ABSTRACT
Background and objective
We recently noted a dramatic increase in the number of patients with accelerated silicosis associated with exposure to artificial stone dust. Therefore, the natural history of artificial stone‐associated silicosis was compared with that of natural stone‐associated silicosis.
Methods
A total of 18 patients with artificial stone‐associated silicosis and 63 with natural stone‐associated silicosis were diagnosed sequentially in 2018 and followed up for a period of 6–12 months. Data were collected from clinical charts.
Results
The median duration of exposure prior to onset of symptoms of silicosis was shorter for patients who had been exposed to artificial stone dust (6.4 vs 29.3 years, P < 0.01). Four of the 18 patients experienced rapid deterioration in lung function over the follow‐up period, with declines in pre‐bronchodilator FVC of 587 (210–960) mL/year and FEV1 of 625 (360–860) mL/year. GGO, PMF, emphysema and pulmonary artery widening were more frequently observed on computed tomography scans of patients with artificial stone‐associated silicosis than of those with natural stone‐associated silicosis. Approximately 38.9% of the patients with artificial stone‐associated silicosis were lung transplant candidates and 27.8% died, both rates being significantly higher than in patients with natural stone‐associated silicosis (3.2% and 0%, both P < 0.01).
Conclusion
Compared to natural stone‐associated silicosis, artificial stone‐associated silicosis was characterized by short latency, rapid radiological progression, accelerated decline in lung function and high mortality.
Keywords: artificial stone, progressive massive fibrosis, pulmonary function, respirable crystalline silica, silicosis
High silica content of artificial stone and uncontrolled dry cutting and grinding presents as a high risk of developing accelerated silicosis. Compared to natural stone‐associated silicosis, artificial stone‐associated silicosis was characterized by short latency, rapid radiological progression, accelerated decline in lung function and high mortality in this study.
See related https://onlinelibrary.wiley.com/doi/10.1111/resp.13766
See https://onlinelibrary.wiley.com/doi/10.1111/resp.13594
INTRODUCTION
Silicosis is a progressive, fibrotic, occupational lung disease, which results from inhalation of respirable crystalline silica (RCS).1, 2 Occupations traditionally associated with increased risk of silicosis include glass and pottery making, mining and quarrying, sandblasting and construction trades that generate silica dust through stone or concrete work.3 Silicosis in workers exposed to artificial quartz conglomerates containing high proportions of crystalline silica particles has been a topic of investigation in recent years.4, 5, 6, 7, 8, 9
Artificial stone, also termed engineered, agglomerated or reconstituted stone or quartz conglomerate, is a relatively new, increasingly popular building material that is used primarily to fabricate kitchen and bathroom countertops.10, 11 Artificial stone is a composite of quartz, the major filler, and coloured glass, shells, metals or mirrors bound together by a polymer resin.12 Artificial stone became commercially available in 1986, and the first report of artificial stone‐associated silicosis caused by stonemasonry was published in Italy in 2010.13 Since then, increasing numbers of cases have been reported from Spain, Israel, Australia, Turkey and the USA.4, 5, 6, 7, 8, 9 Artificial stone has been available in China since the early 2000s, but no reports of artificial stone‐associated silicosis in China have been published. We recently noted a marked increase in the number of patients with artificial stone‐associated silicosis.
At 70–90%, the silica content of artificial stone is far higher than that of natural stone (approximately 3% in marble and 30% in granite).4, 14 Thus, the risk of exposure to artificial quartz conglomerates appears to be higher than that of exposure to natural stone dust, and the associated silicosis may differ significantly in its latency and clinical characteristics. To determine whether artificial stone‐associated silicosis differs from natural stone‐associated silicosis, we studied a series of patients with newly diagnosed silicosis who attended our hospital, including 18 who were exposed to artificial stone dust and 63 who were exposed to natural stone dust.
METHODS
Study cohort
From January to December 2018, 81 patients with newly diagnosed silicosis were sequentially recruited from the Department of Occupational Medicine and Toxicology, Beijing Chao‐Yang Hospital. All patients completed a standardized questionnaire to collect information on their occupational histories (Table S1 in Supplementary Information). No workplace exposure monitoring data were available. All patients were diagnosed according to the criteria for pneumoconiosis of the 2011 International Labour Organization classification and the International Classification of HRCT for Occupational Environmental Respiratory Diseases (ICOERD).15, 16 The patients were divided into two groups: 18 with artificial stone‐associated silicosis and 63 with natural stone‐associated silicosis.
This work was conducted at Beijing Chao‐Yang Hospital with approval from the human research ethics committee of Beijing Chao‐Yang Hospital, Capital Medical University. Informed consent was documented in writing.
Pulmonary function tests
The pulmonary function examination was standardized according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) recommendations and included spirometry, body plethysmography and the measurement of carbon monoxide transfer factor of the lungs utilizing the single‐breath method.17
High‐resolution computed tomography
High‐resolution computed tomography (HRCT) scans in patients were performed with 0.625‐mm sections, a 1‐s scan time and a 10‐mm interval in the apex base scans with inclusion of both lungs in the field of view.
Statistical analysis
The mean values for relevant patient characteristics and pulmonary function variables are expressed as mean ± SD. The Mann–Whitney U‐test was used to assess differences between the two groups, as appropriate. The distribution of radiological opacities was evaluated using the chi‐square test. A P‐value of < 0.05 denotes statistical significance. All data were analysed with SPSS version 21.0 (IBM SPSS, Armonk, NY, USA).
RESULTS
Characteristics of the study population
Table 1 displays the relevant clinical and other characteristics of patients, stratified by type of silicosis. Patients with artificial stone‐associated silicosis were younger than patients with natural stone‐associated silicosis and had shorter exposure durations. The decision to place a patient on the waiting list for lung transplantation was made in accordance with a consensus document for selection of lung transplant candidates18 and a previous study.19 However, candidates in both groups failed to undergo lung transplantation within the study period for lack of medical insurance. During a follow‐up period of 6–12 months, five patients (27.8%) with artificial stone‐associated silicosis died. A higher incidence of spontaneous pneumothorax, lung transplant candidates and mortality was found in patients with artificial stone‐associated silicosis than in those with natural stone‐associated silicosis (all P < 0.05).
Table 1.
Demographics and clinical characteristics of individuals with artificial stone‐associated versus natural stone‐associated silicosis
| Parameters | Artificial stone‐associated silicosis | Natural stone‐associated silicosis | P‐value |
|---|---|---|---|
| No. of patients | 18 | 63 | |
| Male, n (%) | 18 (100.0) | 52 (82.5) | 0.145 |
| Age, mean ± SD | 36.1 ± 9.6 | 52.8 ± 8.6 | 0.000*** |
| Current or ex‐smoker, n (%) | 12 (66.7) | 43 (68.3) | 0.950 |
| Years of exposure | 6.1 ± 2.8 | 14.1 ± 7.3 | 0.000*** |
| Time from exposure to illness (years) | 6.4 ± 2.9 | 29.3 ± 11.7 | 0.000*** |
| Simple silicosis, n (%) | 4 (22.2) | 38 (60.3) | 0.004** |
| Complicated silicosis, n (%) | 14 (77.8) | 25 (39.7) | 0.004** |
| PFT at initial evaluation | |||
| FVC (% predicted) | 69.0 ± 29.5 | 97.5 ± 16.0 | 0.004** |
| FEV1 (% predicted) | 65.2 ± 29.4 | 84.4 ± 19.4 | 0.049 |
| FEV1/FVC ratio (%) | 80.7 ± 11.5 | 71.6 ± 12.6 | 0.031* |
| TLC (% predicted) | 68.0 ± 18.4 | 96.0 ± 14.5 | 0.001*** |
| DLCO (% predicted) | 47.5 ± 22.2 | 80.1 ± 18.6 | 0.001*** |
| CPI | 56.4 ± 27.4 | 15.9 ± 13.9 | 0.000*** |
| PaO2 (mm Hg) | 64.8 ± 9.5 | 88.2 ± 11.1 | 0.000*** |
| Spontaneous pneumothorax, n (%) | 3 (16.7) | 1 (1.6) | 0.033* |
| Lung transplant candidates, n (%) | 7 (38.9) | 2 (3.2) | 0.000*** |
| Mortality, n (%) | 5 (27.8) | 0 (0.0) | 0.000*** |
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.001.
Data are presented as mean ± SD, unless otherwise stated.
CPI, composite physiological index; DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PaO2, partial pressure of oxygen; PFT, pulmonary function test; TLC, total lung capacity.
Occupational dust exposure
The 63 patients with natural stone‐associated silicosis were all local residents. Twenty‐nine patients had been exposed to silica dust in the processing of jade and 34 patients engaged in mining and quarrying. The jade‐processing factories were functional from the 1970s to the 1990s, and the mining plants had been open from the 1970s to the 2000s. These enterprises used lower level production technologies and occupational protection strategies. Eighteen patients (28.6%) with natural stone‐associated silicosis reported using disposable masks during all working hours, 27 (42.8%) reported using disposable masks 3–5 h per day during working hours and 18 (28.6%) reported working without any personal respiratory protection.
Of the 18 patients with artificial stone‐associated silicosis, 5 were from Fujian province, 4 from Guangdong province, 4 from Anhui province, 2 from Shanghai, 2 from Jiangsu province and 1 from Zhejiang province. Four patients (22.2%) from the same artificial stone factory reported using disposable masks during cutting, polishing and grinding activities and applying water curtains. Fourteen patients (77.8%) performed cutting and home installation of kitchen and bathroom countertops without any respiratory protection.
HRCT findings
The patients were classified into groups with complicated or simple silicosis on the basis of disease extent, as determined by HRCT.3 Complicated silicosis, or accelerated silicosis with progressive massive fibrosis (PMF), was defined as the presence of a large opacity or coalescence larger than 1.5 cm in diameter.20, 21 On that basis, 14 patients (77.8%) exposed to artificial stone were diagnosed as having complicated silicosis (Table 1). The incidence of ground‐glass opacities (GGO), large opacities, emphysema and pulmonary artery widening was significantly higher in patients with artificial stone‐associated silicosis than in those with natural stone‐associated silicosis (all P < 0.05). In contrast, the incidence of rounded opacities and mediastinal lymphadenopathy was significantly lower in patients with artificial stone‐associated silicosis (all P < 0.05) (Table 2).
Table 2.
HRCT findings in patients with artificial stone‐associated versus natural stone‐associated silicosis
| HRCT findings | Artificial stone‐associated silicosis | Natural stone‐associated silicosis | P‐value |
|---|---|---|---|
| No. of patients | 18 | 63 | |
| Rounded opacities, n (%) | 6 (33.3) | 59 (93.7) | 0.000*** |
| Irregular opacities, n (%) | 5 (27.8) | 23 (36.5) | 0.492 |
| Large opacities, n (%) | 14 (77.8) | 25 (39.7) | 0.004** |
| GGO, n (%) | 16 (88.9) | 8 (12.7) | 0.000*** |
| Emphysema, n (%) | 10 (55.6) | 17 (27.0) | 0.023* |
| Pleural abnormalities, n (%) | 15 (83.3) | 41 (65.1) | 0.139 |
| Mediastinal and hilar lymphadenopathy, n (%) | 11 (61.1) | 63 (100) | 0.000*** |
| Pulmonary artery widening, n (%) | 7 (38.9) | 4 (6.3) | 0.002** |
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.001.
GGO, ground‐glass opacity; HRCT, high‐resolution computed tomography.
Well‐defined rounded opacity and mediastinal lymphadenopathy were more frequently identified in patients with natural stone‐associated silicosis, and during a follow‐up period of 6–12 months, no change was found in the size, density or distribution of the opacities on HRCT in the 63 patients with natural stone‐associated silicosis (Fig. 1A,B). Two patients with artificial stone‐associated silicosis had extensive faint ground‐glass nodules, similar to hypersensitivity pneumonia (Fig. 1C). Severe radiological abnormalities in 13 patients with artificial stone‐associated silicosis were characterized by bilateral upper lobe fibrosis and volume loss with bilateral patchy consolidation, indistinct boundaries and ground‐glass attenuation, consistent with PMF (Fig. 1D). In addition, during the follow‐up of 6–12 months, HRCT showed rapid radiological progression in 13 patients (72.2%) with artificial stone‐associated silicosis (Fig. 2).
Figure 1.
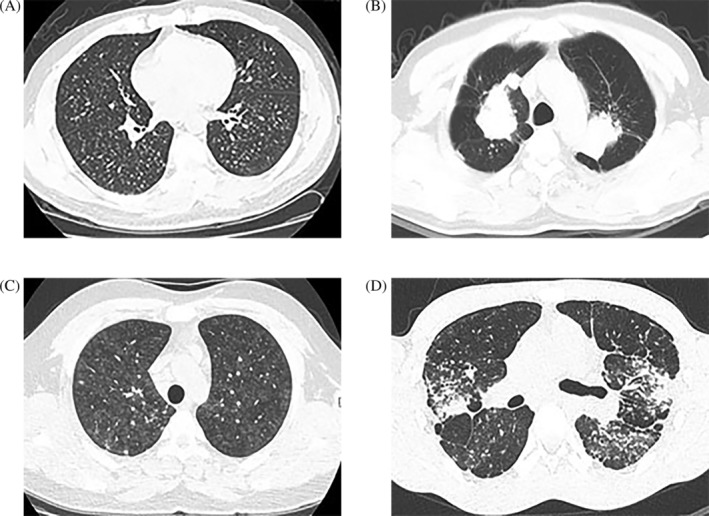
Chest high‐resolution computed tomography (HRCT) of four patients with differing findings. (A, B) Patients with natural stone‐associated silicosis. (C, D) Patients with artificial stone‐associated silicosis. (A) A 52‐year‐old man had been handling raw materials containing silica for 15 years. HRCT showed small, diffuse, well‐defined nodules in both lungs. (B) A 59‐year‐old man had been mining for 20 years. HRCT showed large well‐defined opacities with distinct borders surrounded by radiating lines. (C) A 22‐year‐old man had been cutting artificial stone 6 years. HRCT showed extensive centrilobular ground‐glass nodules, similar to those seen in hypersensitivity pneumonia. (D) A 25‐year‐old man had been cutting and polishing bathroom and kitchen countertops for 5 years. HRCT showed bilateral patchy consolidation, indistinct boundaries and ground‐glass attenuation, consistent with progressive massive fibrosis.
Figure 2.
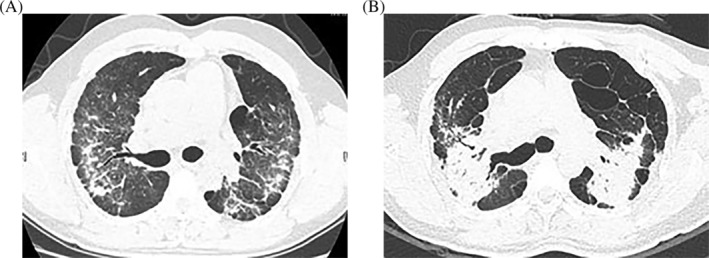
A 37‐year‐old man had been cutting, polishing and home installing artificial stone for 4 years. high‐resolution computed tomography (HRCT) showed rapid radiological progression. (A) HRCT image showed patchy, irregular opacities of varying density. (B) HRCT showed a reduction in the volume of the lungs and features characteristic of progressive massive fibrosis 7 months later.
Changes in pulmonary function
Pulmonary function was tested again in four patients with artificial stone‐associated silicosis, and rapid deterioration in lung function was found over the follow‐up period of 6–12 months, with a decline in pre‐bronchodilator forced vital capacity (FVC) of 587 (210–960) mL/year and forced expiratory volume in 1 s (FEV1) of 625 (360–860) mL/year (Table 3). Nine patients underwent pulmonary function tests at the time of the initial diagnosis but did not undergo them again 6 months later, when they experienced severe respiratory distress.
Table 3.
Deterioration of lung function in patients with artificial stone‐associated silicosis
| Patient | Respiratory function (most recent) | Pre‐bronchodilator FVC decline (mL/year) | Pre‐bronchodilator FEV1 decline (mL/year) | Follow‐up (months) |
|---|---|---|---|---|
| (1) | Restrictive defect (FVC: 76.7%), reduced gas transfer (DLCO: 68.3%) | 960 | 860 | 6 |
| (2) | Mixed obstructive/restrictive defect (FEV1: 56.6%, FVC: 68.5%), reduced gas transfer (DLCO: 35.1%) | 210 | 360 | 12 |
| (3) | Restrictive defect (FVC: 36.7%), reduced gas transfer (DLCO: 39.9%) | 670 | 780 | 9 |
| (4) | Normal spirometry, reduced gas transfer (DLCO: 70.8%) | 510 | 500 | 10 |
DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
During the follow‐up period, pulmonary function in 51 patients with natural stone‐associated silicosis declined to a pre‐bronchodilator FVC of 94.7 (−210 to 320) mL/year and FEV1 of 84.3 (−80 to 300) mL/year (Tables 4,S2 in Supplementary Information).
Table 4.
Deterioration of lung function of individuals with artificial stone‐associated vs. natural stone‐associated silicosis
| Parameters | Artificial stone‐associated silicosis | Natural stone‐associated silicosis | P‐value |
|---|---|---|---|
| No. of patients | 4 | 51 | |
| FVC decline (mL/year) | 587 (210 to 960) | 94.7 (−210 to 320) | 0.001*** |
| FEV1 decline (mL/year) | 625 (360 to 860) | 84.3 (−80 to 300) | 0.001*** |
P ≤ 0.001.
Data are presented as median (range).
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Particle analysis
Four artificial stone dust samples collected from one of the factories were analysed using X‐ray powder diffraction. All samples were found to have a high silica content of above 90%. The particles derived from the samples had a high proportion of small particles (diameter < 5 μm), and some were even smaller than 2 μm (Table S3 in Supplementary Information). No particle size data were available from the natural stone cases for comparison.
DISCUSSION
In the present study, we report an outbreak of accelerated silicosis caused by artificial stone dust, which is different from natural stone‐associated silicosis. All patients with artificial stone‐associated silicosis had performed cutting or polishing without adequate ventilation or respiratory protection, which inevitably leads to high‐level exposure to RCS.
In our cohort, the 18 patients diagnosed with artificial stone‐associated silicosis had a mean age of 36.1 ± 9.6 years, a median exposure time of 6.1 years and median duration of exposure to artificial stone‐associated dust prior to onset of symptoms of 6.4 years. This is significantly shorter than times reported for patients with chronic silicosis caused by exposure in other industries. Previous studies reported durations of occupational exposure to artificial stone‐derived silica of 4–10 years.4 The manifestations of artificial stone‐associated silicosis were nodular centrilobular GGO, indistinct boundaries, ground‐glass attenuation, a high prevalence of PMF and emphysema. These features differ from those of traditional silicosis nodules, which are usually well circumscribed, round and fairly uniform in size and density.22 In addition, some, but not all, patients with artificial stone‐associated silicosis had mediastinal lymphadenopathy. These HRCT imaging features suggest early‐stage silicosis. Rapid radiological progression occurred in 13 patients with artificial stone‐associated silicosis over a period of 6–12 months, while no change was found on HRCT in the size, density or distribution of the opacities in 63 patients with natural stone‐associated silicosis.
Respiratory function tests in patients with artificial stone‐associated silicosis primarily demonstrated restrictive defect patterns and reduced gas transfer, while the respiratory function of patients with natural stone‐associated silicosis mainly demonstrated obstructive ventilatory dysfunction. Four patients with artificial stone‐associated silicosis experienced rapid deterioration in lung function over the following 6–12 months, with declines in FVC and FEV1. In patients with natural stone‐associated silicosis, deterioration in FVC and FEV1 was slower. In addition, the composite physiological index (CPI)23 was significantly higher in patients with artificial stone‐associated silicosis than in those with natural stone‐associated silicosis. Fourteen of the 18 patients with artificial stone‐associated silicosis had complicated silicosis and 7 required lung transplantation. Our data suggest that exposure to artificial stone dust is more harmful to the lungs than exposure to natural stone dust.
A recent study reported that dry cutting artificial stone using a hand‐operated circular saw generated a high concentration of respirable silica dust (44 mg/m3 over a 30‐min sampling period); cutting artificial stone with a wet blade was reported to generate 4.9 mg/m3 concentration of respirable silica dust; the concentration decreased to 0.6 mg/m3 when a wet blade was used in combination with local exhaust ventilation.14 Particles derived from artificial stone dust have been reported to contain a very high proportion (94.0 ± 5.6%) of small particles (<5 μm) and 4.3 ± 3.7% of particles with a diameter between 5 and 10 μm.12 Intratracheal instillation of ultrafine particles can lead to significantly more severe pulmonary inflammatory responses than instillation of larger particles.24, 25, 26 Accumulation of small particles has been negatively correlated with the single‐breath diffusing capacity of the lung for diffusing capacity of the lung for carbon monoxide (DLCO), FEV1 and FVC.12 In our study, particles derived from the samples we collected contained a very high proportion of small particles, accounting for the severe deterioration in lung function and poor outcomes of the patients with artificial stone‐associated silicosis.
Various publications have reported clusters of silicosis cases caused by exposure to artificial stone dust.27 In 2016, China reported 10 072 newly diagnosed patients with silicosis, among which 43.78% were concentrated in Sichuan, Hunan and Chongqing provinces and Beijing.28 However, to our knowledge, there have been no reports of artificial stone‐associated silicosis in China until now. The artificial stone industry in China began in the early 2000s, and is mainly localized in the Pearl River Delta and Yangtze River Delta. The cities of Zhongshan, Heshan and Foshan are the earliest and the largest production areas. The 18 patients we treated for artificial stone‐associated silicosis were mainly from these areas. There are currently insufficient epidemiological data on artificial stone‐associated silicosis in China; further investigation is required.
Our findings highlight the severity of silicosis associated with artificial stone. More cases are likely to occur unless effective preventive measures are taken and safety practices are enforced. Occupational dust control is one of the key preventative measures against silicosis. The US National Institute of Occupational Safety and Health (NIOSH) recommends limiting exposures to RCS is 0.05 mg/m3.29 Currently, the RCS exposure standard in China is 0.2 mg/m3 as an 8‐h time‐weighted average.30 We recommend strict enforcement in artificial stone production. It is important to frequently monitor RCS exposures to detect overexposures in a timely manner. Collective protective measures should be applied in artificial stone workshops, including both wet cutting and local exhaust ventilation. Workers should be made aware of the risk associated with fabricating and cutting artificial stone. Personal protective equipment such as a protective mask, goggles, gloves, special footwear and a helmet should be used while working. Occupational health surveillance should be conducted regularly to detect silicosis early and intervene to prevent progression by halting further exposure.29
Several limitations of this study should be noted. First, our patients were recruited from a single medical centre and the incidence of artificial stone‐associated silicosis in other areas of China is as yet unknown. Second, the patients with artificial stone‐associated silicosis had been referred from various provinces to our medical centre because we specialize in occupational medicine; thus, there may have been a selection bias. Third, because the study period was recent, the follow‐up period was relatively short. Finally, monitoring of workplace exposure was not possible.
In conclusion, artificial stone‐associated silicosis is characterized by a shorter latency, rapid radiological progression and accelerated loss of lung function. More individuals who have been exposed to artificial stone dust are likely to be diagnosed as having accelerated silicosis. Urgent action is required to increase awareness of the risk of silicosis in the artificial stone fabrication industry, and measures to control dust levels and monitor and protect workers in this industry are needed.
Author contributions
Conceptualization: Q.Y. Data curation: N.W. Formal analysis: N.W. Funding acquisition: Q.Y. Investigation: N.W., C.X., S.Y. Supervision: Q.Y. Writing—original draft: N.W., C.X., S.Y. Writing—review and editing: Q.Y.
Abbreviations
- DLCO
diffusing capacity of the lung for carbon monoxide
- FEV1
forced expiratory volume in 1 s
- FVC
forced vital capacity
- GGO
ground‐glass opacity
- PMF
progressive massive fibrosis
- RCS
respirable crystalline silica
Supporting information
Table S1 An occupational history questionnaire.
Table S2 Deterioration of lung function in patients with natural stone‐associated silicosis.
Table S3 The size of particulate matter in the four artificial stone samples.
Visual Abstract Artificial stone‐associated silicosis in China: A prospective comparison with natural stone‐associated silicosis.
Acknowledgements
We thank all doctors and nurses from the Department of Occupational Diseases and Clinical Toxicology of Beijing Chao‐Yang Hospital for their dedicated efforts in the management of the patients. The work was supported by National Natural Science Foundation of China (81970061) and Application of Clinical Characteristics in Capital (Z181100001718118).
Wu, N , Xue, C , Yu, S , Ye, Q . Artificial stone‐associated silicosis in China: A prospective comparison with natural stone‐associated silicosis. Respirology. 2020;25:518–524. 10.1111/resp.13744
(Associate Editor: Chi Chiu Leung; Senior Editor: Chris Grainge)
REFERENCES
- 1. Scarisbrick D. Silicosis and coal workers' pneumoconiosis. Practitioner 2002; 246: 117–9. [PubMed] [Google Scholar]
- 2. Rimal B, Greenberg AK, Rom WN. Basic pathogenetic mechanisms in silicosis: current understanding. Curr. Opin. Pulm. Med. 2005; 11: 169–73. [DOI] [PubMed] [Google Scholar]
- 3. Leung CC, Yu IT, Chen W. Silicosis. Lancet 2012; 379: 2008–18. [DOI] [PubMed] [Google Scholar]
- 4. Hoy RF, Baird T, Hammerschlag G, Hart D, Johnson AR, King P, Putt M, Yateset DH. Artificial stone‐associated silicosis: a rapidly emerging occupational lung disease. Occup. Environ. Med. 2018; 75: 3–5. [DOI] [PubMed] [Google Scholar]
- 5. Shtraichman O, Blanc PD, Ollech JE, Fridel L, Fuks L, Fireman E, Kramer MR. Outbreak of autoimmune disease in silicosis linked to artificial stone. Occup. Med. 2015; 65: 444–50. [DOI] [PubMed] [Google Scholar]
- 6. Pérez‐Alonso A, Córdoba‐Doña JA, Millares‐Lorenzo JL, Figueroa‐Murillo E, García‐Vadillo C, Romero‐Morill J. Outbreak of silicosis in Spanish quartz conglomerate workers. Int. J. Occup. Environ. Health 2014; 20: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pascual S, Urrutia I, Ballaz A, Arrizubieta I, Altube L, Salinas C. Prevalence of silicosis in a marble factory after exposure to quartz conglomerates. Arch. Bronconeumol. 2011; 47: 50–1. [DOI] [PubMed] [Google Scholar]
- 8. Paolucci V, Romeo R, Sisinni AG, Bartoli D, Mazzei MA, Sartorelli P. Silicosis in workers exposed to artificial quartz conglomerates: does it differ from chronic simple silicosis? Arch. Bronconeumol. 2015; 51: e57–60. [DOI] [PubMed] [Google Scholar]
- 9. Pascual DPYF, Garcia SR, Garcia RM, Barroso ME, Flores RE, Carbonell JG. Silicosis: a former occupational disease with new occupational exposure scenarios. Rev. Clin. Esp. 2019; 219: 26–9. [DOI] [PubMed] [Google Scholar]
- 10. Kramer MR, Blanc PD, Fireman E, Amital A, Guber A, Rhahman NA, Shitrit D. Artificial stone silicosis. Chest 2012; 142: 419–24. [DOI] [PubMed] [Google Scholar]
- 11. Hering KG, Jacobsen M, Bosch‐Galetke E, Elliehausen HJ, Hieckel HG, Hofmann‐Preiss K, Jacques W, Jeremie U, Kotschy‐Lang N, Kraus T et al Further development of the International Pneumoconiosis Classification – from ILO 1980 to ILO 2000 and to ILO 2000/German Federal Republic version. Pneumologie 2003; 57: 576–84. [DOI] [PubMed] [Google Scholar]
- 12. Ophir N, Shai AB, Alkalay Y, Israeli S, Korenstein R, Kramer MR, Fireman E. Artificial stone dust‐induced functional and inflammatory abnormalities in exposed workers monitored quantitatively by biometrics. ERJ Open Res. 2016; 2: 86–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martinez C, Prieto A, Garcia L, Quero A, Gonzalez S, Casan P. Silicosis: a disease with an active present. Arch. Bronconeumol. 2010; 46: 97–100. [DOI] [PubMed] [Google Scholar]
- 14. Cooper JH, Johnson DL, Phillips ML. Respirable silica dust suppression during artificial stone countertop cutting. Ann. Occup. Hyg. 2015; 59: 122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suganuma N, Kusaka Y, Hering KG, Vehmas T, Kraus T, Arakawa H, Parker JE, Kivisaari L, Letourneux M, Gevenois PA et al Reliability of the proposed International Classification of High‐Resolution Computed Tomography for Occupational and Environmental Respiratory Diseases. J. Occup. Health 2009; 51: 210–22. [DOI] [PubMed] [Google Scholar]
- 16. ILO (International Labour Office) . International Classification of Radiographs of Pneumoconiosis. 2011. [Accessed 2 Sep 2016.] Available from URL: http://www.ilo.org/wcmsp5/groups/public/---ed_protect/---protrav/---safework/documents/publication/wcms_168260.pdf
- 17. Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP et al Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005; 26: 511–22. [DOI] [PubMed] [Google Scholar]
- 18. Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, Lederer DJ, Mulligan MJ, Patterson GA, Singer LG et al A consensus document for the selection of lung transplant candidates: 2014—An update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2015; 34: 1–15. [DOI] [PubMed] [Google Scholar]
- 19. Blackley DJ, Halldin CN, Cummings KJ, Laney AS. Lung transplantation is increasingly common among patients with coal workers' pneumoconiosis. Am. J. Ind. Med. 2016; 59: 175–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ooi GC, Tsang KW, Cheung TF, Khong PL, Ho IW, Ip MS, Tam CM, Ngan H, Lam WK, Chan FL et al Silicosis in 76 men: qualitative and quantitative CT evaluation – clinical‐radiologic correlation study. Radiology 2003; 228: 816–25. [DOI] [PubMed] [Google Scholar]
- 21. Takahashi M, Nitta N, Kishimoto T, Ohtsuka Y, Honda S, Ashizawa K. Computed tomography findings of arc‐welders' pneumoconiosis: comparison with silicosis. Eur. J. Radiol. 2018; 107: 98–104. [DOI] [PubMed] [Google Scholar]
- 22. Kim JS, Lynch DA. Imaging of nonmalignant occupational lung disease. J. Thorac. Imaging 2002; 17: 238–60. [DOI] [PubMed] [Google Scholar]
- 23. Wells AU, Desai SR, Rubens MB, Goh NS, Cramer D, Nicholson AG, Colby TV, du Bois RM, Hansell DM. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am. J. Respir. Crit. Care Med. 2003; 167: 962–9. [DOI] [PubMed] [Google Scholar]
- 24. López‐Lilao A, Escrig A, Orts MJ, Mallol G, Monfort E. Quartz dustiness: a key factor in controlling exposure to crystalline silica in the workplace. J. Occup. Environ. Hyg. 2016; 13: 817–28. [DOI] [PubMed] [Google Scholar]
- 25. Oberdorster G, Ferin J, Lehnert BE. Correlation between particle size, in vivo particle persistence, and lung injury. Environ. Health Perspect. 1994; 102(Suppl. 5): 173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang J, Fan Y. Lung injury induced by TiO2 nanoparticles depends on their structural features: size, shape, crystal phases, and surface coating. Int. J. Mol. Sci. 2014; 15: 22258–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leso V, Fontana L, Romano R, Gervetti P, Iavicoli I. Artificial stone associated silicosis: a systematic review. Int. J. Environ. Res. Public Health 2019; 16: 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. National Health Commission of the People's Republic of China . Bulletin on the work of occupational disease prevention and treatment in 2016. 2017. [Accessed 28 Dec 2017.] Available from URL: http://www.nhc.gov.cn/zyjks/zcwj2/201712/90667a5571e44ccca42e317b68f50c40.shtml.
- 29. Laney AS, Weissman DN. Respiratory diseases caused by coal mine dust. J. Occup. Environ. Med. 2014; 56(Suppl. 10): S18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Health Commission of the People's Republic of China . Occupational exposure limits for hazardous agents in the workplace. Part 1: Chemical hazardous agents: GBZ2.1‐2007. Beijing: People's Medical Publishing House, 2007.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 An occupational history questionnaire.
Table S2 Deterioration of lung function in patients with natural stone‐associated silicosis.
Table S3 The size of particulate matter in the four artificial stone samples.
Visual Abstract Artificial stone‐associated silicosis in China: A prospective comparison with natural stone‐associated silicosis.


