Abstract
Background:
Metabolic syndrome (MetS) is one of the world's largest health epidemics, and its management is a major challenge worldwide. The aim of this 10-year follow-up study was to assess the most important predictors of MetS persistence among an Iranian adult population.
Methods:
In this cohort study, 887 out of 2000 participants with MetS aged 20–74 years in the central part of Iran were followed-up for about 10 years from 2005–2006 to 2015–2016. MetS was defined based on the criteria of NCEP-ATP III adopted for the Iranian population. Cox proportional hazards regression was conducted to evaluate the predictors of MetS persistence in crude- and multivariate-adjusted models.
Results:
Our analyses showed that 648 out of 887 participants (73%) completed the follow-up and 565 (87.2%) of them had persistence of MetS after 10-year follow-up. There was a significant association between age, weight, body mass index, triglyceride, and waist circumference in participants who had MetS compared to those without MetS after 10-year follow-up (P < 0.05). There was a direct association between increases in the mean changes of systolic/diastolic blood pressure, waist circumference, and low HDL-C and risk of MetS persistence after adjusting the model for sex and age in the total population (Ptrend < 0.05). The trends were the same for women except in diastolic blood pressure. After adjustment for potential confounders, the risk of MetS persistence in men was significantly higher than women (HR = 1.98, 95% CI: 1.38–2.85, Ptrend = 0.001).
Conclusions:
Most of the risk factors of MetS were positively associated with persistence of MetS. Therefore, modification of lifestyle is recommended to reduce MetS.
Keywords: Cohort studies, metabolic syndrome, persistence
Introduction
Metabolic syndrome (MetS) is a cluster of risk factors including high fasting blood glucose, elevated blood pressure, large waist circumference, high triglyceride levels, and low HDL cholesterol (HDL-C). MetS is one of the main predictors of the onset of type 2 diabetes and cardiovascular diseases.[1,2] The prevalence of MetS ranges has been reported from 10% to 40% worldwide.[3] In 2007, the prevalence of MetS was 35.9%, and it decreased to about 33% in 2011 among Iranian adult population.[4] Based on a systematic review and meta-analysis study, the prevalence of MetS was 34.6% in Iran in 2015.[5] Using a modified version of the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATPIII), the cumulative incidence of MetS was more than 56% in the central part of Iran in a 10-year follow-up study.[6] However, the prevalence of MetS and its components are changing over time according to age,[7] lifestyle such as smoking, physical activity, socioeconomic status, education,[8,9] migration from rural to urban area,[10] diet,[11] and decreased or increased risk of MetS components.[12] Some risk factors cause remission, temporary, or permanent MetS in affected people. The evidence showed that decreased incidence of HDL-C levels, triglyceride levels, and hypertension could decrease the risk of MetS significantly.[12] A longitudinal study in China reported an increasing trend in the components of MetS including fasting plasma glucose and triglyceride (TG) levels and eventually that resulted in an increase in the prevalence of MetS.[10] To the best our knowledge, limited cohort studies have reported about the risk factors that could be affected on persistence or disappearance of MetS. Understanding the factors that predict the persistence of MetS and its associated components will provide an opportunity for interventions aiming to reduce diabetes and cardiovascular events. This study aims to determine the most important predictors of MetS persistence and changes of MetS and its components in participants who were affected by MetS at the baseline and were followed-up after 10 years in the central part of Iran.
Methods
Study population
This cohort study is based on Yazd Healthy Heart Project (YHHP) started in 2005–2006 and then followed-up to 2015–2016. Two thousand participants (1000 males and 1000 females) 20–74 years were recruited from the urban area of Yazd City using multistage cluster random sampling method.[13] Based on the modified version of NCEP-ATP III, 887 out of 2000 of YHHP participants were diagnosed as having MetS at entering to the cohort. The methodology of this study has been reported in detail elsewhere.[6] Participants with MetS at the baseline were included in this study and followed up for about 10 years. Those who completed the follow-up were and revisited in 2015–2016 (Phase II of the study). Demographic data, anthropometric measurements (height, weight, and waist circumference), smoking habits, economic status, physical activity, and educational levels and finally, biochemical blood tests such as fasting blood glucose and lipid profiles were collected at the baseline and Phase II. Participants who did not complete the follow-up due to different reasons including death or migration were excluded from the study. The process of MetS persistence after 10-year follow-up is shown in Figure 1.
Figure 1.
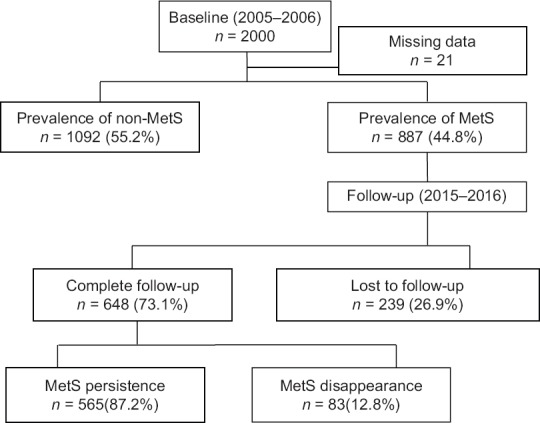
Flow diagram showing the process of MetS persistence after 10-year follow-up
Ethical approval
The ethical committee of the Shahid Sadoughi University of Medical Sciences (No: IR.SSU.MEDICINE.REC.1395.287) approved the study. In addition, informed consents from all participants were obtained at the first and second phases of the study. To report the cohort study, we used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.[14]
Criteria for metabolic syndrome
A modified version of NCEP-ATP III was used to define the MetS.[3,15] Participants who had three out of five components of MetS were considered with MetS. The components include elevated waist circumference (WC), elevated TG, reduced HDL-C, elevated systolic/diastolic blood pressure (SBP/DBP), and elevated fasting blood glucose (FBG). In this study, the criteria for elevated WC were considered based on the adoption for the Iranian population including waist circumference ≥91.5 cm in males and ≥85.5 cm in females.[16] Elevated triglyceride was considered when triglyceride level ≥150 mg/dL or drug treatment for elevated triglyceride. The criteria for reduced HDL-C was HDL <40 mg/dL in males and <50 mg/dL in females or drug treatment for reduced HDL-C. Elevated blood pressure was considered as SBP ≥130 and/or DBP ≥85 mmHg, or antihypertensive drug treatment in participants with a history of hypertension was an alternate indicator. The criterion for elevated FBG was defined as FBG ≥100 mg/dL or drug treatment for elevated FBG.
Anthropometric measurements
Anthropometric measurement includes measuring height, weight, and WC. Height was measured by a standard Stadiometer fixed on the wall, while participants were standing without shoes and measurement performed to the nearest 0.5 cm. Weight was measured with a calibrated digital scale (Model BF511, Omron Co. Karada body scan, Osaka, Japan) to the nearest 0.1 kg. To measure weight, participants were asked to take out shoes and heavy clothes. WC was measured in a horizontal plane, midway between the lowest rib and the upper border of the iliac crest by a nonstretchable tape to the nearest 0.1 cm.
Blood pressure assessment
An automatic digital blood pressure device (Omron, M6 comfort, Osaka, Japan) was used to assess systolic/diastolic blood pressure in the standard method, while participants were in a sitting position. Blood pressure assessment was performed twice on the right arm with 5 min apart and then averaged for analysis.
Biochemical blood analysis
A venous blood sample was drawn after 9–12 h of fasting. The blood samples were centrifuged, and their serum was separated. Biochemical analyses were performed by a biochemical auto-analyzer (BT 3000, Italy). Pars Azmoon Kits (Pars Azmoon Inc., Tehran, Iran) was used to assess fasting blood glucose and triglyceride levels. In addition, the HDL-C level was assessed by standard kits (Bionic Company, Tehran, Iran).
Assessment of covariates
Information about participants’ smoking habit, economic status, physical activity, and educational levels was collected at the baseline by three trained interviewers. Three criteria were used to evaluate economic status including family income, having a private care and home size (m2). The criteria were scored, and the participants were categorized into low, moderate, and high economic status based on tertiles of total economic scores. In addition, participants were classified into three educational levels including primary school completed (low), high school completed (moderate), and academic completed (high) levels. Physical activity was assessed based on the International Physical Activity Questionnaires (IPAQ).[17] Participants were categorized into three groups of low, moderate, and vigorous physical activity based on energy expenditure cut points of <600, 600–1200, and >1200 kcal/week, respectively. Based on current smoking habits, the participants were categorized into two groups of smokers and nonsmokers.
Statistical analysis
For categorical variables (age, BMI, smoking, economic status, physical activity, and education categories), frequency and percentage were reported and Chi-square test was used to compare them between two groups of persistence and disappearance. After testing data distribution status, mean and standard deviation were reported for each numerical variable. Student's t-test was used to compare MetS components at baseline between two groups of completed and noncompleted follow-up. Similarly, to find any significant differences of age, weight, BMI, systolic/diastolic blood pressure, triglyceride, HDL-cholesterol, and waist circumference between two groups of persistence and disappearance, Student's t-test was used. To find any statistically significant differences of MetS components among participants in persistence at Phase II compared to baseline (Phase I), paired t-test was used. Cox proportional hazard regression models were conducted to examine any association between categorized variables, and also categorized changes of components of MetS and the risk of persistence of MetS. For the Cox regression model, numerical variables were reformatted to categorical ones and new categorical variables for changes from baseline to Phase II (value in Phase II – value at baseline) were created. Each individual regression analysis was developed in two models for men, women, and total population, separately. In model I, any confounding effect of age and sex and in model II and the effect of other potential covariates such as smoking habits, economic status, physical activity, and educational level in addition to sex and age were adjusted. All results were interpreted significant at a P value of <0.05. Data were analyzed using Statistical Package for Social Sciences (SPSS) version 19 (IBM Corporation, New York, USA).
Results
The prevalence of MetS at the start of the cohort study was 44.8% (n = 887). Therefore, 887 participants with MetS and mean age of 54.7 ± 12.5 years were included in the study. They were followed-up for about 10 years (with mean age of 9.97 ± 1.24 years and median age of 10.24 years); 239 out of 887 (26.9%) participants were lost to follow-up. Finally, 648 participants with MetS (305 men with mean age of 52.4 ± 12.4; 343 women with mean age of 54.4 ± 11.6 years) completed the follow-up and were included in the current analyses. The prevalence of MetS components at the baseline and at Phase II is reported in Table 1. The persistence and disappearance rate of MetS among participants who completed the follow-up in total population were 87.2% and 12.8%, respectively. The prevalence of disappearance status in men and women was 13.8% (42/305) and 12% (41/343), respectively. In addition, the cumulative rate of disappearance of high FBG 13.3% (56/422), elevated SBP 14.4% (87/606), elevated DBP 18.8% (108/573), elevated BP 20.3% (146/720), high TG 24% (181/755), low HDL-C 18.5% (84/454), and high WC 2.5% (21/829) is reported in total population.
Table 1.
Prevalence of MetS components at baseline and Phase II, by gender
| MetS and its components | Men | Women | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (2005-2006) | After 10-year follow-up (2015-2016) | Baseline (2005-2006) | After 10-year follow-up (2015-2016) | Baseline (2005-2006) | After 10-year follow-up (2015-2016) | |||||||
| Lost to follow-up | MetS Status, n (%) | Lost to follow-up | MetS Status, n (%) | Lost to follow-up | MetS Status, n (%) | |||||||
| Yes | No | Yes | No | Yes | No | |||||||
| High FBG | 285 | 79 | 180 (87.4) | 26 (12.6) | 313 | 97 | 186 (86.1) | 30 (13.9) | 598 | 176 | 366 (86.7) | 56 (13.3) |
| Elevated SBP | 475 | 148 | 268 (82) | 59 (18) | 430 | 151 | 251 (90) | 28 (10) | 905 | 299 | 519 (85.6) | 87 (14.4) |
| Elevated DBP | 441 | 138 | 244 (73.9) | 79 (26.1) | 415 | 145 | 241 (89.3) | 29 (10.7) | 856 | 283 | 465 (81.2) | 108 (18.8) |
| Elevated BP | 540 | 149 | 298 (76.2) | 93 (17.2) | 479 | 150 | 276 (83.9) | 53 (16.1) | 1019 | 299 | 574 (79.7) | 146 (20.3) |
| High TG | 514 | 118 | 282 (71.2) | 114 (28.8) | 507 | 148 | 292 (81.3) | 67 (18.7) | 1021 | 266 | 574 (76) | 181 (24) |
| Low HDL-C | 232 | 61 | 127 (74.3) | 44 (25.7) | 407 | 124 | 243 (85.9) | 40 (14.1) | 639 | 185 | 370 (81.5) | 84 (18.5) |
| High WC | 551 | 163 | 374 (96.4) | 14 (3.6) | 745 | 304 | 434 (98.4) | 7 (1.6) | 1296 | 467 | 808 (97.5) | 21 (2.5) |
| MetS | 403 | 98 | 263 (86.2) | 42 (13.8) | 484 | 141 | 302 (88) | 41 (12) | 887 | 239 | 565 (87.2) | 83 (12.8) |
Data presented as n (%) subjects. FBG, fasting blood glucose; SBP, systolic blood pressure; DBP, diastolic blood pressure; BP, blood pressure; TG, triglyceride; HDL-C, high-density lipoprotein-cholesterol; WC, waist circumference; MetS, metabolic syndrome
There was a statistically significant difference for each FBG, SBP, DBP, TG, HDL-C, and WC before and after 10-year follow-up in total population and among men [Table 2]. The significant difference was found in women only for FBG, TG, HDL-C, and WC. The results indicate that the total participants and men with MetS had higher means of FBG, SBP, DBP, TG, and WC and lower HDL-C, and women with MetS had higher means of FBG, TG, and WC and lower HDL-C after 10-year follow-up.
Table 2.
The comparison of MetS components at baseline and follow-up, by gender
| MetS and its components | Men | Women | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (2005-2016) | Follow-up (2015-2016) | P | Baseline (2005-2006) | Follow-up (2015-2016) | P | Baseline (2005-2006) | Follow-up (2015-2016) | P | |
| FBG (mg/dL) | 116.1±49.5 | 133.7±60.7 | P<0.001 | 116.5±52.7 | 128.7±49.3 | P<0.001 | 116.3±51 | 131.2±55.4 | P<0.001 |
| SBP (mmHg) | 135.6±13.9 | 151.1±78.4 | P<0.001 | 133.1±15.5 | 139.1±56 | 0.481 | 134.2±14.8 | 145.1±68.3 | 0.001 |
| DBP (mmHg) | 86.6±9 | 85.3±29.7 | 0.003 | 84.9±9.3 | 85.1±35.1 | 0.055 | 85.8±9.2 | 85.2±32.4 | P<0.001 |
| TG (mg/dL) | 238.2±117.9 | 182.2±95.8 | P<0.001 | 226.1±108.4 | 187.2±102.9 | P<0.001 | 232.2±113.4 | 184.7±99.3 | P<0.001 |
| HDL-C (mg/dL) | 47.8±13.3 | 37.7±6.9 | P<0.001 | 52.8±13.5 | 43.1±7.9 | P<0.001 | 50.2±13.6 | 40.3±7.9 | P<0.001 |
| WC (cm) | 100.4±8.7 | 104.4±9.2 | P<0.001 | 100.3±10.1 | 107.3±11.2 | P<0.001 | 100.3±9.4 | 105.8±10.3 | P<0.001 |
Data presented as mean±SD. Mean differences between components of MetS in Phase I and Phase II analyzed using paired t-test. FBG, fasting blood glucose; SBP, systolic blood pressure; DBP, diastolic blood pressure; BP, blood pressure; TG, triglyceride; HDL-C, high-density lipoprotein-cholesterol; WC, waist circumference; MetS, metabolic syndrome
The comparison of participants’ characteristics based on existence or absence (persistence vs. disappearance) of MetS after follow-up is reported in Table 3. Participants who had MetS compared to those without MetS after 10-year follow-up showed statistically significant higher levels of BMI, TG, and WC in the total population. Men who had MetS compared to those without MetS were statistically significantly older and more obese, and women who had MetS compared to those without MetS were statistically significantly older, more obese, and had a higher level of TG as well.
Table 3.
Comparison of the participants’ characteristics based on persistence and disappearance of MetS after 10-year follow-up, by gender
| Variable | MetS status after 10-year follow-up in men | MetS status after 10-year follow-up in women | MetS status after 10-year follow-up in Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes n=263 (46.5) | No n=42 (50.6) | P | Yes n=302 (53.5) | No n=41 (49.4) | P | Yes n=565 | No n=83 | P | ||
| Age (year) | 52.8±12.1 | 49.4±13.5 | 0.093 | 54.7±10.9 | 52±15.6 | 0.295 | 53.8±11.5 | 50.7±14.5 | 0.06 | |
| ≤39 | 41 (15.6) | 14 (33.3) | 0.039 | 29 (9.6) | 11 (26.8) | 0.005 | 70 (12.4) | 25 (30.1) | P<0.001 | |
| 40-49 | 63 (24) | 7 (16.7) | 71 (23.5) | 5 (12.2) | 134 (23.7) | 12 (14.5) | ||||
| 50-59 | 74 (28.1) | 8 (19) | 90 (29.8) | 8 (19.5) | 164 (29) | 16 (19.3) | ||||
| 60-69 | 64 (24.3) | 12 (28.6) | 89 (29.5) | 11 (26.8) | 153 (27.1) | 23 (27.7) | ||||
| ≥70 | 21 (8) | 1 (2.4) | 23 (7.6) | 6 (14.6) | 44 (7.8) | 7 (8.4) | ||||
| Weight (kg) | 80.8±10.6 | 76.4±11.9 | 0.013 | 71.3±11.8 | 68.8±11.6 | 0.19 | 75.8±12.2 | 72.6±12.3 | 0.03 | |
| BMI (kg/m2) | 27.2±3.3 | 26.1±2.8 | 0.044 | 28.8±4.4 | 27.7±4.2 | 0.129 | 28±4 | 26.9±3.6 | 0.013 | |
| <25 | 59 (23.2) | 12 (29.3) | 0.179 | 56 (18.9) | 9 (23.7) | 0.209 | 115 (20.8) | 21 (26.6) | 0.03 | |
| 25-29.9 | 148 (58) | 26 (63.4) | 127 (42.8) | 20 (52.6) | 275 (49.8) | 46 (58.2) | ||||
| >30 | 48 (18.8) | 3 (7.3) | 114 (38.4) | 9 (23.7) | 162 (29.3) | 12 (15.2) | ||||
| SBP (mmHg) | 135.6±14 | 132.1±12.8 | 0.135 | 134.1±15.6 | 131.1±13.4 | 0.234 | 134.8±14.8 | 131.6±13 | 0.064 | |
| DBP (mmHg) | 86.3±9.1 | 84.9±7.8 | 0.343 | 85.1±8.8 | 85.3±10 | 0.886 | 85.7±9 | 85.1±8.9 | 0.596 | |
| FBG (mg/dL) | 116.8±49.7 | 110.8±42.3 | 0.463 | 122.3±56 | 107.5±48.5 | 0.109 | 119.7±53.2 | 109.2±45.2 | 0.087 | |
| TG (mg/dL) | 245.5±129.6 | 222±114.2 | 0.267 | 235.5±113.2 | 170.9±72.8 | P<0.001 | 240.1±121.1 | 196.7±98.8 | P<0.001 | |
| HDL-C (mg/dL) | 47.5±13.3 | 48.6±13.3 | 0.615 | 53.8±13.9 | 51.7±12.2 | 0.347 | 50.9±13.9 | 50.1±12.8 | 0.642 | |
| WC (cm) | 100.8±8.8 | 97.8±8.1 | 0.043 | 100.3±10.3 | 95.7±11.8 | 0.008 | 100.5±9.6 | 96.8±10.1 | 0.001 | |
| Current smokers (%) | 85 (32.3) | 16 (38.1) | 0.711 | 6 (2) | 1 (2.4) | 0.848 | 91 (16.1%) | 17 (20.5%) | 0.57 | |
| Socioeconomic (%) | ||||||||||
| Low | 33 (24.1) | 2 (9.1) | 0.221 | 53 (44.9) | 5 (33.3)) | 0.261 | 86 (33.7%) | 7 (18.9%) | 0.074 | |
| Moderate | 59 (43.1) | 13 (59.1) | 38 (32.2) | 8 (53.3) | 97 (38%) | 21 (56.8%) | ||||
| High | 45 (32.8) | 7 (31.8) | 27 (22.9) | 2 (13.3) | 72 (28.2%) | 9 (24.3%) | ||||
| Physical activity (%) | ||||||||||
| Low | 149 (73.8) | 25 (75.8) | 0.335 | 142 (74.7) | 15 (60) | 0.187 | 291 (74.2%) | 40 (69%) | 0.121 | |
| Moderate | 41 (20.3) | 8 (24.2) | 45 (23.7) | 10 (40) | 86 (21.9%) | 18 (31%) | ||||
| Vigorous | 12 (5.9) | 0 | 3 (1.6) | 0 | 15 (3.8%) | 0 | ||||
| Education (%) | ||||||||||
| Low | 147 (56.5) | 21 (52.5) | 0.592 | 258 (86.3) | 32 (82.1) | 0.447 | 405 (72.5%) | 53 (67.1%) | 0.52 | |
| Moderate | 78 (30) | 15 (37.5) | 39 (13) | 6 (15.4) | 117 (20.9%) | 21 (26.6%) | ||||
| High | 35 (13.5) | 4 (10) | 2 (0.7) | 1 (2.6) | 37 (6.6%) | 5 (6.3%) | ||||
Data presented as mean±SD, otherwise indicated n%. P value for Student t-test and Chi-square test for numerical and categorical variables, respectively
Table 4 shows the association of some demographic characteristics with MetS persistence (hazard ratio, 95% confidence interval) in men, women, and total population computed from crude- and multivariable-adjusted model. The analysis revealed that the risk of persistence of MetS in the age group of 40–49 years was higher compared to the reference group in crude status in the total population (HR = 1.51, 95% CI: 1.12–2). In women, the risk of persistence of MetS in current smokers was higher than noncurrent smokers (HR = 3.53, 95% CI: 1.3–9.6, Ptrend = 0.013). A separate analysis showed that the risk of persistence of MetS was higher in men compared to women (HR = 1.98, 95% CI: 1.38–2.85, Ptrend = 0.001). The result is not shown in Table 4.
Table 4.
The risk of MetS persistence (hazard ratio, 95% confidence interval) based on age, BMI, smoking, economic status, physical activity, and education after 10-year follow-up stratified by gender
| Variable | Men | Women | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude HR (95% CI) | Model I HR (95% CI) | Model II HR (95% CI) | Crude HR (95% CI) | Model I HR (95% CI) | Model II HR (95% CI) | Crude HR (95% CI) | Model I HR (95% CI) | Model II HR (95% CI) | |
| Age (year) | |||||||||
| ≤39 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 40-49 | 1.03 (0.4-2.9) | 1.43 (0.8-2.7) | 1.45 (0.97-2.2) | 1.02 (0.18-5.5) | 1.26 (0.66-2.4) | 1.65 (1.1-2.6) | 1.51 (1.12-2) | 1.31 (0.84-2) | 1.06 (0.45-2.5) |
| 50-59 | 1.07 (0.22-5.3) | 1.37 (0.53-3.5) | 1.41 (0.95-2.1) | 0.5 (0.05-5.1) | 0.83 (0.32-2.1) | 1.33 (0.87-2) | 1.31 (0.99-1.76) | 1.03 (0.53-2) | 0.83 (0.23-3) |
| 60-69 | 0.79 (0.08-7.4) | 1.34 (0.37-4.9) | 1.4 (0.93-2.1) | 0.35 (0.01-8.2) | 0.57 (0.16-2.1) | 1.13 (0.74-1.7) | 1.19 (0.89-1.59) | 0.83 (0.34-2) | 0.63 (0.11-3.6) |
| ≥70 | 0.82 (0.04-15.3) | 1.4 (0.28-7.2) | 1.5 (0.87-2.6) | 0.35 (0.01-13.8) | 0.49 (0.1-2.4) | 1.13 (0.65-2) | 1.24 (0.84-1.81) | 0.79 (0.25-2.42) | 0.55 (0.06-4.8) |
| P for trend | 0.16 | 0.998 | 0.778 | 0.391 | 0.074 | 0.296 | 0.916 | 0.193 | 0.36 |
| BMI (kg/m2) | |||||||||
| <25 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 25-29.9 | 1.14 (0.84-1.55) | 1.15 (0.85-1.56) | 1.01 (0.6-1.72) | 1.03 (0.75-1.42) | 1.02 (0.74-1.4) | 0.92 (0.45-1.9) | 1.09 (0.87-1.35) | 1.09 (0.87-1.36) | 0.97 (0.65-1.5) |
| >30 | 0.99 (0.66-1.48) | 1 (0.67-1.5) | 0.83 (0.4-1.7) | 1.18 (0.85-1.63) | 1.16 (0.84-1.6) | 1.6 (0.77-3.3) | 1.11 (0.83-1.42) | 1.15 (0.9-1.5) | 1.19 (0.7-1.9) |
| P for trend | 0.921 | 0.88 | 0.632 | 0.264 | 0.306 | 0.107 | 0.402 | 0.273 | 0.441 |
| Smoking | |||||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Yes | 0.86 (0.66-1.12) | 0.89 (0.37-2.2) | 0.69 (0.43-1.1) | 0.88 (0.36-2.1) | 0.86 (0.66-1.1) | 3.53 (1.3-9.6) | 0.94 (0.75-1.18) | 0.85 (0.66-1.1) | 0.8 (0.53-1.22) |
| P for trend | 0.272 | 0.256 | 0.108 | 0.777 | 0.807 | 0.013 | 0.614 | 0.217 | 0.307 |
| Economic (%) | |||||||||
| Low | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Moderate | 0.99 (0.64-1.5) | 1.08 (0.69-1.68) | 1.14 (0.68-1.9) | 0.86 (0.56-1.32) | 0.86 (0.55-1.3) | 0.77 (0.44-1.4) | 0.99 (0.73-1.3) | 0.96 (0.7-1.3) | 1 (0.7-1.45) |
| High | 0.92 (0.58-1.45) | 1.05 (0.64- | 1.1 (0.6-2) | 0.98 (0.61-1.58) | 0.99 (0.61-1.6) | 1.35 (0.7-2.8) | 1.03 (0.75-1.4) | 1 (0.72-1.4) | 1.13 (0.73-1.7) |
| P for trend | 0.705 | 0.875 | 0.77 | 0.833 | 0.982 | 0.658 | 0.861 | 0.988 | 0.602 |
| Physical activity (%) | |||||||||
| Low | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Moderate | 0.75 (0.52-1.1) | 0.75 (0.52-1.1) | 0.8 (0.49-1.3) | 1.2 (0.86-1.7) | 1.2 (0.86-1.7) | 1.6 (0.96-2.8) | 1.08 (0.61-1.9) | 0.95 (0.54-1.69) | 1.1 (0.54-2.4) |
| Vigorous | 0.79 (0.44-1.4) | 0.79 (0.44-1.4) | 0.8 (0.33-1.9) | 1.5 (0.38-6.3) | 1.6 (0.38-6.4) | 2.8 (0.64-12.2) | 1.03 (0.81-1.32) | 1.05 (0.82-1.34) | 1.1 (0.8-1.5) |
| P for trend | 0.091 | 0.096 | 0.343 | 0.266 | 0.265 | 0.067 | 0.811 | 0.667 | 0.623 |
| Education (%) | |||||||||
| Low | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Moderate | 1.05 (0.79-1.4) | 1.2 (0.87-1.7) | 0.95 (0.51-1.8) | 0.9 (0.63-1.27) | 0.83 (0.57-1.2) | 0.67 (0.33-1.3) | 1 (0.81-1.24) | 0.99 (0.77-1.2) | 0.84 (0.54-1.3) |
| High | 1.06 (0.7-1.5) | 1.21 (0.8-1.8) | 1.37 (0.74-2.6) | 0.83 (0.2-3.3) | 0.75 (0.98-1) | 1 (0.98-1) | 1.08 (0.77-1.52) | 1.03 (0.71-1.5) | 1.18 (0.68-2) |
| P for trend | 0.71 | 0.274 | 0.319 | 0.52 | 0.33 | 0.26 | 0.703 | 0.959 | 0.776 |
The risk of persistence of MetS (hazard ratio, 95% confidence interval) based on changes of MetS components from baseline to Phase II (that was divided into quartiles) in men, women, and total population in crude- and multivariable-adjusted models is shown in Table 5. There was a significant direct association between the highest quartiles of SBP changes (an increase ≥ 15.6 mmHg) and risk of MetS persistence in crude and model I in women and total population. Also, there was a linear trend between the mean changes of SBP and the risk of MetS persistence in women and total population (Ptrend < 0.05). An increase in DBP of − 2 to 6.5 mmHg in crude and model I, and DBP ≥6.6 mmHg in model I, and an increase in WC of 6–10 cm and WC ≥11 cm significantly increased the risk of MetS persistence and also, its trend for DBP and WC were significant in the total population. In addition, an increase in the mean in HDL-C of ≥−1 mg/dL significantly increased the persistence of MetS in crude and model I, but its trend was significant for increase in the changes of HDL-C in the crude analysis in the total population. In men, an increase in TG from −101 to −43 mg/dL and − 42 to −9.25 significantly reduced risk of persistence of MetS in crude, model I, and model II, but its trend was not significant. In women, an increase in DBP of −2 to 6.5 mmHg significantly increased persistence of MetS in crude and model I, but its trend was significant. In addition, an increase of HDL-C of −9 to −2 mg/dL significantly increased the persistence of MetS in crude, and its trend was significant in crude and model I in women. In addition, an increase in WC of 6–10 cm and ≥11 cm significantly increased the persistence of MetS but its trend was significant in the crude analysis in women.
Table 5.
Risk of persistence of MetS (hazard ratio, 95% confidence interval) based on changes in its components from baseline to Phase II of the study, by gender
| Variables | Men | Women | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude HR (95% CI) | Model I HR (95% CI) | Model II HR (95% CI) | Crude HR (95% CI) | Model I HR (95% CI) | Model II HR (95% CI) | Crude HR (95% CI) | Model I HR (95% CI) | Model II HR (95% CI) | |
| FBG mg/dL | |||||||||
| ≤−19.9 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| −20-5.9 | 0.76 (0.52-1.1) | 0.75 (0.51-1.1) | 1.03 (0.54-1.9) | 1.2 (0.84-1.7) | 1.2 (0.84-1.72) | 1.39 (0.61-3.1) | 0.98 (0.76-1.27) | 0.96 (0.75-1.25) | 1.24 (0.77-2) |
| 6-33.9 | 0.9 (0.63-1.28) | 0.93 (0.65-1.3) | 1 (0.56-1.78) | 1.1 (0.78-1.6) | 1.1 (0.78-1.6) | 0.87 (0.4-1.89) | 1.02 (0.79-1.31) | 1.03 (0.8-1.33) | 0.97 (0.62-1.53) |
| ≥34 | 0.9 (0.63-1.28) | 0.9 (0.64-1.29) | 0.96 (0.54-1.7) | 1.07 (0.75-1.5) | 1.07 (0.74-1.5) | 0.88 (0.42-1.87) | 0.98 (0.77-1.26) | 0.98 (0.77-1.27) | 0.96 (0.62-1.48) |
| P for trend | 0.783 | 0.994 | 0.885 | 0.814 | 0.81 | 0.571 | 0.977 | 0.964 | 0.635 |
| SBP (mmHg) | |||||||||
| ≤−10 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| −9.7-2.5 | 0.74 (0.49-1.1) | 0.74 (0.49-1.1) | 0.7 (0.36-1.38) | 1.27 (0.88-1.8) | 1.28 (0.88-1.8) | 1.18 (0.5-2.65) | 1.04 (0.79-1.36) | 1.03 (0.78-1.35) | 1 (0.62-1.6) |
| 2.6-15.5 | 0.99 (0.66-1.49) | 0.99 (0.66-1.5) | 1.42 (0.71-2.8) | 1.18 (0.8-1.7) | 1.24 (0.84-1.8) | 0.94 (0.37-2.38) | 1.1 (0.84-1.45) | 1.12 (0.85-1.48) | 1.1 (0.64-1.79) |
| ≥15.6 | 1 (0.7-1.48) | 1 (0.69-1.47) | 1.1 (0.58-2) | 1.7 (1.15-2.55) | 1.72 (1.16-2.6) | 1.56 (0.62-3.9) | 1.35 (1.03-1.76) | 1.32 (1.002-1.7) | 0.99 (0.61-1.6) |
| P for trend | 0.506 | 0.529 | 0.509 | 0.023 | 0.016 | 0.505 | 0.028 | 0.039 | 0.973 |
| DBP (mmHg) | |||||||||
| ≤−10.5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| −10 to−2.5 | 1.05 (0.7-1.55) | 1.08 (0.73-1.6) | 0.79 (0.42-1.5) | 1.46 (0.99-2.1) | 1.46 (0.99-2.2) | 1.59 (0.77-3.2) | 1.24 (0.95-1.64) | 1.27 (0.97-1.68) | 0.95 (0.6-1.5) |
| −2 to 6.5 | 1.41 (0.97-2.1) | 1.45 (0.98-2.1) | 1.26 (0.66-2.4) | 1.64 (1.12-2.4) | 1.69 (1.15-2.5) | 1.01 (0.42-2.4) | 1.5 (1.14-1.95) | 1.56 (1.19-2.04) | 1.12 (0.67-1.87) |
| ≥ 6.6 | 1.23 (0.84-1.8) | 1.26 (0.85-1.9) | 1.14 (0.63-2) | 1.35 (0.92-2) | 1.43 (0.96-2.1) | 1.13 (0.45-2.8) | 1.27 (0.97-1.67) | 1.34 (1.02-1.78) | 1.02 (0.63-1.63) |
| P for trend | 0.141 | 0.116 | 0.421 | 0.101 | 0.51 | 0.735 | 0.034 | 0.013 | 0.816 |
| TG (mg/dL) | |||||||||
| ≤−101 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| −100 to−43 | 0.65 (0.45-0.92) | 0.62 (0.43-0.9) | 0.39 (0.21-0.7) | 1.23 (0.85-1.8) | 1.23 (0.85-1.8) | 0.93 (0.36-2.3) | 0.9 (0.7-1.16) | 0.88 (0.68-1.13) | 0.5 (0.3-0.83) |
| −42-9.25 | 0.61 (0.43-0.87) | 0.6 (0.42-0.9) | 0.46 (0.25-0.8) | 1.26 (0.87-1.8) | 1.26 (0.87-1.8) | 1.47 (0.71-3.1) | 0.89 (0.68-1.14) | 0.87 (0.67-1.13) | 0.75 (0.47-1.2) |
| ≥9.26 | 1 (0.7-1.43) | 1 (0.7-1.43) | 0.58 (0.3-1.1) | 1.25 (0.88-1.8) | 1.25 (0.88-1.8) | 0.97 (0.48-2) | 1.11 (0.87-1.43) | 1.11 (0.86-1.42) | 0.7 (0.44-1.12) |
| P for trend | 0.789 | 0.822 | 0.309 | 0.219 | 0.853 | 0.773 | 0.462 | 0.458 | 0.526 |
| HDL-C (mg/dL) | |||||||||
| ≤−19 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| −18.9 to−10 | 0.77 (0.55-1.1) | 0.77 (0.55-1.1) | 0.87 (0.49-1.6) | 0.93 (0.66-1.3) | 0.93 (0.65-1.3) | 1.01 (0.4-2.51) | 0.85 (0.66-1.09) | 0.85 (0.67-1.09) | 0.83 (0.52-1.32) |
| −9 to−2 | 1.29 (0.9-1.87) | 1.28 (0.88-1.8) | 1.25 (0.66-2.3) | 0.79 (0.56-1.1) | 0.79 (0.55-1.1) | 0.92 (0.43-2) | 0.96 (0.74-1.23) | 0.97 (0.75-1.25) | 1.14 (0.71-1.83) |
| ≥−1 | 0.75 (0.52-1.1) | 0.78 (0.54-1.1) | 1.34 (0.7-2.52) | 0.66 (0.46-09) | 0.66 (0.45-1) | 0.69 (0.32-1.5) | 0.72 (0.56-0.93) | 0.74 (0.57-0.96) | 0.94 (0.59-1.49) |
| P for trend | 0.468 | 0.645 | 0.211 | 0.016 | 0.017 | 0.297 | 0.036 | 0.06 | 0.894 |
| WC (cm) | |||||||||
| ≤1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2-5 | 0.94 (0.67-1.31) | 0.97 (0.69-1.4) | 0.73 (0.43-1.3) | 1.17 (0.79-1.7) | 1.17 (0.79-1.7) | 0.86 (0.36-2.1) | 1.07 (0.82-1.38) | 1.07 (0.83-1.38) | 0.76 (0.49-1.16) |
| 6-10 | 1.18 (0.84-1.67) | 1.3 (0.93-1.9) | 1.45 (0.77-2.7) | 1.55 (1.1-2.19) | 1.56 (1.1-2.2) | 1.64 (0.8-3.37) | 1.36 (1.07-1.7) | 1.44 (1.13-1.8) | 1.4 (0.9-2.21) |
| ≥11 | 1 (0.68-1.46) | 1.1 (0.75-1.6) | 1.19 (0.61-2.3) | 1.51 (1.07-2.1) | 1.52 (1.1-2.15) | 1.62 (0.78-3.36) | 1.26 (0.98-1.62) | 1.32 (1.03-1.7) | 1.14 (0.72-1.82) |
| P for trend | 0.644 | 0.28 | 0.335 | 0.007 | 0.007 | 0.104 | 0.018 | 0.005 | 0.248 |
| BMI (kg/m2) | |||||||||
| ≤-0.93 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| -0.9 to 0.7 | 0.78 (0.55-1.1) | 0.79 (0.55-1.1) | 0.8 (0.44-1.5) | 0.88 (0.6-1.3) | 0.89 (0.6-1.3) | 0.76 (0.31-1.81) | 0.83 (0.64-1.07) | 0.83 (0.65-1.09) | 0.87 (0.54-1.4) |
| 0.8-2.6 | 0.92 (0.64-1.3) | 0.94 (0.66-1.3) | 1 (0.55-1.83) | 0.75 (0.51-1.1) | 0.75 (0.51-1.1) | 0.89 (0.37-2.15) | 0.84 (0.65-1.08) | 0.85 (0.66-1.1) | 0.8 (0.5-1.31) |
| ≥2.7 | 0.73 (0.49-1.09) | 0.79 (0.52-1.2) | 0.67 (0.3-1.41) | 0.87 (0.62-1.2) | 0.88 (0.62-1.2) | 0.93 (0.38-2.28) | 0.81 (0.63-1.05) | 0.84 (0.65-1.1) | 0.69 (0.4-1.18) |
| P for trend | 0.242 | 0.433 | 0.504 | 0.391 | 0.409 | 0.957 | 0.143 | 0.233 | 0.166 |
Discussion
We found that participants with increase of age, weight, BMI, WC, and TG had a significant increased chance of MetS persistence after 10-year follow-up. Men had a higher risk of persistence of MetS compared to women, smoker women had a higher risk of persistence of MetS compared to nonsmoker women. In addition, we found that an increase in the mean changes of SBP, DBP, and WC after adjustment for age and sex are significantly associated with persistence of MetS, and an increase of HDL-C was negatively associated with the risk of persistence of MetS.
Similar to this study, a 1-year follow-up study reported a reduction in SBP and DBP and an increase of HDL-C were associated with MetS remission or disappearance. The study did not find any statistically significant association between change in FBG and risk of MetS.[12] However, they reported that there was no association between BMI and WC and remission or cure of MetS. It should be noted that the follow-up duration in the study was much lower than our study. As they noted, weight and BMI reduction for a short period of time might have a small effect on the changes of MetS.[12] Different potential confounders should be considered to interpret and compare these two studies. Because, in our study, some mean changes of MetS components were significant with MetS persistence when adjusted by sex and age, and were not significant with other potential confounders, additionally. In this study after a 10-year follow-up, 12.8% of the participants were MetS free while this index was 30% for another study.[12] The period of follow-up, medical treatment, and lifestyle intervention for MetS components might be a cause of the reported difference in the disappearance of MetS.[12,18] Similar to this study, the assessment of 9-year changes of components of MetS in China showed that the participants had higher FBG and TG, but different from our study, SBP and DBP were lower in both gender in 2014 compared to 2005.[10] It should be noted that, in our study, there was a significant difference in SBP and DBP before and after follow-up in men, but not in women.
In another cohort study with a 6.6-year follow-up that was done in Iranian adults, the increase of WC in men was higher than women.[19] In our study, the trend of hazard ratio over the increase of WC was significant only in women and the total population in crude and after adjustment for age and sex, but not in multivariable-adjusted model. Therefore, some factors such as economic status, education, and physical activity might have a protective effect on the risk of MetS persistence and the effect might be different in men and women.[1,19] However, changes in lifestyle and nutritional pattern,[20] having bad dietary habit behaviors,[21] industrialization,[22] as well as physical inactivity due to changes of transportation systems and change in nature of jobs in Iran cause increase of abdominal obesity,[19] and subsequently might increase the risk of MetS persistence. Our study showed that the risk of MetS persistence in men is significantly higher (1.98 times) than women. Similar to our study, Chang et al. reported that the risk of MetS persistence in men was more than women, but there was no significant difference between the two groups.[12] However, limited cohort studies have been done to report the relationship between MetS persistence and its predictors in men and women. The increase of educational level and income in Iran might be a cause of decreasing MetS and its components in women rather than men.[1,23]
In this study after adjustment for age and sex, the increase in mean changes of WC, SBP, and DBP was associated with increased risk of MetS persistence after 10-year follow-up. In another study, the increase of low HDL-C, WC, and TG was the most important predictor for MetS over 10 years in Korea.[24] It seems that the tendency to have a high-fat diet and low physical activities were causes of the MetS changes in Korea. In addition, our previous study showed that lack of having body weight control program, a diet without salad and vegetables, and added extra salt to food when eating increase the risk of MetS.[21] In the study, FBS at the baseline was not associated with the persistence of MetS. However, it seems that the risk factors due to obesity and hyperlipidemia were associated with the persistence of MetS. In the other hand, participants with higher BMI and WC are more in the risk of MetS persistence rather than FBS. Therefore, decreasing obesity is more important to decrease the risk of MetS persistence and other problems.
To the best our knowledge, this study is the first cohort study in Iranian adults with MetS persistence that was followed for a relatively long period of time. However, some limitation might be considered for the interpretation of the results of the study. After repeated measurement during 2015–2016, 26.9% (n = 239) of participants with MetS at the baseline were lost to follow-up due to migration, mortality, disability, and no response to the phone calls. We tried to decrease the attrition by selecting an expert in psychology with good communication to keep participants in the study. In addition, Yazd Telecommunication Company cooperated with us to find the participants who did not answer the phone call that was recorded during 2005–2006. Estimation of the exact time for recurrence of MetS might be another limitation of the study because the second MetS components measurement was done only in Phase II (at the end of 10 years of follow-up). In addition, lifestyle changes during the 10 years of follow-up were not assessed. In addition, the lack of energy intake in the analyses is another limitation of the study. In the study, some differences between follow-up and lost to follow-up group should be considered to interpret the results. The comparison of the baseline characteristics of follow-up and lost to follow-up showed that there were significant differences in age, weight, fasting blood glucose, systolic blood pressure, and socioeconomic status. In other words, lost to follow-up group were older, lower weight, with higher systolic blood pressure and fasting blood glucose than the follow-up group.
Conclusions
In conclusion, we found evidence indicating that age, gender, weight, BMI, WC, and TG, and also increase in the mean changes of SBP, DBP, WC, and low HDL-C were positively associated with persistence of MetS after adjusted with age and sex, but not other potential confounders. Changing lifestyle behaviors to reduce the risk of MetS persistence is recommended. To confirm the findings of this study, further studies are required in Iranian adults.
Financial support and sponsorship
Shahid Sadoughi University of Medical Sciences in Yazd, Iran and Iran National Science Foundation (INSF) supported the study.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We are thankful to all participants of YHHP for their cooperation. In addition, we appreciate Shahid Sadoughi University of Medical Sciences in Yazd, Iran and Iran National Science Foundation (INSF) for supporting the study.
References
- 1.Hadaegh F, Hasheminia M, Lotfaliany M, Mohebi R, Azizi F, Tohidi M. Incidence of metabolic syndrome over 9 years follow-up; the importance of sex differences in the role of insulin resistance and other risk factors. PloS One. 2013;8:e76304. doi: 10.1371/journal.pone.0076304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: A global public health problem and a new definition. J Atheroscler Thromb. 2005;12:295–300. doi: 10.5551/jat.12.295. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Metabolic syndrome update. Trends Cardiovasc Med. 2016;26:364–73. doi: 10.1016/j.tcm.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Noshad S, Abbasi M, Etemad K, Meysamie A, Afarideh M, Khajeh E, et al. Prevalence of metabolic syndrome in Iran: A 2011 update. J Diabetes. 2017;9:518–25. doi: 10.1111/1753-0407.12438. [DOI] [PubMed] [Google Scholar]
- 5.Amirkalali B, Fakhrzadeh H, Sharifi F, Kelishadi R, Zamani F, Asayesh H, et al. Prevalence of metabolic syndrome and its components in the Iranian adult population: A systematic review and meta-analysis. Iranian Red Crescent Med J. 2015;17:e24723. doi: 10.5812/ircmj.24723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarebanhassanabadi M, Jalil Mirhosseini S, Mirzaei M, Namayandeh SM, Soltani MH, Pedarzadeh A, et al. The incidence of metabolic syndrome and the most powerful components as predictors of metabolic syndrome in central Iran: A 10-year follow-up in a cohort study. Iranian Red Crescent Med J. 2017;19:e14934. [Google Scholar]
- 7.Ponciano-Rodriguez G, Paez-Martinez N, Villa-Romero A, Gutierrez-Grobe Y, Mendez-Sanchez N. Early changes in the components of the metabolic syndrome in a group of smokers after tobacco cessation. Metab Syndr Relat Disord. 2014;12:242–50. doi: 10.1089/met.2014.0007. [DOI] [PubMed] [Google Scholar]
- 8.Babio N, Bulló M, Salas-Salvadó J. Mediterranean diet and metabolic syndrome: The evidence. Public Health Nutr. 2009;12:1607–17. doi: 10.1017/S1368980009990449. [DOI] [PubMed] [Google Scholar]
- 9.Brouwer BG, van der Graaf Y, Soedamah-Muthu SS, Wassink AMJ, Visseren FLJ. Leisure-time physical activity and risk of type 2 diabetes in patients with established vascular disease or poorly controlled vascular risk factors. Diabetes Res Clin Pract. 2010;87:372–8. doi: 10.1016/j.diabres.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Jiang B, Li B, Wang Y, Han B, Wang N, Li Q, et al. The nine-year changes of the incidence and characteristics of metabolic syndrome in China: Longitudinal comparisons of the two cross-sectional surveys in a newly formed urban community. Cardiovasc Diabetol. 2016;15:84. doi: 10.1186/s12933-016-0402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panagiotakos DB, Pitsavos C, Skoumas Y, Stefanadis C. The association between food patterns and the metabolic syndrome using principal components analysis: The ATTICA Study. J Am Diet Assoc. 2007;107:979–87. doi: 10.1016/j.jada.2007.03.006. quiz 97. [DOI] [PubMed] [Google Scholar]
- 12.Chang HC, Horng JT, Chau TT, Chen YW, Hsieh CF, Chang CW, et al. Relationship between changes in components associated with metabolic syndrome and disappearance, or remission, of metabolic syndrome during 1 year. J Int Med Res. 2012;40:2311–20. doi: 10.1177/030006051204000629. [DOI] [PubMed] [Google Scholar]
- 13.Namayandeh S, Sadr S, Ansari Z, Rafiei M. A cross-sectional study of the prevalence of coronary artery disease traditional risk factors in Yazd urban population, Yazd Healthy Heart Project. Int Cardiovasc Res J. 2011;5:7–13. [Google Scholar]
- 14.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. International J Surgery. 2014;12:1495–9. [Google Scholar]
- 15.Chackrewarthy S, Gunasekera D, Pathmeswaren A, Wijekoon CN, Ranawaka UK, Kato N, et al. A comparison between revised NCEP ATP III and IDF definitions in diagnosing metabolic syndrome in an urban Sri Lankan population: The Ragama health study. ISRN Endocrinol 2013. 2013 doi: 10.1155/2013/320176. doi: 101155/2013/320176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esteghamati A, Ashraf H, Rashidi A, Meysamie A. Waist circumference cut-off points for the diagnosis of metabolic syndrome in Iranian adults. Diabetes Res Clin Pract. 2008;82:104–7. doi: 10.1016/j.diabres.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Maddison R, Ni Mhurchu C, Jiang Y, Vander Hoorn S, Rodgers A, Lawes CM, et al. International Physical Activity Questionnaire (IPAQ) and New Zealand Physical Activity Questionnaire (NZPAQ): A doubly labelled water validation. Int J Behav Nutr Phys Act. 2007;4:62. doi: 10.1186/1479-5868-4-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiuve SE, McCullough ML, Sacks FM, Rimm EB. Healthy lifestyle factors in the primary prevention of coronary heart disease among men: Benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation. 2006;114:160–7. doi: 10.1161/CIRCULATIONAHA.106.621417. [DOI] [PubMed] [Google Scholar]
- 19.Hosseinpanah F, Barzin M, Eskandary PS, Mirmiran P, Azizi F. Trends of obesity and abdominal obesity in Tehranian adults: A cohort study. BMC Public Health. 2009;9:426. doi: 10.1186/1471-2458-9-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esmaillzadeh A, Azadbakht L. Major dietary patterns in relation to general obesity and central adiposity among Iranian women. J Nutr. 2008;138:358–63. doi: 10.1093/jn/138.2.358. [DOI] [PubMed] [Google Scholar]
- 21.Sarebanhassanabadi M, Mirhosseini SJ, Mirzaei M, Namayandeh SM, Soltani MH, Pakseresht M, et al. Effect of dietary habits on the risk of metabolic syndrome: Yazd Healthy Heart Project. Public Health Nutr. 2018;21:1139–46. doi: 10.1017/S1368980017003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirmiran P, Mohammadi F, Allahverdian S, Azizi F. Estimation of energy requirements for adults: Tehran lipid and glucose study. Int J Vitam Nutr Res. 2003;73:193–200. doi: 10.1024/0300-9831.73.3.193. [DOI] [PubMed] [Google Scholar]
- 23.Santos AC, Severo M, Barros H. Incidence and risk factors for the metabolic syndrome in an urban South European population. Prev Med. 2010;50:99–105. doi: 10.1016/j.ypmed.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Lim S, Shin H, Song JH, Kwak SH, Kang SM, Won Yoon J, et al. Increasing prevalence of metabolic syndrome in Korea: The Korean National Health and Nutrition Examination Survey for 1998–2007. Diabetes Care. 2011;34:1323–8. doi: 10.2337/dc10-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]


