0001.
Among the 2597 women in the study, 2101 had given birth of whom 2080 had the number of births recorded and 21 did not.
BMI, body mass index. CVD, chronic venous disease.
Bowel habits
Constipation was reported by 2685 (49.3%) subjects, and its presence influenced hemorrhoid grade (Table 2). Duration of constipation was <18 months in 1334 (49.7%) subjects and chronic (>18 months) in 1351 (50.3%) subjects.
Duration of evacuation was described as <5 min in 41.5%, between 6 and 30 min in 54.7%, and ≥31 min in 3.9%. In those with a duration of evacuation ≥31 min the proportion of Grade III and IV hemorrhoids was 28.6% and 10.7%, respectively, compared with 14.0% and 2.0%, respectively, in patients with a duration <5 min.
Bowel habits were also assessed using the Bristol stool scale, which allows a classification of the form of human feces, albeit not an accurate grading of the severity of constipation or diarrhea. Nearly two thirds of subjects with hemorrhoidal complaints (64.4%) had normal stools (stool Type 3, 4, and 5). The proportions of Type 1 and 2 stools (suggesting constipation) were 9.2% and 21.4%, respectively, and the proportions of Type 6 and 7 (suggesting diarrhea) were 3.9% and 1.1%, respectively. The proportion of patients with more severe constipation (Type 1 stool) increased with age group, from 7.0% in those aged 18–34 years to 11.9% in those aged ≥65 years. BMI had no impact on stool type.
According to the Bristol stool scale, a greater proportion of people with constipation had more severe hemorrhoid grades compared with patients with normal stools (Table S2; P = 0.0236, Fisher's exact test). The numbers of patients with diarrhea were too low for conclusions to be drawn.
CVD
Coexistence with CVD was more frequent as HD severity increased: concomitant CVD was present in 43.1%, 54.0%, 54.8%, and 58.2% of those with hemorrhoid grades of I, II, III, and IV, respectively. The severity of CVD was strongly associated with hemorrhoid severity (P < 0.0001) (Table 3).
Table 3.
Proportion of patients with concomitant CVD defined by CEAP classification according to hemorrhoid grade
| Subjects with CVD by CEAP class (%) | |||||||
| C0A (N = 2739) | C0S (N = 552) | C1 (N = 847) | C2 (N = 708) | C3 (N = 472) | C4 (N = 263) | C5–C6 (N = 36) | |
| Grade of hemorrhoid | |||||||
| I (N = 1631) | 56.9 | 11.0 | 14.3 | 8.9 | 5.8 | 2.6 | 0.6 |
| II (N = 2398) | 46.0 | 11.5 | 16.6 | 12.9 | 8.4 | 4.2 | 0.5 |
| III (N = 1301) | 45.2 | 6.0 | 14.1 | 15.7 | 11.1 | 7.1 | 0.8 |
| IV (N = 287) | 41.8 | 6.6 | 11.1 | 17.8 | 11.1 | 9.8 | 1.7 |
CEAP, Clinical manifestations, Etiologic factors, Anatomic distribution of disease, and underlying Pathophysiology; CVD, chronic venous disease.
Influence of subject characteristics on HD recurrence
Univariate analyses indicated a significant association between previous consultations for HD (hemorrhoid recurrence) and older age (56.6% in 35–50 year‐olds, 65.7% in 51–64 year‐olds, and 67.8% in ≥65 year‐olds, P < 0.0001); elevated BMI (49.1% normal BMI, 58.2% overweight category, and 68.3% obese category, P < 0.0001); and constipation (46.0% no constipation, 67.6% with constipation, P < 0.0001). In women, risk was higher in those who had given birth (58.8% vs 41.7% in those who had not given birth, P < 0.0001), and increased with number of births (P < 0.0001). Risk of hemorrhoid recurrence was also more likely in those with concomitant CVD (63.6% vs 49.1% in those without CVD, P < 0.0001) and increased with CVD CEAP class (P < 0.0001). Gender had no influence on likelihood of hemorrhoid recurrence in univariate analysis (P = 0.1539).
A higher proportion of patients enrolled in Thailand, Pakistan, India, and Mexico (1693 out of 2702, 62.8%) had previously consulted for HD compared with those enrolled in Russia, Belgium, and Slovenia (1445 out of 2915, 49.6%).
Multivariate analyses confirmed univariate findings. Variables with the strongest association with hemorrhoid recurrence were in descending order: constipation (OR 2.20, 95%CI 1.96, 2.46), age group (OR 2.11, 95%CI 1.68, 2.65 for the comparison 18–34 years vs >65 years), CVD CEAP class (OR 3.75, 95%CI 1.30, 10.90 CEAP C0a vs C6) (Fig. 2), BMI category (OR 2.34, 95%CI 1.51, 3.64 for the comparison 12–18 years vs ≥31 kg/m2), and male gender (OR 1.25, 95%CI 1.11, 1.41). Among women, having given birth and number of births were the most important risk factors (P < 0.0001), followed by constipation (P < 0.001), age group (P < 0.0001), presence of CVD (P = 0.0089), and BMI category (P = 0.0123). Among men, constipation was the most important risk factor (P < 0.0001) followed by age group (P < 0.0001), BMI category (P = 0.0011), and presence of CVD (P < 0.0001).
Figure 2.
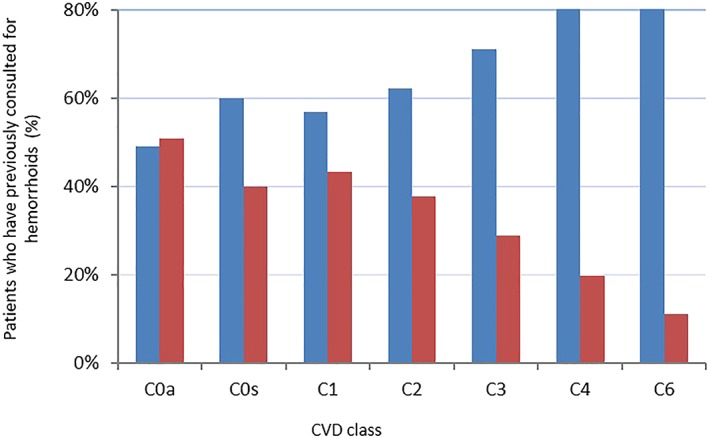
Distribution of patients with and without previous consultation for hemorrhoids according to stage of chronic venous disease (CVD) based on the Clinical manifestations, Etiologic factors, Anatomic distribution of disease, and underlying Pathophysiology (CEAP) classification.  , Yes;
, Yes;  , No. [Color figure can be viewed at http://wileyonlinelibrary.com]
, No. [Color figure can be viewed at http://wileyonlinelibrary.com]
Characteristics of subjects with concomitant CVD
In the CHORUS survey, 51.2% of participants with hemorrhoidal complaints also presented with signs and/or symptoms of CVD: C0s–C2 (73.2%), C3 (16.4%), C4 (9.1%), and C5–C6 (1.3%).
Patients with CVD were on average 7 years older and were more frequently women (59.3% vs 32.5%, respectively), overweight (21.8% vs 12.9%), constipated (53.1% vs 42.2%), and had more severe and recurrent hemorrhoids than subjects without CVD (Table 4).
Table 4.
Characteristics of patients with hemorrhoids according to concomitant CVD
|
Patients without CVD (n = 2739) |
Patients with CVD (n = 2878) |
|
| Male/female (%) | 67.5/32.5 | 40.7/59.3 |
| Age years (mean ± SD) | 41.9 ± 13.1 | 48.6 ± 13.6 |
| Age class (%) | ||
| 18–34 (N = 1416) | 33.8 | 17.1 |
| 35–50 (N = 2199) | 40.6 | 37.7 |
| 51–64 (N = 1465) | 19.6 | 32.3 |
| ≥ 65 (N = 537) | 6.0 | 12.9 |
| BMI (kg/m2) (mean ± SD) | 26.1 ± 4.5 | 27.3 ± 5.0 |
| BMI class (%) | ||
| ≤18 (N = 112) | 2.6 | 1.4 |
| 19–24 (N = 1825) | 35.9 | 29.3 |
| 25–30 (N = 2696) | 48.5 | 47.5 |
| ≥31 (N = 984) | 13.0 | 21.8 |
| Constipation (%) | 42.2 | 53.1 |
| 0–18 months | 25.5 | 22.1 |
| 19 months to 5 years | 8.7 | 14.4 |
| >5 years | 8.0 | 16.7 |
| Use of laxatives (%) | 20.4 | 29.4 |
| Duration of evacuation (%) | ||
| <5 min | 46.1 | 37.0 |
| ≥6 min | 53.9 | 63.0 |
| Standing position (%) | ||
| ≤2 h (N = 1727) | 31.3 | 30.2 |
| 3–6 h (N = 2780) | 49.5 | 49.4 |
| ≥7 h (N = 1110) | 19.1 | 20.4 |
| Smoking status (%) | ||
| Never smoked (N = 3222) | 56.7 | 58.0 |
| Current smoker (N = 1468) | 27.5 | 24.9 |
| Previous smoker (N = 927) | 15.9 | 17.1 |
| Hemorrhoid grade (%) | ||
| I | 33.9 | 24.4 |
| II | 40.3 | 45.0 |
| III | 21.5 | 24.5 |
| IV | 4.4 | 5.8 |
| Previous consultation for hemorrhoids (%) | 42.1 | 57.9 |
| Among women | N = 890 | N = 1707 |
| Use of hormonal contraception (%) | 21.9 | 15.8 |
| Use of HRT (%) | 5.6 | 6.3 |
| Previous pregnancy (%) | 71.9 | 85.6 |
BMI, body mass index; CVD, chronic venous disease; HRT, hormone replacement therapy.
According to multivariate analysis, variables with the strongest association with the presence of CVD in individuals with HD were in descending order: female gender, older age, elevated BMI, constipation, and severe hemorrhoid grade (all P < 0.0001). Among women, number of births ranked second after age group, followed by constipation and BMI class, while among men hemorrhoid grade ranked second after age group, followed by BMI class and constipation.
Treatment patterns
Nearly all patients (99.8%) were prescribed at least one treatment for their HD: venoactive drug (94.3%), dietary fiber (71.4%), topical treatment (70.3%), analgesics (26.3%), surgical procedures (23.5%), or other (14.7%). Most patients (97.9%) were prescribed at least one of the following: venoactive drug, dietary fiber, topical treatment, or analgesic; 1037 (18.8%) received all four treatments, 2248 (40.9%) received three, 1627 (29.6%) received two, and 590 (10.7%) received one. Among the venoactive drugs the most commonly prescribed was micronized purified flavonoid fraction (93.5%), followed by diosmin (3.5%). For these agents, the mean prescription duration was ≥4 weeks in 61.0% of participants, 2–3 weeks in 27.6%, and ≤1 week in 10.0%. Other venoactive drug classes such as calcium dobesilate, horse chestnut seed extract (escin), rutosides, ruscus extracts, proanthocyanidines, and ginkgo biloba accounted for <3.0% of prescriptions.
Venoactive drugs were prescribed in over 94.0% of subjects with Grade I to III hemorrhoids and in 86.8% of subjects with Grade IV hemorrhoids (Fig. 3). Dietary fiber and topical treatments were prescribed in around two thirds of patient across hemorrhoid grades of I to III, and slightly less in Grade IV (65.5% and 63.4%, respectively). Prescription of analgesic drugs increased with hemorrhoid grade.
Figure 3.
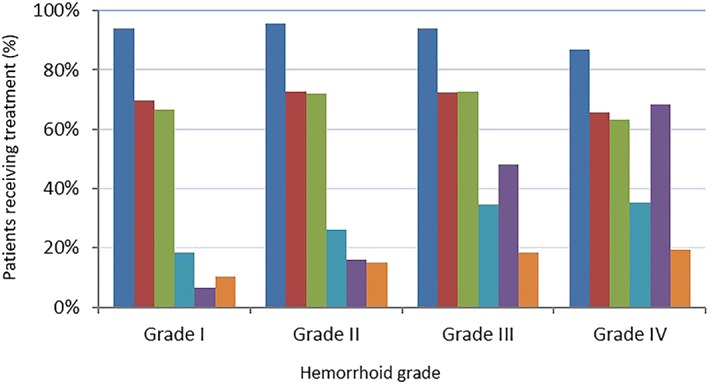
Distribution of prescribed treatments for hemorrhoidal disease according to the hemorrhoid severity stage. VAD, venoactive drug.  , VAD;
, VAD;  , Dietary fiber;
, Dietary fiber;  , Tropical treatment;
, Tropical treatment;  , Analgesic;
, Analgesic;  , Surgery;
, Surgery;  , Other. [Color figure can be viewed at http://wileyonlinelibrary.com]
, Other. [Color figure can be viewed at http://wileyonlinelibrary.com]
Surgical procedures (including minimally invasive procedures) were prescribed in 48.1% of Grade III individuals and 68.3% of Grade IV. Among the 1319 patients who underwent surgical procedures, 1159 (87.8%) were also prescribed a venoactive drug, 847 (64.2%) dietary fiber, 838 (63.5%) topical treatment, and 363 (27.5%) an analgesic.
Discussion
Almost all CHORUS participants (99.2%) underwent a clinical examination, either digital and/or anoscopic. Although a digital examination cannot always confirm a diagnosis of lower grade HD, it is important to eliminate any local malignancies. Anoscopy is necessary for the diagnosis of Grade I hemorrhoids and was performed in the majority of patients with low‐grade hemorrhoids, thus confirming the high level of diagnostic evidence in this study.
CHORUS confirmed that HD affects both sexes with peak presentation in those aged 35–50 years. An unexpected finding was the male predominance (53.8% vs 46.2% women predominance), but this may reflect the large proportion of male subjects enrolled in India and, cultural habits making women participants with HD less likely to come forward.
Most female participants presenting with hemorrhoids (80.7%) had had at least one full‐term pregnancy, and pregnancy was a risk factor for all hemorrhoid grades. Hemorrhoids are common during pregnancy, particularly the last trimester,11, 12 and although they generally resolve after delivery these women are at greater risk of hemorrhoids later in life. Hemorrhoids were also more common in those with a BMI in the overweight or obese category. Previous studies have hypothesized that this may be because of increased intraabdominal pressure and increased stress on rectal muscles.13
Multiple logistic regression analysis identified male gender, older age, pregnancy and number of births, constipation, and CVD presence and severity as being significantly associated with HD grade. Several studies, including those that have used colonoscopy to confirm hemorrhoids, have shown an increased risk in patients with chronic constipation.14, 15, 16 Whether causal or a contributory factor in the presence of a primary cause prolonged straining increases intraabdominal pressure and raises venous pressure in the hemorrhoidal tissue. In the current study, half the respondents (50.9%) were suffering from constipation according to the Bristol stool scale, and 54.7% reported a straining duration of 6–30 min. Severe constipation was also observed more frequently in individuals with Grade IV HD than less severe forms of the disease.
Hemorrhoid recurrence rates have been reported to range from 4% to 30% after treatment.17 In CHORUS, 56.5% of patients had previously consulted for hemorrhoids. Constipation, older age, higher CVD CEAP class, elevated BMI, and male gender had a statistically significant association with hemorrhoid recurrence. Among women, pregnancy and number of births had the strongest association. Constipation was an important risk factor for recurrence in both men and women. BMI was associated with HD recurrence even though it was not linked to HD severity. Recurrence rates were higher in countries that typically have a greater consumption of spices. This may indicate a possible influence of diet on likelihood of hemorrhoid recurrence, but could also be related to the stage of HD at which patients were enrolled in the study.
HD and CVD may have a common cause in the form of loss of vascular integrity,7 but there are few published papers on the coexistence of the two disorders and recent data are lacking.18 An early review of epidemiologic evidence for a link between HD and CVD hypothesized that chronic constipation associated with a low fiber diet was involved by increasing intraabdominal pressure.18 It was suggested that this pressure would easily be transmitted to the hemorrhoidal plexus, which have no valves. The valves of the lower limb veins would offer initial protection but would eventually become incompetent and expose the veins to elevated pressure.18 This hypothesis is supported by data from a Hungarian epidemiologic study of pregnant women, which found that around half with a diagnosis of HD also had constipation. Varicose veins were also more common in pregnant women with hemorrhoids compared with those without.19
Just over half the CHORUS subjects also presented with signs and/or symptoms of CVD, the majority having a CEAP classification of C0s to C2. In multivariate analyses, independent risk factors for CVD among patients with HD were female gender, older age, elevated BMI, constipation, and more severe hemorrhoid grade. Among women, number of births was also an importan risk factor. The finding that HD coexists with CVD in over half the study population should prompt physicians treating patients with a hemorrhoid diagnosis to also ask about CVD and vice versa, particularly in the presence of the risk factors above.
Nonsurgical approaches are preferred for lower grade hemorrhoids because of the physiologic importance of the hemorrhoid cushions and potential self‐limiting nature of many hemorrhoid symptoms.12, 17, 20, 21 When conservative therapy fails, clinical practice guidelines recommend office‐based procedures such as banding, sclerotherapy, and infrared coagulation for Grade I to III hemorrhoids.20, 22 Surgical options such as hemorrhoidectomy, stapled hemorrhoidopexy, or hemorrhoidal artery ligation may be the initial step in patients with Grade III or IV hemorrhoids or in those who are refractory to or cannot tolerate office procedures.20, 22 However, conservative therapy can still play a role, creating favorable conditions for a smooth postoperative recovery.23 Adherence to these guideline recommendations is important to avoid repetitive and prolonged treatment and severe complications in Grade IV disease.
Among pharmacological approaches, venoactive drugs can address the underlying causes of both symptomatic hemorrhoids and CVD via their beneficial effects on venous tone, inflammatory processes, and microcirculatory permeability.24 Early use of such agents may play a role in preventing or slowing the development and recurrence of both hemorrhoidal and CVD signs and symptoms.25, 26 Hemorrhoid recurrence was more likely in those with concurrent CVD. This association is an indirect argument for the use of a targeted medical treatment on the vein in both pathologies.
This study was subject to the inherent limitations of observational surveys, which include sample bias, incomplete survey response data, as well as the potential inaccuracy of self‐reported behavior. A comparison of the demographic data for per protocol and total enrolled population illustrates their similarity and indicates that a selection bias was unlikely to have been introduced by restricting the study to patients with no relevant protocol deviations. However, the authors acknowledge that representativeness is also linked to whether participating sites reflect the management of disease in that country in terms of specialty and place of practice. During this investigation, symptoms of pain were defined by the patients themselves. Pain intensity was not evaluated as the use of a visual analogue scale in such a population was not considered feasible. The use of a broad definition of pain inevitably led to a relatively high percentage of pain symptoms in this survey. The link with HD nevertheless remains plausible as the incidence of pain increased with the grade of disease (Fig. 1).
While neither HD nor CVD is likely to have a single causative agent, some risk factors may contribute or exacerbate disease in predisposed patients. Further studies are now warranted to determine the proportion of patients with hemorrhoids who subsequently develop CVD and whether this is more likely in patients with certain risk factors. Any changes in lifestyle or pharmacologic treatment that target these conditions in their early stages would be advantageous to prevent progression to more severe forms.
Conclusion
CHORUS provides large‐scale data on the profiles of patients presenting with HD in clinical practice. Factors identified as having a statistically significant association with both hemorrhoid grade and recurrence were older age, CVD CEAP class, constipation, and male gender. Among women, additional factors were pregnancy and number of births. Physicians should be aware of these risk factors and be proactive in questioning patients about potential hemorrhoid symptoms and conduct the appropriate examinations. As symptoms affect patients from Grade I disease, a conservative treatment should be the cornerstone of care. Further studies are needed to confirm the correlation between HD and CVD so that a common targeted therapy can be developed for both these diseases.
Supporting information
Table S1. CVD of the lower extremities graded according to the clinical classification of the CEAP (Clinical manifestations, Etiologic factors, Anatomic distribution of disease, and underlying Pathophysiology).10
Table S2. Distribution of Bristol stool types according to hemorrhoid grade.
Acknowledgment
The authors thanks Les Laboratoires Servier for their material support in carrying that original study.
Godeberge, P. , Sheikh, P. , Zagriadskiĭ, E. , Lohsiriwat, V. , Montaño, A. J. , Košorok, P. , and De Schepper, H. (2020) Hemorrhoidal disease and chronic venous insufficiency: Concomitance or coincidence; results of the CHORUS study (Chronic venous and HemORrhoidal diseases evalUation and Scientific research). Journal of Gastroenterology and Hepatology, 35: 577–585. 10.1111/jgh.14857.
Declaration of conflict of interest: Philippe Godeberge reports consulting fees from Les Laboratoires Servier/Norgine Pharma and lecture fees from Les Laboratoires Servier/Tillotts Pharma/Pfizer PFE. Parvez Sheikh has received lecture fees from Servier. Abel Jalife‐Montaño reports consulting fees from Servier, grants from Servier, and lecture fees from Servier. Evgeny Zagriadskiĭ, Varut Lohsiriwat, Pavle Košorok, and Heiko De Schepper declare no conflicts of interest.
Author contributions: All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
The corresponding author had full access to the data and final responsibility for the decision to submit for publication.
Financial support: The trial was sponsored and funded by Servier, which was responsible for the study design, study management, data collection, and data analysis and was involved in the writing of the report. Editorial assistance for this paper was provided by Jenny Grice and funded by Servier Affaires Médicales.
References
- 1. Abramowitz L, Benabderrahmane M, Pospait D, Philip D, Laouénan C. The prevalence of proctological symptoms amongst patients who see general practitioners in France. Eur. J. Gen. Pract. 2014; 20: 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tournu G, Abramowitz L, Couffignal C et al GREP study group; MG‐PREVAPROCT study group. Prevalence of anal symptoms in general practice: a prospective study. BMC Fam. Pract. 2017; 18: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation. An epidemiologic study. Gastroenterology 1990; 98: 380–386. [DOI] [PubMed] [Google Scholar]
- 4. Siproudhis L, Pigot F, Godeberge P, Damon H, Soudan D, Bigard MA. Defecation disorders: a French population survey. Dis. Colon Rectum 2006; 49: 219–227. [DOI] [PubMed] [Google Scholar]
- 5. Riss S, Weiser FA, Schwameis K et al The prevalence of hemorrhoids in adults. Int. J. Colorectal Dis. 2012; 27: 215–220. [DOI] [PubMed] [Google Scholar]
- 6. Pigot F, Siproudhis L, Allaert FA. Risk factors associated with hemorrhoidal symptoms in specialized consultation. Gastroenterol. Clin. Biol. 2005; 29: 1270–1274. [DOI] [PubMed] [Google Scholar]
- 7. MacKay D. Hemorrhoids and varicose veins: a review of treatment options. Altern. Med. Rev. 2001; 6: 126–140. [PubMed] [Google Scholar]
- 8. Goligher JC. Haemorrhoids or piles In: Surgery of the Anus, Rectum and Colon, 4th edn. London: Baillere Tindall, 1980; 93–135. [Google Scholar]
- 9. Eklof B, Rutherford RB, Bergan JJ et al American venous forum international ad hoc committee for revision of the CEAP classification. Revision of the CEAP classification for chronic venous disorders: consensus statement. J. Vasc. Surg. 2004; 40: 1248–1252. [DOI] [PubMed] [Google Scholar]
- 10. Heaton KW, Radvan J, Cripps H, Mountford RA, Braddon FE, Hughes AO. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut 1992; 33: 818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Avsar AF, Keskin HL. Haemorrhoids during pregnancy. J. Obstet. Gynaecol. 2010; 30: 231–237. [DOI] [PubMed] [Google Scholar]
- 12. Hollingshead JR, Phillips RK. Haemorrhoids: modern diagnosis and treatment. Postgrad. Med. J. 2016; 92: 4–8. [DOI] [PubMed] [Google Scholar]
- 13. Chang SS, Sung FC, Lin CL, Hu WS. Association between hemorrhoid and risk of coronary heart disease: a nationwide population‐based cohort study. Medicine (Baltimore) 2017; 96: e7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh G, Lingala V, Wang H et al Use of health care resources and cost of care for adults with constipation. Clin. Gastroenterol. Hepatol. 2007; 5: 1053–1058. [DOI] [PubMed] [Google Scholar]
- 15. Riss S, Weiser FA, Schwameis K, Mittlböck M, Stift A. Haemorrhoids, constipation and faecal incontinence: is there any relationship? Colorectal Dis. 2011; 13: e227–e233. [DOI] [PubMed] [Google Scholar]
- 16. Peery AF, Sandler RS, Galanko JA et al Risk factors for hemorrhoids on screening colonoscopy. PLoS ONE 2015; 10: e0139100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanchez C, Chinn BT. Hemorrhoids. Clin. Colon Rectal Surg. 2011; 24: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burkitt DP. Varicose veins, deep vein thrombosis, and haemorrhoids: epidemiology and suggested aetiology. Br. Med. J. 1972; 2: 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Acs N, Bánhidy F, Czeizel AE. Hemorrhoids In: Acs N, Bánhidy F, Czeizel AE, eds. Congenital Abnormalities and Preterm Birth Related to Maternal Illnesses During Pregnancy. Springer, 2010;Chapter 10.12; 234–237. [Google Scholar]
- 20. Higuero T, Abramowitz L, Castinel A et al Guidelines for the treatment of hemorrhoids (short report). J. Visc. Surg. 2016; 153: 213–218. [DOI] [PubMed] [Google Scholar]
- 21. Davis BR, Lee‐Kong SA, Migaly J, Feingold DL, Steele SR. The American society of colon and rectal surgeons clinical practice guidelines for the management of hemorrhoids. Dis. Colon Rectum 2018; 61: 284–292. [DOI] [PubMed] [Google Scholar]
- 22. Wald A, Bharucha AE, Cosman BC, Whitehead WE. ACG clinical guideline: management of benign anorectal disorders. Am. J. Gastroenterol. 2014; 109: 1141–1157. [DOI] [PubMed] [Google Scholar]
- 23. Zagriadskiĭ EA. Conservative treatment of hemorrhoids: results of an observational multicenter atudy. Adv. Ther. 2018; 35: 1979–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katsenis K. Micronized purified flavonoid fraction (MPFF): a review of its pharmacological effects, therapeutic efficacy and benefits in the management of chronic venous insufficiency. Curr. Vasc. Pharmacol. 2005; 3: 1–9. [DOI] [PubMed] [Google Scholar]
- 25. Misra MC, Parshad R. Randomized clinical trial of micronized flavonoids in the early control of bleeding from acute internal haemorrhoids. Br. J. Surg. 2000; 87: 868–872. [DOI] [PubMed] [Google Scholar]
- 26. Nicolaides AN, Allegra C, Bergan J et al Management of chronic venous disorders of the lower limbs: guidelines according to scientific evidence. Int. Angiol. 2008; 27: 1–59. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. CVD of the lower extremities graded according to the clinical classification of the CEAP (Clinical manifestations, Etiologic factors, Anatomic distribution of disease, and underlying Pathophysiology).10
Table S2. Distribution of Bristol stool types according to hemorrhoid grade.


